Full-Scale Demonstration of Nitrogen Removal from Mature Landfill Leachate Using a Two-Stage Partial Nitritation and Anammox Process
Abstract
:1. Introduction
2. Method and Materials
2.1. Full-Scale System Setup, Operation, and Monitoring
2.2. Chemical Analysis
2.3. Microbial Analysis
2.4. Statistical Analysis
3. Results and Discussion
3.1. Long-Term Operation of PN Process
3.2. Long-Term Operation of Anammox Process
3.3. Microbial Community Composition Assessment
3.4. Implications and Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schindler, D.W. Eutrophication and Recovery in Experimental Lakes: Implications for Lake Management. Science 1974, 184, 897–899. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhu, Y.; Zhu, J.; Li, C.; Chen, G. A comprehensive review on wastewater nitrogen removal and its recovery processes. Int. J. Environ. Res. Public Health 2023, 20, 3429. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, S.; Modin, O.; Mijakovic, I. Technologies for biological removal and recovery of nitrogen from wastewater. Biotechnol. Adv. 2020, 43, 107570. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, M.; Duan, H.; Yuan, Z.; Hu, S. A 20-year journey of partial nitritation and anammox (PN/A): From sidestream toward mainstream. Environ. Sci. Technol. 2022, 56, 7522–7531. [Google Scholar] [CrossRef]
- Hu, Z.; Hu, S.; Ye, L.; Duan, H.; Wu, Z.; Hong, P.-Y.; Yuan, Z.; Zheng, M. Novel Use of a Ferric Salt to Enhance Mainstream Nitrogen Removal from Anaerobically Pretreated Wastewater. Environ. Sci. Technol. 2023, 57, 6712–6722. [Google Scholar] [CrossRef]
- Strous, M.; Heijnen, J.J.; Kuenen, J.G.; Jetten, M.S.M. The sequencing batch reactor as a powerful tool for the study of slowly growing anaerobic ammonium-oxidizing microorganisms. Appl. Microbiol. Biotechnol. 1998, 50, 589–596. [Google Scholar] [CrossRef]
- Hu, Z.; Liu, T.; Wang, Z.; Meng, J.; Zheng, M. Toward energy neutrality: Novel wastewater treatment incorporating acidophilic ammonia oxidation. Environ. Sci. Technol. 2023, 57, 4522–4532. [Google Scholar] [CrossRef] [PubMed]
- Blackburne, R.; Yuan, Z.; Keller, J. Demonstration of nitrogen removal via nitrite in a sequencing batch reactor treating domestic wastewater. Water Res. 2008, 42, 2166–2176. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dai, X.; Cao, D.; Wang, S.; Hu, X.; Liu, W.; Yang, D. Performance and microbial ecology of a nitritation sequencing batch reactor treating high-strength ammonia wastewater. Sci. Rep. 2016, 6, 35693. [Google Scholar] [CrossRef]
- Anthonisen, A.C.; Loehr, R.C.; Prakasam, T.B.S.; Srinath, E.G. Inhibition of nitrification by ammonia and nitrous acid. J. Water Pollut. Control. Fed. 1976, 48, 835–852. [Google Scholar]
- Bae, W.; Baek, S.; Chung, J.; Lee, Y. Optimal operational factors for nitrite accumulation in batch reactors. Biodegradation 2001, 12, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zeng, Y.; Cheng, Y.; He, D.; Pan, X. Recent advances in municipal landfill leachate: A review focusing on its characteristics, treatment, and toxicity assessment. Sci. Total Environ. 2020, 703, 135468. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Liang, H.; Wang, Z.; Wu, D.; Zhou, J.; Peng, Y. Attaining superior nitrogen removal from integrated mature landfill leachate and kitchen waste digestion liquid via a two-stage partial nitrification/anammox (PN/A) process. Chem. Eng. J. 2024, 480, 148352. [Google Scholar] [CrossRef]
- Jiang, H.; Li, X.; Zhang, F.; Wang, Z.; Ren, S.; Qiu, J.; Wang, S.; Peng, Y. Advanced nitrogen removal from mature landfill leachate based on novel step-draining partial nitrification-denitrification and Anammox process: Significance of low volume exchange ratio. Bioresour. Technol. Biomass Bioenergy Biowastes Convers. Technol. Biotransform. Prod. Technol. 2022, 364, 128025. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments—A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Peng, Y.; Wang, Z.; Jiang, H.; Ren, S.; Qiu, J.; Zhang, L. An innovative process for mature landfill leachate and waste activated sludge simultaneous treatment based on partial nitrification, in situ fermentation, and anammox (PNFA). Environ. Sci. Technol. 2021, 56, 1310–1320. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Wang, Z.; Jiang, H.; Li, X.; Zhang, Q.; Peng, Y. Efficient nitrogen removal from mature landfill leachate in a step feed continuous plug-flow system based on one-stage anammox process. Bioresour. Technol. 2022, 347, 126676. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yang, P.; Wang, Z.; Ren, S.; Qiu, J.; Liang, H.; Peng, Y.; Li, X.; Zhang, Q. Efficient and advanced nitrogen removal from mature landfill leachate via combining nitritation and denitritation with Anammox in a single sequencing batch biofilm reactor. Bioresour. Technol. 2021, 333, 125138. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, F.; Xu, D.; Xie, B. Advances in biological nitrogen removal of landfill leachate. Sustainability 2021, 13, 6236. [Google Scholar] [CrossRef]
- Li, X.; Tao, R.-j.; Tian, M.-j.; Yuan, Y.; Huang, Y.; Li, B.-l. Recovery and dormancy of nitrogen removal characteristics in the pilot-scale denitrification-partial nitrification-Anammox process for landfill leachate treatment. J. Environ. Manag. 2021, 300, 113711. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, Y.; Yin, J.; Luo, A.; Tian, Y.; Liu, Y.; Xu, J.; Peng, Y. Achieving advanced nitrogen removal from mature landfill leachate in continuous flow system involving partial nitrification-anammox and denitrification. Bioresour. Technol. 2024, 399, 130553. [Google Scholar] [CrossRef]
- Weifeng, D.; Litao, W.; Lang, C.; Wenbo, Y.; Dawen, G. Nitrogen Removal from Mature Landfill Leachate via Anammox Based Processes: A Review. Sustainability 2022, 14, 995. [Google Scholar] [CrossRef]
- Wenbo, Y.; Lang, C.; Hong, L.; Ao, X.; Yuqi, L.; Mohammad, N.; Huan, W.; Jiachen, H.; Dawen, G. Efficient nitrogen removal from mature landfill leachate by single-stage partial-nitritation anammox using expanded granular sludge bed. J. Environ. Manag. 2023, 344, 118460. [Google Scholar]
- Young, J.C.; Clesceri, L.S.; Kamhawy, S.M. Changes in the biochemical oxygen demand procedure in the 21st edition of Standard Methods for the Examination of Water and Wastewater. Water Environ. Res. A Res. Publ. Water Environ. Fed. 2005, 77, 404–410. [Google Scholar]
- Fan, Z.; Hong, Y.; Jiawei, W.; Ziqi, L.; Qingkun, G. Effect of free ammonia inhibition on NOB activity in high nitrifying performance of sludge. RSC Adv. 2018, 8, 31987–31995. [Google Scholar]
- Jaroszynski, L.W.; Cicek, N.; Sparling, R.; Oleszkiewicz, J.A. Impact of free ammonia on anammox rates (anoxic ammonium oxidation) in a moving bed biofilm reactor. Chemosphere 2012, 88, 188–195. [Google Scholar] [CrossRef]
- Teske, A.; Alm, E.; Regan, J.M.; Toze, S.; Rittmann, B.E.; Stahl, D.A. Evolutionary relationships among ammonia- and nitrite-oxidizing bacteria. J. Bacteriol. 1994, 176, 6623–6630. [Google Scholar] [CrossRef]
- Ibrahim, M.; Yusof, N.; Mohd Yusoff, M.Z.; Hassan, M.A. Enrichment of anaerobic ammonium oxidation (anammox) bacteria for short start-up of the anammox process: A review. Desalination Water Treat. 2016, 57, 13958–13978. [Google Scholar] [CrossRef]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; Loosdrecht, M.C.M.v. Full-scale partial nitritation/anammox experiences—An application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Li, J.; Liu, J.; Zhang, Q.; Li, X.; Zhang, L. Rapid initiation and stable maintenance of municipal wastewater nitritation during the continuous flow anaerobic/oxic process with an ultra-low sludge retention time. Water Res. 2021, 197, 117091. [Google Scholar] [CrossRef]
- Van Dongen, U.; Jetten, M.S.; van Loosdrecht, M. The SHARON®-Anammox® process for treatment of ammonium rich wastewater. Water Sci. Technol. 2001, 44, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Rong, C.; Luo, Z.; Wang, T.; Qin, Y.; Wu, J.; Guo, Y.; Hu, Y.; Kong, Z.; Hanaoka, T.; Sakemi, S. Biomass retention and microbial segregation to offset the impacts of seasonal temperatures for a pilot-scale integrated fixed-film activated sludge partial nitritation-anammox (IFAS-PN/A) treating anaerobically pretreated municipal wastewater. Water Res. 2022, 225, 119194. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.M.; Agrawal, S.; Schwartz, T.; Horn, H.; Lackner, S. Comparing different reactor configurations for Partial Nitritation/Anammox at low temperatures. Water Res. 2015, 81, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Kleerebezem, R.; Van Loosdrecht, M. Effect of temperature change on anammox activity. Biotechnol. Bioeng. 2015, 112, 98–103. [Google Scholar] [CrossRef]
- Winkler, M.K.; Kleerebezem, R.; Kuenen, J.G.; Yang, J.; van Loosdrecht, M.C. Segregation of biomass in cyclic anaerobic/aerobic granular sludge allows the enrichment of anaerobic ammonium oxidizing bacteria at low temperatures. Environ. Sci. Technol. 2011, 45, 7330–7337. [Google Scholar] [CrossRef]
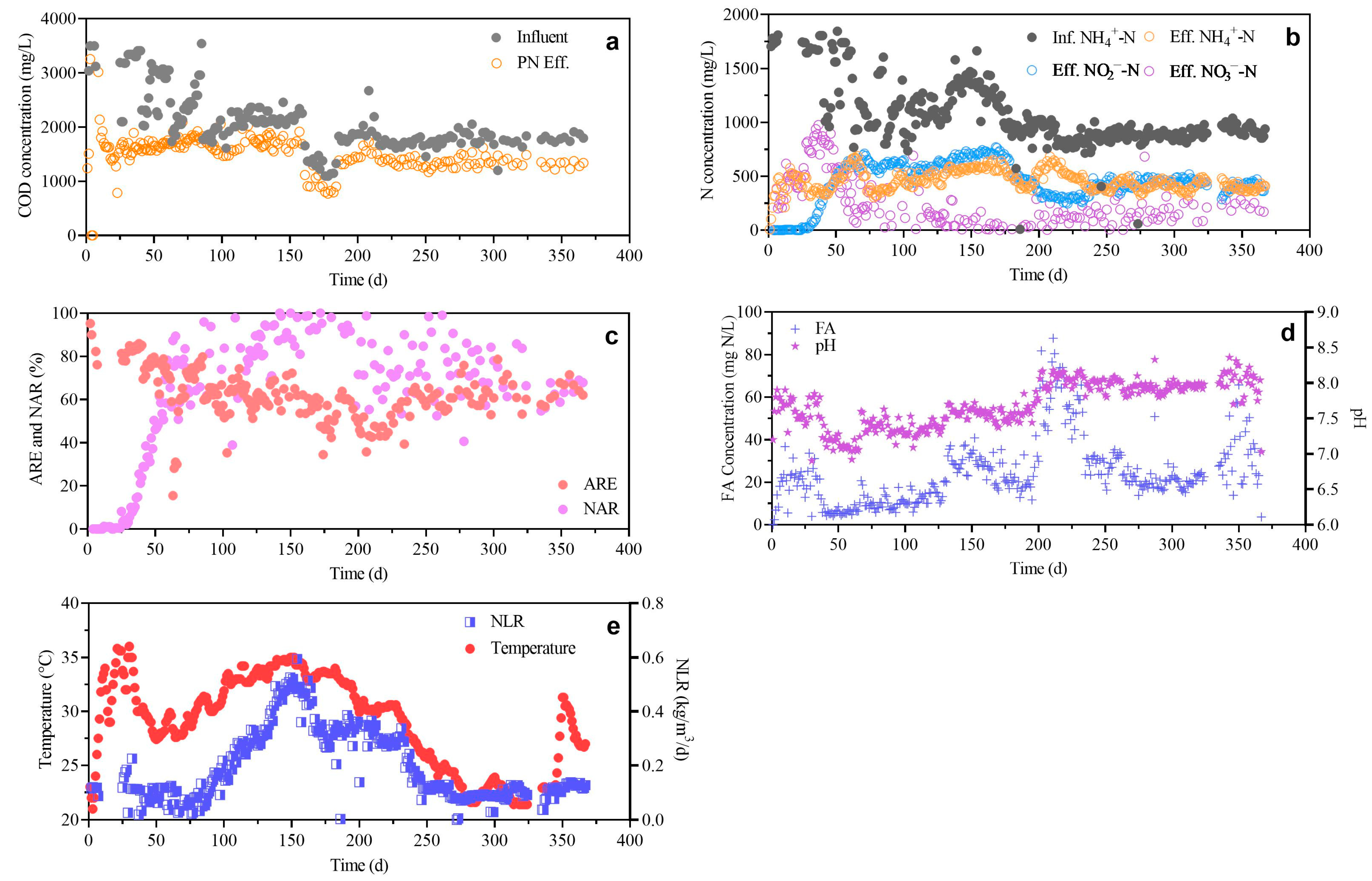
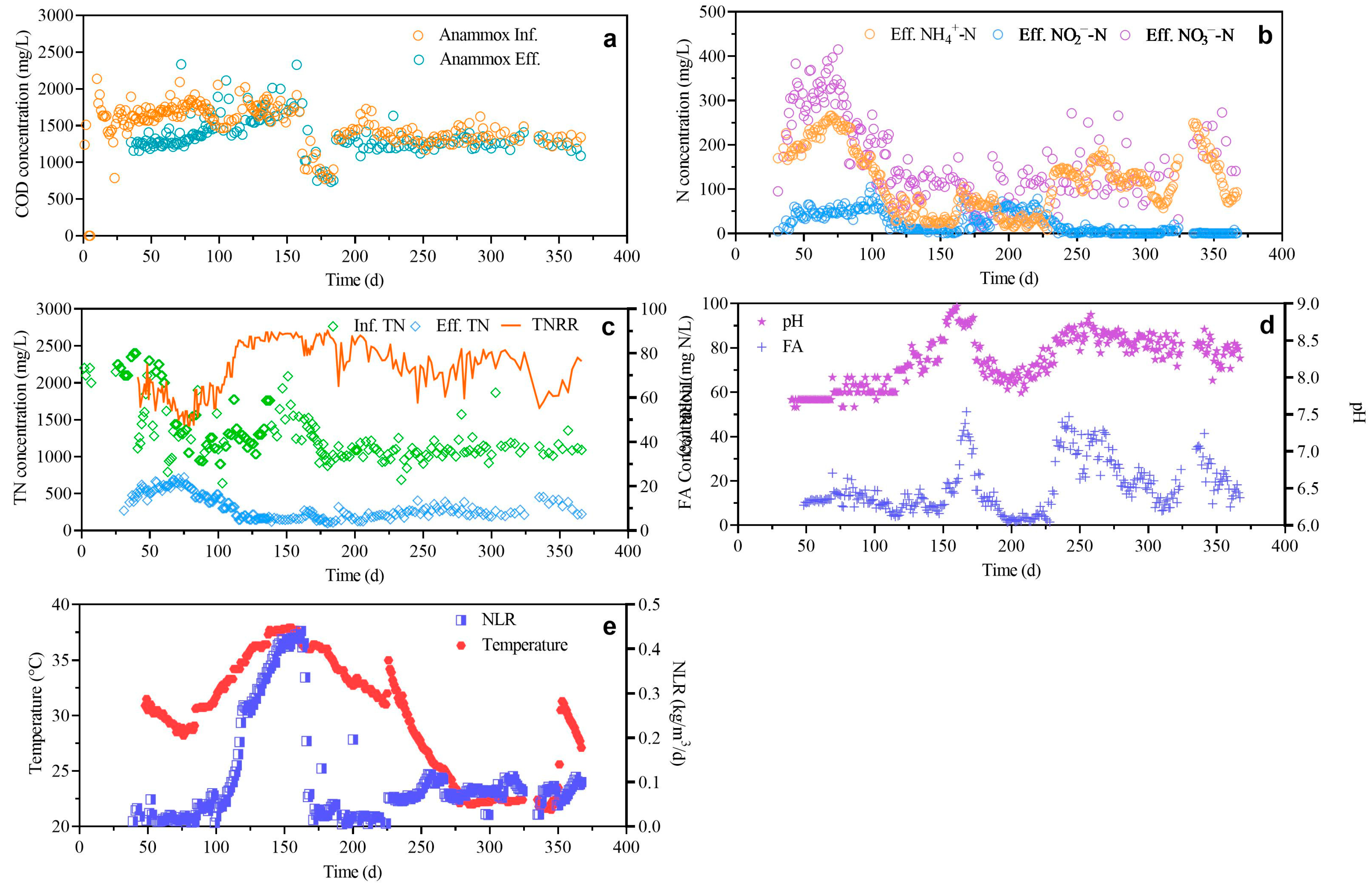
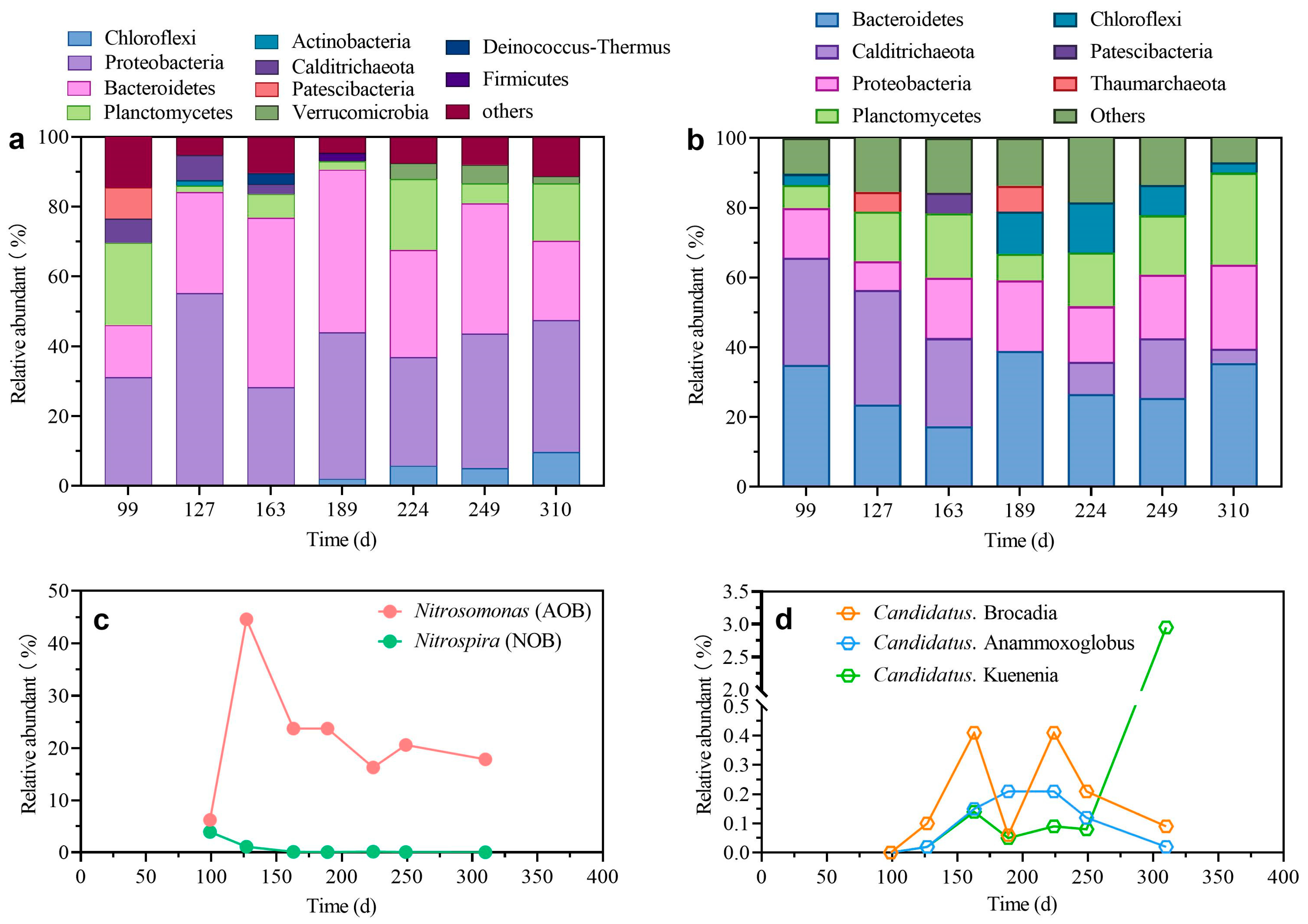
| Parameter | Unit | Value |
|---|---|---|
| Ammonia nitrogen | mg N/L | 1042.7 ± 267.1 |
| Total nitrogen | mg N/L | 1311.0 ± 375.5 |
| COD | mg O2/L | 2082.7 ± 506.0 |
| pH | 8.5 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, R.; Lu, D.; Zuo, Z.; Zhang, R.; Lu, X.; Zhu, C.; Hu, Z. Full-Scale Demonstration of Nitrogen Removal from Mature Landfill Leachate Using a Two-Stage Partial Nitritation and Anammox Process. Processes 2024, 12, 1307. https://doi.org/10.3390/pr12071307
Du R, Lu D, Zuo Z, Zhang R, Lu X, Zhu C, Hu Z. Full-Scale Demonstration of Nitrogen Removal from Mature Landfill Leachate Using a Two-Stage Partial Nitritation and Anammox Process. Processes. 2024; 12(7):1307. https://doi.org/10.3390/pr12071307
Chicago/Turabian StyleDu, Rui, Dandan Lu, Zhiqiang Zuo, Renfu Zhang, Xi Lu, Chunshen Zhu, and Zhetai Hu. 2024. "Full-Scale Demonstration of Nitrogen Removal from Mature Landfill Leachate Using a Two-Stage Partial Nitritation and Anammox Process" Processes 12, no. 7: 1307. https://doi.org/10.3390/pr12071307




