Abstract
Catalytic studies hydrogen production via steam reforming of ethanol (SRE) are essential for process optimization. Likewise, selecting the ideal support for the active phase can be critical to achieve high conversion rates during the catalytic steam reforming process. In this work, copper-based catalysts were synthesized using two different supports, NaY zeolite and Nb2O5/Al2O3 mixed oxides. The materials were prepared using wet impregnation and characterized for their physicochemical properties using different analytical techniques. Differences in the catalyst morphologies were readily attributed to the characteristics of the support. The Cu/NaY catalyst exhibited a higher specific surface area (210.40 m2 g−1) compared to the Cu/Nb2O5/Al2O3 catalyst (26.00 m2 g−1), resulting in a homogeneous metal dispersion over the support surface. The obtained results showed that, at 300 °C, both the Cu/Nb2O5/Al2O3 and Cu/NaY catalysts produced approximately 50% hydrogen and 40% acetaldehyde, but with significant differences in conversion (6% and 56%, respectively). At 450 °C, a greater product distribution and a 10% higher conversion were observed when the catalyst was supported on NaY compared to Nb2O5/Al2O3. Hence, the performance of copper-based catalysts was influenced significantly by the textural properties of the support.
1. Introduction
The increasing global demand for energy, coupled with the socioenvironmental impacts of an energy matrix that is still heavily reliant on traditional fossil fuels such as coal, crude oil, and natural gas [1,2], requires the development of sustainable production processes to support the transition to a new and more diversified energy matrix. Among the sustainable alternatives for this energy transition, hydrogen (H2) stands out as one of the most prominent.
H2 can be obtained through different pathways such as electrolysis [3,4], biological reactions [5], biomass gasification [6], steam reforming [7,8], aqueous phase reforming [9], and partial oxidation of hydrocarbons and alcohols [10]. Among these possibilities, the use of ethanol as feedstock for H2 production in fuel cells has considerable advantages. These include easier storage, handling, and safe transportation due to its low toxicity and volatility. Additionally, ethanol is a renewable feedstock when obtained through biomass fermentation, is rich in H2, and has a nearly closed carbon cycle that helps in the abatement of greenhouse gas emissions [11,12]. Thus, the steam reforming of ethanol (SRE) emerges as an attractive solution for H2 production due to its high H2 yield and thermodynamic feasibility [13].
SRE for H2 production is a catalytic process. Therefore, H2 yield depends on the properties of the catalyst to be employed [14]. This includes the catalytic support for the active phase [15] and the method used for catalyst preparation [16]. In general, the catalyst design is crucial for a successful SRE process. Different catalytic systems have been investigated for SRE using noble and non-noble metal-based catalysts [17]. Among them, copper-based catalysts [18,19,20] have the advantage of being cost-effective and widely available compared to other metals. Copper as an active phase is widely used in commercial catalysts for methanol reforming. Additionally, the presence of copper active sites promotes ethanol steam reforming to produce H2 and CO or its dehydrogenation to acetaldehyde followed by decarbonylation, producing CH4 and CO [21,22,23]. Hence, copper-based catalysts have potential for SRE applications.
The choice of support for the active phase is extremely important for SRE because it plays a significant role in H2 selectivity and catalyst stability [24,25], as already demonstrated in previous studies [26,27]. In general, efficient SRE supports must have favorable textural properties and moderate acidity, in addition to being relatively cheap, readily available, and easily accessible. In this study, two supports were selected and evaluated for their effect on H2 production by SRE using copper as the catalytic active phase.
The first selected support was NaY, a commercially available zeolite that is known for its high heat resistance, unique ordered three-dimensional porous structure, and larger pores compared to the dimensions of the ethanol molecule, as well as low production costs [28,29]. For instance, NaY can be synthesized from alternative, abundant, and inexpensive materials such as rice husks [30,31] and wheat straw [32] ashes. Several studies have already demonstrated the application of NaY as a catalyst support for SRE [33,34,35]. The other selected support was a Nb2O5/Al2O3 mixed oxide. Alumina is widely used as a support in heterogeneous catalysis [36,37,38,39,40] due to its large surface area, good stability, and wide commercial availability [41]. Nb2O5 is also a notable material in the field of catalysis, known for its non-toxic nature, suitable acid properties [42,43], excellent chemical stability, high thermodynamic stability, low cost, and high commercial availability [44,45]. The combination of Nb2O5 with alumina is favorable because, being an n-type semiconductor, Nb2O5 can interact with copper in catalytic active reaction sites [46,47]. Additionally, Nb2O5 is structurally similar to commercial catalysts for methanol reforming (Cu/ZnO/Al2O3). Since ZnO is also an n-type semiconductor oxide, Nb2O5 may have similar catalytic properties.
This study aimed at evaluating the effect of NaY and Nb2O5/Al2O3 as the catalyst support for SRE. H2 production was carried out using copper as the active phase under the same reaction conditions. To the best of our knowledge, these supports have never been compared in SRE reactions with copper used as the active phase. Copper was anchored on the support surface by wet impregnation. Then, the resulting catalytic systems were characterized using various analytical techniques and subjected to SRE using an experimental reaction module.
2. Materials and Methods
2.1. Material
Materials were synthesized using copper nitrate (Cu(NO3)2·3H2O, 98%) from Sigma-Aldrich, commercial alumina (Al2O3, 90%) from Merck, NaY zeolite from Sigma-Aldrich, and niobic acid (HY-340) from the Brazilian Metallurgy and Mining Company (CBMM). HY-340 was heat-treated to obtain niobium pentoxide (Nb2O5).
2.2. Methods
2.2.1. Catalyst Preparation
The catalysts were prepared using a simple wet impregnation methodology under solvent excess [48]. For the Cu/NaY catalyst, 57.25 g Cu(NO3)2·3H2O were dissolved in water and mixed with 14.94 g of the NaY support. For the Cu/Nb2O5/Al2O3 catalyst, 57.25 g Cu(NO3)2·3H2O were dissolved in water and mixed with 9.27 g Nb2O5 and 5.67 g Al2O3. The mixtures were placed in a rotary evaporator and evaporated for 2 h at 343 K for the complete elimination of water. After this, the materials were placed in an oven at 353 K for about 10 h. Then, the dried materials were crushed and placed in a muffle furnace in ambient atmosphere. The temperature was raised to 773.15 K using a 10 K min−1 heating rate, and the catalysts remained at this plateau for 5 h. At the end of the process, two catalysts, named Cu/NaY and Cu/ Nb2O5/Al2O3, were obtained.
2.2.2. Catalyst Characterization
Scanning Electron Microscopy (SEM) was performed using a Zeiss EVO MA15 microscope coupled with an X-Max 20 mm2 Energy Dispersive Spectrometer (EDS). X-ray Diffraction (XRD) analyses were conducted on a Shimadzu XDR-7000 diffractometer using CuKα radiation. Measurements were taken at 40 kV and 30 mA using a Cu tube with a wavelength of 1.54 nm, with a scan rate of 2° min−1 and an interval of 5° ≤ 2θ ≤ 80°. The FWHM of the XRD peaks was used to estimate the average particle size using the Scherrer equation (Equation (1)).
The textural properties of the catalysts were determined by N2 adsorption/desorption at 77 K using a NOVA-4000-Quantachrome adsorption analyzer. Infrared spectroscopy analyses (FTIR) were performed using a Varian 640-IR spectrometer with potassium bromide (KBr) as the dispersing agent in the region from 4000 to 400 cm−1. Temperature-programmed desorption of NH3 (TPD-NH3) and temperature-programmed reduction (TPR) were carried out using a Quantachrome Chembet-3000 multi-use unit coupled with a ThermoStar-GSD 301 mass spectrometer. In both analyses, 0.1 g sample was placed in a “U”-shaped quartz reactor, which was first subjected to a 20 cm³ min−1 N2 flow at 300 °C for 1 h to remove humidity and possibly adsorbed materials. For TPD-NH3 analysis, the samples were reduced with 1.75% H2 diluted in N2 for 1 h using a heating rate of 10 °C min−1 from room temperature to 500 °C and remaining at this temperature for another 1 h. NH3 adsorption was performed at 100 °C for 30 min with a flow rate of 15 cm³ min−1 of 5% NH3 diluted in N2. Subsequently, the system was purged for 2 h with a flow rate of 20 cm³ min−1 N2. Finally, the sample was heated to 700 °C at a heating rate of 10 °C min−1 under N2 flow for NH3 desorption. TPR was performed with a reducing gas feed containing 1.75% H2 in N2 at a flow rate of 20 cm³ min−1, progressing from room temperature to 1000 °C with a heating rate of 10 °C min−1.
2.2.3. Catalytic Performance Evaluation
SRE was carried out using two catalysts, Cu/NaY and Cu/Nb2O5/Al2O3. Tests were performed in an experimental unit consisting of a preheating system, a 20 cm-long stainless-steel reactor with an internal diameter of 2.54 cm, a condenser, and a phase collector/separator. The reactant mixture was introduced through the system inlet using a peristaltic pump.
Before the catalytic tests, the catalysts were activated in situ with an 85 cm³ min−1 N2 flow rate containing 40% H2 by volume using the following heating steps: 30 min at 100 °C, 1 h at 200 °C, and 4 h at 500 °C. After this, the H2 flow was stopped, and the N2 flow was adjusted to 85 mL min−1 for 4 h to purge H2 from the entire reaction system. Subsequently, catalytic tests were conducted at 300 °C and 450 °C using a mass hourly space velocity of 40 dm³ h−1 gcat−1, 5 g catalyst (20–40 mesh, positioned at the center with the reactor ends filled with silica of the same particle size), and a H2O/C2H5OH molar ratio of 10/1 without the presence of an inert. The pellet size distribution was determined according to Trimm [49] to minimize both mass and heat diffusion effects in packed bed reactors (L/dpart ≥ 100 and D/dpart ≥ 30), resulting in a mean particle size distribution of 0.6 mm. The carbon balance was determined as described in Equation (2),
where is the molar flow rate of carbon in the products, is the corresponding number of carbons, and is the molar flow rate at the inlet.
The gaseous products were analyzed in a Trace GC ThermoQuest gas chromatograph with a Carboxen 1010 PLOT column, with argon as carrier gas, detection by TCD, and the following temperature program: 7 min at 45 °C, 25 °C min−1 to 180 °C, and 5 min at 180 °C. The liquid phase was analyzed in a Varian 3300 gas chromatograph using a 10% Carbowax 20M CHR W HP column, helium as carrier gas, detection by TCD, and the following temperature program: 2 min at 50 °C, 25 °C min−1 to 100 °C, and 2 min at 100 °C.
Evaluation of catalytic performance for H2 production was based on ethanol conversion () and product selectivity () on dry basis following Equations (3) and (4),
where is the molar flow rate of ethanol at the inlet, is the molar flow rate of ethanol at the outlet, and is the molar flow rate of i-species.
3. Results and Discussion
3.1. Catalyst Characterization
3.1.1. Morphology
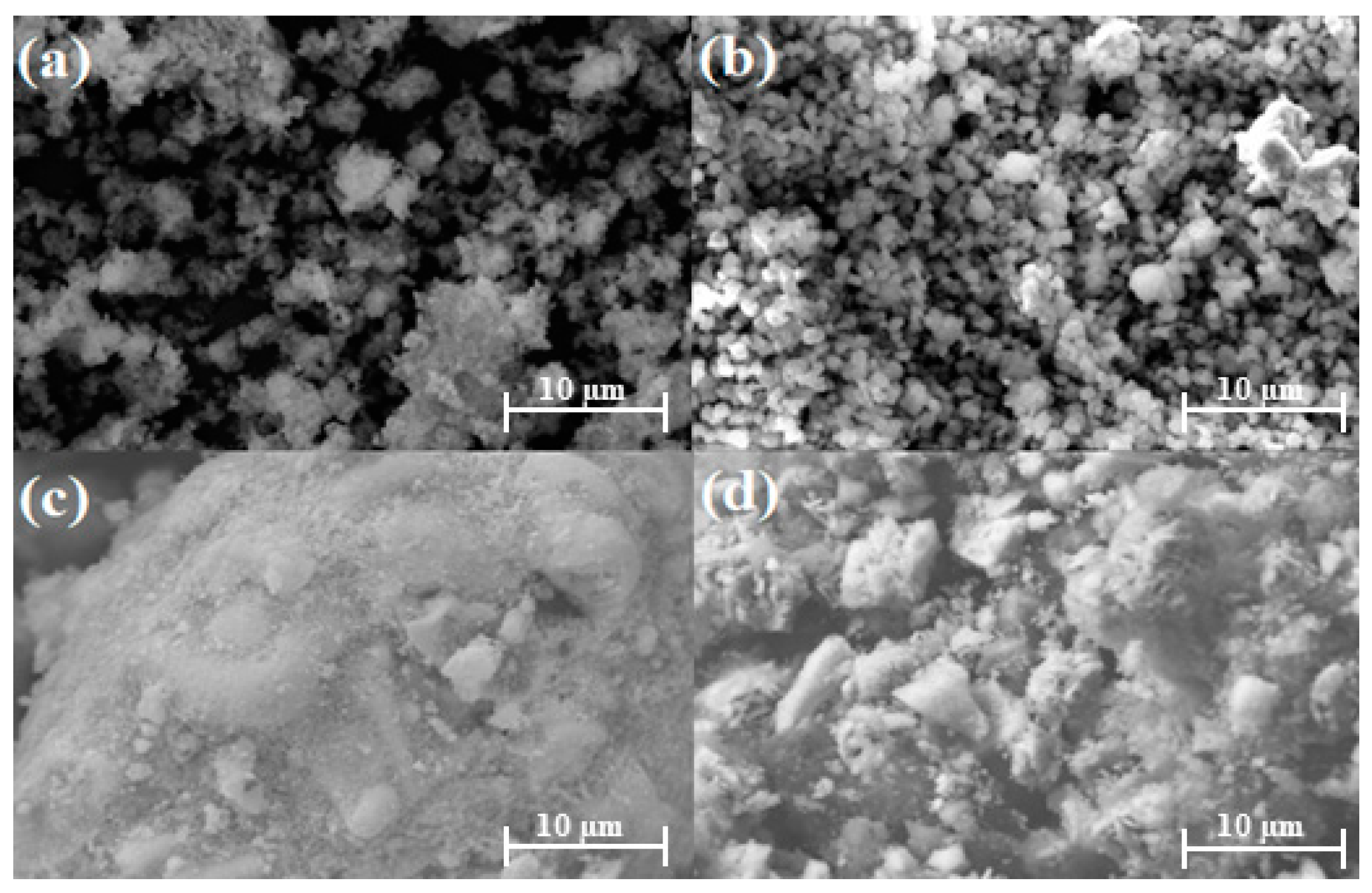
SEM was used to analyze the microstructural morphology of the catalysts. Figure 1a shows the NaY support, and Figure 1b shows the synthesized Cu/NaY catalyst. Even after the wet impregnation of copper on the NaY support, the polyhedral shapes of the zeolite remained regular and homogeneous, indicating no change in its morphological structure. By contrast, Figure 1c shows the Nb2O5 support, while Figure 1d shows the synthesized Cu/Nb2O5/Al2O3 catalyst. Unlike Cu/NaY, the particles are randomly distributed with various geometries and distinct sizes. These morphological properties were attributed to the nature of the support used, which does not present a well-defined microstructural morphology and evenly distributed particle sizes.
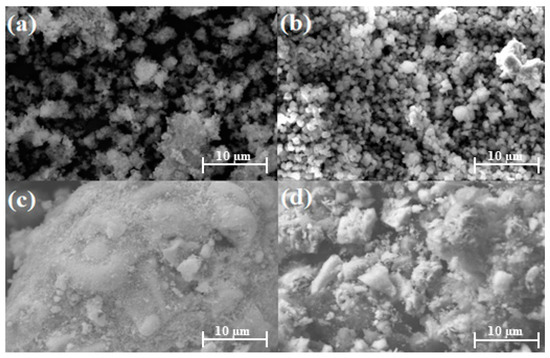
Figure 1.
SEM at 4000× of (a) NaY, (b) Cu/NaY, (c) Nb2O5, and (d) Cu/Nb2O5/Al2O3.
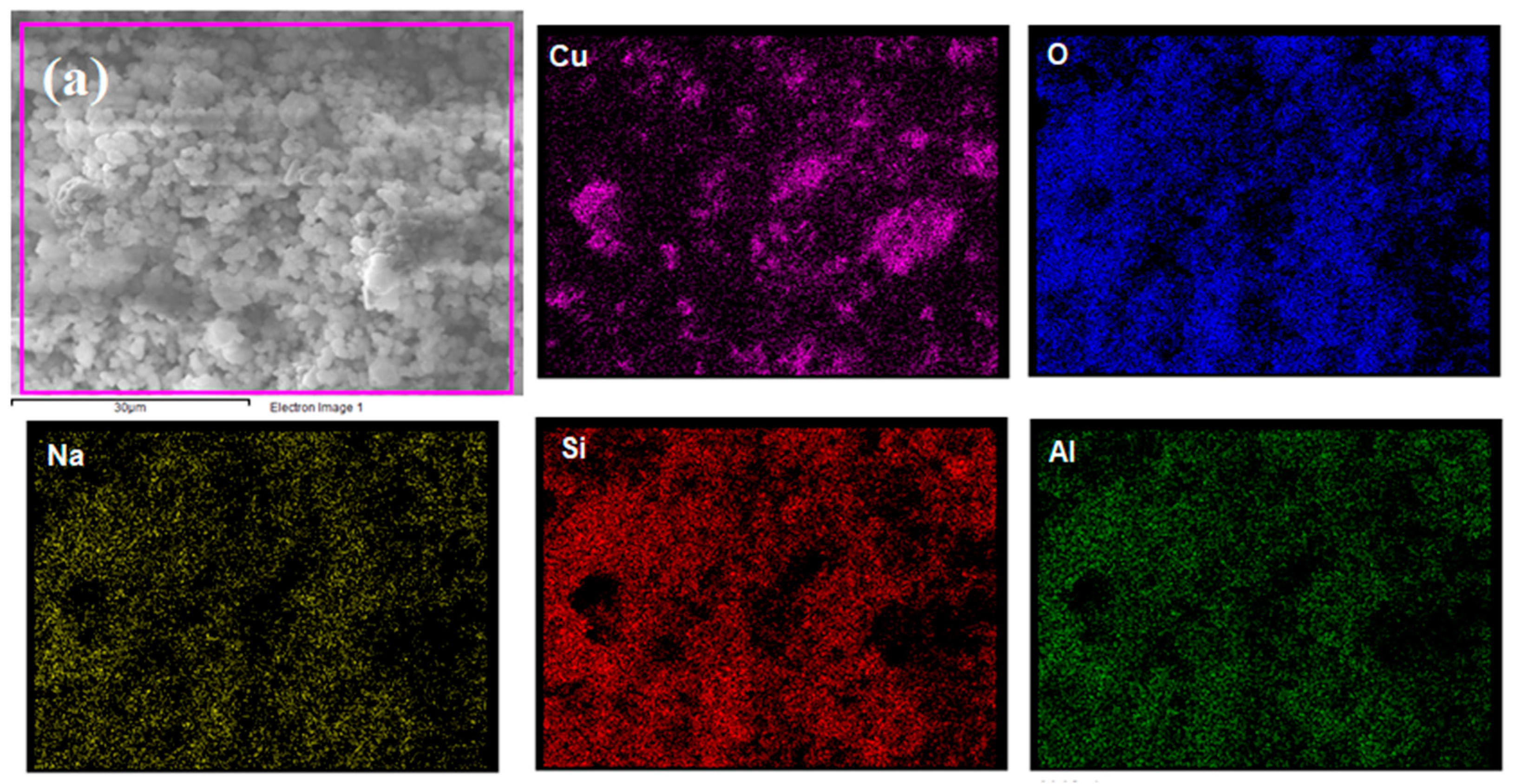
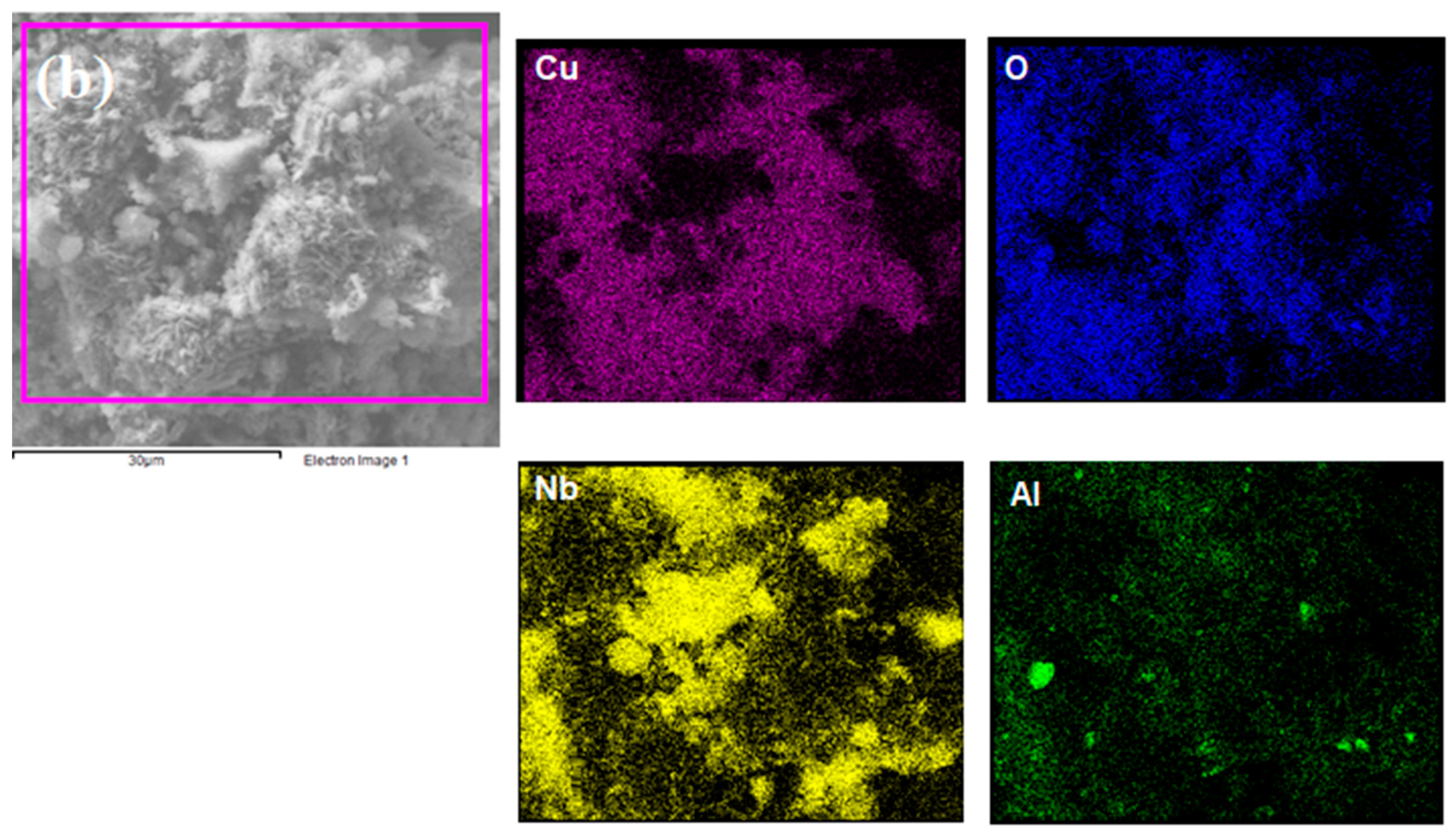
Elemental mapping was performed by EDS to identify chemical elements at the catalyst surface using a magnification of 4000×. Figure 2 shows that all proposed elements were detected, indicating the effectiveness of the proposed wet impregnation process. Additionally, Cu seemed to be better dispersed on NaY compared to Nb2O5/Al2O3, which presented higher Cu concentrations in some regions. This is strongly linked to the superior textural properties of the zeolite, which allowed for a better distribution of the metal particles.
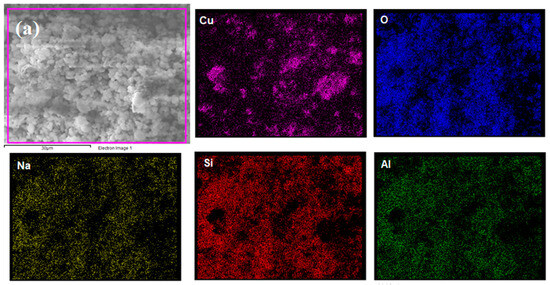
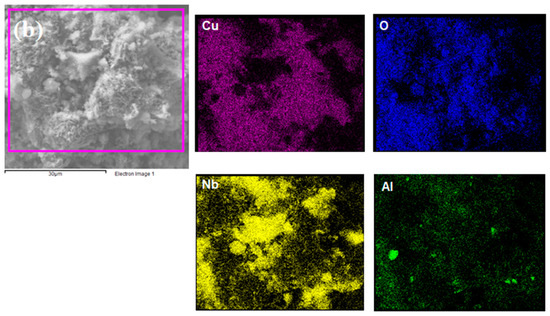
Figure 2.
Elemental mapping of (a) Cu/NaY and (b) Cu/Nb2O5/Al2O3.
Table 1 presents the EDS mass composition of the catalysts. Cu was present in both catalysts in very similar percentages. Furthermore, a Si/Al ratio of 2.58 confirmed the presence of an unmodified NaY zeolite after the wet impregnation synthesis.

Table 1.
Elemental analysis of Cu/NaY and Cu/Nb2O5/Al2O3.
3.1.2. Crystallinity
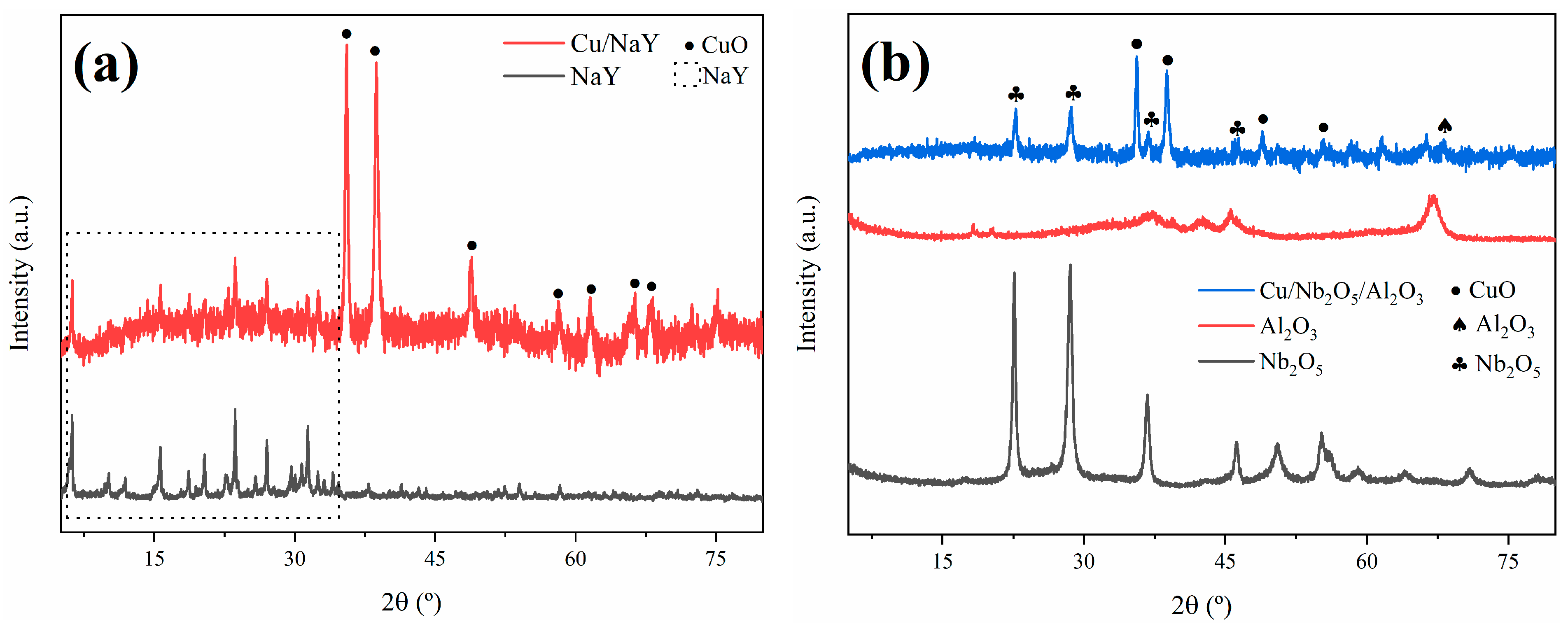
The XRD technique was employed to determine the crystallinity and purity of the synthesized catalysts. The experimental XRD patterns obtained for Cu/NaY and Cu/Nb2O5/Al2O3 are shown in Figure 3. For the Cu/NaY catalyst, the diffraction peaks at 2θ = 6.18°, 15.61°, 18.66°, 23.64°, 26.98°, and 31.36° are indexed to the cubic crystal structure of NaY, which corresponds to the Fd-3m space group in card #00-039-1380. The diffraction peaks at 2θ = 35.57°, 38.72°, 48.81°, 58.30°, 61.60°, 66.32°, and 68.12° are indexed to the monoclinic crystal system of CuO, referring to card #01-089-5895. The X-ray diffractogram of the Cu/NaY catalyst indicated that there were no modifications in the original crystal structure of the zeolite, probably due to the fine distribution of copper on the zeolite structure [50].
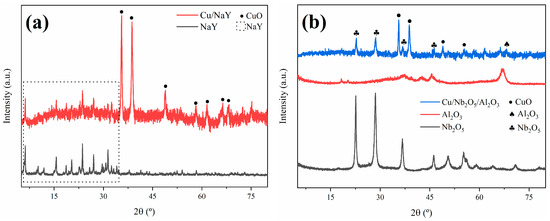
Figure 3.
DRX patterns of (a) Cu/NaY and (b) Cu/Nb2O5/Al2O3.
The Cu/Nb2O5/Al2O3 catalyst presented diffraction peaks at 2θ = 35.57°, 38.72°, 48.81°, and 56.72°, which are indexed to the monoclinic crystal system of CuO in card #01-089-5895. The diffraction peak at 2θ = 67.03° is indexed to the hexagonal crystal system of Al2O3 in card #00-013-0373, while the diffraction peaks at 2θ = 22.60°, 28.58°, 36.71°, and 46.23° are indexed to the hexagonal crystal system of Nb2O5 in card #00-028-0317. Both copper oxide and niobium pentoxide are present in the diffractogram with significant intensities, indicating that the precursors maintained their defined crystal lattice even after the catalyst synthesis. Also, alumina diffraction peaks are almost imperceptible, indicating a high dispersion of alumina in niobium oxide [51].
The crystallite size has important implications for the rate of molecular diffusion and the contribution of the external surface area to adsorption and desorption rates. For the calculation of the crystallite size of the active phase in both catalysts, the (−111) diffraction peak at 2θ = 35.57° of copper oxide was considered. The result was approximately 55 nm for both catalysts, indicating that the change of support did not cause an alteration in the crystallite size of the active phase. For the calculation of the support crystallite sizes, the (533) diffraction peak at 2θ = 23.64° of NaY and the (100) diffraction peak at 2θ = 28.58° of Nb2O5 were considered, resulting in 114 nm and 43 nm, respectively. Kugai et al. [52] concluded that the smaller the crystallite size of the support, the greater the dispersion of the active phase and consequently the higher the catalytic activity, indicating that the catalyst performance strongly depends on the surface area and crystallite sizes.
3.1.3. Textural Parameters
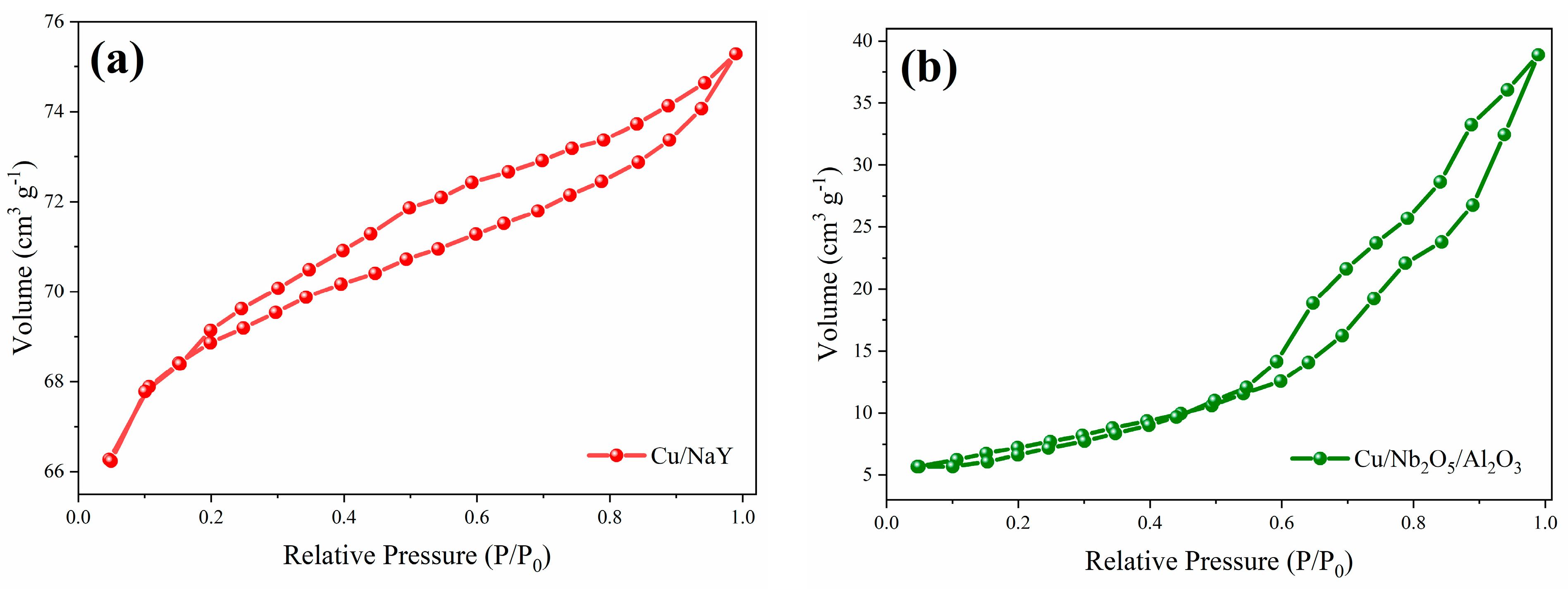
The determination of the textural parameters of the synthesized materials and their precursors is relevant to understand their SRE catalytic performance. The parameters obtained through N2 physisorption are presented in Table 2, while the N2 adsorption/desorption isotherms are shown in Figure 4. For the Cu/NaY catalyst, the isotherm exhibits characteristics of type IV according to I.U.P.A.C. [53], which is attributed to the presence of micropores associated with mesopores. The formation of a round knee-like feature at the beginning of the isotherm is related to the formation of adsorbed N2 monolayers inside micropores [54], while the increase in relative pressure improves adsorption as the material mesopores are filled. For the Cu/Nb2O5/Al2O3 catalyst, the obtained isotherm is of type V, which is also associated with mesoporous materials with weak adsorbate–adsorbent interactions. In both isotherms, there was hysteresis in N2 desorption, which is characteristic of capillary condensation in mesoporous materials [55]. For Cu/NaY, the hysteresis of type H4 is associated with narrow slit-like pores, while for Cu/Nb2O5/Al2O3, the hysteresis of type H3 refers to non-rigid aggregates of plate-like particles forming slit pores, typical of non-uniform pore sizes and shapes as observed by SEM.

Table 2.
Textural parameters of the catalysts and precursors.
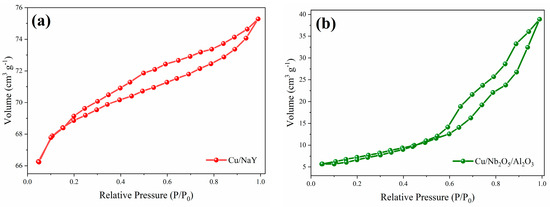
Figure 4.
N2 adsorption/desorption isotherms of the catalysts (a) Cu/NaY and (b) Cu/Nb2O5/Al2O3.
Concerning the obtained values for the textural parameters, the surface areas of the catalytic supports were similar to other studies involving NaY [56,57], Nb2O5 [11,45], and Al2O3 [58,59]. For the catalysts, a noticeable reduction in surface area and pore volume was observed, compared to their corresponding precursors. This effect is strongly associated with the possible obstruction of smaller pore diameters (micropores) by deposition of copper oxides inside the catalyst structure, as evidenced by the reduction in micropore volume. However, despite this reduction, a significant variation in textural properties was detected among our catalysts. This reinforces the possible influence of the support type on the catalyst performance, as it plays a fundamental role in the reaction. Cu/NaY has a significantly higher surface area than Cu/Nb2O5/Al2O3, which is expected to result in a superior catalytic performance of the former compared to the latter. This advantageous characteristic of Cu/NaY has been attributed to the three-dimensional pore structure of zeolite NaY [60].
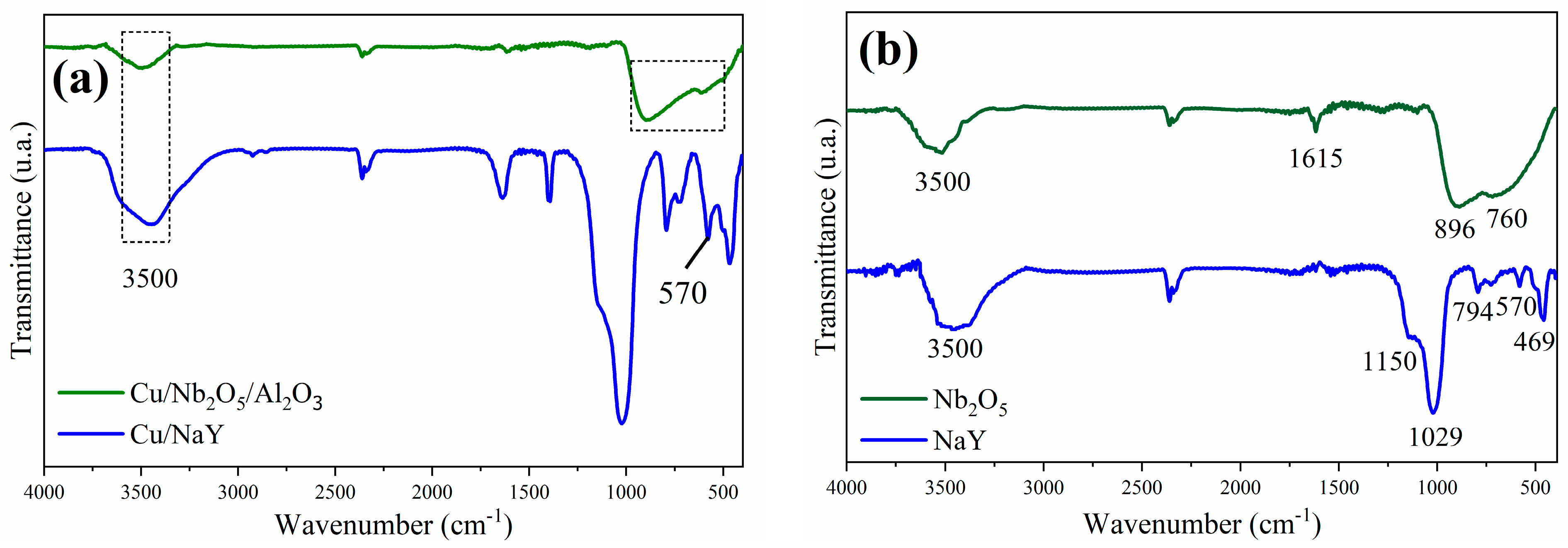
The FTIR spectra of the synthesized catalysts and both Nb2O5 and NaY supports are shown in Figure 5a and Figure 5b, respectively. The band at approximately 3500 cm−1, observed in all spectra, reveals the presence of surface hydroxyl groups in these materials [29,61]. The main NaY vibration modes were identified as the strong band located at 1029 cm−1 and the lower intensity band at 469 cm−1, which correspond to the internal vibrations of the tetrahedral units of the zeolite, while the bands identified at 1150, 794, and 570 cm−1 were attributed to the external linkages between the (Si/Al)O4 tetrahedra [62]. Sensitive bands did not show significant changes in the Cu/NaY spectrum compared to those of NaY. On the other hand, the band at 570 cm−1, attributed to the polyhedral ring in the zeolite structure, showed a slight alteration that may be associated with the binding of copper. According to previous studies, copper oxide (CuO) bands are present between the wavenumbers of 610 and 500 cm−1 [62,63].
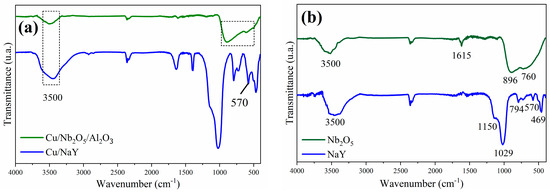
Figure 5.
Infrared spectra of the catalysts (a) Cu/NaY and Cu/Nb2O5/Al2O3 and of the precursors (b) NaY and Nb2O5.
The FTIR spectrum of Nb2O5 (Figure 5b) shows strong and broad bands in the region between 500 and 900 cm−1. The band centered at 896 cm−1 is attributed to the Nb-O stretching vibration and the band at 760 cm−1 to the Nb-O-Nb vibration [64]. The narrower band at 1622 cm−1 is attributed to water molecules adsorbed on the Nb2O5 surface [65]. The presence of niobium pentoxide was confirmed by the presence of its main vibration modes in the FTIR spectrum of the Cu/Nb2O5/Al2O3 catalyst (Figure 5a). Alterations were also noted in the intensity of the two bands between 500 and 900 cm−1, and in the resolution between them, when compared to the Nb2O5 spectrum. These changes are attributed to the binding with copper, as we are once again referring to the region of copper oxide bands.
3.1.4. Temperature-Programmed Desorption (TPD)
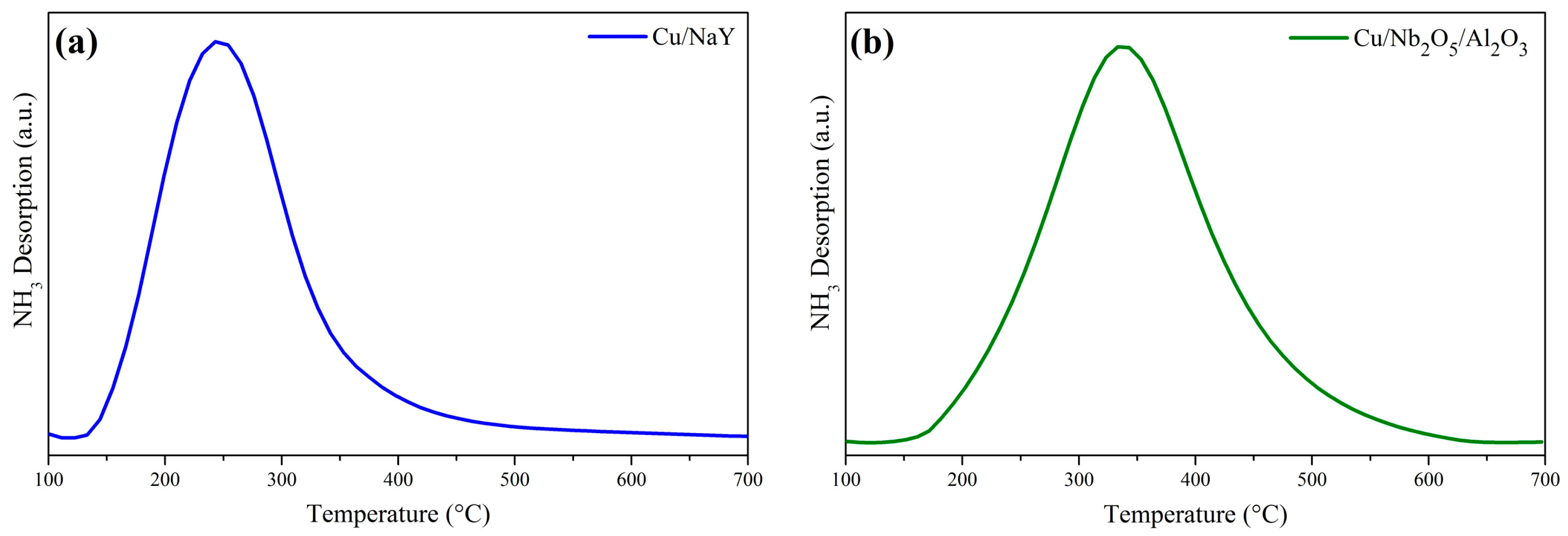
Analyses of both catalysts by TPD-NH3 are shown in Figure 6. The NH3 desorption profiles for Cu/NaY and Cu/Nb2O5/Al2O3 were confined to the 140 °C to 460 °C and 150 °C to 620 °C temperatures ranges, respectively, showing that the support type influenced the acidity of the catalyst. The peak location and wide temperature range for Cu/NaY suggest the presence of sites of weak and intermediate acid strength. By contrast, the higher temperature range for Cu/Nb2O5/Al2O3 is attributable to the presence of intermediate to strong acid sites. Also, Cu/NaY had a higher concentration of acid sites compared to Cu/Nb2O5/Al2O3 (Table 3). In general, the higher acidity of Cu/NaY may be justified by its higher surface area.
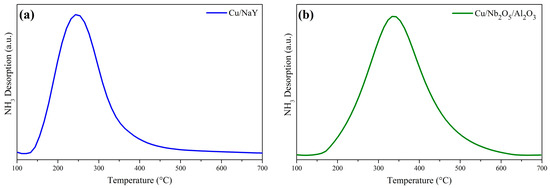
Figure 6.
NH3 desorption curves of the catalysts (a) Cu/NaY and (b) Cu/Nb2O5/Al2O3.

Table 3.
Acidity of the synthesized catalysts by TPD-NH3.
The maximum NH3 desorption temperature for the Cu/NaY catalyst was slightly higher than that for NaY alone, which typically ranges between 150 °C and 250 °C [66,67,68]. This is an indication that the incorporation of Cu into the zeolite structure increased the strength of its acid sites. On the other hand, the impregnation of Cu onto Nb2O5/Al2O3 did not have a significant influence on the desorption temperature range.
3.1.5. Temperature-Programmed Reduction (TPR)
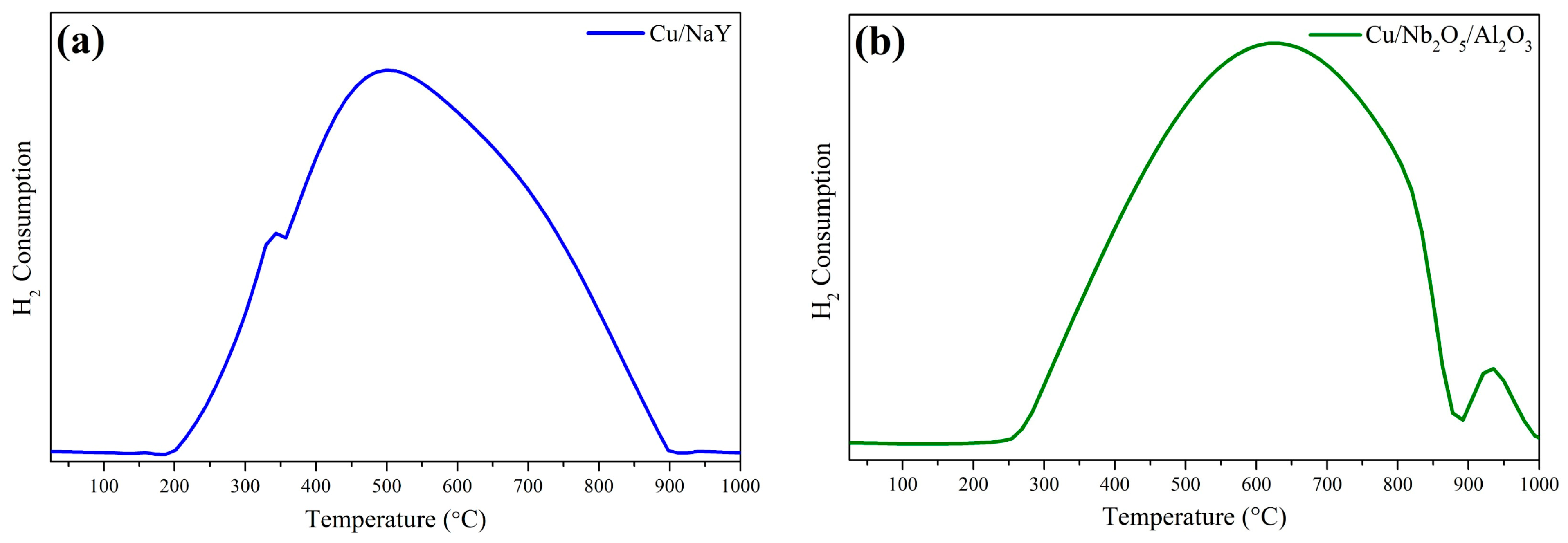
Both catalysts displayed a wide reduction range in their TPR profiles (Figure 7). However, the position of the maximum reduction temperature of CuO for Cu/Nb2O5/Al2O3 was shifted to higher values compared to Cu/NaY, which may indicate a greater interaction of Cu with Nb2O5/Al2O3 compared to NaY. Reduction at temperatures below 300 °C indicated that CuO was dispersed on the catalyst surface with little interaction with the support. Above this temperature, total copper reduction was prevented in both catalytic systems by the interaction between CuO and the support surface [18,69]. Additionally, Cu/Nb2O5/Al2O3 showed a reduction peak with a maximum around 928 °C, which was attributed to the partial reduction of Nb2O5 to NbO2 [46,70]. The hydrogen consumption of the Cu/NaY catalyst was 6.16 mmol gcat−1, while that of Cu/Nb2O5/Al2O3 was 6.38 mmol gcat−1—a slightly higher value due to the partial reduction of Nb2O5.
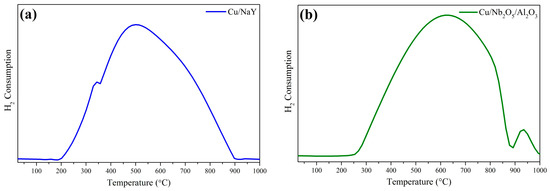
Figure 7.
TPR profiles of (a) Cu/NaY and (b) Cu/Nb2O5/Al2O3 catalysts.
3.2. Catalytic Performance Evaluation
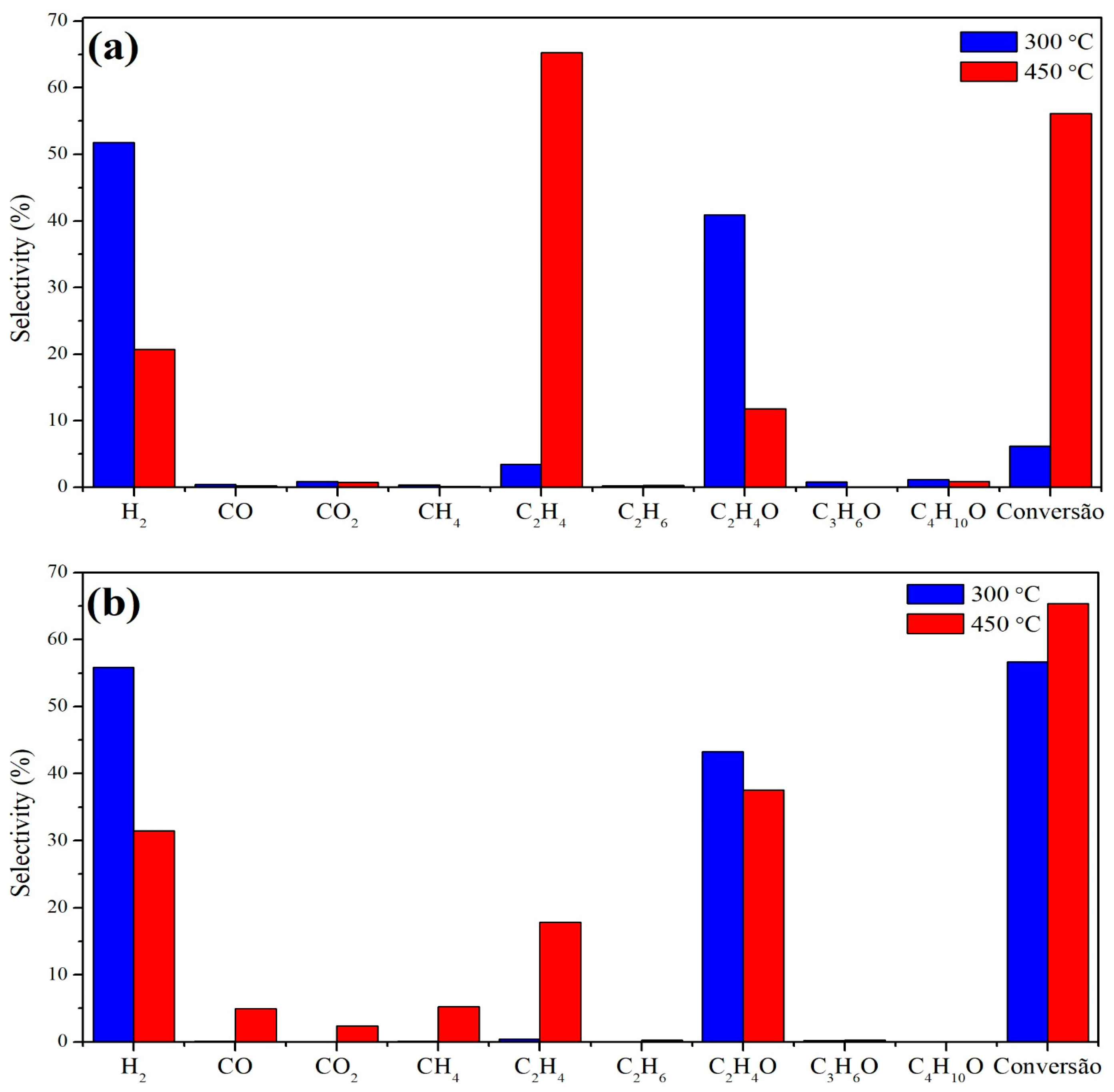
The main products generated with the Cu/Nb2O5/Al2O3 catalyst at 300 °C were hydrogen and acetaldehyde, but with a conversion below 10%. Increasing the temperature resulted in an increase in the average conversion, reaching approximately 55%, but the selectivity for hydrogen and acetaldehyde decreased while the selectivity for ethylene increased. For the Cu/NaY catalyst, a similar result was obtained at 300 °C regarding the selectivity for hydrogen and acetaldehyde, but with a higher average conversion close to 55%. Finally, with an increase in the reaction temperature to 450 °C, the conversion increased to 65% but the product distribution was greater, with CO, CO2, CH4, and C2H4 in detectable quantities.
Figure 8 shows the selectivity data for both Cu/Nb2O5/Al2O3 and Cu/NaY at 300 °C and 450 °C. The differences between the catalytic performances show that both support and temperature influenced the reaction efficiency. Conversion increased with increasing temperature for both catalysts, but the effect was more pronounced for the Cu/Nb2O5/Al2O3 catalyst. On the other hand, the selectivity for H2 and acetaldehyde decreased with increasing temperature, and the quantity of by-products (especially ethylene) increased, with this effect being greater for the Cu/Nb2O5/Al2O3 catalyst.
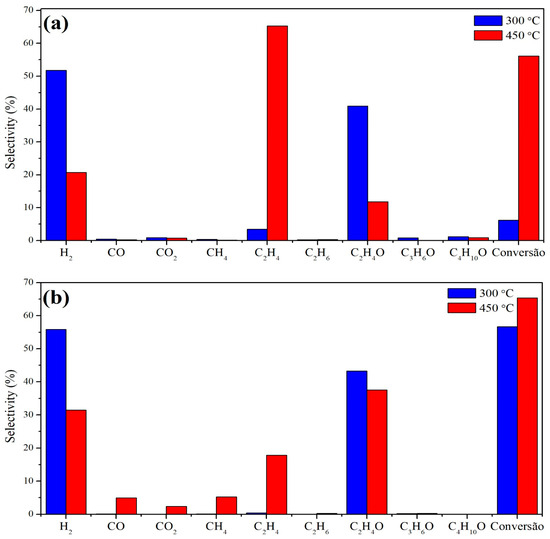
Figure 8.
Average selectivity of (a) Cu/Nb2O5/Al2O3 and (b) Cu/NaY at 300 °C and 450 °C.
For both catalysts at 300 °C, the main reaction was ethanol dehydrogenation (Equation (5)) forming acetaldehyde and H2, while higher temperatures favored dehydration forming ethylene (Equation (6)). Also, the use of NaY at 450 °C favored parallel reactions forming CO and CH4 [33]. This is probably due to the presence of more pronounced acid sites in the zeolite at lower temperatures, as observed by TPD-NH3 analysis of the Cu/NaY catalyst, where acid sites are found between 100 and 450 °C (Figure 6). Also, the high surface area of this catalyst contributed to the greater exposure of acid sites, as well as the catalytically active copper sites on the catalyst surface, promoting parallel reactions that are part of the ethanol reforming reaction pathway. This explains the detection of reaction intermediates such as acetaldehyde.
C2H5OH → C2H4O + H2
C2H5OH → C2H4 + H2O
The selectivity of Cu/Nb2O5/Al2O3 to C2H4 was higher than that of Cu/NaY. The TPD-NH3 of this catalyst showed acid sites of greater strength due to the desorption of NH3 at higher temperatures, which may be connected to the higher formation of C2H4 [70]. Lorenzut et al. [71] observed the same low selectivity behavior for Cu catalysts supported on ZnO/Al2O3. Also, the support seemed to have influenced the reaction pathway, since Nb2O5 is an n-type semiconductor that may have driven the selectivity toward ethylene. For the Cu/Nb2O5/Al2O3 catalyst, the formation of CO2 was limited at both temperatures, while for Cu/NaY, CO2 formation was more pronounced at 450 °C, indicating the occurrence of ethanol reforming.
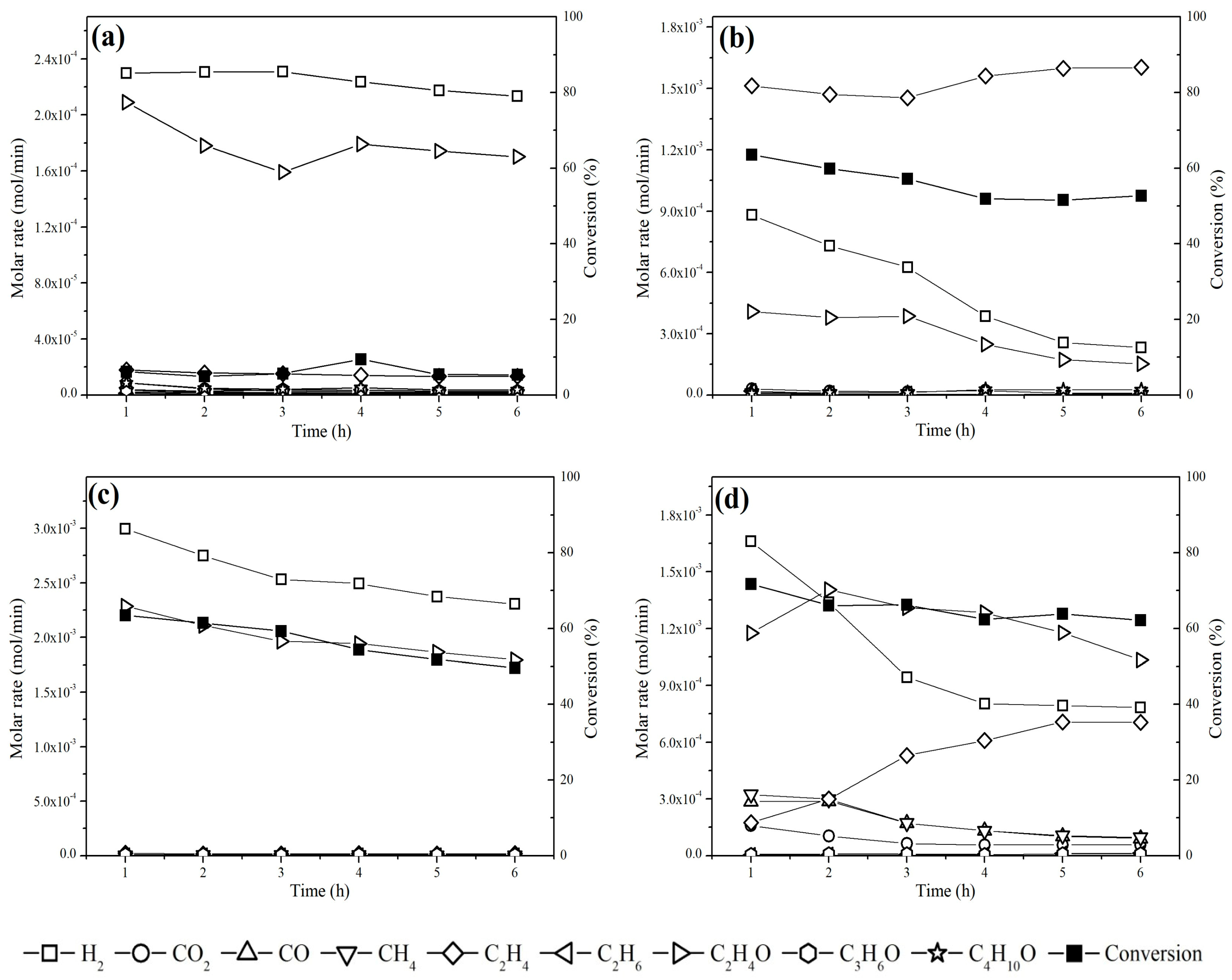
Both catalysts exhibited more stability at 300 °C (Figure 9) because, at 450 °C, C2H4 formation accelerated deactivation by coke deposition [72]. For the Cu/NaY catalyst, the carbon balance at 300 °C and 450 °C was 87% and 79%, respectively, whereas for Cu/Nb2O5/Al2O3, the carbon balance was 99% at 300 °C and 83% at 450 °C. In general, values below 100% may suggest the formation of coke on the catalyst surface [73]. Overall, the results highlight the strong influence of the catalytic support on the steam reforming of ethanol for H2 production.
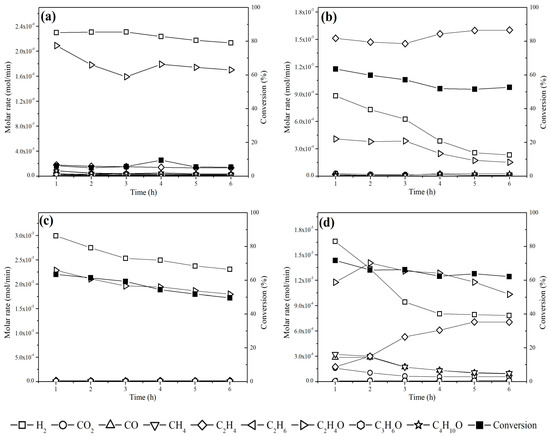
Figure 9.
Molar rate as a function of time for Cu/Nb2O5/Al2O3 at (a) 300 °C and (b) 450 °C and Cu/NaY at (c) 300 °C and (d) 450 °C.
The Cu/NaY catalyst showed a higher ethanol conversion rate compared to Cu/Nb2O5/Al2O3, suggesting that support properties such as surface area, pore volume, and pore size significantly influenced the catalytic performance. The porous structure and higher surface area of NaY may have facilitated copper dispersion and interaction with the reactants, enhancing the catalytic activity. Additionally, zeolites have a structure with a regular arrangement of uniform micropores that promote greater selectivity for H2 formation. Furthermore, the acidity measurements revealed that the incorporation of copper into NaY increases the strength of the acid sites, as indicated by the higher desorption temperature found for Cu/NaY compared to pure NaY in other studies [74,75,76]. This increased acidity may have contributed to the improvement of the catalytic activity by influencing the adsorption and breakdown of ethanol molecules.
The catalytic performance of Cu/SiO [77] and CuO/ZrO2 [73] in SRE reactions was evaluated at lower temperatures, e.g., 300 °C and 350 °C. The former showed no catalytic activity, while the latter produced hydrogen (Y = 36%), acetaldehyde (S = 58%), and acetic acid (S = 24%) as the main products, achieving a conversion of 90%. Only at 500 °C did the Cu/SiO catalyst produce acetaldehyde with a conversion of 12%. Cu/Nb2O5 resulted in catalytic conversions below 10% at 450 °C [18], producing 60% H2 and 35% C2H4O, on average, along with co-products such as ethylene and ethyl ether (<5%). These results, along with those obtained in this work, highlight the role of the catalytic support in SRE. Therefore, a careful selection of the support is crucial for optimizing the performance of copper catalysts in H2 production by SRE.
4. Conclusions
The steam reforming of ethanol was viable using copper-based catalysts supported on a NaY zeolite (Cu/NaY) and niobium-aluminum oxides (Cu/Nb2O5/Al2O3). The physicochemical properties of the two catalysts were different due to differences in the support properties. Nevertheless, copper particles were well dispersed in both catalysts, contributing to achieving better catalytic performances. Both catalysts were active in the steam reforming of ethanol, but Cu/NaY was best for H2 production at 450 °C, with CO2 formation remaining constant throughout the reaction course. Therefore, this catalyst has potential for large-scale operations and, with the addition of a small amount of acidity dopants, it may become even more selective for H2 production by ethanol reforming. Finally, the support effect was demonstrated as a relevant parameter for optimal SRE catalytic performance. The greatest limitation of this work was catalyst shortage, and this leads to the emergence of synthesizing larger quantities of the most efficient NaY-supported copper catalyst for further optimization studies including the addition of acidity dopants to enhance the selectivity for hydrogen production. Additionally, studies on catalyst recovery and reuse must be performed, as well as a detailed economic analysis for process development on an industrial scale.
Author Contributions
Conceptualization, R.C.P.R.-D., L.P.R. and M.d.S.; methodology, R.C.P.R.-D., A.D.G. and B.R.F.; investigation, A.D.G. and B.R.F.; resources, L.P.R., M.d.S. and R.C.P.R.-D.; data curation, R.P.N., P.D.M., A.D.G. and B.R.F.; writing—original draft preparation, P.D.M., R.P.N., R.C.P.R.-D. and A.D.G.; writing—review and editing, P.D.M., R.P.N., A.D.G., L.P.R. and R.C.P.R.-D.; supervision, R.C.P.R.-D. and L.P.R.; funding acquisition, L.P.R. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the financial support of Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil—grant number 315930/2021-7), Fundação Araucária (grant agreement 002/2021, process #17.521.887-4—NAPI-HCR project), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Brazil—Finance Code 001).
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
The support of the Multi-User Laboratories for Materials Characterization (CMCM) and Chemical Analysis (LAMAQ) at the Federal Technological University of Paraná (UTFPR), the Department of Soils at the Agrarian Sciences Campus of the Federal University of Paraná (UFPR), the Companhia Brasileira de Metalurgia e Mineração (CBMM, Minas Gerais, Brazil), and the Catalysis Laboratory (LabCat) at the State University of Maringá (UEM) are greatly acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, M.; Li, S.; Di, Z.; Yang, Z.; Chou, W.; Shi, B. Fischer-Tropsch Synthesis: Effect of Nitric Acid Pretreatment on Graphene-Supported Cobalt Catalyst. Appl. Catal. A Gen. 2020, 599, 117608. [Google Scholar] [CrossRef]
- Yousefian, F.; Babatabar, M.A.; Eshaghi, M.; Poor, S.M.; Tavasoli, A. Pyrolysis of Rice Husk, Coconut Shell, and Cladophora Glomerata Algae and Application of the Produced Biochars as Support for Cobalt Catalyst in Fischer–Tropsch Synthesis. Fuel Process. Technol. 2023, 247, 107818. [Google Scholar] [CrossRef]
- Liu, Y.; Zou, R.; Qin, B.; Gan, J.; Peng, X. Energy-Efficient Monosaccharides Electrooxidation Coupled with Green Hydrogen Production by Bifunctional Co9S8/Ni3S2 Electrode. Chem. Eng. J. 2022, 446, 136950. [Google Scholar] [CrossRef]
- Mert, M.E.; Edis, C.; Akyıldız, Ş.; Demir, B.N.; Nazligul, H.; Gurdal, Y.; Doğru Mert, B. Design and Performance Analysis of a PV-Assisted Alkaline Electrolysis for Hydrogen Production: An Experimental and Theoretical Study. Fuel 2024, 355, 129497. [Google Scholar] [CrossRef]
- Vadalà, M.; Kröll, E.; Küppers, M.; Lupascu, D.C.; Brunstermann, R. Hydrogen Production via Dark Fermentation by Bacteria Colonies on Porous PDMS-Scaffolds. Int. J. Hydrogen Energy 2023, 48, 25274–25284. [Google Scholar] [CrossRef]
- Öztan, H.; Çapoğlu, İ.K.; Uysal, D.; Doğan, Ö.M. A Parametric Study to Optimize the Temperature of Hazelnut and Walnut Shell Gasification for Hydrogen and Methane Production. Bioresour. Technol. Rep. 2023, 23, 101581. [Google Scholar] [CrossRef]
- Guan, D.; Wang, F.; Zhang, X.; Dou, W.; Sun, Y. Comprehensive Study on Catalytic Coating Tubular Reactor with Electromagnetic Induction Heating for Hydrogen Production through Methanol Steam Reforming. Int. J. Hydrogen Energy 2024, 50, 1–17. [Google Scholar] [CrossRef]
- Hu, Y.; He, W.; Shen, Y. Recyclable NiMnOx/NaF Catalysts: Hydrogen Generation via Steam Reforming of Formaldehyde. Fuel 2023, 354, 129311. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Gruber, H.; Weber, G.; Reichhold, A.; Pirone, R.; Bensaid, S. Aqueous Phase Reforming of Pilot-Scale Fischer-Tropsch Water Effluent for Sustainable Hydrogen Production. Catal. Today 2021, 367, 239–247. [Google Scholar] [CrossRef]
- Levikhin, A.A.; Boryaev, A.A. High-Temperature Reactor for Hydrogen Production by Partial Oxidation of Hydrocarbons. Int. J. Hydrogen Energy 2023, 48, 28187–28204. [Google Scholar] [CrossRef]
- da Silva, F.A.; Dancini-Pontes, I.; DeSouza, M.; Fernandes, N.R.C. Kinetics of Ethanol Steam Reforming over Cu–Ni/NbxOy Catalyst. React. Kinet. Mech. Catal. 2017, 122, 557–574. [Google Scholar] [CrossRef]
- Shtyka, O.; Dimitrova, Z.; Ciesielski, R.; Kedziora, A.; Mitukiewicz, G.; Leyko, J.; Maniukewicz, W.; Czylkowska, A.; Maniecki, T. Steam Reforming of Ethanol for Hydrogen Production: Influence of Catalyst Composition (Ni/Al2O3, Ni/Al2O3–CeO2, Ni/Al2O3–ZnO) and Process Conditions. React. Kinet. Mech. Catal. 2021, 132, 907–919. [Google Scholar] [CrossRef]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current Status of Hydrogen Production Techniques by Steam Reforming of Ethanol: A Review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Zanchet, D.; Santos, J.B.O.; Damyanova, S.; Gallo, J.M.R.; Bueno, J.M.C. Toward Understanding Metal-Catalyzed Ethanol Reforming. ACS Catal. 2015, 5, 3841–3863. [Google Scholar] [CrossRef]
- Trane-Restrup, R.; Dahl, S.; Jensen, A.D. Steam Reforming of Ethanol: Effects of Support and Additives on Ni-Based Catalysts. Int. J. Hydrogen Energy 2013, 38, 15105–15118. [Google Scholar] [CrossRef]
- Wurzler, G.T.; Rabelo-Neto, R.C.; Mattos, L.V.; Fraga, M.A.; Noronha, F.B. Steam Reforming of Ethanol for Hydrogen Production over MgO—Supported Ni-Based Catalysts. Appl. Catal. A Gen. 2016, 518, 115–128. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Castaldo, F.; Ricca, A.; Boettge, D. Ethanol Steam Reforming over Bimetallic Coated Ceramic Foams: Effect of Reactor Configuration and Catalytic Support. Int. J. Hydrogen Energy 2015, 40, 12650–12662. [Google Scholar] [CrossRef]
- Alonso, C.G.; Furtado, A.C.; Cantão, M.P.; Andreo dos Santos, O.A.; Camargo Fernandes-Machado, N.R. Reactions over Cu/Nb2O5 Catalysts Promoted with Pd and Ru during Hydrogen Production from Ethanol. Int. J. Hydrogen Energy 2009, 34, 3333–3341. [Google Scholar] [CrossRef]
- Chen, F.; Tao, Y.; Ling, H.; Zhou, C.; Liu, Z.; Huang, J.; Yu, A. Ni-Cu Bimetallic Catalysts on Yttria-Stabilized Zirconia for Hydrogen Production from Ethanol Steam Reforming. Fuel 2020, 280, 118612. [Google Scholar] [CrossRef]
- Ranjekar, A.M.; Yadav, G.D. Steam Reforming of Ethanol for Hydrogen Production: Efficacy of Ceria Promoted Cu–Co on Mesoporous Cellular Foam Silica. Int. J. Hydrogen Energy 2023, 48, 31550–31570. [Google Scholar] [CrossRef]
- Guarido, C.E.M.; Cesar, D.V.; Souza, M.M.V.M.; Schmal, M. Ethanol Reforming and Partial Oxidation with Cu/Nb2O5 Catalyst. Catal. Today 2009, 142, 252–257. [Google Scholar] [CrossRef]
- Hou, T.; Zhang, S.; Chen, Y.; Wang, D.; Cai, W. Hydrogen Production from Ethanol Reforming: Catalysts and Reaction Mechanism. Renew. Sustain. Energy Rev. 2015, 44, 132–148. [Google Scholar] [CrossRef]
- Mariño, F.J.; Cerrella, E.G.; Duhalde, S.; Jobbagy, M.; Laborde, M.A. Hydrogen from Steam Reforming of Ethanol. Characterization and Performance of Copper-Nickel Supported Catalysts. Int. J. Hydrogen Energy 1998, 23, 1095–1101. [Google Scholar] [CrossRef]
- Ni, M.; Leung, D.Y.C.; Leung, M.K.H. A Review on Reforming Bio-Ethanol for Hydrogen Production. Int. J. Hydrogen Energy 2007, 32, 3238–3247. [Google Scholar] [CrossRef]
- Snytnikov, P.V.; Badmaev, S.D.; Volkova, G.G.; Potemkin, D.I.; Zyryanova, M.M.; Belyaev, V.D.; Sobyanin, V.A. Catalysts for Hydrogen Production in a Multifuel Processor by Methanol, Dimethyl Ether and Bioethanol Steam Reforming for Fuel Cell Applications. Int. J. Hydrogen Energy 2012, 37, 16388–16396. [Google Scholar] [CrossRef]
- Furtado, A.C.; Alonso, C.G.; Cantão, M.P.; Fernandes-Machado, N.R.C. Bimetallic Catalysts Performance during Ethanol Steam Reforming: Influence of Support Materials. Int. J. Hydrogen Energy 2009, 34, 7189–7196. [Google Scholar] [CrossRef]
- Denis, A.; Grzegorczyk, W.; Gac, W.; Machocki, A. Steam Reforming of Ethanol over Ni/Support Catalysts for Generation of Hydrogen for Fuel Cell Applications. Catal. Today 2008, 137, 453–459. [Google Scholar] [CrossRef]
- Inokawa, H.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Difference in the Catalytic Activity of Transition Metals and Their Cations Loaded in Zeolite Y for Ethanol Steam Reforming. Int. J. Hydrogen Energy 2010, 35, 11719–11724. [Google Scholar] [CrossRef]
- Nippes, R.P.; Frederichi, D.; Olsen Scaliante, E.M.H.N. Enhanced Photocatalytic Performance under Solar Radiation of ZnO through Hetero-Junction with Iron Functionalized Zeolite. J. Photochem. Photobiol. A Chem. 2021, 418, 113373. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Barakat, M.A. Rice Husk Ash as a Renewable Source for the Production of Zeolite NaY and Its Characterization. Arab. J. Chem. 2015, 8, 48–53. [Google Scholar] [CrossRef]
- Tolentino, C.M.C.; de Luna, M.D.G.; Futalan, C.M.; Choi, A.E.S.; Manegdeg, F.G.; Grisdanurak, N. Influence of Hydrocarbons on Hydrogen Chloride Removal from Refinery Off-Gas by Zeolite NaY Derived from Rice Husks. Sci. Total Environ. 2020, 728, 138782. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.M.; Ahmed, M.J.; Hameed, B.H. NaY Zeolite from Wheat (Triticum aestivum L.) Straw Ash Used for the Adsorption of Tetracycline. J. Clean. Prod. 2018, 172, 602–608. [Google Scholar] [CrossRef]
- Campos-Skrobot, F.C.; Rizzo-Domingues, R.C.P.; Fernandes-Machado, N.R.C.; Cantão, M.P. Novel Zeolite-Supported Rhodium Catalysts for Ethanol Steam Reforming. J. Power Sources 2008, 183, 713–716. [Google Scholar] [CrossRef]
- Kwak, B.S.; Lee, J.S.; Lee, J.S.; Choi, B.-H.; Ji, M.J.; Kang, M. Hydrogen-Rich Gas Production from Ethanol Steam Reforming over Ni/Ga/Mg/Zeolite Y Catalysts at Mild Temperature. Appl. Energy 2011, 88, 4366–4375. [Google Scholar] [CrossRef]
- Lee, J.-S.; Kim, J.-E.; Kang, M.-S. Hydrogen Production from Ethanol Steam Reforming over SnO2-K2O/Zeolite Y Catalyst. Bull. Korean Chem. Soc. 2011, 32, 1912–1920. [Google Scholar] [CrossRef]
- Androulakis, A.; Yentekakis, I.V.; Panagiotopoulou, P. Dry Reforming of Methane over Supported Rh and Ru Catalysts: Effect of the Support (Al2O3, TiO2, ZrO2, YSZ) on the Activity and Reaction Pathway. Int. J. Hydrogen Energy 2023, 48, 33886–33902. [Google Scholar] [CrossRef]
- Kharaji, A.G.; Shariati, A.; Takassi, M.A. A Novel γ-Alumina Supported Fe-Mo Bimetallic Catalyst for Reverse Water Gas Shift Reaction. Chin. J. Chem. Eng. 2013, 21, 1007–1014. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Shah, M.; le Saché, E.; Ramirez Reina, T. Improving Fe/Al2O3 Catalysts for the Reverse Water-Gas Shift Reaction: On the Effect of Cs as Activity/Selectivity Promoter. Catalysts 2018, 8, 608. [Google Scholar] [CrossRef]
- Ranjbar, A.; Aghamiri, S.F.; Irankhah, A. Effect of MgO/Al2O3 Ratio in the Support of Mesoporous Ni/MgO–Al2O3 Catalysts for CO2 Utilization via Reverse Water Gas Shift Reaction. Int. J. Hydrogen Energy 2023, 48, 19115–19125. [Google Scholar] [CrossRef]
- Xiao, T.; Xie, J.; Cheng, J.; Dai, X.; Lu, S.; Zuo, R.; Li, Z.; Yang, Z. Al2O3 Supported NiCu Alloy as a Stable Catalyst for Selective Hydrogenation of Phthalic Anhydride to Phthalide. Appl. Catal. A Gen. 2023, 660, 119189. [Google Scholar] [CrossRef]
- Yao, X.; Gao, F.; Dong, L. The Application of Incorporation Model in γ-Al2O3 Supported Single and Dual Metal Oxide Catalysts: A Review. Chin. J. Catal. 2013, 34, 1975–1985. [Google Scholar] [CrossRef]
- Ji, N.; Yin, J.; Rong, Y.; Li, H.; Yu, Z.; Lei, Y.; Wang, S.; Diao, X. More than a Support: The Unique Role of Nb2O5 in Supported Metal Catalysts for Lignin Hydrodeoxygenation. Catal. Sci. Technol. 2022, 12, 3751–3766. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, J.; Hua, C.; Zhu, L.; Qiu, K.; Wang, S. Hydrodeoxygenation Performance of Lignin-Derived Phenolics to Cycloalkanes: Insights into the Crystal Structures of the Nb2O5 Support. Energy Fuels 2023, 37, 14006–14020. [Google Scholar] [CrossRef]
- Nippes, R.P.; Gomes, A.D.; Macruz, P.D.; de Souza, M. Photocatalytic Removal of 17β-Estradiol from Water Using a Novel Bimetallic NiCu/Nb2O5 Catalyst. Environ. Sci. Pollut. Res. 2023, 30, 103731–103742. [Google Scholar] [CrossRef] [PubMed]
- Nippes, R.P.; Macruz, P.D.; Gomes, A.D.; Girotto, C.P.; Scaliante, M.H.N.O.; de Souza, M. Removal of Reactive Blue 250 Dye from Aqueous Medium Using Cu/Fe Catalyst Supported on Nb2O5 through Oxidation with H2O2. React. Kinet. Mech. Catal. 2022, 135, 2697–2717. [Google Scholar] [CrossRef]
- Dancini-Pontes, I.; DeSouza, M.; Silva, F.A.; Scaliante, M.H.N.O.; Alonso, C.G.; Bianchi, G.S.; Medina Neto, A.; Pereira, G.M.; Fernandes-Machado, N.R.C. Influence of the CeO2 and Nb2O5 Supports and the Inert Gas in Ethanol Steam Reforming for H2 Production. Chem. Eng. J. 2015, 273, 66–74. [Google Scholar] [CrossRef]
- Menezes, J.P.D.S.Q.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen Production from Glycerol Steam Reforming over Nickel Catalysts Supported on Alumina and Niobia: Deactivation Process, Effect of Reaction Conditions and Kinetic Modeling. Int. J. Hydrogen Energy 2018, 43, 15064–15082. [Google Scholar] [CrossRef]
- Pelissari Rizzo-Domingues, B.; Carolina, R.; Cantão, P.; Fernandes Machado, C.; Regina, N. Estudo de Catalisadores a Base de Cobre e Nióbia Na Reação de Reforma a Vapor de Etanol. Acta Sci. Technol. 2007, 29, 1–7. [Google Scholar] [CrossRef][Green Version]
- Trimm, D.L. Design of Industrial Catalysts; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1980; Volume 11, ISBN 0444419063. [Google Scholar]
- Mortezaei, Z.; Zendehdel, M.; Bodaghifard, M.A. Cu Complex Grafted on the Porous Materials: Synthesis, Characterization and Comparison of Their Antibacterial Activity with Nano-Cu/NaY Zeolite. J. Iran. Chem. Soc. 2020, 17, 283–295. [Google Scholar] [CrossRef]
- Gonçalves, J.F.; Souza, M.M.V.M. Ni/X%Nb2O5/Al2O3 Catalysts Prepared via Coprecipitation-Wet Impregnation Method for Methane Steam Reforming. Curr. Catal. 2020, 9, 80–89. [Google Scholar] [CrossRef]
- Kugai, J.; Subramani, V.; Song, C.; Engelhard, M.; Chin, Y. Effects of Nanocrystalline CeO2 Supports on the Properties and Performance of Ni–Rh Bimetallic Catalyst for Oxidative Steam Reforming of Ethanol. J. Catal. 2006, 238, 430–440. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Beltrame, K.K.; Cazetta, A.L.; de Souza, P.S.C.; Spessato, L.; Silva, T.L.; Almeida, V.C. Adsorption of Caffeine on Mesoporous Activated Carbon Fibers Prepared from Pineapple Plant Leaves. Ecotoxicol. Environ. Saf. 2018, 147, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, X.; He, G.; Zhang, X.; Bao, S.; Hu, W. Bioinspired Synthesis of Nitrogen/Sulfur Co-Doped Graphene as an Efficient Electrocatalyst for Oxygen Reduction Reaction. J. Power Sources 2015, 279, 252–258. [Google Scholar] [CrossRef]
- Patdhanagul, N.; Srithanratana, T.; Rangsriwatananon, K.; Hengrasmee, S. Ethylene Adsorption on Cationic Surfactant Modified Zeolite NaY. Microporous Mesoporous Mater. 2010, 131, 97–102. [Google Scholar] [CrossRef]
- Zeng, Y.; Walker, H.; Zhu, Q. Reduction of Nitrate by NaY Zeolite Supported Fe, Cu/Fe and Mn/Fe Nanoparticles. J. Hazard. Mater. 2017, 324, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-Y.; Lai, G.-H.; Tsai, D.-H. Aerosol Route Synthesis of Ni-CeO2-Al2O3 Hybrid Nanoparticle Cluster for Catalysis of Reductive Amination of Polypropylene Glycol. Adv. Powder Technol. 2019, 30, 2293–2298. [Google Scholar] [CrossRef]
- Fawaz, A.; Bizreh, Y.W.; Al-Hamoud, L. (NiO, Ag /Fe2O3-Al2O3-Bentonite) as Promising Catalyst for CO and HC Removal from Single-Cylinder Engine Exhaust Emissions. Catal. Commun. 2022, 171, 106521. [Google Scholar] [CrossRef]
- Morales-Pacheco, P.; Alvarez, F.; Bucio, L.; Domínguez, J.M. Synthesis and Structural Properties of Zeolitic Nanocrystals II: FAU-Type Zeolites. J. Phys. Chem. C 2009, 113, 2247–2255. [Google Scholar] [CrossRef]
- Ameri, A.; Faramarzi, M.A.; Tarighi, S.; Shakibaie, M.; Ameri, A.; Ramezani-Sarbandi, A.; Forootanfar, H. Removal of Dyes by Trametes Versicolor Laccase Immobilized on NaY-Zeolite. Chem. Eng. Res. Des. 2023, 197, 240–253. [Google Scholar] [CrossRef]
- El-Bahy, Z.M. Oxidation of Carbon Monoxide over Cu- and Ag-NaY Catalysts with Aqueous Hydrogen Peroxide. Mater. Res. Bull. 2007, 42, 2170–2183. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, D.; Jiao, X. Fabrication of CuO Pricky Microspheres with Tunable Size by a Simple Solution Route. J. Phys. Chem. B 2005, 109, 13561–13566. [Google Scholar] [CrossRef]
- Khan, I.; Baig, N.; Qurashi, A. Graphitic Carbon Nitride Impregnated Niobium Oxide (g-C3N4/Nb2O5) Type (II) Heterojunctions and Its Synergetic Solar-Driven Hydrogen Generation. ACS Appl. Energy Mater. 2019, 2, 607–615. [Google Scholar] [CrossRef]
- da Conceição, L.R.V.; Carneiro, L.M.; Rivaldi, J.D.; de Castro, H.F. Solid Acid as Catalyst for Biodiesel Production via Simultaneous Esterification and Transesterification of Macaw Palm Oil. Ind. Crops Prod. 2016, 89, 416–424. [Google Scholar] [CrossRef]
- Fan, D.; Jiang, S.; Qiao, K.; Zhang, S.; Wang, H.; Bo, D.; Zhang, Y.; Yu, T.; Zhai, D.; Ren, G.; et al. Cuprous Species Distribution over CuCl/NaY Dependent on Acidity and Their CO Adsorption/Desorption Performance Study. Chem. Eng. J. 2022, 433, 133763. [Google Scholar] [CrossRef]
- Padró, C.L.; Rey, E.A.; González Peña, L.F.; Apesteguía, C.R. Activity, Selectivity and Stability of Zn-Exchanged NaY and ZSM5 Zeolites for the Synthesis of o-Hydroxyacetophenone by Phenol Acylation. Microporous Mesoporous Mater. 2011, 143, 236–242. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, R.; Song, W. Influence of HSAPO-34, HZSM-5, and NaY on Pyrolysis of Corn Straw Fermentation Residue via Py-GC/MS. J. Anal. Appl. Pyrolysis 2016, 122, 183–190. [Google Scholar] [CrossRef]
- Ungureanu, A.; Dragoi, B.; Chirieac, A.; Ciotonea, C.; Royer, S.; Duprez, D.; Mamede, A.S.; Dumitriu, E. Composition-Dependent Morphostructural Properties of Ni–Cu Oxide Nanoparticles Confined within the Channels of Ordered Mesoporous SBA-15 Silica. ACS Appl. Mater. Interfaces 2013, 5, 3010–3025. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.A.; Pontes, I.D.; Wurzler, G.T.; Alonso, C.G.; Neto, A.M.; Scaliante, M.H.N.O.; Desouza, M.; Fernandes-Machado, N.R.C. Production of Hydrogen from Bioethanol in Cu-Ni/NbxOy Catalysts Obtained by Different Preparation Methods. Int. J. Hydrogen Energy 2016, 41, 8111–8119. [Google Scholar] [CrossRef]
- Lorenzut, B.; Montini, T.; De Rogatis, L.; Canton, P.; Benedetti, A.; Fornasiero, P. Hydrogen Production through Alcohol Steam Reforming on Cu/ZnO-Based Catalysts. Appl. Catal. B 2011, 101, 397–408. [Google Scholar] [CrossRef]
- Mattos, L.V.; Jacobs, G.; Davis, B.H.; Noronha, F.B. Production of Hydrogen from Ethanol: Review of Reaction Mechanism and Catalyst Deactivation. Chem. Rev. 2012, 112, 4094–4123. [Google Scholar] [CrossRef] [PubMed]
- Śliwa, M.; Samson, K. Steam Reforming of Ethanol over Copper-Zirconia Based Catalysts Doped with Mn, Ni, Ga. Int. J. Hydrogen Energy 2021, 46, 555–564. [Google Scholar] [CrossRef]
- Tang, S.; Li, F.; Liu, J.; Guo, B.; Tian, Z.; Lv, J. MgO/NaY as Modified Mesoporous Catalyst for Methanolysis of Polyethylene Terephthalate Wastes. J. Environ. Chem. Eng. 2022, 10, 107927. [Google Scholar] [CrossRef]
- Boonyoung, P.; Thongratkaew, S.; Rungtaweevoranit, B.; Pengsawang, A.; Praserthdam, P.; Sanpitakseree, C.; Faungnawakij, K. Formic Acid as a Sacrificial Agent for Byproduct Suppression in Glucose Dehydration to 5-Hydroxymethylfurfural Using NaY Zeolite Catalyst. Bioresour. Technol. 2024, 392, 130010. [Google Scholar] [CrossRef]
- Nagpure, A.S.; Mohture, V.M.; Kayarkar, A. Green Synthesis of Highly Dispersed Cu Metal Nanoparticles Catalysts. Inorg. Chem. Commun. 2022, 146, 110118. [Google Scholar] [CrossRef]
- Rossetti, I.; Lasso, J.; Nichele, V.; Signoretto, M.; Finocchio, E.; Ramis, G.; Di Michele, A. Silica and Zirconia Supported Catalysts for the Low-Temperature Ethanol Steam Reforming. Appl. Catal. B 2014, 150–151, 257–267. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).