Spent Lithium-Ion Battery Recycling Using Flotation Technology: Effect of Material Heterogeneity on Separation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterisation of Feed Materials
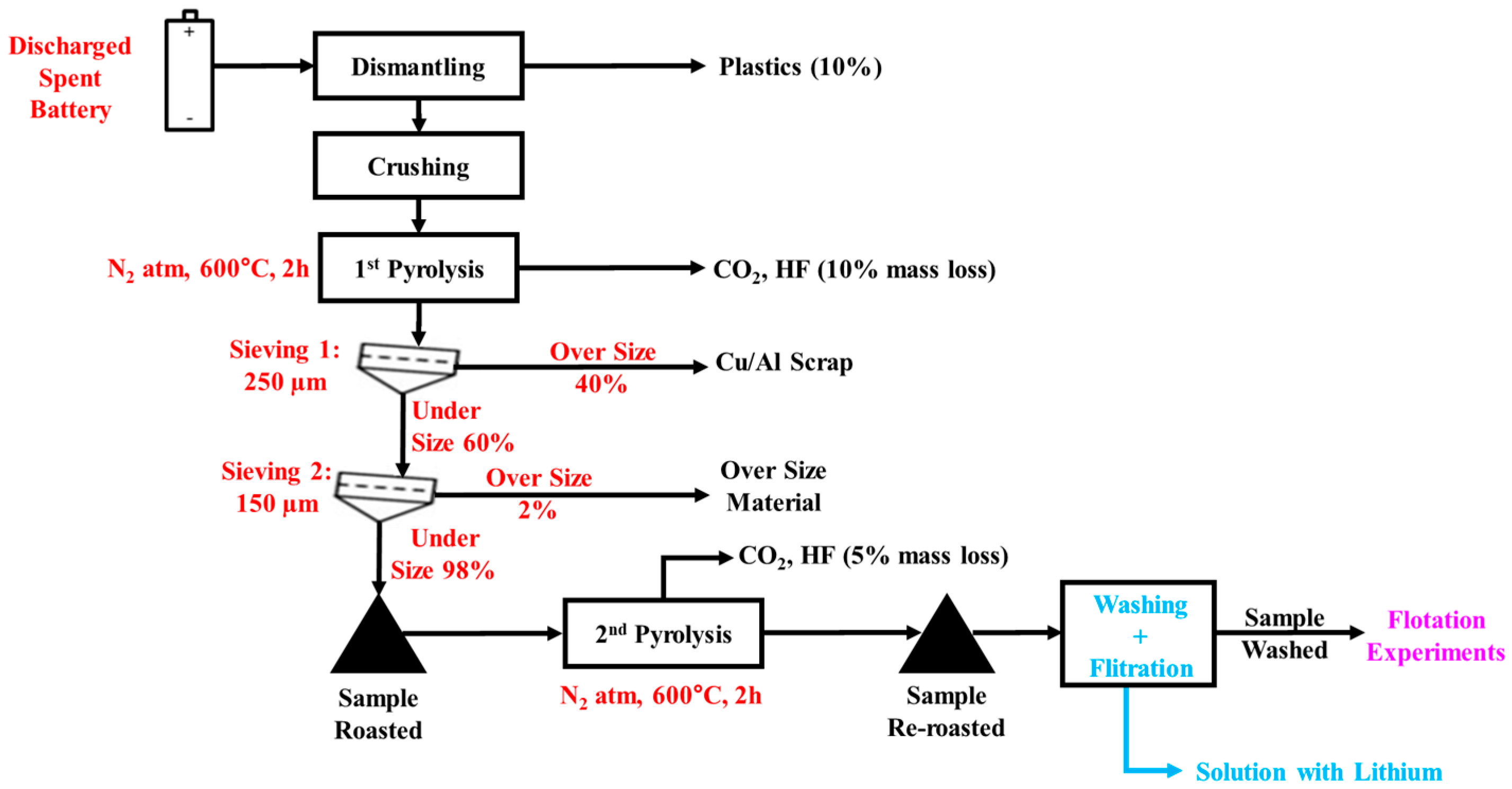
2.2. Sample Preparation Diagram of Feed Materials
2.3. Experimental Procedure of Froth Flotation Trials
2.4. Characterisation of Flotation Products
2.5. Flotation Data Processing Methodology
2.5.1. Flotation Kinetics
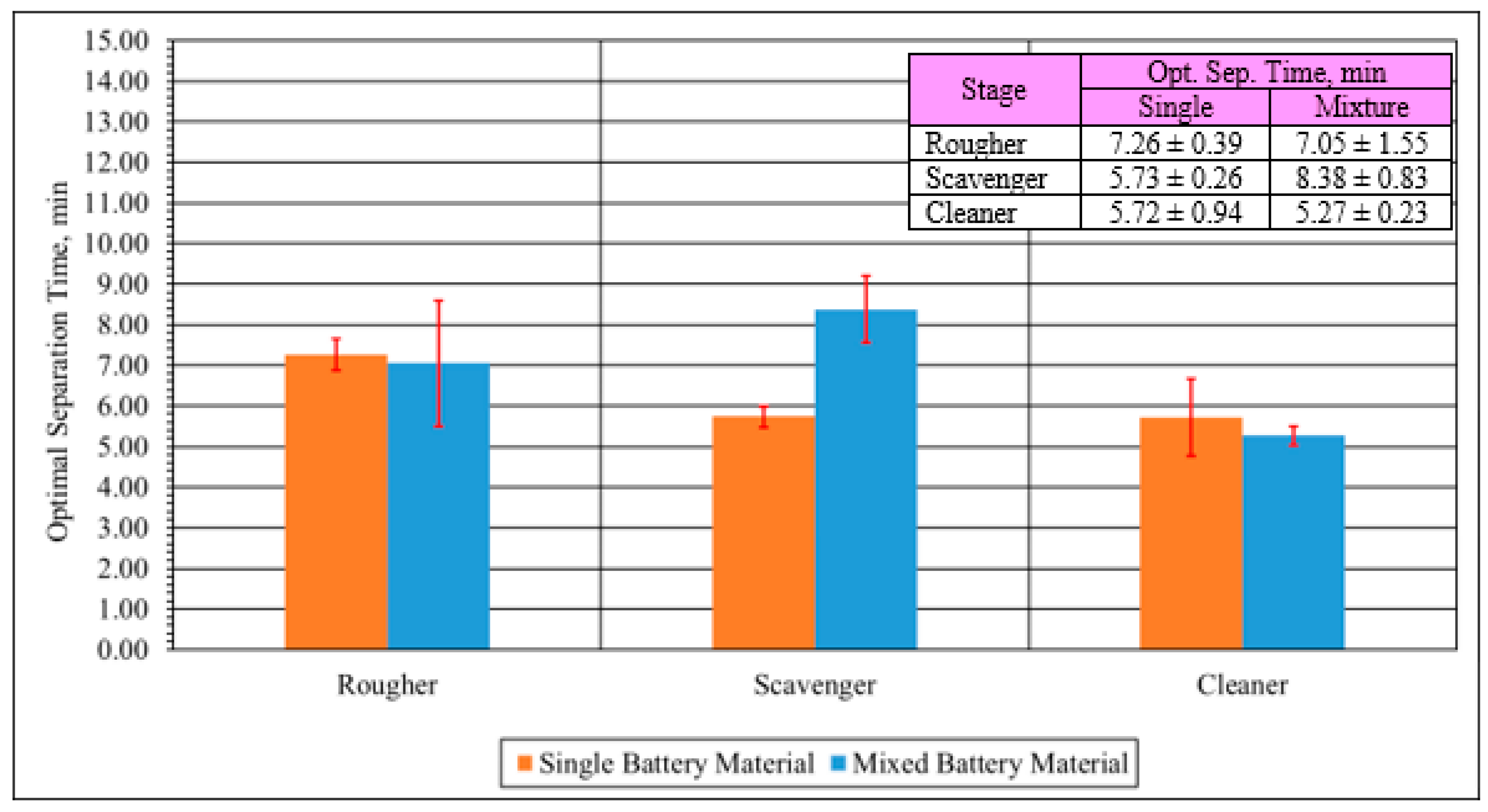
2.5.2. Optimum Flotation Time
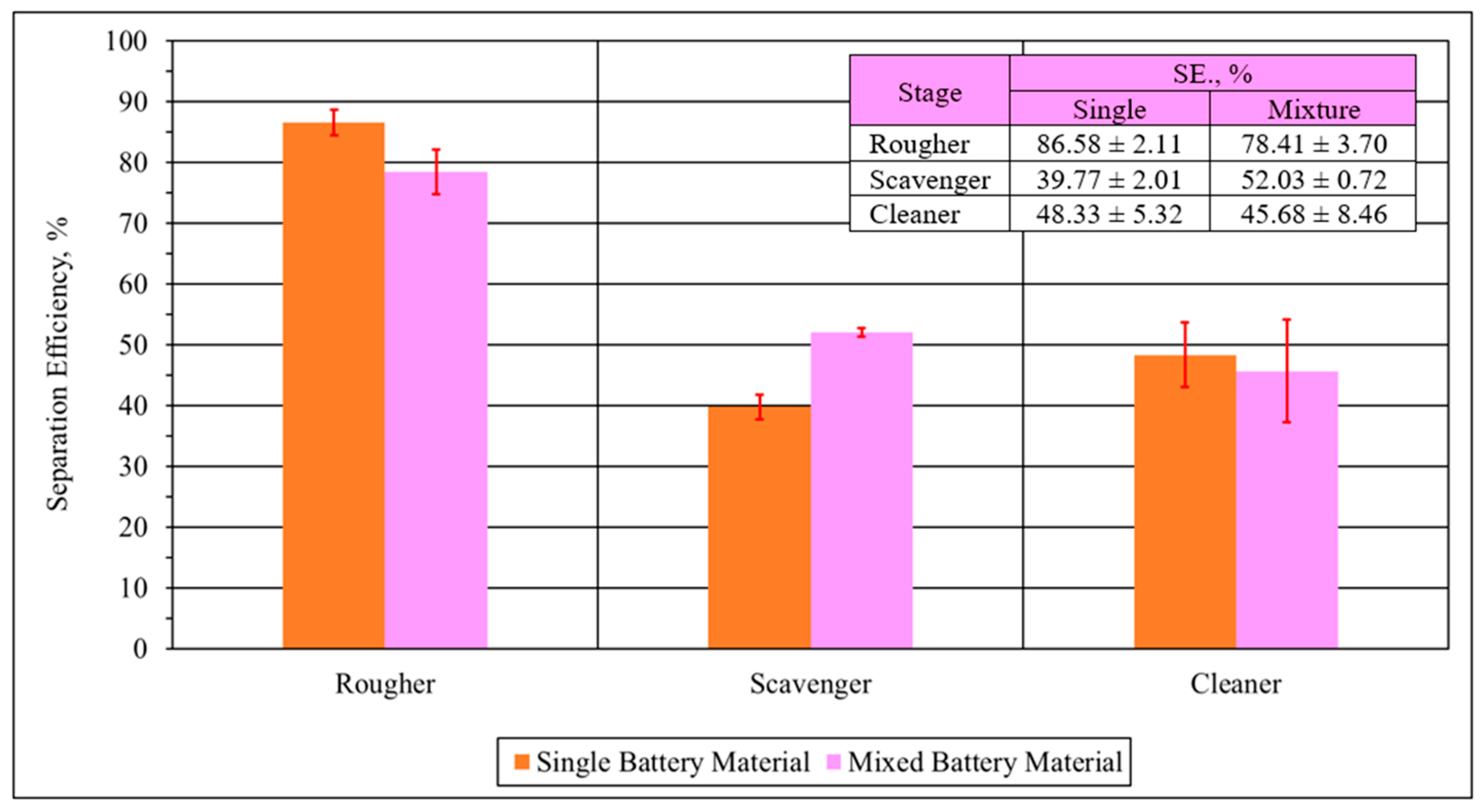
2.5.3. Separation Efficiency
2.5.4. Selectivity Index
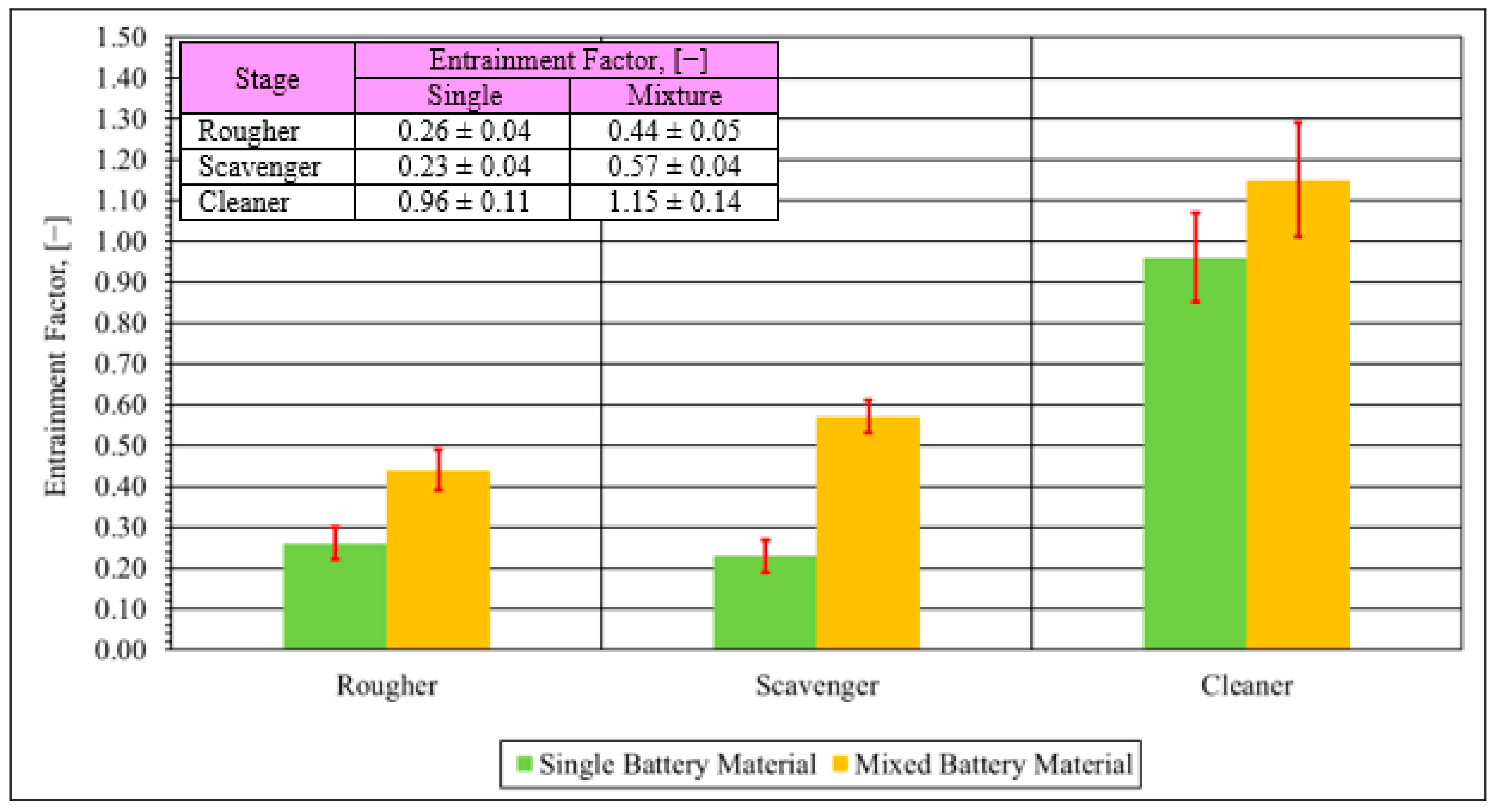
2.5.5. Degree of Entrainment
3. Results
3.1. Characterisation of Feed Materials
3.1.1. Chemical and Physical Analysis of Feed Materials
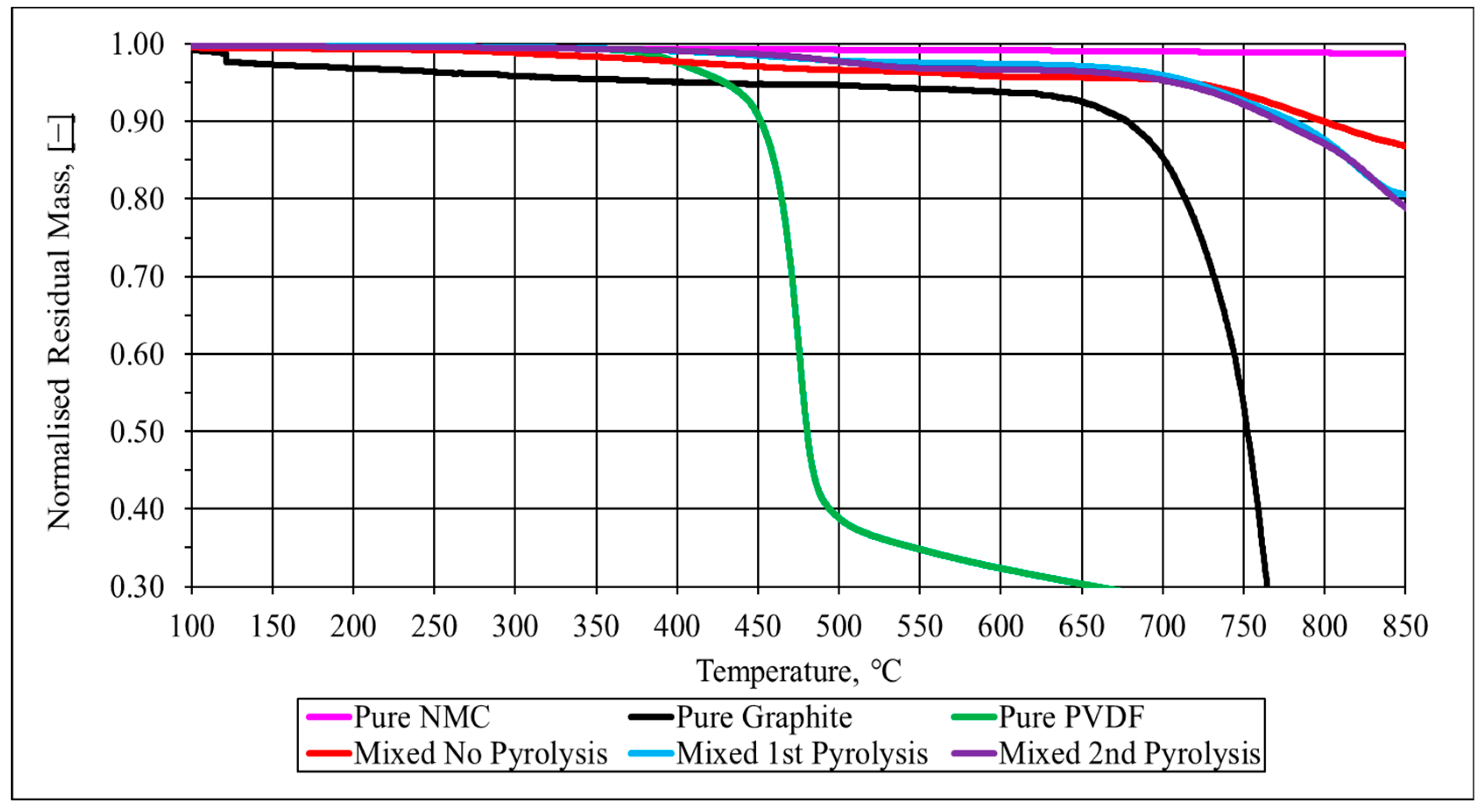
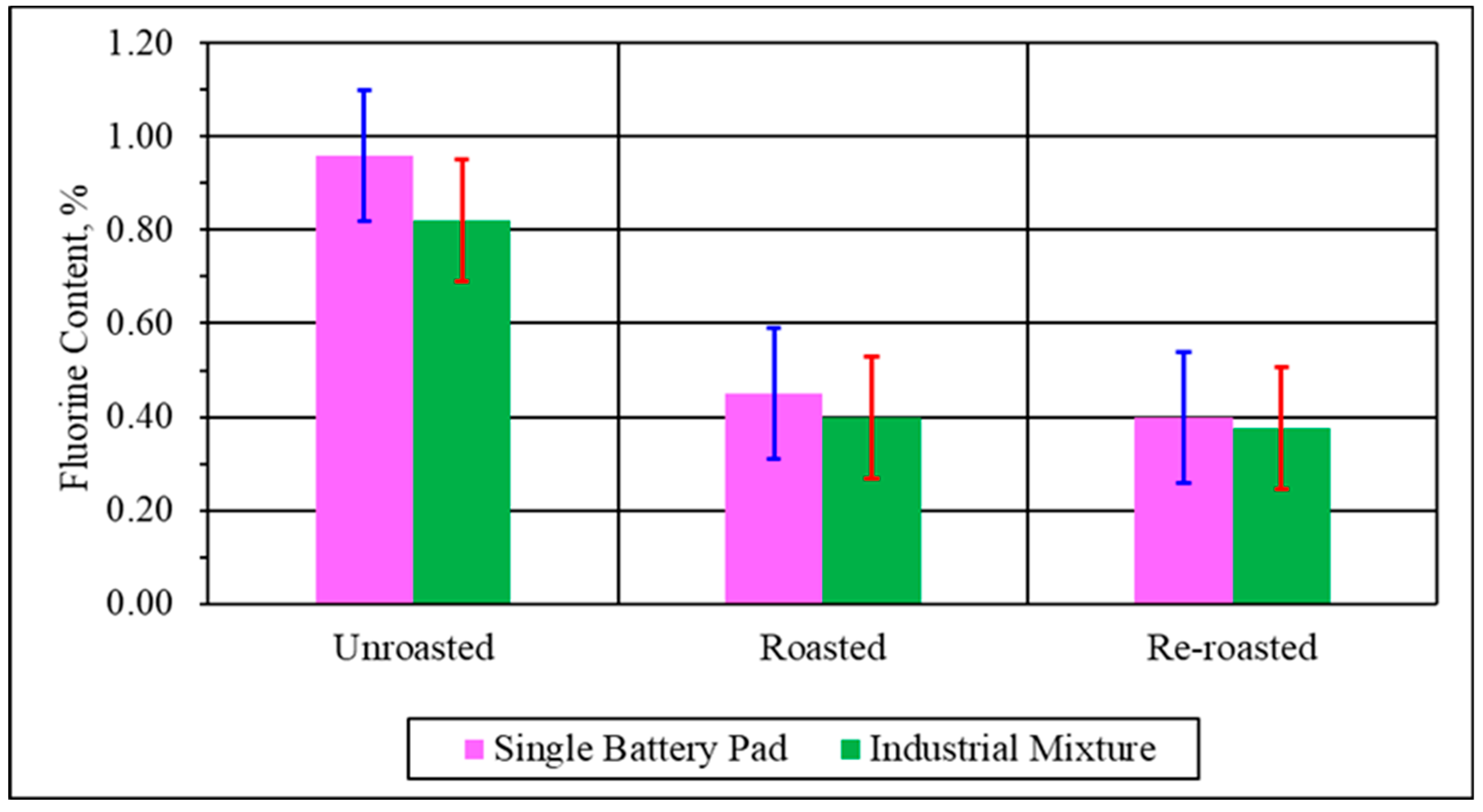
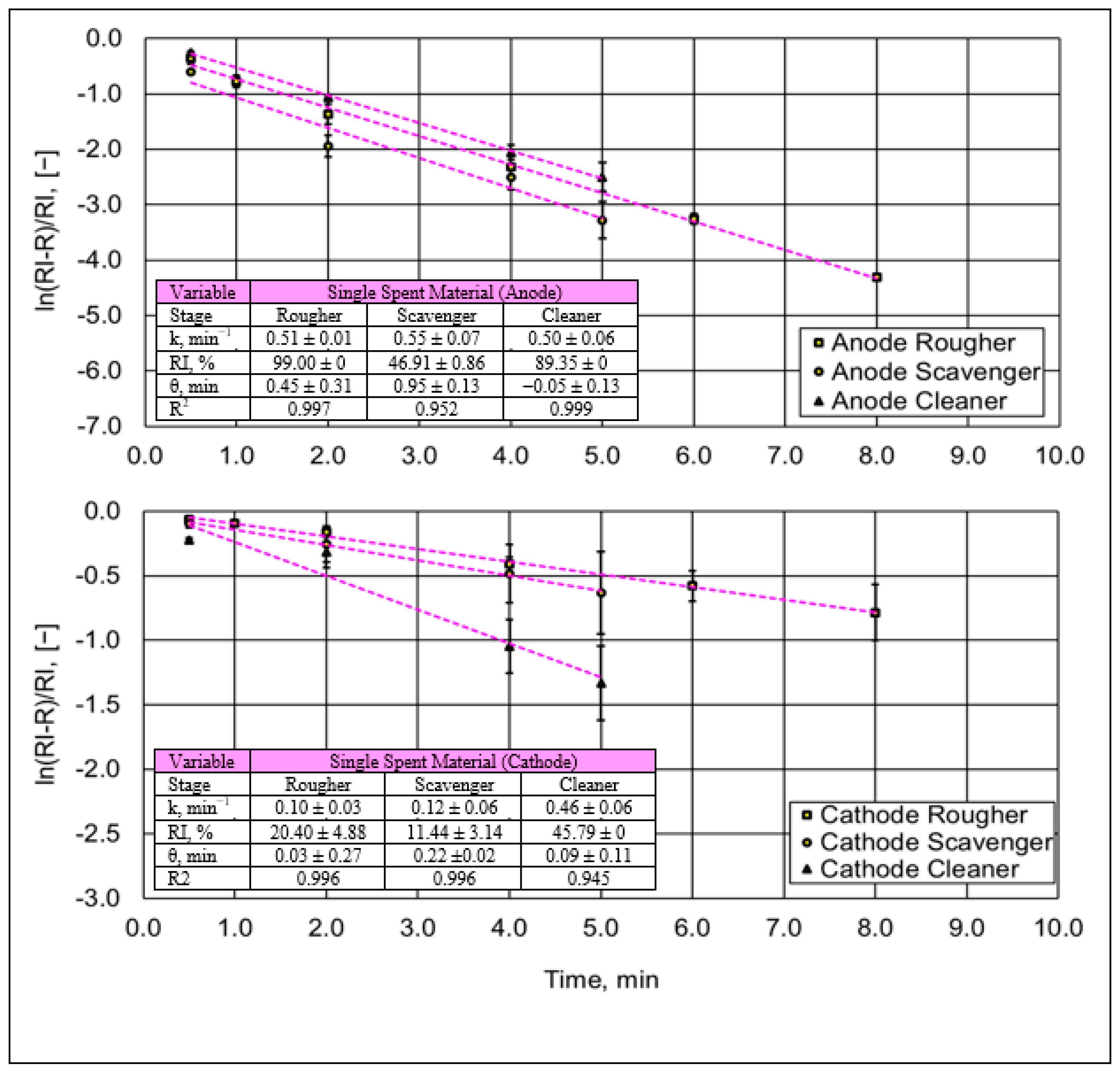
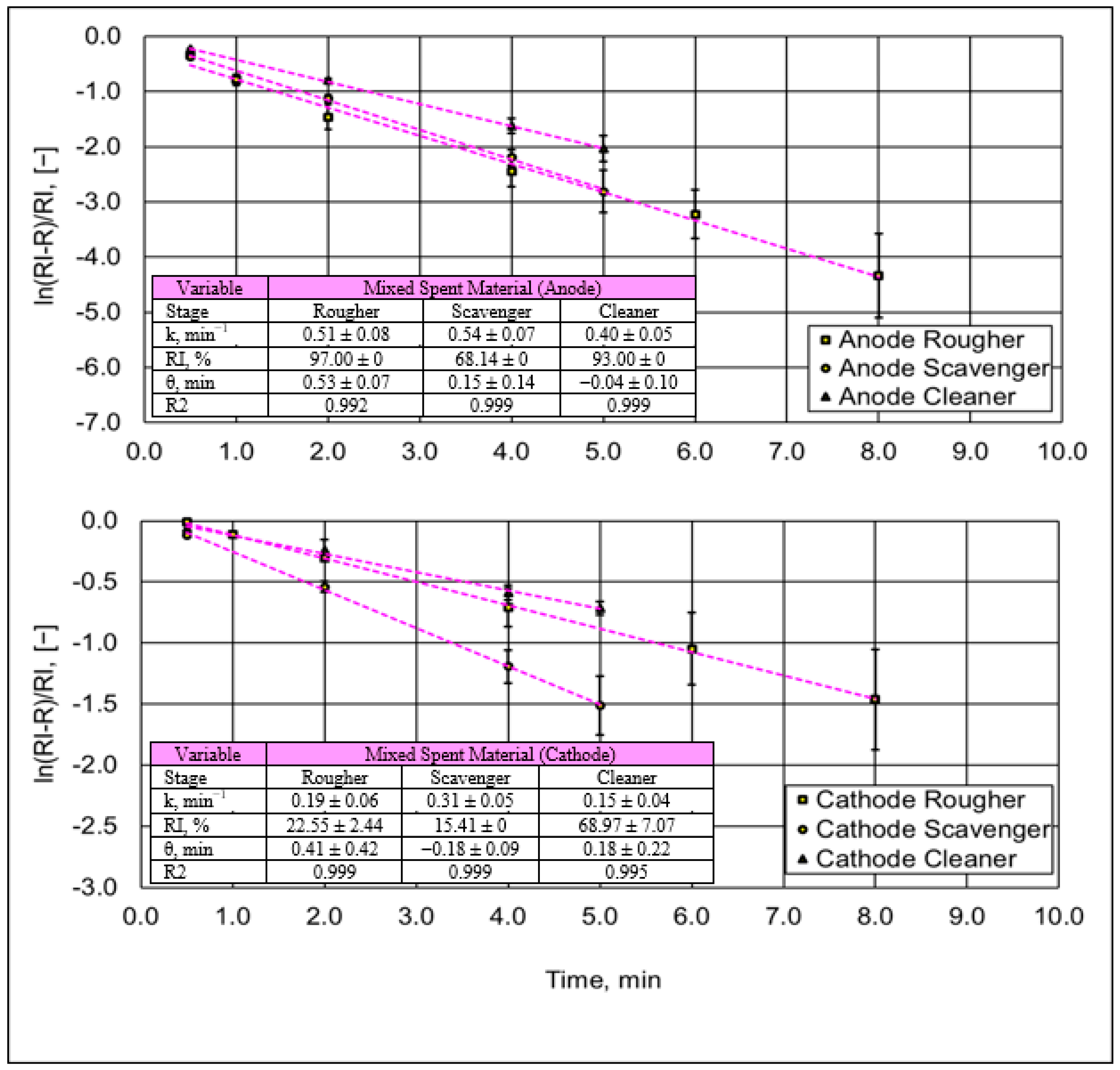
3.1.2. Pyrolysis Analysis in Spent Single Battery Material
3.2. Flotation Trials on Single Spent Lithium-Ion Battery Materials
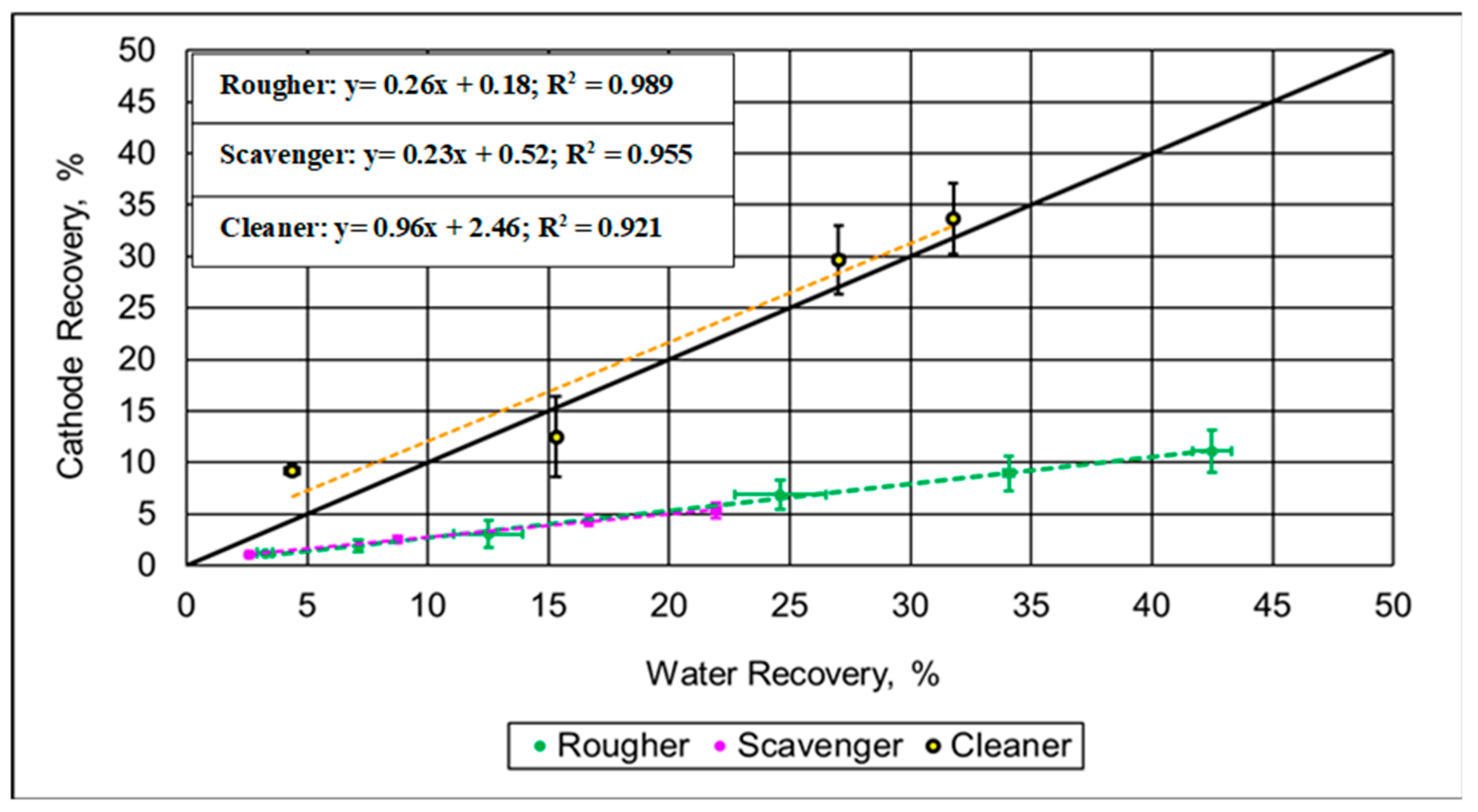
3.2.1. Flotation Trials on Single Spent Battery Materials
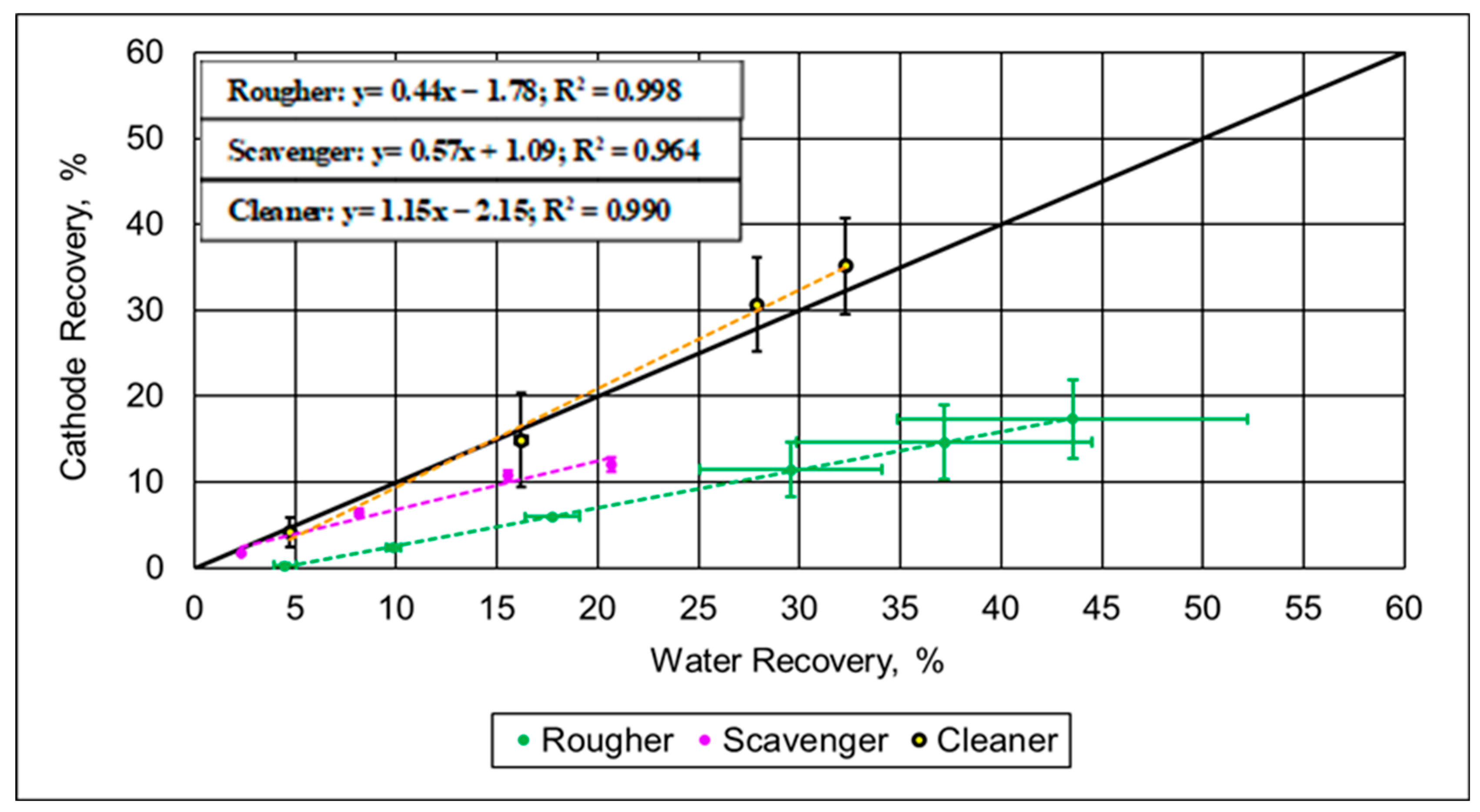
3.2.2. Flotation Trials on Spent Battery Mixed Materials
4. Discussion
5. Conclusions
- The graphite grade in the concentrate of the cleaner stage is higher for the single battery material, with grades over 90 wt% of anode material achieved. In the case of mixed battery materials, the maximum graphite grade is over 81 wt%. The difference is attributed to the impurities in the feed for the mixed material.
- The entrainment results for the rougher and scavenger stages were relatively low for both types of materials, indicating that water entrainment is the main entrainment mechanism.
- A much higher entrainment factor was observed for both materials for the cleaner stage. This indicates an interaction between cathode material particles with the anode materials when cathode materials have a comparatively low concentration.
- The impurity content of the recycled electrode material negatively impacts the flotation performance. Therefore, if possible, battery materials should be processed separately according to their cathode chemistry in order to achieve the best subsequent separation. The selectivity index of the single material was double that of the mixed materials for both the rougher and scavenger stages.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WEF. A Vision for a Sustainable Battery Value Chain in 2030; World Economic Forum: Cologny, Switzerland, 2019; 52p. [Google Scholar]
- Lei, Y.; Zhang, J.; Chen, X.; Min, W.; Wang, R.; Yan, M.; Xu, J. From spent lithium-ion batteries to high performance sodium-ion batteries: A case study. Mater. Today Energy 2022, 26, 100997. [Google Scholar] [CrossRef]
- Parka, Y.; Leea, K. Evaluation of low-purity natural graphite as a cost-effective anode active material for lithium ion batteries. J. Ceram. Process. Res. 2016, 17, 348–351. [Google Scholar]
- Jara, A.D.; Betemariam, A.; Woldetinsae, G.; Kim, J.Y. Purification, application and current market trend of natural graphite: A review. Int. J. Min. Sci. Technol. 2019, 29, 671–689. [Google Scholar] [CrossRef]
- Boxall, N.J.; King, S.; Cheng, K.Y.; Gumulya, Y.; Bruckard, W.; Kaksonen, A.H. Urban mining of lithium-ion batteries in Australia: Current state and future trends. Miner. Eng. 2018, 128, 45–55. [Google Scholar] [CrossRef]
- Baum, Z.J.; Bird, R.E.; Yu, X.; Ma, J. Lithium-Ion Battery Recycling—Overview of Techniques and Trends. ACS Energy Lett. 2022, 7, 712–719. [Google Scholar] [CrossRef]
- Gaines, L.; Sullivan, J.; Burnham, A. Life-Cycle Analysis for Lithium-Ion Battery Production and Recycling. In Proceedings of the Transportation Research Board 90th Annual Meeting, Washington, DC, USA, 23–27 January 2011. [Google Scholar]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Dunn, J.B.; Gaines, L.; Sullivan, J.; Wang, M.Q. Impact of Recycling on Cradle-to-Gate Energy Consumption and Greenhouse Gas Emissions of Automotive Lithium-Ion Batteries. Environ. Sci. Technol. 2012, 46, 12704–12710. [Google Scholar] [CrossRef]
- Held, M.; Tuchschmid, M.; Zennegg, M.; Figi, R.; Schreiner, C.; Mellert, L.D.; Welte, U.; Kompatscher, M.; Hermann, M.; Nachef, L. Thermal runaway and fire of electric vehicle lithium-ion battery and contamination of infrastructure facility. Renew. Sustain. Energy Rev. 2022, 165, 112474. [Google Scholar] [CrossRef]
- Folayan, T.-O.; Lipson, A.L.; Durham, J.L.; Pinegar, H.; Liu, D.; Pan, L. Direct Recycling of Blended Cathode Materials by Froth Flotation. Energy Technol. 2021, 9, 2100468. [Google Scholar] [CrossRef]
- Kim, Y.; Matsuda, M.; Shibayama, A.; Fujita, T. Recovery of LiCoO2 from Wasted Lithium Ion Batteries by using Mineral Processing Technology. Resour. Process. 2004, 51, 3–7. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Saito, K.; Bruckard, W.; Menacho, J.; Hoadley, A. Flotation behavior of the most common electrode materials in lithium ion batteries. Sep. Purif. Technol. 2022, 301, 121885. [Google Scholar] [CrossRef]
- Kaya, M. State-of-the-art lithium-ion battery recycling technologies. Circ. Econ. 2022, 1, 100015. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, D.S. Chapter 16—Flotation. In Mineral Processing Design and Operation; Gupta, A., Yan, D.S., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2006; pp. 555–603. [Google Scholar] [CrossRef]
- Chehreh Chelgani, S.; Rudolph, M.; Kratzsch, R.; Sandmann, D.; Gutzmer, J. A Review of Graphite Beneficiation Techniques. Miner. Process. Extr. Metall. Rev. 2016, 37, 58–68. [Google Scholar] [CrossRef]
- Vanderbruggen, A.; Sygusch, J.; Rudolph, M.; Serna-Guerrero, R. A contribution to understanding the flotation behavior of lithium metal oxides and spheroidized graphite for lithium-ion battery recycling. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127111. [Google Scholar] [CrossRef]
- Verdugo, L.; Zhang, L.; Etschmann, B.; Bruckard, W.; Menacho, J.; Hoadley, A. Effect of lithium ion on the separation of electrode materials in spent lithium ion batteries using froth flotation. Sep. Purif. Technol. 2023, 311, 123241. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Numata, Y. Flotation of graphite. Miner. Eng. 1991, 4, 975–982. [Google Scholar] [CrossRef]
- Dunne, R.C.; Lane, G.S.; Richmond, G.D.; Dioses, J. Flotation data for the design of process plants Part 2—case studies. Miner. Process. Extr. Metall. 2013, 119, 205–215. [Google Scholar] [CrossRef]
- Muzenda, E.; Abdulkareem, A.; Afolabi, A. Reagent Optimization across a UG2 Plant. Lect. Notes Eng. Comput. Sci. 2013, 1, 575–579. [Google Scholar]
- Zhang, S.; Zhao, T.; Wang, J.; Qu, X.; Chen, W.; Han, Y. Determination of fluorine, chlorine and bromine in household products by means of oxygen bomb combustion and ion chromatography. J. Chromatogr. Sci. 2013, 51, 65–69. [Google Scholar] [CrossRef]
- Zhan, R.; Yang, Z.; Bloom, I.; Pan, L. Significance of a Solid Electrolyte Interphase on Separation of Anode and Cathode Materials from Spent Li-Ion Batteries by Froth Flotation. ACS Sustain. Chem. Eng. 2021, 9, 531–540. [Google Scholar] [CrossRef]
- Henry, C.L.; Karakashev, S.I.; Nguyen, P.T.; Nguyen, A.V.; Craig, V.S. Ion specific electrolyte effects on thin film drainage in nonaqueous solvents propylene carbonate and formamide. Langmuir 2009, 25, 9931–9937. [Google Scholar] [CrossRef] [PubMed]
- Michaux, B.; Rudolph, M.; Reuter, M.A. Challenges in predicting the role of water chemistry in flotation through simulation with an emphasis on the influence of electrolytes. Miner. Eng. 2018, 125, 252–264. [Google Scholar] [CrossRef]
- Wang, B.; Peng, Y. The effect of saline water on mineral flotation—A critical review. Miner. Eng. 2014, 66–68, 13–24. [Google Scholar] [CrossRef]
- Murphy, O.; Haji, M.N. A review of technologies for direct lithium extraction from low Li+ concentration aqueous solutions. Front. Chem. Eng. 2022, 4, 1008680. [Google Scholar] [CrossRef]
- Agar, G.E.; Barrett, J. The use of flotation rate data to evaluate reagents. CIM Bull. 1983, 76, 157–162. [Google Scholar]
- Agar, G.E.; Stratton-Crawley, R.; Bruce, T.J. Optimizing the Design of Flotation Circuits; Canadian Institute of Mining, Metallurgy and Petroleum: Westmount, QC, Canada, 1980. [Google Scholar]
- Schulz, N.F. Separation Efficiency; Society for Mining, Metallurgy & Exploration: Englewood, CO, USA, 1969. [Google Scholar]
- Xu, M. Modified flotation rate constant and selectivity index. Miner. Eng. 1998, 11, 271–278. [Google Scholar] [CrossRef]
- Gutierrez, L.; Betancourt, F.; Uribe, L.; Maldonado, M. Influence of Seawater on the Degree of Entrainment in the Flotation of a Synthetic Copper Ore. Minerals 2020, 10, 615. [Google Scholar] [CrossRef]
- Warren, L.J. Determination of the contributions of true flotation and entrainment in batch flotation tests. Int. J. Miner. Process. 1985, 14, 33–44. [Google Scholar] [CrossRef]
- Norori-McCormac, A.; Brito-Parada, P.R.; Hadler, K.; Cole, K.; Cilliers, J.J. The effect of particle size distribution on froth stability in flotation. Sep. Purif. Technol. 2017, 184, 240–247. [Google Scholar] [CrossRef]
- Trahar, W.J.; Warren, L.J. The flotability of very fine particles—A review. Int. J. Miner. Process. 1976, 3, 103–131. [Google Scholar] [CrossRef]
- de Jesus Silva, A.J.; Contreras, M.M.; Nascimento, C.R.; da Costa, M.F. Kinetics of thermal degradation and lifetime study of poly(vinylidene fluoride) (PVDF) subjected to bioethanol fuel accelerated aging. Heliyon 2020, 6, e04573. [Google Scholar] [CrossRef] [PubMed]
- Montaudo, G.; Puglisi, C.; Scamporrino, E.; Vitalini, D. Correlation of thermal degradation mechanisms: Polyacetylene and vinyl and vinylidene polymers. J. Polym. Sci. Part A Polym. Chem. 1986, 24, 301–316. [Google Scholar] [CrossRef]
- Konopacka, Z.; Drzymala, J. Types of particles recovery—Water recovery entrainment plots useful in flotation research. Adsorption 2010, 16, 313–320. [Google Scholar] [CrossRef]





| Processing Condition | Units | Rougher | Scavenger | Cleaner |
|---|---|---|---|---|
| Cell Volume | L | 0.50 | ||
| Stirring Speed | rpm | 900 | ||
| Sample Mass | g | 76 | 68 | 68 |
| Solid Content, Cw | % | 13 | 12 | 12 |
| pH | [−] | 11 | 11 | 11 |
| Collector Dosage * [19] | g/t | 500 | 250 | 167 |
| Flotation Time * [20,21] | min | 8 | 5 | 5 |
| Frother Concentration | mg/L | 30 | ||
| Superficial Gas Rate | cm/s | 1.05 | ||
| Variable | Units | Feed Sample to Flotation Stage | ||
|---|---|---|---|---|
| Rougher | Scavenger | Cleaner | ||
| Anode Content * | % | 46.21 ± 1.68 | 2.40 ± 0.14 | 84.81 ± 0.27 |
| Cathode Content * | % | 49.79 ± 1.65 | 92.43 ± 0.37 | 10.56 ± 0.24 |
| Impurities | % | 4.00 ± 2.00 | 5.16 ± 0.25 | 4.64 ± 0.52 |
| Particle Size D50 | µm | 21.49 ± 1.20 | 15.17 ± 1.10 | 19.48 ± 1.21 |
| Variable | Units | Feed Sample to Flotation Stage | ||
|---|---|---|---|---|
| Rougher | Scavenger | Cleaner | ||
| Anode Content * | % | 36.61 ± 3.35 | 5.62 ± 0.15 | 69.15 ± 0.92 |
| Cathode Content * | % | 45.91 ± 2.45 | 78.98 ± 1.30 | 15.62 ± 0.53 |
| Impurities | % | 17.47 ± 2.60 | 15.40 ± 1.55 | 15.23 ± 1.80 |
| Particle Size D50 | µm | 16.51 ± 1.15 | 14.95 ± 1.45 | 18.09 ± 1.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verdugo, L.; Zhang, L.; Etschmann, B.; Brugger, J.; Bruckard, W.; Menacho, J.; Molina, L.; Hoadley, A. Spent Lithium-Ion Battery Recycling Using Flotation Technology: Effect of Material Heterogeneity on Separation Performance. Processes 2024, 12, 1363. https://doi.org/10.3390/pr12071363
Verdugo L, Zhang L, Etschmann B, Brugger J, Bruckard W, Menacho J, Molina L, Hoadley A. Spent Lithium-Ion Battery Recycling Using Flotation Technology: Effect of Material Heterogeneity on Separation Performance. Processes. 2024; 12(7):1363. https://doi.org/10.3390/pr12071363
Chicago/Turabian StyleVerdugo, Luis, Lian Zhang, Barbara Etschmann, Joël Brugger, Warren Bruckard, Jorge Menacho, Lorena Molina, and Andrew Hoadley. 2024. "Spent Lithium-Ion Battery Recycling Using Flotation Technology: Effect of Material Heterogeneity on Separation Performance" Processes 12, no. 7: 1363. https://doi.org/10.3390/pr12071363






