High-Surface-Area Co-Cu-B Monolithic Self-Supported Catalyst for Efficient Sodium Borohydride Hydrolysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Standards and Reagents
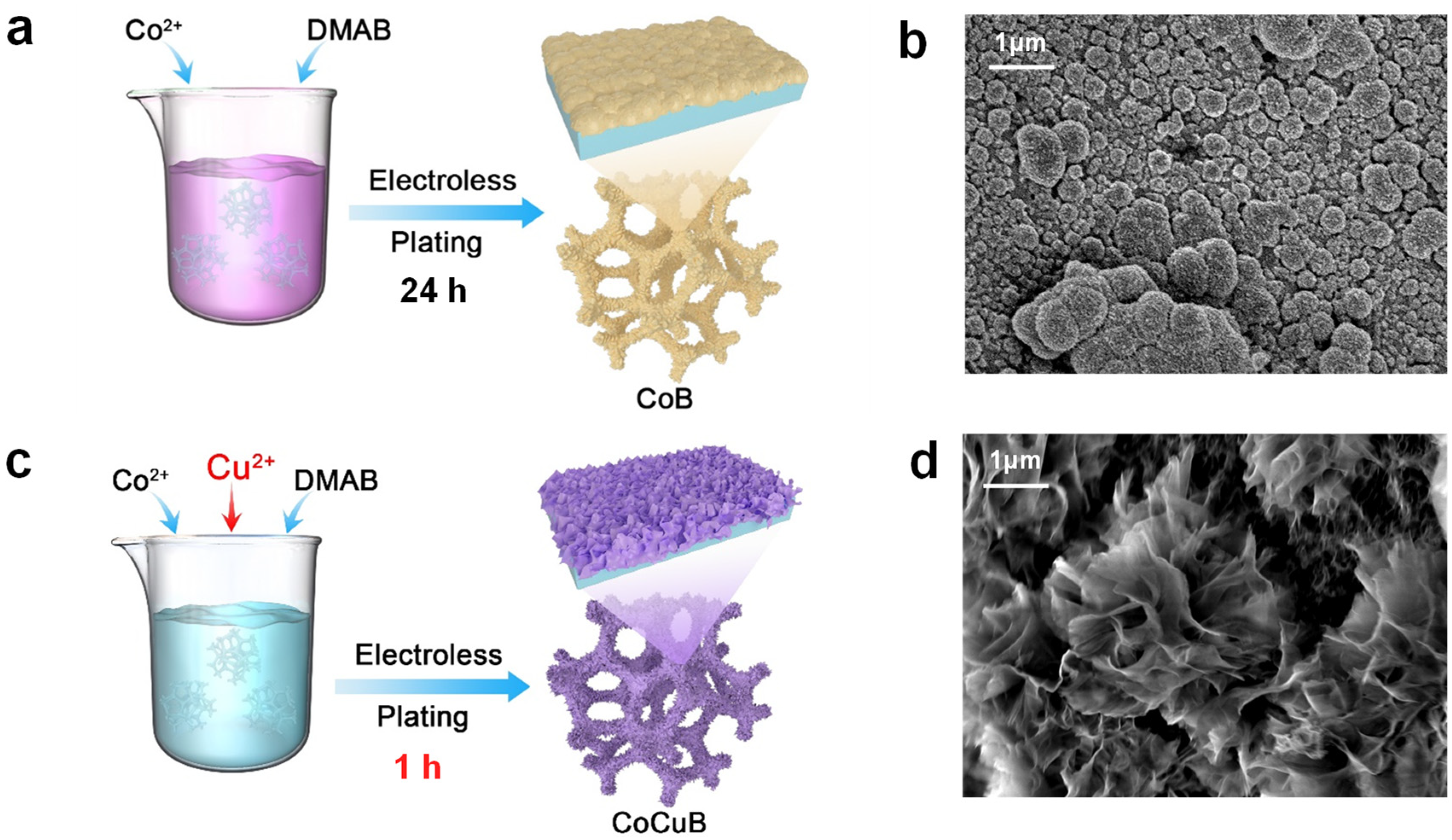
2.2. Synthesis of Co-B Catalyst
2.3. Synthesis of Co-Cu-B Catalyst
2.4. Characterization of Materials
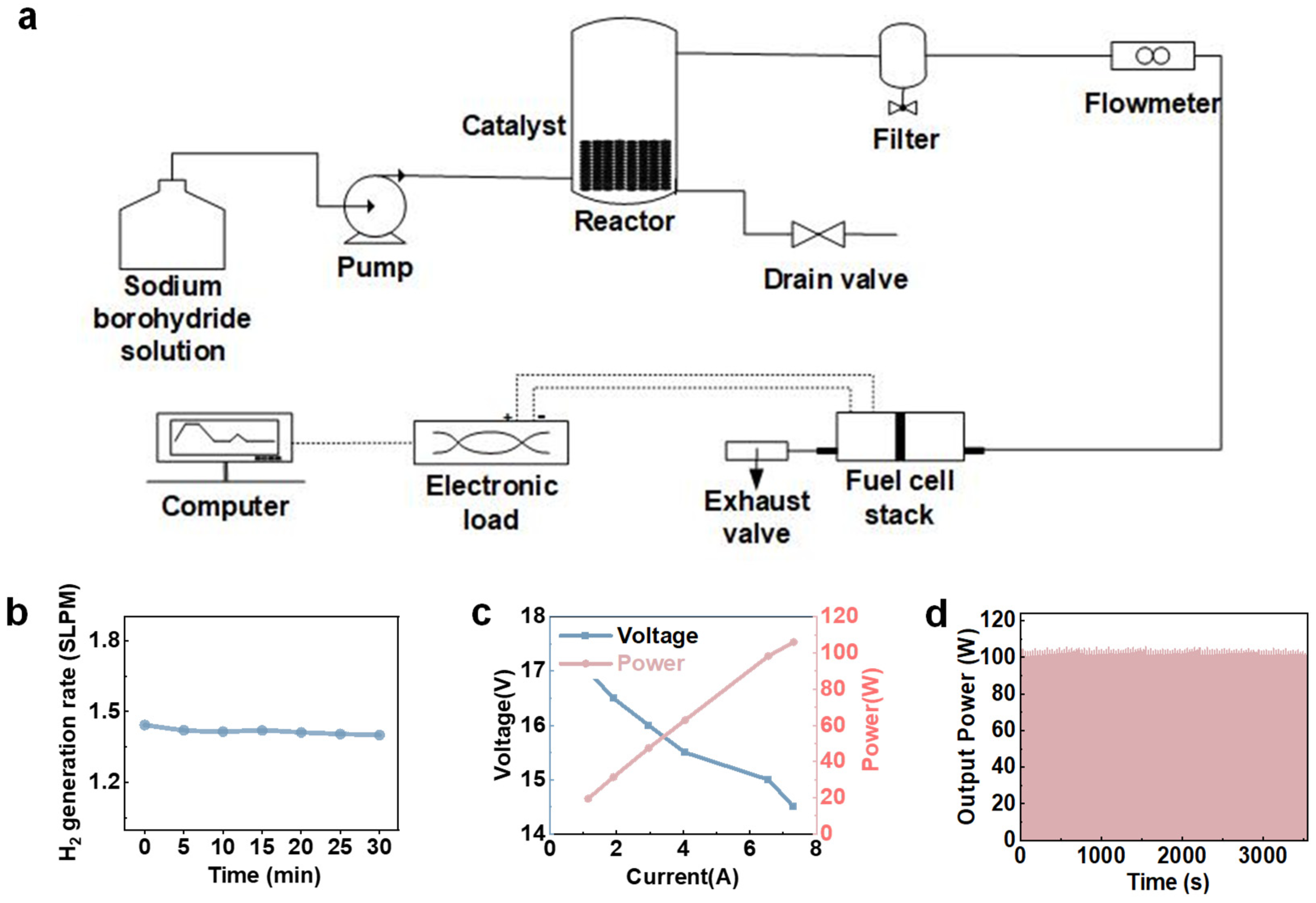
2.5. Reaction Parameter Evaluation
3. Results and Discussion
3.1. Material Characterization
3.2. Catalytic Performance
3.3. H2 Supply System Application
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruslan, N.; Yahya, M.S.; Siddique, M.N.I.; Yengantiwar, A.P.; Ismail, M.; Awal, M.R.; Mohd Yusoff, M.Z.; Abdul Halim Yap, M.F.A.; Mustafa, N.S. Review on Magnesium Hydride and Sodium Borohydride Hydrolysis for Hydrogen Production. Crystals 2022, 12, 1376. [Google Scholar] [CrossRef]
- Abdelhamid, H.N. A Review on Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2021, 46, 726–765. [Google Scholar] [CrossRef]
- Yao, Q.; Zhang, X.; Lu, Z.-H.; Xu, Q. Metal-Organic Framework-Based Catalysts for Hydrogen Production from Liquid-Phase Chemical Hydrides. Coord. Chem. Rev. 2023, 493, 215302. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, Y.; Guo, Q. Research Progress on Catalysts for Hydrogen Generation through Sodium Borohydride Alcoholysis. Int. J. Hydrogen Energy 2021, 47, 5929–5946. [Google Scholar] [CrossRef]
- Long, B.; Chen, J.; Sharshir, S.W.; Ibrahim, L.; Zhou, W.; Wang, C.; Wang, L.; Yuan, Z. The Mechanism and Challenges of Cobalt-Boron-Based Catalysts in the Hydrolysis of Sodium Borohydride. J. Mater. Chem. A 2024, 12, 5606–5625. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, T.; Zhang, H.; Zhao, Y. Advances in Catalysts for Hydrogen Production by Methanolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2022, 47, 14589–14610. [Google Scholar] [CrossRef]
- Schlesinger, H.I.; Brown, H.C.; Finholt, A.E.; Gilbreath, J.R.; Hoekstra, H.R.; Hyde, E.K. Sodium Borohydride, Its Hydrolysis and Its Use as a Reducing Agent and in the Generation of Hydrogen1. J. Am. Chem. Soc. 1953, 75, 215–219. [Google Scholar] [CrossRef]
- Baytar, O.; Şahin, Ö.; Ekinci, A. Effect of Environmentally Friendly and Efficient Metal-Free Hydrochars as Catalysts on Sodium Borohydride Hydrolysis. Fuel 2023, 346, 128308. [Google Scholar] [CrossRef]
- Rong Weiren, Q.D. Decomposition of Borohydride Solution and Stabilization Mechanism of NaOH. J. Fudan Univ. Sci. 1998, 3, 276–278. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, N.; Xu, Q.; Yu, J. Nanopore-Supported Metal Nanocatalysts for Efficient Hydrogen Generation from Liquid-Phase Chemical Hydrogen Storage Materials. Adv. Mater. 2020, 32, 2001818. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Luo, Y.; Wang, F. Study on Preparation and Performance of Ru-Fe/GO Catalyst for Sodium Borohydride Alcoholysis to Produce Hydrogen. Fuller. Nanotub. Carbon Nanostructures 2020, 28, 786–793. [Google Scholar] [CrossRef]
- Arzac, G.M.; Paladini, M.; Godinho, V.; Beltrán, A.M.; Jiménez de Haro, M.C.; Fernández, A. Strong Activation Effect on a Ru-Co-c Thin Film Catalyst for the Hydrolysis of Sodium Borohydride. Sci. Rep. 2018, 8, 9755. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Agnihotri, N.; De, A. Hydrolysis of Sodium Borohydride Using Ru–Co-PEDOT Nanocomposites as Catalyst. Chem. Eng. J. 2015, 264, 531–537. [Google Scholar] [CrossRef]
- Rakap, M.; Özkar, S. Intrazeolite Cobalt(0) Nanoclusters as Low-Cost and Reusable Catalyst for Hydrogen Generation from the Hydrolysis of Sodium Borohydride. Appl. Catal. B Environ. 2009, 91, 21–29. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; von Colbe, J.B.; Blanchard, D.; Bowman, R.C., Jr.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P.; et al. Materials for Hydrogen-Based Energy Storage—Past, Recent Progress and Future Outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Li, J.; Hong, X.; Wang, Y.; Luo, Y.; Huang, P.; Li, B.; Zhang, K.; Zou, Y.; Sun, L.; Xu, F.; et al. Encapsulated Cobalt Nanoparticles as a Recoverable Catalyst for the Hydrolysis of Sodium Borohydride. Energy Storage Mater. 2020, 27, 187–197. [Google Scholar] [CrossRef]
- Ganesan, K.; Hayagreevan, C.; Rahul, R.; Jeevagan, A.J.; Adinaveen, T.; Bhuvaneshwari, D.S.; Muthukumar, P.; Amalraj, M. Catalytic Hydrolysis of Sodium Borohydride for Hydrogen Production Using Phosphorylated Silica Particles. Environ. Sci. Pollut. Res. 2022, 30, 21199–21212. [Google Scholar] [CrossRef] [PubMed]
- Eugénio, S.; Demirci, U.B.; Silva, T.M.; Carmezim, M.J.; Montemor, M.F. Copper-Cobalt Foams as Active and Stable Catalysts for Hydrogen Release by Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2016, 41, 8438–8448. [Google Scholar] [CrossRef]
- Obligacion, J.V.; Chirik, P.J. Earth-Abundant Transition Metal Catalysts for Alkene Hydrosilylation and Hydroboration. Nat. Rev. Chem. 2018, 2, 15–34. [Google Scholar] [CrossRef]
- Wang, L.; Nitopi, S.; Wong, A.B.; Snider, J.L.; Nielander, A.C.; Morales-Guio, C.G.; Orazov, M.; Higgins, D.C.; Hahn, C.; Jaramillo, T.F. Electrochemically Converting Carbon Monoxide to Liquid Fuels by Directing Selectivity with Electrode Surface Area. Nat. Catal. 2019, 2, 702–708. [Google Scholar] [CrossRef]
- Koh, J.S.; Kim, D.H.; Lee, S.H.; Kim, M.S. Hydrogen Generation System for Fuel Cells Based on High Pressure Hydrolysis of Solid-State Sodium Borohydride. Energy Convers. Manag. 2023, 281, 116850. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu, S.F.; Dizaji, A.K.; Altıntaş, Z.; Khoshsima, S.; Uzun, A.; Balcı, Ö. Nanocrystalline Cobalt–Nickel–Boron (Metal Boride) Catalysts for Efficient Hydrogen Production from the Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2021, 46, 7974–7988. [Google Scholar] [CrossRef]
- Shen, X.; Dai, M.; Gao, M.; Zhao, B.; Ding, W. Solvent Effects in the Synthesis of CoB Catalysts on Hydrogen Generation from Hydrolysis of Sodium Borohydride. Chin. J. Catal. 2013, 34, 979–985. [Google Scholar] [CrossRef]
- Paksoy, A.; Kurtoğlu-Öztulum, S.F.; Yağcı, M.B.; Balcı-Çağıran, Ö. Low-Cost and Reusable Iron- and Nickel-Based Metal Boride Nanoparticles for Efficient Catalytic Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2022, 47, 36898–36913. [Google Scholar] [CrossRef]
- Liu, J.; Li, B.; Dong, Y.; Liu, Q.; Song, Y.; Guo, Y.; Zhao, Y.; Li, X.; Xiong, J. Hydrolysis of Ammonia Borane for Hydrogen Generation on Bimetallic CoCu Catalysts: Regulation of Synergistic Effect. Catal. Lett. 2024, 154, 461–472. [Google Scholar] [CrossRef]
- Ding, X.-L.; Yuan, X.; Jia, C.; Ma, Z.-F. Hydrogen Generation from Catalytic Hydrolysis of Sodium Borohydride Solution Using Cobalt–Copper–Boride (Co–Cu–B) Catalysts. Int. J. Hydrogen Energy 2010, 35, 11077–11084. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, K.; Zhang, D.; Cao, Z.; Zhang, K.; Xie, Y.; Zhou, G.; Li, G.; Bai, S. Cobalt–Copper–Boron Nanoparticles as Catalysts for the Efficient Hydrolysis of Alkaline Sodium Borohydride Solution. Int. J. Hydrogen Energy 2020, 45, 9845–9853. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Q.; Yang, Y.; Yu, Y.-N.; Zhang, Y.; Zhang, M.; Wei, L.; Lu, Y. CoO-Co2P Composite Nanosheets as Highly Active Catalysts for Sodium Borohydride Hydrolysis to Generate Hydrogen. Funct. Mater. Lett. 2020, 13, 2051025. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Qi, K.; Cao, Z.; Zhang, K.; Wu, S. Nanostructured Cobalt–Phosphorous Catalysts for Hydrogen Generation from Hydrolysis of Sodium Borohydride Solution. Renew. Energy 2016, 13, 6. [Google Scholar] [CrossRef]
- Han, J.; Yuan, W.; Wei, M.; Zhang, B.; Guo, Y. Real-Time Tunable Hydrogen Generation from Hydrolysis of Borohydrides Using 3D Magnetic Catalysts. Inorg. Chem. Front. 2023, 10, 1876–1886. [Google Scholar] [CrossRef]
- Paladini, M.; Arzac, G.M.; Godinho, V.; Haro, M.C.J.D.; Fernández, A. Supported Co Catalysts Prepared as Thin Films by Magnetron Sputtering for Sodium Borohydride and Ammonia Borane Hydrolysis. Appl. Catal. B Environ. 2014, 158, 400–409. [Google Scholar] [CrossRef]
- Faghihi, M.; Akbarbandari, F.; Zabihi, M.; Pazouki, M. Synthesis and Characterization of the Magnetic Supported Metal-Organic Framework Catalysts (CuCoBTC@MAC and CuBTC@MAC) for the Hydrogen Production from Sodium Borohydride. Mater. Chem. Phys. 2021, 267, 124599. [Google Scholar] [CrossRef]
- Wei, M.; Han, J.; Wu, Q.; Li, J.; Ji, Z.; Li, Z.; Liang, J.; Guo, Y. Monolithic CoMoB Based Catalyst Enabling High Water-Sources Adaptive Hydrolysis of Borohydrides. Chem. Eng. J. 2024, 482, 148879. [Google Scholar] [CrossRef]
- Han, J.; Niu, W.; Yuan, W.; Wei, M.; Zhang, B.; Guo, Y. Rational 3D Structure of Monolithic Catalysts for Enhanced Hydrolytic Dehydrogenation of Ammonia Borane. ACS Sustain. Chem. Eng. 2023, 11, 8462–8473. [Google Scholar] [CrossRef]
- Zhuang, D.-W.; Dai, H.-B.; Zhong, Y.-J.; Sun, L.-X.; Wang, P. A New Reactivation Method towards Deactivation of Honeycomb Ceramic Monolith Supported Cobalt–Molybdenum–Boron Catalyst in Hydrolysis of Sodium Borohydride. Int. J. Hydrogen Energy 2015, 40, 9373–9381. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Tan, B.; Li, Z.; Cui, Y.; He, G. Ce-Co Catalyst with High Surface Area and Uniform Mesoporous Channels Prepared by Template Method for Hg0 Oxidation. Catal. Commun. 2017, 98, 5–8. [Google Scholar] [CrossRef]
- Lu, C.; Geng, H.; Zhu, Y.; Ma, J.; Wang, R.; Liu, X.; Tu, G. 3D Flower-Shape Co/Cu Bimetallic Nanocomposites with Excellent Wideband Electromagnetic Microwave Absorption. Appl. Surf. Sci. 2023, 615, 156219. [Google Scholar] [CrossRef]
- Wu, K.-J.; Tse, E.C.M.; Shang, C.; Guo, Z. Nucleation and Growth in Solution Synthesis of Nanostructures—From Fundamentals to Advanced Applications. Prog. Mater. Sci. 2022, 123, 100821. [Google Scholar] [CrossRef]
- Ji, L.; Lin, J.; Zeng, H.C. Metal−Support Interactions in Co/Al 2 O 3 Catalysts: A Comparative Study on Reactivity of Support. J. Phys. Chem. B 2000, 104, 1783–1790. [Google Scholar] [CrossRef]
- Balčiūnaitė, A.; Sukackienė, Z.; Antanavičiūtė, K.; Vaičiūnienė, J.; Naujokaitis, A.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Investigation of Hydrogen Generation from Sodium Borohydride Using Different Cobalt Catalysts. Int. J. Hydrogen Energy 2021, 46, 1989–1996. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, J.; Cheng, F.; Liang, J.; Tao, Z.; Chen, J. Electroless-Deposited Co–P Catalysts for Hydrogen Generation from Alkaline NaBH4 Solution. Int. J. Hydrog. Energy 2010, 35, 8363–8369. [Google Scholar] [CrossRef]
- Oh, T.H.; Kwon, S. Effect of Manufacturing Conditions on Properties of Electroless Deposited Co–P/Ni Foam Catalyst for Hydrolysis of Sodium Borohydride Solution. Int. J. Hydrog. Energy 2012, 37, 15925–15937. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, M.; Fu, W.; Si, S.; Wei, L.; Zhao, X.; Wang, Y. Mn Doped CoP/Ni Foam Catalyst for Hydrogen Generation from Hydrolysis of Sodium Borohydride. Mater. Lett. 2022, 308, 131166. [Google Scholar] [CrossRef]
- Wei, Y.; Huang, X.; Wang, J.; Yu, H.; Zhao, X.; Cheng, D. Synthesis of Bifunctional Non-Noble Monolithic Catalyst Co-W-P/Carbon Cloth for Sodium Borohydride Hydrolysis and Reduction of 4-Nitrophenol. Int. J. Hydrog. Energy 2017, 42, 25860–25868. [Google Scholar] [CrossRef]
- Guo, Y.; Dong, Z.; Cui, Z.; Zhang, X.; Ma, J. Promoting Effect of W Doped in Electrodeposited Co–P Catalysts for Hydrogen Generation from Alkaline NaBH4 Solution. Int. J. Hydrog. Energy 2012, 37, 1577–1583. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, R.; Meng, L.; Wang, Y.; Li, G.; Xin, S.; Zhao, X.; Zhang, K. Hydrogen Generation from Alkaline NaBH4 Solution Using a Dandelion-like Co–Mo–B Catalyst Supported on Carbon Cloth. Int. J. Hydrog. Energy 2017, 42, 9945–9951. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, W.; Yang, X.; Liu, C.; Xue, L.; Niu, W.; Yan, Q.; Zhu, Y.; Han, J.; Guo, W.; Zhang, B. High-Surface-Area Co-Cu-B Monolithic Self-Supported Catalyst for Efficient Sodium Borohydride Hydrolysis. Processes 2024, 12, 1384. https://doi.org/10.3390/pr12071384
Yuan W, Yang X, Liu C, Xue L, Niu W, Yan Q, Zhu Y, Han J, Guo W, Zhang B. High-Surface-Area Co-Cu-B Monolithic Self-Supported Catalyst for Efficient Sodium Borohydride Hydrolysis. Processes. 2024; 12(7):1384. https://doi.org/10.3390/pr12071384
Chicago/Turabian StyleYuan, Wuning, Xiao Yang, Cheng Liu, Liangyao Xue, Wenzhe Niu, Qisheng Yan, Yajie Zhu, Junchao Han, Wen Guo, and Bo Zhang. 2024. "High-Surface-Area Co-Cu-B Monolithic Self-Supported Catalyst for Efficient Sodium Borohydride Hydrolysis" Processes 12, no. 7: 1384. https://doi.org/10.3390/pr12071384
APA StyleYuan, W., Yang, X., Liu, C., Xue, L., Niu, W., Yan, Q., Zhu, Y., Han, J., Guo, W., & Zhang, B. (2024). High-Surface-Area Co-Cu-B Monolithic Self-Supported Catalyst for Efficient Sodium Borohydride Hydrolysis. Processes, 12(7), 1384. https://doi.org/10.3390/pr12071384






