Application of Anti-Solvent Crystallization for High-Purity Potash Production from K-Feldspar Leaching Solution
Abstract
:1. Background and Objectives
1.1. Background
1.2. Objectives
2. Materials and Method
2.1. Materials
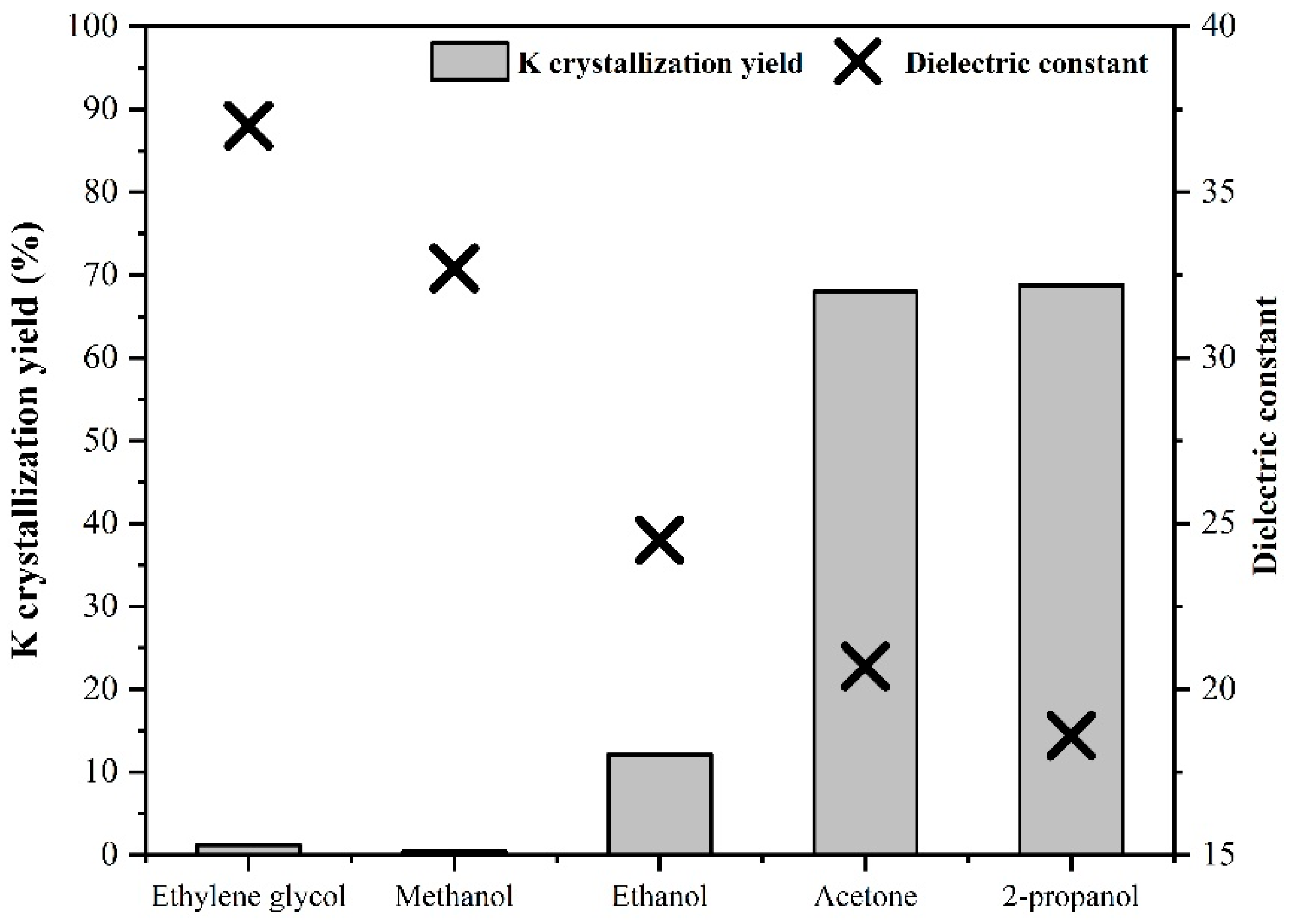
2.2. Anti-Solvents Selection
| Anti-Solvent | Molecular Structure | Molecular Weight (g·mol−1) | Density (g·mL−1) | Boiling Point (°C) | Dielectric Constant | Viscosity (cP) |
|---|---|---|---|---|---|---|
| Methanol |  | 32 | 0.792 | 64.6 | 32.7 | 0.59 |
| Ethanol | 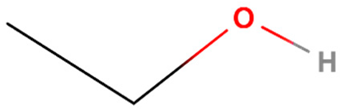 | 46 | 0.789 | 78 | 24.5 | 1.1 |
| Acetone | 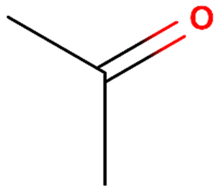 | 58 | 0.784 | 56 | 20.7 | 0.36 |
| 2-propanol |  | 60 | 0.786 | 82.4 | 18.6 | 2.4 |
| Ethylene glycol | 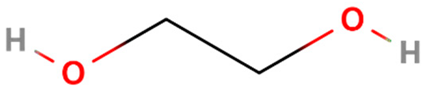 | 62 | 1.11 | 197 | 37 | 18.4 |
2.3. Experimental Procedure
3. Results and Discussion
3.1. Results of Anti-Solvent Screening Tests
- “Energy required to separate water molecules where they cannot form hydrogen bonds (the energy reference by which hydration is characterized)”.
- “Energy required for a hydrated ion to similarly be separated from its bound water, known as the heat of hydration”.
| Ion | Oxidation State | Ionic Radius (pm) | Heat of Hydration (kcalg−1·ion) |
|---|---|---|---|
| K | +1 | 138 | 75 |
| Na | +1 | 102 | 95 |
| Ca | +2 | 114 | 410 |
| Fe | +2 | 75 | 500 |
| Al | +3 | 67 | 1149 |
| Fe | +3 | 69 | 1200 |
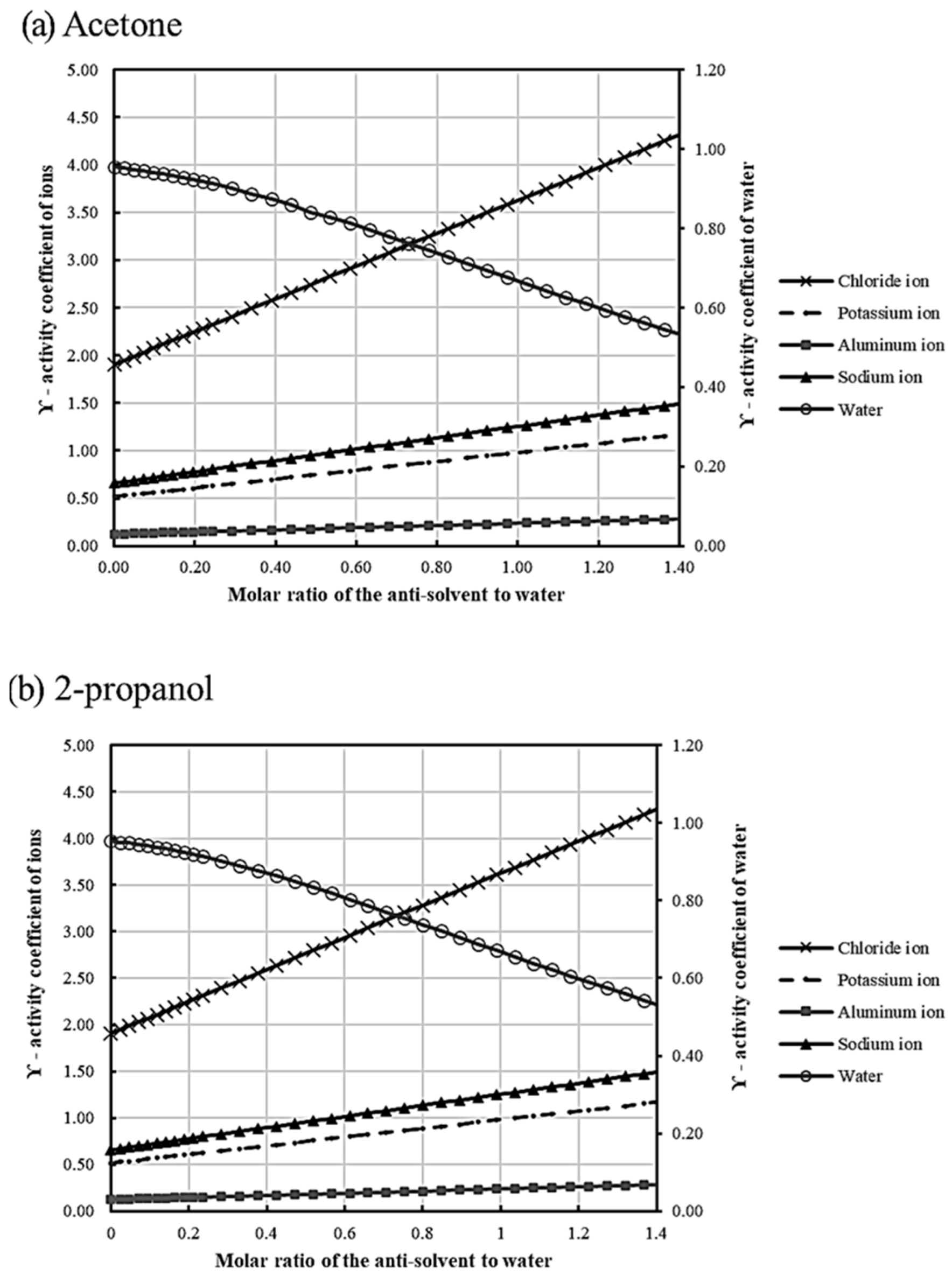
3.2. Influence of Anti-Solvent Ratio and Time on Potassium Crystallization
3.3. Progressive Addition of Anti-Solvents
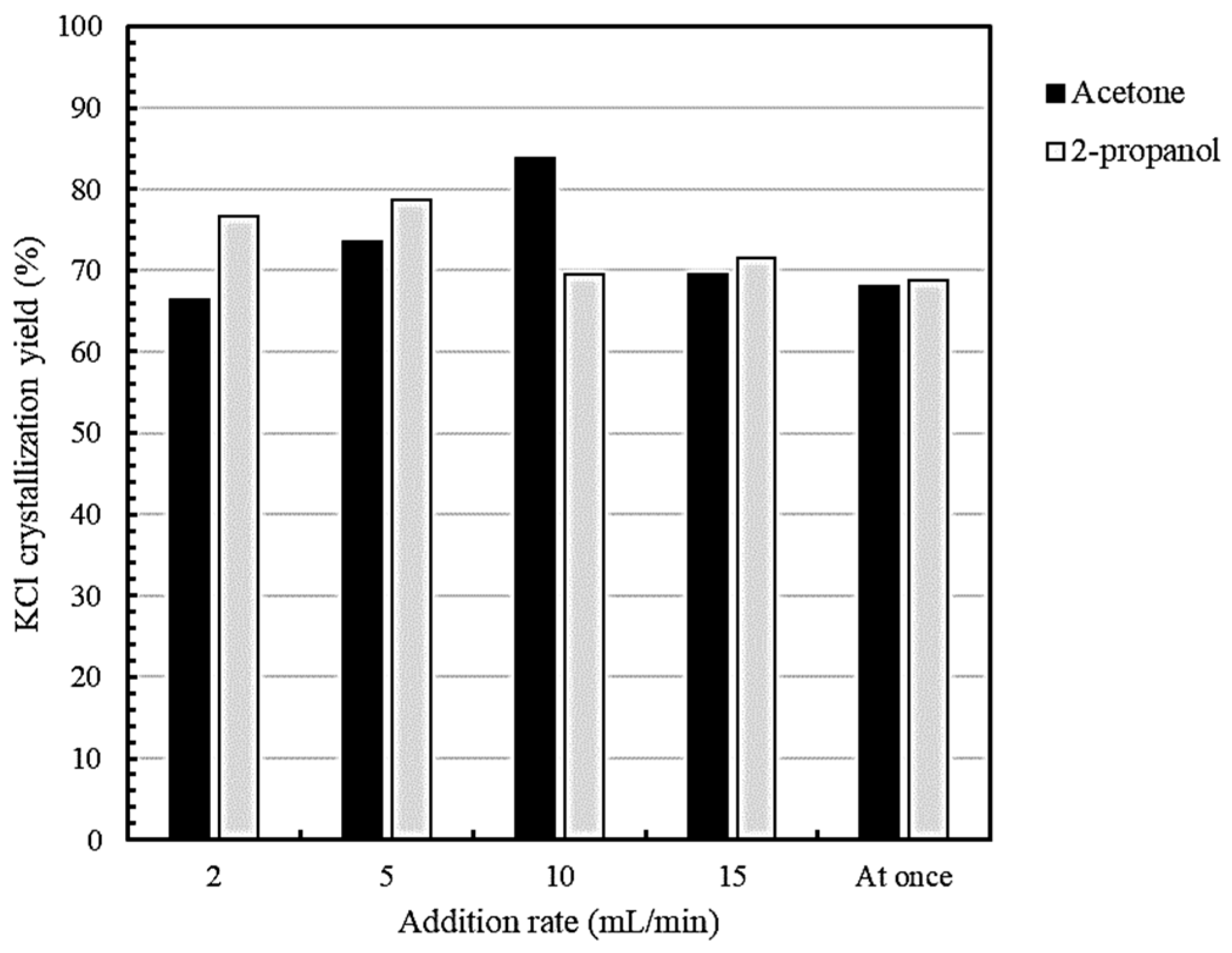
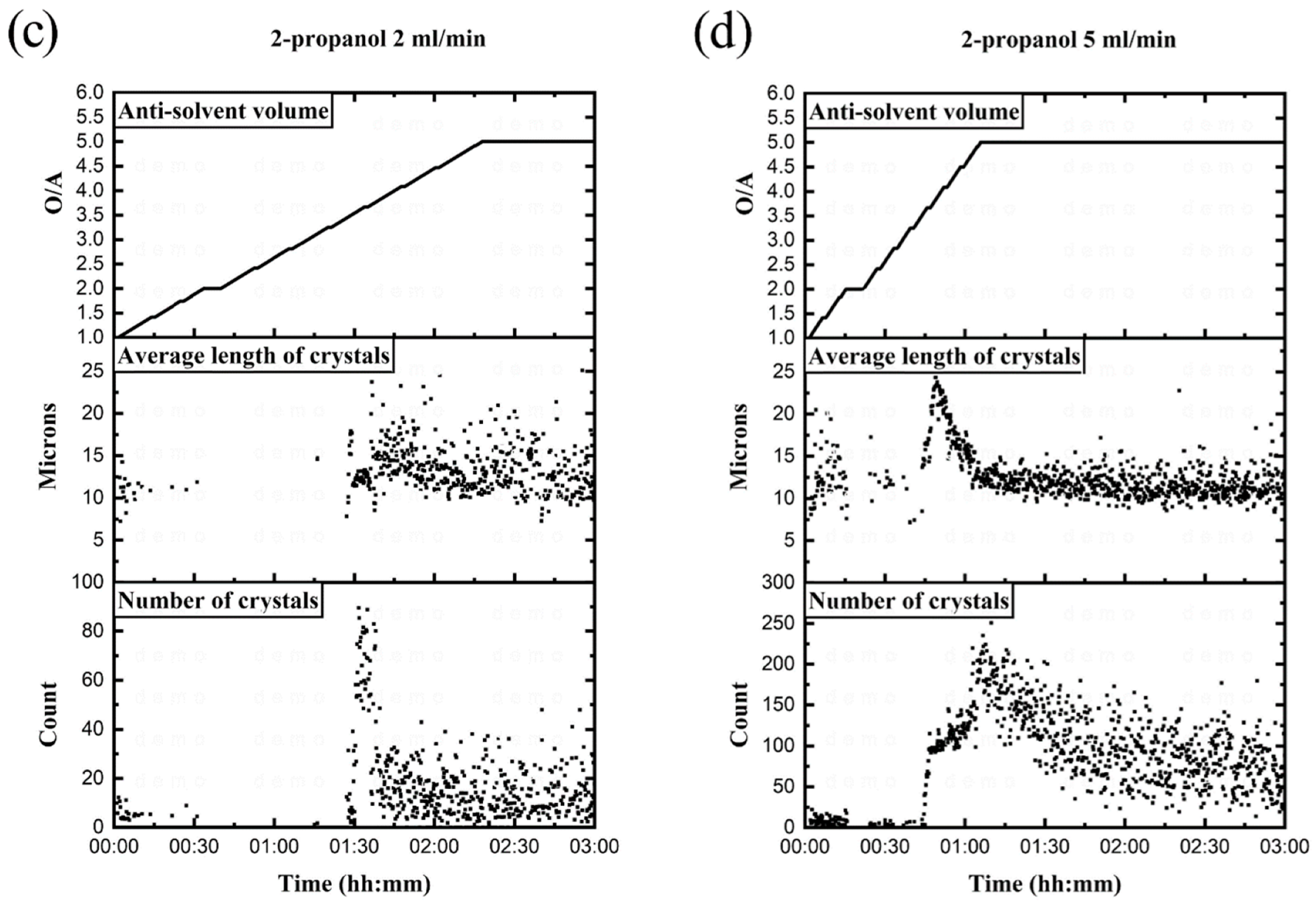
3.3.1. Effect of Addition Rate on Potassium Crystallization Yield
3.3.2. Controlling Parameters through Addition Rate
Degree of Supersaturation
Nucleation Rate
Growth Rate
3.3.3. Impact of Addition Rate on Nucleation and Growth
3.4. Process Considerations
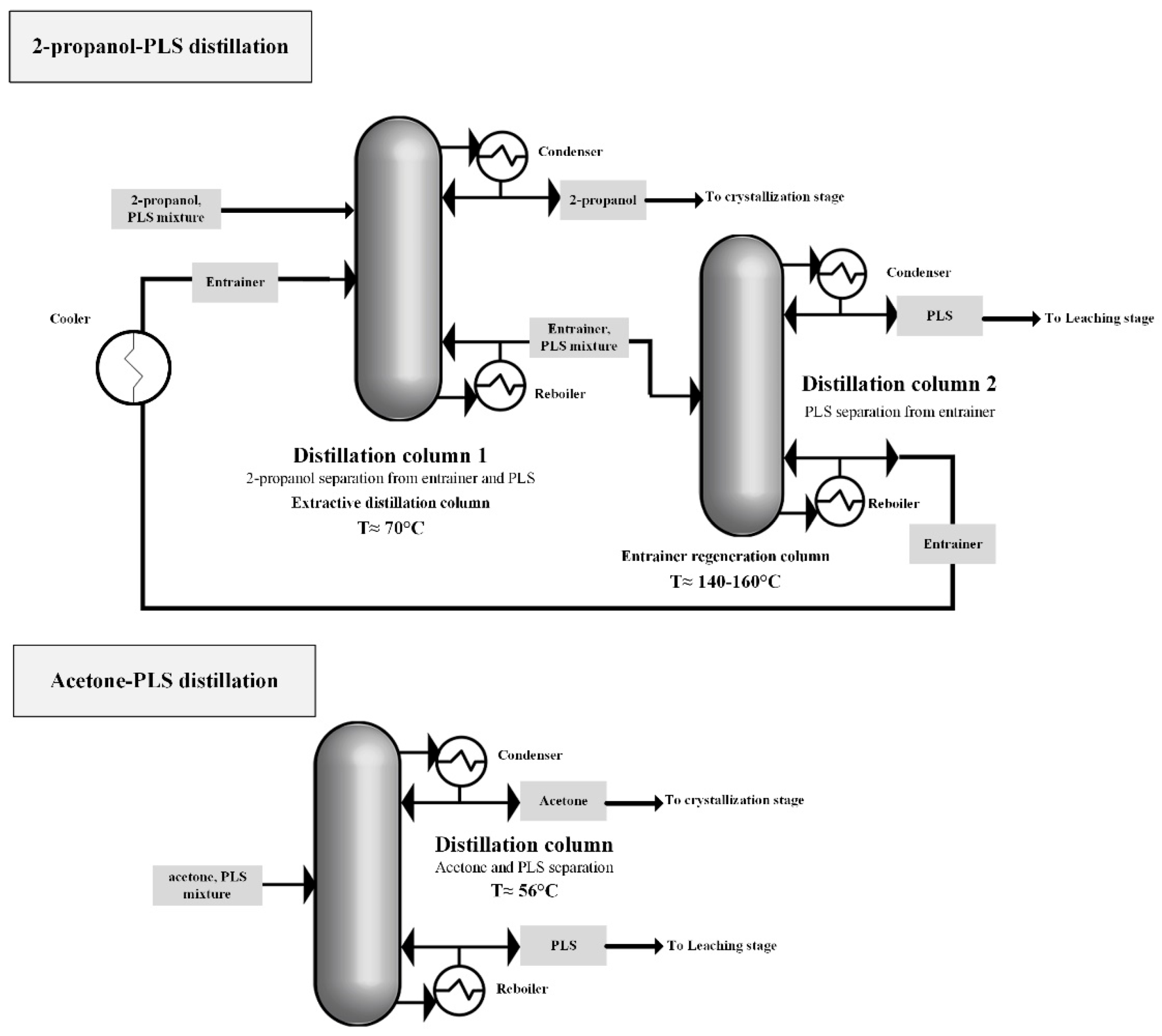
3.4.1. Anti-Solvent Recovery Method
3.4.2. Limitations and Potential Improvements
4. Conclusions
- The highest crystallization yields for potassium in these experiments were obtained using acetone and 2-propanol at around 68% and 69%, respectively.
- The potash products obtained in these experiments had a purity of over 99.9%.
- For efficient crystallization of potassium using either anti-solvent, at least an O/A of 5 is required and all chemical and physical interactions during the process happen in less than 24 h.
- The highest potassium crystallization yields were 83% using acetone at an addition rate of 10 mL/min and 79% using 2-propanol at an addition rate of 5 mL/min.
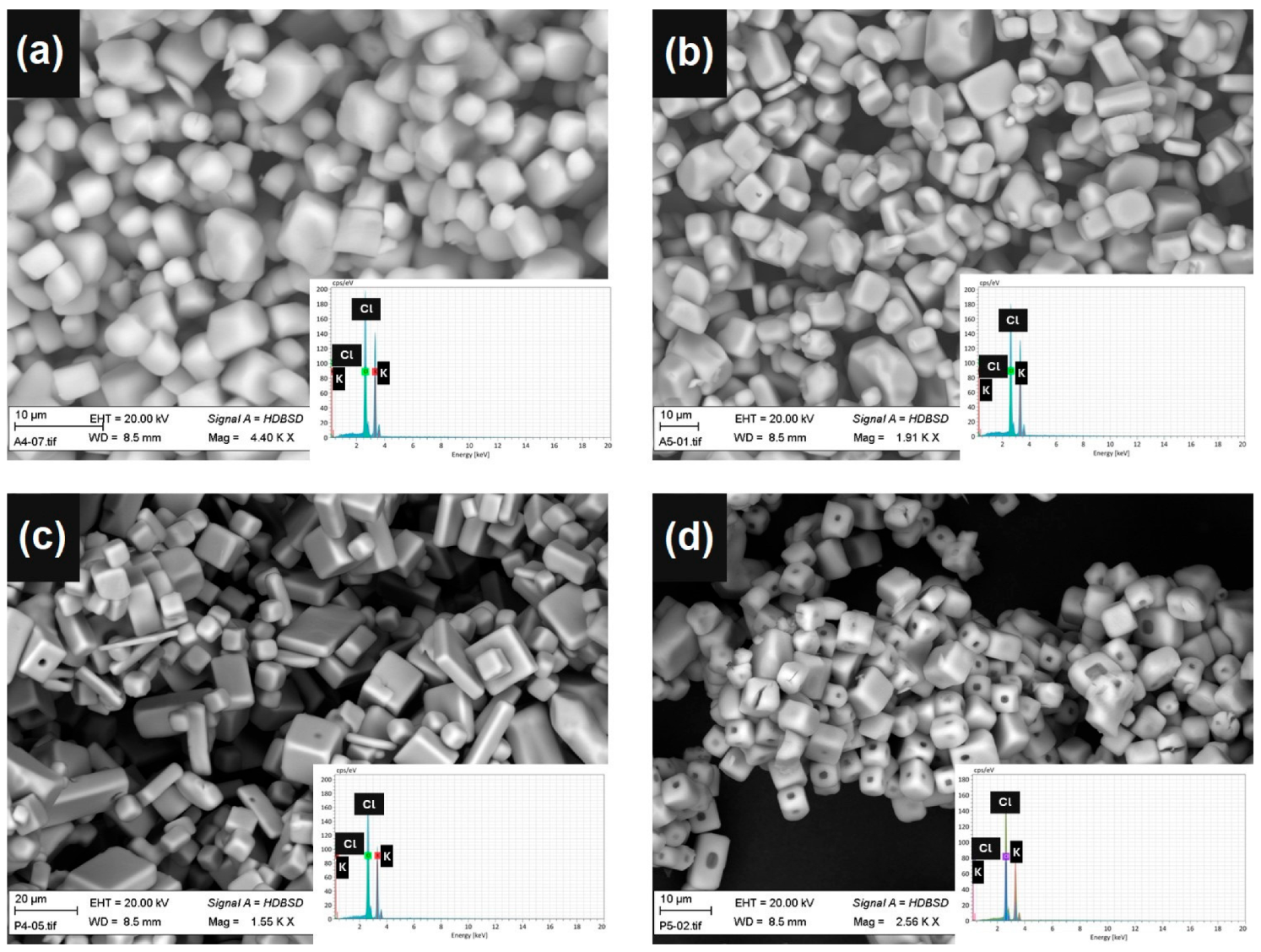
- In all the experiments, selective crystallization of the potassium was observed. The final potash product has a purity of over 99.9% with sodium as the major impurity.
- The application of acetone reduced the size of potash crystals as compared to 2-propanol.
- When acetone was applied, crystal aggregation was detected.
- The separation of acetone from a PLS-antisolvent mixture turned out to be less complex requiring less energy for distillation.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- King, H. Feldspar a Large Group of Silicate Minerals. The Most Abundant Group of Minerals in Earth’s Crust. Available online: https://geology.com/minerals/feldspar.shtml (accessed on 15 June 2023).
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Production of potassium chloride from K-feldspar through roast–leach–solvent extraction route. Trans. Indian Inst. Met. 2019, 72, 2613–2622. [Google Scholar] [CrossRef]
- Zahradník, J.; Jirásek, J.; Starý, J.; Sivek, M. Production, Reserves, and Processing of Feldspar and Feldspathoid Rocks in the Czech Republic from 2005 to 2019—An Overview. Minerals 2020, 10, 722. [Google Scholar] [CrossRef]
- Salimkhani, H.; Joodi, T.; Bordbar-Khiabani, A.; Dizaji, A.M.; Abdolalipour, B.; Azizi, A. Surface and structure characteristics of commercial K-Feldspar powders: Effects of temperature and leaching media. Chin. J. Chem. Eng. 2020, 28, 307–317. [Google Scholar] [CrossRef]
- Samantray, J.; Anand, A.; Dash, B.; Ghosh, M.K.; Behera, A.K. Silicate minerals-Potential source of potash—A review. Miner. Eng. 2022, 179, 107463. [Google Scholar] [CrossRef]
- Kumanan, M.; Sathya, G.; Nandakumar, V.; Berchmans, L.J. Extraction of potash from K-Feldspar mineral by acid and molten salt leaching processes. Chem. Eng. 2016, 7, 1–10. [Google Scholar]
- Heffer, P.; Prud’homme, M. Fertilizer Outlook 2016–2020. In Proceedings of the 84th IFA Annual Conference, Moscow, Russia, 30 May–1 June 2016; pp. 1–5. [Google Scholar]
- Jena, S.K. A review on potash recovery from different rock and mineral sources. Min. Met. Explor. 2021, 38, 47–68. [Google Scholar] [CrossRef]
- National Minerals Information Center, Potash Statistics and Information, U.S. Geological Survey. 2023. Available online: https://www.usgs.gov/centers/national-minerals-information-center/potash-statistics-and-information (accessed on 15 June 2023).
- Ciceri, D.; Allanore, A. Microfluidic leaching of soil minerals: Release of K+ from K feldspar. PLoS ONE 2015, 10, e0139979. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.-H.; Wang, L.-S.; Jin, Z.-M. Leaching process of potassium sulfate from roasts of potash feldspar, gypsum and calcium carbonate. J. Chem. Eng. Chin. Univ. 2000, 14, 465–469. [Google Scholar]
- Feng, W.; Ma, H. Thermodynamic analysis and experiments of thermal decomposition for potassium feldspar at intermediate temperatures. Guisuanyan Xuebao (J. Chin. Ceram. Soc.) 2004, 32, 789–799. [Google Scholar]
- Jena, S.K.; Dhawan, N.; Rath, S.S.; Rao, D.S.; Das, B. Investigation of microwave roasting for potash extraction from nepheline syenite. Sep. Purif. Technol. 2016, 161, 104–111. [Google Scholar] [CrossRef]
- Jena, S.K.; Misra, P.K.; Das, B. Studies on extraction of potassium from feldspar by roast-leach method using phosphogypsum and sodium chloride. Miner. Process. Extr. Metall. Rev. 2016, 37, 323–332. [Google Scholar] [CrossRef]
- Jena, S.K.; Dash, N.; Samal, A.K.; Misra, P.K. Competency of chlorination roasting coupled water leaching process for potash recovery from K-feldspar: Mechanism and kinetics aspects. Korean J. Chem. Eng. 2019, 36, 2060–2073. [Google Scholar] [CrossRef]
- Ma, H.W.; Su, S.Q.; Yang, J.; Cai, B.Y.; Liu, M.T.; Yao, W.G.; Peng, H. Preparation of potassium sulfate from K-feldspar by hydrothermal alkaline method: Reaction principle and process evaluation. CIESC J. 2014, 65, 2363–2371. [Google Scholar]
- Yuan, B.; Li, C.; Liang, B.; Lü, L.; Yue, H.; Sheng, H.; Ye, L.; Xie, H. Extraction of potassium from K-feldspar via the CaCl2 calcination route. Chin. J. Chem. Eng. 2015, 23, 1557–1564. [Google Scholar] [CrossRef]
- Nie, Y.; Ma, H.; Liu, H.; Zhang, P.; Qiu, M.Y.; Wang, L. Reactive mechanism of potassium feldspar dissolution under hydrothermal condition. Guisuanyan Xuebao (J. Chin. Ceram. Soc.) 2006, 34, 846. [Google Scholar]
- Wang, C.; Yue, H.; Li, C.; Liang, B.; Zhu, J.; Xie, H. Mineralization of CO2 using natural K-feldspar and industrial solid waste to produce soluble potassium. Ind. Eng. Chem. Res. 2014, 53, 7971–7978. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Qin, Y.; Wu, Z.; Wang, T.; Wang, C. The leaching kinetics of K-feldspar in sulfuric acid with the aid of ultrasound. Ultrason. Sonochem. 2017, 35, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.; Peng, Q.; He, Q.; Peng, Q. Isolation and identification of potash feldspar-solubilizing bacteria and their potassium-releasing activities. Zhongguo Weishengtaxixue Zazhi/Chin. J. Microecol. 2012, 24, 773–785. [Google Scholar]
- Ciceri, D.; de Oliveira, M.; Chen, D.P.; Allanore, A. Role of processing temperature and time on the hydrothermal alteration of K-feldspar rock in autoclave. Min. Met. Explor. 2020, 37, 955–963. [Google Scholar] [CrossRef]
- Fogler, H.S.; Lund, K.; McCune, C.C. Acidization III—The kinetics of the dissolution of sodium and potassium feldspar in HF/HCl acid mixtures. Chem. Eng. Sci. 1975, 30, 1325–1332. [Google Scholar] [CrossRef]
- Xue, N.; Zhang, Y.; Liu, T.; Huang, J. Study of the dissolution behavior of muscovite in stone coal by oxygen pressure acid leaching. Metall. Mater. Trans. B 2016, 47, 694–701. [Google Scholar] [CrossRef]
- Rossi, G. Potassium recovery through leucite bioleaching possibilities and limitations. In Metallurgical Applications of Bacterial Leaching and Related Microbiological Phenomena; Academic Press: New York, NY, USA, 1978; ISBN 0125111509. [Google Scholar]
- Štyriaková, I.; Štyriak, I.; Malachovský, P.; Lovás, M. Biological, chemical and electromagnetic treatment of three types of feldspar raw materials. Miner. Eng. 2006, 19, 348–354. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. Production of mechanically activated rock flour fertilizer by high intensive ultrafine grinding. Miner. Eng. 2007, 20, 334–341. [Google Scholar] [CrossRef]
- Nojiri, H.; Okuno, M.; Okudera, H.; Mizukami, T.; Arai, S. Structural change of alkali feldspar by ball milling. J. Mineral. Petrol. Sci. 2013, 108, 267–277. [Google Scholar] [CrossRef]
- Ribbe, P.H. Feldspar Mineralogy; Mineralogical Society of America: Chantilly, VA, USA, 1983; ISBN 0-939950-14-6. [Google Scholar]
- Vernon, R.H. K-feldspar megacrysts in granites—Phenocrysts, not porphyroblasts. Earth Sci. Rev. 1986, 23, 1–63. [Google Scholar] [CrossRef]
- Dye, J.L.; DeBacker, M.G. Physical and chemical properties of alkalides and electrides. Annu. Rev. Phys. Chem. 1987, 38, 271–299. [Google Scholar] [CrossRef]
- Elseewi, A.A.; Straughan, I.R.; Page, A.L. Sequential cropping of fly ash-amended soils: Effects on soil chemical properties and yield and elemental composition of plants. Sci. Total Environ. 1980, 15, 247–259. [Google Scholar] [CrossRef]
- Lee, G.S.; Uchikoshi, M.; Mimura, K.; Isshiki, M. Separation of major impurities Ce, Pr, Nd, Sm, Al, Ca, Fe, and Zn from La using bis (2-ethylhexyl) phosphoric acid (D2EHPA)-impregnated resin in a hydrochloric acid medium. Sep. Purif. Technol. 2010, 71, 186–191. [Google Scholar] [CrossRef]
- Fritsch, E.; Allard, T.; Benedetti, M.F.; Bardy, M.; Nascimento, N.R.D.; Li, Y.; Calas, G. Organic complexation and translocation of ferric iron in podzols of the Negro River watershed. Separation of secondary Fe species from Al species. Geochim. Cosmochim. Acta 2009, 73, 1813–1825. [Google Scholar] [CrossRef]
- Ma, Y.; Svärd, M.; Xiao, X.; Gardner, J.M.; Olsson, R.T.; Forsberg, K. Precipitation and crystallization used in the production of metal salts for Li-ion battery materials: A review. Metals 2020, 10, 1609. [Google Scholar] [CrossRef]
- Moldoveanu, G.A.; Demopoulos, G.P. Organic solvent-assisted crystallization of inorganic salts from acidic media. J. Chem. Technol. Biotechnol. 2015, 90, 686–692. [Google Scholar] [CrossRef]
- Sinha, B.; Müller, R.H.; Möschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharm. 2013, 453, 126–141. [Google Scholar] [CrossRef]
- Jia, S.; Yang, P.; Gao, Z.; Li, Z.; Fang, C.; Gong, J. Recent progress in antisolvent crystallization. CrystEngComm 2022, 24, 3122–3135. [Google Scholar] [CrossRef]
- Tulcidas, A.; Nascimento, S.; Santos, B.; Alvarez, C.; Pawlowski, S.; Rocha, F. Statistical methodology for scale-up of an anti-solvent crystallization process in the pharmaceutical industry. Sep. Purif. Technol. 2019, 213, 56–62. [Google Scholar] [CrossRef]
- Demirel, H.S.; Svärd, M.; Uysal, D.; Doğan, Ö.M.; Uysal, B.Z.; Forsberg, K. Antisolvent crystallization of battery grade nickel sulphate hydrate in the processing of lateritic ores. Sep. Purif. Technol. 2022, 286, 120473. [Google Scholar] [CrossRef]
- Korkmaz, K.; Alemrajabi, M.; Rasmuson, Å.C.; Forsberg, K.M. Separation of valuable elements from NiMH battery leach liquor via antisolvent precipitation. Sep. Purif. Technol. 2020, 234, 115812. [Google Scholar] [CrossRef]
- Xuan, W.; Chagnes, A.; Xiao, X.; Olsson, R.T.; Forsberg, K. Antisolvent Precipitation for Metal Recovery from Citric Acid Solution in Recycling of NMC Cathode Materials. Metals 2022, 12, 607. [Google Scholar] [CrossRef]
- Peters, E.M.; Svärd, M.; Forsberg, K. Impact of process parameters on product size and morphology in hydrometallurgical antisolvent crystallization. CrystEngComm 2022, 24, 2851–2866. [Google Scholar] [CrossRef]
- Peters, E.M.; Kaya, Ş.; Dittrich, C.; Forsberg, K. Recovery of scandium by crystallization techniques. J. Sustain. Metall. 2019, 5, 48–56. [Google Scholar] [CrossRef]
- Kaya, Ş.; Peters, E.M.; Forsberg, K.; Dittrich, C.; Stopic, S.; Friedrich, B. Scandium recovery from an ammonium fluoride strip liquor by anti-solvent crystallization. Metals 2018, 8, 767. [Google Scholar] [CrossRef]
- Alimohammadizadeh, H.; Behrad-Vakylabad, A.; Ghader, S. On the optimization of the crystallization related to an aqueous copper sulfate (CuSO4. 5H2O). Miner. Process. Extr. Metall. 2021, 130, 50–58. [Google Scholar]
- Ma, C.; Svärd, M.; Forsberg, K. Recycling cathode material LiCo1/3Ni1/3Mn1/3O2 by leaching with a deep eutectic solvent and metal recovery with antisolvent crystallization. Resour. Conserv. Recycl. 2022, 186, 106579. [Google Scholar] [CrossRef]
- Djamali, E.; Tomson, M.B.; Chapman, W.G. Thermodynamic Properties and Solubility of Sodium and Potassium Chloride in Ethane-1, 2-diol/Water Mixed Solvent Systems to High Temperatures. J. Chem. Eng. Data 2017, 62, 1326–1334. [Google Scholar] [CrossRef]
- Seidell, A. Solubilities of Inorganic and Metal Organic Compounds, 3rd ed.; National Institute of Health: Washington, DC, USA, 1940. [Google Scholar]
- ERA-MIN3, Zero-Waste Valorisation of Feldspathic Ores: Green Application and Sustainable Sourcing of Strategic Raw Materials. 2021. Available online: https://www.era-learn.eu/network-information/networks/era-min3/eu-co-funded-era-min-joint-call-2021/zero-waste-valorisation-of-feldspathic-ores-green-application-and-sustainable-sourcing-of-strategic-raw-materials (accessed on 15 June 2023).
- Peeters, D. Hydrogen bonds in small water clusters: A theoretical point of view. J. Mol. Liq. 1995, 67, 49–61. [Google Scholar] [CrossRef]
- Schneider, H. The selective solvation of ions in mixed solvents. J. Chim. Phys. 1969, 66, 2. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Solvents; ChemTec Publishing: Toronto, ON, Canada, 2001; ISBN 1-895198-24-0. [Google Scholar]
- Pitzer, K.S.; Press, C.R.C. Activity Coefficients in Electrolyte Solutions; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Price, E. Solvation of electrolytes and solution equilibria. Chem. Non-Aqueous Sol. Princ. Techn. 1966, 1, 67. [Google Scholar]
- Desnoyers, J.E.; Jolicoeur, C. Hydration effects and thermodynamic properties of ions. In Modern Aspects of Electrochemistry; Bockris, J.O.M., Conway, B.E., Eds.; Plenum: New York, NY, USA, 1969; Volume 5, p. 26. [Google Scholar]
- Atomistic Simulation Group in the Materials Department of Imperial College, Database of Ionic Radii. Available online: http://abulafia.Mt.Ic.Ac.Uk/Shannon/Ptable.Php (accessed on 15 June 2023).
- Fonseca, T.L.; Coutinho, K.; Canuto, S. Hydrogen bond interactions between acetone and supercritical water. Phys. Chem. Chem. Phys. 2010, 12, 6660–6665. [Google Scholar] [CrossRef] [PubMed]
- Daniele, P.G.; De Robertis, A.; De Stefano, C.; Sammartano, S.; Rigano, C. On the possibility of determining the thermodynamic parameters for the formation of weak complexes using a simple model for the dependence on ionic strength of activity coefficients: Na+, K+, and Ca2+ complexes of low molecular weight ligands in aqueous solution. J. Chem. Soc. Dalton Trans. 1985, 11, 2353–2361. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Mukerjee, P. Ionic strength effects on the activity coefficient of methylene blue and its self-association. J. Am. Chem. Soc. 1970, 92, 6413–6415. [Google Scholar] [CrossRef]
- Sarig, S.; Glasner, A.; Epstein, J.A.; Eidelman, N. Growth of potassium chloride crystals. J. Cryst. Growth 1977, 39, 255–266. [Google Scholar] [CrossRef]
- Jin, S.; Chen, M.; Li, Z.; Wu, S.; Du, S.; Xu, S.; Rohani, S.; Gong, J. Design and mechanism of the formation of spherical KCl particles using cooling crystallization without additives. Powder Technol. 2018, 329, 455–462. [Google Scholar] [CrossRef]
- MacFhionnghaile, P.; Svoboda, V.; McGinty, J.; Nordon, A.; Sefcik, J. Crystallization diagram for antisolvent crystallization of lactose: Using design of experiments to investigate continuous mixing-induced supersaturation. Cryst. Growth Des. 2017, 17, 2611–2621. [Google Scholar] [CrossRef]
- Wang, L.; Bao, Y.; Sun, Z.; Pinfield, V.J.; Yin, Q.; Yang, H. Investigation of Agglomeration in the Presence of Oiling out in the Antisolvent Crystallization Process. Ind. Eng. Chem. Res. 2021, 60, 4110–4119. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, J.; Feng, D.; Du, Q.; Wu, S.; Zhao, Y. Optimization of the process of antisolvent crystallization of carbonized ammonia with a low carbon-to-nitrogen ratio. Fuel Process. Technol. 2017, 155, 59–67. [Google Scholar] [CrossRef]
- Lu, C.; Liu, X.; Xia, Y.; Li, Q.; Dang, L.; He, X.; Wang, Z. Determination of Metastable Zone Width and Nucleation Induction Period of Palm Oil and its Olein/Stearin in Melting Layer Crystallization. Chem. Eng. Technol. 2020, 43, 422–428. [Google Scholar] [CrossRef]
- Si, Z.; Li, A.; Yan, Y.; Zhang, X.; Yang, H. Interaction of metastable zone width and induction time based on nucleation potential. Ind. Eng. Chem. Res. 2020, 59, 22597–22604. [Google Scholar] [CrossRef]
- Smith, J.M. Introduction to Chemical Engineering Thermodynamics; McGraw Hill Publisher: New York, NY, USA, 1950; ISBN 1259696529. [Google Scholar]
- Thakur, A.K.; Kumar, R.; Kumar, V.K.V.; Kumar, A.; Gaurav, G.K.; Gupta, K.N. A critical review on thermodynamic and hydrodynamic modeling and simulation of liquid antisolvent crystallization of pharmaceutical compounds. J. Mol. Liq. 2022, 362, 119663. [Google Scholar] [CrossRef]
- Kalikmanov, V.I. Classical nucleation theory. In Nucleation Theory; Springer: Berlin/Heidelberg, Germany, 2012; pp. 17–41. [Google Scholar]
- Mullin, J.W. Nucleation. Crystallization 2001, 4, 181–215. [Google Scholar]
- Kwon, S.G.; Hyeon, T. Formation mechanisms of uniform nanocrystals via hot-injection and heat-up methods. Small 2011, 7, 2685–2702. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.; Seckler, M.; Kramer, H.; Van Rosmalen, G. Industrial Crystallization: Fundamentals and Applications; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Panagiotou, T.; Mesite, S.V.; Fisher, R.J. Production of norfloxacin nanosuspensions using microfluidics reaction technology through solvent/antisolvent crystallization. Ind. Eng. Chem. Res. 2009, 48, 1761–1771. [Google Scholar] [CrossRef]
- Kadam, S.S.; Kramer, H.J.M.; Horst, J.H.T. Combination of a single primary nucleation event and secondary nucleation in crystallization processes. Cryst. Growth Des. 2011, 11, 1271–1277. [Google Scholar] [CrossRef]
- Jain, V.K. Defects in Solids. In Solid State Physics; Springer: Berlin/Heidelberg, Germany, 2022; pp. 81–96. [Google Scholar]
- Benisek, A.; Dachs, E. Calorimetric study of the entropy relation in the NaCl–KCl system. J. Chem. Thermodyn. 2013, 62, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Barrett, W.T.; Wallace, W.E. Studies of NaCl-KCl solid solutions. I. Heats of formation, lattice spacings, densities, Schottky defects and mutual solubilities. J. Am. Chem. Soc. 1954, 76, 366–369. [Google Scholar] [CrossRef]
- Saidur, R.; Elcevvadi, E.T.; Mekhilef, S.; Safari, A.; Mohammed, H.A. An overview of different distillation methods for small scale applications. Renew. Sustain. Energy Rev. 2011, 15, 4756–4764. [Google Scholar] [CrossRef]
- De Guido, G.; Monticelli, C.; Spatolisano, E.; Pellegrini, L.A. Separation of the Mixture 2-Propanol+ Water by Heterogeneous Azeotropic Distillation with Isooctane as an Entrainer. Energies 2021, 14, 5471. [Google Scholar] [CrossRef]
- Weires, N.A.; Johnston, A.; Warner, D.L.; McCormick, M.M.; Hammond, K.; McDougal, O.M. Recycling of waste acetone by fractional distillation. J. Chem. Educ. 2011, 88, 1724–1726. [Google Scholar] [CrossRef]
- De, D.; Sai, M.S.N.; Aniya, V.; Jyothi, K.N.; Satyavathi, B. Economic and environmental impact assessment of extractive distillation with renewable entrainers for reprocessing aqueous 2-Propanol. Chem. Eng. Process.-Process Intensif. 2019, 143, 107616. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Zhang, Q.; Sun, D.; Pan, F.; Dai, S.; Li, W. Separation of 2-propanol and water azeotropic system using ionic liquids as entrainers. Fluid Phase Equilibria 2016, 412, 94–100. [Google Scholar] [CrossRef]
- DDBST GmbH, Dortmund Data Bank. Available online: http://www.ddbst.com/ (accessed on 15 June 2023).
- E-Commerce of Chemical. Available online: https://www.echemi.com/ (accessed on 15 June 2023).
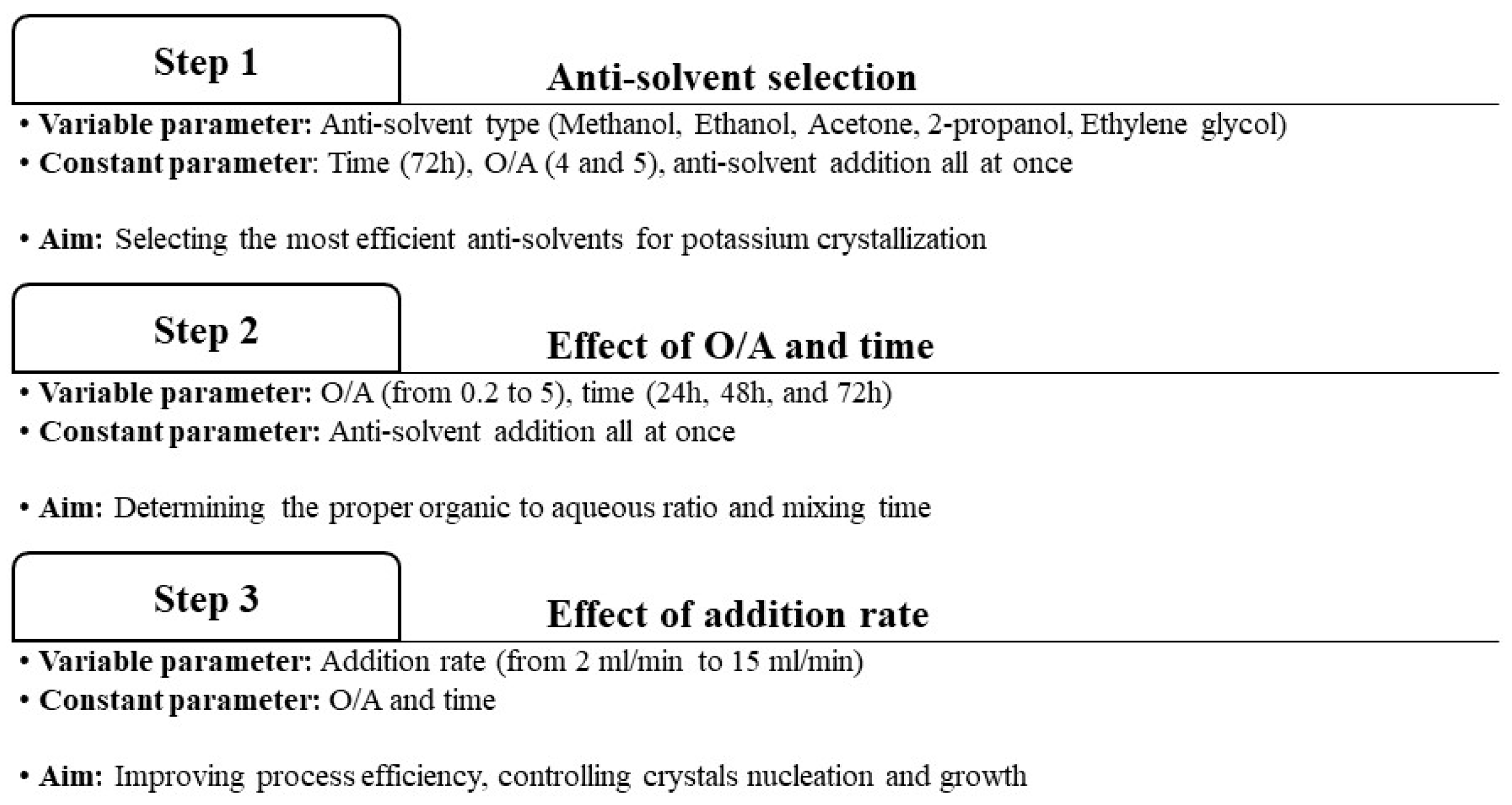




| Element/Compound | K | Al | Na | Ca | Fe | HCl |
|---|---|---|---|---|---|---|
| Concentration | 9 g/L | 9 g/L | 1.5 g/L | 0.2 g/L | 0.1 g/L | 2 M |
| Anti-Solvents | Methanol | Ethanol | Acetone | 2-Propanol | Ethylene Glycol | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| O/A ratio | 4 | 5 | 4 | 5 | 4 | 5 | 4 | 5 | 4 | 5 |
| K recovery (%) | 0.0 | 0.4 | 0.1 | 12.1 | 50.6 | 68.0 | 51.0 | 68.8 | 1.2 | 1.2 |
| Na recovery (%) | 0.0 | 0.0 | 0.0 | 0.3 | 0.0 | 0.5 | 0.0 | 0.5 | 0.4 | 0.0 |
| Al recovery (%) | 1.8 | 0.9 | 4.3 | 0.5 | 2.6 | 0.0 | 0.4 | 0.0 | 3.9 | 1.3 |
| Fe recovery (%) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.1 | 0.0 | 1.0 | 0.0 | 0.3 |
| Anti-Solvent | O/A | Na (%) | Al (%) | Ca (%) |
|---|---|---|---|---|
| Acetone | 4 | 0.028 | 0.032 | 0.018 |
| Acetone | 5 | 0.054 | 0.034 | 0.016 |
| 2-propanol | 4 | 0.036 | 0.030 | 0.010 |
| 2-propanol | 5 | 0.089 | 0.057 | 0.019 |
| O/A | 0.1 | 0.5 | 1 | 2 | 3 | 3.6 | 4.4 | 5 | 5.4 | 6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Acetone | 0.5276 | 0.5730 | 0.6297 | 0.7205 | 0.8567 | 0.9247 | 1.0155 | 1.0836 | 1.1290 | 1.1970 |
| 2-propanol | 0.5300 | 0.5712 | 0.6262 | 0.7361 | 0.8460 | 0.9120 | 0.9999 | 1.0659 | 1.1098 | 1.1758 |
| Anti-Solvent | Anti-Solvent Addition Rate (mL/min) | Na (%) | Al (%) | Ca (%) |
|---|---|---|---|---|
| Acetone | 5 | 0.0125 | 0.0218 | 0.0102 |
| Acetone | 10 | 0.0255 | 0.0198 | 0.0099 |
| 2-propanol | 2 | 0.0322 | 0.0204 | 0.0139 |
| 2-propanol | 5 | 0.0341 | 0.0390 | 0.0178 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shakibania, S.; Sundqvist-Öqvist, L.; Rosenkranz, J.; Ghorbani, Y. Application of Anti-Solvent Crystallization for High-Purity Potash Production from K-Feldspar Leaching Solution. Processes 2024, 12, 1385. https://doi.org/10.3390/pr12071385
Shakibania S, Sundqvist-Öqvist L, Rosenkranz J, Ghorbani Y. Application of Anti-Solvent Crystallization for High-Purity Potash Production from K-Feldspar Leaching Solution. Processes. 2024; 12(7):1385. https://doi.org/10.3390/pr12071385
Chicago/Turabian StyleShakibania, Sina, Lena Sundqvist-Öqvist, Jan Rosenkranz, and Yousef Ghorbani. 2024. "Application of Anti-Solvent Crystallization for High-Purity Potash Production from K-Feldspar Leaching Solution" Processes 12, no. 7: 1385. https://doi.org/10.3390/pr12071385








