Determination of High Concentration Copper Ions Based on Ultraviolet—Visible Spectroscopy Combined with Partial Least Squares Regression Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Sample Preparation
2.2. The Collection of UV-Visible Spectra
2.3. Data Processing
3. Results and Discussion
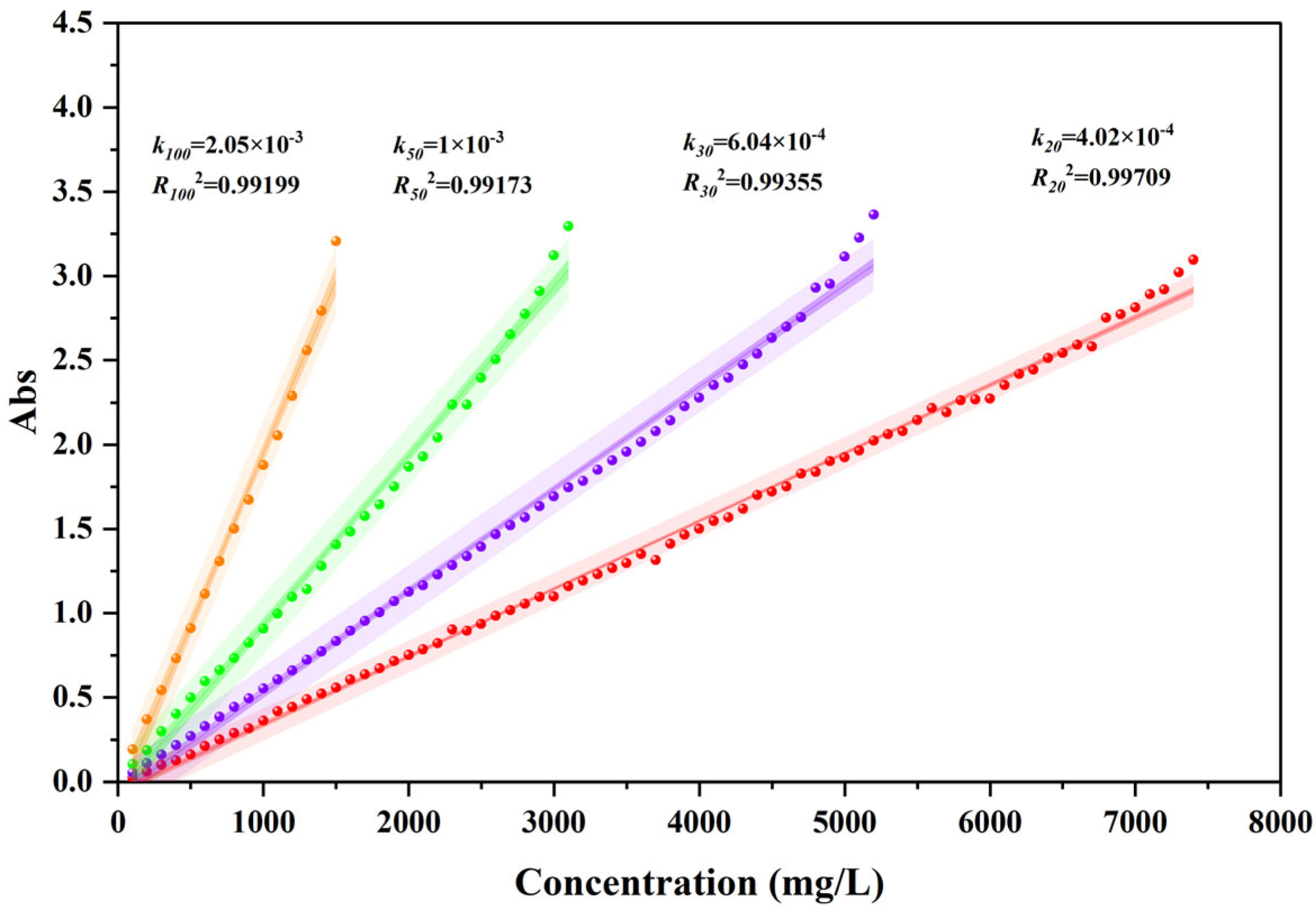
3.1. Determination of Optical Parameters
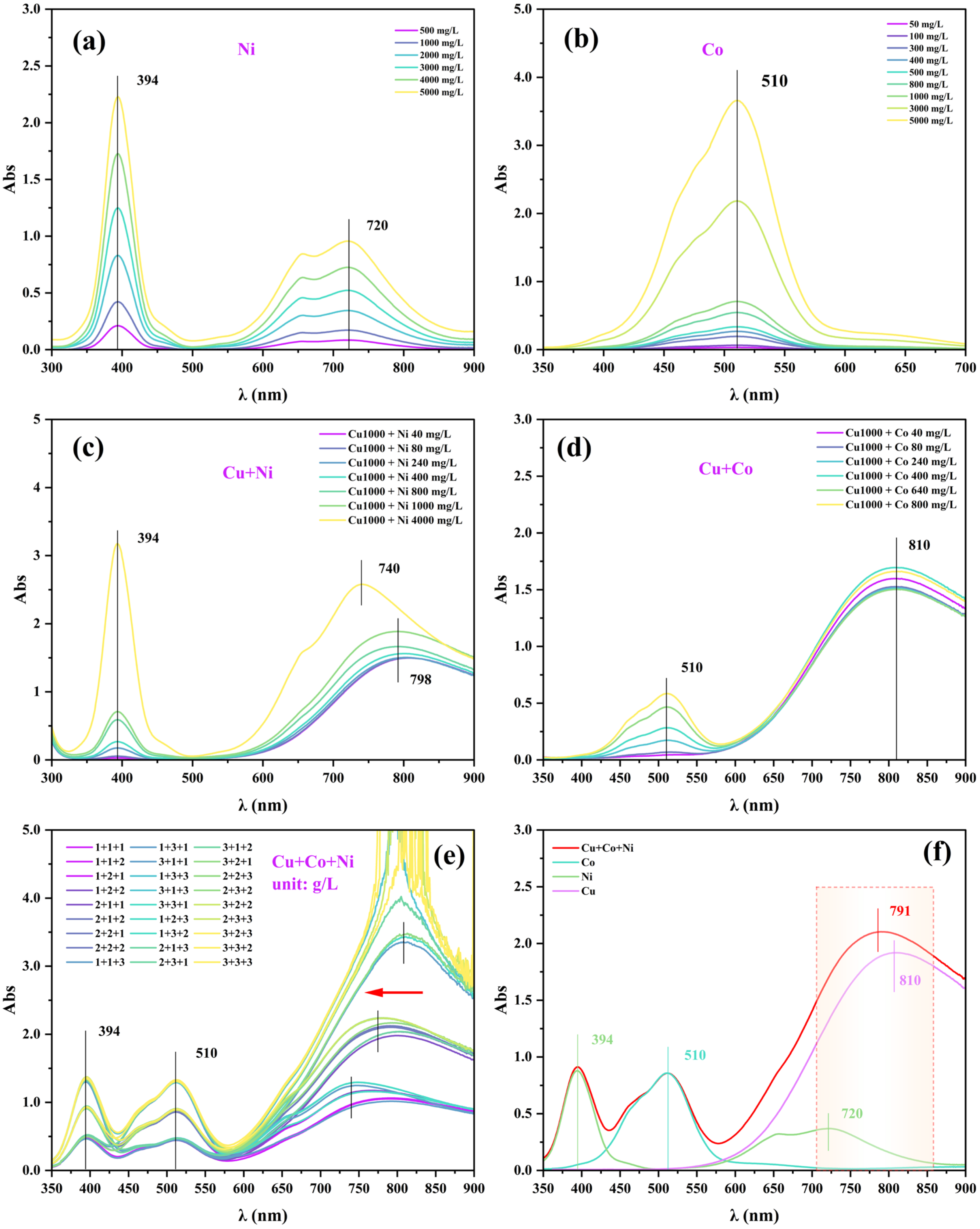
3.2. Influence of Interfering Factors
3.3. Establishment of Monitoring Model
- i.
- Extract the first components u1 and v1 from the absorbance matrix and concentration matrix.
- ii.
- Establish regressions of on and on . Assume the regression model is as follows:
- iii.
- Substitute the residual matrices and for and , and repeat the aforementioned steps.
- iv.
- Given the rank of the data matrix is , there exist components that make
- v.
- Cross validation test.
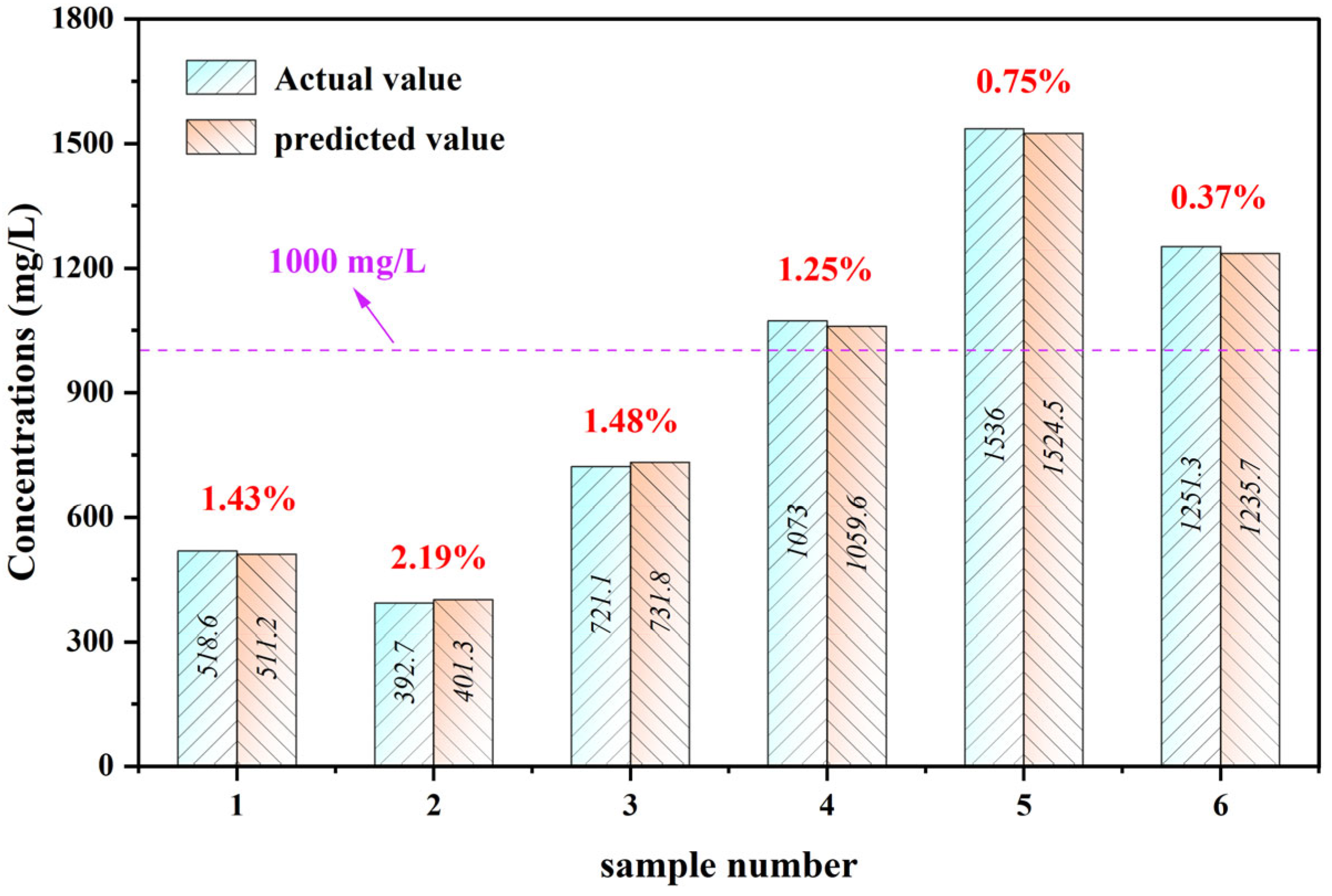
3.4. Validation of the Model
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feng, S.; Che, J.; Zhang, W.; Zuo, Y.; Wang, C.; Ma, B.; Chen, Y. A sustainable approach for recovering copper and zinc from copper smelting flue dust: Paving the path for waste reduction. Sep. Purif. Technol. 2024, 342, 127037. [Google Scholar] [CrossRef]
- Li, Q.; Lai, X.; Liu, Z.; Chai, F.; Zhao, F.; Peng, C.; Liang, Y. Thiourea-assisted selective removal of arsenic from copper smelting flue dusts in NaOH solution. Hydrometallurgy 2024, 224, 106246. [Google Scholar] [CrossRef]
- Tian, Q.H.; Li, Z.C.; Wang, Q.M.; Guo, X.Y. Synergistic recovery of copper, lead and zinc via sulfurization–reduction method from copper smelting slag. Trans. Nonferrous Met. Soc. China 2023, 33, 3847–3859. [Google Scholar] [CrossRef]
- Wen, X.; Yang, Q.; Yan, Z.; Deng, Q. Determination of cadmium and copper in water and food samples by dispersive liquid–liquid microextraction combined with UV–vis spectrophotometry. Microchem. J. 2011, 97, 249–254. [Google Scholar] [CrossRef]
- Pourbasheer, E.; Morsali, S.; Banaei, A.; Aghabalazadeh, S.; Ganjali, M.R.; Norouzi, P. Design of a novel optical sensor for determination of trace amounts of copper by UV/vis spectrophotometry in the real samples. J. Ind. Eng. Chem. 2015, 26, 370–374. [Google Scholar] [CrossRef]
- Racheva, P.V.; Milcheva, N.P.; Genc, F.; Gavazov, K.B. A centrifuge-less cloud point extraction-spectrophotometric determination of copper(II) using 6-hexyl-4-(2-thiazolylazo)resorcinol. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2021, 262, 120106. [Google Scholar] [CrossRef] [PubMed]
- Drosaki, E.; Anthemidis, A.N. A novel automatic flow-batch extraction induced by emulsion breaking platform for on-line copper determination in edible oil samples by atomic absorption spectrometry. Talanta 2022, 244, 123423. [Google Scholar] [CrossRef] [PubMed]
- Duran, C.; Gundogdu, A.; Bulut, V.N.; Soylak, M.; Elci, L.; Sentürk, H.B.; Tüfekci, M. Solid-phase extraction of Mn(II), Co(II), Ni(II), Cu(II), Cd(II) and Pb(II) ions from environmental samples by flame atomic absorption spectrometry (FAAS). J. Hazard. Mater. 2007, 146, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Şaylan, M.; Metin, B.; Akbıyık, H.; Turak, F.; Çetin, G.; Bakırdere, S. Microwave assisted effective synthesis of CdS nanoparticles to determine the copper ions in artichoke leaves extract samples by flame atomic absorption spectrometry. J. Food Compos. Anal. 2023, 115, 104965. [Google Scholar] [CrossRef]
- Ebrar Karlidağ, N.; Toprak, M.; Demirel, R.; Tuğba Zaman, B.; Bakirdere, S. Development of copper nanoflowers based dispersive solid-phase extraction method for cadmium determination in shalgam juice samples using slotted quartz tube atomic absorption spectrometry. Food Chem. 2022, 396, 133669. [Google Scholar] [CrossRef]
- Karbowska, B.; Włódarzewska, E.; Zembrzuski, W.; Zembrzuska, J.; Janeba-Bartoszewicz, E.; Bartoszewicz, J.; Selech, J. Determination of Some Heavy Metals in European and Polish Coal Samples. Molecules 2023, 28, 8055. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Kirkbright, G.F.; Ranson, L.; West, T.S. The simultaneous determination of traces of cobalt, chromium, copper, iron, manganese and zinc by atomic fluorescence spectrometry with preconcentration by an automated solvent extraction procedure. Anal. Chim. Acta 1973, 63, 210–215. [Google Scholar] [CrossRef]
- Takara, E.A.; Pasini-Cabello, S.D.; Cerutti, S.; Gásquez, J.A.; Martinez, L.D. On-line preconcentration/determination of copper in parenteral solutions using activated carbon by inductively coupled plasma optical emission spectrometry. J. Pharm. Biomed. Anal. 2005, 39, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Feng, J.; Tang, L.; Yu, C.; Mo, G.; Deng, B. A highly efficient introduction system for single cell- ICP-MS and its application to detection of copper in single human red blood cells. Talanta 2020, 206, 120174. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, J.-B.; Wang, X.; Wang, Z.-H.; Zhao, R.-S. Simultaneous determination of copper, cobalt, and mercury ions in water samples by solid-phase extraction using carbon nanotube sponges as adsorbent after chelating with sodium diethyldithiocarbamate prior to high performance liquid chromatography. Anal. Bioanal. Chem. 2016, 408, 4445–4453. [Google Scholar] [CrossRef]
- El-Raheem, H.A.; Hassan, R.Y.A.; Khaled, R.; Farghali, A.; El-Sherbiny, I.M. Polyurethane-doped platinum nanoparticles modified carbon paste electrode for the sensitive and selective voltammetric determination of free copper ions in biological samples. Microchem. J. 2020, 155, 104765. [Google Scholar] [CrossRef]
- Fan, Y.; Xu, C.; Wang, R.; Hu, G.; Miao, J.; Hai, K.; Lin, C. Determination of copper(II) ion in food using an ionic liquids-carbon nanotubes-based ion-selective electrode. J. Food Compos. Anal. 2017, 62, 63–68. [Google Scholar] [CrossRef]
- Birinci, A.; Eren, H.; Coldur, F.; Coskun, E.; Andac, M. Rapid determination of trace level copper in tea infusion samples by solid contact ion selective electrode. J. Food Drug Anal. 2016, 24, 485–492. [Google Scholar] [CrossRef]
- Gismera, M.J.; Hueso, D.; Procopio, J.R.; Sevilla, M.T. Ion-selective carbon paste electrode based on tetraethyl thiuram disulfide for copper(II) and mercury(II). Anal. Chim. Acta 2004, 524, 347–353. [Google Scholar] [CrossRef]
- Bishara, S.W.; El-Samman, F.M. Polarographic microdetermination of iron, manganese, lead, copper, bismuth, and tin in organic compounds: Application to analysis of pharmaceuticals. Microchem. J. 1977, 22, 442–450. [Google Scholar] [CrossRef]
- Ni, Y.; Jin, L. Simultaneous polarographic chemometric determination of lead, copper, vanadium, cadmium and nickel. Chemometrics Intellig. Lab. Syst. 1999, 45, 105–111. [Google Scholar] [CrossRef]
- Shi, T.; Xie, Z.; Mo, X.; Feng, Y.; Peng, T.; Wu, F.; Yu, M.; Zhao, J.; Zhang, L.; Guo, J. Synthesis and Application of Salicylhydrazone Probes with High Selectivity for Rapid Detection of Cu2+. Molecules 2024, 29, 2032. [Google Scholar] [CrossRef] [PubMed]
- Shpigun, L.K.; Shushenachev, Y.V.; Kamilova, P.M. Kinetic separation in flow injection spectrophotometry: Simultaneous determination of copper and zinc in a single run. Anal. Chim. Acta 2006, 573–574, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.C.; Mesquita, R.B.R.; Rangel, A.O.S.S. Micro solid phase spectrophotometry in a sequential injection lab-on-valve platform for cadmium, zinc, and copper determination in freshwaters. Anal. Chim. Acta 2015, 891, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N. Development of vortex-assisted ionic liquid-dispersive microextraction methodology for vanillin monitoring in food products using ultraviolet-visible spectrophotometry. LWT 2018, 93, 9–15. [Google Scholar] [CrossRef]
- Bosch Ojeda, C.; Sanchez Rojas, F. Recent developments in derivative ultraviolet/visible absorption spectrophotometry. Anal. Chim. Acta 2004, 518, 1–24. [Google Scholar] [CrossRef]
- Mörschbächer, A.P.; Dullius, A.; Dullius, C.H.; Bandt, C.R.; Kuhn, D.; Brietzke, D.T.; Malmann Kuffel, F.J.; Etgeton, H.P.; Altmayer, T.; Gonçalves, T.E.; et al. Validation of an analytical method for the quantitative determination of selenium in bacterial biomass by ultraviolet–visible spectrophotometry. Food Chem. 2018, 255, 182–186. [Google Scholar] [CrossRef]
- HJ 486-2009; Water Quality—Determination of Copper-2,9-Dimethy-1,10-phenanthroline Spectrophotometric Method. MEP, State Environmental Protection Standards of the Peoples Republic of China: Beijing, China, 2009. (In Chinese)
- HJ 485-2009; Water Quality―Determination of Copper―Sodium Diethydlthiocabamate Spectrophotometric Method. MEP, State Environmental Protection Standards of the Peoples Republic of China: Beijing, China, 2009. (In Chinese)
- Garcia Rodriguez, A.M.; Garcia de Torres, A.; Cano Pavon, J.M.; Bosch Ojeda, C. Simultaneous determination of iron, cobalt, nickel and copper by UV-visible spectrophotometry with multivariate calibration. Talanta 1998, 47, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, C.; Yang, C.; Zhu, H.; Li, Y. A spectrophotometric method for simultaneous determination of trace ions of copper, cobalt, and nickel in the zinc sulfate solution by ultraviolet-visible spectrometry. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2019, 223, 117370. [Google Scholar] [CrossRef]
- Zhou, F.; Li, C.; Zhu, H.; Li, Y. Determination of trace ions of cobalt and copper by UV–vis spectrometry in purification process of zinc hydrometallurgy. Optik 2019, 184, 227–233. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, W.; Sukjairungwattana, P.; Yu, Z. Learners’ continuance intention in multimodal language learning education: An innovative multiple linear regression model. Heliyon 2024, 10, e28104. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Luo, Q.; Feng, M.; Zhou, L.; Qiu, Y.; Li, C.; Song, D.; Tan, Q.; Yang, F. Enhanced nitrate contribution to light extinction during haze pollution in Chengdu: Insights based on an improved multiple linear regression model. Environ. Pollut. 2023, 323, 121309. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Lu, Y.; Wang, Y.; Yang, L.; Yang, Z.; Li, H. Predicting the formation of disinfection by-products using multiple linear and machine learning regression. J. Environ. Chem. Eng. 2023, 11, 110612. [Google Scholar] [CrossRef]
- Meng, X.; Chen, S.; Li, D.; Song, Y.; Sun, L. Identification of marine microplastics based on laser-induced fluorescence and principal component analysis. J. Hazard. Mater. 2024, 465, 133352. [Google Scholar] [CrossRef] [PubMed]
- Shrivastav, A.M.; Ali, N.; Singh, N.; Lunenfeld, E.; Abdulhalim, I.; Huleihel, M. Identification of spermatogenesis in individual seminiferous tubules and testicular tissue of adult normal and busulfan-treated mice employing Raman spectroscopy and principal component analysis. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2024, 315, 124232. [Google Scholar] [CrossRef] [PubMed]
- Teng, Q.; Zhou, K.; Yu, K.; Zhang, X.; Li, Z.; Wang, H.; Zhu, C.; Wang, Z.; Dai, Z. Principal component analysis-assisted zirconium-based metal-organic frameworks/DNA biosensor for the analysis of various phosphates. Talanta 2024, 271, 125733. [Google Scholar] [CrossRef]
- Andaryani, S.; Nourani, V.; Abbasnejad, H.; Koch, J.; Stisen, S.; Klöve, B.; Haghighi, A.T. Spatio-temporal analysis of climate and irrigated vegetation cover changes and their role in lake water level depletion using a pixel-based approach and canonical correlation analysis. Sci. Total Environ. 2023, 873, 162326. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Xiu, X.; Yang, Y. Towards efficient state of charge estimation of lithium-ion batteries using canonical correlation analysis. Energy 2022, 254, 124415. [Google Scholar] [CrossRef]
- Perera, K.D.C.; Weragoda, G.K.; Haputhanthri, R.; Rodrigo, S.K. Study of concentration dependent curcumin interaction with serum biomolecules using ATR-FTIR spectroscopy combined with Principal Component Analysis (PCA) and Partial Least Square Regression (PLS-R). Vib. Spectrosc. 2021, 116, 103288. [Google Scholar] [CrossRef]
- Wang, Z.; Deng, J.; Ding, Z.; Jiang, H. Comparison of optimization algorithms for variable selection to enhance the predictive performance of PLS regression model in determining the concentration of heavy metal Cd in peanut oil. Infrared Phys. Technol. 2024, 138, 105264. [Google Scholar] [CrossRef]
- Borràs, E.; Ferré, J.; Boqué, R.; Mestres, M.; Aceña, L.; Calvo, A.; Busto, O. Prediction of olive oil sensory descriptors using instrumental data fusion and partial least squares (PLS) regression. Talanta 2016, 155, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Numata, Y. Simultaneous quantitative analysis of quercetin and rutin in Tartary buckwheat flour by Raman spectroscopy and partial least square regression. J. Food Compos. Anal. 2024, 128, 105991. [Google Scholar] [CrossRef]
- Sun, X.; Jiang, L.; Duan, N.; Zhu, G.; Xu, Y.; Jin, H.; Liu, Y.; Zhang, R. Efficient recovery of copper resources from copper smelting waste acid based on Cu(Ⅱ)/As(Ⅲ) competitive sulfuration mechanism. J. Clean Prod. 2024, 451, 141975. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, X.; Duan, N.; Jiang, L.; Xu, Y.; Chen, Y.; Liu, Y.; Fan, P. Direct-determination of high-concentration sulfate by serial differential spectrophotometry with multiple optical pathlengths. Sci. Total Environ. 2022, 811, 152121. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhao, H.; Ni, N.; Wang, Y.; Gao, J.; Gao, Q.; Zhang, Y.; Zhang, Y. Study on the origin of linear deviation with the Beer-Lambert law in absorption spectroscopy by measuring sulfur dioxide. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2022, 275, 121192. [Google Scholar] [CrossRef] [PubMed]
- Kafle, B.P. Chemical Analysis and Material Characterization by Spectrophotometry; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Cheng, W.; Duan, N.; Jiang, L.; Xu, Y.; Zhu, G.; Zhang, X.; Liu, Y.; Chen, Y.; Zhang, R.; Xu, F. The characteristics of ultraviolet absorption and electronic excitation of sulfate at high concentrations. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2023, 293, 122455. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Duan, N.; Jiang, L.; Xu, F.; Cheng, W.; Zhu, G.; Gao, W. Electronic Excitation Characteristics and Spectral Behavior of Phosphate Anions. Langmuir 2023, 39, 9144–9153. [Google Scholar] [CrossRef]
- Alsharif, S.T.; Almalki, A.H.; Ramzy, S.; Sultan Alqahtani, A.; Abduljabbar, M.H.; Algarni, M.A.; Serag, A. Derivative spectroscopy and wavelet transform as green spectrophotometric methods for abacavir and lamivudine measurement. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2024, 310, 123913. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.-P.; Zhou, F.-Y.; Liang, J.; Kuang, H.-X.; Xia, Y.-G. Distinction and quantification of Panax polysaccharide extracts via attenuated total reflectance-Fourier transform infrared spectroscopy with first-order derivative processing. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2024, 313, 124124. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Gao, J.; Tu, Y.; Zhang, Y.; Gao, J. Estimating low concentration heavy metals in water through hyperspectral analysis and genetic algorithm-partial least squares regression. Sci. Total Environ. 2024, 916, 170225. [Google Scholar] [CrossRef]
- Lv, S.; Wang, J.; Wang, S.; Wang, Q.; Wang, Z.; Fang, Y.; Zhai, W.; Wang, F.; Qu, G.; Ma, W. Quantitative analysis of chlorophyll in Catalpa bungei leaves based on partial least squares regression and spectral reflectance index. Sci. Hortic. 2024, 329, 113019. [Google Scholar] [CrossRef]
- Ghasemi, J.; Ahmadi, S.; Torkestani, K. Simultaneous determination of copper, nickel, cobalt and zinc using zincon as a metallochromic indicator with partial least squares. Anal. Chim. Acta 2003, 487, 181–188. [Google Scholar] [CrossRef]
- Safavi, A.; Abdollahi, H.; Mirzajani, R. Simultaneous spectrophotometric determination of Fe(III), Al(III) and Cu(II) by partial least-squares calibration method. Spectroc. Acta Pt. A-Molec. Biomolec. Spectr. 2006, 63, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Li, C.; Zhu, H.; Li, Y. A novel method for simultaneous determination of zinc, nickel, cobalt and copper based on UV–vis spectrometry. Optik 2019, 182, 58–64. [Google Scholar] [CrossRef]




| Component | Content | Units |
|---|---|---|
| Cu | 200–3500 | mg/L |
| Ni | 1000–8000 | mg/L |
| Co | 50–200 | mg/L |
| Fe | 500–2000 | mg/L |
| Bi | 0–200 | mg/L |
| As | 500–20,000 | mg/L |
| H2SO4 | 35–240 | g/L |
| Principal Components | PRESS(h) | Cumulative Y-Squared R2 (%) |
|---|---|---|
| 0 | 1.05882 | / |
| 1 | 0.94094 | 33.18 |
| 2 | 0.75196 | 66.44 |
| 3 | 0.06412 | 99.76 |
| 4 | 0.02855 | 99.96 |
| 5 | 0.03386 | |
| 6 | 0.03742 | |
| 7 | 0.04131 | |
| 8 | 0.04311 | |
| 9 | 0.04355 | |
| 10 | 0.04299 | |
| 11 | 0.04337 | |
| 12 | 0.04574 | |
| 13 | 0.0473 | |
| 14 | 0.04766 | |
| 15 | 0.04773 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Jiang, L.; Zheng, J.; Duan, N. Determination of High Concentration Copper Ions Based on Ultraviolet—Visible Spectroscopy Combined with Partial Least Squares Regression Analysis. Processes 2024, 12, 1408. https://doi.org/10.3390/pr12071408
Liang Q, Jiang L, Zheng J, Duan N. Determination of High Concentration Copper Ions Based on Ultraviolet—Visible Spectroscopy Combined with Partial Least Squares Regression Analysis. Processes. 2024; 12(7):1408. https://doi.org/10.3390/pr12071408
Chicago/Turabian StyleLiang, Qian, Linhua Jiang, Jiwu Zheng, and Ning Duan. 2024. "Determination of High Concentration Copper Ions Based on Ultraviolet—Visible Spectroscopy Combined with Partial Least Squares Regression Analysis" Processes 12, no. 7: 1408. https://doi.org/10.3390/pr12071408




