Efficient, Facile, and Green Synthesis of Ruthenium Carboxylate Complexes by Manual Grinding
Abstract
:1. Introduction
2. Materials and Methods
3. Results
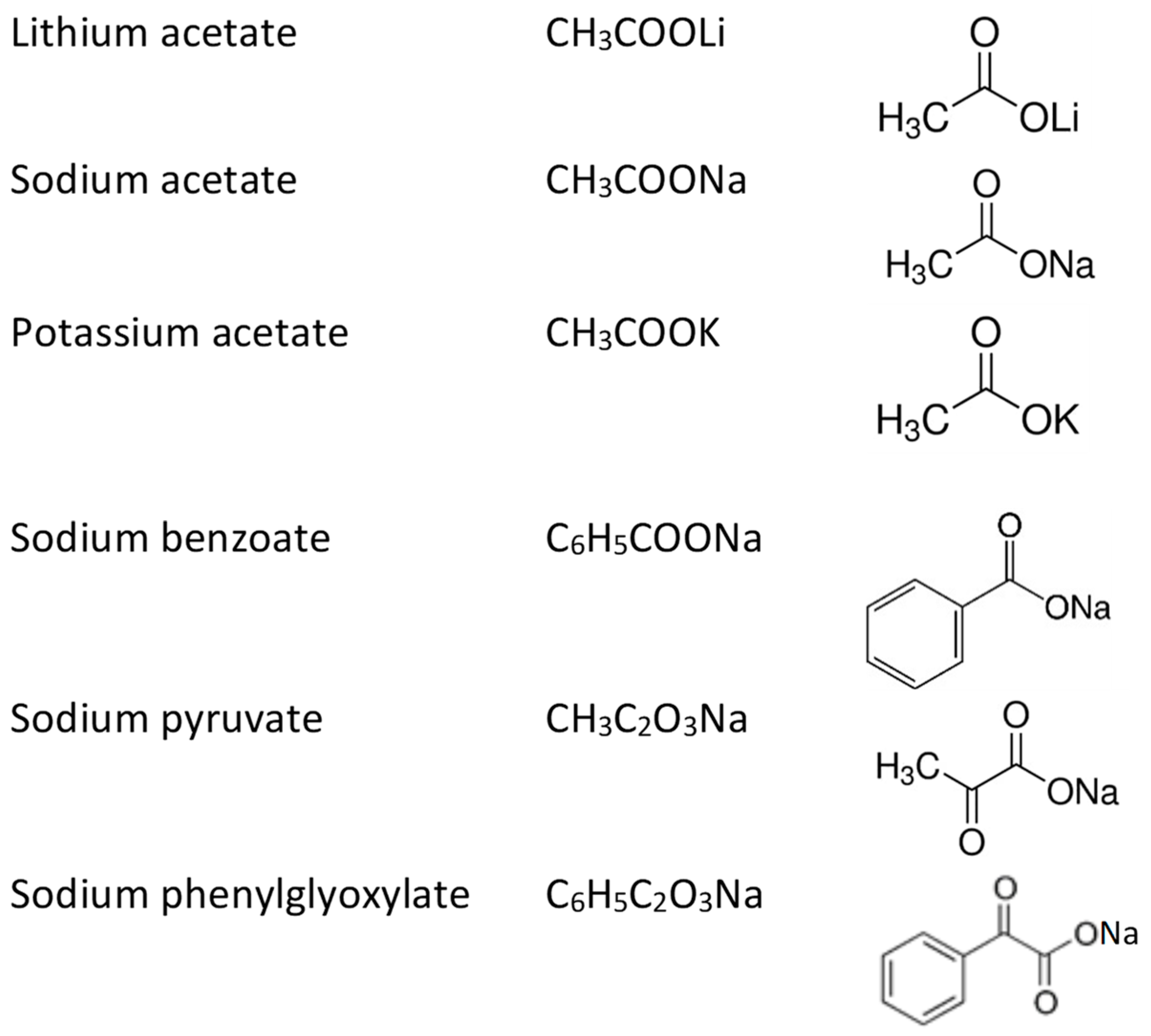
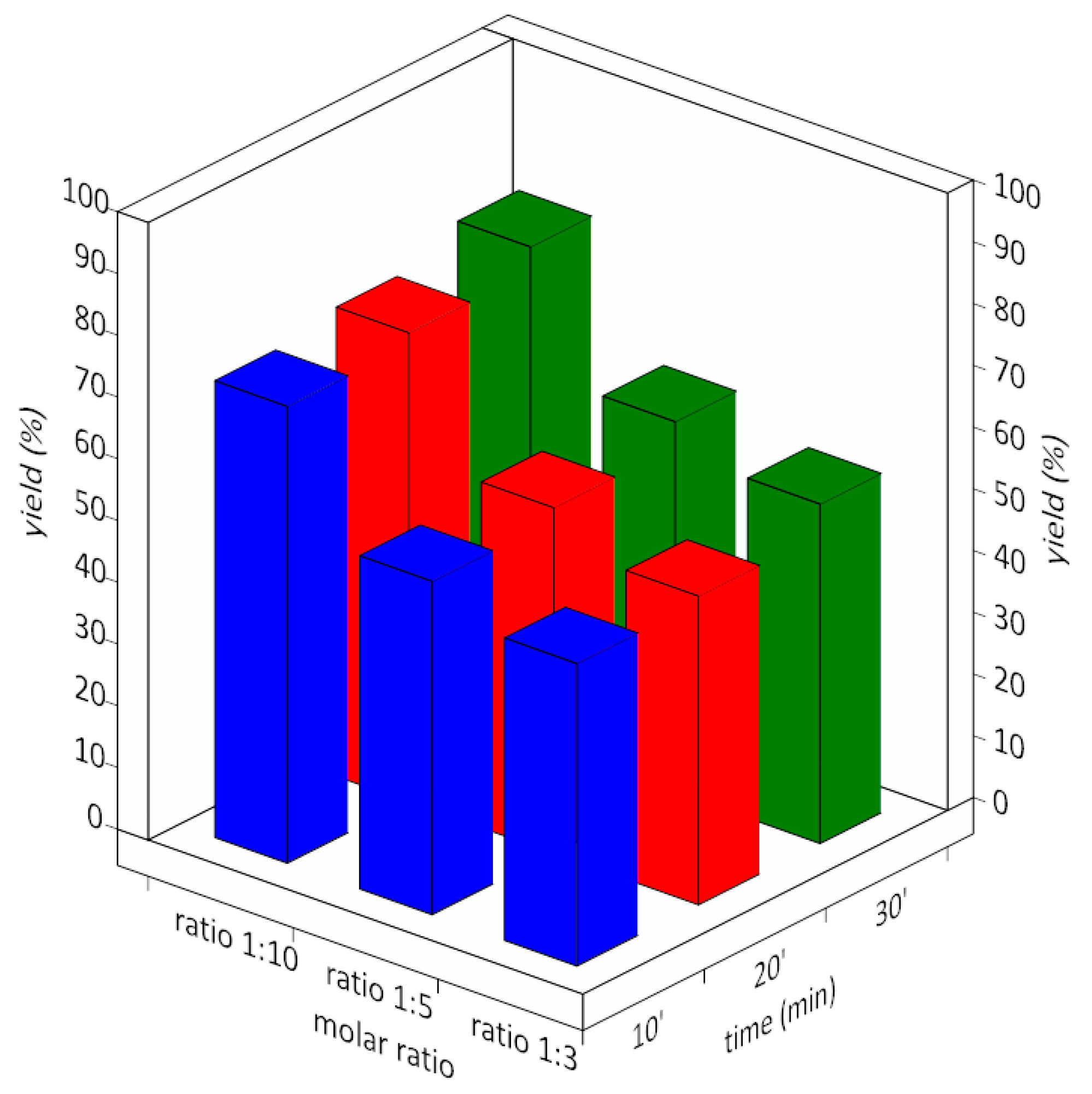
3.1. Alkali Metal Acetates
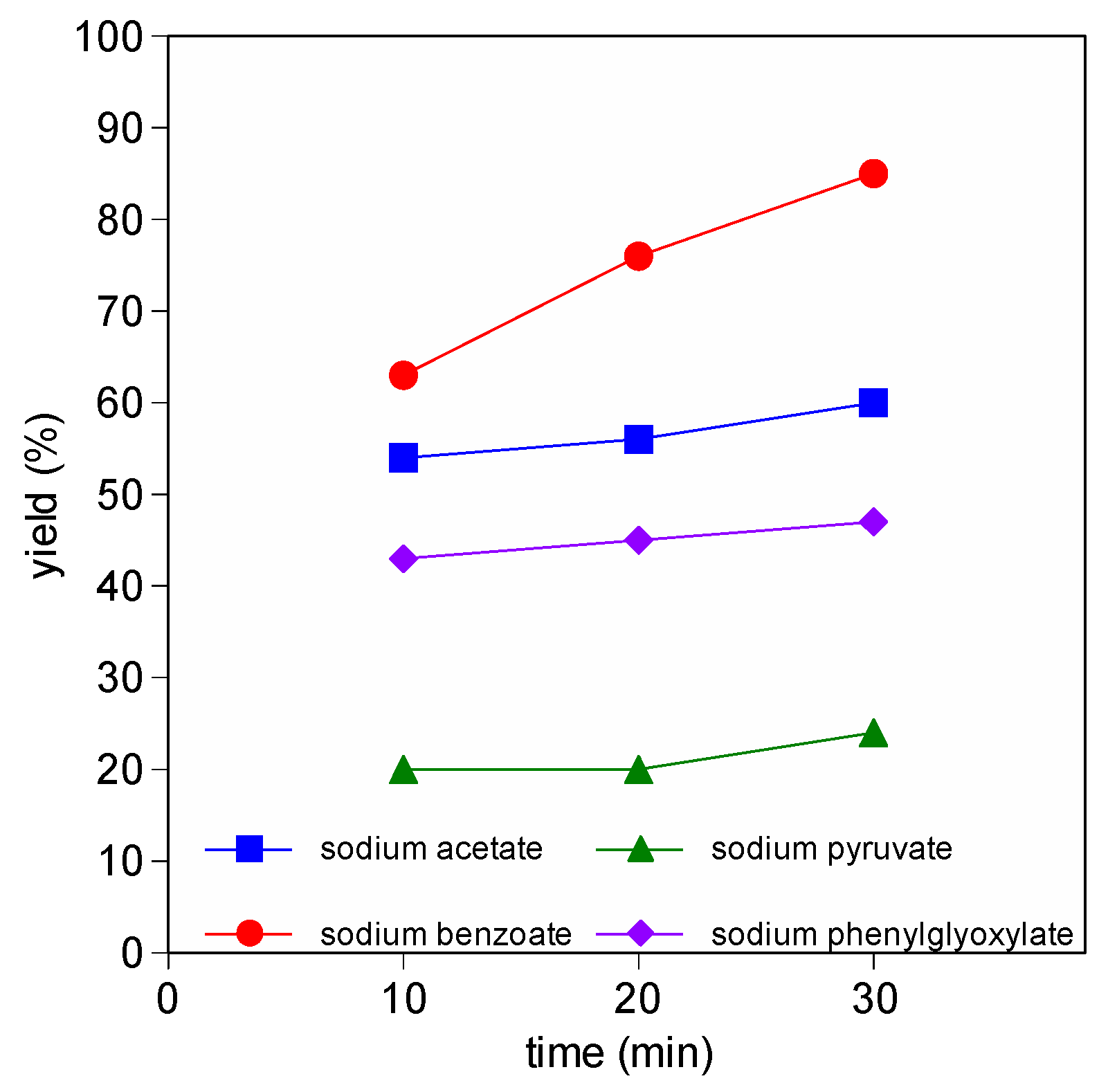
3.2. Sodium Carboxylates
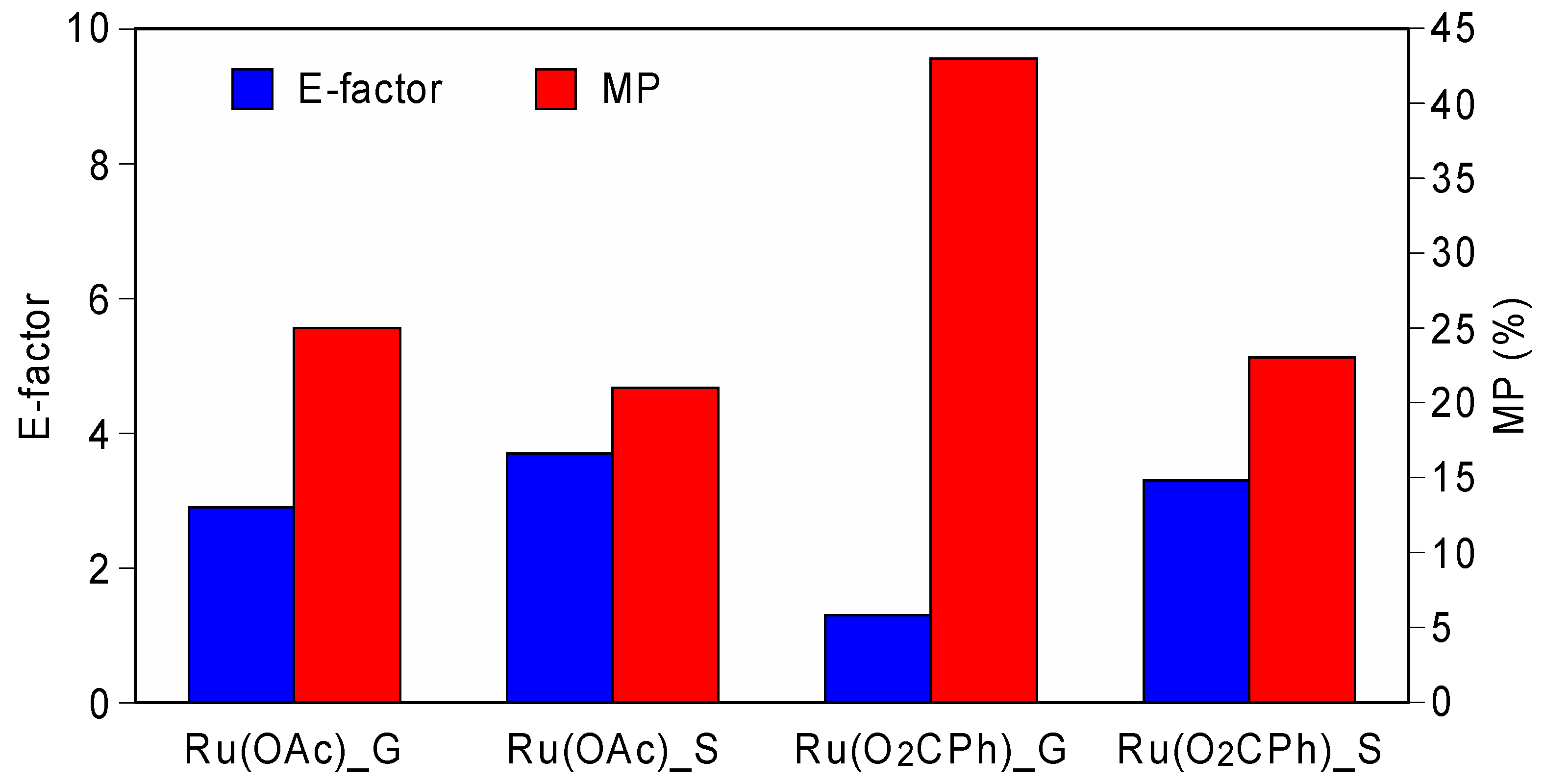
3.3. Comparison of Green Chemistry Metrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 2000. [Google Scholar]
- Porcheddu, A.; Colacino, E.; De Luca, L.; Delogu, F. Metal-Mediated and Metal-Catalyzed Reactions Under Mechanochemical Conditions. ACS Catal. 2020, 10, 8344–8394. [Google Scholar] [CrossRef]
- Ennas, G.; Scano, A.; Porcheddu, A.; Halasz, I.; Colacino, E. 1 Mechanochemistry: An Overview and a Historical Account. In Mechanochemistry; Evelina, C., Guido, E., Ivan, H., Andrea, P., Alessandra, S., Eds.; De Gruyter: Berlin, Germany; Boston, MA, USA, 2021; pp. 1–8. [Google Scholar] [CrossRef]
- James, S.L.; Adams, C.J.; Bolm, C.; Braga, D.; Collier, P.; Friščić, T.; Grepioni, F.; Harris, K.D.M.; Hyett, G.; Jones, W.; et al. Mechanochemistry: Opportunities for new and cleaner synthesis. Chem. Soc. Rev. 2012, 41, 413–447. [Google Scholar] [CrossRef]
- Do, J.-L.; Friščić, T. Mechanochemistry: A Force of Synthesis. ACS Cent. Sci. 2017, 3, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Friščić, T.; Mottillo, C.; Titi, H.M. Mechanochemistry for Synthesis. Angew. Chem. Int. Ed. 2020, 59, 1018–1029. [Google Scholar] [CrossRef]
- Bolm, C.; Hernández, J.G. Mechanochemistry of Gaseous Reactants. Angew. Chem. Int. Ed. 2019, 58, 3285–3299. [Google Scholar] [CrossRef] [PubMed]
- Fantozzi, N.; Volle, J.-N.; Porcheddu, A.; Virieux, D.; García, F.; Colacino, E. Green metrics in mechanochemistry. Chem. Soc. Rev. 2023, 52, 6680–6714. [Google Scholar] [CrossRef] [PubMed]
- Cuccu, F.; De Luca, L.; Delogu, F.; Colacino, E.; Solin, N.; Mocci, R.; Porcheddu, A. Mechanochemistry: New Tools to Navigate the Uncharted Territory of “Impossible” Reactions. ChemSusChem 2022, 15, e202200362. [Google Scholar] [CrossRef] [PubMed]
- Beillard, A.; Bantreil, X.; Métro, T.-X.; Martinez, J.; Lamaty, F. Alternative Technologies That Facilitate Access to Discrete Metal Complexes. Chem. Rev. 2019, 119, 7529–7609. [Google Scholar] [CrossRef]
- Hernández, J.G.; Bolm, C. Altering Product Selectivity by Mechanochemistry. J. Org. Chem. 2017, 82, 4007–4019. [Google Scholar] [CrossRef]
- Ballico, M.; Zuccaccia, D.; Figliolia, R.; Baratta, W. Bulky Diphosphine Acetate Ruthenium Complexes: Synthesis and Catalytic Activity in Ketone Transfer Hydrogenation and Alkyne Dimerization. Organometallics 2020, 39, 3180–3193. [Google Scholar] [CrossRef]
- Jia, G.; Rheingold, A.L.; Haggerty, B.S.; Meek, D.W. Synthesis and characterization of ruthenium acetate complexes containing triphosphines. Inorg. Chem. 1992, 31, 900–904. [Google Scholar] [CrossRef]
- Nicholls, L.D.M.; Alcarazo, M. Applications of α-Cationic Phosphines as Ancillary Ligands in Homogeneous Catalysis. Chem. Lett. 2018, 48, 1–13. [Google Scholar] [CrossRef]
- Scherpf, T.; Schwarz, C.; Scharf, L.T.; Zur, J.-A.; Helbig, A.; Gessner, V.H. Ylide-Functionalized Phosphines: Strong Donor Ligands for Homogeneous Catalysis. Angew. Chem. Int. Ed. 2018, 57, 12859–12864. [Google Scholar] [CrossRef]
- Scholl, M.; Ding, S.; Lee, C.W.; Grubbs, R.H. Synthesis and Activity of a New Generation of Ruthenium-Based Olefin Metathesis Catalysts Coordinated with 1,3-Dimesityl-4,5-dihydroimidazol-2-ylidene Ligands. Org. Lett. 1999, 1, 953–956. [Google Scholar] [CrossRef]
- Bano, T.; Zahoor, A.F.; Rasool, N.; Irfan, M.; Mansha, A. Recent trends in Grubbs catalysis toward the synthesis of natural products: A review. J. Iran. Chem. Soc. 2022, 19, 2131–2170. [Google Scholar] [CrossRef]
- Montgomery, T.P.; Johns, A.M.; Grubbs, R.H. Recent Advancements in Stereoselective Olefin Metathesis Using Ruthenium Catalysts. Catalysts. 2017, 7, 87. [Google Scholar] [CrossRef]
- Xie, X.; Lu, B.; Li, W.; Zhang, Z. Coordination determined chemo- and enantioselectivities in asymmetric hydrogenation of multi-functionalized ketones. Coord. Chem. Rev. 2018, 355, 39–53. [Google Scholar] [CrossRef]
- Noyori, R.; Ohkuma, T. Asymmetric catalysis by architectural and functional molecular engineering: Practical chemo and stereoselective hydrogenation of ketones. Angew. Chem. Int. Ed. 2001, 40, 40–73. [Google Scholar] [CrossRef]
- Noyori, R. Asymmetric Catalysis: Science and Opportunities (Nobel Lecture). Angew. Chem. Int. Ed. 2002, 41, 2008–2022. [Google Scholar] [CrossRef]
- Sandoval, C.A.; Ohkuma, T.; Muñiz, K.; Noyori, R. Mechanism of Asymmetric Hydrogenation of Ketones Catalyzed by BINAP/1,2-Diamine−Ruthenium(II) Complexes. J. Am. Chem. Soc. 2003, 125, 13490–13503. [Google Scholar] [CrossRef]
- Noyori, R.; Yamakawa, M.; Hashiguchi, S. Metal−Ligand Bifunctional Catalysis: A Nonclassical Mechanism for Asymmetric Hydrogen Transfer between Alcohols and Carbonyl Compounds. J. Org. Chem. 2001, 66, 7931–7944. [Google Scholar] [CrossRef] [PubMed]
- Baldino, S.; Giboulot, S.; Lovison, D.; Nedden, H.G.; Pöthig, A.; Zanotti-Gerosa, A.; Zuccaccia, D.; Ballico, M.; Baratta, W. Preparation of Neutral trans—Cis [Ru(O2CR)2P2(NN)], Cationic [Ru(O2CR)P2(NN)](O2CR) and Pincer [Ru(O2CR)(CNN)P2] (P = PPh3, P2= diphosphine) Carboxylate Complexes and their Application in the Catalytic Carbonyl Compounds Reduction. Organometallics 2021, 40, 1086–1103. [Google Scholar] [CrossRef]
- Lovison, D.; Berghausen, T.; Thomas, S.R.; Robson, J.; Drees, M.; Jandl, C.; Pöthig, A.; Mollik, P.; Halter, D.P.; Baratta, W.; et al. Beyond Metal-Arenes: Monocarbonyl Ruthenium(II) Catalysts for Transfer Hydrogenation Reactions in Water and in Cells. ACS Catal. 2023, 13, 10798–10823. [Google Scholar] [CrossRef]
- Alessi, D.; Del Mestre, P.; Aneggi, E.; Ballico, M.; Beltrami, A.P.; Busato, M.; Cesselli, D.; Heidecker, A.A.; Zuccaccia, D.; Baratta, W. Cyclometalated C^N diphosphine ruthenium catalysts for Oppenauer-type oxidation/transfer hydrogenation reactions and cytotoxic activity. Catal. Sci. Technol. 2023, 13, 5267–5279. [Google Scholar] [CrossRef]
- Hey, D.A.; Sauer, M.J.; Fischer, P.J.; Esslinger, E.-M.H.J.; Kühn, F.E.; Baratta, W. Acetate Acetylacetonate Ampy Ruthenium(II) Complexes as Efficient Catalysts for Ketone Transfer Hydrogenation. ChemCatChem 2020, 12, 3537–3544. [Google Scholar] [CrossRef]
- Hey, D.A.; Fischer, P.J.; Baratta, W.; Kühn, F.E. Ru(O2CCF3)2(PPh3)2 and ruthenium phosphine complexes bearing fluoroacetate ligands: Synthesis, characterization and catalytic activity. Dalton Trans. 2019, 48, 4625–4635. [Google Scholar] [CrossRef]
- Dupau, P.; Bonomo, L.; Kermorvan, L. Unexpected Role of Anionic Ligands in the Ruthenium-Catalyzed Base-Free Selective Hydrogenation of Aldehydes. Angew. Chem. Int. Ed. 2013, 52, 11347–11350. [Google Scholar] [CrossRef]
- Bennett, M.A.; Byrnes, M.J.; Chung, G.; Edwards, A.J.; Willis, A.C. Bis(acetylacetonato)ruthenium(II) complexes containing bulky tertiary phosphines. Formation and redox behaviour of Ru(acac)2 (PR3) (R=iPr, Cy) complexes with ethene, carbon monoxide, and bridging dinitrogen. Inorg. Chim. Acta 2005, 358, 1692–1708. [Google Scholar] [CrossRef]
- Manimaran, T.; Wu, T.C.; Klobucar, W.D.; Kolich, C.H.; Stahly, G.P.; Fronczek, F.R.; Watkins, S.E. In situ generation of ruthenium-chiral phosphine complexes and their use in asymmetric hydrogenation. Organometallics 1993, 12, 1467–1470. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Alberico, E.; Junge, K.; Junge, H.; Beller, M. Towards a general ruthenium-catalyzed hydrogenation of secondary and tertiary amides to amines. Chem. Sci. 2016, 7, 3432–3442. [Google Scholar] [CrossRef]
- van Buijtenen, J.; Meuldijk, J.; Vekemans, J.A.J.M.; Hulshof, L.A.; Kooijman, H.; Spek, A.L. Dinuclear Ruthenium Complexes Bearing Dicarboxylate and Phosphine Ligands. Acceptorless Catalytic Dehydrogenation of 1-Phenylethanol. Organometallics 2006, 25, 873–881. [Google Scholar] [CrossRef]
- Lynam, J.M.; Welby, C.E.; Whitwood, A.C. Exploitation of a Chemically Non-innocent Acetate Ligand in the Synthesis and Reactivity of Ruthenium Vinylidene Complexes. Organometallics 2009, 28, 1320–1328. [Google Scholar] [CrossRef]
- Welby, C.E.; Eschemann, T.O.; Unsworth, C.A.; Smith, E.J.; Thatcher, R.J.; Whitwood, A.C.; Lynam, J.M. Ruthenium Acetate Complexes as Versatile Probes of Metal–Ligand Interactions: Insight into the Ligand Effects of Vinylidene, Carbene, Carbonyl, Nitrosyl and Isocyanide. Eur. J. Inorg. Chem. 2012, 2012, 1493–1506. [Google Scholar] [CrossRef]
- Noyori, R.; Takaya, H. BINAP: An efficient chiral element for asymmetric catalysis. Acc. Chem. Res. 1990, 23, 345–350. [Google Scholar] [CrossRef]
- Tang, W.; Zhang, X. New Chiral Phosphorus Ligands for Enantioselective Hydrogenation. Chem. Rev. 2003, 103, 3029–3070. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Zhang, X.; Matsumura, K.; Sayo, N.; Kumobayashi, H.; Ohta, T.; Nozaki, K.; Takaya, H. Highly Efficient Enantioselective Synthesis of Optically Active Carboxylic Acids by Ru(OCOCH3)2[(S)-H8-BINAP]. J. Org. Chem. 1996, 61, 5510–5516. [Google Scholar] [CrossRef]
- Ohta, T.; Takaya, H.; Noyori, R. (BINAP)-ruthenium (II) dicarboxylate complexes: New, highly efficient catalysts for asymmetric hydrogenations. Inorg. Chem. 1988, 27, 566–569. [Google Scholar] [CrossRef]
- Kitamura, M.; Tokunaga, M.; Noyori, R. Practical synthesis of BINAP-ruthenium(II) dicarboxylate complexes. J. Org. Chem. 1992, 57, 4053–4054. [Google Scholar] [CrossRef]
- Ohta, T.; Takaya, H.; Kitamura, M.; Nagai, K.; Noyori, R. Asymmetric hydrogenation of unsaturated carboxylic acids catalyzed by BINAP-ruthenium(II) complexes. J. Org. Chem. 1987, 52, 3174–3176. [Google Scholar] [CrossRef]
- Baratta, W.; Ballico, M.; Del Zotto, A.; Herdtweck, E.; Magnolia, S.; Peloso, R.; Siega, K.; Toniutti, M.; Zangrando, E.; Rigo, P. Pincer CNN Ruthenium(II) Complexes with Oxygen-Containing Ligands (O2CR, OAr, OR, OSiR3, O3SCF3): Synthesis, Structure, and Catalytic Activity in Fast Transfer Hydrogenation. Organometallics 2009, 28, 4421–4430. [Google Scholar] [CrossRef]
- Pagola, S. Outstanding Advantages, Current Drawbacks, and Significant Recent Developments in Mechanochemistry: A Perspective View. Crystals 2023, 13, 124. [Google Scholar] [CrossRef]
- Hernández, J.G.; Friščić, T. Metal-catalyzed organic reactions using mechanochemistry. Tetrahedron Lett. 2015, 56, 4253–4265. [Google Scholar] [CrossRef]
- Sheldon, R.A. Organic synthesis–Past, present and future. Chem. Ind. 1992, 23, 903–906. [Google Scholar]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Constable, D.J.C.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘green’ chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Mulvihill, M.J.; Beach, E.S.; Zimmerman, J.B.; Anastas, P.T. Green Chemistry and Green Engineering: A Framework for Sustainable Technology Development. Annu. Rev. Environ. Resour. 2011, 36, 271–293. [Google Scholar] [CrossRef]
- Hallman, P.S.; Stephenson, T.A.; Wilkinson, G. Tetrakis(triphenylphosphine)dichlororuthenium(II) and Tris(triphenylphosphine)dichlororuthenium(II). In Inorganic Syntheses; John Wiley & Sons: Hoboken, NJ, USA, 1970; pp. 237–240. [Google Scholar] [CrossRef]
- Akoka, S.; Barantin, L.; Trierweiler, M. Concentration Measurement by Proton NMR Using the ERETIC Method. Anal. Chem. 1999, 71, 2554–2557. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, R.W.; Spencer, A.; Wilkinson, G. Carboxylato-triphenylphosphine complexes of ruthenium, cationic triphenylphosphine complexes derived from them, and their behaviour as homogeneous hydrogenation catalysts for alkenes. J. Chem. Soc. Dalton Trans. 1973, 8, 846–854. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2004; Volume 85. [Google Scholar]
- Hiett, N.P.; Lynam, J.M.; Welby, C.E.; Whitwood, A.C. Ruthenium carboxylate complexes as easily prepared and efficient catalysts for the synthesis of β-oxopropyl esters. J. Organomet. Chem. 2011, 696, 378–387. [Google Scholar] [CrossRef]
- Yan, Z.; Ma, Z.; Deng, J.; Luo, G. Mechanism and kinetics of epoxide ring-opening with carboxylic acids catalyzed by the corresponding carboxylates. Chem. Eng. Sci. 2021, 242, 116746. [Google Scholar] [CrossRef]
- Bunting, J.W.; Thong, K.M. Stability constants for some 1:1 metal–carboxylate complexes. Can. J. Chem. 1970, 48, 1654–1656. [Google Scholar] [CrossRef]

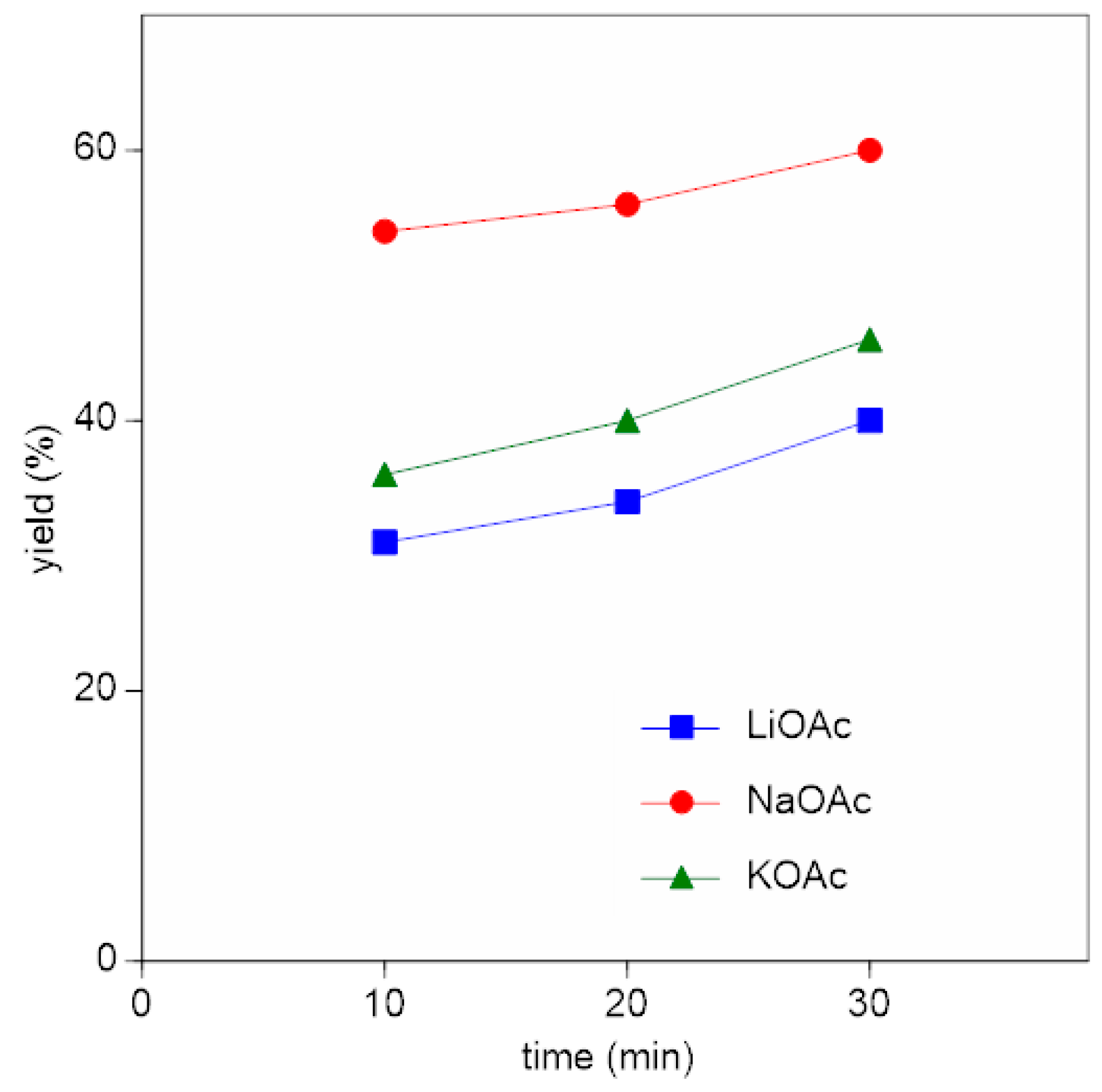
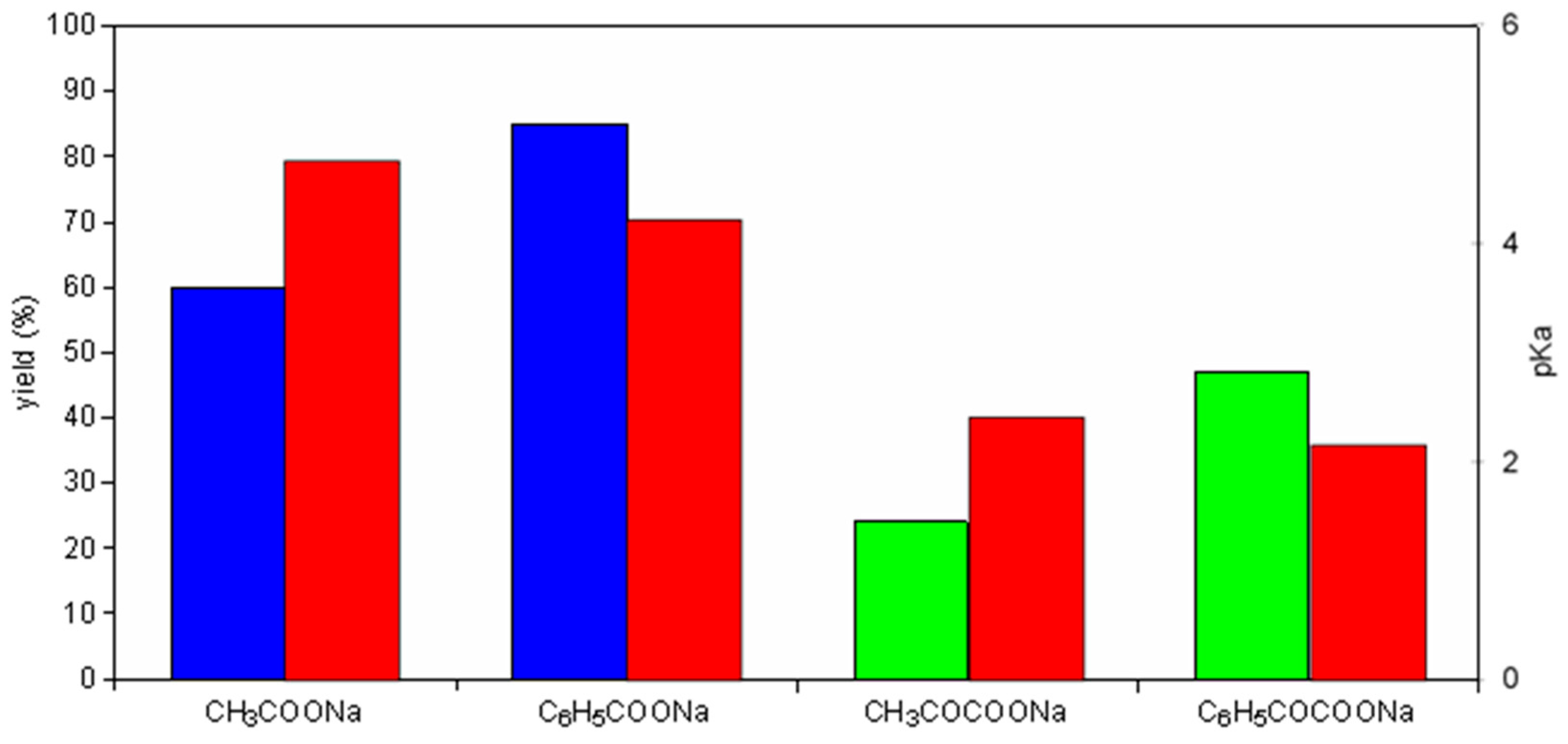
| Alkali Metal Acetate | ΔfH0 MOAc (kJ/mol) | ΔfH0 MCl (kJ/mol) | Product Yield (%) |
|---|---|---|---|
| LiOAc | −741 | −409 | 40 |
| NaOAc | −709 | −411 | 60 |
| KOAc | −723 | −436 | 46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aneggi, E.; Zuccaccia, D.; Porcheddu, A.; Baratta, W. Efficient, Facile, and Green Synthesis of Ruthenium Carboxylate Complexes by Manual Grinding. Processes 2024, 12, 1413. https://doi.org/10.3390/pr12071413
Aneggi E, Zuccaccia D, Porcheddu A, Baratta W. Efficient, Facile, and Green Synthesis of Ruthenium Carboxylate Complexes by Manual Grinding. Processes. 2024; 12(7):1413. https://doi.org/10.3390/pr12071413
Chicago/Turabian StyleAneggi, Eleonora, Daniele Zuccaccia, Andrea Porcheddu, and Walter Baratta. 2024. "Efficient, Facile, and Green Synthesis of Ruthenium Carboxylate Complexes by Manual Grinding" Processes 12, no. 7: 1413. https://doi.org/10.3390/pr12071413
APA StyleAneggi, E., Zuccaccia, D., Porcheddu, A., & Baratta, W. (2024). Efficient, Facile, and Green Synthesis of Ruthenium Carboxylate Complexes by Manual Grinding. Processes, 12(7), 1413. https://doi.org/10.3390/pr12071413











