Fabrication of NiO-CuO/RGO Composite for Lithium Storage Property
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Characterization
2.3. Electrochemical Measurements
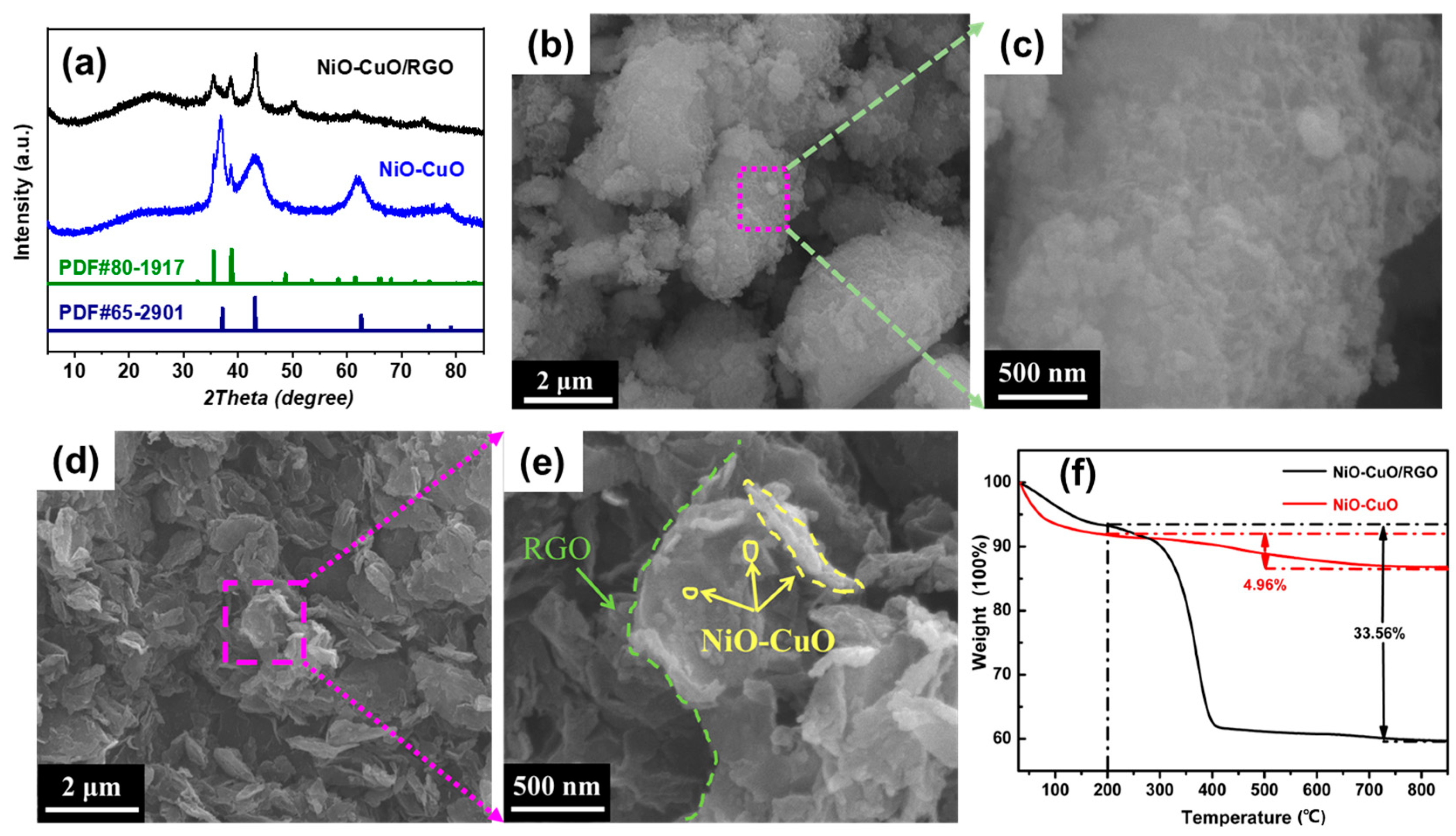
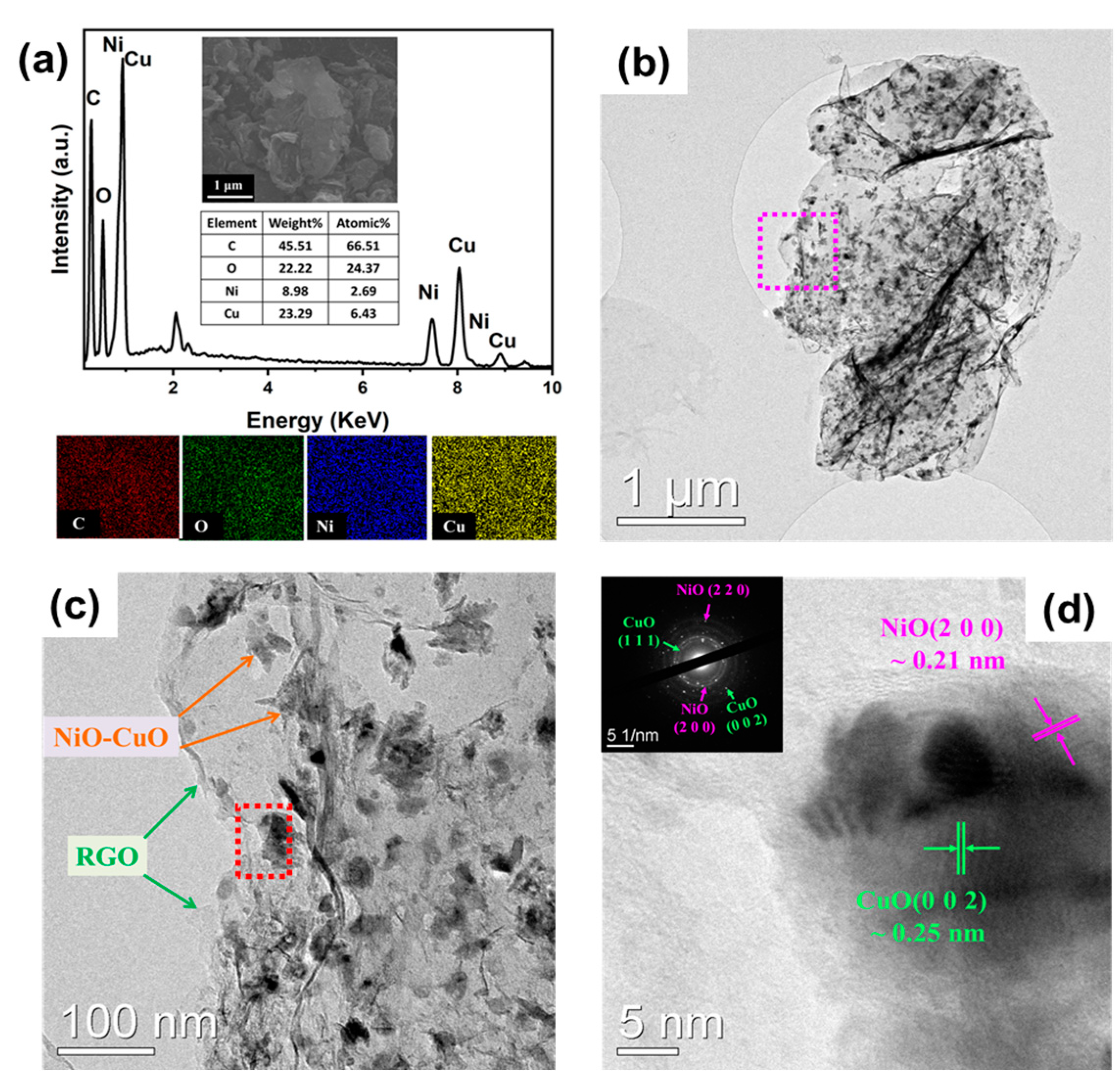
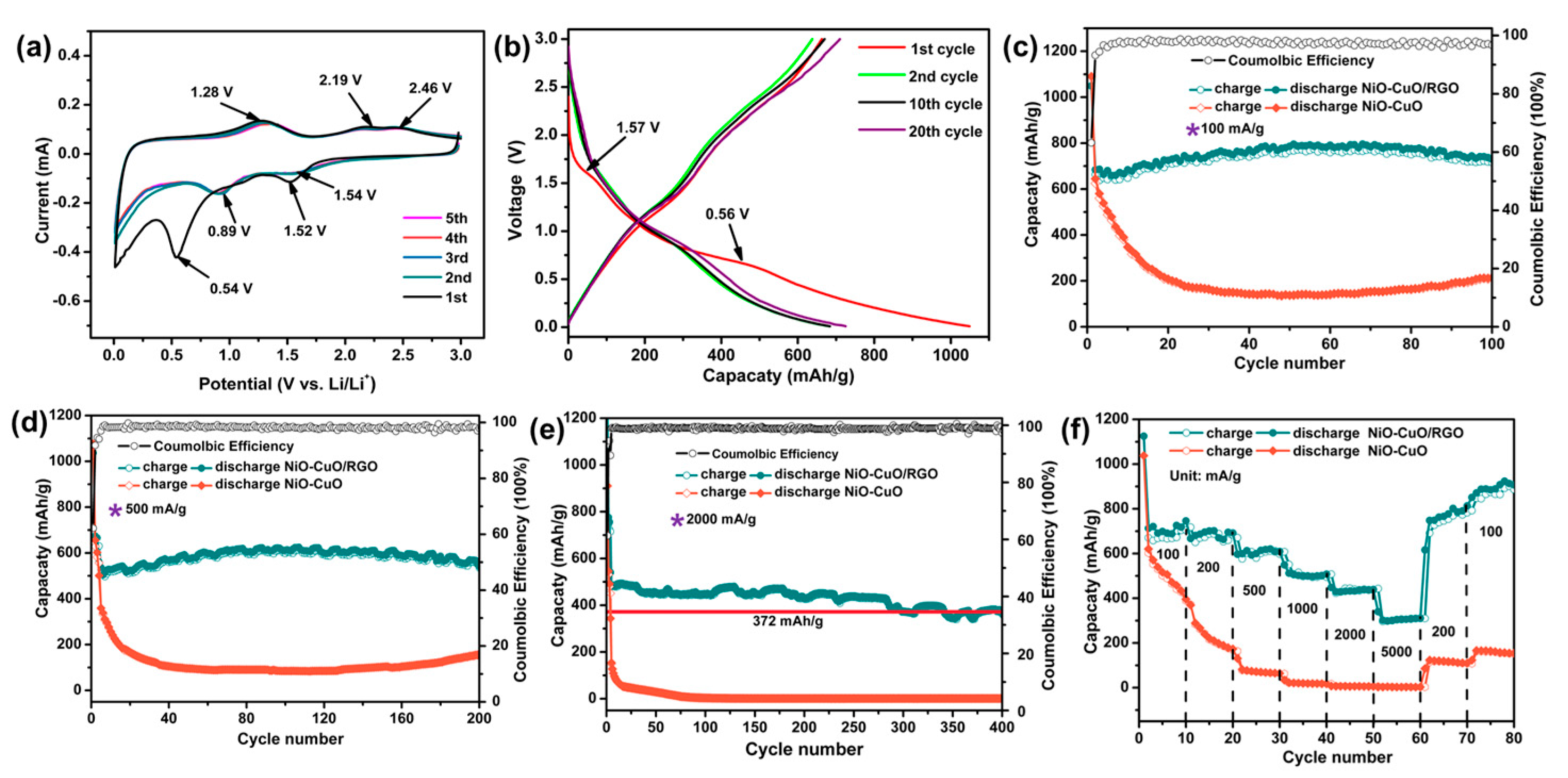
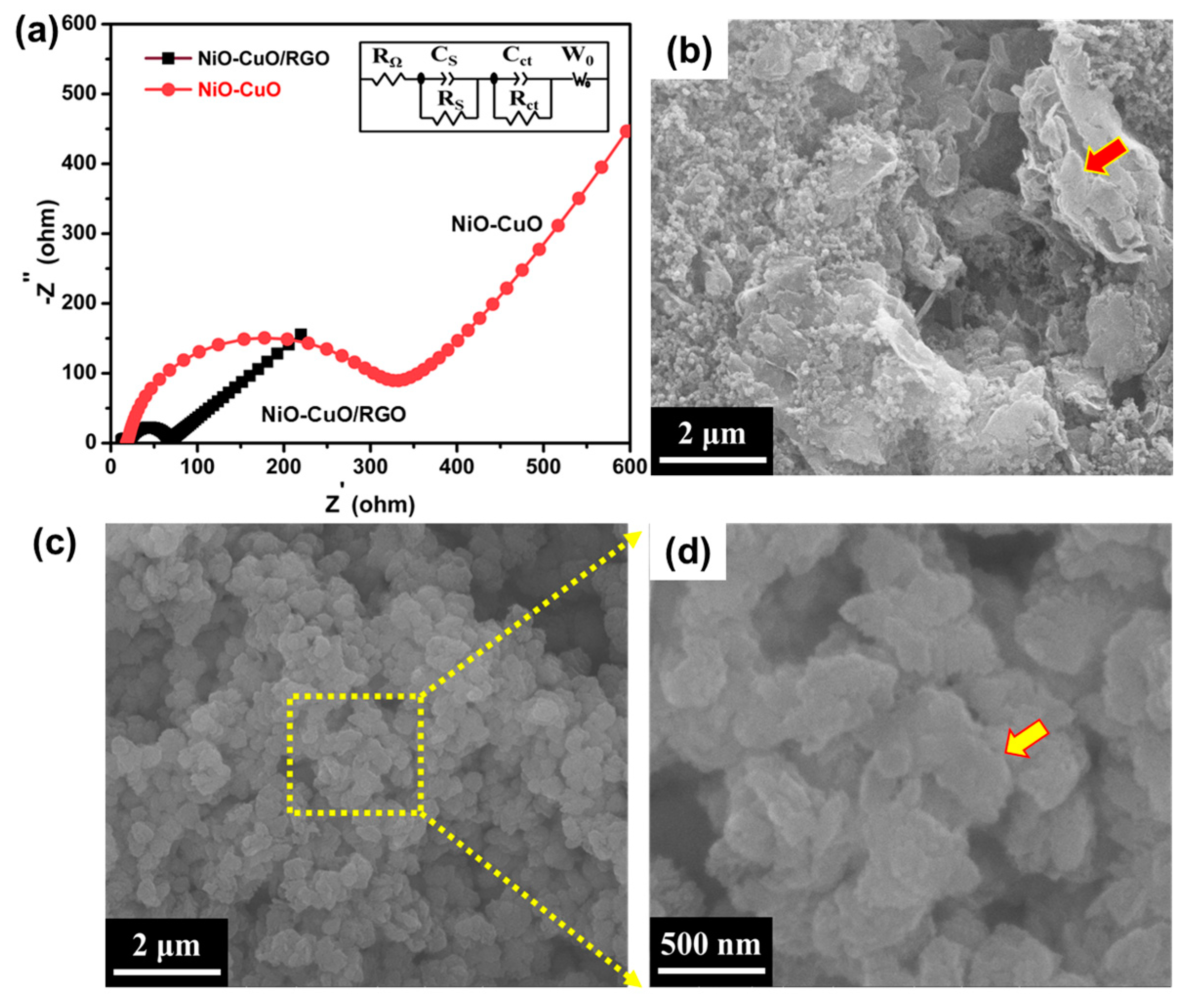
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, M.G.; Cho, J. Reversible and High-Capacity Nanostructured Electrode Materials for Li-Ion Batteries. Adv. Funct. Mater. 2009, 19, 1497–1514. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.X.; Lee, J.T.; Yushin, G.; Lee, G. Yushin, Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Fang, J.; Yuan, Y.F.; Wang, L.K.; Ni, H.L.; Zhu, H.L.; Gui, J.S.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Hierarchical ZnO@NiO core–shell nanorod array as high performance anode material for lithium-ion batteries. Mater. Lett. 2013, 111, 1–4. [Google Scholar] [CrossRef]
- Cao, T.; Fang, D.; Liu, L.; Luo, Z.; Wang, Q.; Dong, L.; Xiong, C. Nanosheets-based ZnO–NiO microspheres for lithium-ion batteries. J. Mater. Sci. Mater. Electron. 2015, 26, 5279–5286. [Google Scholar] [CrossRef]
- Xiong, Q.Q.; Tu, J.P.; Xia, X.H.; Zhao, X.Y.; Gu, C.D.; Wang, X.L. A three-dimensional hierarchical Fe2O3@NiO core/shell nanorod array on carbon cloth: A new class of anode for high-performance lithium-ion batteries. Nanoscale 2013, 5, 7906–7912. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, R.; Yang, Y.; Liu, C.; Yuan, A.; Yang, H.; Shen, X. Cyanometallic frameworks derived hierarchical porous Fe2O3/NiO microflowers with excellent lithium-storage property. J. Alloys Compd. 2017, 698, 469–475. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhuo, Q.; Lv, X.; Ma, Y.; Zhong, J.; Sun, X. NiO-Co3O4 nanoplate composite as efficient anode in Li-ion battery. Electrochim. Acta 2015, 178, 590–596. [Google Scholar] [CrossRef]
- Zhong, Y.; Huang, H.; Wang, K.; He, Z.; Zhu, S.; Chang, L.; Shao, H.; Wang, J.; Cao, C.-N. NiO@MnO2 core–shell composite microtube arrays for high-performance lithium ion batteries. RSC Adv. 2017, 7, 4840–4847. [Google Scholar] [CrossRef]
- Chen, H.; Hu, Z.-L.; Li, C.-L.; Li, N.; Xiang, K.-X. Facile synthesis of CuO–NiO nanocomposites with high surface areas and their application for lithium-ion batteries. Micro Nano Lett. 2013, 8, 544–548. [Google Scholar] [CrossRef]
- Gu, X.; Chen, L.; Ju, Z.; Xu, H.; Yang, J.; Qian, Y. Controlled Growth of Porous α-Fe2O3 Branches on β-MnO2 Nanorods for Excellent Performance in Lithium-Ion Batteries. Adv. Funct. Mater. 2013, 23, 4049–4056. [Google Scholar] [CrossRef]
- Ren, W.; Liu, D.; Sun, C.; Yao, X.; Tan, J.; Wang, C.; Zhao, K.; Wang, X.; Li, Q.; Mai, L. Nonhierarchical Heterostructured Fe2O3/Mn2O3 Porous Hollow Spheres for Enhanced Lithium Storage. Small 2018, 14, e1800659. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.D.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Molitor, F.; Guttinger, J.; Stampfer, C.; Droscher, S.; Jacobsen, A.; Ihn, T.; Ensslin, K. Electronic properties of graphene nanostructures. J. Phys-Condens. Matter 2011, 23, 212–215. [Google Scholar] [CrossRef]
- Wang, Z.; Mu, J.C.; Li, Y.; Chen, J.; Zhang, L.P.; Li, D.G.; Zhao, P.P. Preparation and lithium storage properties of NiO-SnO2/graphene nanosheet ternary composites. J. Alloys Compd. 2017, 695, 2909–2915. [Google Scholar] [CrossRef]
- Du, D.J.; Yue, W.B.; Fan, X.L.; Tang, K.; Yang, X.J. Ultrathin NiO/NiFe2O4 Nanoplates Decorated Graphene Nanosheets with Enhanced Lithium Storage Properties. Electrochim. Acta 2016, 194, 17–25. [Google Scholar] [CrossRef]
- Ma, L.; Pei, X.-Y.; Mo, D.-C.; Lyu, S.-S.; Fu, Y.-X. Fabrication of NiO-ZnO/RGO composite as an anode material for lithium-ion batteries. Ceram. Int. 2018, 44, 22664–22670. [Google Scholar] [CrossRef]
- Abbas, S.M.; Hussain, S.T.; Ali, S.; Ahmad, N.; Ali, N.; Abbas, S.; Ali, Z. Modification of carbon nanotubes by CuO-doped NiO nanocomposite for use as an anode material for lithium-ion batteries. J. Solid. State Chem. 2013, 202, 43–50. [Google Scholar] [CrossRef]
- Hummer, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. Acs Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Song, X.; Sun, S.; Zhang, W.; Yu, H.; Fan, W. Synthesis of Cu(OH)2 Nanowires at Aqueous-Organic Interfaces. J. Phys. Chem. B 2004, 108, 5200–5205. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Sun, W.; Ning, G.; Wei, T.; Zhang, Q.; Zhang, R.; Zhi, L.; Wei, F. Advanced Asymmetric Supercapacitors Based on Ni(OH)2/Graphene and Porous Graphene Electrodes with High Energy Density. Adv. Funct. Mater. 2012, 22, 2632–2641. [Google Scholar] [CrossRef]
- Yang, W.F.; Cheng, G.H.; Dong, C.Q.; Bai, Q.G.; Chen, X.T.; Peng, Z.Q.; Zhang, Z.H. NiO nanorod array anchored Ni foam as a binder-free anode for high-rate lithium ion batteries. J. Mater. Chem. A 2014, 2, 20022–20029. [Google Scholar] [CrossRef]
- Yang, J.R.; Zeng, D.Q.; Zheng, H.F.; Xie, Q.S.; Huang, J.; Xiao, L.; Peng, D.L. 3D graphene encapsulated ZnO-NiO-CuO double-shelled hollow microspheres with enhanced lithium storage properties. J. Alloys Compd. 2018, 765, 1158–1166. [Google Scholar] [CrossRef]
- Sun, L.; Deng, Q.; Li, Y.; Deng, L.; Wang, Y.; Ren, X.; Zhang, P. Solvothermal synthesis of ternary Cu2O-CuO-RGO composites as anode materials for high performance lithium-ion batteries. Electrochim. Acta 2016, 222, 1650–1659. [Google Scholar] [CrossRef]
- Hu, L.; Huang, Y.; Zhang, F.; Chen, Q. CuO/Cu2O composite hollow polyhedrons fabricated from metal-organic framework templates for lithium-ion battery anodes with a long cycling life. Nanoscale 2013, 5, 4186–4190. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Wang, Y. Multilayer CuO@NiO Hollow Spheres: Microwave-Assisted Metal Organic-Framework Derivation and Highly Reversible Structure-Matched Stepwise Lithium Storage. ACS Nano 2015, 9, 11462–11471. [Google Scholar] [CrossRef]
- Yin, H.; Yu, X.-X.; Li, Q.-W.; Cao, M.-L.; Zhang, W.; Zhao, H.; Zhu, M.-Q. Hollow porous CuO/C composite microcubes derived from metal-organic framework templates for highly reversible lithium-ion batteries. J. Alloys Compd. 2017, 706, 97–102. [Google Scholar] [CrossRef]
- Fan, Z.; Liang, J.; Yu, W.; Ding, S.; Cheng, S.; Yang, G.; Wang, Y.; Xi, Y.; Xi, K.; Kumar, R.V. Ultrathin NiO nanosheets anchored on a highly ordered nanostructured carbon as an enhanced anode material for lithium ion batteries. Nano Energy 2015, 16, 152–162. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Q.; Lu, Z.; Yang, C.; Qian, W.; Yu, F. Porous nanocomposites by cotton-derived carbon/NiO with high performance for lithium-ion storage. J. Alloys Compd. 2021, 874, 159788. [Google Scholar] [CrossRef]
- Deng, W.; Chen, X.; Hub, A.; Zhang, S. Graphitic carbon-wrapped NiO embedded three dimensional nitrogen doped aligned carbon nanotube arrays with long cycle life for lithium ion batteries. Rsc Adv. 2018, 8, 28440–28446. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, Y.; Su, D.; Ding, C.; Wang, L.; Yan, D.; Li, J.; Jin, H. Synthesis of NiO Nano Octahedron Aggregates as High-Performance Anode Materials for Lithium Ion Batteries. Electrochim. Acta 2017, 231, 272–278. [Google Scholar] [CrossRef]
- Cui, X.; Song, B.; Cheng, S.; Xie, Y.; Shao, Y.; Sun, Y. Synthesis of carbon nanotube (CNT)-entangled CuO nanotube networks via CNT-catalytic growth and in situ thermal oxidation as additive-free anodes for lithium ion batteries. Nanotechnology 2018, 29, 035603. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, L.; Li, X.; Shan, H.; Chen, C.; Yan, B.; Xiong, D.; Li, D. Enhanced anode performance of flower-like NiO/RGO nanocomposites for lithium-ion batteries. Mater. Chem. Phys. 2018, 217, 547–552. [Google Scholar] [CrossRef]
- Yin, X.; Chen, H.; Zhi, C.; Sun, W.; Lv, L.P.; Wang, Y. Functionalized Graphene Quantum Dot Modification of Yolk-Shell NiO Microspheres for Superior Lithium Storage. Small 2018, 14, e1800589. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-X.; Zhao, D.-L.; Yao, R.-R.; Li, C.; Wang, X.-J.; Sun, F.-F. Hedgehog-like CuO/nitrogen-doped graphene nanocomposite for high-performance lithium-ion battery anodes. J. Alloys Compd. 2017, 714, 419–424. [Google Scholar] [CrossRef]
- Wang, Q.; Zhao, J.; Shan, W.; Xia, X.; Xing, L.; Xue, X. CuO nanorods/graphene nanocomposites for high-performance lithium-ion battery anodes. J. Alloys Compd. 2014, 590, 424–427. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, Y.; Key, J.; Shen, P.K. Three-dimensional graphene sheets with NiO nanobelt outgrowths for enhanced capacity and long term high rate cycling Li-ion battery anode material. J. Power Sources 2018, 379, 362–370. [Google Scholar] [CrossRef]
- Park, J.C.; Kim, J.; Kwon, H.; Song, H. Gram-Scale Synthesis of Cu2O Nanocubes and Subsequent Oxidation to CuO Hollow Nanostructures for Lithium-Ion Battery Anode Materials. Adv. Mater. 2009, 21, 803–807. [Google Scholar] [CrossRef]
- Yuan, W.; Yan, Z.; Pan, B.; Qiu, Z.; Luo, J.; Tan, Z.; Tang, Y.; Li, Z. Hierarchical MCMB/CuO/Cu anode with super-hydrophilic substrate and blind-hole structures for lithium-ion batteries. J. Alloys Compd. 2017, 719, 353–364. [Google Scholar] [CrossRef]
- Ranjbar-Azad, M.; Behpour, M. Facile in situ co-precipitation synthesis of CuO–NiO/rGO nanocomposite for lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 2021, 32, 18043–18056. [Google Scholar] [CrossRef]
- Liang, J.; Jiang, J.; Xu, M.; Huo, X.; Ye, D.; Zhang, S.; Wu, X.; Wu, W. Improved lithium storage performance of urchin-like CuO microspheres by stereotaxically constructed graphene mediating synergistic effect. J. Mater. Sci. Mater. Electron. 2021, 32, 8557–8569. [Google Scholar] [CrossRef]
- Fu, J.; He, H.; Zeng, T.; Zhang, C. Tunable surface pseudocapacitance assisted fast and flexible lithium storage of graphene wrapped NiO nano-arrays on nitrogen-doped carbon foams. Electrochim. Acta 2022, 407, 139875. [Google Scholar] [CrossRef]
- Bajorowicz, B.; Wilamowska-Zawłocka, M.; Lisowski, W.; Żak, A.; Klimczuk, T. N-doped graphene quantum dot-decorated MOF-derived yolk-shell ZnO/NiO hybrids to boost lithium and sodium ion battery performance. Appl. Surf. Sci. 2024, 655, 159702. [Google Scholar] [CrossRef]
- Ma, L.; Pei, X.Y.; Mo, D.C.; Heng, Y.; Lyu, S.S.; Fu, Y.X. Facile fabrication of NiO flakes and reduced graphene oxide (NiO/RGO) composite as anode material for lithium-ion batteries. J. Mater. Sci.-Mater. 2019, 30, 5874–5880. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Chen, Y.; Wang, F.; Zhou, G. Fabrication of NiO-CuO/RGO Composite for Lithium Storage Property. Processes 2024, 12, 1422. https://doi.org/10.3390/pr12071422
Fu Y, Chen Y, Wang F, Zhou G. Fabrication of NiO-CuO/RGO Composite for Lithium Storage Property. Processes. 2024; 12(7):1422. https://doi.org/10.3390/pr12071422
Chicago/Turabian StyleFu, Yuanxiang, Yuxin Chen, Fan Wang, and Guoyong Zhou. 2024. "Fabrication of NiO-CuO/RGO Composite for Lithium Storage Property" Processes 12, no. 7: 1422. https://doi.org/10.3390/pr12071422



