Impacts of CO2-CH4 Mixed Gas on Property of Formation Oil from the Bohai Oilfield
Abstract
:1. Introduction
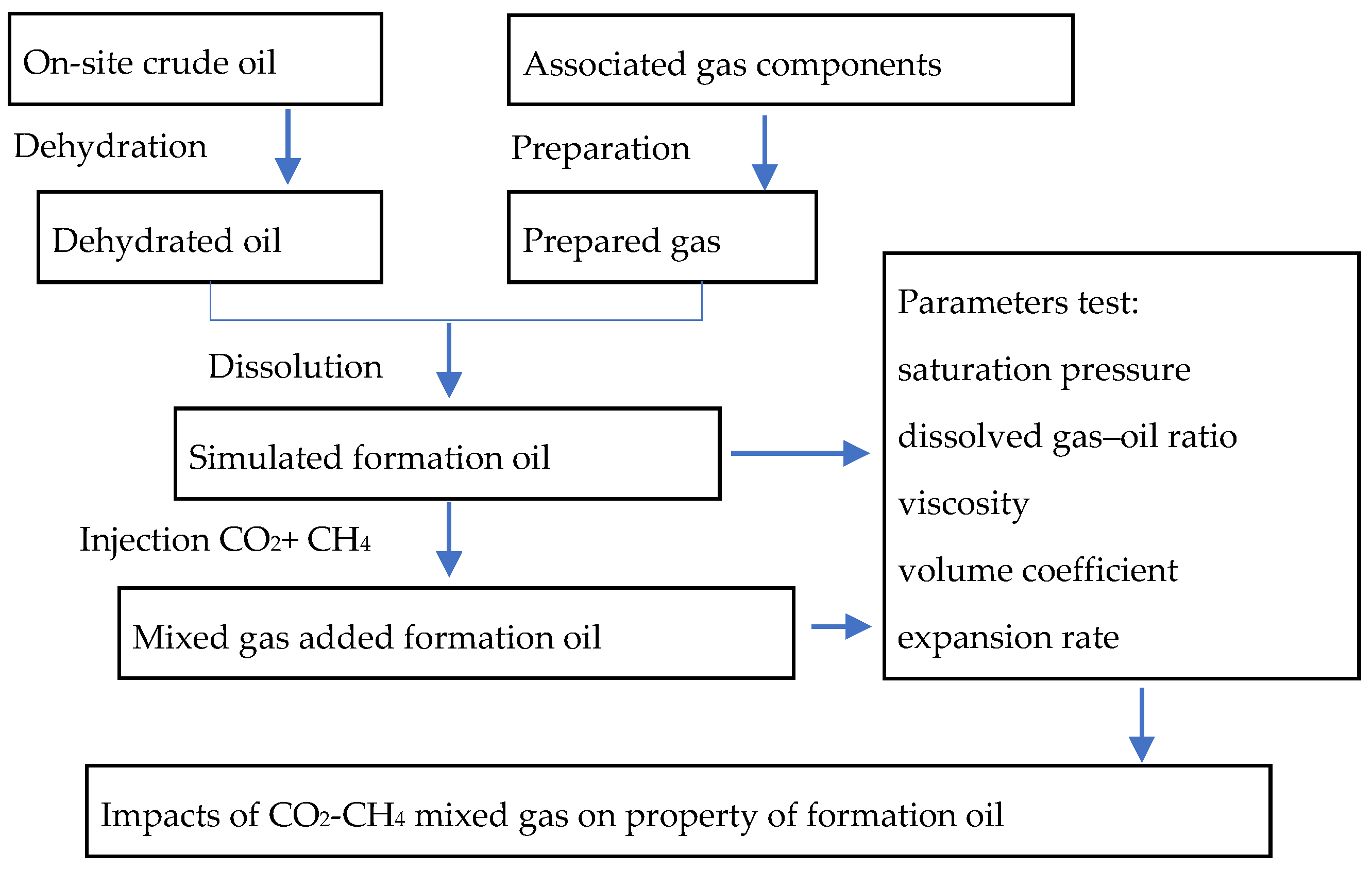
2. Experimental Description
2.1. Experimental Installation
- (1)
- PVT cell: TC-II-70 type high temperature and high-pressure formation fluid property analyzer with an operating pressure of 0–70 MPa, an operating temperature of room temperature −200 °C, and a maximum volume of 316 mL.
- (2)
- Viscometer: HXND-2 falling ball viscometer with an operating pressure of 0–50 MPa and an operating temperature of room temperature −200 °C.
2.2. Experimental Samples
2.3. Experimental Process
- (1)
- Prepare the simulated formation oil sample with dehydrated crude oil and natural gas in the sample container;
- (2)
- Transfer the oil sample from the sample container to the PVT cell to test the physical property parameters of the oil sample before adding CO2 + CH4 mixed gas;
- (3)
- Inject the designed amount of mixed gas according to the scheme, and increase the pressure at the experimental temperature to fully dissolve the mixed gas and oil sample;
- (4)
- Test the physical property parameters of crude oil after adding the mixed gas.
3. Results and Discussions
4. Conclusions
- (1)
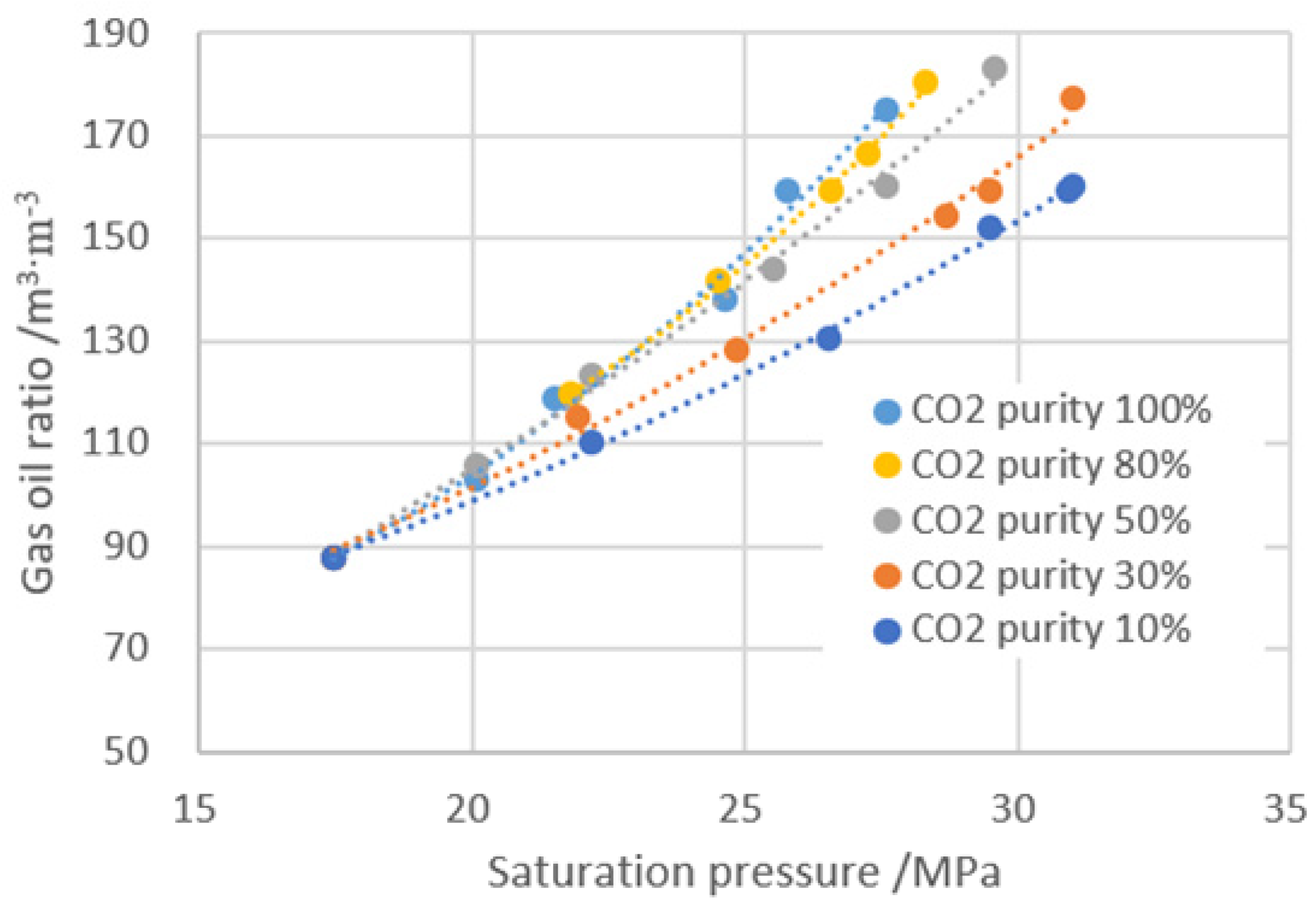
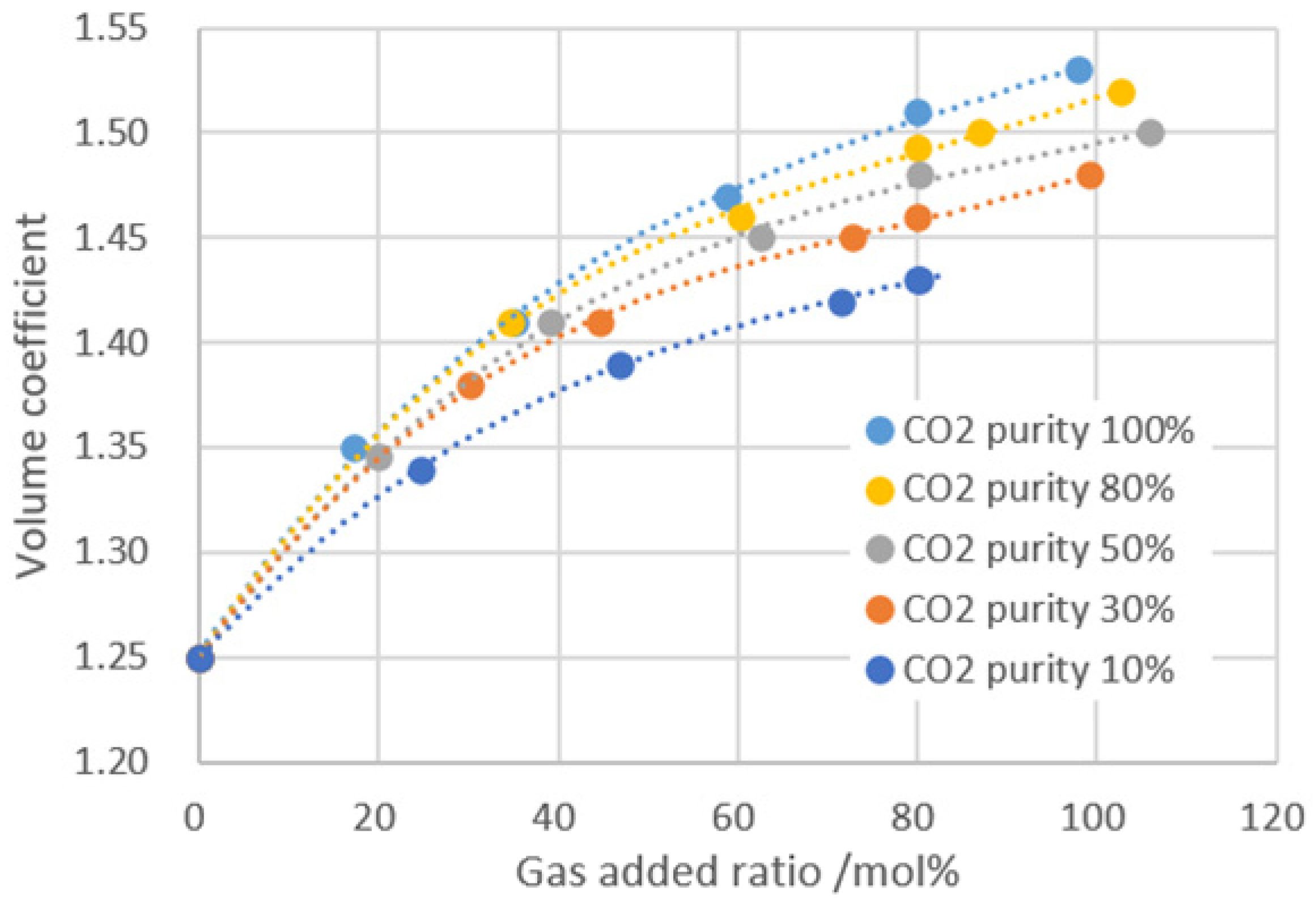
- With the increase of gas injection, the saturation pressure and dissolved gas–oil ratio, increases the volume coefficient and expansion rate, and reduces the viscosity, which is helpful in improving the exploitation of crude oil.
- (2)
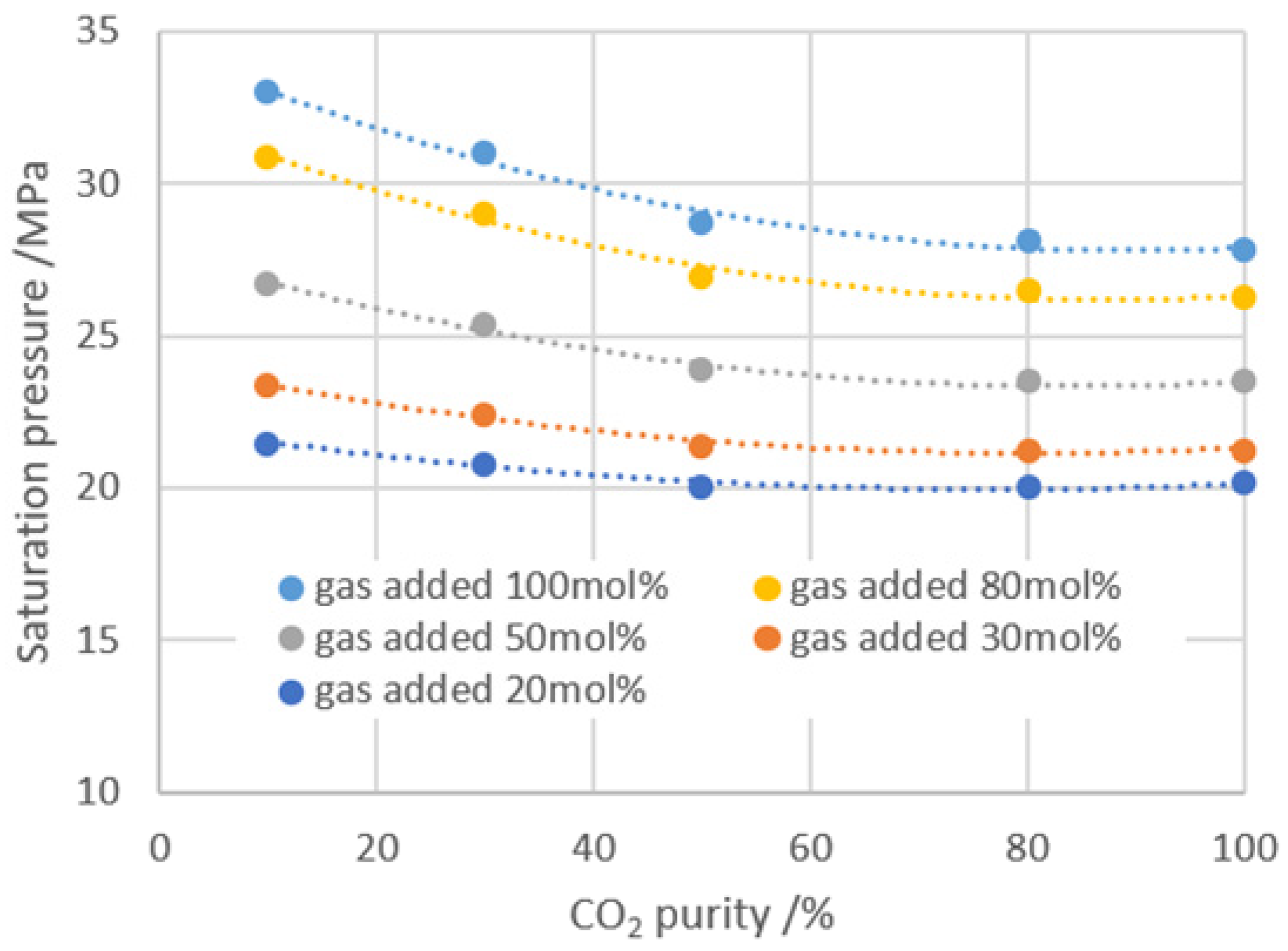
- Increasing the dissolution pressure can increase the dissolution amount of gas into formation oil; on the other hand, the pressure increase enhances the compression effect on the system. From the effect of gas dissolution to improve the physical properties of crude oil, the suitable gas injection amount is about 80 mol% for the BZ 25-1 block.
- (3)
- High-purity CO2 is beneficial to improving the physical properties of crude oil, while mixing with CH4 can elevate the formation energy, and CO2 purity of 60% can well balance the considerations on improving the oil properties and increasing the formation energy.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.; Pan, S.Y.; Li, H.; Cai, J.; Olabi, A.G.; Anthony, E.J.; Manovic, V. Recent advances in carbon dioxide utilization. Renew. Sustain. Energy Rev. 2020, 125, 109799. [Google Scholar] [CrossRef]
- Godin, J.; Liu, W.; Ren, S.; Xu, C.C. Advances in recovery and utilization of carbon dioxide: A brief review. J. Environ. Chem. Eng. 2021, 9, 105644. [Google Scholar] [CrossRef]
- Wang, M.; Yao, Y. Development situation and countermeasures of the oil and gas industry facing the challenge of carbon neutrality. Pet. Drill. Tech. 2021, 49, 1–6. [Google Scholar]
- Hou, J.; Song, K.; Wen, Y. Development Trend of Enhanced Oil Recovery Technology in Old Oilfields after Polymer Flooding. Sci. Technol. Foresight 2023, 2, 47–61. [Google Scholar]
- Hartono, K.F.; Permadi, A.K.; Siagian, U.W.R.; Hakim, A.L.L.; Paryoto, S.; Resha, A.H.; Adinugraha, Y.; Pratama, E.A. The impacts of CO2 flooding on crude oil stability and recovery performance. J. Petrol. Explor. Prod. Technol. 2024, 14, 107–123. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Dong, W.; Li, Q.; Wang, F.; Bai, H.; Zhang, R.; Owusu, A.B. Effect of different factors on the yield of epoxy-terminated polydimethylsiloxane and evaluation of CO2 thickening. RSC Adv. 2018, 8, 39787–39796. [Google Scholar] [CrossRef] [PubMed]
- Lashkarbolooki, M.; Ayatollahi, S. Experimental investigation on CO2-light crude oil interfacial and swelling behavior. Chin. J. Chem. Eng. Engl. Ed. 2018, 26, 7. [Google Scholar] [CrossRef]
- Li, L.; Jia, C.; Yao, J.; Sepehrnoori, K.; Abushaikha, A.; Liu, Y. An Investigation of Gas-Fingering Behavior during CO2 Flooding in Acid Stimulation Formations. SPE J. 2024. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wang, X.G.; Dong, R.C.; Teng, W.C.; Zhan, S.Y.; Zeng, G.Y.; Jia, C.Q. Reservoir heterogeneity controls of CO2-EOR and storage potentials in residual oil zones: Insights from numerical simulations. Pet. Sci. 2023, 20, 2879–2891. [Google Scholar] [CrossRef]
- Wang, T.; Wang, L.; Meng, X. Key parameters and dominant EOR mechanism of CO2 miscible flooding applied in low-permeability oil reservoirs. Geoenergy Sci. Eng. 2023, 225, 211724. [Google Scholar] [CrossRef]
- Nascimento, F.P.; Pereira, V.J.; Souza, R.P.; Lima, R.C.; Costa, G.M.; Rosa, P.T.; Forca, A.F.; de Melo, S.A.B.V. An experimental and theoretical investigation of asphaltene precipitation in a crude oil from the Brazilian pre-salt layer under CO2 injection. Fuel 2021, 284, 118968. [Google Scholar] [CrossRef]
- Qian, K.; Yang, S.; Dou, H.; Wang, Q.; Huang, Y.; Wan, T.; Zhang, Y. Interaction of the CO2-oil system and displacement mechanisms during CO2 flooding. Pet. Sci. Bull. 2019, 4, 69–82. [Google Scholar]
- Alghawi, Y.; Rahnema, H.; Parra, E. Laboratory measurement of oil viscosity reduction due to CO2 injection at high-pressure, high-temperature conditions. Int. J. Oil Gas Coal Technol. 2022, 31, 242–262. [Google Scholar] [CrossRef]
- Rezk, M.G.; Foroozesh, J. Phase behavior and fluid interactions of a CO2-Light oil system at high pressures and temperatures. Heliyon 2019, 5, E02057. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Ma, K.; Chai, S.; Tian, X.; Li, N.; Peng, S.; Li, N.; Zheng, W.; Zhang, L.; Hao, C. Study on Dynamic Phase Behavior of Oil and Gas System during CO2 Flooding. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 15–18 July 2019. [Google Scholar]
- Ruan, H. Study on phase characteristics of CO2 injection in condensate gas reservoirs rich in condensate oil. J. Chongqing Univ. Sci. Technol. (Nat. Sci. Ed.) 2022, 24, 26–29. [Google Scholar]
- Zuo, M.; Chen, H.; Xu, C.; Stephenraj, I.R.; Qi, X.; Yu, H.; Liu, X.Y. Study on Dynamic Variation Characteristics of Reservoir Fluid Phase Behavior during CO2 Injection in CO2 Based Enhanced Oil Recovery Process. In Proceedings of the IADC/SPE Asia Pacific Drilling Technology Conference and Exhibition, Bangkok, Thailand, 9–10 August 2022. [Google Scholar]
- Song, Z.; Deng, S.; Song, Y.; Liu, Y.; Xian, C.; Zhang, J.; Han, X.; Cao, S.; Fu, L.; Cui, H. High pressure phase and mass transfer law of Gulong shale oil -CO2 in Daqing Oilfield. Acta Pet. Sin. 2024, 45, 390–402. [Google Scholar]
- Ariza-Quiroga, C.; Aristizabal, J.D.; Martinez Vertel, J.J.; Cundar, C.; Delgadillo, C.; Trujillo-Portillo, M.L.; Sandoval, J.; Maya, G.A.; Osorio, R. Effect of Phase Behavior and Mass Transfer Mechanisms on Crude Oil Recovery and CO2 Storage in a CO2 Injection Process in Colombian Reservoirs. In Proceedings of the SPE Latin American and Caribbean Petroleum Engineering Conference, Port of Spain, Trinidad and Tobago, 14–15 June 2023. [Google Scholar]
- Li, J.; Gao, H.; Yan, C.; Wang, S.; Wang, L. Molecular dynamics simulation of interaction mechanism between crude oil and CO2. Oil Gas Reserv. Eval. Dev. 2024, 14, 26–34. [Google Scholar]





| Layer | Reservoir Pressure /MPa | Oil Layer Temperature /°C | Saturation Pressure /MPa | Gas–Oil Ratio /m3·m−3 | Volume Coefficient at Formation Pressure | Underground Crude Oil Density /g·cm−3 | Ground Crude Oil Density /g·cm−3 | Viscosity under Formation Pressure/mPa·s |
|---|---|---|---|---|---|---|---|---|
| SA3 | 51.0 | 127.0 | 17.0 | 89.0 | 1.29 | 0.744 | 0.870 | 1.13 |
| Layer | Relative Density | Components of Natural Gas/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | C4H10 | C5H12 | C6H14 | N2 | CO2 | ||
| SA3 | 0.735 | 75.5 | 11.24 | 3.85 | 1.03 | 0.2 | - | 1.51 | 6.64 |
| Component | Percentage/mol% | Component | Percentage/mol% | Component | Percentage/mol% |
|---|---|---|---|---|---|
| CO2 | 3.734186 | nC12 | 1.123333 | nC25 | 0.639754 |
| N2 | 0.566727 | nC13 | 1.171444 | nC26 | 0.485013 |
| nC1 | 53.9555 | nC14 | 1.385151 | nC27 | 0.438839 |
| nC2 | 10.53491 | nC15 | 1.518425 | nC28 | 0.395537 |
| nC3 | 3.943798 | nC16 | 1.360843 | nC29 | 0.348941 |
| nC4 | 3.229567 | nC17 | 1.214582 | nC30 | 0.280766 |
| nC5 | 1.164507 | nC18 | 1.024728 | nC31 | 0.207071 |
| nC6 | 0.621071 | nC19 | 1.010208 | nC32 | 0.199959 |
| nC7 | 1.147974 | nC20 | 0.947291 | nC33 | 0.146798 |
| nC8 | 1.157135 | nC21 | 0.827465 | nC34 | 0.122372 |
| nC9 | 0.946416 | nC22 | 0.775117 | nC35 | 0.06786 |
| nC10 | 0.98429 | nC23 | 0.72877 | nC36 | 0.026 |
| nC11 | 0.951419 | nC24 | 0.616234 | nC37+ | 4.300913 |
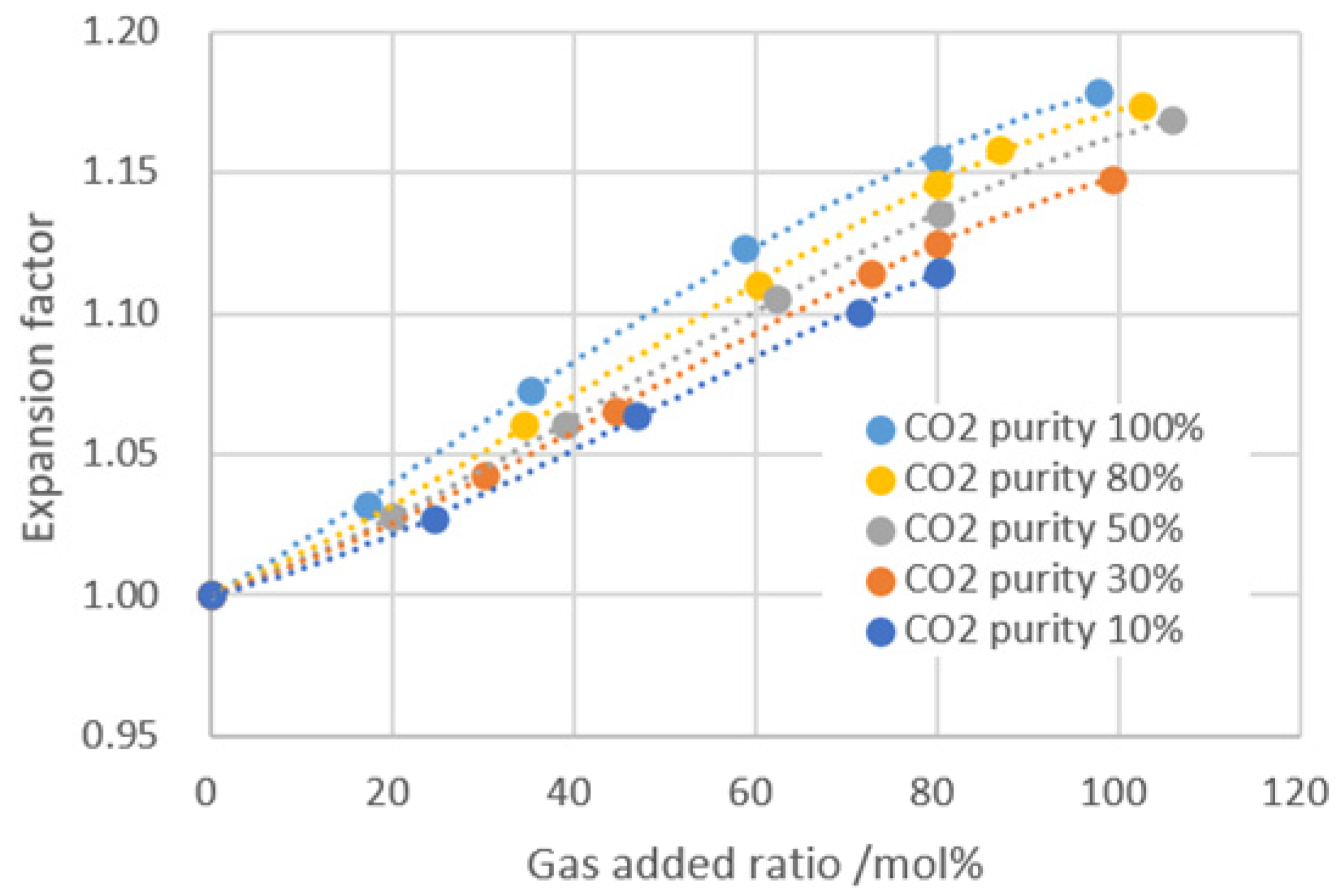
| CO2 Purity/% | Gas Added Ratio/mol% | GOR /cm3·cm−3 | Saturation Pressure /MPa | Viscosity /mPa·s | Expansion Factor | Volume Coefficient |
|---|---|---|---|---|---|---|
| / | 0 | 88.00 | 17.46 | 1.13 | 1.00 | 1.25 |
| 10 | 24.53 | 110.28 | 22.20 | 1.08 | 1.03 | 1.34 |
| 46.83 | 130.12 | 26.50 | 1.03 | 1.06 | 1.39 | |
| 71.36 | 151.96 | 29.50 | 0.99 | 1.10 | 1.42 | |
| 80.28 | 160.32 | 31.00 | 0.98 | 1.12 | 1.43 | |
| 30 | 30.11 | 115.20 | 21.90 | 1.05 | 1.04 | 1.38 |
| 44.60 | 128.46 | 24.80 | 1.01 | 1.07 | 1.41 | |
| 72.64 | 154.25 | 28.70 | 0.94 | 1.11 | 1.45 | |
| 99.24 | 177.33 | 31.00 | 0.92 | 1.15 | 1.48 | |
| 50 | 39.00 | 123.38 | 22.20 | 0.98 | 1.06 | 1.41 |
| 62.44 | 144.23 | 25.50 | 0.89 | 1.11 | 1.45 | |
| 80.25 | 160.33 | 27.60 | 0.80 | 1.14 | 1.48 | |
| 105.92 | 182.96 | 29.60 | 0.75 | 1.17 | 1.50 | |
| 80 | 34.55 | 119.32 | 21.80 | 0.98 | 1.06 | 1.41 |
| 60.21 | 142.33 | 24.50 | 0.85 | 1.11 | 1.46 | |
| 86.97 | 166.27 | 27.30 | 0.75 | 1.16 | 1.50 | |
| 102.58 | 180.48 | 28.30 | 0.71 | 1.17 | 1.52 | |
| 100 | 17.19 | 102.98 | 20.10 | 1.05 | 1.03 | 1.35 |
| 35.1 | 118.65 | 21.50 | 0.95 | 1.07 | 1.41 | |
| 78.76 | 138.25 | 24.60 | 0.79 | 1.12 | 1.47 | |
| 97.78 | 175.23 | 27.60 | 0.68 | 1.18 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Zhang, L.; Tan, X.; Tian, X.; Yang, X.; Shu, X.; Zou, G.; Yang, E.; Jiang, C.; Hu, S. Impacts of CO2-CH4 Mixed Gas on Property of Formation Oil from the Bohai Oilfield. Processes 2024, 12, 1480. https://doi.org/10.3390/pr12071480
Yang R, Zhang L, Tan X, Tian X, Yang X, Shu X, Zou G, Yang E, Jiang C, Hu S. Impacts of CO2-CH4 Mixed Gas on Property of Formation Oil from the Bohai Oilfield. Processes. 2024; 12(7):1480. https://doi.org/10.3390/pr12071480
Chicago/Turabian StyleYang, Renfeng, Lijun Zhang, Xianhong Tan, Xiaofeng Tian, Xugang Yang, Xiaohan Shu, Guodong Zou, Erlong Yang, Changdong Jiang, and Shaobin Hu. 2024. "Impacts of CO2-CH4 Mixed Gas on Property of Formation Oil from the Bohai Oilfield" Processes 12, no. 7: 1480. https://doi.org/10.3390/pr12071480





