Exploring the Potential of Silicon Tetrachloride as an Additive in CO2-Based Binary Mixtures in Transcritical Organic Rankine Cycle—A Comparative Study with Traditional Hydrocarbons
Abstract
:1. Introduction
2. Additive Selection for CO2-Based Binary Mixtures
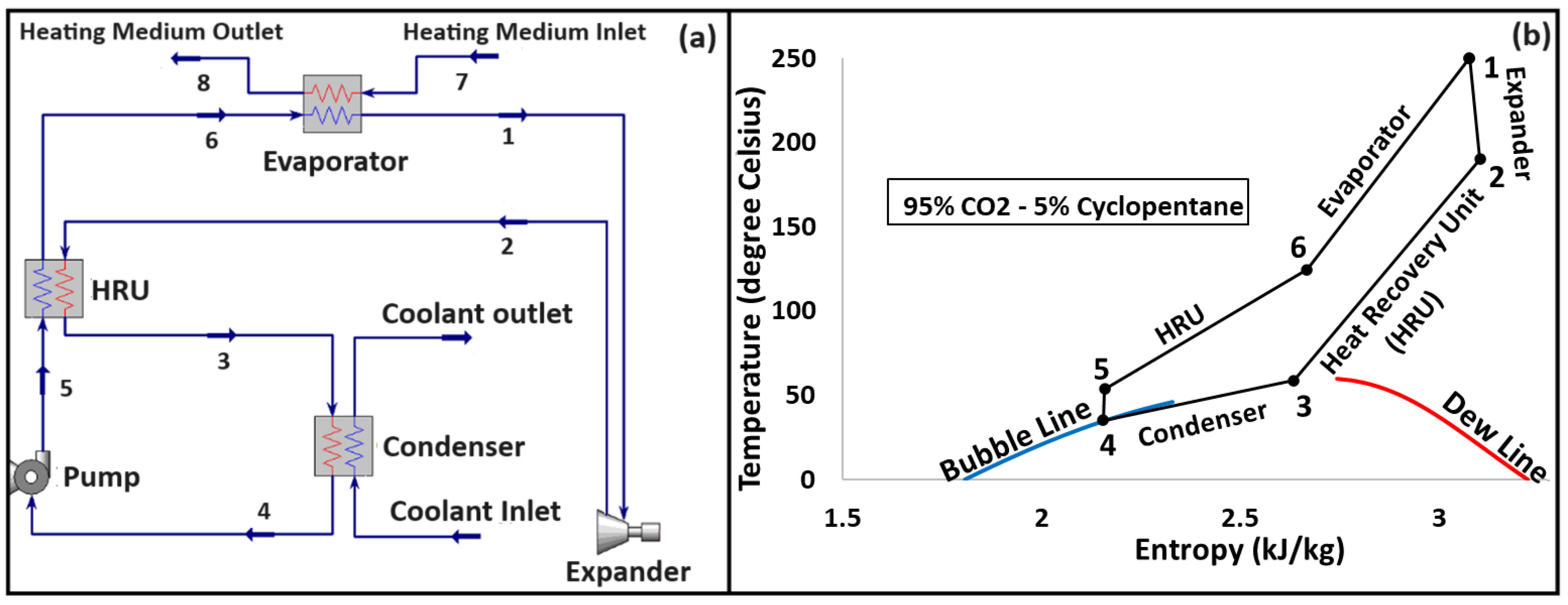
3. Organic Rankine Cycle: System Configuration and Operating Conditions
4. Quantitative Framework: A Mathematical Model for the System
4.1. Energy Model
4.2. Exergy Model
5. Energy Conversion Analysis
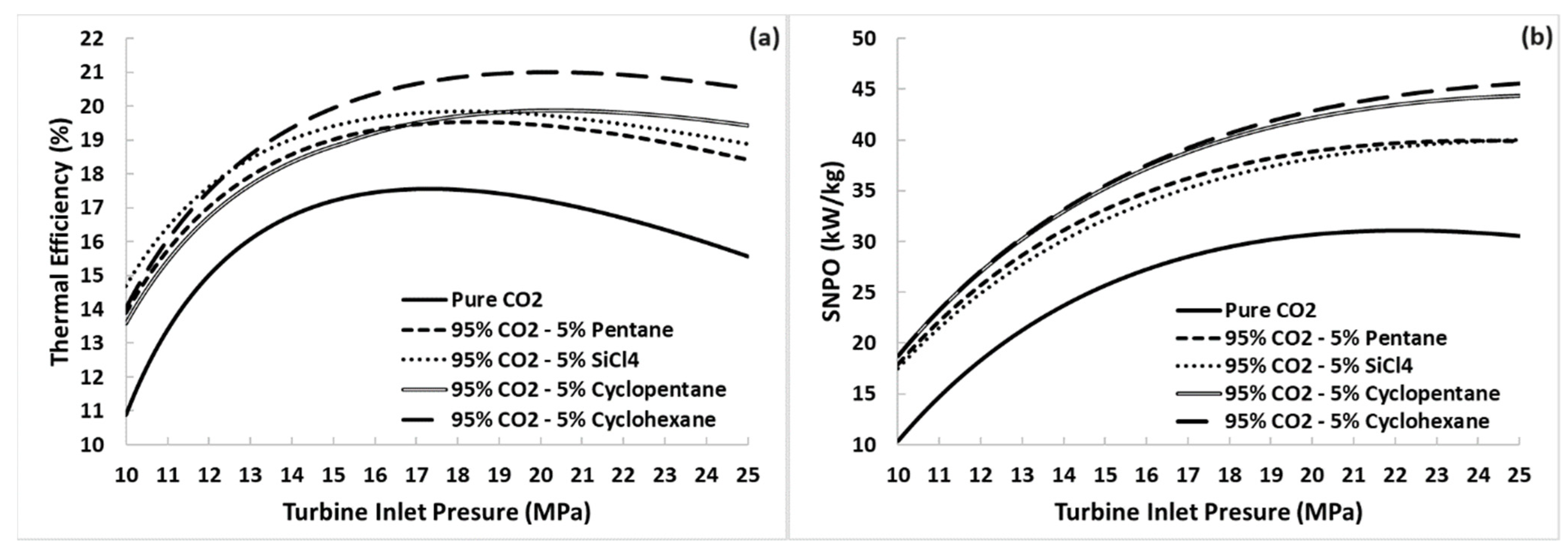
5.1. Thermal Efficiency and SNPO versus Turbine Inlet Pressure
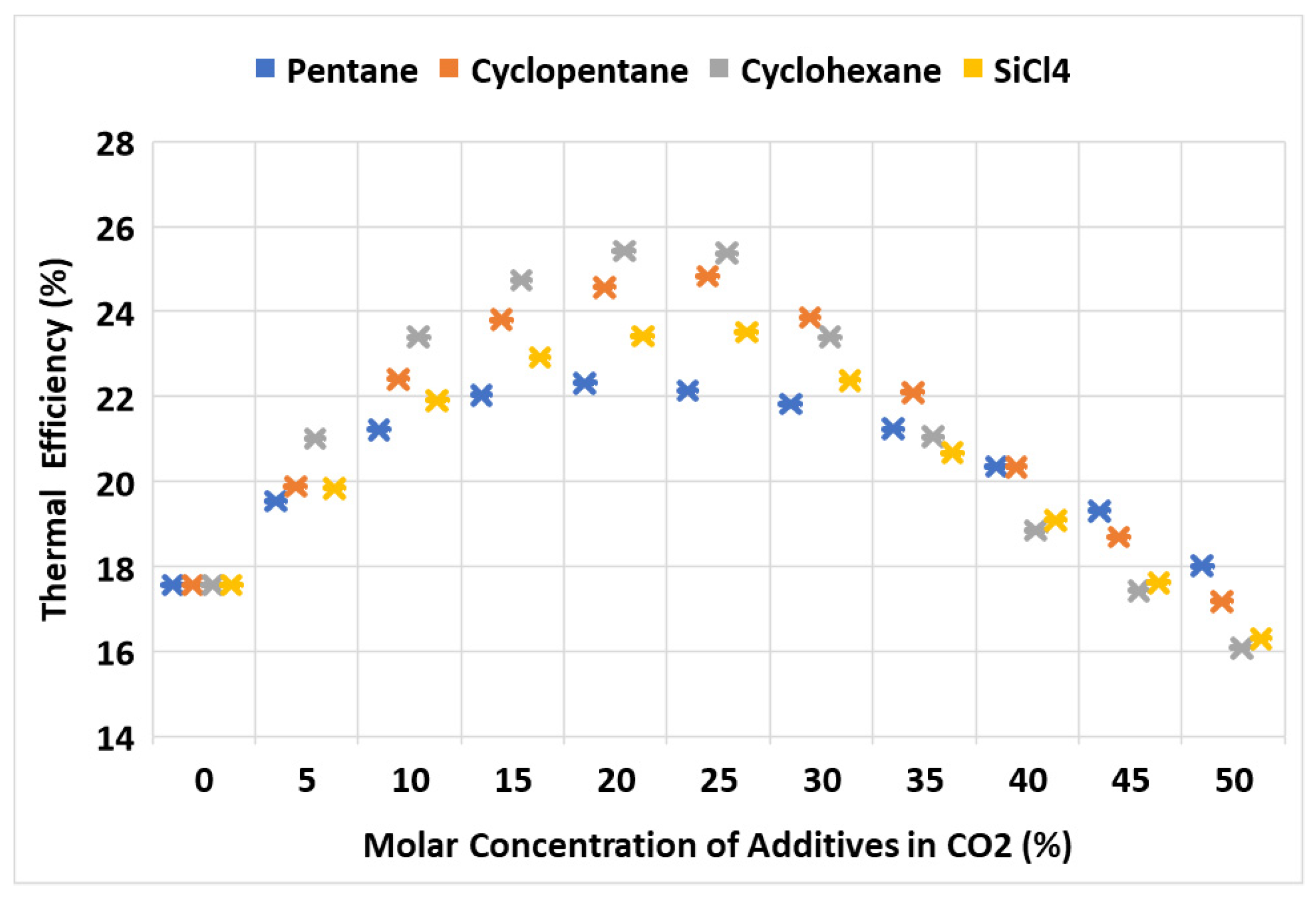
5.2. Thermal Efficiency versus Mixture Concentration
5.3. SNPO and Flow Ratio versus Mixture Concentration
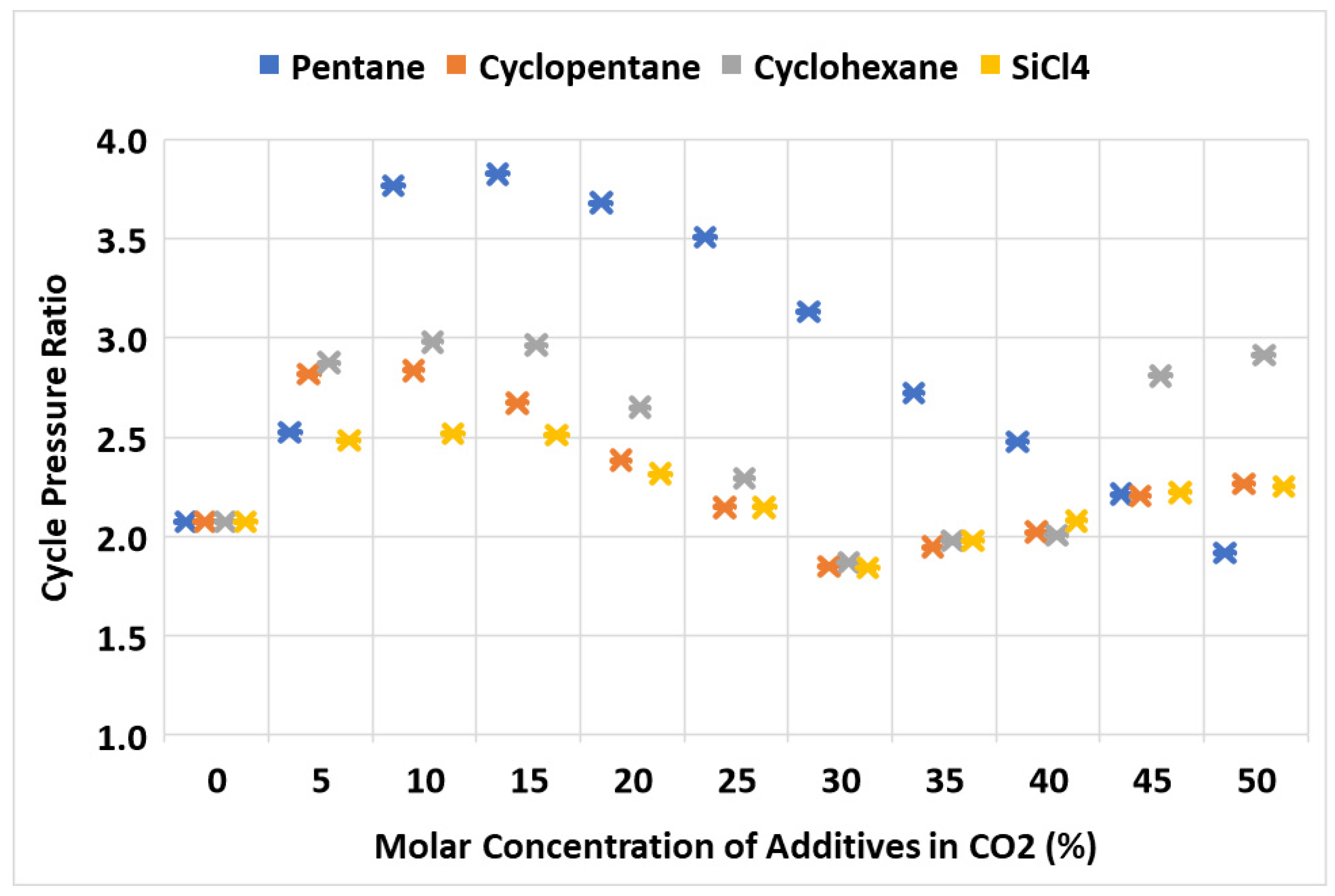
5.4. Cycle’s Optimal Pressure Ratio versus Mixture Concentration
6. Exergy Performance of the Cycle
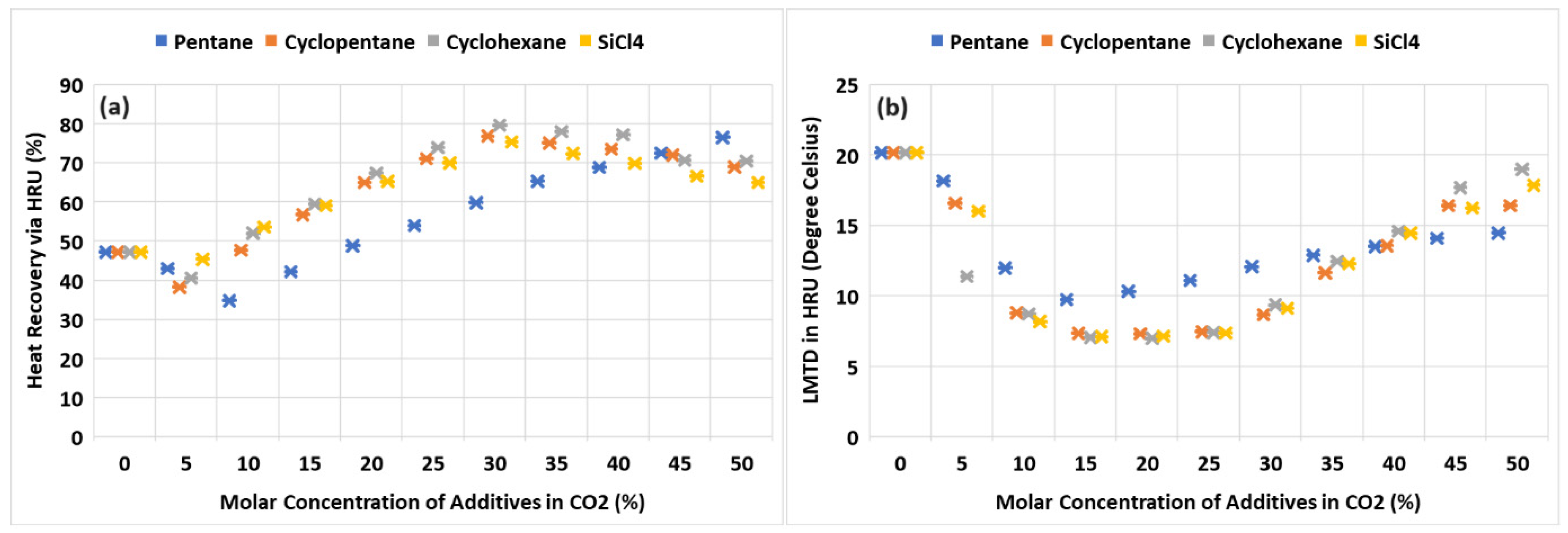
7. Heat Recovery Unit Performance in the Cycle
8. Conclusions
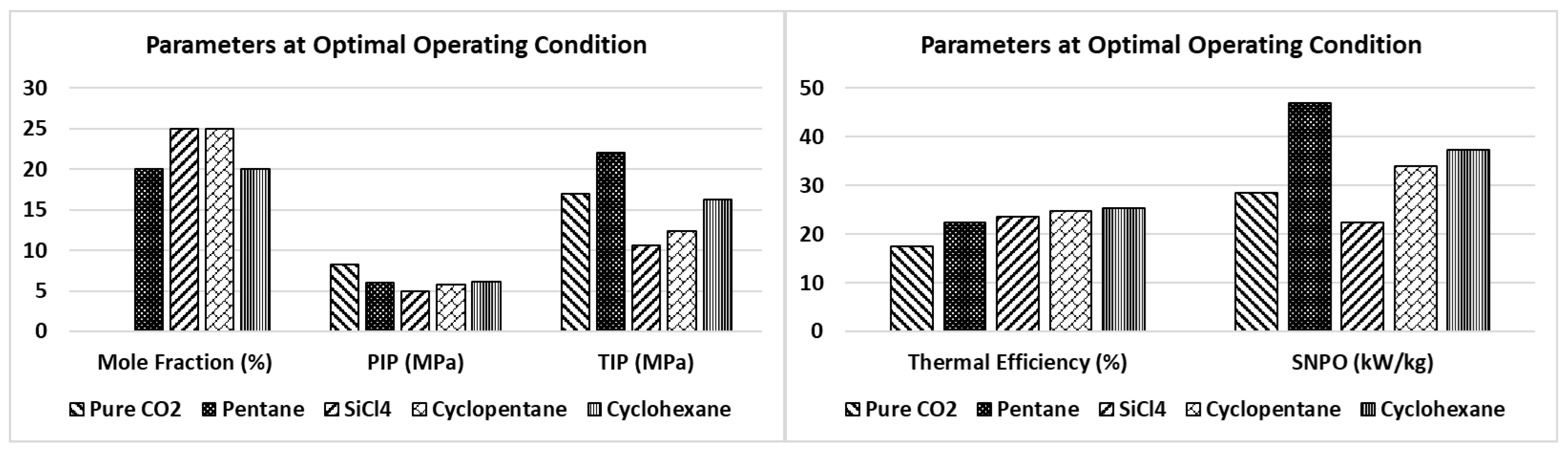
- The optimal molar concentration of the additives in CO2, which produced the maximum thermal efficiency, was between 20% to 25%.
- In comparison to pure CO2, the thermal efficiency of the cycle saw significant improvements of 4.8, 7.3, 7.8, and 6.0 percentage points with the addition of pentane, cyclopentane, cyclohexane, and SiCl4 as additives, respectively.
- Pentane, cyclopentane, cyclohexane, and SiCl4 as additives also yielded considerable enhancements in the exergetic efficiency of the cycle, with respective improvements of 12.4, 17.3, 18.3, and 13.8 percentage points.
- In terms of SNPO, cyclohexane and cyclopentane produced similar results (disregarding pentane due to its low energy and exergy performance) and significantly higher results in comparison to SiCl4 (refer to Figure 4).
- Silicon tetrachloride demonstrated comparable results in the thermal and exergy efficiency of the cycle compared to cyclopentane and cyclohexane. Furthermore, SiCl4 offers advantages over these additives as it is non-flammable and possesses superior thermal and chemical stability. The heat recovery from the cycle via the heat recovery unit (HRU) using SiCl4 was nearly equivalent to that of cyclopentane and cyclohexane. Notably, the required turbine inlet pressure and the corresponding cycle pressure ratio for optimal operation were the lowest for SiCl4 among the evaluated additives. This lower operating pressure requirement could potentially lead to reduced material and overall plant costs.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| CO2 | carbon dioxide |
| C5H12 | pentane |
| C5H10 | cyclopentane |
| C5H12 | cyclohexane |
| exergy of ith state point in the cycle | |
| exergy loss in turbine | |
| exergy loss in pump | |
| exergy loss in evaporator | |
| exergy loss in heat recovery unit | |
| exergy loss in condenser | |
| net exergy loss in condenser | |
| exergy input to the cycle | |
| h | specific enthalpy |
| GWP | global warming potential |
| HRU | heat recovery unit |
| heat recovery unit effectiveness | |
| LMTD | log mean temperature difference |
| mass flow rate of cycle working fluid | |
| mass flow rate of heat source (air) | |
| ORC | organic Rankine cycle |
| heat input to the cycle | |
| heat recovered from heat recovery unit | |
| s | specific entropy |
| SiCl4 | silicon tetrachloride |
| SNPO | specific net power output |
| turbine power output | |
| pump power input | |
| exergy efficiency of the cycle | |
| thermal efficiency of the cycle | |
| cycle to heat source mass flow ratio |
References
- Bell, I.H.; Lemmon, E.W. Organic Fluids for Organic Rankine Cycle Systems: Classification and Calculation of Thermodynamic and Transport Properties. In Organic Rankine Cycle (ORC) Power Systems: Technologies and Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 91–119. [Google Scholar] [CrossRef]
- Astolfi, M.; Martelli, E.; Pierobon, L. Thermodynamic and Technoeconomic Optimization of Organic Rankine Cycle Systems. In Organic Rankine Cycle (ORC) Power Systems: Technologies and Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 173–249. [Google Scholar] [CrossRef]
- Nemati, A.; Nami, H.; Ranjbar, F.; Yari, M. A Comparative Thermodynamic Analysis of ORC and Kalina Cycles for Waste Heat Recovery: A Case Study for CGAM Cogeneration System. Case Stud. Therm. Eng. 2017, 9, 1–13. [Google Scholar] [CrossRef]
- Bao, J.; Zhao, L. A Review of Working Fluid and Expander Selections for Organic Rankine Cycle. Renew. Sustain. Energy Rev. 2013, 24, 325–342. [Google Scholar] [CrossRef]
- Wieland, C.; Dawo, F.; Schifflechner, C.; Astolfi, M. Market Report on Organic Rankine Cycle Power Systems: Recent Developments and Outlook. In Proceedings of the 6th International Seminar on ORC Power Systems, 11–13 October 2021; Technical University of Munich: Munich, Germany.
- Siddiqui, M.E.; Almatrafi, E.; Saeed, U. Performance Analysis of Organic Rankine Cycle with Internal Heat Regeneration: Comparative Study of Binary Mixtures and Pure Constituents in Warm Regions. Processes 2023, 11, 2267. [Google Scholar] [CrossRef]
- Safarian, S.; Aramoun, F. Energy and Exergy Assessments of Modified Organic Rankine Cycles (ORCs). Energy Rep. 2015, 1, 1–7. [Google Scholar] [CrossRef]
- Zhou, N.; Wang, X.; Chen, Z.; Wang, Z. Experimental Study on Organic Rankine Cycle for Waste Heat Recovery from Low-Temperature Flue Gas. Energy 2013, 55, 216–225. [Google Scholar] [CrossRef]
- Meinel, D.; Wieland, C.; Spliethoff, H. Economic Comparison of ORC (Organic Rankine Cycle) Processes at Different Scales. Energy 2014, 74, 694–706. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, L.; Mao, S.S.; Deng, S. Towards Novel Low Temperature Thermodynamic Cycle: A Critical Review Originated from Organic Rankine Cycle. Appl. Energy 2020, 270, 115186. [Google Scholar] [CrossRef]
- Stijepovic, M.Z.; Papadopoulos, A.I.; Linke, P.; Stijepovic, V.; Grujic, A.S.; Kijevčanin, M.; Seferlis, P. Organic Rankine Cycle System Performance Targeting and Design for Multiple Heat Sources with Simultaneous Working Fluid Selection. J. Clean. Prod. 2017, 142, 1950–1970. [Google Scholar] [CrossRef]
- Kishore, R.A.; Priya, S. A Review on Low-Grade Thermal Energy Harvesting: Materials, Methods and Devices. Materials 2018, 11, 1433. [Google Scholar] [CrossRef]
- Stijepovic, M.Z.; Linke, P.; Papadopoulos, A.I.; Grujic, A.S. On the Role of Working Fluid Properties in Organic Rankine Cycle Performance. Appl. Therm. Eng. 2012, 36, 406–413. [Google Scholar] [CrossRef]
- Park, B.S.; Usman, M.; Imran, M.; Pesyridis, A. Review of Organic Rankine Cycle Experimental Data Trends. Energy Convers. Manag. 2018, 173, 679–691. [Google Scholar] [CrossRef]
- Wang, E.H.; Zhang, H.G.; Fan, B.Y.; Ouyang, M.G.; Zhao, Y.; Mu, Q.H. Study of Working Fluid Selection of Organic Rankine Cycle (ORC) for Engine Waste Heat Recovery. Energy 2011, 36, 3406–3418. [Google Scholar] [CrossRef]
- Invernizzi, C.M.; Bonalumi, D. Thermal Stability of Organic Fluids for Organic Rankine Cycle Systems. In Organic Rankine Cycle (ORC) Power Systems: Technologies and Applications; Woodhead Publishing: Sawston, UK, 2017; pp. 121–151. [Google Scholar] [CrossRef]
- Siddiqui, M.E.; Almatrafi, E.; Bamasag, A.; Saeed, U. Adoption of CO2-Based Binary Mixture to Operate Transcritical Rankine Cycle in Warm Regions. Renew. Energy 2022, 199, 1372–1380. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, R.; Deng, S.; Zhao, L.; Mao, S.S. Is Zeotropic Working Fluid a Promising Option for Organic Rankine Cycle: A Quantitative Evaluation Based on Literature Data. Renew. Sustain. Energy Rev. 2021, 148, 111267. [Google Scholar] [CrossRef]
- Bamorovat Abadi, G.; Kim, K.C. Investigation of Organic Rankine Cycles with Zeotropic Mixtures as a Working Fluid: Advantages and Issues. Renew. Sustain. Energy Rev. 2017, 73, 1000–1013. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, Y.; Luo, X.; Chen, J.; Yang, Z.; Wang, C.; Chen, Y. Synthesis and Simultaneous Optimization of Multi-Heat Source Multi-Pressure Evaporation Organic Rankine Cycle with Mixed Working Fluid. Energy Convers. Manag. 2022, 251, 114930. [Google Scholar] [CrossRef]
- Ganjehsarabi, H. Mixed Refrigerant as Working Fluid in Organic Rankine Cycle for Hydrogen Production Driven by Geothermal Energy. Int. J. Hydrog. Energy 2019, 44, 18703–18711. [Google Scholar] [CrossRef]
- Dai, B.; Li, M.; Ma, Y. Thermodynamic Analysis of Carbon Dioxide Blends with Low GWP (Global Warming Potential) Working Fluids-Based Transcritical Rankine Cycles for Low-Grade Heat Energy Recovery. Energy 2014, 64, 942–952. [Google Scholar] [CrossRef]
- Wu, Y.; Zhu, Y.; Yu, L. Thermal and Economic Performance Analysis of Zeotropic Mixtures for Organic Rankine Cycles. Appl. Therm. Eng. 2016, 96, 57–63. [Google Scholar] [CrossRef]
- Oyewunmi, O.A.; Markides, C.N. Thermo-Economic and Heat Transfer Optimization of Working-Fluid Mixtures in a Low-Temperature Organic Rankine Cycle System. Energies 2016, 9, 448. [Google Scholar] [CrossRef]
- Desai, N.B.; Bandyopadhyay, S. Thermo-Economic Analysis and Selection of Working Fluid for Solar Organic Rankine Cycle. Appl. Therm. Eng. 2016, 95, 471–481. [Google Scholar] [CrossRef]
- Wang, X.; Levy, E.K.; Pan, C.; Romero, C.E.; Banerjee, A.; Rubio-Maya, C.; Pan, L. Working Fluid Selection for Organic Rankine Cycle Power Generation Using Hot Produced Supercritical CO2 from a Geothermal Reservoir. Appl. Therm. Eng. 2019, 149, 1287–1304. [Google Scholar] [CrossRef]
- Hærvig, J.; Sørensen, K.; Condra, T.J. Guidelines for Optimal Selection of Working Fluid for an Organic Rankine Cycle in Relation to Waste Heat Recovery. Energy 2016, 96, 592–602. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Li, Q.; Liu, L.; Huo, E.; Zhang, C. Selection Principle of Working Fluid for Organic Rankine Cycle Based on Environmental Benefits and Economic Performance. Appl. Therm. Eng. 2020, 178, 115598. [Google Scholar] [CrossRef]
- Vitale Di Maio, D.; Boccitto, A.; Caruso, G. Supercritical Carbon Dioxide Applications for Energy Conversion Systems. Energy Procedia 2015, 82, 819–824. [Google Scholar] [CrossRef]
- Crespi, F.; Gavagnin, G.; Sánchez, D.; Martínez, G.S. Supercritical Carbon Dioxide Cycles for Power Generation: A Review. Appl. Energy 2017, 195, 152–183. [Google Scholar] [CrossRef]
- Kumar, P.; Srinivasan, K. Carbon Dioxide Based Power Generation in Renewable Energy Systems. Appl. Therm. Eng. 2016, 109, 831–840. [Google Scholar] [CrossRef]
- Kulhánek, M.; Dostál, V. Thermodynamic Analysis and Comparison of Supercritical Carbon Dioxide Cycles. In Proceedings of the Supercritical CO2 Power Cycle Symposium, Boulder, CO, USA, 24–25 May 2011. [Google Scholar]
- Al-Sulaiman, F.A.; Atif, M. Performance Comparison of Different Supercritical Carbon Dioxide Brayton Cycles Integrated with a Solar Power Tower. Energy 2015, 82, 61–71. [Google Scholar] [CrossRef]
- Shamsi, S.S.M.; Barberis, S.; Maccarini, S.; Traverso, A. Large Scale Energy Storage Systems Based on Carbon Dioxide Thermal Cycles: A Critical Review. Renew. Sustain. Energy Rev. 2024, 192, 114245. [Google Scholar] [CrossRef]
- Wu, C.; Wang, S.S.; Jiang, X.; Li, J. Thermodynamic Analysis and Performance Optimization of Transcritical Power Cycles Using CO2-Based Binary Zeotropic Mixtures as Working Fluids for Geothermal Power Plants. Appl. Therm. Eng. 2017, 115, 292–304. [Google Scholar] [CrossRef]
- Shu, G.; Yu, Z.; Tian, H.; Liu, P.; Xu, Z. Potential of the Transcritical Rankine Cycle Using CO2-Based Binary Zeotropic Mixtures for Engine’s Waste Heat Recovery. Energy Convers. Manag. 2018, 174, 668–685. [Google Scholar] [CrossRef]
- Sánchez, C.J.N.; da Silva, A.K. Technical and Environmental Analysis of Transcritical Rankine Cycles Operating with Numerous CO2 Mixtures. Energy 2018, 142, 180–190. [Google Scholar] [CrossRef]
- Niu, X.; Ma, N.; Bu, Z.; Hong, W.; Li, H. Thermodynamic Analysis of Supercritical Brayton Cycles Using CO2-Based Binary Mixtures for Solar Power Tower System Application. Energy 2022, 254, 124286. [Google Scholar] [CrossRef]
- Ma, N.; Bu, Z.; Fu, Y.; Hong, W.; Li, H.; Niu, X. An Operation Strategy and Off-Design Performance for Supercritical Brayton Cycle Using CO2-Propane Mixture in a Direct-Heated Solar Power Tower Plant. Energy 2023, 278, 127882. [Google Scholar] [CrossRef]
- Doninelli, M.; Di Marcoberardino, G.; Iora, P.; Gelfi, M.; Invernizzi, C.M.; Manzolini, G. Silicon Tetrachloride as Innovative Working Fluid for High Temperature Rankine Cycles: Thermal Stability, Material Compatibility, and Energy Analysis. Appl. Therm. Eng. 2024, 249, 123239. [Google Scholar] [CrossRef]
- Doninelli, M.; Morosini, E.; Di Marcoberardino, G.; Invernizzi, C.M.; Iora, P.; Riva, M.; Stringari, P.; Manzolini, G. Experimental Investigation of the CO2+SiCl4 Mixture as Innovative Working Fluid for Power Cycles: Bubble Points and Liquid Density Measurements. Energy 2024, 299, 131197. [Google Scholar] [CrossRef]
- Siddiqui, M.E.; Almatrafi, E.; Saeed, U.; Taimoor, A.A. Selection of Organic Fluid Based on Exergetic Performance of Subcritical Organic Rankine Cycle (ORC) for Warm Regions. Energies 2023, 16, 5149. [Google Scholar] [CrossRef]
- Bahrami, M.; Pourfayaz, F.; Kasaeian, A. Low Global Warming Potential (GWP) Working Fluids (WFs) for Organic Rankine Cycle (ORC) Applications. Energy Rep. 2022, 8, 2976–2988. [Google Scholar] [CrossRef]
- Toffolo, A.; Lazzaretto, A.; Manente, G.; Paci, M. A Multi-Criteria Approach for the Optimal Selection of Working Fluid and Design Parameters in Organic Rankine Cycle Systems. Appl. Energy 2014, 121, 219–232. [Google Scholar] [CrossRef]
- Aboelwafa, O.; Fateen, S.E.K.; Soliman, A.; Ismail, I.M. A Review on Solar Rankine Cycles: Working Fluids, Applications, and Cycle Modifications. Renew. Sustain. Energy Rev. 2018, 82, 868–885. [Google Scholar] [CrossRef]
- Chen, H.; Goswami, D.Y.; Stefanakos, E.K. A Review of Thermodynamic Cycles and Working Fluids for the Conversion of Low-Grade Heat. Renew. Sustain. Energy Rev. 2010, 14, 3059–3067. [Google Scholar] [CrossRef]
- Dai, X.; Shi, L.; An, Q.; Qian, W. Thermal Stability of Some Hydrofluorocarbons as Supercritical ORCs Working Fluids. Appl. Therm. Eng. 2018, 128, 1095–1101. [Google Scholar] [CrossRef]
- Dai, X.; Shi, L.; An, Q.; Qian, W. Chemical Kinetics Method for Evaluating the Thermal Stability of Organic Rankine Cycle Working Fluids. Appl. Therm. Eng. 2016, 100, 708–713. [Google Scholar] [CrossRef]
- Invernizzi, C.M.; Iora, P.; Manzolini, G.; Lasala, S. Thermal Stability of N-Pentane, Cyclo-Pentane and Toluene as Working Fluids in Organic Rankine Engines. Appl. Therm. Eng. 2017, 121, 172–179. [Google Scholar] [CrossRef]
- Krempus, D.; Bahamonde, S.; van der Stelt, T.P.; Klink, W.; Colonna, P.; De Servi, C.M. On Mixtures as Working Fluids of Air-Cooled ORC Bottoming Power Plants of Gas Turbines. Appl. Therm. Eng. 2024, 236, 121730. [Google Scholar] [CrossRef]
- Shahrooz, M.; Lundqvist, P.; Nekså, P. Performance of Binary Zeotropic Mixtures in Organic Rankine Cycles (ORCs). Energy Convers. Manag. 2022, 266, 115783. [Google Scholar] [CrossRef]
- Castelli, A.F.; Elsido, C.; Scaccabarozzi, R.; Nord, L.O.; Martelli, E. Optimization of Organic Rankine Cycles for Waste Heat Recovery from Aluminum Production Plants. Front. Energy Res. 2019, 7, 44. [Google Scholar] [CrossRef]


| Name | Chemical Formula | Critical Pressure (MPa) | Critical Temperature (°C) | Standard Boiling Point (°C) | GWP (ASHRAE Safety Group) | Thermal Stability Threshold (°C) | |
|---|---|---|---|---|---|---|---|
| 1 | Carbon dioxide | CO2 | 7.4 | 31 | −78.5 | 1 (A1) | |
| 2 | Pentane | C5H12 | 3.4 | 197 | 36.1 | 5 (A3) | 280–320 |
| 3 | Cyclopentane | C5H10 | 4.5 | 239 | 49.2 | 6 (A3) | 350 |
| 4 | Cyclohexane | C6H12 | 4.1 | 281 | 80.8 | - (A3) | - |
| 5 | Silicon Tetrachloride | SiCl4 | 3.6 | 235 | 57.7 | 0 (-) | >650 |
| Cycle Components/Parameters | Operating Conditions |
|---|---|
| Source | Air at 300 °C and 101 kPa with mass flow rate of 1 kg/s. |
| Sink | Water at 30 °C and 101 kPa. |
| Turbine | The inlet temperature is 250 °C and pressure is optimized for maximum thermal efficiency. Adiabatic efficiency is 80%. |
| Pump | Saturated liquid with inlet temperature fixed at 35 °C. Adiabatic efficiency is 90%. |
| Dead State (Ambient Condition) | 101 kPa; 35 °C. |
| Evaporator | The minimum pinch temperature is 10 °C. |
| Condenser and HRU | The minimum pinch temperature is 5 °C. |
| Parameters | 80% CO2/20% C5H12 | 75% CO2/25% SiCl4 | 75% CO2/25% C5H10 | 80% CO2/20% C6H12 |
|---|---|---|---|---|
| Cricondentherm (°C) | 105 | 148 | 142 | 168 |
| Cricondenbar (MPa) | 9.7 | 11.4 | 12.6 | 14.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alazwari, M.A.; Siddiqui, M.E. Exploring the Potential of Silicon Tetrachloride as an Additive in CO2-Based Binary Mixtures in Transcritical Organic Rankine Cycle—A Comparative Study with Traditional Hydrocarbons. Processes 2024, 12, 1507. https://doi.org/10.3390/pr12071507
Alazwari MA, Siddiqui ME. Exploring the Potential of Silicon Tetrachloride as an Additive in CO2-Based Binary Mixtures in Transcritical Organic Rankine Cycle—A Comparative Study with Traditional Hydrocarbons. Processes. 2024; 12(7):1507. https://doi.org/10.3390/pr12071507
Chicago/Turabian StyleAlazwari, Mashhour A., and Muhammad Ehtisham Siddiqui. 2024. "Exploring the Potential of Silicon Tetrachloride as an Additive in CO2-Based Binary Mixtures in Transcritical Organic Rankine Cycle—A Comparative Study with Traditional Hydrocarbons" Processes 12, no. 7: 1507. https://doi.org/10.3390/pr12071507





