Review of Hydrogen-Driven Power-to-X Technology and Application Status in China
Abstract
:1. Introduction
2. Hydrogen Energy Policies in China
3. Power-to-Hydrogen
3.1. Typical Water Electrolysis Techniques
3.2. Chinese Electrolyzer Manufacturers
3.3. Trends and Challenges
4. Power-to-Methanol
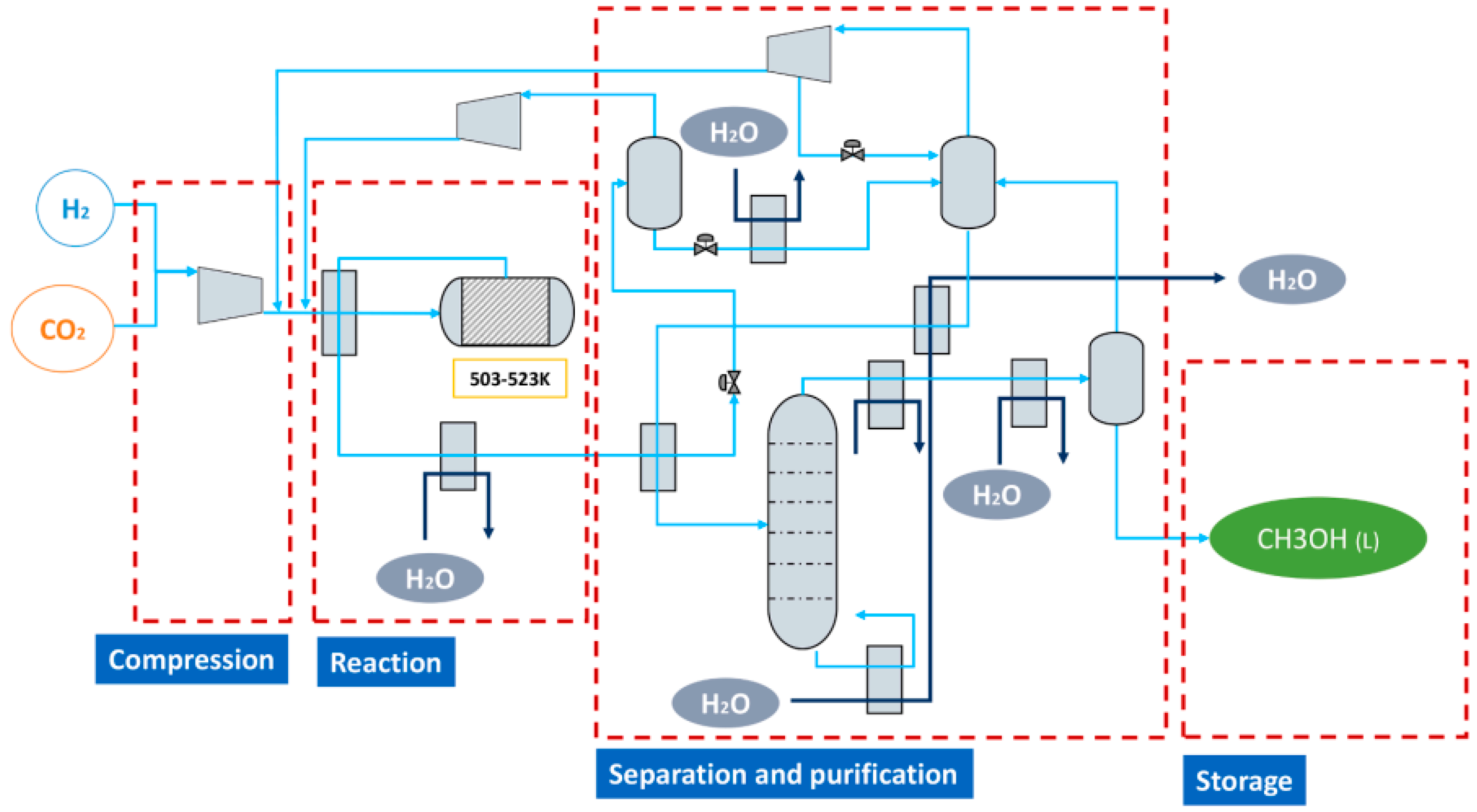
4.1. Methanol Synthetic Reaction
4.2. Methanol Synthesis Process System
4.3. Trends and Challenges
5. Power-to-Ammonia
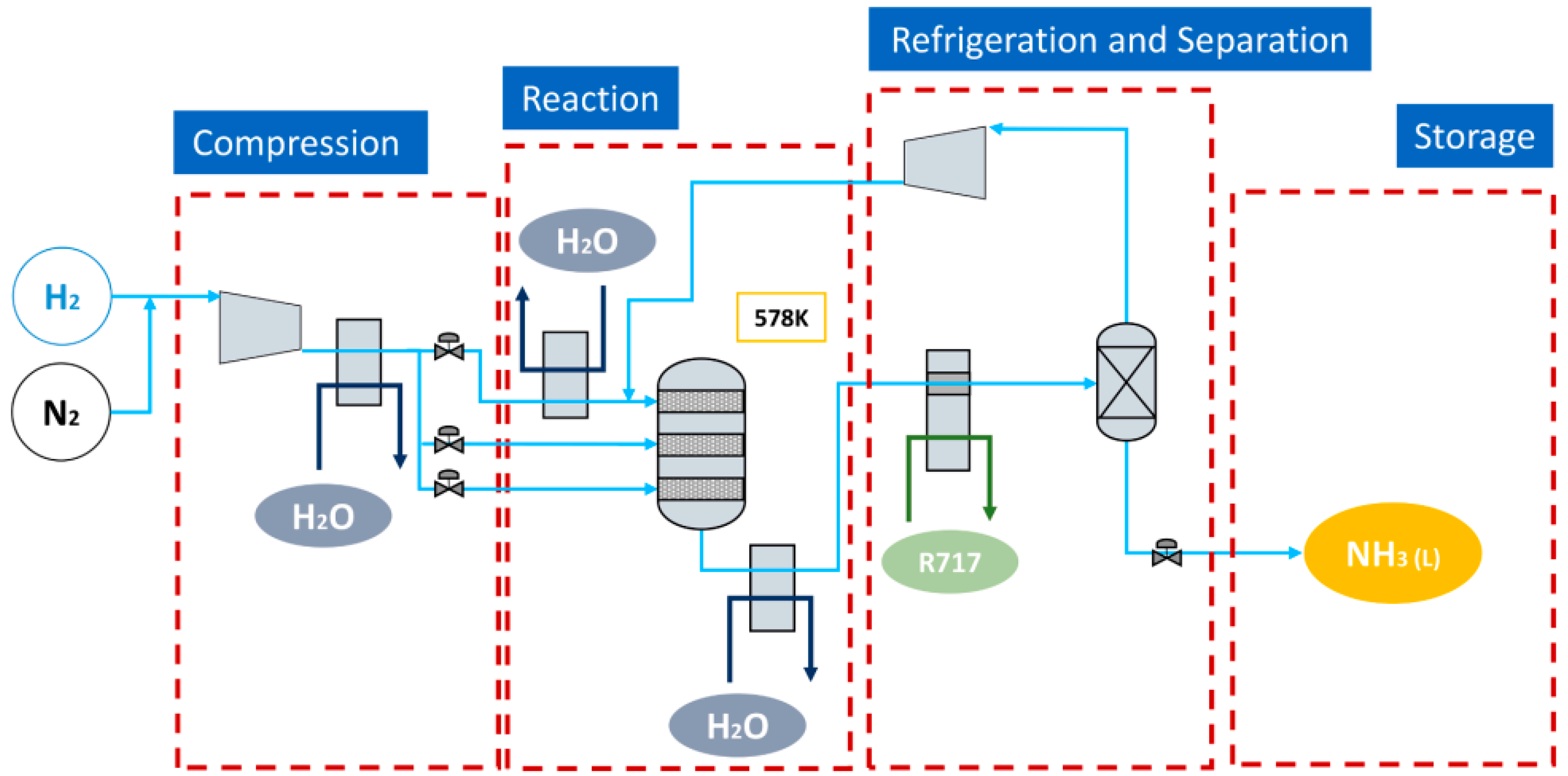
5.1. Ammonia Synthetic Reaction
5.2. Ammonia Synthesis Process System
5.3. Trends and Challenges
6. Typical Commercial or Demonstration Projects
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- BP Energy Outlook. 2023. Available online: https://www.bp.com/en/global/corporate/energy-economics/energy-outlook.html (accessed on 5 July 2023).
- Hota, P.; Das, A.; Maiti, D.K. A short review on generation of green fuel hydrogen through water splitting. Int. J. Hydrogen Energy 2023, 48, 523–541. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen production through renewable and non-renewable energy processes and their impact on climate change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Sharma, S.; Agarwal, S.; Jain, A. Significance of hydrogen as economic and environmentally friendly fuel. Energies 2021, 14, 7389. [Google Scholar] [CrossRef]
- Li, Z.; Guo, P.; Han, R.; Sun, H. Current status and development trend of wind power generation-based hydrogen production technology. Energy Explor. Exploit. 2018, 37, 5–25. [Google Scholar] [CrossRef]
- Available online: https://www.gov.cn/lianbo/bumen/202307/content_6895756.htm (accessed on 22 December 2023).
- 14th Five-Year Plan for Renewable Energy Development. Available online: https://chinaenergyportal.org/14th-five-year-plan-for-renewable-energy-development/ (accessed on 1 June 2022).
- Gan, W.; Yan, M.; Yao, W.; Wen, J. Peer to peer transactive energy for multiple energy hub with the penetration of high-level renewable energy. Appl. Energy 2021, 295, 117027. [Google Scholar] [CrossRef]
- Meng, X.; Chen, M.; Gu, A.; Wu, X.; Liu, B.; Zhou, J.; Mao, Z. China’s hydrogen development strategy in the context of double carbon targets. Nat. Gas Ind. B 2022, 9, 521–547. [Google Scholar] [CrossRef]
- Zheng, K.; Gao, X.; Fan, Y.; Luo, Z.; Li, Z.; Zheng, Y.; Liu, Y. Comparison and Application Prospects of Ammonia and Methanol Technologies Supporting Large-Scale Development of Green Hydrogen Energy. South. Energy Constr. 2023, 10, 63–73. [Google Scholar] [CrossRef]
- Zhong, Z.; Fang, J.; Hu, K.; Huang, D.; Ai, X.; Yang, X.; Wen, J.; Pan, Y.; Cheng, S. Power-to-Hydrogen by Electrolysis in Carbon Neutrality: Technology Overview and Future Development. CSEE J. Power Energy Syst. 2023, 9, 1266–1283. [Google Scholar] [CrossRef]
- Hu, G.; Chen, C.; Lu, H.T.; Wu, Y.; Liu, C.; Tao, L.; Men, Y.; He, G.; Li, K.G. A Review of Technical Advances, Barriers, and Solutions in the Power to Hydrogen (P2H) Roadmap. Engineering 2020, 6, 1364–1380. [Google Scholar] [CrossRef]
- Liu, W.; Sun, L.; Li, Z.; Fujii, M.; Geng, Y.; Dong, L.; Fujita, T. Trends and future challenges in hydrogen production and storage research. Environ. Sci. Pollut. Res. Int. 2020, 27, 31092–31104. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Ayodele, T.R.; Munda, J.L. Potential and economic viability of green hydrogen production by water electrolysis using wind energy resources in South Africa. Int. J. Hydrogen Energy 2019, 44, 17669–17687. [Google Scholar] [CrossRef]
- Huang, Y.-S.; Liu, S.-J. Chinese Green Hydrogen Production Potential Development: A Provincial Case Study. IEEE Access 2020, 8, 171968–171976. [Google Scholar] [CrossRef]
- Griffiths, S.; Sovacool, B.K.; Kim, J.; Bazilian, M.; Uratani, J.M. Industrial decarbonization via hydrogen: A critical and systematic review of developments, socio-technical systems and policy options. Energy Res. Soc. Sci. 2021, 80, 102208. [Google Scholar] [CrossRef]
- Pramuanjaroenkij, A.; Kakaç, S. The fuel cell electric vehicles: The highlight review. Int. J. Hydrogen Energy 2023, 48, 9401–9425. [Google Scholar] [CrossRef]
- Zhou, P.; Gao, S.; Wang, B.; Wang, Y.; Li, C.; Wang, Y.; Sun, B. Influence of hydrogen fuel cell temperature safety on bus driving characteristics and stack heating mode. Int. J. Hydrogen Energy 2023, 48, 11541–11554. [Google Scholar] [CrossRef]
- Wang, J.; An, Q.; Zhao, Y.; Pan, G.; Song, J.; Hu, Q.; Tan, C.-W. Role of electrolytic hydrogen in smart city decarbonization in China. Appl. Energy 2023, 336, 120699. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2020, 13, 298. [Google Scholar] [CrossRef]
- Reda, B.; Elzamar, A.A.; AlFazzani, S.; Ezzat, S.M. Green hydrogen as a source of renewable energy: A step towards sustainability, an overview. Environ. Dev. Sustain. 2024. [Google Scholar] [CrossRef]
- Saha, P.; Akash, F.A.; Shovon, S.M.; Monir, M.U.; Ahmed, M.T.; Khan, M.F.H.; Sarkar, S.M.; Islam, M.K.; Hasan, M.M.; Vo, D.-V.N.; et al. Grey, blue, and green hydrogen: A comprehensive review of production methods and prospects for zero-emission energy. Int. J. Green Energy 2023, 21, 1383–1397. [Google Scholar] [CrossRef]
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Yang, X.; Wang, S.; He, Y. Review of catalytic reforming for hydrogen production in a membrane-assisted fluidized bed reactor. Renew. Sustain. Energy Rev. 2022, 154, 111832. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Pal, D.B.; Singh, A.; Bhatnagar, A. A review on biomass based hydrogen production technologies. Int. J. Hydrogen Energy 2022, 47, 1461–1480. [Google Scholar] [CrossRef]
- Liu, W.; Wan, Y.; Xiong, Y.; Gao, P. Green hydrogen standard in China: Standard and evaluation of low-carbon hydrogen, clean hydrogen, and renewable hydrogen. Int. J. Hydrogen Energy 2022, 47, 24584–24591. [Google Scholar] [CrossRef]
- Lakhera, S.K.; Rajan, A.; Rugma, T.P.; Bernaurdshaw, N. A review on particulate photocatalytic hydrogen production system: Progress made in achieving high energy conversion efficiency and key challenges ahead. Renew. Sustain. Energy Rev. 2021, 152, 111694. [Google Scholar] [CrossRef]
- Daiyan, R.; MacGill, I.; Amal, R. Opportunities and Challenges for Renewable Power-to-X. ACS Energy Lett. 2020, 5, 3843–3847. [Google Scholar] [CrossRef]
- Chehade, Z.; Mansilla, C.; Lucchese, P.; Hilliard, S.; Proost, J. Review and analysis of demonstration projects on power-to-X pathways in the world. Int. J. Hydrogen Energy 2019, 44, 27637–27655. [Google Scholar] [CrossRef]
- Cormos, C.-C. Deployment of integrated Power-to-X and CO2 utilization systems: Techno-economic assessment of synthetic natural gas and methanol cases. Appl. Therm. Eng. 2023, 231, 120943. [Google Scholar] [CrossRef]
- Qi, M.; Vo, D.N.; Yu, H.; Shu, C.-M.; Cui, C.; Liu, Y.; Park, J.; Moon, I. Strategies for flexible operation of power-to-X processes coupled with renewables. Renew. Sustain. Energy Rev. 2023, 179, 113282. [Google Scholar] [CrossRef]
- Watanabe, M.D.B.; Hu, X.; Ballal, V.; Cavalett, O.; Cherubini, F. Climate change mitigation potentials of on grid-connected Power-to-X fuels and advanced biofuels for the European maritime transport. Energy Convers. Manag. X 2023, 20, 100418. [Google Scholar] [CrossRef]
- Breyer, C.; Lopez, G.; Bogdanov, D.; Laaksonen, P. The role of electricity-based hydrogen in the emerging power-to-X economy. Int. J. Hydrogen Energy 2024, 49, 351–359. [Google Scholar] [CrossRef]
- Sterner, M.; Specht, M. Power-to-Gas and Power-to-X—The History and Results of Developing a New Storage Concept. Energies 2021, 14, 6594. [Google Scholar] [CrossRef]
- Palys, M.J.; Daoutidis, P. Power-to-X: A review and perspective. Comput. Chem. Eng. 2022, 165, 107948. [Google Scholar] [CrossRef]
- Arnaiz del Pozo, C.; Cloete, S.; Jiménez Álvaro, Á. Techno-economic assessment of long-term methanol production from natural gas and renewables. Energy Convers. Manag. 2022, 266, 115785. [Google Scholar] [CrossRef]
- Sonthalia, A.; Kumar, N.; Tomar, M.; Edwin Geo, V.; Thiyagarajan, S.; Pugazhendhi, A. Moving ahead from hydrogen to methanol economy: Scope and challenges. Clean Technol. Environ. Policy 2023, 25, 551–575. [Google Scholar] [CrossRef]
- Alrebei, O.F.; Le Page, L.M.; McKay, G.; El-Naas, M.H.; Amhamed, A.I. Recalibration of carbon-free NH3/H2 fuel blend process: Qatar’s roadmap for blue ammonia. Int. J. Hydrogen Energy 2023, 48, 23716–23736. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, G.; Liu, K.; Zhang, G.; Huang, Z.; Liu, M.; Zhou, J.; Wang, S. Application of CuNi–CeO2 fuel electrode in oxygen electrode supported reversible solid oxide cell. Int. J. Hydrogen Energy 2023, 48, 9565–9573. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Su, L.; Zhang, H.; Miao, X. High efficient trifunctional electrocatalyst based on bimetallic oxide with rich oxygen vacancies towards electrochemical oxidation of small molecules at high current density. Int. J. Hydrogen Energy 2023, 48, 9669–9681. [Google Scholar] [CrossRef]
- Ye, J.; Cui, J.; Hua, Z.; Xie, J.; Peng, W.; Wang, W. Study on the high-pressure hydrogen gas flow characteristics of the needle valve with different spool shapes. Int. J. Hydrogen Energy 2023, 48, 11370–11381. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Zhong, R.; Wei, C.; Zhu, B. Hydrogen energy development in China: Potential assessment and policy implications. Int. J. Hydrogen Energy 2024, 49, 659–669. [Google Scholar] [CrossRef]
- Xiaoyu, H.; Weiwei, C.; Hao, Y.; Yingying, R.; Guiping, D.; Lei, P. Development Status of Hydrogen Energy Industry Chain in China. Electr. Power Surv. Des. 2024, 3, 12–17. [Google Scholar] [CrossRef]
- Chai, S.; Zhang, G.; Li, G.; Zhang, Y. Industrial hydrogen production technology and development status in China: A review. Clean Technol. Environ. Policy 2021, 23, 1931–1946. [Google Scholar] [CrossRef]
- Li, T.; Liu, W.; Wan, Y.; Wang, Z.; Zhang, M.; Zhang, Y. China’s Green Hydrogen New Era 2030: China’s Renewable Hydrogen 100GW Roadmap; RMI China Energy Alliance Research Institute: Snowmass, CO, USA, 2022. [Google Scholar]
- Anwar, S.; Khan, F.; Zhang, Y.; Djire, A. Recent development in electrocatalysts for hydrogen production through water electrolysis. Int. J. Hydrogen Energy 2021, 46, 32284–32317. [Google Scholar] [CrossRef]
- Yan, D.; Mebrahtu, C.; Wang, S.; Palkovits, R. Innovative Electrochemical Strategies for Hydrogen Production: From Electricity Input to Electricity Output. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214333. [Google Scholar] [CrossRef]
- Madadi Avargani, V.; Zendehboudi, S.; Cata Saady, N.M.; Dusseault, M.B. A comprehensive review on hydrogen production and utilization in North America: Prospects and challenges. Energy Convers. Manag. 2022, 269, 115927. [Google Scholar] [CrossRef]
- Varela, C.; Mostafa, M.; Zondervan, E. Modeling alkaline water electrolysis for power-to-x applications: A scheduling approach. Int. J. Hydrogen Energy 2021, 46, 9303–9313. [Google Scholar] [CrossRef]
- Epelle, E.I.; Desongu, K.S.; Obande, W.; Adeleke, A.A.; Ikubanni, P.P.; Okolie, J.A.; Gunes, B. A comprehensive review of hydrogen production and storage: A focus on the role of nanomaterials. Int. J. Hydrogen Energy 2022, 47, 20398–20431. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrogen Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Lange, H.; Klose, A.; Lippmann, W.; Urbas, L. Technical evaluation of the flexibility of water electrolysis systems to increase energy flexibility: A review. Int. J. Hydrogen Energy 2023, 48, 15771–15783. [Google Scholar] [CrossRef]
- Xiaofeng, M.; Shuhan, Z.; Yong, H.; Yanqun, Z.; Zhihua, W. Research status and application prospect of PEM electrolysis water technology for hydrogen production. Acta Energiae Solaris Sin. 2022, 43, 420. [Google Scholar] [CrossRef]
- Jang, D.; Cho, H.-S.; Kang, S. Numerical modeling and analysis of the effect of pressure on the performance of an alkaline water electrolysis system. Appl. Energy 2021, 287, 116554. [Google Scholar] [CrossRef]
- Hu, S.; Guo, B.; Ding, S.; Yang, F.; Dang, J.; Liu, B.; Gu, J.; Ma, J.; Ouyang, M. A comprehensive review of alkaline water electrolysis mathematical modeling. Appl. Energy 2022, 327, 120099. [Google Scholar] [CrossRef]
- Alanazi, H.E.; Emran, K.M. Nd-Gd–Platinum doped TiO2 nanotube arrays catalyst for water splitting in Alkaline Medium. Int. J. Electrochem. Sci. 2023, 18, 100112. [Google Scholar] [CrossRef]
- Marelli, E.; Lyu, J.; Morin, M.; Lemenager, M.; Shang, T.; Yuzbasi, N.S.; Aegerter, D.; Huang, J.; Daffe, N.D.; Clark, A.H.; et al. Cobalt-free layered perovskites RBaCuFeO(5+delta) (R = 4f lanthanide) as electrocatalysts for the oxygen evolution reaction. EES Catal. 2024, 2, 335–350. [Google Scholar] [CrossRef]
- Dubouis, N.; Aymé-Perrot, D.; Degoulange, D.; Grimaud, A.; Girault, H. Alkaline electrolyzers: Powering industries and overcoming fundamental challenges. Joule 2024, 8, 883–898. [Google Scholar] [CrossRef]
- de Groot, M.T.; Vreman, A.W. Ohmic resistance in zero gap alkaline electrolysis with a Zirfon diaphragm. Electrochim. Acta 2021, 369, 137684. [Google Scholar] [CrossRef]
- Petrov, Y.; Schosger, J.-P.; Stoynov, Z.; de Bruijn, F. Hydrogen evolution on nickel electrode in synthetic tap water—Alkaline solution. Int. J. Hydrogen Energy 2011, 36, 12715–12724. [Google Scholar] [CrossRef]
- Haverkort, J.W.; Rajaei, H. Voltage losses in zero-gap alkaline water electrolysis. J. Power Sources 2021, 497, 229864. [Google Scholar] [CrossRef]
- Mucci, S.; Mitsos, A.; Bongartz, D. Power-to-X processes based on PEM water electrolyzers: A review of process integration and flexible operation. Comput. Chem. Eng. 2023, 175, 108260. [Google Scholar] [CrossRef]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Maier, M.; Smith, K.; Dodwell, J.; Hinds, G.; Shearing, P.R.; Brett, D.J.L. Mass transport in PEM water electrolysers: A review. Int. J. Hydrogen Energy 2022, 47, 30–56. [Google Scholar] [CrossRef]
- Aizaz Ud Din, M.; Irfan, S.; Dar, S.U.; Rizwan, S. Synthesis of 3D IrRuMn Sphere as a Superior Oxygen Evolution Electrocatalyst in Acidic Environment. Chemistry 2020, 26, 5662–5666. [Google Scholar] [CrossRef] [PubMed]
- Wallnöfer-Ogris, E.; Grimmer, I.; Ranz, M.; Höglinger, M.; Kartusch, S.; Rauh, J.; Macherhammer, M.-G.; Grabner, B.; Trattner, A. A review on understanding and identifying degradation mechanisms in PEM water electrolysis cells: Insights for stack application, development, and research. Int. J. Hydrogen Energy 2024, 65, 381–397. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A short review. Renew. Sustain. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Lo Basso, G.; Mojtahed, A.; Pastore, L.M.; De Santoli, L. High-temperature green hydrogen production: A innovative– application of SOEC coupled with AEC through sCO2 HP. Int. J. Hydrogen Energy 2024, 52, 978–993. [Google Scholar] [CrossRef]
- Alamiery, A. Advancements in materials for hydrogen production: A review of cutting-edge technologies. ChemPhysMater 2024, 3, 64–73. [Google Scholar] [CrossRef]
- Liu, S.; Li, B.; Mohite, S.V.; Devaraji, P.; Mao, L.; Xing, R. Ultrathin MoS2 nanosheets in situ grown on rich defective Ni0.96S as heterojunction bifunctional electrocatalysts for alkaline water electrolysis. Int. J. Hydrogen Energy 2020, 45, 29929–29937. [Google Scholar] [CrossRef]
- Alshehri, F.; Suárez, V.G.; Torres, J.L.R.; Perilla, A.; van der Meijden, M.A.M.M. Modelling and evaluation of PEM hydrogen technologies for frequency ancillary services in future multi-energy sustainable power systems. Heliyon 2019, 5, e01396. [Google Scholar] [CrossRef] [PubMed]
- Koponen, J.; Kosonen, A.; Ruuskanen, V.; Huoman, K.; Niemela, M.; Ahola, J. Control and energy efficiency of PEM water electrolyzers in renewable energy systems. Int. J. Hydrogen Energy 2017, 42, 29648–29660. [Google Scholar] [CrossRef]
- Scheller, F.; Wald, S.; Kondziella, H.; Gunkel, P.A.; Bruckner, T.; Keles, D. Future role and economic benefits of hydrogen and synthetic energy carriers in Germany: A review of long-term energy scenarios. Sustain. Energy Technol. Assess. 2023, 56, 103037. [Google Scholar] [CrossRef]
- Nemmour, A.; Inayat, A.; Janajreh, I.; Ghenai, C. Green hydrogen-based E-fuels (E-methane, E-methanol, E-ammonia) to support clean energy transition: A literature review. Int. J. Hydrogen Energy 2023, 48, 29011–29033. [Google Scholar] [CrossRef]
- Nyári, J. Techno-Economic Feasibility Study of a Methanol Plant Using Carbon Dioxide and Hydrogen. Master’s Thesis, Aalto University, Espoo, Finland, 2018. Available online: https://aaltodoc.aalto.fi/items/9af1d544-fc0c-4a80-bc68-5b68a0c6dd9f (accessed on 14 July 2024).
- Leonzio, G. Methanol Synthesis: Optimal Solution for a Better Efficiency of the Process. Processes 2018, 6, 20. [Google Scholar] [CrossRef]
- Shih, C.F.; Zhang, T.; Li, J.; Bai, C. Powering the Future with Liquid Sunshine. Joule 2018, 2, 1925–1949. [Google Scholar] [CrossRef]
- Van-Dal, É.S.; Bouallou, C. Design and simulation of a methanol production plant from CO2 hydrogenation. J. Clean. Prod. 2013, 57, 38–45. [Google Scholar] [CrossRef]
- Huang, C.; Wen, J.; Sun, Y.; Zhang, M.; Bao, Y.; Zhang, Y.; Liang, L.; Fu, M.; Wu, J.; Ye, D.; et al. CO2 hydrogenation to methanol over Cu/ZnO plate model catalyst: Effects of reducing gas induced Cu nanoparticle morphology. Chem. Eng. J. 2019, 374, 221–230. [Google Scholar] [CrossRef]
- Fang, X.; Men, Y.; Wu, F.; Zhao, Q.; Singh, R.; Xiao, P.; Du, T.; Webley, P.A. Improved methanol yield and selectivity from CO2 hydrogenation using a novel Cu-ZnO-ZrO2 catalyst supported on Mg-Al layered double hydroxide (LDH). J. CO2 Util. 2019, 29, 57–64. [Google Scholar] [CrossRef]
- Wang, J.; Song, Y.; Li, J.; Liu, F.; Wang, J.; Lv, J.; Wang, S.; Li, M.; Bao, X.; Ma, X. Modulation of Al2O3 and ZrO2 composite in Cu/ZnO-based catalysts with enhanced performance for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2024, 674, 119618. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, J.; Wang, S.; Zhao, N.; Xiao, F.; Wang, Y. Effect of Ni content on Cu–Mn/ZrO2 catalysts for methanol synthesis from CO2 hydrogenation. Catal. Sci. Technol. 2024, 14, 2153–2165. [Google Scholar] [CrossRef]
- Sharma, S.K.; Khan, T.S.; Singha, R.K.; Paul, B.; Poddar, M.K.; Sasaki, T.; Bordoloi, A.; Samanta, C.; Gupta, S.; Bal, R. Design of highly stable MgO promoted Cu/ZnO catalyst for clean methanol production through selective hydrogenation of CO2. Appl. Catal. A Gen. 2021, 623, 118239. [Google Scholar] [CrossRef]
- Vergara, T.; Gómez, D.; Lacerda de Oliveira Campos, B.; Herrera Delgado, K.; Concepción, P.; Jiménez, R.; Karelovic, A. Combined role of Ce promotion and TiO2 support improves CO2 hydrogenation to methanol on Cu catalysts: Interplay between structure and kinetics. J. Catal. 2023, 426, 200–213. [Google Scholar] [CrossRef]
- Ren, M.; Zhang, Y.; Wang, X.; Qiu, H. Catalytic Hydrogenation of CO2 to Methanol: A Review. Catalysts 2022, 12, 403. [Google Scholar] [CrossRef]
- Wu, H.; Xiong, S.; Liu, C.-J. Preparation of In2O3/ZrO2 catalyst via DBD plasma decomposition of Zr(OH)4 for CO2 hydrogenation to methanol. Catal. Today 2023, 423, 114024. [Google Scholar] [CrossRef]
- Tsoukalou, A.; Serykh, A.I.; Willinger, E.; Kierzkowska, A.; Abdala, P.M.; Fedorov, A.; Müller, C.R. Hydrogen dissociation sites on indium-based ZrO2-supported catalysts for hydrogenation of CO2 to methanol. Catal. Today 2022, 387, 38–46. [Google Scholar] [CrossRef]
- Vourros, A.; Garagounis, I.; Kyriakou, V.; Carabineiro, S.A.C.; Maldonado-Hódar, F.J.; Marnellos, G.E.; Konsolakis, M. Carbon dioxide hydrogenation over supported Au nanoparticles: Effect of the support. J. CO2 Util. 2017, 19, 247–256. [Google Scholar] [CrossRef]
- Kuwahara, Y.; Mihogi, T.; Hamahara, K.; Kusu, K.; Kobayashi, H.; Yamashita, H. A quasi-stable molybdenum sub-oxide with abundant oxygen vacancies that promotes CO2 hydrogenation to methanol. Chem. Sci. 2021, 12, 9902–9915. [Google Scholar] [CrossRef]
- Darji, H.R.; Kale, H.B.; Shaikh, F.F.; Gawande, M.B. Advancement and State-of-art of heterogeneous catalysis for selective CO2 hydrogenation to methanol. Coord. Chem. Rev. 2023, 497, 215409. [Google Scholar] [CrossRef]
- Wang, Y.; Kattel, S.; Gao, W.; Li, K.; Liu, P.; Chen, J.G.; Wang, H. Exploring the ternary interactions in Cu-ZnO-ZrO(2) catalysts for efficient CO2 hydrogenation to methanol. Nat. Commun. 2019, 10, 1166. [Google Scholar] [CrossRef]
- Gao, P.; Zhang, L.; Li, S.; Zhou, Z.; Sun, Y. Novel Heterogeneous Catalysts for CO2 Hydrogenation to Liquid Fuels. ACS Cent. Sci. 2020, 6, 1657–1670. [Google Scholar] [CrossRef] [PubMed]
- Martin, O.; Martin, A.J.; Mondelli, C.; Mitchell, S.; Segawa, T.F.; Hauert, R.; Drouilly, C.; Curulla-Ferre, D.; Perez-Ramirez, J. Indium Oxide as a Superior Catalyst for Methanol Synthesis by CO2 Hydrogenation. Angew. Chem. Int. Ed. Engl. 2016, 55, 6261–6265. [Google Scholar] [CrossRef] [PubMed]
- Pontzen, F.; Liebner, W.; Gronemann, V.; Rothaemel, M.; Ahlers, B. CO2-based methanol and DME—Efficient technologies for industrial scale production. Catal. Today 2011, 171, 242–250. [Google Scholar] [CrossRef]
- Toyir, J.; Miloua, R.; Elkadri, N.E.; Nawdali, M.; Toufik, H.; Miloua, F.; Saito, M. Sustainable process for the production of methanol from CO2 and H2 using Cu/ZnO-based multicomponent catalyst. Phys. Procedia 2009, 2, 1075–1079. [Google Scholar] [CrossRef]
- Doss, B.; Ramos, C.; Atkins, S. Optimization of Methanol Synthesis from Carbon Dioxide and Hydrogen: Demonstration of a Pilot-Scale Carbon-Neutral Synthetic Fuels Process. Energy Fuels 2009, 23, 4647–4650. [Google Scholar] [CrossRef]
- Choi, E.J.; Lee, Y.H.; Lee, D.-W.; Moon, D.-J.; Lee, K.-Y. Hydrogenation of CO2 to methanol over Pd–Cu/CeO2 catalysts. Mol. Catal. 2017, 434, 146–153. [Google Scholar] [CrossRef]
- Tan, Q.; Shi, Z.; Wu, D. CO2 Hydrogenation to Methanol over a Highly Active Cu–Ni/CeO2–Nanotube Catalyst. Ind. Eng. Chem. Res. 2018, 57, 10148–10158. [Google Scholar] [CrossRef]
- Zabilskiy, M.; Sushkevich, V.L.; Palagin, D.; Newton, M.A.; Krumeich, F.; van Bokhoven, J.A. The unique interplay between copper and zinc during catalytic carbon dioxide hydrogenation to methanol. Nat. Commun. 2020, 11, 2409. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, P.; Tang, Z.; Lv, M.; Sun, Y. Three dimensional porous Cu-Zn/Al foam monolithic catalyst for CO2 hydrogenation to methanol in microreactor. J. CO2 Util. 2017, 21, 191–199. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Wang, N.; Chen, B. The copper size effect of CuZn/CeO2 catalyst in CO2 hydrogenation to methanol. Catal. Today 2024, 436, 114773. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Zhou, H.; Xiao, F.; Zhao, N. Performance of Cu-Mn-Zn/ZrO2 catalysts for methanol synthesis from CO2 hydrogenation: The effect of Zn content. J. Fuel Chem. Technol. 2024, 52, 293–303. [Google Scholar] [CrossRef]
- Rui, N.; Wang, Z.; Sun, K.; Ye, J.; Ge, Q.; Liu, C.-J. CO2 hydrogenation to methanol over Pd/In2O3: Effects of Pd and oxygen vacancy. Appl. Catal. B Environ. 2017, 218, 488–497. [Google Scholar] [CrossRef]
- Chou, C.-Y.; Lobo, R.F. Direct conversion of CO2 into methanol over promoted indium oxide-based catalysts. Appl. Catal. A Gen. 2019, 583, 117144. [Google Scholar] [CrossRef]
- Wang, J.; Tang, C.; Li, G.; Han, Z.; Li, Z.; Liu, H.; Cheng, F.; Li, C. High-Performance MaZrOx (Ma = Cd, Ga) Solid-Solution Catalysts for CO2 Hydrogenation to Methanol. ACS Catal. 2019, 9, 10253–10259. [Google Scholar] [CrossRef]
- Li, X.; Liu, G.; Xu, D.; Hong, X.; Edman Tsang, S.C. Confinement of subnanometric PdZn at a defect enriched ZnO/ZIF-8 interface for efficient and selective CO2 hydrogenation to methanol. J. Mater. Chem. A 2019, 7, 23878–23885. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Patiño-Arévalo, L.J.; Tsatsaronis, G.; Morosuk, T. Hydrogen-driven Power-to-X: State of the art and multicriteria evaluation of a study case. Energy Convers. Manag. 2022, 266, 115814. [Google Scholar] [CrossRef]
- Chen, C.; Yang, A. Power-to-methanol: The role of process flexibility in the integration of variable renewable energy into chemical production. Energy Convers. Manag. 2021, 228, 113673. [Google Scholar] [CrossRef]
- Dieterich, V.; Buttler, A.; Hanel, A.; Spliethoff, H.; Fendt, S. Power-to-liquidviasynthesis of methanol, DME or Fischer–Tropsch-fuels: A review. Energy Environ. Sci. 2020, 13, 3207–3252. [Google Scholar] [CrossRef]
- Iliuta, I.; Larachi, F. Enhanced Methanol Synthesis Process via an Integrated Process Involving CO2 Hydrogenation under Plasma Conditions. Ind. Eng. Chem. Res. 2019, 59, 6815–6827. [Google Scholar] [CrossRef]
- Leonzio, G. State of art and perspectives about the production of methanol, dimethyl ether and syngas by carbon dioxide hydrogenation. J. CO2 Util. 2018, 27, 326–354. [Google Scholar] [CrossRef]
- Chen, C.; Yang, A.; Bañares-Alcántara, R. Renewable methanol production: Understanding the interplay between storage sizing, renewable mix and dispatchable energy price. Adv. Appl. Energy 2021, 2, 100021. [Google Scholar] [CrossRef]
- Tso, W.W.; Demirhan, C.D.; Lee, S.; Song, H.; Powell, J.B.; Pistikopoulos, E.N. Energy Carrier Supply Chain Optimization: A Texas Case Study. In Proceedings of the 9th International Conference on Foundations of Computer-Aided Process Design, Copper Mountain, CO, USA, 14–18 July 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Martín, M.; Grossmann, I.E. Optimal integration of a self sustained algae based facility with solar and/or wind energy. J. Clean. Prod. 2017, 145, 336–347. [Google Scholar] [CrossRef]
- Kiss, A.A.; Pragt, J.J.; Vos, H.J.; Bargeman, G.; de Groot, M.T. Novel efficient process for methanol synthesis by CO2 hydrogenation. Chem. Eng. J. 2016, 284, 260–269. [Google Scholar] [CrossRef]
- Atsonios, K.; Panopoulos, K.D.; Kakaras, E. Thermocatalytic CO2 hydrogenation for methanol and ethanol production: Process improvements. Int. J. Hydrogen Energy 2016, 41, 792–806. [Google Scholar] [CrossRef]
- Kim, J.; Qi, M.; Park, J.; Moon, I. Revealing the impact of renewable uncertainty on grid-assisted power-to-X: A data-driven reliability-based design optimization approach. Appl. Energy 2023, 339, 121015. [Google Scholar] [CrossRef]
- Moioli, E.; Wötzel, A.; Schildhauer, T. Feasibility assessment of small-scale methanol production via power-to-X. J. Clean. Prod. 2022, 359, 132071. [Google Scholar] [CrossRef]
- Ye, D.; Tsang, S.C.E. Prospects and challenges of green ammonia synthesis. Nat. Synth. 2023, 2, 612–623. [Google Scholar] [CrossRef]
- Aziz, M.; Wijayanta, A.T.; Nandiyanto, A.B.D. Ammonia as Effective Hydrogen Storage: A Review on Production, Storage and Utilization. Energies 2020, 13, 3062. [Google Scholar] [CrossRef]
- Bennani, Y.; Perl, A.; Patil, A.C.; Someren, C.V.; Heijne, L.; Steenis, M.V. Power-to-Ammonia: Rethinking the Role of Ammonia—From a Value Product to a Flexible Energy Carrier (FlexNH3); Hanzehogeschool Groningen: Groningen, The Netherlands, 2016. [Google Scholar]
- Kyriakou, V.; Garagounis, I.; Vourros, A.; Vasileiou, E.; Stoukides, M. An Electrochemical Haber-Bosch Process. Joule 2020, 4, 142–158. [Google Scholar] [CrossRef]
- Liu, H. Ammonia synthesis catalyst 100 years: Practice, enlightenment and challenge. Chin. J. Catal. 2014, 35, 1619–1640. [Google Scholar] [CrossRef]
- Mortensen, J.J.; Hansen, L.B.; Hammer, B.; Nørskov, J.K. Nitrogen Adsorption and Dissociation on Fe(111). J. Catal. 1999, 182, 479–488. [Google Scholar] [CrossRef]
- Moghadam, M.R.; Bazmandegan-Shamili, A.; Bagheri, H. The current methods of ammonia synthesis by Haber-Bosch process. In Progresses in Ammonia: Science, Technology and Membranes; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–32. [Google Scholar] [CrossRef]
- Humphreys, J.; Lan, R.; Tao, S. Development and Recent Progress on Ammonia Synthesis Catalysts for Haber–Bosch Process. Adv. Energy Sustain. Res. 2020, 2, 2000043. [Google Scholar] [CrossRef]
- Weiss, W.; Ranke, W. Surface chemistry and catalysis on well-defined epitaxial iron-oxide layers. Prog. Surf. Sci. 2002, 70, 1–151. [Google Scholar] [CrossRef]
- Hu, L.Z.N. Development of novel low temperature and low pressure ammonia synthesis catalyst. Appl. Catal. A Gen. 1996, 142, 209–222. [Google Scholar]
- Berwal, P.; Kumar, S.; Khandelwal, B. A comprehensive review on synthesis, chemical kinetics, and practical application of ammonia as future fuel for combustion. J. Energy Inst. 2021, 99, 273–298. [Google Scholar] [CrossRef]
- Ghoreishian, S.M.; Shariati, K.; Huh, Y.S.; Lauterbach, J. Recent advances in ammonia synthesis over ruthenium single-atom-embedded catalysts: A focused review. Chem. Eng. J. 2023, 467, 143533. [Google Scholar] [CrossRef]
- Inoue, Y.; Kitano, M.; Kim, S.-W.; Yokoyama, T.; Hara, M.; Hosono, H. Highly Dispersed Ru on Electride [Ca24Al28O64]4+(e−)4 as a Catalyst for Ammonia Synthesis. ACS Catal. 2014, 4, 674–680. [Google Scholar] [CrossRef]
- Kitano, M.; Inoue, Y.; Sasase, M.; Kishida, K.; Kobayashi, Y.; Nishiyama, K.; Tada, T.; Kawamura, S.; Yokoyama, T.; Hara, M.; et al. Self-organized Ruthenium-Barium Core-Shell Nanoparticles on a Mesoporous Calcium Amide Matrix for Efficient Low-Temperature Ammonia Synthesis. Angew. Chem. Int. Ed. Engl. 2018, 57, 2648–2652. [Google Scholar] [CrossRef]
- Han, W.; Huang, S.; Cheng, T.; Tang, H.; Li, Y.; Liu, H. Promotion of Nb2O5 on the wustite-based iron catalyst for ammonia synthesis. Appl. Surf. Sci. 2015, 353, 17–23. [Google Scholar] [CrossRef]
- Xiujin, Y.U.; Lin, B.; Lin, J.; Wang, R.; Wei, K. A novel fused iron catalyst for ammonia synthesis promoted with rare earth gangue. J. Rare Earths 2008, 26, 6. [Google Scholar]
- Czekajło, Ł.; Lendzion-Bieluń, Z. Wustite based iron-cobalt catalyst for ammonia synthesis. Catal. Today 2017, 286, 114–117. [Google Scholar] [CrossRef]
- Jafari, A.; Ebadi, A.; Sahebdelfar, S. Effect of iron oxide precursor on the properties and ammonia synthesis activity of fused iron catalysts. React. Kinet. Mech. Catal. 2018, 126, 307–325. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Tang, Y.; Kageyama, T.; Yamashita, H.; Masuda, N.; Hosokawa, S.; Kageyama, H. Titanium-Based Hydrides as Heterogeneous Catalysts for Ammonia Synthesis. J. Am. Chem. Soc. 2017, 139, 18240–18246. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Li, Z.; Liu, H. La2Ce2O7 supported ruthenium as a robust catalyst for ammonia synthesis. J. Rare Earths 2019, 37, 492–499. [Google Scholar] [CrossRef]
- Lin, B.; Guo, Y.; Cao, C.; Ni, J.; Lin, J.; Jiang, L. Carbon support surface effects in the catalytic performance of Ba-promoted Ru catalyst for ammonia synthesis. Catal. Today 2018, 316, 230–236. [Google Scholar] [CrossRef]
- Karolewska, M.; Truszkiewicz, E.; Wściseł, M.; Mierzwa, B.; Kępiński, L.; Raróg-Pilecka, W. Ammonia synthesis over a Ba and Ce-promoted carbon-supported cobalt catalyst. Effect of the cerium addition and preparation procedure. J. Catal. 2013, 303, 130–134. [Google Scholar] [CrossRef]
- Zybert, M.; Wyszyńska, M.; Tarka, A.; Patkowski, W.; Ronduda, H.; Mierzwa, B.; Kępiński, L.; Sarnecki, A.; Moszyński, D.; Raróg-Pilecka, W. Surface enrichment phenomenon in the Ba-doped cobalt catalyst for ammonia synthesis. Vacuum 2019, 168, 108831. [Google Scholar] [CrossRef]
- Ye, T.N.; Park, S.W.; Lu, Y.; Li, J.; Sasase, M.; Kitano, M.; Hosono, H. Contribution of Nitrogen Vacancies to Ammonia Synthesis over Metal Nitride Catalysts. J. Am. Chem. Soc. 2020, 142, 14374–14383. [Google Scholar] [CrossRef]
- Morgan, E.R. Techno-Economic Feasibility Study of Ammonia Plants Powered by Offshore Wind. Ph.D. Thesis, University of Massachusetts Amherst, Provo, UT, USA, 2013. [Google Scholar]
- Cheema, I.I.; Krewer, U. Operating envelope of Haber-Bosch process design for power-to-ammonia. RSC Adv. 2018, 8, 34926–34936. [Google Scholar] [CrossRef]
- Folke, J.; Song, H.; Schittkowski, J.; Schlögl, R.; Ruland, H. Oxygen Poisoning in Laboratory Testing of Iron-Based Ammonia Synthesis Catalysts and its Potential Sources. Chem. Ing. Tech. 2020, 92, 1567–1573. [Google Scholar] [CrossRef]
- Rouwenhorst, K.H.R.; Krzywda, P.M.; Benes, N.E.; Mul, G.; Lefferts, L. Ammonia Production Technologies. In Techno-Economic Challenges of Green Ammonia as an Energy Vector; Academic Press: New York, NY, USA, 2021; pp. 41–83. [Google Scholar] [CrossRef]
- Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 1 September 2023).

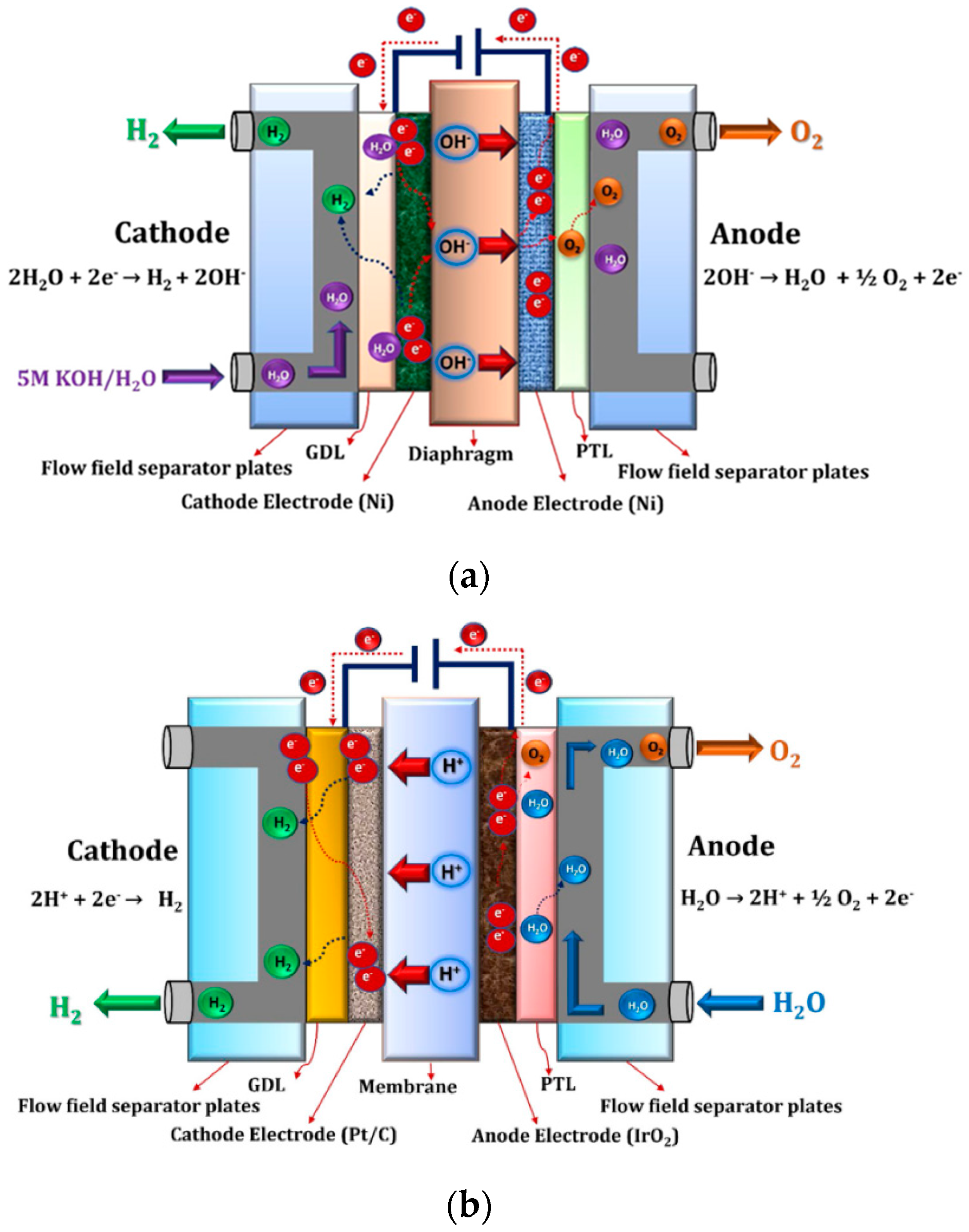
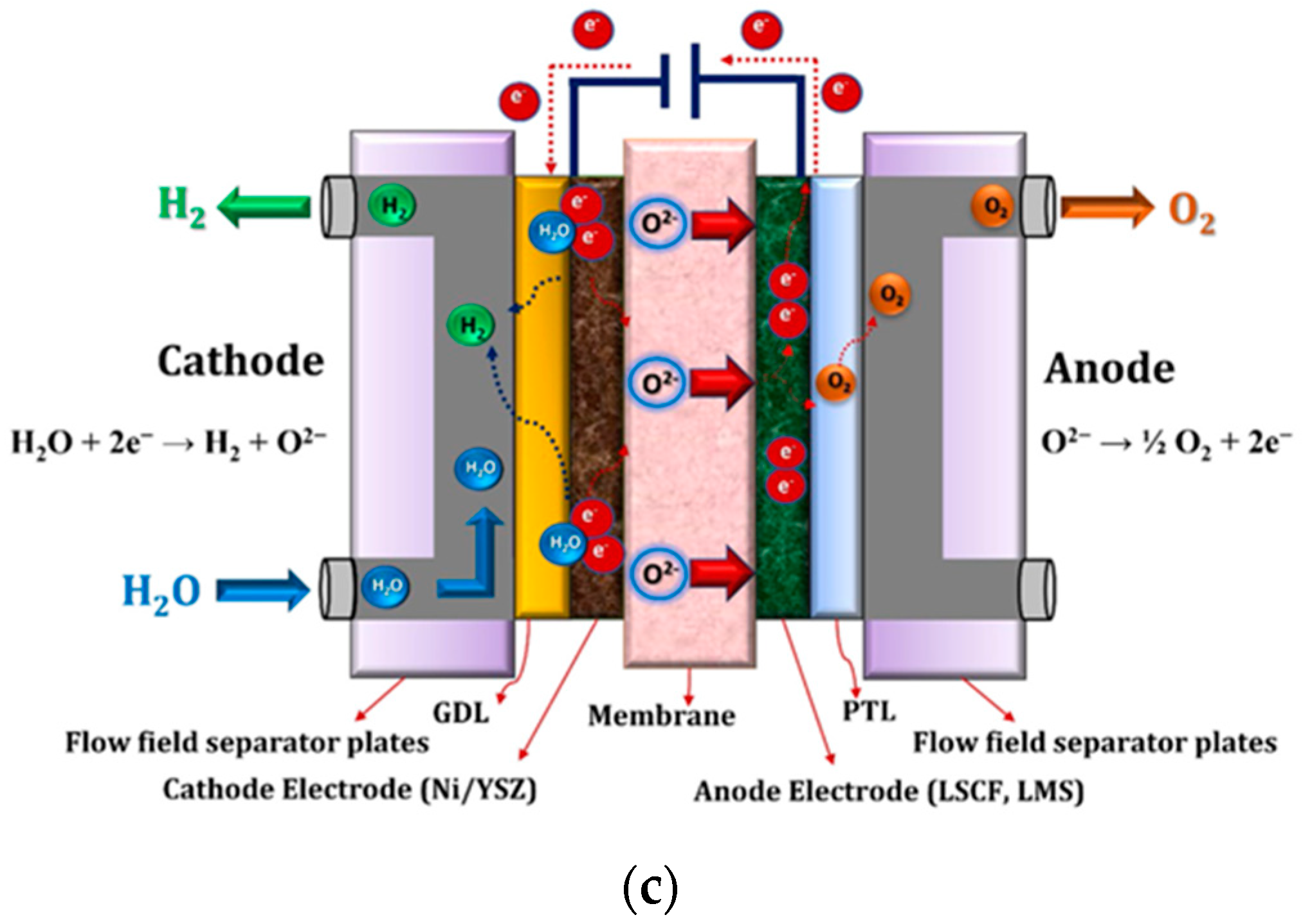
| Gravimetric Energy Density (MJ/kg) | Gravimetric Energy Density (kWh/kg) | Volumetric Energy Density (MJ/L) | Volumetric Energy Density (kWh/L) | |
|---|---|---|---|---|
| H2 (35 Mpa) | 120.0 | 33.3 | 2.8 | 0.8 |
| H2 (70 Mpa) | 120.0 | 33.3 | 4.8 | 1.3 |
| H2 (liquid) | 120.0 | 33.3 | 8.5 | 2.2 |
| CH4 (25 Mpa) | 50.1 | 13.9 | 9.5 | 2.6 |
| Standard coal | 29.3 | 8.1 | ||
| Gasoline | 43.1 | 12.2 | 32.0 | 8.9 |
| Diesel | 46.0 | 12.8 | 38.6 | 10.7 |
| Year | Main Policy | Key Points |
|---|---|---|
| 2006 | Outline of the National Medium- and Long-term Planning for Development of Science and Technology (From 2006 to 2020) | Hydrogen energy and fuel cell technologies were incorporated into the advanced energy technologies. |
| 2012 | Plan for Development of the Energy Efficient and New Energy Automobile Industry (2012–2020) | The government proposed to drive the development of hydrogen production, storage, transportation, and refueling technology by means of fuel cell application demonstration. |
| 2014 | Energy Development Strategy Action Plan (2014–2020) | Hydrogen energy and fuel cell were determined as strategic directions for energy technology innovation |
| 2016 | China Energy Technology Innovation Action Plan 2016–2030 | The section on hydrogen energy has significantly expanded, covering hydrogen production from renewable energy and nuclear energy, fuel cell distributed power generation, etc. |
| 2019 | Government Work Report | For the first time, hydrogen was included in the government work report, which emphasized the need to promote the implementation of charging and hydrogen refueling facilities. |
| 2020 | The energy law of the people’s republic of China (Exposure Draft) | At the level of national law, hydrogen energy was incorporated in the energy management. |
| 2021 | Action Plan for Carbon Dioxide Peaking Before 2030 | The plan proposed to explore the large-scale application of hydrogen energy in industry, transportation, construction, and other fields. |
| 2022 | New Energy Storage Development Implementation during the 14th Five-year Plan | It clarified the position of hydrogen energy and ammonia energy as a new type of energy storage. |
| 2022 | 14th Five-year plan for renewable energy development | It was emphasized that large-scale hydrogen production from renewable energy sources was promoted. |
| 2022 | China maps 2021–2035 plan on hydrogen energy development | The plan defined the energy properties of hydrogen, and proposed that hydrogen energy would play an important supporting role in the country’s green energy transformation. |
| 2023 | Guidelines on Hydrogen Energy Industry Standard System Construction (2023) | The guidelines systematically established a standard system for the entire industrial chain of hydrogen energy, including hydrogen production, storage, transportation, and utilization. |
| Type | ALK | PEM | SOEC |
|---|---|---|---|
| Current density (A/cm2) | 0.2~0.7 | 0.1~2.2 | 1.0~2.0 |
| Temperature (°C) | 50~80 | 40~80 | 700~900 |
| H2 Purity (%) | 99.5~99.9 | 99.99 | 99.90 |
| Pressure (MPa) | 1.0~3.0 | 2.0~5.0 | 0.1~1.5 |
| Energy consumption (kWh/Nm3) | 4.5~5.5 | 3.4~4.4 | 2.23~2.27 |
| Efficiency (%) | 56~80 | 76~85 | 90~100 |
| Load range (%) | 15~100 | 0~150 | 0~120 |
| Respond speed | Dozens of seconds | Several seconds | Several minutes |
| Lifetime (h) | 90,000 | 20,000~50,000 | ~7000 |
| Brand | Province | Technology | Hydrogen Production Rate (Nm3/h) | Hydrogen Pressure (MPa) | Power Consumption (kWh/Nm3) |
|---|---|---|---|---|---|
| Peric Hydrogen | Hebei | ALK | 20–2000 | 1.5–2.5 | ≤4.3–4.5 |
| PEM | 0.01–200 | 0.1–3.2 | ≤5.4 | ||
| Longi Hydrogen | Shannxi | ALK | 1–3000 | 1.6 | 4.1–4.3 (ALK Hi1) 4.3–4.5 (ALK G) |
| Sungrow | Anhui | ALK | ≤1000 | 1.8 | - |
| PEM | ≤250 | 3 | - | ||
| Tianjin Mainland Hydrogen Equipment | Tianjin | ALK | 0.1–1000 | 5 | ≤4.4–4.9 |
| PEM | 0.4–10 | 7 | - | ||
| Sany | Hunan | ALK | 500–2000 | 1.8 | 4.3–4.7 |
| PEM | ≤200 | 3 | 4.3 | ||
| Guofuhee | Jiangsu | ALK | 50–1000 | - | - |
| PEM | 4–200 | - | - | ||
| Kohodo Hydrogen Energy | Guangdong | ALK | 0.5–1000 | 1.6 | 4.0 |
| Changchun lvdong | Jilin | PEM | ≤200 | - | - |
| Shandong Saikesaisi Hydrogen Energy | Shandong | PEM | 0.5–200 | 3 | - |
| BPEG | Beijing | ALK | 2–2000 | - | 4.3 |
| C hySA | Guangdong | PEM | 0.2–300 | 3.5 | - |
| H2-Bank | Zhejiang | SOEC | 2 | - | 3.5 |
| Reference | Catalysts | H2:CO2 | Temperature (°C) | Pressure (MPa) | GHSV (h−1) | CO2 Conversion (%) | Methanol Selectivity (%) |
|---|---|---|---|---|---|---|---|
| Florian et al. [99] | Standard commercial catalyst (Süd-Chemie) | 3.1:1 | 250 | 8 | 10,500 | ||
| Toyir et al. [100] | Cu/ZnO/ZrO2/Al2O3/SiO2 | 3.7:1 | 250 | 7 | 10,000 | ||
| Doss et al. [101] | commercial catalyst (Johnson Matthey) Cu/ZnO/Al2O3 | 3:1 | 240 | 6.9 | 3300 | 5.81 | |
| Choi et al. [102] | Cu-Pd/CeO2 | 3:1 | 270 | 3 | 16.1 | 26.7 | |
| Tan et al. [103] | Cu/ZnO/Al2O3 | 3:1 | 260 | 3 | 6000 | 23.1 | 31.2 |
| Tan et al. [103] | CuNi2/CeO2−NT | 3:1 | 260 | 3 | 6000 | 17.8 | 78.8 |
| Zabilskiy et al. [104] | Standard Cu/ZnO/Al2O3 catalyst (Alfa Aesar) | 3:1 | 260 | 1.5 | 2 | 43 | |
| Liang et al. [105] | Cn-Zn/Al foam | 3:1 | 250 | 3 | 13.6 | 64.5 | |
| Wang et al. [96] | Cu/ZnO/ZrO2 | 3:1 | 220 | 3 | 18.9 | 80.2 | |
| Zhang et al. [106] | CuZn/CeO | 260 | 3 | 68.5 | |||
| Wang et al. [107] | Cu-Mn-Zn/ZrO | 3:1 | 250 | 5 | 4000 | 6.5 | 73.7 |
| Martin et al. [98] | In2O3/ZrO2 | 4:1 | 300 | 5 | 20,000 | 5.2 | 99.8 |
| Rui et al. [108] | In2O3 | 4:1 | 300 | 5 | 21,000 | 8.2 | 71 |
| Chou et al. [109] | In2O3/ZrO2 | 4:1 | 300 | 4 | 52,000 | 10.5 | 53 |
| Chou et al. [109] | 1.5YIn2O3/ZrO2 | 4:1 | 300 | 4 | 52,000 | 7.6 | 69 |
| Wang et al. [110] | MaZrOx (Ma = Cd, Ga) solid-solution catalysts | 3:1 | 300 | 5 | 24,000 | 4.3–12.4 | 80 |
| Li et al. [111] | Pd/ZnO-ZIF-8 | 3:1 | 270 | 4.5 | 9.3 | 74 |
| Reference | Catalysts | Compositions (wt.%) | H2:N3 | Temperature (°C) | Pressure (MPa) | GHSV (h−1) | NH3 Concentration (%) |
|---|---|---|---|---|---|---|---|
| Han et al. [138] | WBC | 80.46FeO + 12.66Fe2O3 + 1.8Al2O3 + 0.6K2O + 1.8CaO + 2.68others | 3:1 | 350–475 | 5 | 30,000 | 10.4–18.5 |
| Han et al. [138] | Nb-WBC | 80FeO + 12.52Fe2O3 + 1.8Al2O3 + 0.6K2O + 1.8CaO + 0.6Nb2O5 + 2.08others | 3:1 | 350–475 | 5 | 30,000 | 9.6–17.9 |
| Yu et al. [139] | A110-3 | 68Fe(total) + 2.2Al2O3 + 0.59K2O + 1.2CaO + 0.36SiO2 | 3:1 | 425 | 15 | 10,000 | 19.5 |
| Yu et al. [139] | FA401 | 68Fe(total) + 2.3Al2O3 + 0.58K2O + 1.1CaO + 0.33SiO2 + 0.3MgO + 0.38others | 3:1 | 425 | 15 | 10,000 | 20.8 |
| Czekajło et al. [140] | ZBRW-10 | 2.18Al2O3 + 0.44K2O + 1.3CaO + 2.01CoO2 | 3:1 | 475 | 10 | 20,000 | 10.8 |
| Jafari et al. [141] | Wustite | 65.7O + 30.62Fe + 2.4Al + 0.31K + 1Ca | 3:1 | 350–530 | 3 | ||
| Jafari et al. [141] | Magnetite | 65.7O + 30.24Fe + 3.3Al + 0.23K + 0.54Ca | 3:1 | 350–530 | 3 | ||
| Kobayashi et al. [142] | Ru/BaTiO2.5H0.5 | 0.9Ru | 3:1 | 400 | 5 | 0.17 | |
| Han et al. [143] | Ru/La2Ce2O7 | 4Ru | 3:1 | 425 | 10 | 10,000 | 12.94 |
| Lin et al. [144] | Ba-Ru/AC-G | 9Ba | 3:1 | 400 | 10 | ||
| Karolewska et al. [145] | (Co-Ce)-Ba/C | 9.34Co | 3:1 | 400 | 9 | ||
| Zybert et al. [146] | Co/Ba(CP) | 3:1 | 400 | 6.3 | 70,000 | ||
| Ye et al. [147] | Ni/CeN NPs | 10Ni | 3:1 | 400 | 0.1 | 36,000 |
| Power-to-Hydrogen | Power-to-Methanol | Power-to-Ammonia | |||
|---|---|---|---|---|---|
| ALK | PEM | SOEC | |||
| Cost | CNY 5437~9425/kW | CNY 14,500/kW | CNY 25,375/kW | CNY 7.91~15.81/kgCH3OH | CNY 7.12/kgNH3 |
| Project Name | Location | Scale | Status |
|---|---|---|---|
| Guyuan Wind Energy Hydrogen Production Industrial Application Project | Guyuan, Hebei | 200 MW wind 10 MW H2 | 2022 start operation |
| Kuqa Green Hydrogen Pilot Project | Kuqa, Xinjiang | 200 MW solar 20,000 t/a H2 | 2023.06 the first phase start operation |
| Ordos Green Hydrogen Pilot Project | Ordos, Inner Mongolia | 450 MW wind 250 MW solar 30,000 t/a H2 | Under construction |
| Lanzhou Liquid Solar Fuel Production Demonstration Project | Lanzhou, Gansu | 10 MW solar 1000 t/a CH3OH | 2020.01 start operation |
| The Da’an Wind and Solar Green Hydrogen Synthesis Ammonia Integration Demonstration Project | Da’an, Jilin | 700 MW wind power 100 MW solar 32,000 t/a H2 180,000 t/a NH3 | Under construction |
| Songwon Hydrogen Energy Industrial Park Project | Songyuan, Jilin | 800 MW wind power 100 MW solar 45,000 t/a H2 200,000 t/a NH3 20,000 t/a CH3OH | Under construction |
| Liaoyuan Power-to-X Project | Liaoyuan, Jilin | 1.4 GW wind power 0.4 GW solar 100,000 t/a H2 38,000 t/a NH3 620,000 t/a CH3OH | Under construction |
| Qianguo Power-to-X Project | Songyuan, Jilin | 1.3 GW wind power 20,000 t/a NH3 400,000 t/a CH3OH | Planning |
| Xingan League Energy Carbon- zero Hydrogen Production Project | Ulanhot, Inner Mongolia | 1.25 GW wind power 56,200 t/a H2 300,000 t/a NH3 | Planning |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; He, Y.; Shao, J.; Zhang, W.; Tong, X.; Wang, Z.; Weng, W. Review of Hydrogen-Driven Power-to-X Technology and Application Status in China. Processes 2024, 12, 1518. https://doi.org/10.3390/pr12071518
Zhai Y, He Y, Shao J, Zhang W, Tong X, Wang Z, Weng W. Review of Hydrogen-Driven Power-to-X Technology and Application Status in China. Processes. 2024; 12(7):1518. https://doi.org/10.3390/pr12071518
Chicago/Turabian StyleZhai, Yunchu, Yong He, Jiaming Shao, Weiling Zhang, Xiaofan Tong, Zhihua Wang, and Wubin Weng. 2024. "Review of Hydrogen-Driven Power-to-X Technology and Application Status in China" Processes 12, no. 7: 1518. https://doi.org/10.3390/pr12071518
APA StyleZhai, Y., He, Y., Shao, J., Zhang, W., Tong, X., Wang, Z., & Weng, W. (2024). Review of Hydrogen-Driven Power-to-X Technology and Application Status in China. Processes, 12(7), 1518. https://doi.org/10.3390/pr12071518








