Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis
Abstract
1. Introduction
2. Materials and Fabrications
2.1. Biodegradable Materials
2.1.1. Metals and Inorganic Materials
2.1.2. Organic Materials
2.2. Nanomaterials
2.3. Fabrications
3. Recent Developments in Implantable and Semi-Implantable Biosensing
3.1. Chemical and Biomarker Sensors
3.1.1. Glucose Sensors
3.1.2. Sensors for Other Chemicals and Biomarkers
3.2. pH Sensors
3.3. Temperature and Thermal Sensors
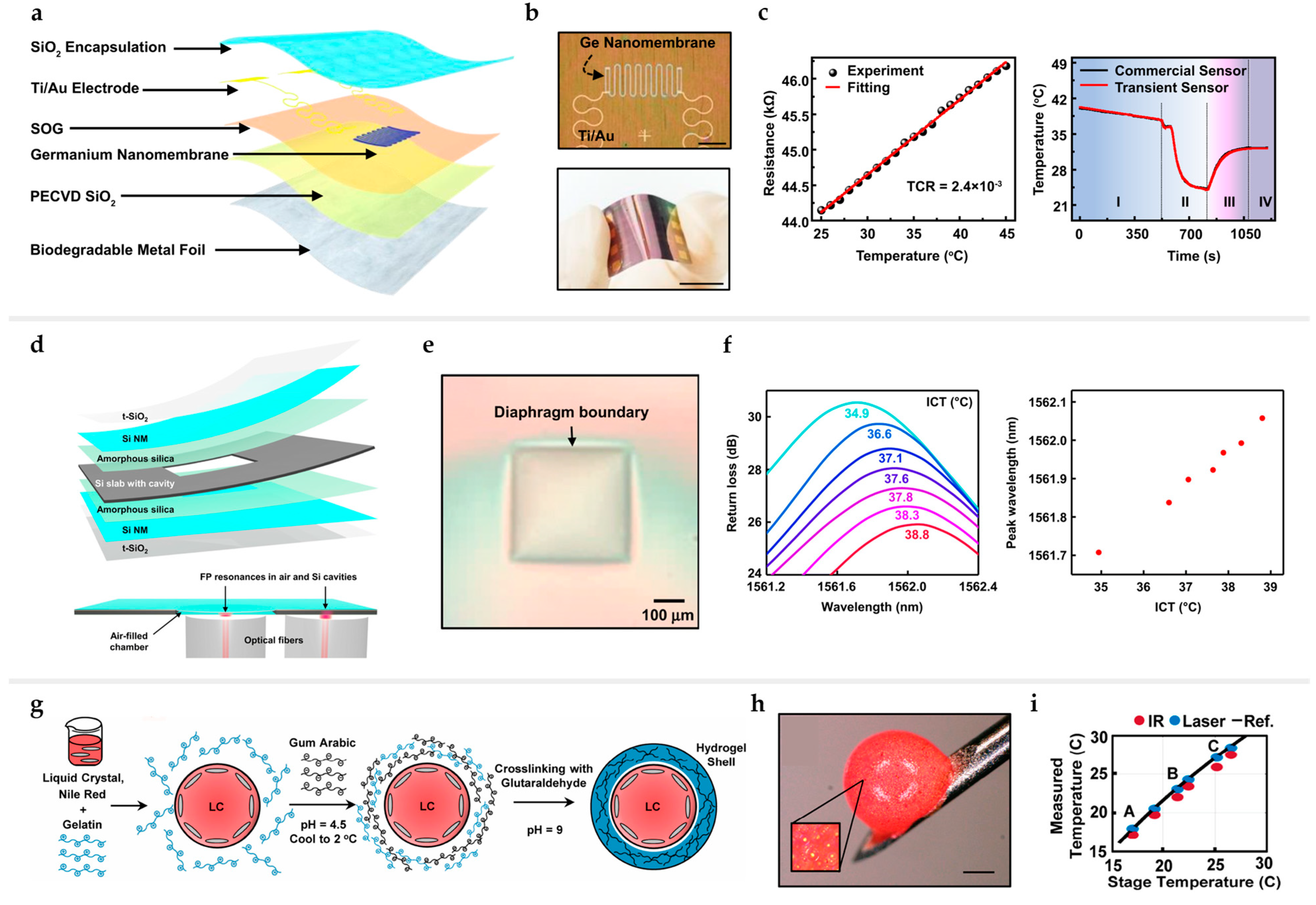
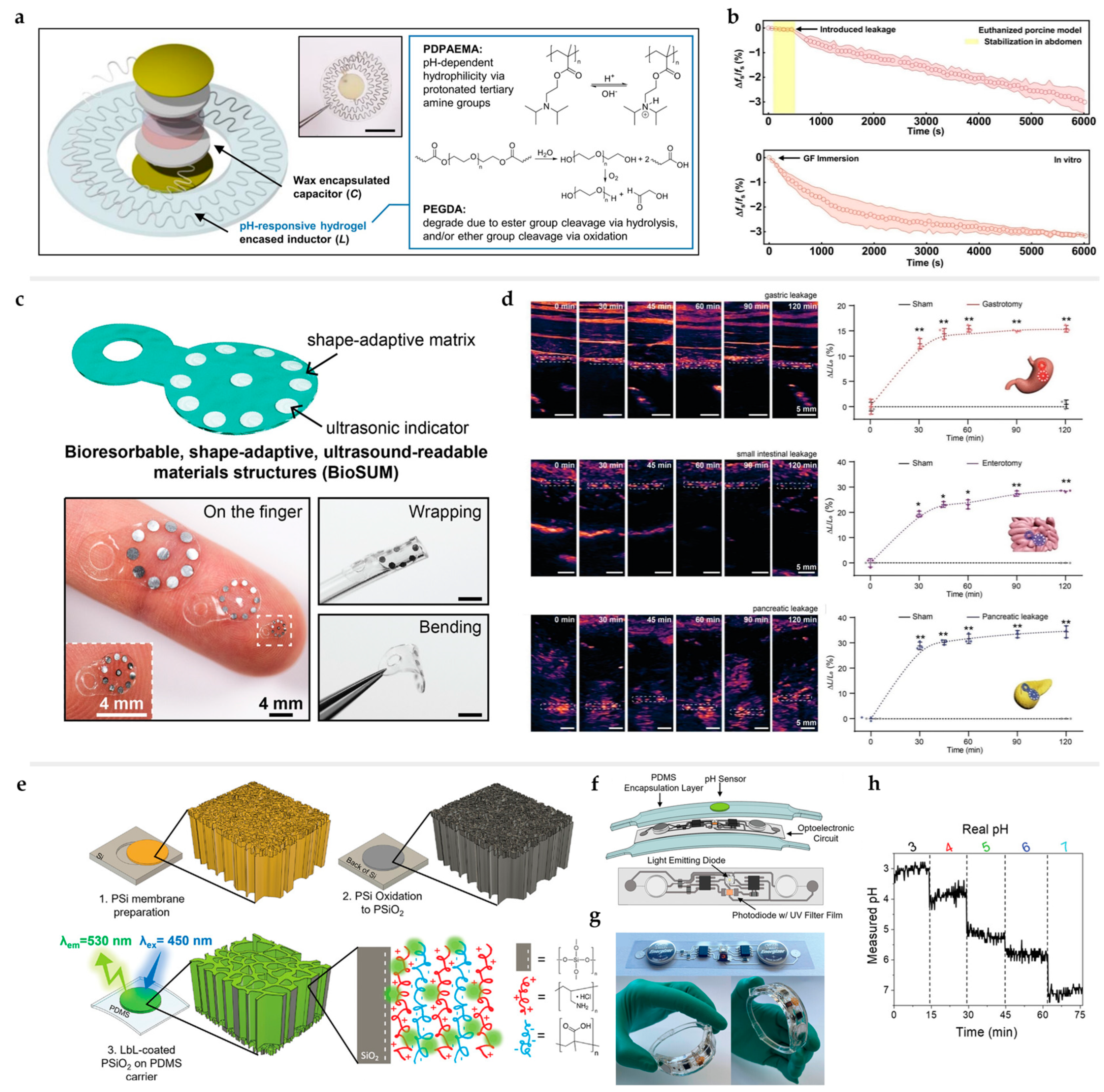
3.4. Pressure and Strain Sensors
3.5. Sensors for Nervous System Diagnosis
4. Challenge and Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| Ag | silver |
| AJP | aerosol jet printing |
| Au | gold |
| BGF | biodegradable glucose-responsive fluorescent monomer |
| BPS | biodegradable piezoelectric sensors |
| BTS | biodegradable triboelectric sensors |
| Ca | calcium |
| CE | counter electrodes |
| CLC | cholesteric liquid crystal |
| CT | computed tomography |
| DBR | distributed Bragg reflector |
| DMAEMA | 2-(dimethylamino)ethyl methacrylate |
| DPAEMA | 2-(diisopropylamino)ethyl methacrylate |
| ECoG | electrocorticogram |
| FPI | Fabry–Pérot interferometer |
| GelMA | gelatin methacrylate |
| GF | gauge factor |
| GluOx | glutamate oxidase |
| GO | graphene oxide |
| GOx | glucose oxidase |
| H2O2 | hydrogen peroxide |
| Hb | deoxyhemoglobin |
| HbO2 | oxyhemoglobin |
| HEDN | highly entangled double network |
| HPU | hydrophilic polyurethanes |
| ICP | intracranial pressure |
| IrO2 | iridium(IV) oxide |
| LED | light-emitting diode |
| MBM | microencapsulated biomarkers |
| Mg | magnesium |
| Mo | molybdenum |
| MRI | magnetic resonance imaging |
| mPD | 1,3-phenylenediamine |
| m-Si | monocrystalline silicon |
| MWCNT | multiwalled carbon nanotube |
| NM | nanomembrane |
| NP | nanoparticle |
| nPSi | nanostructured porous silicon |
| NR | nanorod |
| NT | nanotube |
| OE | organic electrodes |
| OECT | organic electrochemical transistors |
| PA | phytic acid |
| PAA | polyacrylic acid |
| PAH | poly-allylamine-hydrochloride |
| PANI | polyaniline |
| PCL | poly(ɛ-caprolactone) |
| PD | photodetector |
| PdBMAP | Pd (II) tetramethacrylated benzoporphyrin |
| PDMS | polydimethylsiloxane |
| PDO | polydioxanone |
| PDTEC | poly(desaminotyrosyl-tyrosine ethyl ester carbonate) |
| PEDOT:PSS | poly(3,4-ethylene dioxythiophene) polystyrene sulfonate |
| PEG | polyethylene glycol |
| PEGDA | poly(ethylene glycol)diacrylate |
| PEG-LA-DA | polyethylene glycol-lactide acid diacrylate |
| PGA | polyglycolide |
| PGCL | poly(glycolide-co-ε-caprolactone) |
| PGS | poly(glycerol-co-sebacate) |
| PLA | poly(lactic acid) |
| PLGA | poly lactic-co-glycolic acid |
| PLLA | poly-l-lactic acid |
| PMAA | poly-methacrylic-acid |
| PPMM | polypropylene micromembrane |
| Pt | platinum |
| PVA | polyvinyl alcohol |
| QD | quantum dot |
| RE | reference electrodes |
| Rh | Rhodamine-B |
| SA | sodium alginate |
| Si | silicon |
| SPCE | screen-printed carbon electrodes |
| SPRi | surface plasmon resonance image |
| StO2 | oxygen saturation |
| TCR | temperature coefficient of resistance |
| VEGF | vascular endothelial growth factor |
| W | tungsten |
| WHO | World Health Organization |
| Zn | zinc |
References
- Ayres, J.S. The Biology of Physiological Health. Cell 2020, 181, 250–269. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-C.; Lee, A.-R. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015, 23, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.; Cho, M.; Lim, S.; Min, I.S.; Han, Y.; Lee, C.; Shin, J.; Yoon, K.; Yeo, W.-H.; Lee, T.; et al. Fluorescent-based biodegradable microneedle sensor array for tether-free continuous glucose monitoring with smartphone application. Sci. Adv. 2023, 9, eadh1765. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, J.; Wu, Z.; Shang, X.; Li, Y.; Huo, W.; Huang, X. Fully printed and self-compensated bioresorbable electrochemical devices based on galvanic coupling for continuous glucose monitoring. Sci. Adv. 2023, 9, eadi3839. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Bai, W.; Ouyang, W.; Liu, Y.; Wu, C.; Xu, Y.; Weng, Y.; Zang, H.; Liu, Y.; Jacobson, L.; et al. Wireless implantable optical probe for continuous monitoring of oxygen saturation in flaps and organ grafts. Nat. Commun. 2022, 13, 3009. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.; Shin, J.; Fu, R.; Kandela, I.; Lu, D.; Ni, X.; Park, Y.; Liu, Z.; Hang, T.; Wu, D.; et al. Bioresorbable photonic devices for the spectroscopic characterization of physiological status and neural activity. Nat. Biomed. Eng. 2019, 3, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch. Comput. Methods Eng. 2020, 27, 1187–1230. [Google Scholar] [CrossRef]
- Hunt, K.D.; O‘Loughlin, V.D.; Fitting, D.W.; Adler, L. Ultrasonic determination of the elastic modulus of human cortical bone. Med. Biol. Eng. Comput. 1998, 36, 51–56. [Google Scholar] [CrossRef]
- Liu, J.; Liu, N.; Xu, Y.; Wu, M.; Zhang, H.; Wang, Y.; Yan, Y.; Hill, A.; Song, R.; Xu, Z.; et al. Bioresorbable shape-adaptive structures for ultrasonic monitoring of deep-tissue homeostasis. Science 2024, 383, 1096–1103. [Google Scholar] [CrossRef]
- Hormuzdi, S.G.; Filippov, M.A.; Mitropoulou, G.; Monyer, H.; Bruzzone, R. Electrical synapses: A dynamic signaling system that shapes the activity of neuronal networks. Biochim. Biophys. Acta 2004, 1662, 113–137. [Google Scholar] [CrossRef]
- Kandel, E.R.; Schwartz, J.H.; Jessell, T.M. Principles of Neural Science; McGraw-Hill Professional: New York, NY, USA, 2000. [Google Scholar]
- Feiner, R.; Dvir, T. Tissue–electronics interfaces: From implantable devices to engineered tissues. Nat. Rev. Mater. 2017, 3, 17076. [Google Scholar] [CrossRef]
- Mistral, J.; Ve Koon, K.T.; Fernando Cotica, L.; Sanguino Dias, G.; Aparecido Santos, I.; Alcouffe, P.; Milhau, N.; Pin, D.; Chapet, O.; Serghei, A.; et al. Chitosan-Coated Superparamagnetic Fe3O4 Nanoparticles for Magnetic Resonance Imaging, Magnetic Hyperthermia, and Drug Delivery. ACS Appl. Nano Mater. 2024, 7, 7097–7110. [Google Scholar] [CrossRef]
- Handali, P.R.; Webb, L.J. Quantifying Bound Proteins on Pegylated Gold Nanoparticles Using Infrared Spectroscopy. ACS Appl. Bio Mater. 2024, 7, 2338–2345. [Google Scholar] [CrossRef] [PubMed]
- Mamidi, N.; Delgadillo, R.M.V.; González-Ortiz, A. Engineering of carbon nano-onion bioconjugates for biomedical applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 120, 11. [Google Scholar] [CrossRef] [PubMed]
- Herbert, R.; Elsisy, M.; Rigo, B.; Lim, H.-R.; Kim, H.; Choi, C.; Kim, S.; Ye, S.-H.; Wagner, W.R.; Chun, Y.; et al. Fully implantable batteryless soft platforms with printed nanomaterial-based arterial stiffness sensors for wireless continuous monitoring of restenosis in real time. Nano Today 2022, 46, 101557. [Google Scholar] [CrossRef] [PubMed]
- O‘Brien, D.J.; Mills, D.; Farina, J.; Paranjape, M. A Needle-Free Transdermal Patch for Sampling Interstitial Fluid. IEEE Trans. Biomed. Eng. 2023, 70, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Schartner, E.; Musolino, S.; Quirk, B.C.; Kirk, R.W.; Ebendorff-Heidepriem, H.; McLaughlin, R.A. Miniaturized single-fiber-based needle probe for combined imaging and sensing in deep tissue. Opt. Lett. 2018, 43, 1682–1685. [Google Scholar] [CrossRef]
- Hu, C.; Wang, L.; Liu, S.; Sheng, X.; Yin, L. Recent Development of Implantable Chemical Sensors Utilizing Flexible and Biodegradable Materials for Biomedical Applications. ACS Nano 2024, 18, 3969–3995. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Xue, Z.; Wu, X.; Wei, Z.; Guo, Q.; Xu, M.; Qu, C.; You, C.; Mei, Y.; Zhang, M.; et al. Biodegradable germanium electronics for integrated biosensing of physiological signals. npj Flex. Electron. 2022, 6, 63. [Google Scholar] [CrossRef]
- Ryu, H.; Seo, M.-H.; Rogers, J.A. Bioresorbable Metals for Biomedical Applications: From Mechanical Components to Electronic Devices. Adv. Healthc. Mater. 2021, 10, 2002236. [Google Scholar] [CrossRef]
- Kang, S.-K.; Koo, J.; Lee, Y.K.; Rogers, J.A. Advanced Materials and Devices for Bioresorbable Electronics. Acc. Chem. Res. 2018, 51, 988–998. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, H.; Mao, S.; Haasch, R.; Liu, Y.; Xie, X.; Hwang, S.-W.; Jain, H.; Kang, S.-K.; Su, Y.; et al. Dissolvable Metals for Transient Electronics. Adv. Funct. Mater. 2014, 24, 645–658. [Google Scholar] [CrossRef]
- Kang, S.-K.; Park, G.; Kim, K.; Hwang, S.-W.; Cheng, H.; Shin, J.; Chung, S.; Kim, M.; Yin, L.; Lee, J.C.; et al. Dissolution Chemistry and Biocompatibility of Silicon- and Germanium-Based Semiconductors for Transient Electronics. ACS Appl. Mater. Interfaces 2015, 7, 9297–9305. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Choi, Y.; Koo, J.; Rogers, J.A. Inorganic materials for transient electronics in biomedical applications. MRS Bull. 2020, 45, 103–112. [Google Scholar] [CrossRef]
- Hosseini, E.S.; Dervin, S.; Ganguly, P.; Dahiya, R. Biodegradable Materials for Sustainable Health Monitoring Devices. ACS Appl. Bio Mater. 2021, 4, 163–194. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, D.; Yuan, Z.; Xie, W.; Wu, Y.; Li, R.; Zhao, Y.; Luo, D.; Cen, L.; Chen, B.; et al. A Fully Biodegradable Battery for Self-Powered Transient Implants. Small 2018, 14, 1800994. [Google Scholar] [CrossRef]
- Kim, H.-S.; Yang, S.M.; Jang, T.-M.; Oh, N.; Kim, H.-S.; Hwang, S.-W. Bioresorbable Silicon Nanomembranes and Iron Catalyst Nanoparticles for Flexible, Transient Electrochemical Dopamine Monitors. Adv. Healthc. Mater. 2018, 7, 1801071. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qi, H.; Ma, Y.; Deng, Y.; Liu, S.; Jie, Y.; Jing, J.; He, J.; Zhang, X.; Wheatley, L.; et al. A flexible and physically transient electrochemical sensor for real-time wireless nitric oxide monitoring. Nat. Commun. 2020, 11, 3207. [Google Scholar] [CrossRef]
- Fernandes, C.; Loukopoulos, V.; Smets, J.; Franceschini, F.; Deschaume, O.; Bartic, C.; Ameloot, R.; Ustarroz, J.; Taurino, I. Unraveling the Potential of a Nanostructured Tungsten–Tungsten Oxide Thin Film Electrode as a Bioresorbable Multichemical Wound Healing Monitor. Adv. Mater. Technol. 2024, 9, 2302007. [Google Scholar] [CrossRef]
- Park, J.-H.; Gu, L.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Biodegradable luminescent porous silicon nanoparticles for in vivo applications. Nat. Mater. 2009, 8, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kim, D.-J.; Jang, T.-M.; Han, W.B.; Lee, J.H.; Ko, G.-J.; Yang, S.M.; Rajaram, K.; Han, S.; Kang, H.; et al. Highly Elastic, Bioresorbable Polymeric Materials for Stretchable, Transient Electronic Systems. Nano-Micro Lett. 2024, 16, 102. [Google Scholar] [CrossRef] [PubMed]
- Bezwada, R.S.; Jamiolkowski, D.D.; Lee, I.-Y.; Agarwal, V.; Persivale, J.; Trenka-Benthin, S.; Erneta, M.; Suryadevara, J.; Yang, A.; Liu, S. Monocryl® suture, a new ultra-pliable absorbable monofilament suture. Biomaterials 1995, 16, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, B.S.; Kim, S.H.; Choi, S.W.; Jeong, S.I.; Kwon, I.K.; Kang, S.W.; Nikolovski, J.; Mooney, D.J.; Han, Y.K.; et al. Elastic biodegradable poly (glycolide-co-caprolactone) scaffold for tissue engineering. J. Biomed. Mater. Res. Part A 2003, 66, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Yoon, H.; Kwak, Y.J.; Wang, C.; Alzahrani, K.M.; Wang, S.; Alzahrani, F.D.H.; Kim, H.M.; Koo, E.; Yoo, J.E. Feasibility and safety of inserting transient biodegradable stents in the pylorus during pylorus-preserving gastrectomy for gastric cancer: A preliminary study in a porcine for proof of concept. Gastric Cancer 2023, 26, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Concagh, D.; Core, L.; Kuang, Y.; You, C.; Pham, Q.; Zugates, G.; Busold, R.; Webber, S.; Merlo, J.; et al. The development of bioresorbable composite polymeric implants with high mechanical strength. Nat. Mater. 2018, 17, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Feili, D.; Schuettler, M.; Doerge, T.; Kammer, S.; Stieglitz, T. Encapsulation of organic field effect transistors for flexible biomedical microimplants. Sens. Actuators A Phys. 2005, 120, 101–109. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.; Choi, Y. Organic encapsulants for bioresorbable medical electronics. Mrs Bull. 2024, 49, 247–255. [Google Scholar] [CrossRef]
- Lim, D.; Hong, I.; Park, S.U.; Chae, J.W.; Lee, S.; Baac, H.W.; Shin, C.; Lee, J.; Roh, Y.; Im, C.; et al. Functional Encapsulating Structure for Wireless and Immediate Monitoring of the Fluid Penetration. Adv. Funct. Mater. 2022, 32, 202201854. [Google Scholar] [CrossRef]
- Fumeaux, N.; Almeida, C.P.; Demuru, S.; Briand, D. Organic electrochemical transistors printed from degradable materials as disposable biochemical sensors. Sci. Rep. 2023, 13, 11467. [Google Scholar] [CrossRef]
- Xu, M.; Jiang, Y.; Pradhan, S.; Yadavalli, V.K. Use of Silk Proteins to Form Organic, Flexible, Degradable Biosensors for Metabolite Monitoring. Front. Mater. 2019, 6, 331. [Google Scholar] [CrossRef]
- Gao, H.N.; Zhang, J.H.; Yu, W.L.; Li, Y.F.; Zhu, S.J.; Li, Y.; Wang, T.Q.; Yang, B. Monolithic polyaniline/polyvinyl alcohol nanocomposite actuators with tunable stimuli-responsive properties. Sens. Actuators B Chem. 2010, 145, 839–846. [Google Scholar] [CrossRef]
- Altin-Yavuzarslan, G.; Sadaba, N.; Brooks, S.M.; Alper, H.S.; Nelson, A. Engineered Living Material Bioreactors with Tunable Mechanical Properties using Vat Photopolymerization. Small 2023, 20, e2306564. [Google Scholar] [CrossRef]
- Guo, B.C.; Chen, Y.W.; Lei, Y.D.; Zhang, L.Q.; Zhou, W.Y.; Rabie, A.B.M.; Zhao, J.Q. Biobased Poly(propylene sebacate) as Shape Memory Polymer with Tunable Switching Temperature for Potential Biomedical Applications. Biomacromolecules 2011, 12, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Lang, W.Y.; Huang, H.; Yang, L.; Luo, R.F.; Wang, Y.B.; Xue, B.; Yang, S.G. Polymer Complex Multilayers for Drug Delivery and Medical Devices. ACS Appl. Bio Mater. 2023, 6, 3555–3565. [Google Scholar] [CrossRef] [PubMed]
- Hearon, K.; Wierzbicki, M.A.; Nash, L.D.; Landsman, T.L.; Laramy, C.; Lonnecker, A.T.; Gibbons, M.C.; Ur, S.; Cardinal, K.O.; Wilson, T.S.; et al. A Processable Shape Memory Polymer System for Biomedical Applications. Adv. Healthc. Mater. 2015, 4, 1386–1398. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, I.; De Maria, C.; Ceccarini, M.R.; Mussolin, L.; Coletta, R.; Morabito, A.; Tonin, R.; Calamai, M.; Morrone, A.; Beccari, T.; et al. 3D Printing Silk-Based Bioresorbable Piezoelectric Self-Adhesive Holey Structures for In Vivo Monitoring on Soft Tissues. ACS Appl. Mater. Interfaces 2022, 14, 19253–19264. [Google Scholar] [CrossRef] [PubMed]
- Molinnus, D.; Janus, K.A.; Fang, A.C.; Drinic, A.; Achtsnicht, S.; Köpf, M.; Keusgen, M.; Schöning, M.J. Thick-Film Carbon Electrode Deposited onto a Biodegradable Fibroin Substrate for Biosensing Applications. Phys. Status Solidi 2022, 219, 2200100. [Google Scholar] [CrossRef]
- Presley, K.F.; Falcucci, T.; Shaidani, S.; Fitzpatrick, V.; Barry, J.; Ly, J.T.; Dalton, M.J.; Grusenmeyer, T.A.; Kaplan, D.L. Engineered porosity for tissue-integrating, bioresorbable lifetime-based phosphorescent oxygen sensors. Biomaterials 2023, 301, 122286. [Google Scholar] [CrossRef]
- Kim, H.-J.; Kim, J.-H.; Jun, K.-W.; Kim, J.-H.; Seung, W.-C.; Kwon, O.H.; Park, J.-Y.; Kim, S.-W.; Oh, I.-K. Silk Nanofiber-Networked Bio-Triboelectric Generator: Silk Bio-TEG. Adv. Energy Mater. 2016, 6, 1502329. [Google Scholar] [CrossRef]
- Kaveti, R.; Lee, J.H.; Youn, J.K.; Jang, T.-M.; Han, W.B.; Yang, S.M.; Shin, J.-W.; Ko, G.-J.; Kim, D.-J.; Han, S.; et al. Soft, Long-Lived, Bioresorbable Electronic Surgical Mesh with Wireless Pressure Monitor and On-Demand Drug Delivery. Adv. Mater. 2024, 36, 2307391. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.-S.; Rogers, J.A.; Kang, S.-K. Physically transient electronic materials and devices. Mater. Sci. Eng. R Rep. 2021, 145, 100624. [Google Scholar] [CrossRef]
- Xie, S.; Ren, B.; Gong, G.; Zhang, D.; Chen, Y.; Xu, L.; Zhang, C.; Xu, J.; Zheng, J. Lanthanide-Doped Upconversion Nanoparticle-Cross-Linked Double-Network Hydrogels with Strong Bulk/Interfacial Toughness and Tunable Full-Color Fluorescence for Bioimaging and Biosensing. ACS Appl. Nano Mater. 2020, 3, 2774–2786. [Google Scholar] [CrossRef]
- Cao, Y.; Uhrich, K.E. Biodegradable and biocompatible polymers for electronic applications: A review. J. Bioact. Compat. Polym. 2019, 34, 3–15. [Google Scholar] [CrossRef]
- Falcucci, T.; Presley, K.F.; Choi, J.; Fizpatrick, V.; Barry, J.; Kishore Sahoo, J.; Ly, J.T.; Grusenmeyer, T.A.; Dalton, M.J.; Kaplan, D.L. Degradable Silk-Based Subcutaneous Oxygen Sensors. Adv. Funct. Mater. 2022, 32, 2202020. [Google Scholar] [CrossRef]
- Chae, J.W.; Lee, D.; Osman, A.; Kang, B.; Hwang, J.; Kim, W.; Kim, D.; Lee, W.H.; Won, S.M. Silk Fibroin, Sericin, and Conductive Silk Composites for Skin-Attachable Transient Electronics. ACS Appl. Electron. Mater. 2024, 6, 1746–1756. [Google Scholar] [CrossRef]
- Ko, G.-J.; Han, S.D.; Kim, J.-K.; Zhu, J.; Han, W.B.; Chung, J.; Yang, S.M.; Cheng, H.; Kim, D.-H.; Kang, C.-Y.; et al. Biodegradable, flexible silicon nanomembrane-based NOx gas sensor system with record-high performance for transient environmental monitors and medical implants. NPG Asia Mater. 2020, 12, 71. [Google Scholar] [CrossRef]
- Soomro, A.M.; Jabbar, F.; Ali, M.; Lee, J.-W.; Mun, S.W.; Choi, K.H. All-range flexible and biocompatible humidity sensor based on poly lactic glycolic acid (PLGA) and its application in human breathing for wearable health monitoring. J. Mater. Sci. Mater. Electron. 2019, 30, 9455–9465. [Google Scholar] [CrossRef]
- Ma, X.; Hu, Q.; Dai, Y.; He, P.; Zhang, X. Disposable sensors based on biodegradable polylactic acid piezoelectret films and their application in wearable electronics. Sens. Actuators A Phys. 2022, 346, 113834. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, P.; Yang, G.; Lei, H. Single Polylactic Acid Nanowire for Highly Sensitive and Multifunctional Optical Biosensing. ACS Appl. Mater. Interfaces 2021, 13, 27983–27990. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, Y.M.; Langer, R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res. Part A 2003, 66A, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Zanjanizadeh Ezazi, N.; Ajdary, R.; Correia, A.; Mäkilä, E.; Salonen, J.; Kemell, M.; Hirvonen, J.; Rojas, O.J.; Ruskoaho, H.J.; Santos, H.A. Fabrication and Characterization of Drug-Loaded Conductive Poly(glycerol sebacate)/Nanoparticle-Based Composite Patch for Myocardial Infarction Applications. ACS Appl. Mater. Interfaces 2020, 12, 6899–6909. [Google Scholar] [CrossRef] [PubMed]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Park, S.C.; Kim, H.K.; Koh, Y.J.; Lee, J.-H.; Lee, M.C.; Lee, J.H. Degradation Behavior of 3D Porous Polydioxanone-b-Polycaprolactone Scaffolds Fabricated Using the Melt-Molding Particulate-Leaching Method. J. Biomater. Sci. Polym. Ed. 2011, 22, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Soto, R.J.; Hall, J.R.; Brown, M.D.; Taylor, J.B.; Schoenfisch, M.H. In Vivo Chemical Sensors: Role of Biocompatibility on Performance and Utility. Anal. Chem. 2017, 89, 276–299. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, B.; Xi, Z.; Zhao, L.; Cen, L.; Yang, Y. A comparable study of polyglycolic acid’s degradation on macrophages’ activation. Mater. Sci. Eng. C 2020, 109, 110574. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, H.; Yasuzawa, M. Fabrication of an Implantable Fine Needle-Type Glucose Sensor Using γ-Polyglutamic Acid. Anal. Sci. 2010, 26, 551–555. [Google Scholar] [CrossRef][Green Version]
- Özcan, B.; Sezgintürk, M.K. A novel and disposable GP- based impedimetric biosensor using electropolymerization process with PGA for highly sensitive determination of leptin: Early diagnosis of childhood obesity. Talanta 2021, 225, 121985. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Lee, C.H.; Cheng, H.; Jeong, J.-W.; Kang, S.-K.; Kim, J.-H.; Shin, J.; Yang, J.; Liu, Z.; Ameer, G.A.; et al. Biodegradable Elastomers and Silicon Nanomembranes/Nanoribbons for Stretchable, Transient Electronics, and Biosensors. Nano Lett. 2015, 15, 2801–2808. [Google Scholar] [CrossRef]
- Beyene, A.G.; Demirer, G.S.; Landry, M.P. Nanoparticle-Templated Molecular Recognition Platforms for Detection of Biological Analytes. Curr. Protoc. Chem. Biol. 2016, 8, 197–223. [Google Scholar] [CrossRef]
- Piacenti da Silva, M.; Fernandes, J.C.; de Figueiredo, N.B.; Congiu, M.; Mulato, M.; de Oliveira Graeff, C.F. Melanin as an active layer in biosensors. AIP Adv. 2014, 4, 037120. [Google Scholar] [CrossRef]
- Ozlu, B.; Shim, B.S. Highly Conductive Melanin-like Polymer Composites for Nonenzymatic Glucose Biosensors with a Wide Detection Range. ACS Appl. Polym. Mater. 2022, 4, 2527–2535. [Google Scholar] [CrossRef]
- Lin, S.; Yuk, H.; Zhang, T.; Parada, G.A.; Koo, H.; Yu, C.; Zhao, X. Stretchable Hydrogel Electronics and Devices. Adv. Mater. 2016, 28, 4497–4505. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Zhou, H.; Li, W.; Chen, M.; Zhou, M.; Zhao, L. An Environment-Tolerant Ion-Conducting Double-Network Composite Hydrogel for High-Performance Flexible Electronic Devices. Nano-Micro Lett. 2024, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, Z.; Shi, D.; Zhou, T.; Kaneko, D.; Chen, M. High strength and toughness of double physically cross-linked hydrogels composed of polyvinyl alcohol and calcium alginate. J. Appl. Polym. Sci. 2021, 138, 49987. [Google Scholar] [CrossRef]
- Gong, J.P.; Katsuyama, Y.; Kurokawa, T.; Osada, Y. Double-Network Hydrogels with Extremely High Mechanical Strength. Adv. Mater. 2003, 15, 1155–1158. [Google Scholar] [CrossRef]
- Sun, J.Y.; Zhao, X.; Illeperuma, W.R.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, Y.; Cao, Y.; Xue, B. Tough Hydrogels with Different Toughening Mechanisms and Applications. Int. J. Mol. Sci. 2024, 25, 2675. [Google Scholar] [CrossRef]
- Zhao, X. Multi-scale multi-mechanism design of tough hydrogels: Building dissipation into stretchy networks. Soft Matter 2014, 10, 672–687. [Google Scholar] [CrossRef]
- Zhu, R.; Zhu, D.; Zheng, Z.; Wang, X. Tough double network hydrogels with rapid self-reinforcement and low hysteresis based on highly entangled networks. Nat. Commun. 2024, 15, 1344. [Google Scholar] [CrossRef]
- Yang, Q.; Wei, T.; Yin, R.T.; Wu, M.; Xu, Y.; Koo, J.; Choi, Y.S.; Xie, Z.; Chen, S.W.; Kandela, I.; et al. Photocurable bioresorbable adhesives as functional interfaces between flexible bioelectronic devices and soft biological tissues. Nat. Mater. 2021, 20, 1559–1570. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Park, J.; Lee, J.Y. Conductive double-network hydrogel composed of sodium alginate, polyacrylamide, and reduced graphene oxide. Korean J. Chem. Eng. 2023, 40, 352–360. [Google Scholar] [CrossRef]
- Zhang, X.; Deng, J.-N.; Yang, K.; Li, Q.; Meng, S.-Y.; Sun, X.-X.; Song, Z.-Z.; Tian, Y.-D.; Zhang, S.-A.; Liu, X.-J.; et al. High-strength and self-degradable sodium alginate/polyacrylamide preformed particle gels for conformance control to enhance oil recovery. Pet. Sci. 2022, 19, 3149–3158. [Google Scholar] [CrossRef]
- Cao, Q.; Shu, Z.; Zhang, T.; Ji, W.; Chen, J.; Wei, Y. Highly Elastic, Sensitive, Stretchable, and Skin-Inspired Conductive Sodium Alginate/Polyacrylamide/Gallium Composite Hydrogel with Toughness as a Flexible Strain Sensor. Biomacromolecules 2022, 23, 2603–2613. [Google Scholar] [CrossRef] [PubMed]
- Darvishi, S.; Souissi, M.; Kharaziha, M.; Karimzadeh, F.; Sahara, R.; Ahadian, S. Gelatin methacryloyl hydrogel for glucose biosensing using Ni nanoparticles-reduced graphene oxide: An experimental and modeling study. Electrochim. Acta 2018, 261, 275–283. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, Y.; Ferracci, G.; Zheng, J.; Cho, N.-J.; Lee, B.H. Gelatin methacryloyl and its hydrogels with an exceptional degree of controllability and batch-to-batch consistency. Sci. Rep. 2019, 9, 6863. [Google Scholar] [CrossRef] [PubMed]
- Naficy, S.; Oveissi, F.; Patrick, B.; Schindeler, A.; Dehghani, F. Printed, Flexible pH Sensor Hydrogels for Wet Environments. Adv. Mater. Technol. 2018, 3, 1800137. [Google Scholar] [CrossRef]
- Teo, M.Y.; Lim, K.; Aw, K.C.; Kee, S.; Stringer, J. Towards biodegradable conducting polymers by incorporating seaweed cellulose for decomposable wearable heaters. RSC Adv. 2023, 13, 26267–26274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bing, Y.; Chen, Q.; Pang, B.; Li, J.; Zhang, T. Nonenzymatic Flexible Wearable Biosensors for Vitamin C Monitoring in Sweat. ACS Appl. Mater. Interfaces 2023, 15, 19384–19392. [Google Scholar] [CrossRef]
- Ballabio, M.; Zhang, T.; Chen, C.; Zhang, P.; Liao, Z.; Hambsch, M.; Mannsfeld, S.C.B.; Zschech, E.; Sirringhaus, H.; Feng, X.; et al. Band-Like Charge Transport in Phytic Acid-Doped Polyaniline Thin Films. Adv. Funct. Mater. 2021, 31, 2105184. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Xu, H.; Huang, C.; Lu, Y.; Cui, H.; Tan, Y. Chitosan-driven biocompatible hydrogel based on water-soluble polypyrrole for stable human-machine interfaces. Carbohydr. Polym. 2022, 295, 119890. [Google Scholar] [CrossRef]
- Mill, T.; Mabey, W. Hydrolysis of Organic Chemicals. In Reactions and Processes; Barraclough, P.B., Crossland, N.O., Herrmann, R., Mabey, W., Menzie, C.M., Mill, T., Tinker, P.B., Waldichuk, M., Wolff, C.J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 1988; pp. 71–111. [Google Scholar]
- Mabey, W.; Mill, T. Critical review of hydrolysis of organic compounds in water under environmental conditions. J. Phys. Chem. Ref. Data 1978, 7, 383–415. [Google Scholar] [CrossRef]
- Verma, H.; Aggarwal, M.; Kumar, S. Opportunities and Significance of Nanoparticle-DNA Binding in Medical Biotechnology: A Review. Cureus 2022, 14, e31005. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef]
- Lin, J.; Song, T.; Liu, Z.; Yang, D.; Xiang, R.; Hua, W.; Wan, H. Effects of biodegradable biomedical porous MnO2 nanoparticles on blood components and functions. Colloids Surf. B Biointerfaces 2022, 217, 112667. [Google Scholar] [CrossRef]
- Rahman, B.M.A.; Viphavakit, C.; Chitaree, R.; Ghosh, S.; Pathak, A.K.; Verma, S.; Sakda, N. Optical Fiber, Nanomaterial, and THz-Metasurface-Mediated Nano-Biosensors: A Review. Biosensors 2022, 12, 42. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Barhoum, A.; Chan, Y.S.; Dufresne, A.; Danquah, M.K. Review on nanoparticles and nanostructured materials: History, sources, toxicity and regulations. Beilstein J. Nanotechnol. 2018, 9, 1050–1074. [Google Scholar] [CrossRef] [PubMed]
- Carlander, U.; Midander, K.; Hedberg, Y.S.; Johanson, G.; Bottai, M.; Karlsson, H.L. Macrophage-Assisted Dissolution of Gold Nanoparticles. ACS Appl. Bio Mater. 2019, 2, 1006–1016. [Google Scholar] [CrossRef] [PubMed]
- Oldak, L.; Zielinska, Z.; Socha, K.; Bogdan, S.; Gorodkiewicz, E. Phospho-Tau 181 quantification method for Alzheimer’s disease based on an array 2D biosensor combined with surface plasmon resonance imaging. Talanta 2024, 271, 125736. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, B.; Wei, H.; Li, Y.; Hu, F.; Du, X.; Chen, J. Investigating immunosensor for determination of depression marker-Apo-A4 based on patterning AuNPs and N-Gr nanomaterials onto ITO-PET flexible electrodes with amplifying signal. Anal. Chim. Acta 2022, 1224, 340217. [Google Scholar] [CrossRef]
- Jayakumar, K.; Bennett, R.; Leech, D. Electrochemical glucose biosensor based on an osmium redox polymer and glucose oxidase grafted to carbon nanotubes: A design-of-experiments optimisation of current density and stability. Electrochim. Acta 2021, 371, 137845. [Google Scholar] [CrossRef]
- Shrestha, D.; Kang, K.; Nayaju, T.; Bacirhonde, P.M.; Maharjan, B.; Park, C.H.; Kim, C.S. Co1.22xNixO4/fMWCNTs-hybrid nanocomposite-based self-adhesive wearable non-enzymatic electrochemical sensor for continuous glucose monitoring in sweat. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133361. [Google Scholar] [CrossRef]
- Franklin, D.; Ueltschi, T.; Carlini, A.; Yao, S.; Reeder, J.; Richards, B.; Van Duyne, R.P.; Rogers, J.A. Bioresorbable Microdroplet Lasers as Injectable Systems for Transient Thermal Sensing and Modulation. ACS Nano 2021, 15, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zhang, H.; Sun, X.; Hao, S.; Dong, S. A hybrid bioelectrochemical device based on glucose/O2 enzymatic biofuel cell for energy conversion and storage. Electrochim. Acta 2022, 420, 140440. [Google Scholar] [CrossRef]
- Yu, K.J.; Kuzum, D.; Hwang, S.-W.; Kim, B.H.; Juul, H.; Kim, N.H.; Won, S.M.; Chiang, K.; Trumpis, M.; Richardson, A.G.; et al. Bioresorbable silicon electronics for transient spatiotemporal mapping of electrical activity from the cerebral cortex. Nat. Mater. 2016, 15, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, H.; Zhang, Z.; Yao, Y.; Bao, X.; Hu, Q. Ultrastretchable, self-adhesive, strain-sensitive and self-healing GO@DA/Alginate/P(AAc-co-AAm) multifunctional hydrogels via mussel-inspired chemistry. Carbohydr. Polym. 2021, 254, 117316. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, S.; Gu, Z.; Chen, C.; Zhao, Y. Toxicity of manufactured nanomaterials. Particuology 2022, 69, 31–48. [Google Scholar] [CrossRef]
- Zhang, A.; Meng, K.; Liu, Y.; Pan, Y.; Qu, W.; Chen, D.; Xie, S. Absorption, distribution, metabolism, and excretion of nanocarriers in vivo and their influences. Adv. Colloid Interface Sci. 2020, 284, 102261. [Google Scholar] [CrossRef] [PubMed]
- Riaz Ahmed, K.B.; Nagy, A.M.; Brown, R.P.; Zhang, Q.; Malghan, S.G.; Goering, P.L. Silver nanoparticles: Significance of physicochemical properties and assay interference on the interpretation of in vitro cytotoxicity studies. Toxicol. In Vitro 2017, 38, 179–192. [Google Scholar] [CrossRef]
- Hwang, S.-W.; Kim, D.-H.; Tao, H.; Kim, T.-i.; Kim, S.; Yu, K.J.; Panilaitis, B.; Jeong, J.-W.; Song, J.-K.; Omenetto, F.G.; et al. Materials and Fabrication Processes for Transient and Bioresorbable High-Performance Electronics. Adv. Funct. Mater. 2013, 23, 4087–4093. [Google Scholar] [CrossRef]
- Palmroth, A.; Salpavaara, T.; Vuoristo, P.; Karjalainen, S.; Kääriäinen, T.; Miettinen, S.; Massera, J.; Lekkala, J.; Kellomäki, M. Materials and Orthopedic Applications for Bioresorbable Inductively Coupled Resonance Sensors. ACS Appl. Mater. Interfaces 2020, 12, 31148–31161. [Google Scholar] [CrossRef]
- Helmersson, U.; Lattemann, M.; Bohlmark, J.; Ehiasarian, A.P.; Gudmundsson, J.T. Ionized physical vapor deposition (IPVD): A review of technology and applications. Thin Solid Films 2006, 513, 1–24. [Google Scholar] [CrossRef]
- Schuettler, M.; Stiess, S.; King, B.V.; Suaning, G.J. Fabrication of implantable microelectrode arrays by laser cutting of silicone rubber and platinum foil*. J. Neural Eng. 2005, 2, S121. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhu, Y. Printing conductive nanomaterials for flexible and stretchable electronics: A review of materials, processes, and applications. Adv. Mater. Technol. 2019, 4, 1800546. [Google Scholar] [CrossRef]
- Zavanelli, N.; Yeo, W.-H. Advances in screen printing of conductive nanomaterials for stretchable electronics. ACS Omega 2021, 6, 9344–9351. [Google Scholar] [CrossRef] [PubMed]
- Secor, E.B. Electronics, P. Principles of aerosol jet printing. Flex. Print. Electron. 2018, 3, 035002. [Google Scholar] [CrossRef]
- Hales, S.; Tokita, E.; Neupane, R.; Ghosh, U.; Elder, B.; Wirthlin, D.; Kong, Y.L. 3D printed nanomaterial-based electronic, biomedical, and bioelectronic devices. Nanotechnology 2020, 31, 172001. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.; Sen, R.K.; Dwivedi, N.; Khan, R.; Solanki, P.R.; Srivastava, A.K.; Dhand, C. 3D-Printed Microfluidics and Potential Biomedical Applications. Front. Nanotechnol. 2021, 3, 609355. [Google Scholar] [CrossRef]
- Zhu, Y.Z.; Joralmon, D.; Shan, W.T.; Chen, Y.Y.; Rong, J.H.; Zhao, H.Y.; Xiao, S.Q.; Li, X.J. 3D printing biomimetic materials and structures for biomedical applications. Bio-Design Manuf. 2021, 4, 405–428. [Google Scholar] [CrossRef]
- Li, J.; Luo, S.; Liu, J.; Xu, H.; Huang, X. Processing Techniques for Bioresorbable Nanoparticles in Fabricating Flexible Conductive Interconnects. Materials 2018, 11, 1102. [Google Scholar] [CrossRef]
- Shou, W.; Mahajan, B.K.; Ludwig, B.; Yu, X.; Staggs, J.; Huang, X.; Pan, H. Low-Cost Manufacturing of Bioresorbable Conductors by Evaporation–Condensation-Mediated Laser Printing and Sintering of Zn Nanoparticles. Adv. Mater. 2017, 29, 1700172. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Hu, Z.; Seo, M.-H.; Xu, Y.; Yan, Y.; Hsu, Y.-H.; Berkovich, J.; Lee, K.; Liu, T.-L.; McDonald, S. High-speed, scanned laser structuring of multi-layered eco/bioresorbable materials for advanced electronic systems. Nat. Commun. 2022, 13, 6518. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Rytkin, E.; Bimrose, M.; Li, S.; Choi, Y.S.; Lee, G.; Wang, Y.; Tang, L.; Madrid, M.; Wickerson, G.; et al. A Sewing Approach to the Fabrication of Eco/bioresorbable Electronics. Small 2023, 19, 2305017. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Technology. The benefits of implanted glucose sensors. J. Diabetes Sci. Technol. 2007, 1, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, Q.; Liu, Y.; Gai, W.; Ye, L.; Yang, L.; Cui, Y. Closed-Loop Diabetes Minipatch Based on a Biosensor and an Electroosmotic Pump on Hollow Biodegradable Microneedles. ACS Sens. 2022, 7, 1347–1360. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, S.; Liu, Y.; Xu, Z.; Zhang, K.; Guo, Y. Development of Cu nanoflowers modified the flexible needle-type microelectrode and its application in continuous monitoring glucose in vivo. Biosens. Bioelectron. 2018, 110, 44–51. [Google Scholar] [CrossRef]
- Bai, W.; Yang, H.; Ma, Y.; Chen, H.; Shin, J.; Liu, Y.; Yang, Q.; Kandela, I.; Liu, Z.; Kang, S.-K.; et al. Flexible Transient Optical Waveguides and Surface-Wave Biosensors Constructed from Monocrystalline Silicon. Adv. Mater. 2018, 30, 1801584. [Google Scholar] [CrossRef]
- Mehrotra, S.; Rai, P.; Gautam, K.; Saxena, A.; Verma, R.; Lahane, V.; Singh, S.; Yadav, A.K.; Patnaik, S.; Anbumani, S.; et al. Chitosan-carbon nanofiber based disposable bioelectrode for electrochemical detection of oxytocin. Food Chem. 2023, 418, 135965. [Google Scholar] [CrossRef]
- Xu, M.; Yadavalli, V.K. Flexible Biosensors for the Impedimetric Detection of Protein Targets Using Silk-Conductive Polymer Biocomposites. ACS Sens. 2019, 4, 1040–1047. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, W.; Abbaspourrad, A.; Ahn, J.; Bader, A.; Bose, S.; Vegas, A.; Lin, J.; Tao, J.; Hang, T.; et al. Microfluidic Fabrication of Colloidal Nanomaterials-Encapsulated Microcapsules for Biomolecular Sensing. Nano Lett. 2017, 17, 2015–2020. [Google Scholar] [CrossRef]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med Devices Evid. Res. 2014, 7, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Chan, M.M.; Chan, M.M. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir. Med. 2013, 107, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Bai, W.; Zhang, H.; Xu, C.; Chiarelli, A.M.; Vázquez-Guardado, A.; Xie, Z.; Shen, H.; Nandoliya, K.; Zhao, H.; et al. Wireless, implantable catheter-type oximeter designed for cardiac oxygen saturation. Sci. Adv. 2021, 7, eabe0579. [Google Scholar] [CrossRef] [PubMed]
- Kahlert, H. Functionalized carbon electrodes for pH determination. J. Solid State Electrochem. 2008, 12, 1255–1266. [Google Scholar] [CrossRef]
- Andrews, R.J.; Bringas, J.R.; Alonzo, G. Cerebrospinal fluid pH and PCO2 rapidly follow arterial blood pH and PCO2 with changes in ventilation. Neurosurgery 1994, 34, 466–470. [Google Scholar] [CrossRef] [PubMed]
- Wijayaratna, U.N.; Kiridena, S.D.; Adams, J.D.; Behrend, C.J.; Anker, J.N. Synovial Fluid pH Sensor for Early Detection of Prosthetic Hip Infections. Adv. Funct. Mater. 2021, 31, 2104124. [Google Scholar] [CrossRef]
- Swietach, P.; Vaughan-Jones, R.D.; Harris, A.L.; Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130099. [Google Scholar] [CrossRef]
- Ghoneim, M.T.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G.C.; Murzynowski, P.J.; Dagdeviren, C. Recent Progress in Electrochemical pH-Sensing Materials and Configurations for Biomedical Applications. Chem. Rev. 2019, 119, 5248–5297. [Google Scholar] [CrossRef] [PubMed]
- Paghi, A.; Corsi, M.; La Mattina, A.A.; Egri, G.; Dähne, L.; Barillaro, G. Wireless and Flexible Optoelectronic System for In Situ Monitoring of Vaginal pH Using a Bioresorbable Fluorescence Sensor. Adv. Mater. Technol. 2023, 8, 2201600. [Google Scholar] [CrossRef]
- Girard, E.; Messager, M.; Sauvanet, A.; Benoist, S.; Piessen, G.; Mabrut, J.Y.; Mariette, C. Anastomotic leakage after gastrointestinal surgery: Diagnosis and management. J. Visc. Surg. 2014, 151, 441–450. [Google Scholar] [CrossRef]
- Chou, S.-C.; Hsieh, Y.-C.; Cheang, W.-H.; Sun, B.-Y.; Chu, C.-Y.; Chen, S.-Y.; Chiao, J.-C.; Wu, P.-W. A flexible IrO2 membrane for pH sensing. Sci. Rep. 2022, 12, 11712. [Google Scholar] [CrossRef] [PubMed]
- Drozdov, A.D.; deClaville Christiansen, J. Modeling the effects of pH and ionic strength on swelling of polyelectrolyte gels. J. Chem. Phys. 2015, 142, 114904. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.E.; Hourdet, D.; Audebert, R.; Khokhlov, A.R. pH-Responsive Gels of Hydrophobically Modified Poly(acrylic acid). Macromolecules 1997, 30, 8278–8285. [Google Scholar] [CrossRef]
- Li, S.; Lu, D.; Li, S.; Liu, J.; Xu, Y.; Yan, Y.; Rodriguez, J.Z.; Bai, H.; Avila, R.; Kang, S.; et al. Bioresorbable, wireless, passive sensors for continuous pH measurements and early detection of gastric leakage. Sci. Adv. 2024, 10, eadj0268. [Google Scholar] [CrossRef] [PubMed]
- Gurkov, A.; Sadovoy, A.; Shchapova, E.; Teh, C.; Meglinski, I.; Timofeyev, M. Microencapsulated fluorescent pH probe as implantable sensor for monitoring the physiological state of fish embryos. PLoS ONE 2017, 12, e0186548. [Google Scholar] [CrossRef]
- Sadovoy, A.; Teh, C.; Korzh, V.; Escobar, M.; Meglinski, I. Microencapsulated bio-markers for assessment of stress conditions in aquatic organisms in vivo. Laser Phys. Lett. 2012, 9, 542. [Google Scholar] [CrossRef]
- Yu, K.-K.; Li, K.; Hou, J.-T.; Yang, J.; Xie, Y.-M.; Yu, X.-Q. Rhodamine based pH-sensitive “intelligent” polymers as lysosome targeting probes and their imaging applications in vivo. Polym. Chem. 2014, 5, 5804–5812. [Google Scholar] [CrossRef]
- Arman Kuzubasoglu, B.; Kursun Bahadir, S. Flexible temperature sensors: A review. Sens. Actuators A Phys. 2020, 315, 112282. [Google Scholar] [CrossRef]
- Lu, D.; Yan, Y.; Avila, R.; Kandela, I.; Stepien, I.; Seo, M.-H.; Bai, W.; Yang, Q.; Li, C.; Haney, C.R.; et al. Bioresorbable, Wireless, Passive Sensors as Temporary Implants for Monitoring Regional Body Temperature. Adv. Healthc. Mater. 2020, 9, 2000942. [Google Scholar] [CrossRef]
- Bai, W.; Irie, M.; Liu, Z.; Luan, H.; Franklin, D.; Nandoliya, K.; Guo, H.; Zang, H.; Weng, Y.; Lu, D.; et al. Bioresorbable Multilayer Photonic Cavities as Temporary Implants for Tether-Free Measurements of Regional Tissue Temperatures. BME Front. 2021, 2021, 8653218. [Google Scholar] [CrossRef]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y.; et al. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; Li, Z.; Gu, M.; Hu, Y.; Xu, L.; Jiang, D.; Cheng, S.; Zou, Y.; Deng, Y.; Shi, B.; et al. A Bioresorbable Dynamic Pressure Sensor for Cardiovascular Postoperative Care. Adv. Mater. 2021, 33, 2102302. [Google Scholar] [CrossRef]
- Abramson, A.; Chan, C.T.; Khan, Y.; Mermin-Bunnell, A.; Matsuhisa, N.; Fong, R.; Shad, R.; Hiesinger, W.; Mallick, P.; Gambhir, S.S.; et al. A flexible electronic strain sensor for the real-time monitoring of tumor regression. Sci. Adv. 2022, 8, eabn6550. [Google Scholar] [CrossRef]
- Yu, X.; Wang, H.; Ning, X.; Sun, R.; Albadawi, H.; Salomao, M.; Silva, A.C.; Yu, Y.; Tian, L.; Koh, A.; et al. Needle-shaped ultrathin piezoelectric microsystem for guided tissue targeting via mechanical sensing. Nat. Biomed. Eng. 2018, 2, 165–172. [Google Scholar] [CrossRef]
- Li, Z.; Li, C.; Deng, Y. Bioresorbable Pressure Sensor and Its Applications in Abnormal Respiratory Event Identification. ACS Appl. Electron. Mater. 2023, 5, 1761–1769. [Google Scholar] [CrossRef]
- Qiao, Z.; Mieles, M.; Ji, H.-F. Injectable and moldable hydrogels for use in sensitive and wide range strain sensing applications. Biopolymers 2020, 111, e23355. [Google Scholar] [CrossRef]
- Kang, M.; Park, J.; Kim, S.A.; Kim, T.Y.; Kim, J.Y.; Kim, D.W.; Park, K.; Seo, J. Modulus-tunable multifunctional hydrogel ink with nanofillers for 3D-Printed soft electronics. Biosens. Bioelectron. 2024, 255, 116257. [Google Scholar] [CrossRef] [PubMed]
- What Does the Nervous System Do? Available online: https://www.nichd.nih.gov/health/topics/neuro/conditioninfo/functions (accessed on 25 June 2024).
- Lu, D.; Yan, Y.; Deng, Y.; Yang, Q.; Zhao, J.; Seo, M.-H.; Bai, W.; MacEwan, M.R.; Huang, Y.; Ray, W.Z.; et al. Bioresorbable Wireless Sensors as Temporary Implants for In Vivo Measurements of Pressure. Adv. Funct. Mater. 2020, 30, 2003754. [Google Scholar] [CrossRef]
- Shin, J.; Yan, Y.; Bai, W.; Xue, Y.; Gamble, P.; Tian, L.; Kandela, I.; Haney, C.R.; Spees, W.; Lee, Y.; et al. Bioresorbable pressure sensors protected with thermally grown silicon dioxide for the monitoring of chronic diseases and healing processes. Nat. Biomed. Eng. 2019, 3, 37–46. [Google Scholar] [CrossRef]
- Wu, X.; Feng, J.; Deng, J.; Cui, Z.; Wang, L.; Xie, S.; Chen, C.; Tang, C.; Han, Z.; Yu, H.; et al. Fiber-shaped organic electrochemical transistors for biochemical detections with high sensitivity and stability. Sci. China Chem. 2020, 63, 1281–1288. [Google Scholar] [CrossRef]
- Xu, K.; Li, S.; Dong, S.; Zhang, S.; Pan, G.; Wang, G.; Shi, L.; Guo, W.; Yu, C.; Luo, J. Bioresorbable Electrode Array for Electrophysiological and Pressure Signal Recording in the Brain. Adv Healthc. Mater. 2019, 8, e1801649. [Google Scholar] [CrossRef]
- Xie, J.; Dai, Y.; Xing, Y.; Wang, Y.; Yang, G.; He, E.; Xu, Z.; Fan, P.; Mo, F.; Wu, Y.; et al. PtNPs/rGO-GluOx/mPD Directionally Electroplated Dual-Mode Microelectrode Arrays for Detecting the Synergistic Relationship between the Cortex and Hippocampus of Epileptic Rats. ACS Sens. 2023, 8, 1810–1818. [Google Scholar] [CrossRef]
- Macdonald, T.J.; Lanzetta, L.; Liang, X.; Ding, D.; Haque, S.A. Engineering Stable Lead-Free Tin Halide Perovskite Solar Cells: Lessons from Materials Chemistry. Adv. Mater. 2023, 35, e2206684. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Panel on Micronutrients. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; National Academies Press (US): Washington, DC, USA, 2001.
- Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference, Intakes. Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride; National Academies Press (US): Washington, DC, USA, 1997.
- Su, H.; Wang, Y.; Gu, Y.; Bowman, L.; Zhao, J.; Ding, M. Potential applications and human biosafety of nanomaterials used in nanomedicine. J. Appl. Toxicol. JAT 2018, 38, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Sigurdsson, S.A.; Yu, Z.; Lee, J.; Nurmikko, A. A method for large-scale implantation of 3D microdevice ensembles into brain and soft tissue. Microsyst. Nanoeng. 2020, 6, 97. [Google Scholar] [CrossRef]
- Yuk, H.; Varela, C.E.; Nabzdyk, C.S.; Mao, X.; Padera, R.F.; Roche, E.T.; Zhao, X. Dry double-sided tape for adhesion of wet tissues and devices. Nature 2019, 575, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.-K.; Murphy, R.K.J.; Hwang, S.-W.; Lee, S.M.; Harburg, D.V.; Krueger, N.A.; Shin, J.; Gamble, P.; Cheng, H.; Yu, S.; et al. Bioresorbable silicon electronic sensors for the brain. Nature 2016, 530, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Lacava, A.; Zottola, V.; Bonaldo, A.; Cuomo, F.; Basagni, S. Securing Bluetooth Low Energy networking: An overview of security procedures and threats. Comput. Netw. 2022, 211, 108953. [Google Scholar] [CrossRef]
- Cope, P.; Campbell, J.; Hayajneh, T. An investigation of Bluetooth security vulnerabilities. In Proceedings of the 2017 IEEE 7th Annual Computing and Communication Workshop and Conference (CCWC), Las Vegas, NV, USA, 9–11 January 2017; pp. 1–7. [Google Scholar]
- Krzysztoń, M.; Marks, M. Simulation of watchdog placement for cooperative anomaly detection in Bluetooth Mesh Intrusion Detection System. Simul. Model. Pract. Theory 2020, 101, 102041. [Google Scholar] [CrossRef]
- Lacava, A.; Giacomini, E.; Alterio, F.D.; Cuomo, F. Intrusion Detection System for Bluetooth Mesh Networks: Data Gathering and Experimental Evaluations. In Proceedings of the 2021 IEEE International Conference on Pervasive Computing and Communications Workshops and other Affiliated Events (PerCom Workshops), Kassel, Germany, 22–26 March 2021; pp. 661–666. [Google Scholar]



| Material | Electrical Property | Dielectric Constant (Ksp) | Dissolution Rates (nm/h) | Dissolution Product | Applications |
|---|---|---|---|---|---|
| Magnesium (Mg) | Conductor (interconnects) [19] | 8.9 × 10−12 [25] | 70 (in Hank’s solution [22] and PBS (pH 7.4; 37 °C)) [26]. | Mg(OH)2 [27] | Biodegradable battery system [28]. |
| Iron (Fe) | Conductor (electrodes) [19] | 1.8 × 10−15 (Fe(OH)2) & 4.0 × 10−38 (Fe(OH)3) [25] | 3.0 (in PBS (pH 7.4; 37 °C)) [26]. | Fe(OH)2 & Fe(OH)3 [27] | Cerebral dopamine monitoring [29]. |
| Zinc (Zn) | Conductor (electrodes) [19] | 4.5 × 10−17 [25] | 7 (in Hank’s solution) [22]; 146 (in PBS (pH 7.4; 37 °C)) [26]. | Zn(OH)2 [27] | Subcutaneous glucose monitoring [4]. |
| Molybdenum (Mo) | Conductor (interconnects) [19] | N/A | 0.3 (in Hank’s solution) [22] ; 0.8 (in PBS (pH 7.4; 37 °C)) [26]. | H2MoO4 [27] | Nitric oxide monitoring [30]; Biodegradable battery system [28]. |
| Tungsten (W) | Conductor (electrodes) [19] | N/A | 1.7 (in Hank’s solution) [22]; 6 (in PBS (pH 7.4; 37 °C)) [26]. | H2WO4 [27] | Subcutaneous glucose monitoring [4]; Wound healing monitoring [31]. |
| Material | Electrical Roles | Dissolution Rates | Applications |
|---|---|---|---|
| Silk | Insulator [55] | 75% in 14 days [56]. | Oxygen monitoring [56]; Fibroin conductive composites [57]. |
| Poly lactic-co-glycolic acid | Insulator [55] | Degradation in 8 h [58]. | NOx gas monitoring [58]; Humidity monitoring [59]. |
| Poly(lactic acid) | Insulator [55] | 11 h [60]. | Concentration sensors, pH sensors [61]; piezoelectric film for electro sensors [60]. |
| Poly(glycerol-co-sebacate) | Insulator [55] | 80% in 35 days [62]. | Conductive patches in infarcted myocardium [63]. |
| Poly(ɛ-caprolactone) | Insulator [55] | Degradation in 15 h [29]. | Cerebral dopamine monitoring [29]. |
| Poly-l-lactic acid | Insulator [55] | 56 days in 74 °C PBS [64]. | Piezoelectric force sensors [64]. |
| Polydioxanone | Insulator [55] | 10% in 55 days [65]. | Sensors membranes [66]. |
| Polyglycolide | Insulator [55] | 17% to 40% in 55 days [67]. | Glucose monitoring [68]; Leptin monitoring [69]. |
| Poly octanediol-co-citrate | Insulator [55] | Degradation in weeks [70]. | pH monitoring [70]. |
| Poly(glycolide-co-ε-caprolactone) | Insulator [33] | 12 weeks [33]. | Encapsulation layers [33]; Synthetic molecular recognition [71]. |
| Melanin | Conductor [33,55] | N/A | pH monitoring [72]; Glucose monitoring [73]. |
| Hydrogel Material | Electrical Roles | Dissolution Rates | Applications |
|---|---|---|---|
| Alginate/polyacrylamide | Conductor [83] | 501 h at 80 °C, 50,000 ppm NaCl solution [84]. | Pressure sensors, strain sensors [84,85]. |
| HEDN | N/A | N/A | Pressure sensors, strain sensors [81]. |
| crystalline domain cross-linked PVA/Ca2+-cross-linked alginate | Conductor [76] | N/A | Soft adhesive interfaces for electrical sensors [76]. |
| PEG-LA-DA/sodium alginate/chitosan network | Conductor [82] | 20 days to several months in PBS (pH 7.4) at 37 °C [82]. | Soft adhesive interfaces for optical, electrical, chemical sensors [82]. |
| Gelatin methacrylate (GelMA) | Conductor [86] | 2 to 7 h [87]. | Glucose sensors [86]. |
| PEDOT:PSS/HPU | Conductor [88] | 70% in 84 days [89]. | pH sensors [88]. |
| PANI-PA/SPCE | Conductor [90] | N/A | Vitamin C sensors [90]. |
| PANI film doped with phytic acid | Conductor [91] | N/A | Electrical sensors [91]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Zhang, J.; Ray, W.Z.; MacEwan, M.R. Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis. Processes 2024, 12, 1535. https://doi.org/10.3390/pr12071535
Xu Y, Zhang J, Ray WZ, MacEwan MR. Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis. Processes. 2024; 12(7):1535. https://doi.org/10.3390/pr12071535
Chicago/Turabian StyleXu, Yameng, Jingyuan Zhang, Wilson Z. Ray, and Matthew R. MacEwan. 2024. "Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis" Processes 12, no. 7: 1535. https://doi.org/10.3390/pr12071535
APA StyleXu, Y., Zhang, J., Ray, W. Z., & MacEwan, M. R. (2024). Implantable and Semi-Implantable Biosensors for Minimally Invasive Disease Diagnosis. Processes, 12(7), 1535. https://doi.org/10.3390/pr12071535






