Efficient Enzymatic Hydrolysis and Polyhydroxybutyrate Production from Non-Recyclable Fiber Rejects from Paper Mills by Recombinant Escherichia coli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Collection
2.2. Chemical Composition
2.3. Hot Water Pretreatment
2.4. Disk Milling
2.5. Enzymatic Hydrolysis
2.6. HPLC Analysis
2.7. Cellulose Conversion Efficiency
2.8. PHB Production Using Hydrolysate by Recombinant E. coli LSBJ
2.9. Analytical Methods for PHB Extraction and Quantification
2.10. PHB Characterization
2.10.1. Extraction and Purification of PHB
2.10.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.10.3. Fourier-Transform Infrared Spectroscopy with Attenuated Total Reflectance (FTIR-ATR)
2.10.4. Thermogravimetric Analysis (TGA)
2.10.5. Differential Scanning Calorimetry (DSC)
2.11. Statistical Analysis
3. Results and Discussion
3.1. Composition of Fiber Rejects
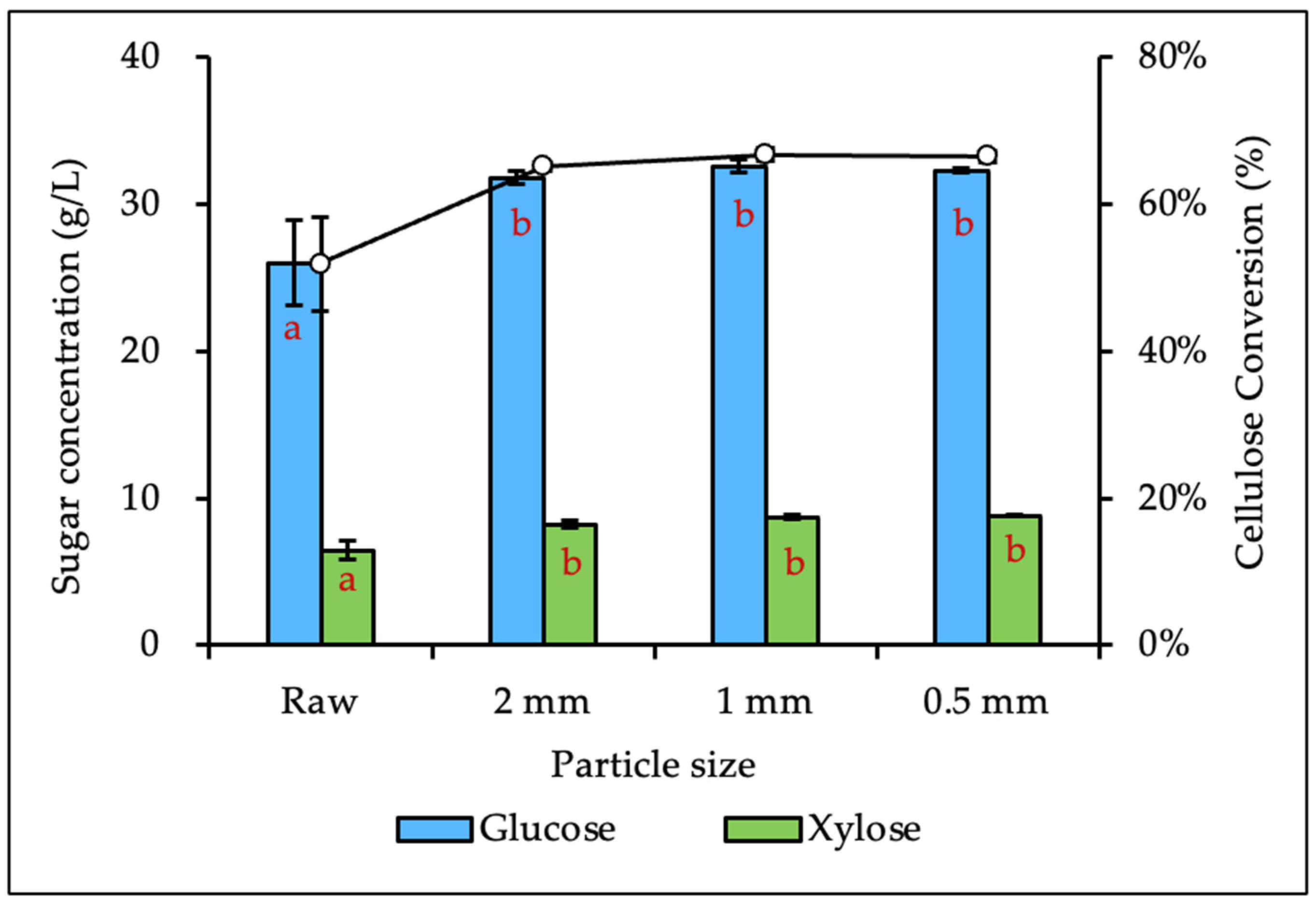
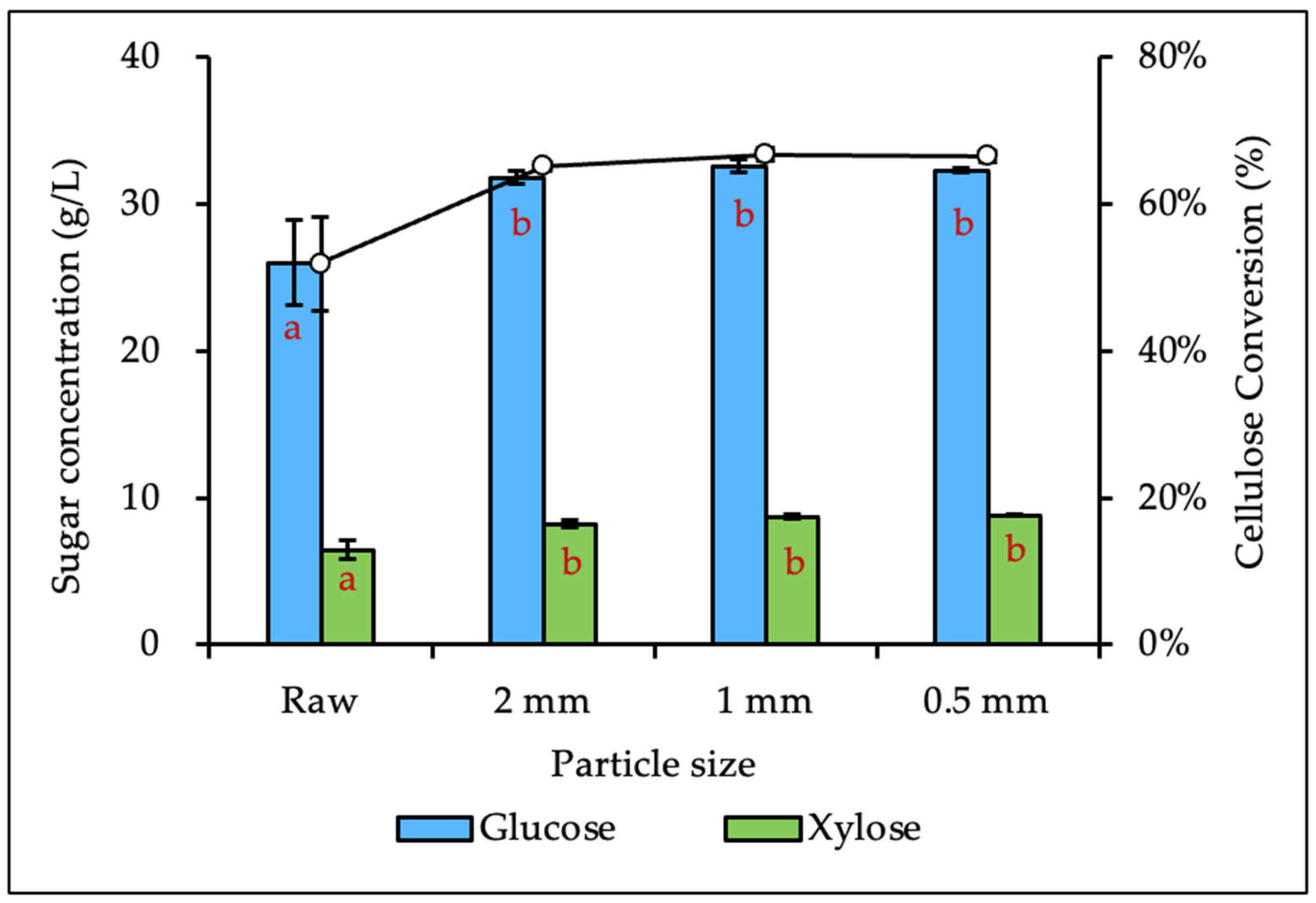
3.2. Effects of Biomass Size Reduction on Enzymatic Hydrolysis
3.3. Effects of Surfactant Addition on Enzymatic Hydrolysis
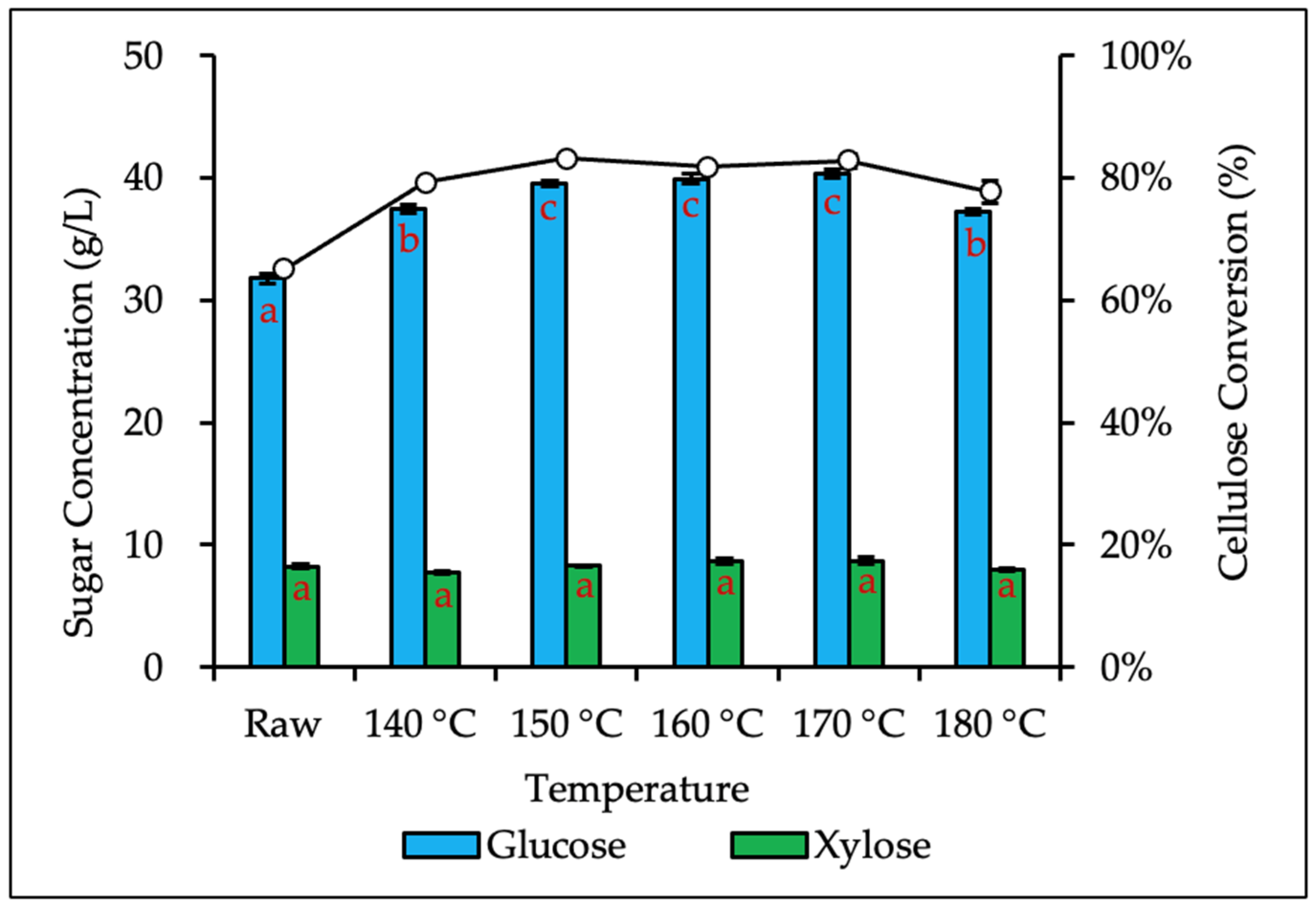
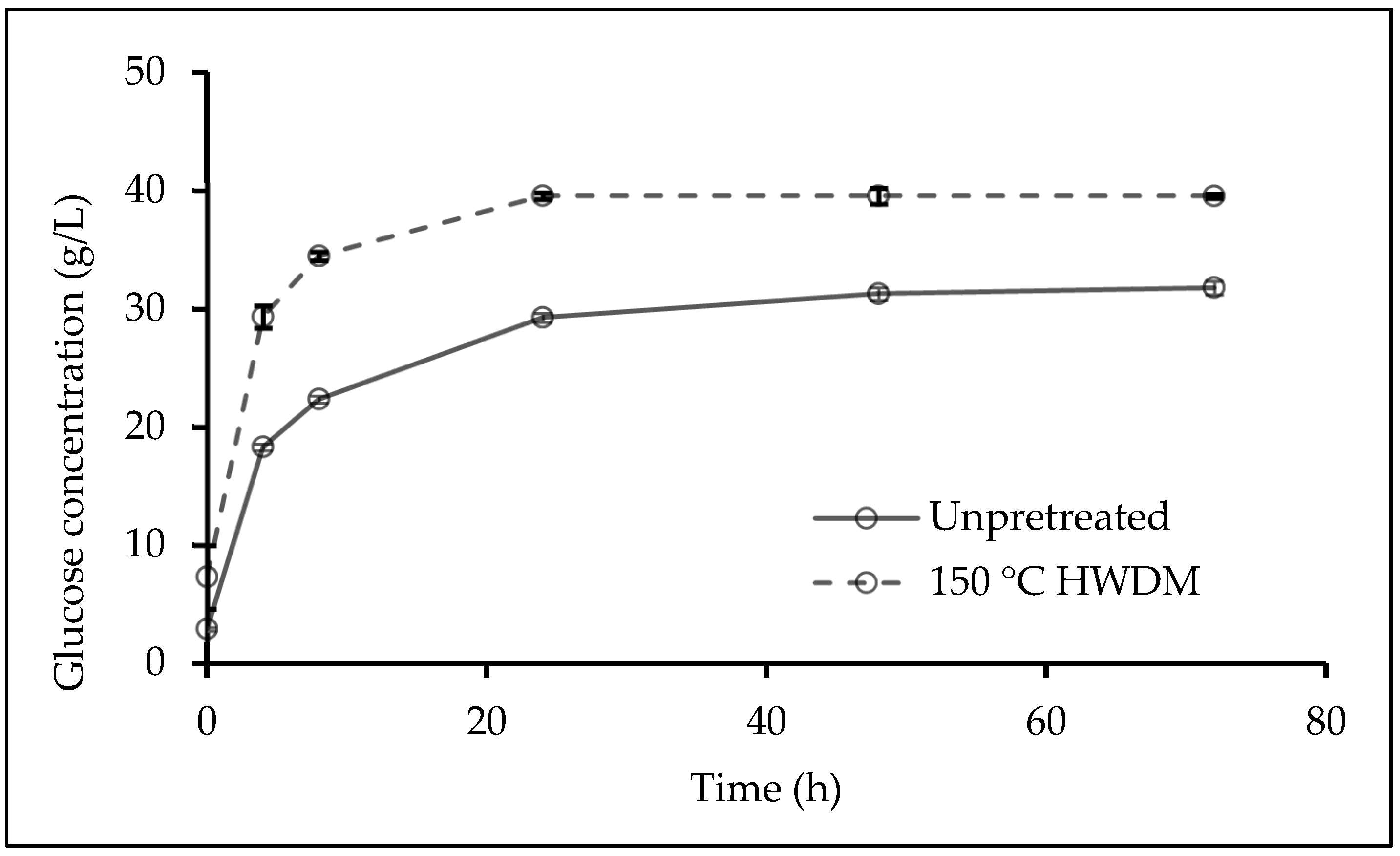
3.4. Effect of Hydrothermal-Mechanical Pretreatment on Enzymatic Hydrolysis
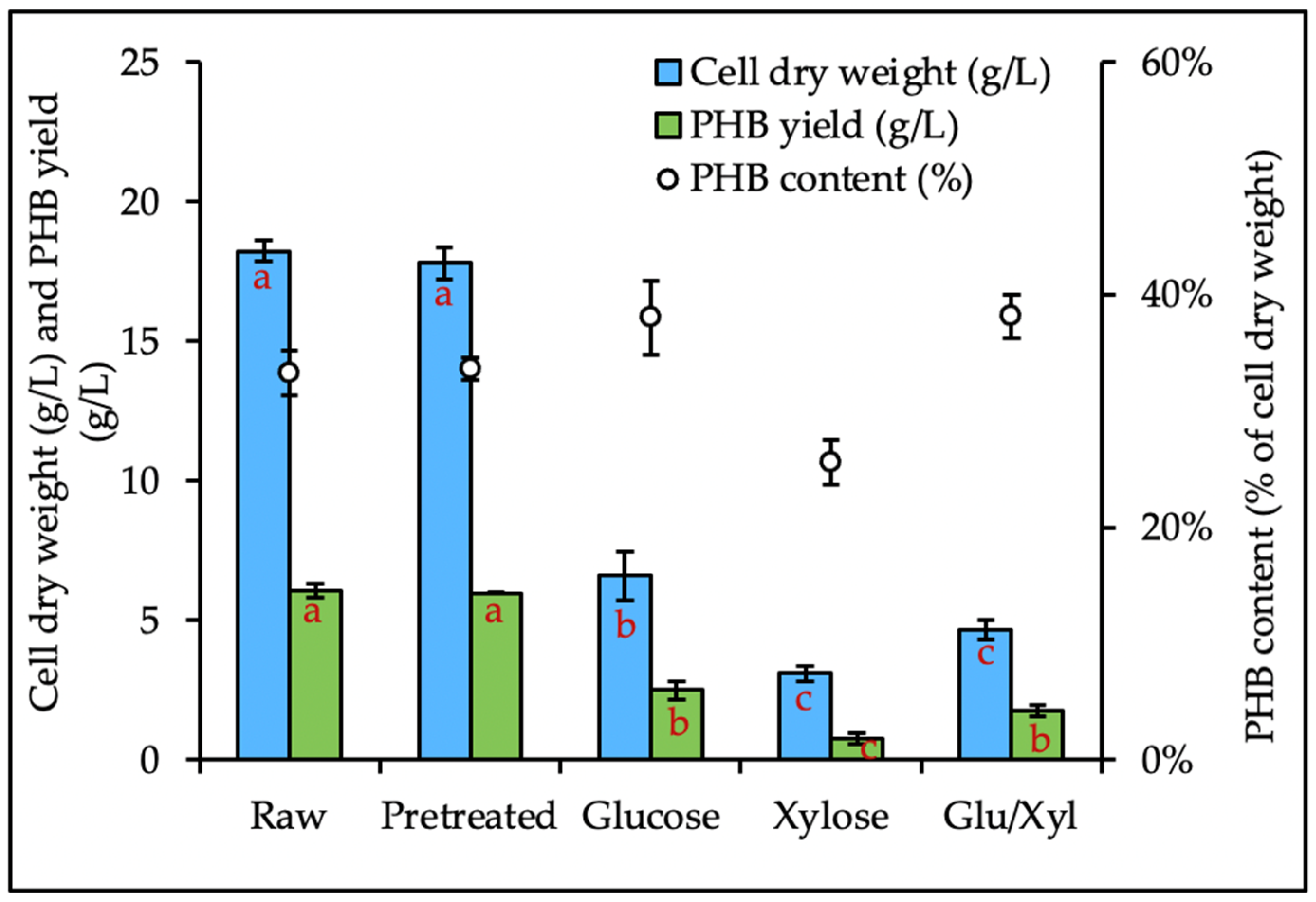
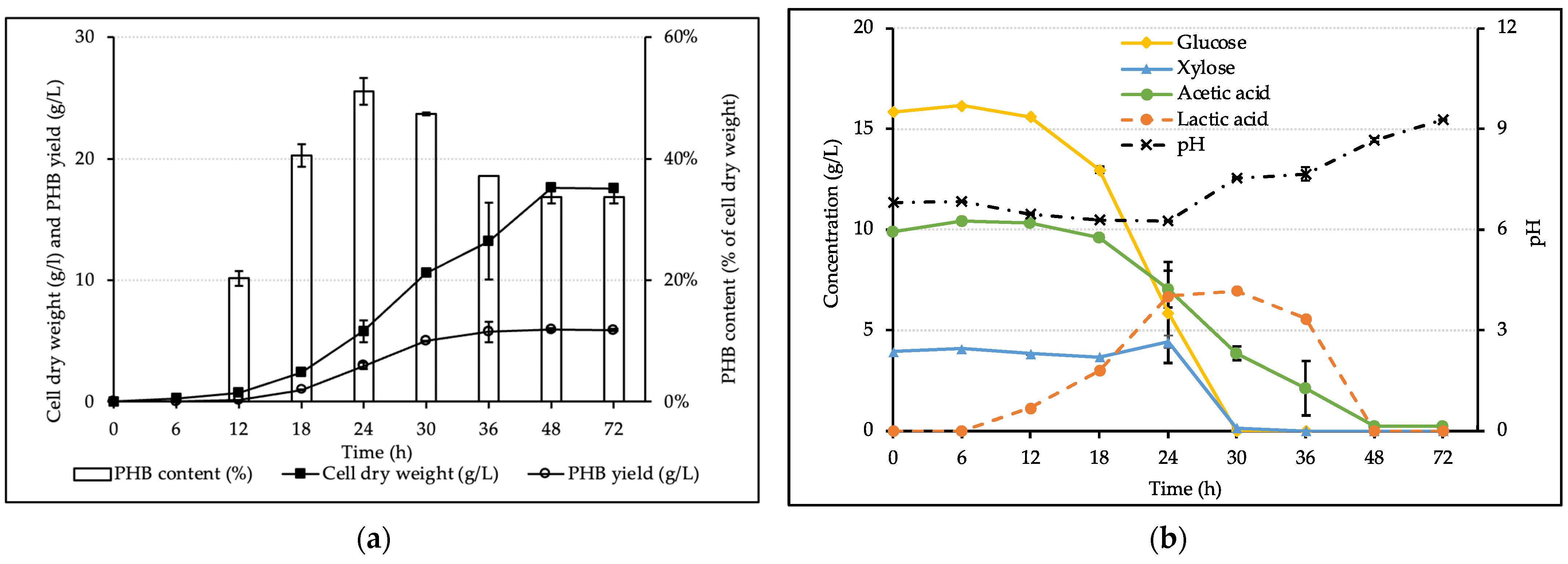
3.5. PHB Production
3.6. PHB Characterization
3.6.1. Nuclear Magnetic Resonance (NMR) Analysis
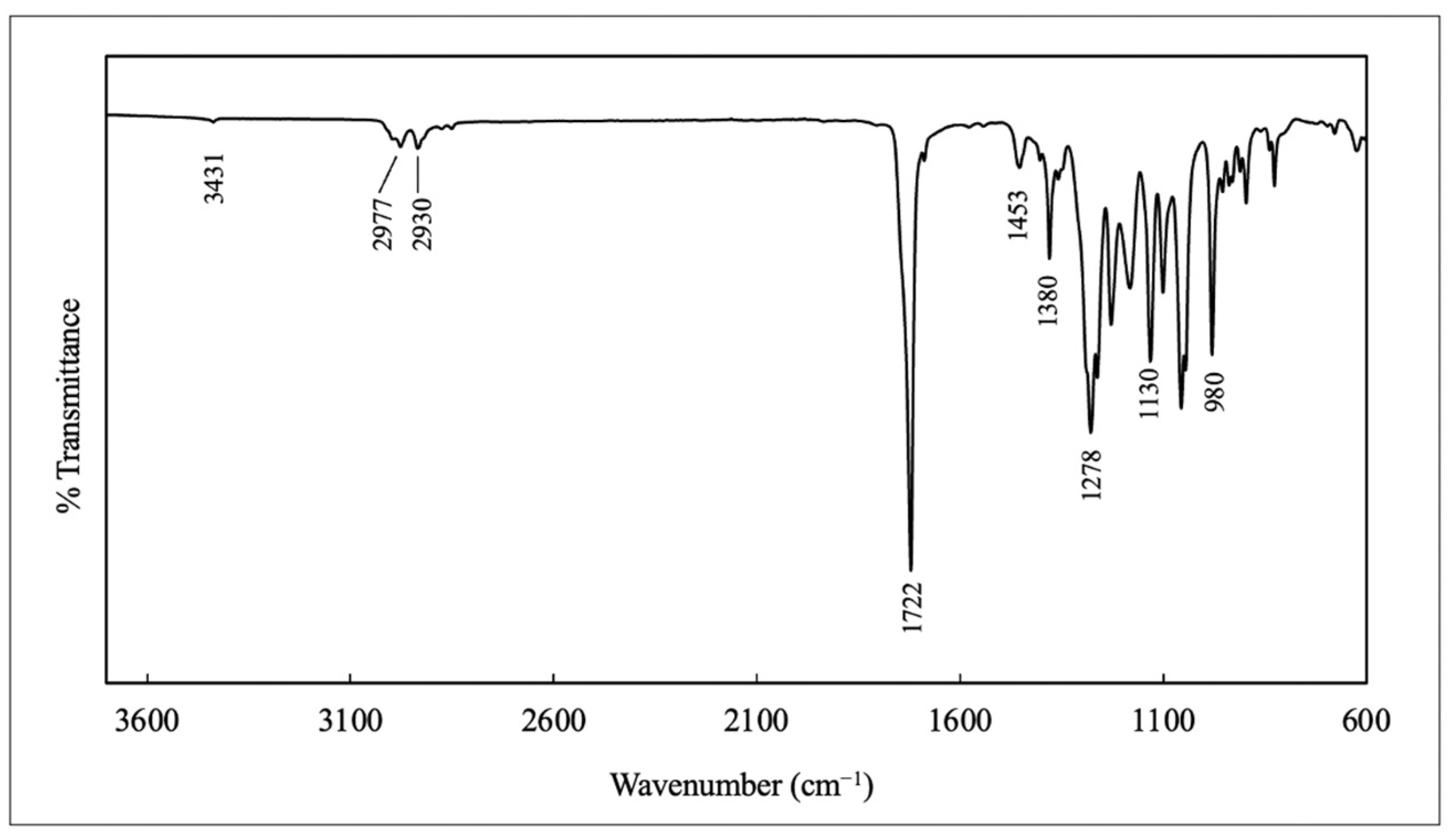
3.6.2. Fourier Transform InfraRed (FTIR) Spectroscopy
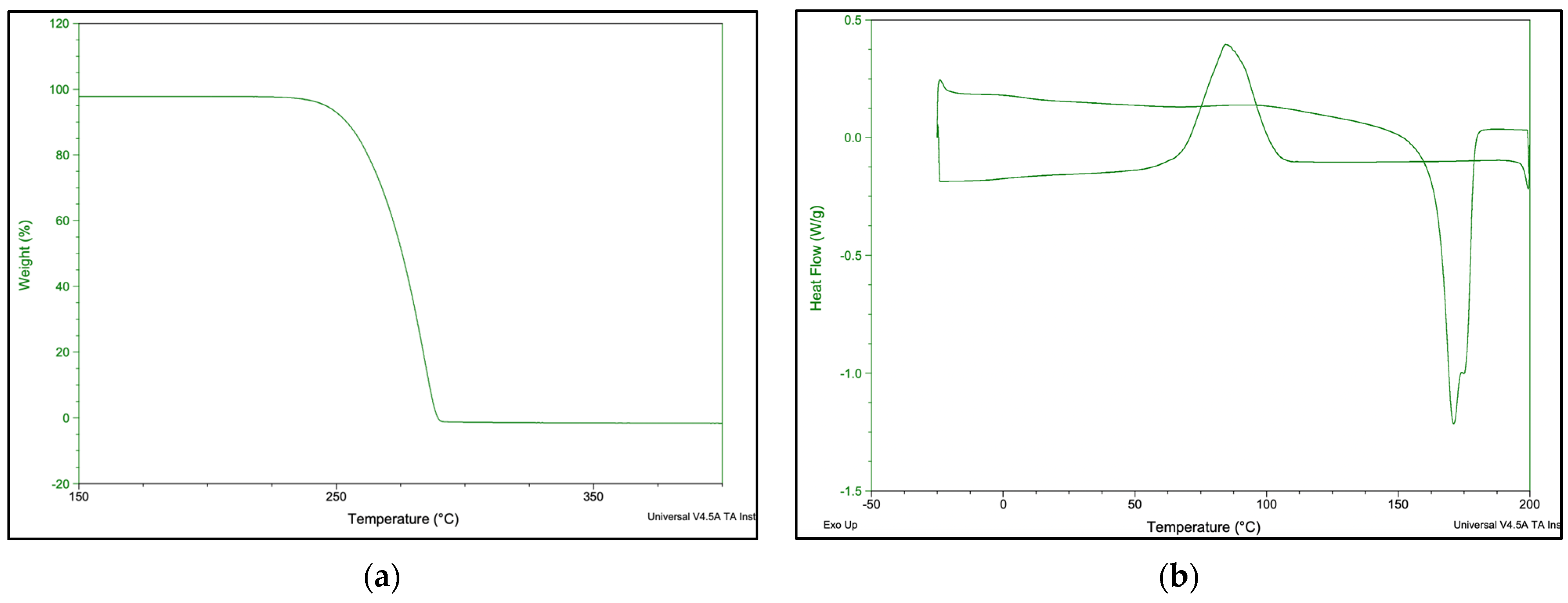
3.6.3. Thermogravimetric Analysis (TGA) and Differential Scanning Calorimetry (DSC)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zytner, P.; Kumar, D.; Elsayed, A.; Mohanty, A.K.; Ramarao, B.; Misra, M. A review on cost-effective polyhydroxyalkanoate (PHA) production through the use of lignocellulosic biomass. RSC Sustain. 2023, 1, 2120–2134. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic pollution in the marine environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Siddiqui, A.J.; Ashraf, S.A.; Snoussi, M.; Badraoui, R.; Ibrahim, A.M.; Alreshidi, M.; Sachidanandan, M.; Patel, M. Characterization and Process Optimization for Enhanced Production of Polyhydroxybutyrate (PHB)-Based Biodegradable Polymer from Bacillus flexus Isolated from Municipal Solid Waste Landfill Site. Polymers 2023, 15, 1407. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A. Perspectives for biotechnological production and utilization of biopolymers: Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 2001, 1, 1–24. [Google Scholar] [CrossRef]
- Thomas, C.M.; Kumar, D.; Scheel, R.A.; Ramarao, B.; Nomura, C.T. Production of Medium Chain Length polyhydroxyalkanoate copolymers from agro-industrial waste streams. Biocatal. Agric. Biotechnol. 2022, 43, 102385. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of polyhydroxybutyrate (PHB) and factors impacting its chemical and mechanical characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef] [PubMed]
- Reis, M.; Serafim, L.; Lemos, P.; Ramos, A.; Aguiar, F.; Van Loosdrecht, M. Production of polyhydroxyalkanoates by mixed microbial cultures. Bioprocess Biosyst. Eng. 2003, 25, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.; Kumar, G.; Yang, Y.-H. Renewable biohydrogen production from lignocellulosic biomass using fermentation and integration of systems with other energy generation technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K. Biopolymer poly-hydroxyalkanoates (PHA) production from apple industrial waste residues: A review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Venditti, R.A.; Rojas, O.J. What happens to cellulosic fibers during papermaking and recycling? A Review. BioResources 2007, 2, 739–788. [Google Scholar]
- Laivins, G.; Scallan, A. The influence of drying and beating on the swelling of fines. J. Pulp Pap. Sci. 1996, 22, J178–J184. [Google Scholar]
- Rauzi, J.; Tschirner, U. Enzymatic Glucose and Xylose Production from Paper Mill Rejects. Recycling 2022, 7, 24. [Google Scholar] [CrossRef]
- Kirui, A.; Zhao, W.; Deligey, F.; Yang, H.; Kang, X.; Mentink-Vigier, F.; Wang, T. Carbohydrate-aromatic interface and molecular architecture of lignocellulose. Nat. Commun. 2022, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, L.; Liu, D. Biomass recalcitrance. Part I: The chemical compositions and physical structures affecting the enzymatic hydrolysis of lignocellulose. Biofuels Bioprod. Biorefining 2012, 6, 465–482. [Google Scholar] [CrossRef]
- Merino-Pérez, O.; Martínez-Palou, R.; Labidi, J.; Luque, R. Microwave-assisted pretreatment of lignocellulosic biomass to produce biofuels and value-added products. In Production of Biofuels and Chemicals with Microwave; Springer: Berlin/Heidelberg, Germany, 2015; pp. 197–224. [Google Scholar]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef] [PubMed]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Paul, A.; Jia, L.; Majumder, E.L.-W.; Yoo, C.G.; Rajendran, K.; Villarreal, E.; Kumar, D. Poly (3-hydroxybuyrate) production from industrial hemp waste pretreated with a chemical-free hydrothermal process. Bioresour. Technol. 2023, 381, 129161. [Google Scholar] [CrossRef] [PubMed]
- Juneja, A.; Kumar, D.; Singh, V.K.; Singh, V. Chemical free two-step hydrothermal pretreatment to improve sugar yields from energy cane. Energies 2020, 13, 5805. [Google Scholar] [CrossRef]
- Wang, Z.; Dien, B.S.; Rausch, K.D.; Tumbleson, M.; Singh, V. Fermentation of undetoxified sugarcane bagasse hydrolyzates using a two stage hydrothermal and mechanical refining pretreatment. Bioresour. Technol. 2018, 261, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Dien, B.S.; Tumbleson, M.; Rausch, K.D.; Singh, V. Improvement of sugar yields from corn stover using sequential hot water pretreatment and disk milling. Bioresour. Technol. 2016, 216, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of extractives in biomass. Lab. Anal. Proced. 2005, 1617, 1–16. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Thomas, C.M.; Scheel, R.A.; Nomura, C.T.; Ramarao, B.; Kumar, D. Production of polyhydroxybutyrate and polyhydroxybutyrate-co-MCL copolymers from brewer’s spent grains by recombinant Escherichia coli LSBJ. Biomass Convers. Biorefinery 2021, 1–12. [Google Scholar] [CrossRef]
- Hou, L.; Jia, L.; Morrison, H.M.; Majumder, E.L.-W.; Kumar, D. Enhanced polyhydroxybutyrate production from acid whey through determination of process and metabolic limiting factors. Bioresour. Technol. 2021, 342, 125973. [Google Scholar] [CrossRef] [PubMed]
- Tappel, R.C.; Wang, Q.; Nomura, C.T. Precise control of repeating unit composition in biodegradable poly (3-hydroxyalkanoate) polymers synthesized by Escherichia coli. J. Biosci. Bioeng. 2012, 113, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Scheel, R.A. Enhancing polyhydroxyalkanoate biosynthesis in Escherichia coli: A genetic engineering and process optimization approach towards functionalized polymeric nanomedicine. State Univ. N.Y. Coll. Environ. Sci. For. 2020. Available online: https://experts.esf.edu/esploro/outputs/graduate/Enhancing-polyhydroxyalkanoate-biosynthesis-in-Escherichia-coli/99870875704826 (accessed on 7 July 2024).
- Thomas, C.M. Brewer’s Spent Grains as a Substrate for Recombinant E. coli LSBJ to Produce Bioplastic Polyhydroxybutyrate and Medium Chain Length Polyhydroxyalkanoate Copolymers. Ph.D. Thesis, College of Environmental Science, Syracuse, NY, USA, 2021. [Google Scholar]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. Preparation and characterization of environmentally safe and highly biodegradable microbial polyhydroxybutyrate (PHB) based graphene nanocomposites for potential food packaging applications. Int. J. Biol. Macromol. 2020, 154, 866–877. [Google Scholar] [CrossRef]
- de Sousa Junior, R.R.; Dos Santos, C.A.S.; Ito, N.M.; Suqueira, A.N.; Lackner, M.; Dos Santos, D.J. PHB processability and property improvement with linear-chain polyester oligomers used as plasticizers. Polymers 2022, 14, 4197. [Google Scholar] [CrossRef]
- Borisova, I.; Stoilova, O.; Manolova, N.; Rashkov, I. Modulating the mechanical properties of electrospun PHB/PCL materials by using different types of collectors and heat sealing. Polymers 2020, 12, 693. [Google Scholar] [CrossRef] [PubMed]
- Min, B.C.; Bhayani, B.; Jampana, V.; Ramarao, B. Enhancement of the enzymatic hydrolysis of fines from recycled paper mill waste rejects. Bioresour. Bioprocess. 2015, 2, 40. [Google Scholar] [CrossRef]
- Jeffries, T.W.; Schartman, R. Bioconversion of secondary fiber fines to ethanol using counter-current enzymatic saccharification and co-fermentation. Appl. Biochem. Biotechnol. 1999, 78, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Cowling, E.B.; Kirk, T.K. Properties of Cellulose and Lignocellulosie Materials as Substrates for Enzymatic Conversion Processes. In Proceedings of the Biotechnology and Bioengineering Symposium, New York, NY, USA, 1 January 1976. [Google Scholar]
- Zhu, J.; Wang, G.; Pan, X.; Gleisner, R. Specific surface to evaluate the efficiencies of milling and pretreatment of wood for enzymatic saccharification. Chem. Eng. Sci. 2009, 64, 474–485. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Fan, C.; Yan, Z. The mechanism of poly (ethylene glycol) 4000 effect on enzymatic hydrolysis of lignocellulose. Colloids Surf. B Biointerfaces 2012, 89, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Zhao, X.; Zheng, Y.; Lin, W.; Lai, C.; Yong, Q.; Ragauskas, A.J.; Meng, X. Revealing the mechanism of surfactant-promoted enzymatic hydrolysis of dilute acid pretreated bamboo. Bioresour. Technol. 2022, 360, 127524. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Zeng, M.; Hu, Q.; Cai, C.; Lin, X.; Qiu, X.; Yang, D.; Pang, Y. Nonionic surfactants enhanced enzymatic hydrolysis of cellulose by reducing cellulase deactivation caused by shear force and air-liquid interface. Bioresour. Technol. 2018, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hideno, A.; Inoue, H.; Tsukahara, K.; Fujimoto, S.; Minowa, T.; Inoue, S.; Endo, T.; Sawayama, S. Wet disk milling pretreatment without sulfuric acid for enzymatic hydrolysis of rice straw. Bioresour. Technol. 2009, 100, 2706–2711. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, R.J. Characterizing refining action in PFI mills. Tappi J. 2005, 4, 9–14. [Google Scholar]
- Uetani, K.; Yano, H. Nanofibrillation of wood pulp using a high-speed blender. Biomacromolecules 2011, 12, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhu, J.; Gleisner, R.; Kuster, T.; Baxa, U.; McNeil, S. Morphological development of cellulose fibrils of a bleached eucalyptus pulp by mechanical fibrillation. Cellulose 2012, 19, 1631–1643. [Google Scholar] [CrossRef]
- Leu, S.-Y.; Zhu, J. Substrate-related factors affecting enzymatic saccharification of lignocelluloses: Our recent understanding. Bioenergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Weiqi, W.; Shubin, W.; Liguo, L. Combination of liquid hot water pretreatment and wet disk milling to improve the efficiency of the enzymatic hydrolysis of eucalyptus. Bioresour. Technol. 2013, 128, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Sediawan, W.B.; Sulistyo, H.; Hidayat, M. Kinetics of sequential reaction of hydrolysis and sugar degradation of rice husk in ethanol production: Effect of catalyst concentration. Bioresour. Technol. 2011, 102, 2062–2067. [Google Scholar]
- Kadhum, H.J.; Mahapatra, D.M.; Murthy, G.S. A novel method for real-time estimation of insoluble solids and glucose concentrations during enzymatic hydrolysis of biomass. Bioresour. Technol. 2019, 275, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.; Taciro, M.; Michelin Ramos, M.; Carter, J.; Pradella, J.; Gomez, J. Poly-3-hydroxybutyrate (P3HB) production by bacteria from xylose, glucose and sugarcane bagasse hydrolysate. J. Ind. Microbiol. Biotechnol. 2004, 31, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Le Bouguénec, C.; Schouler, C. Sugar metabolism, an additional virulence factor in enterobacteria. Int. J. Med. Microbiol. 2011, 301, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kovalcik, A.; Kucera, D.; Matouskova, P.; Pernicova, I.; Obruca, S.; Kalina, M.; Enev, V.; Marova, I. Influence of removal of microbial inhibitors on PHA production from spent coffee grounds employing Halomonas halophila. J. Environ. Chem. Eng. 2018, 6, 3495–3501. [Google Scholar] [CrossRef]
- Pan, W.; Perrotta, J.A.; Stipanovic, A.J.; Nomura, C.T.; Nakas, J.P. Production of polyhydroxyalkanoates by Burkholderia cepacia ATCC 17759 using a detoxified sugar maple hemicellulosic hydrolysate. J. Ind. Microbiol. Biotechnol. 2012, 39, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Walfridsson, M.; Hallborn, J.; Penttil, M.; Kernen, S.; Hahn-Hgerdal, B. Xylose-metabolizing Saccharomyces cerevisiae strains overexpressing the TKL1 and TAL1 genes encoding the pentose phosphate pathway enzymes transketolase and transaldolase. Appl. Environ. Microbiol. 1995, 61, 4184–4190. [Google Scholar] [CrossRef]
- Scheel, R.A.; Fusi, A.D.; Min, B.C.; Thomas, C.M.; Ramarao, B.V.; Nomura, C.T. Increased production of the value-added biopolymers poly (R-3-hydroxyalkanoate) and poly (γ-glutamic acid) from hydrolyzed paper recycling waste fines. Front. Bioeng. Biotechnol. 2019, 7, 409. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, K.; Oliveira-Filho, E.R.; Dumont, M.-J.; Gomez, J.G.; Taciro, M.K.; da Silva, L.F.; Orsat, V.; Del Rio, L.F. Increasing PHB production with an industrially scalable hardwood hydrolysate as a carbon source. Ind. Crops Prod. 2020, 154, 112703. [Google Scholar] [CrossRef]
- Min, B.; Jampana, S.N.; Thomas, C.M.; Ramarao, B.V. Study of buffer substitution using inhibitory compound CaCO3 in enzymatic hydrolysis of paper mill waste fines. J. TAPPI 2018, 50, 77–82. [Google Scholar] [CrossRef]
- Sun, S.; Ding, Y.; Liu, M.; Xian, M.; Zhao, G. Comparison of glucose, acetate and ethanol as carbon resource for production of poly (3-hydroxybutyrate) and other acetyl-CoA derivatives. Front. Bioeng. Biotechnol. 2020, 8, 833. [Google Scholar] [CrossRef] [PubMed]
- Dañez, J.C.A.; Requiso, P.J.; Alfafara, C.G.; Nayve Jr, F.R.P.; Ventura, J.-R.S. Optimization of fermentation factors for polyhydroxybutyrate (PHB) production using Bacillus megaterium PNCM 1890 in simulated glucose-xylose hydrolysates from agricultural residues. Philipp. J. Sci. USA 2020, 149, 163–175. [Google Scholar] [CrossRef]
- Enjalbert, B.; Millard, P.; Dinclaux, M.; Portais, J.-C.; Létisse, F. Acetate fluxes in Escherichia coli are determined by the thermodynamic control of the Pta-AckA pathway. Sci. Rep. 2017, 7, 42135. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Castaño-Cerezo, S.; Cánovas, M. Acetate metabolism regulation in Escherichia coli: Carbon overflow, pathogenicity, and beyond. Appl. Microbiol. Biotechnol. 2016, 100, 8985–9001. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, J.; Keasling, J.D. Engineering cellular metabolism. Cell 2016, 164, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Sekar, K.; Tyo, K.E. Regulatory effects on central carbon metabolism from poly-3-hydroxybutryate synthesis. Metab. Eng. 2015, 28, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Prabisha, T.P.; Sindhu, R.; Binod, P.; Sankar, V.; Raghu, K.G.; Pandey, A. Production and characterization of PHB from a novel isolate Comamonas sp. from a dairy effluent sample and its application in cell culture. Biochem. Eng. J. 2015, 101, 150–159. [Google Scholar] [CrossRef]
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M. Characterization of poly (3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour. Technol. 2007, 98, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Kansiz, M.; Billman-Jacobe, H.; McNaughton, D. Quantitative determination of the biodegradable polymer poly (β-hydroxybutyrate) in a recombinant Escherichia coli strain by use of mid-infrared spectroscopy and multivariative statistics. Appl. Environ. Microbiol. 2000, 66, 3415–3420. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Almaguer-Cantú, V.; Rosas-Flores, W.; Martínez-Gómez, V.J.; Quintero-Zapata, I.; Rivera, G.; Rutiaga-Quiñones, O.M. Efficient recovery of thermostable polyhydroxybutyrate (PHB) by a rapid and solvent-free extraction protocol assisted by ultrasound. Int. J. Biol. Macromol. 2020, 164, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Sirohi, R. Sustainable utilization of food waste: Production and characterization of polyhydroxybutyrate (PHB) from damaged wheat grains. Environ. Technol. Innov. 2021, 23, 101715. [Google Scholar] [CrossRef]
- Martínez-Herrera, R.E.; Alemán-Huerta, M.E.; Flores-Rodríguez, P.; Almaguer-Cantú, V.; Valencia-Vázquez, R.; Rosas-Flores, W.; Medrano-Roldán, H.; Ochoa-Martínez, L.A.; Rutiaga-Quiñones, O.M. Utilization of Agave durangensis leaves by Bacillus cereus 4N for polyhydroxybutyrate (PHB) biosynthesis. Int. J. Biol. Macromol. 2021, 175, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sharma-Shivappa, R.R.; Olson, J.W.; Khan, S.A. Production of polyhydroxybutyrate (PHB) by Alcaligenes latus using sugarbeet juice. Ind. Crops Prod. 2013, 43, 802–811. [Google Scholar] [CrossRef]
- Chaijamrus, S.; Udpuay, N. Production and characterization of polyhydroxybutyrate from molasses and corn steep liquor produced by Bacillus megaterium ATCC 6748. Agric. Eng. Int. CIGR J. 2008, 1–12. [Google Scholar]
- Kerketta, A.; Vasanth, D. Madhuca indica flower extract as cheaper carbon source for production of poly (3-hydroxybutyrate-co-3-hydroxyvalerate) using Ralstonia eutropha. Process Biochem. 2019, 87, 1–9. [Google Scholar] [CrossRef]

| Composition | Dry Matter (%) |
|---|---|
| Glucan | 35.5 ± 1.4 |
| Xylan | 9.0 ± 0.3 |
| Lignin | 16.7 ± 0.3 |
| Ash | 25.0 ± 1.3 |
| Extractives | 9.7 ± 0.7 |
| Others (by difference) | 4.2 |
| Pretreatment | Residence Time during Hot Water Pretreatment (min) | Sugar Concentration (g/L) | Cellulose Conversion (%) | |
|---|---|---|---|---|
| Glucose | Xylose | |||
| Untreated | NA | 31.8 ± 0.4 | 8.2 ± 0.2 | 65.1 ± 0.6 |
| Disk milling only (1 cycle) | NA | 33.1 ± 0.2 | 7.5 ± 0.2 | 69.1 ± 0.7 |
| Disk milling only (3 cycle) | NA | 34.7 ± 0.1 | 7.7 ± 0.0 | 73.5 ± 0.9 |
| Hot water pretreatment (180 °C) | 10 | 33.7 ± 0.7 | 7.9 ± 0.1 | 67.8 ± 1.4 |
| Hot water pretreatment 180 °C and disk milling | 10 | 37.2 ± 0.3 | 9.0 ± 0.2 | 77.7 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Juneja, A.; Majumder, E.L.-W.; Ramarao, B.V.; Kumar, D. Efficient Enzymatic Hydrolysis and Polyhydroxybutyrate Production from Non-Recyclable Fiber Rejects from Paper Mills by Recombinant Escherichia coli. Processes 2024, 12, 1576. https://doi.org/10.3390/pr12081576
Jia L, Juneja A, Majumder EL-W, Ramarao BV, Kumar D. Efficient Enzymatic Hydrolysis and Polyhydroxybutyrate Production from Non-Recyclable Fiber Rejects from Paper Mills by Recombinant Escherichia coli. Processes. 2024; 12(8):1576. https://doi.org/10.3390/pr12081576
Chicago/Turabian StyleJia, Linjing, Ankita Juneja, Erica L.-W. Majumder, Bandaru V. Ramarao, and Deepak Kumar. 2024. "Efficient Enzymatic Hydrolysis and Polyhydroxybutyrate Production from Non-Recyclable Fiber Rejects from Paper Mills by Recombinant Escherichia coli" Processes 12, no. 8: 1576. https://doi.org/10.3390/pr12081576








