Abstract
A strong alkaline catalyst, sodium methoxide (CH3ONa), is commonly used to catalyze the transesterification reaction for biodiesel production. Meanwhile, titanium dioxide (TiO2) anatase with a bandgap of 3.2 eV is a highly competitive photocatalyst after the absorption of sufficient energy from ultraviolet light. There has been no published report on the synergistic catalyst effects of CH3ONa and TiO2 on further facilitating the transesterification reaction. Hence, an impregnating method was used in this study to prepare the heterogeneous photocatalyst comprising TiO2 nanoparticles embedded with a CH3ONa catalyst. The TiO2 nanoparticles were first immersed in an aqueous solution of CH3ONa so that CH3ONa could diffuse into the interior surfaces of the TiO2 porous structure. The mixture of TiO2 and CH3ONa was then calcined in the temperature range from 150 °C to 450 °C for 4 h to produce the TiO2/CH3ONa photocatalyst. Various characteristics of the catalyst were analyzed to determine the optimum preparation conditions. The Fourier transform infrared spectroscopy spectra revealed that the absorption peaks of CH3ONa appeared in the wavelength range of 600 cm−1 and 1500 cm−1. The X-ray diffractometer analysis showed that the calcined CH3ONa did not alter the crystal structure of the catalyst carrier TiO2. At the calcined temperatures between 100 °C and 800 °C, no intermediate or pyrolyzed product of CH3ONa was detected, as revealed by the thermogravimetric analyzer spectra. In addition, about 5~9 wt.% elemental calcium in the CH3ONa solution could be calcined onto the surface of TiO2. In addition, the FTIR spectra confirmed the successful sintering and bonding of CH3ONa onto the TiO2 nanoparticles. The energy dispersive spectrometry result revealed that the interior surface of the TiO2 nanoparticles was filled with the CH3ONa compound.
1. Introduction
Diesel engine-powered vehicles emit large amounts of NOX, toxic gasses, black smoke, and particulate matter that cause serious concerns about environmental pollution and human health. The rapid development of alternative energy sources to replace traditional fossil fuels has become the global goal towards net-zero carbon emission [1]. Fatty acid methyl ester (FAME) is a fuel derived from animal fats, vegetable oils, or microalgae lipids, which is regarded as a promising alternative fuel to mitigate pollutant emissions and provide sustainable fuel resources. Vegetable oils primarily comprise triglycerides (about 98%) and a small amount of monoglycerides and glycerol [2]. Triglycerides are composed of three free fatty acids and a glycerol molecule [3]. Fatty acids vary in the length of their carbon chains and the number of double bonds [4]. Using vegetable oil as a biodiesel feedstock can reduce air pollution and protect the environment. Plants, through the photosynthesis process, can fix carbon dioxide emitted from combustion equipment. The main disadvantages of vegetable oils are higher kinematic viscosity and carbon residue, causing poor fluidity and inferior burning efficiency, and the increased weight of carbon residue deposited inside the engine cylinder [5]. The adequate transesterification reaction of vegetable feedstock oils to produce biodiesel reduces these shortcomings. The transesterification reaction converts those triglycerides to form FAMEs and glycerol. Generally, a strong alkaline catalyst is applied to facilitate the reaction rate for industrial biodiesel production.
The catalysts used in transesterification reactions can be categorized as homogeneous and heterogeneous [6]. At the end of a process, it is hard to separate the catalyst, as the homogeneous catalyst is of the same phase as the reactant mixture. In contrast, heterogeneous catalysts are in different phases than reactants. In heterogeneous catalytic reactions, the reactants are first adsorbed on the catalyst surface, and the original chemical bonds in the structure of the reactants are broken to generate new chemical bonds; finally, the products are desorbed from the surface of the catalyst [7]. The major advantages of heterogeneous catalytic reactions are the ease of separation from the product and the superior recycle and reuse capability of the heterogeneous catalyst. Hence, heterogeneous catalysts are considered green and environmentally friendly catalysts. Another advantage of using heterogeneous alkaline catalysts is their high efficiency in biodiesel production rate [8]. Jan et al. [9] used TiO2 nanoparticles as a heterogeneous catalyst to assist the feedstock Carthamus tinctorius L. oil for biodiesel synthesis. They found that the particle size and surface area of the TiO2 catalyst ranged between 42 nm and 58 nm and 21 and 27 m2/g, respectively. At a molar ratio of methanol to feedstock oil of 10 and the reaction temperature of 65 °C, the highest biodiesel yield was 95%. However, the transesterification took a significantly longer reaction time of 80–120 min to complete compared with other transesterification reactions catalyzed by typical alkaline catalysts, such as potassium hydroxide (KOH) or sodium hydroxide (NaOH), which took less than 1 h [10]. Prabhakar et al. [11] compared the biodiesel yields from palm oil using a catalyst comprising titanium dioxide (TiO2) and iron oxide (Fe2O3) nanoparticles synthesized by the sol–gel method. They observed the maximum biodiesel yield when the transesterification was enhanced upon the activity of the TiO2 synthesized catalyst compared to that of KOH. Besides the sol–gel method, the most commonly used approaches to prepare heterogeneous catalysts are impregnation, gel combustion, hydrothermal, precipitation, co-precipitation, microemulsion, and atomic layer deposition [12].
A small number of precious metal oxides such as TiO2, Fe3O4, SiO2, γ-A2O3, ZrO2, CeO, etc., exhibit great catalytic performance [13] for transesterification or dry reforming to produce gasses such as H2 and CO from methane and glycerol; these metal oxides are used as carriers or supports for acid or alkaline catalysts in the impregnating method [14,15]. These carriers are characterized by high porosity, a large number of holes with large surface areas, and high reaction activity even at atmospheric pressure and temperature. When the photocatalyst is prepared, the carrier of some appropriate metal oxide is immersed in a large amount of aqueous solution. The photocatalyst is then prepared through a serial procedure of filtration, drying, heating, and calcination [16]. Xu et al. [17] proposed a facile combustion–impregnation method to prepare a Ni catalyst supported by SiO2, which was then used as a catalyst to promote CO2 methanation and exhibited excellent activity at low temperatures. They found that the combustion process catalyzed by adding the Ni/SiO2 achieved a much higher selectivity (94.1%) to CH4 and a significantly higher CO2 conversion rate (66.9%) even at a relatively lower temperature of 350 °C. In addition, glycine, citric acid, and glucose were all promising candidate fuels for applying the impregnation method. The common types of non-homogeneous catalysts include metal oxides, loaded and ion-exchanged resins, and hydrotalcites. Soares Dias et al. [18] carried out the transesterification of soybean oil and poultry animal fat with methanol at room temperature using the nano-grade CaO powder (an average particle size of 20 nm) of metal oxides as a catalyst to enhance the catalytic activity. After adding a catalyst of 1 wt.% oil weight, the transesterification rates exceeded 99% when soybean oil to methanol was in the ratio of 1:27. Rizwanul Fattah et al. [19] used the SrO/ZnO catalysts of ion-exchanged resins for the transesterification of soybean oil and methanol. The optimum conditions for transesterification were a methanol reflux temperature of 65 °C, alcohol to oil molar ratio of 12, and adding 5 wt.% catalyst, leading to a biodiesel conversion rate of 94.7%. Osazuwa and Abidin [20] used an ion exchange resin (denoted as PK208) as the catalyst for transesterification and other anion-exchanged resin catalysts (PA308, PA306, PA306s, and HPA25) to enhance the conversion of ethyl oleate to biodiesel. Another heterogeneous catalyst, hydrotalcite, was also used to produce biodiesel from Jatropha oil [21]. The yield of feedstock oil reached 95% biodiesel under the optimal reaction conditions, using methanol to raw oil in a molar ratio of 12, 3 wt.% catalyst loading by the feedstock oil weight, and a reaction temperature of 70 °C. Cui et al. [22] investigated the production of soybean oil biodiesel using a magnesium catalyst with MCM-41 as a carrier (Mg/MCM-41), and magnesium-aluminum hydrotalcite and zirconium oxide impregnated with potassium ions as catalysts. They found the highest activity of Mg-AL hydrotalcite among the tested catalysts, with a conversion rate as high as 97%.
Two different catalysts might be synthesized together to synergize their catalyst functions so that the combined effects of the catalysts can facilitate some specific reactions and improve the product characteristics compared to using a single catalyst [23,24]. Ge et al. [25] synthesized a dispersed CoNi bimetallic catalyst on the α-MoC surface and found a greatly enhanced catalyst activity of the combined catalyst than a single commercial catalyst. In the current study, a novel photocatalyst was manufactured by synthesizing a strong alkaline catalyst CH3ONa with a photocatalyst of TiO2 nanoparticles to leverage its synergistic effects in enhancing transesterification. The TiO2/CH3ONa combined catalyst has not been reported in the previous literature. CH3ONa is toxic if swallowed, inhaled, or in contact with skin. Sodium methoxide (CH3ONa) causes eye damage, skin burns, and respiratory irritation; it is also a flammable liquid [26]. TiO2 is the most popular catalyst carrier or support due to its low cost, abundant, and high photoresponsive [27]. In addition, serious toxicity concerns regarding TiO2 nanoparticles have long existed. Upon intravenous exposure, TiO2 nanoparticles can enter the human body and severely damage the spleen, liver, brain, and kidney. Long-term exposure to TiO2 nanoparticles through the respiratory system might cause lung cancer [28]. Anatase-type TiO2 has a relatively wide energy band gap between 2.28 and 3.25 EV, which requires the irradiation of ultraviolet light to provide sufficient energy jump. Doping TiO2 with different elements is an effective way to modify the energy band gap and, in turn, catalyst characteristics [29]. An impregnation method is often used to synthesize the carrier catalyst embedded with other alkaline or acid catalysts to exert combined catalyst functions. Until now, there are no reports on the preparation of a TiO2 heterogeneous catalyst along with a strong alkaline catalyst CH3ONa to form the TiO2/CH3ONa photocatalyst for manufacturing biodiesel [30].
Moreover, none of the previously published studies have analyzed the characteristics of the photocatalyst TiO2/CH3ONa, such as the spectra of CH3ONa and TiO2 by a Fourier transform infrared spectroscopy (FTIR) analyzer, the crystalline structure of the photocatalyst analyzed by an X-ray diffractometer (XRD), and the weight percentages of the elemental compositions of the calcined catalyst by an energy dispersive spectrometer (EDS). The primary catalyst properties of the synthesized photocatalyst TiO2/CH3ONa were analyzed in this study to confirm the successful preparation of this catalyst. The optimum methods and conditions for preparing the TiO2/CH3ONa catalyst, including the forging temperature in the high-temperature furnace based on the catalyst characteristics, were also pursued.
2. Experiment Details
The impregnation method was used to prepare the TiO2/CH3ONa photocatalyst. The TiO2 nanoparticles primarily consisted of many pores with a large specific surface area to act as a catalyst carrier to accommodate the strong alkaline CH3ONa catalyst inside its pores. TiO2 nanoparticle powder was directly added to the CH3ONa aqueous solution and then forged at six different temperatures, from 150 °C to 450 °C. The aqueous CH3ONa compound was diffused to fill the interior cavities of the pore structure of the TiO2 nanoparticles. The wet TiO2/CH3ONa catalyst was then sintered to form a solid catalyst structure of TiO2 and CH3ONa. The analyzing tools for the sintered catalyst included an FTIR analyzer, an XRD, and an EDS to analyze the characteristics of the photocatalyst produced from the above procedures.
2.1. Preparation of Strong Solid TiO2/CH3ONa Alkaline Photocatalysts
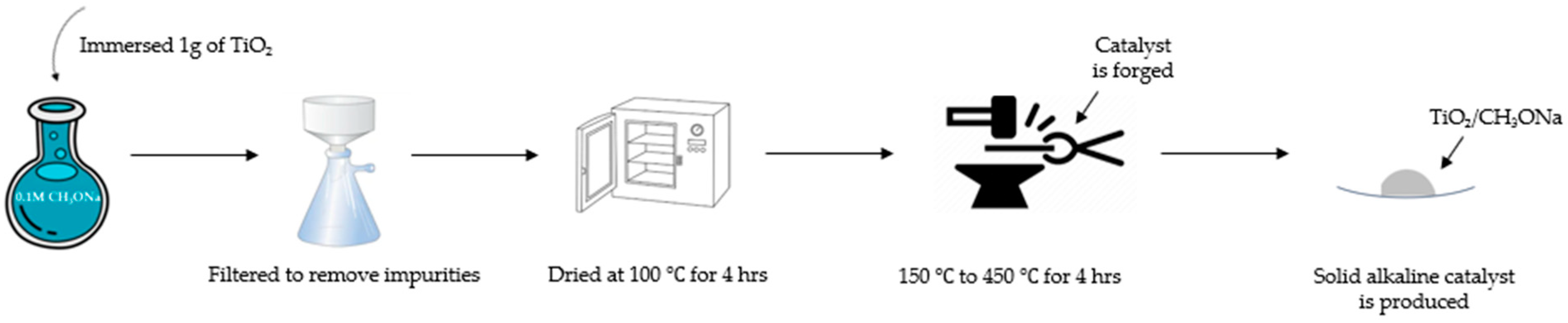
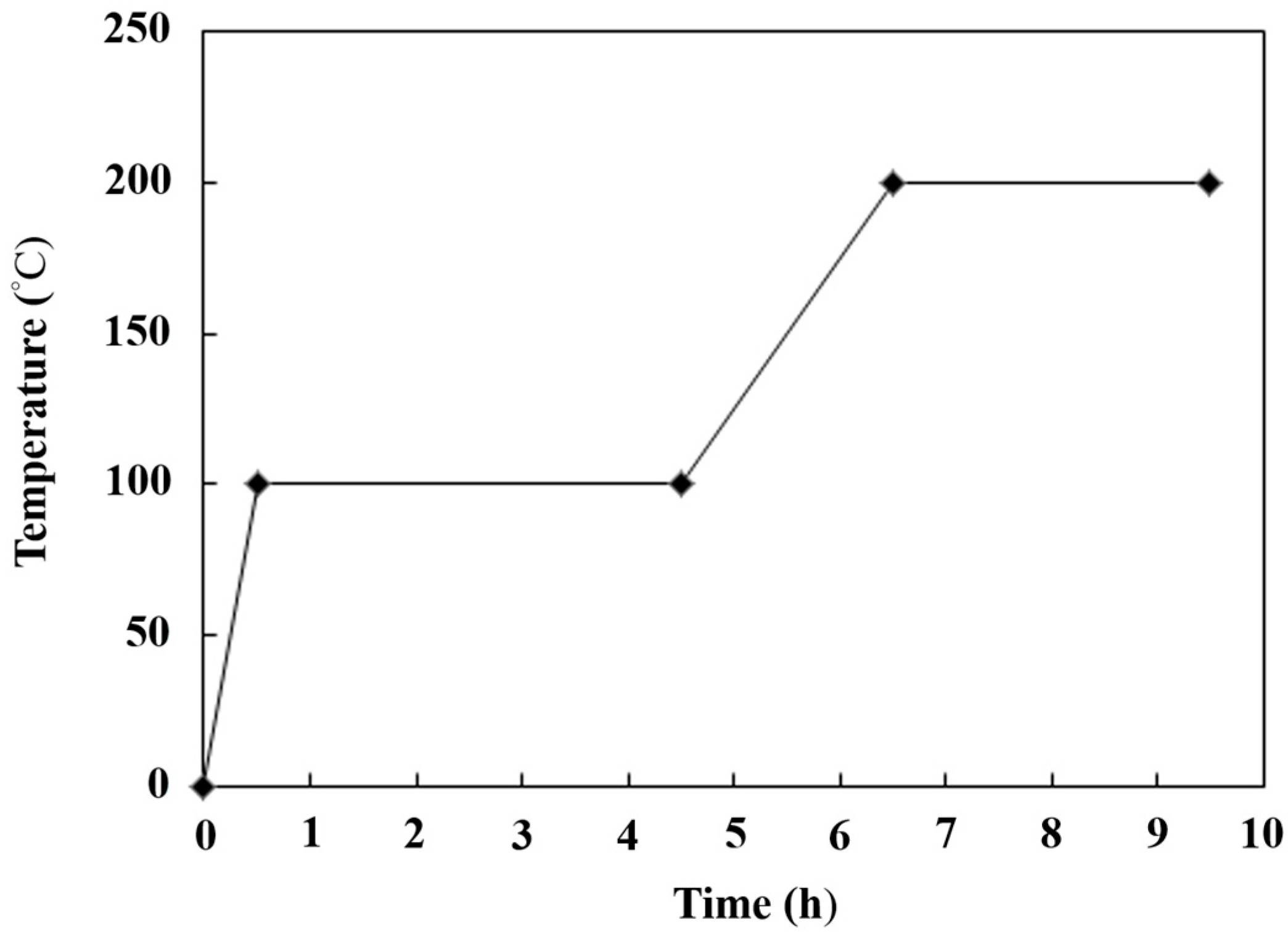
Figure 1 shows the preparation procedures for the synthesized strong alkaline catalyst TiO2/CH3ONa. The TiO2 nanoparticle powder, with its physical structure of full pores with a large specific surface area, acts as a catalyst carrier to encapsulate the strong alkaline catalyst CH3ONa inside its pore cavities. The CH3ONa compound of 5 M concentration was purchased from Merck Taiwan Ltd. (Taipei City, Taiwan). The commercial TiO2 nanoparticles of the anatase type were procured from Uni-Onward Corp., Ltd. (New Taipei City, Taiwan). The purity of the TiO2 nanoparticles was 99.5 wt.%. Table 1 [31] presents the compositions and properties of the TiO2 powder used in this study. The specific surface area (i.e., BET surface area) of the TiO2 nanoparticles could be as high as 50.5 m2/g. The average particle size was about 21 nm. In addition, the TiO2 nanoparticles also contained little impurity, including Al2O3, SiO2, Fe2O3, etc. (Table 1). The amount of 1 g powder of TiO2 with a concentration of 0.1 M was directly immersed in an aqueous CH3ONa solution of 10 mL for 12 h. The aqueous CH3ONa gradually diffused to fill in the interior surface of a large number of the pores of TiO2. Then, this aqueous solution containing a mixture of TiO2 and CH3ONa was poured into a ceramic funnel with a filter and passed through a glass fiber by a vacuum pump to remove impurities in the catalyst. The filtered solution was dried at 100 °C to distill away volatile compounds and water, and then forged at six different high temperatures in the range of 150 °C to 450 °C in a high-temperature furnace (model DF404, Deng Yng Ltd., New Taipei City, Taiwan) associated with a K-type thermocouple thermometer. The steps for heating the combined catalyst of TiO2/CH3ONa under controlled temperature are illustrated in Figure 2. The calcined catalyst was first heated to 100 °C for 0.5 h. Then, the temperature in the furnace was controlled at 100 °C for 4 h. It was then increased to 200 °C in 2 h and allowed to remain constant at this temperature for 3 h to complete the production of the catalyst. The physical structure of TiO2 was then sintered, ligated with CH3ONa, and cooled down naturally to form a firm, solid, strong alkaline photocatalyst structure of TiO2/CH3ONa.
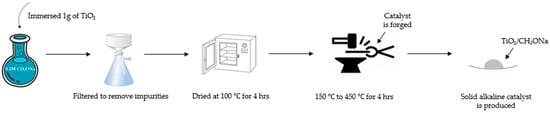
Figure 1.
Preparation procedures for the synthesized photocatalyst TiO2/CH3ONa.

Table 1.
Properties of titanium oxide (TiO2).
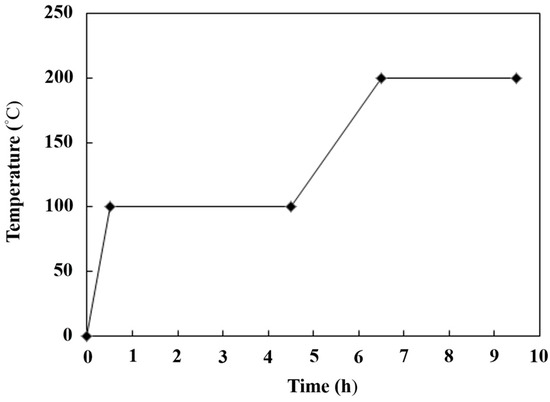
Figure 2.
The temperature-control program for the calcined catalyst in the high-temperature furnace.
2.2. Analysis for Properties of TiO2/CH3ONa Solid Alkaline Catalysts
An FTIR analyzer (model 27, Bruker Ltd., Berlin, Germany) was used to determine the infrared absorption spectra of the organic materials. The structures and compositions of the samples were analyzed by Raman spectroscopy (Horiba Scientific Ltd., Chiyoda-ku, Tokyo, Japan), a method generally used to measure non-polar organic materials. The TiO2/CH3ONa catalyst was forged and sintered and FTIR in this experiment was used to mainly analyze the absorption peaks of CH3ONa on the surface of the TiO2 particles and the absorption peaks of TiO2 itself. The Brunauer–Emmett–Teller (BET) surface areas were acquired by the physisorption of nitrogen using a Microtrac-Belsorp-Max instrument (Haan, Germany). The pore size and surface area influence the selectivity and activity of a heterogeneous porous catalyst [32].
The XRD (model X’Pert Pro MPD, Panalytical Ltd., Almelo, The Netherlands) was used to analyze and measure the X-ray polarization of the metallic and non-metallic materials to obtain information on the crystalline structure compositions and estimate the size and crystallinity by determining the width of the polarization peaks. Here, XRD was primarily employed to analyze the crystalline structure of the photocatalyst after being forged and the structural changes in the TiO2 catalyst upon the adsorption of CH3ONa.
The chemical compositions of the catalysts before and after TiO2 and CH3ONa were sintered together and analyzed by a high-resolution two-shot ion cutting system (model JIB-4500, JEOL Inc., Peabody, MA, USA) associated with EDS. The equipment was also used to verify whether the sodium element had burned onto the interior hole surface of the TiO2 nanoparticle. A scanning electron microscope (SEM, nano science instruments Ltd., Phoenix, AZ, USA) was used to capture the micro image of the surface of the TiO2/CH3ONa catalyst and observe the ligating structure between the catalysts of TiO2 and CH3ONa.
2.3. Transesterification of Palm Oil Using Heterogeneous Nanocatalysts TiO2/CH3ONa
Palm oil, which was supplied from Formosa Oilseed Processing Co., Ltd. (Taichung City, Taiwan) mixed with methanol in a molar ratio from 1:5 to 1:9, was transesterified with the synthesized nanocatalyst TiO2/CH3ONa of amounts 1~3 wt.% of the palm oil. Methanol was purchased from First Chemical Materials Ltd. (Taipei City, Taiwan). Fatty acid methyl esters (FAMEs) from the palm oil were finally produced after the transesterification reaction under 1h of reaction time, and 62 °C of reaction temperatures. After completing the transesterification processes, glacial acetic acid of the corresponding amount to the added strong alkaline CH3ONa was mixed and stirred with the crude product to neutralize its pH value. A centrifuging machine was used to separate the crude product into the biodiesel and glycerol at the upper and lower layers due to their obvious density difference. The crude biodiesel after excluding the glycerol was heated to 70 °C to vaporize methanol and other volatile materials away from the biodiesel for 20 min. An amount of 10 vol.% de-ionized water of the crude biodiesel weight was used to water-wash the biodiesel two times and then the water content was centrifuged away. The biodiesel was finally distilled at 110 °C to remove water to obtain the biodiesel sample.
3. Results and Discussion
3.1. Effect of Forging Temperature on the Characteristic Bonding of TiO2/CH3ONa Alkaline Catalyst
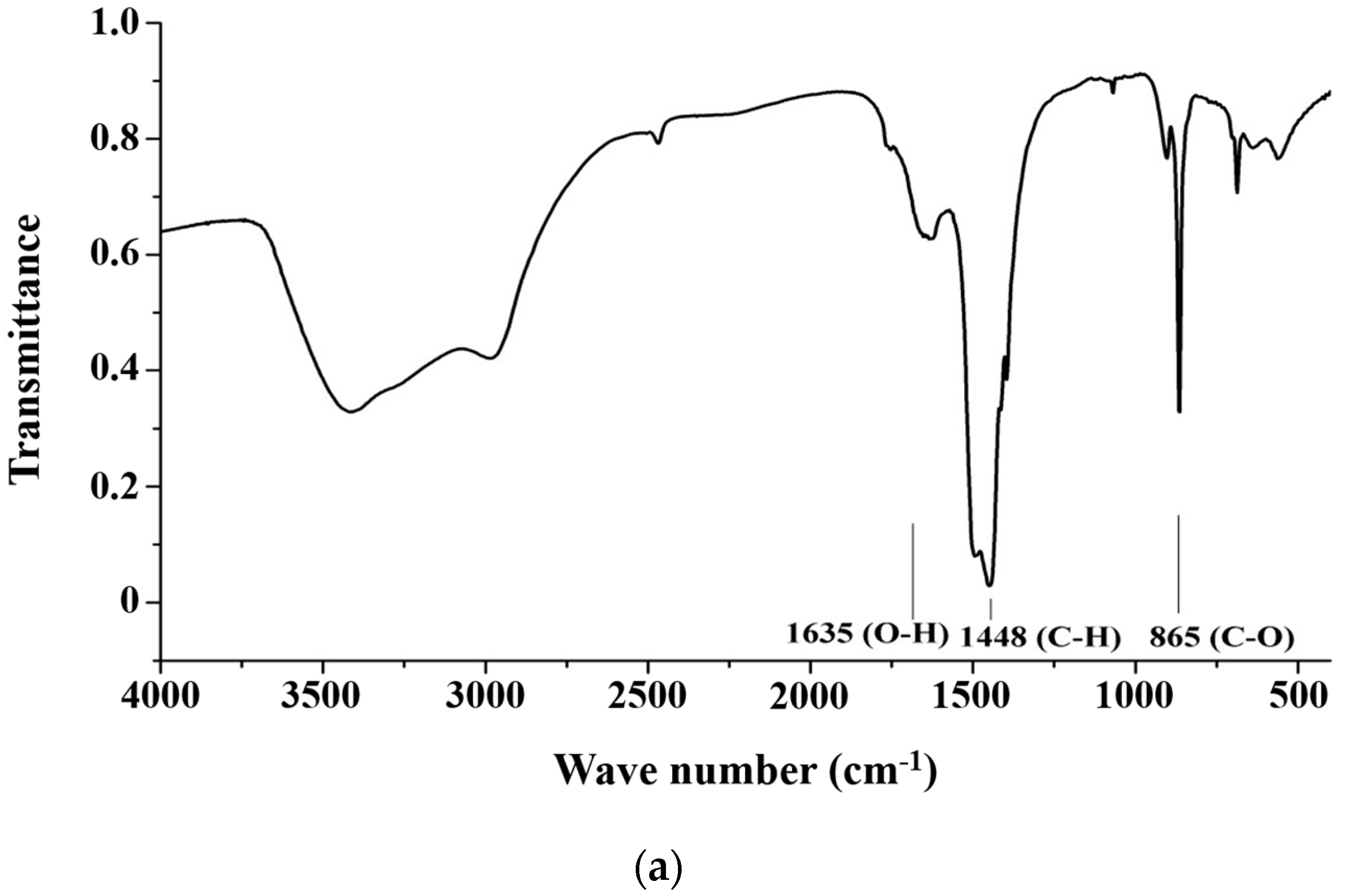
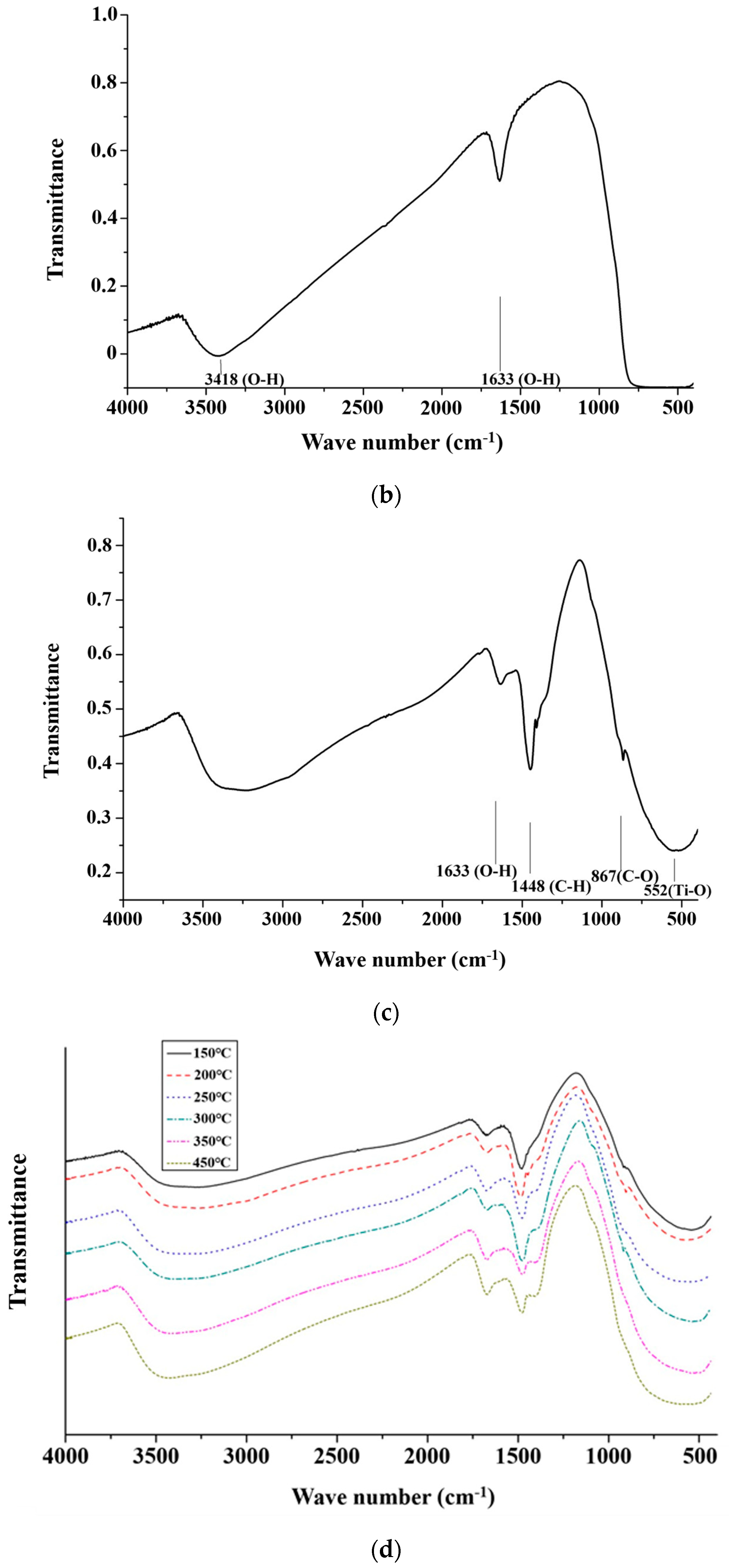
Figure 3a shows the spectra of CH3ONa analyzed by an FTIR. The peaks at the wave numbers 3000 cm−1 and 1450 cm−1 appeared due to the stretching and bending vibration of C-H bonding in methanol, at 3500 cm−1 were attributed to O-H bonding in the water molecule, and those at 1000 cm−1 were attributed to the bending vibration of C-O bonding [33]. Figure 3b shows the spectra of the TiO2 powder derived from the analysis of the FTIR results. The O-H bonding of the water molecule appeared around the wave number of 3500 cm−1 and 1600 cm−1, and the characteristic bonding of TiO2 appeared below 1000 cm−1 [34]. Figure 3c shows the FTIR spectrum of the solid alkaline catalyst TiO2/CH3ONa in which the two catalysts of TiO2 and CH3ONa were sintered at 200 °C. A comparison of the absorption peaks at 560 cm−1 (Ti-O bond), 865 cm−1 (C-O bond), 1448 cm−1 (C-H bond), and 1600 cm−1 (O-H bond) in Figure 3a,b confirm the adsorption of CH3ONa on the surface of TiO2. Hence, the impregnation method could successfully associate CH3ONa with the TiO2 nanoparticles to form the heterogeneous catalyst TiO2/CH3ONa. Thus, the synthesized catalyst TiO2/CH3ONa was successfully prepared, and the photocatalyst is ready to catalyze the transesterification reaction for biodiesel production.
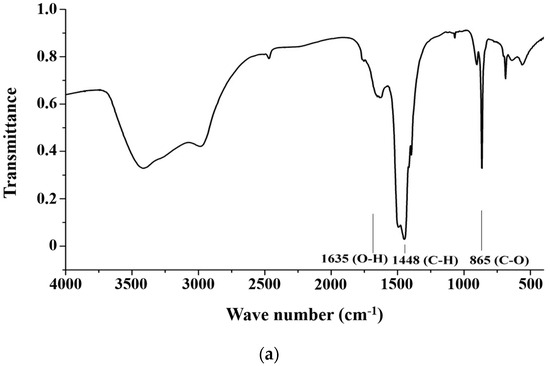
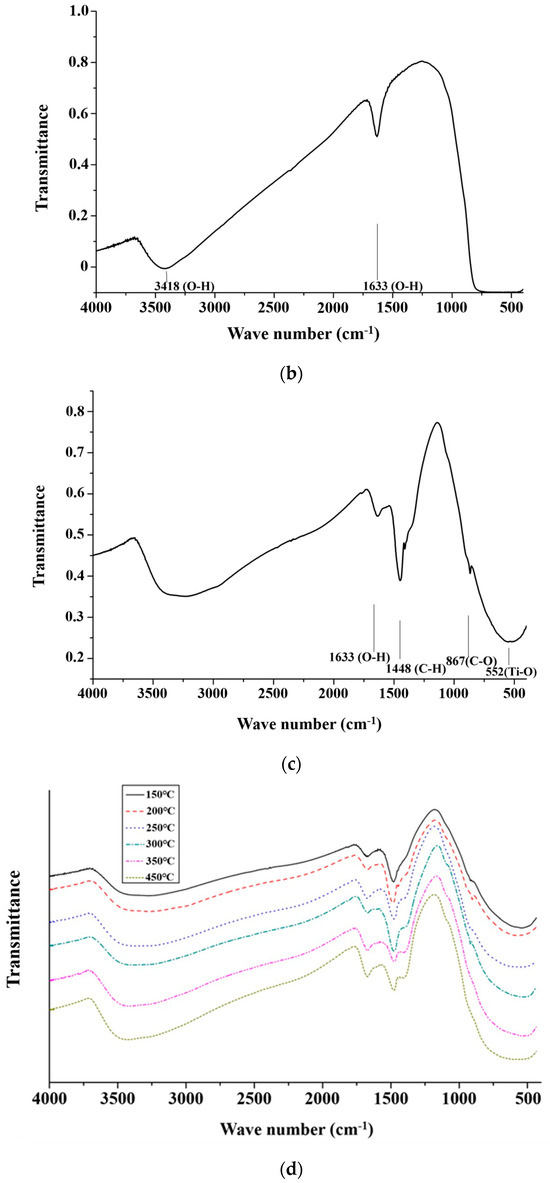
Figure 3.
Spectra of (a) catalyst components of sodium methoxide (CH3Ona), (b) titanium dioxide (TiO2) powder, (c) solid alkaline catalyst TiO2/CH3Ona sintered at 200 °C, and (d) solid alkaline catalyst TiO2/CH3Ona sintered at temperatures between 150 °C and 450 °C analyzed by Fourier transform infrared spectroscopy.
The FTIR spectra of the TiO2/CH3Ona catalyst prepared at the sintered temperatures between 150 °C and 450 °C reveal absorption peaks at 1648 cm−1 representing O-H bonds, and 1448 cm−1 representing C-H bonds (Figure 3d) [35]. When the sintered temperature was increased to 300 °C, those two peaks of the FTIR spectra decreased significantly with the sintered temperatures for the TiO2 and CH3Ona catalysts. Therefore, under the high sintered temperature of greater than 300 °C, the C-H bond reacted with the oxygen in the air to form carbon dioxide and water, leading to a weakened tendency of those two peaks in the FTIR spectra. Under high sintered temperatures, for the peak at 1648 cm−1, the O-H bond was also affected by the solid alkaline catalysts. It was observed that the peaks at the wave numbers of 560 cm−1 and 865 cm−1, which are below 1000 cm−1, corresponded to the absorption peaks of the TiO2 catalyst [36].
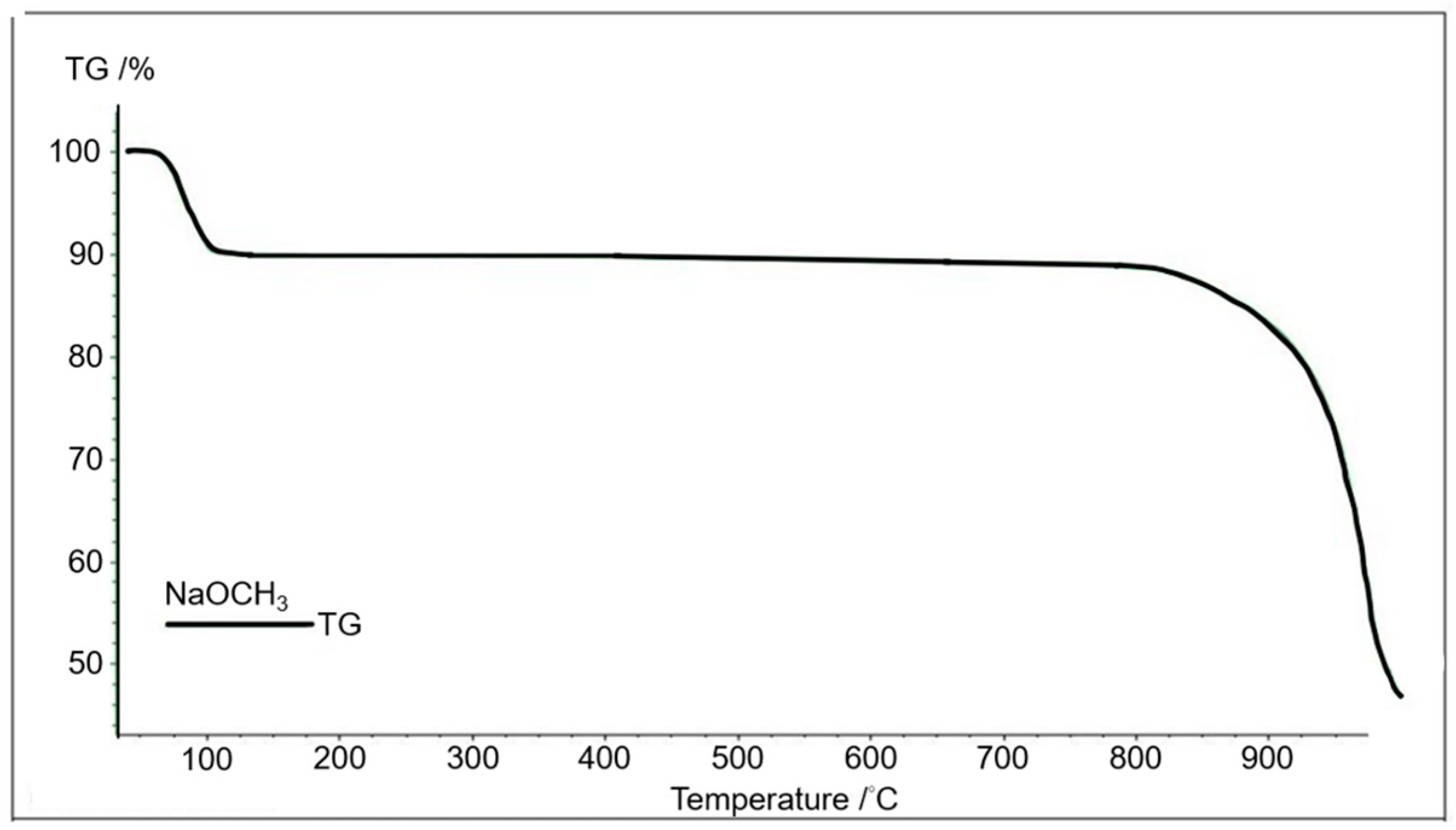
3.2. Thermogravimetric Analysis of CH3Ona Catalysts
Figure 4 shows the relationship of the variations in thermogravimetric weight with the sintering temperature from 40 to approximately 1000 °C of the CH3Ona by the TGA. It is inferred that there was obvious weight loss within this sintering temperature range due to the moisture desorption from the CH3Ona catalyst and the evaporation of methanol at the sintering temperature from 40 °C to 100 °C. In contrast, there was no significant variation in weight in the temperature range of 100 °C and 800 °C. This was ascribed to insignificant thermal cracking or the chemical conversion of CH3Ona, a stable chemical compound. There was no chemical reaction and thermal pyrolysis of CH3Ona within this temperature range. The thermogravimetric weight of CH3Ona decreased significantly with the sintering temperature higher than 800 °C, primarily due to rapid thermal cracking under such high sintering temperatures.
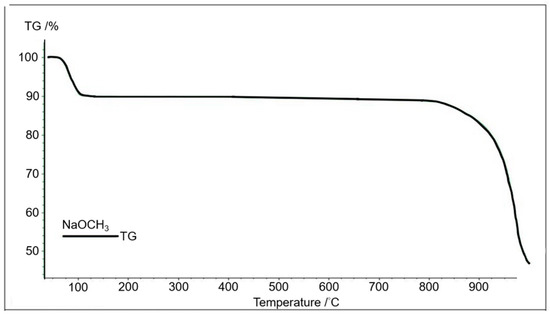
Figure 4.
Thermogravimetric analysis of CH3Ona at the temperature range of 40 °C to 1000 °C.
3.3. Structural Analysis for TiO2/CH3Ona Catalyst
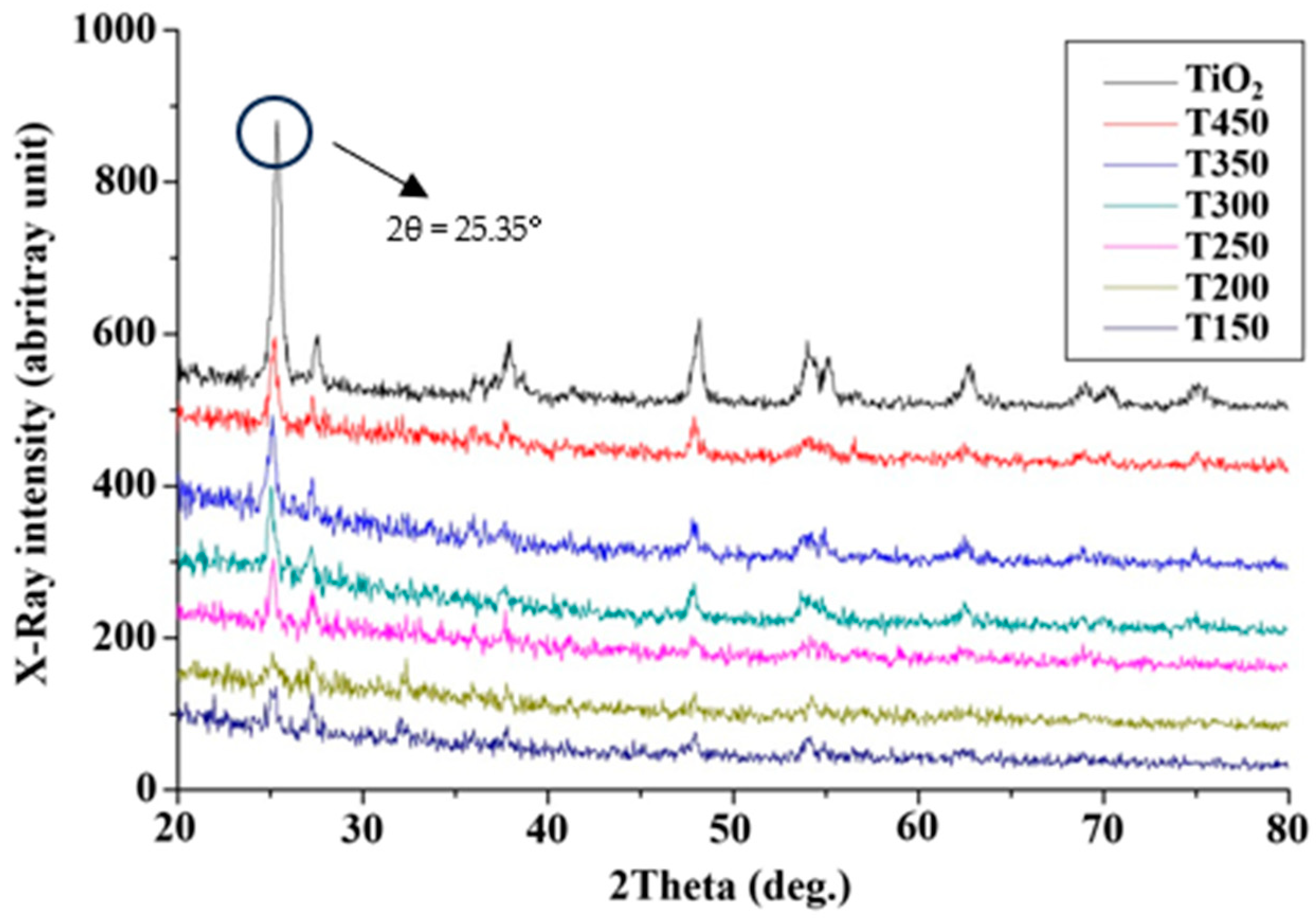
In this experiment, the TiO2 catalyst was analyzed by an XRD within the range of scanning 2θ angle from 20° to 80° (Figure 5). The present solid-state catalyst belonged to the crystalline structure of the anatase-type TiO2 upon comparison with the standard of the Joint Committee on Powder Diffraction Standards database. However, the TiO2 metal oxide has different compositional structures for the TiO2/CH3Ona catalyst depending on the forging temperatures. The TiO2/CH3Ona alkaline catalysts prepared herein did not affect the crystalline phase of TiO2 after the synthesis of TiO2 with CH3Ona. Nevertheless, the micro-crystalline structure of TiO2 after being combined with CH3Ona was not affected and retained the anatase structure of TiO2 when the range of the 2θ angle was between 20° and 80° (Figure 5). The anatase-type TiO2 exhibited the strongest X-ray intensity compared to the TiO2/CH3Ona catalysts calcined at various temperatures. The increase in the forging temperature from 150 °C to 450 °C caused a larger X-ray intensity due to energy increase. X-rays with a much higher energy and shorter wavelengths are a type of electromagnetic radiation [37]. The intensity of the X-ray is an indication of the radiation rate. A higher energy tends to produce a more intense X-ray beam and produce an X-ray of much higher intensity [38]. Manikandan et al. [39] applied XRD to analyze the structural properties of synthesized TiO2 nanoparticles and observed the tetragonal anatase TiO2 structure and fine crystalline nature of the TiO2 nanoparticle. A peak with the highest intensity appeared at the distinct diffraction angle observed at 2θ = 25.35°, with an average crystallite size of 16.81 nm. HRTEM image shows the interplanar spacing of 3.52 Å and 2.38 Å agree well with the (101) and (004) plane of anatase TiO2 (JCPDS 89-4921) [40]. The findings imply that the forging temperatures between 150 °C and 450 °C did not significantly alter the crystalline structure and size of the heterogeneous catalyst of TiO2 and TiO2/CH3Ona.
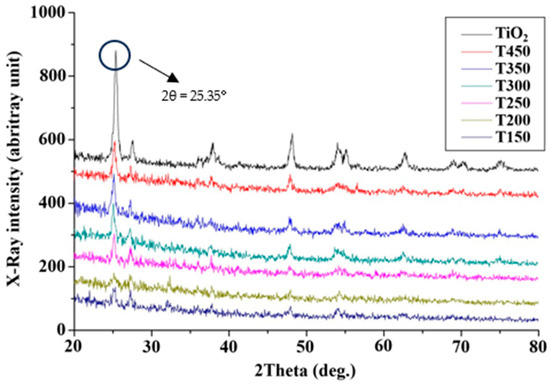
Figure 5.
X-ray intensity spectra of pure TiO2 and TiO2/CH3Ona calcined at 150 °C~450 °C.
3.4. Scanning Electron Microscopy and Elemental Na Analysis for TiO2/CH3Ona Catalyst
The TiO2/CH3Ona catalyst in which CH3Ona was sintered into the particles of TiO2 powder was prepared through the immersion of the TiO2 powder in the aqueous CH3Ona solution and followed by high-temperature forging and sintering. A high-resolution two-shot ion-cutting system along with an EDS was used to analyze the weight percentages of the elemental compositions of TiO2 after an alkalization reaction as shown in Table 2. No elemental Na was detected in the sole TiO2 compound. However, it was confirmed that a significant amount of Na ranging from 5.03 wt.% to 9.26 wt.% was sintered on the TiO2 for the TiO2/CH3Ona catalyst at forging temperatures in the range of 150 °C to 450 °C. Within the sintering temperature range between 150 °C and 450 °C, the amount of Na sintered on the TiO2 surface generally decreased. This is ascribed to the evaporation of a large amount of CH3Ona from the impregnating TiO2/CH3Ona catalyst to remove the Na component under higher sintering temperatures, leading to lower Na content at higher sintering temperatures. The amount of elemental Na sintering on the TiO2 particle surface of the TiO2/CH3Ona catalyst was the highest at 9.26 wt.% under the forging temperature of 200 °C.

Table 2.
Elemental compositions of solid alkaline catalysts analyzed by Energy Dispersive Spectrometer (EDS).
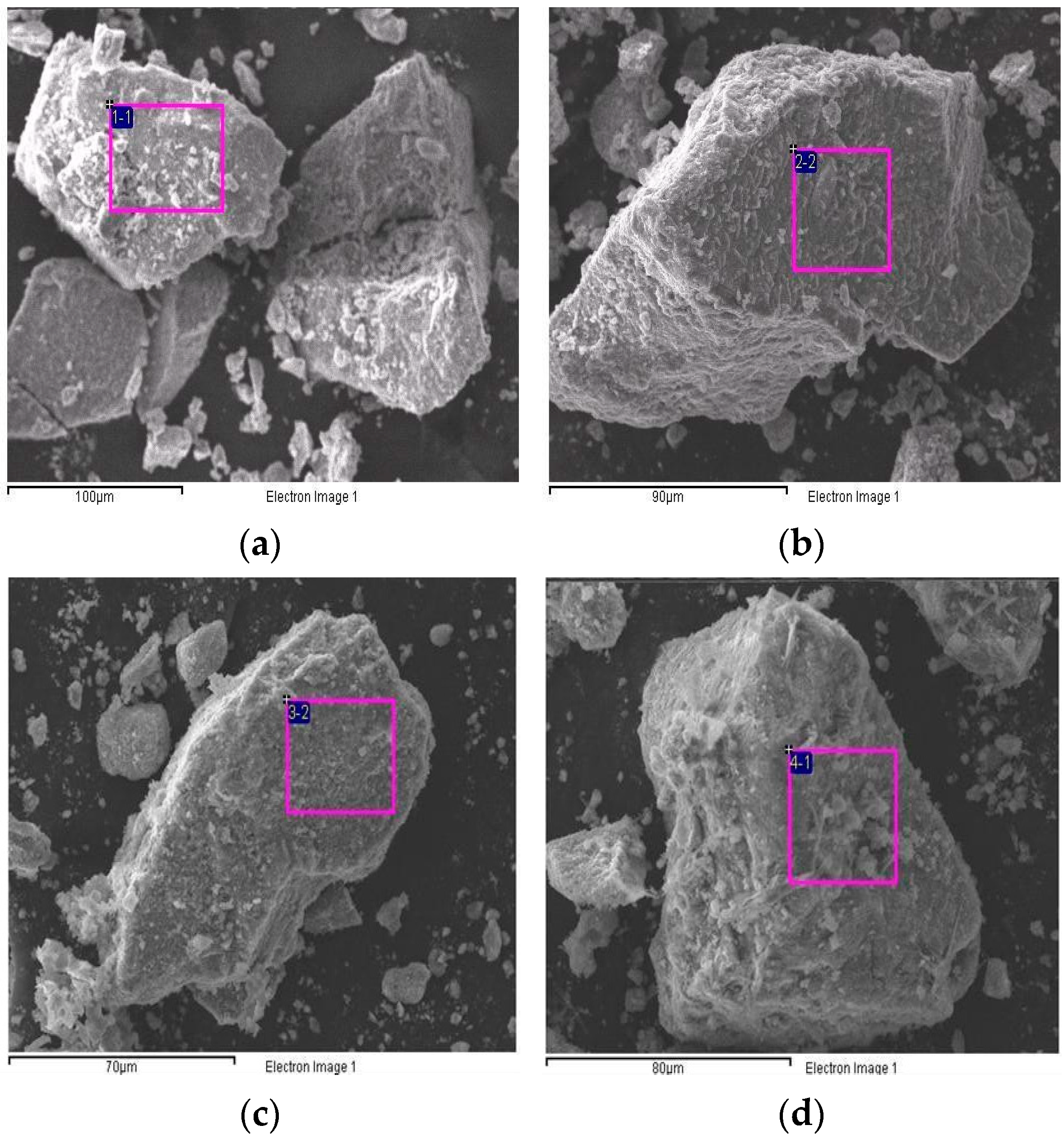
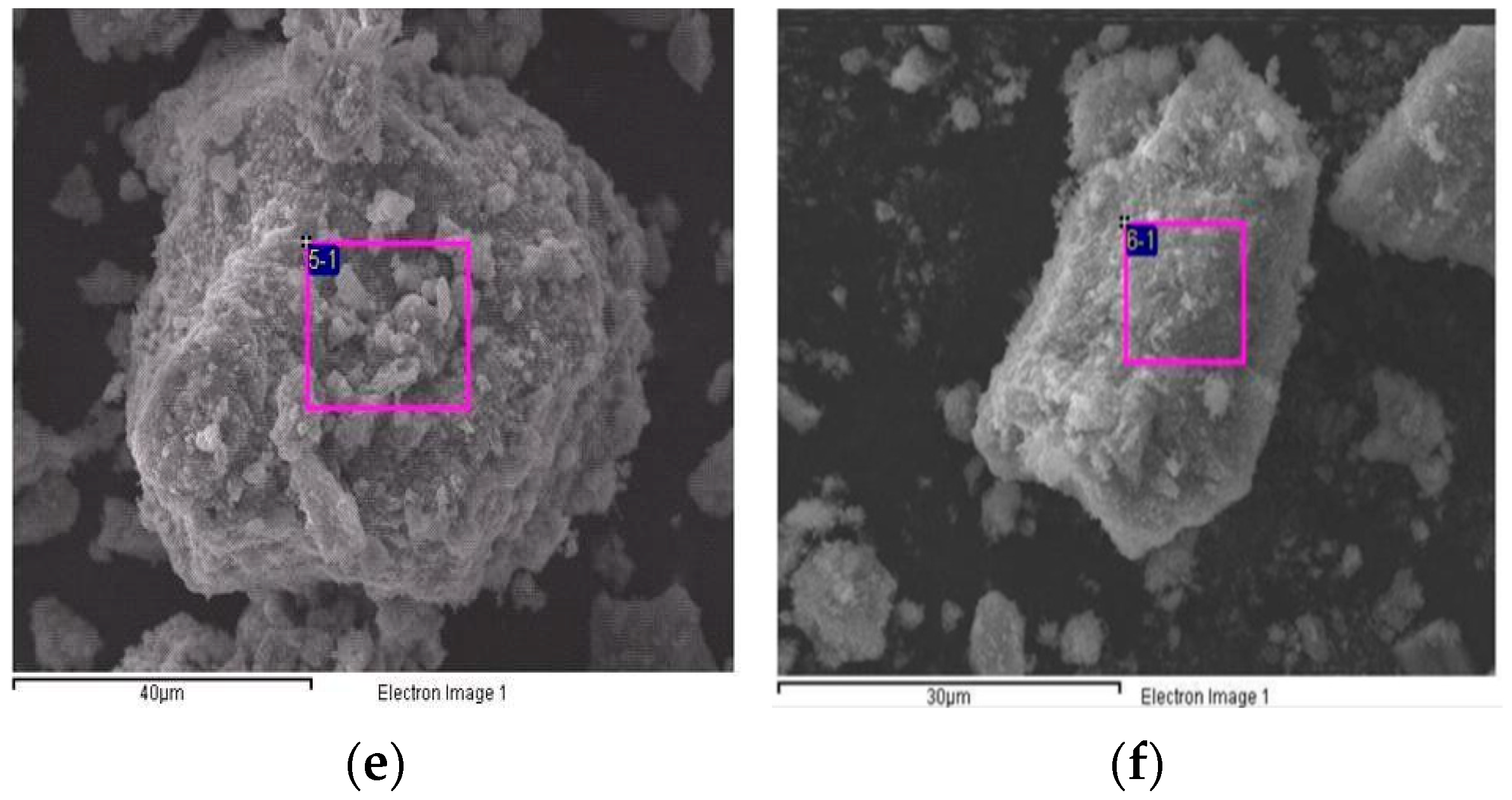
Figure 6 shows the SEM images of the TiO2/CH3Ona catalyst under various forging temperatures from 150 °C to 450 °C. At a higher forging temperature, the TiO2 surface exhibited more obvious roughness and an intense formation of large-sized crystal particles, which can be seen by comparing the appearance of Figure 6f with Figure 6a. When the forging temperature for synthesizing the solid alkaline catalyst TiO2/CH3Ona was increased to 300 °C, the sintering effect of CH3Ona onto the TiO2 surface became much more significant. Singh et al. [41] confirmed that increasing the heat treatment temperature would improve the hardness and other relevant mechanical properties of the sample of engine parts. Hence, more crystal CH3Ona clusters were found to aggregate on the TiO2 nanoparticle surface. In addition, the TiO2 nanoparticles appeared more rugged under higher sintering temperatures. Nabi et al. [42] observed the surface morphology of the TiO2 nanoparticle by SEM. They found that the TiO2 particles are all nanometer-sized and the images exhibited bicycle-chain-like, clustered, and evenly distributed structures over the particle surface. Zhang et al. [43] used the SEM technique to monitor the coarsening process of a polymorgh catalyst (Ni-P/Ni/NF) which proceeded through the cathodic electrodeposition of Ni on the NF. Figure 6 shows that although there was no phase change or thermal cracking phenomenon in the temperature range of 100 °C and 800 °C for the TiO2/CH3Ona catalyst, the addition of CH3Ona at a higher forging temperature did affect the sintering effects of TiO2 with CH3Ona.
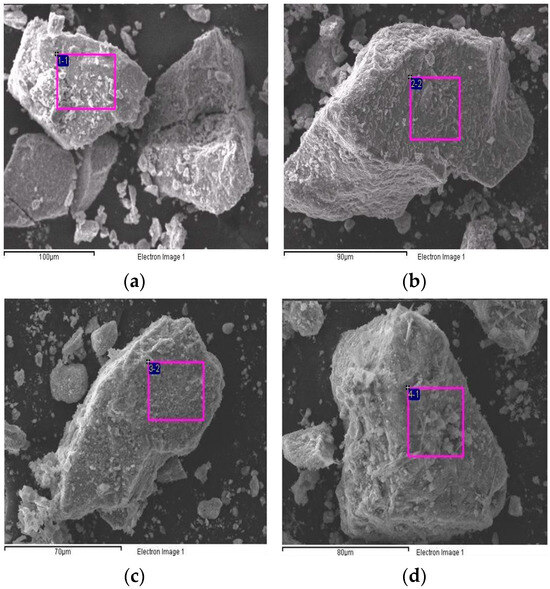
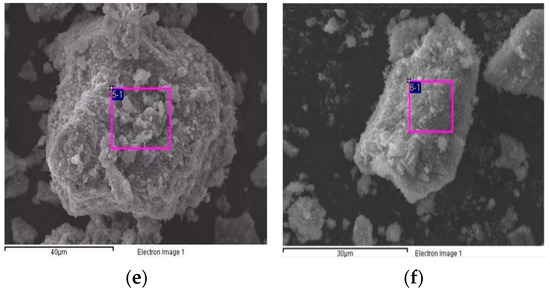
Figure 6.
Photos of scanning electron microscopy of the TiO2/CH3Ona catalyst under various forging temperatures from 150 °C to 450 °C at (a) 150 °C, (b) 200 °C, (c) 250 °C, (d) 300 °C, (e) 350 °C, and (f) 450 °C forging temperatures.
4. Conclusions
TiO2 nanoparticles of the anatase phase were impregnated with a strong alkaline CH3ONa catalyst to prepare a heterogeneous TiO2/CH3ONa photocatalyst to catalyze transesterification for palm oil biodiesel production. Then, the nanometer-sized TiO2 particles were immersed in the aqueous solution of the CH3ONa catalyst and calcined in a high-temperature furnace to synthesize the TiO2/CH3ONa photocatalyst. The catalyst characteristics of the photocatalyst TiO2/CH3ONa were then analyzed by FTIR, XRD, and TGA to derive the optimum preparation conditions for the photocatalyst to have an excellent catalytic effect to improve the transesterification reaction. The experimental results are summarized below.
- (1)
- A FTIR was used to find the wavelengths of sodium methoxide (CH3ONa) bonding in the infrared spectral frequency ranging from 560 cm−1 to 1500 cm−1. The FTIR spectra confirmed that CH3ONa was successfully sintered and bonded onto the TiO2 carrier employing the current catalyst impregnation method.
- (2)
- The elemental analysis by EDS revealed that the solid, strong, alkaline catalyst prepared by the current immersion method contained the CH3ONa catalyst inside the porous surface of the carrier TiO2 nanoparticles.
- (3)
- A TGA was used to analyze the CH3ONa catalyst heated from 40 °C to 1000 °C. There was no significant weight loss of the CH3ONa catalyst when heated from 100 °C to 800 °C, although the weight loss was noticeable when the catalyst was heated from 40 °C to 100 °C. This proved the thermal stability of the CH3ONa catalyst and presented no phase change or thermal cracking upon heating the catalyst from 100 °C to 800 °C.
- (4)
- The TiO2/CH3ONa strong alkaline catalyst produced in this study did not alter the crystalline structure of the TiO2 nanoparticles in the presence of the CH3ONa catalyst. However, the merging of the CH3ONa compound into the nanoparticles of TiO2 crystals did affect the catalyst characteristics of TiO2 after sintering CH3ONa into the TiO2 nanoparticles. In addition, the wavelength intensity of the anatase-type TiO2 was reduced due to the loading of CH3ONa into the TiO2 carrier.
Author Contributions
Conceptualization, C.-Y.L.; methodology, C.-Y.L.; formal analysis, S.-L.T.; investigation, C.-Y.L. and S.-L.T.; data curation, S.-L.T.; writing—original draft preparation, C.-Y.L. and S.-L.T.; writing—review and editing, C.-Y.L.; supervision, C.-Y.L.; project administration, C.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Council of Science and Technology, Taiwan, under the contract number NCST 110-2221-E-019-055-MY2.
Data Availability Statement
The data presented in this study are contained within this article.
Acknowledgments
The authors would like to gratefully acknowledge the financial support from the National Council of Science and Technology, Taiwan, under the contract number NCST 110-2221-E-019-055-MY2.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Lin, C.-Y.; Lin, K.-H. Comparison of the engine performance of soybean oil biodiesel emulsions prepared by phase inversion temperature and mechanical homogenization methods. Processes 2023, 11, 907. [Google Scholar] [CrossRef]
- Nitbani, F.O.; Tjitda, P.J.P.; Nurohmah, B.A.; Wogo, H.E. Preparation of fatty acid and monoglyceride from vegetable oil. J. Oleo Sci. 2020, 69, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Shi, H.; Zhao, Y.; Dai, J.; Zhang, K. Organophosphate ester cresyl diphenyl phosphate disrupts lipid homeostasis in zebrafish embryos. Environ. Pollut. 2024, 342, 123149. [Google Scholar] [CrossRef] [PubMed]
- Maltsev, Y.; Maltseva, K. Fatty acids of microalgae: Diversity and applications. Rev. Environ. Sci. Biotechnol. 2021, 20, 515–547. [Google Scholar] [CrossRef]
- Torres-García, M.; Martin, J.F.G.; Aguilar, F.J.J.-E.; Barbin, D.F.; Álvarez-Mateos, P. Vegetable oils as renewable fuels for power plants based on low and medium speed diesel engines. J. Energy Inst. 2020, 93, 953–961. [Google Scholar] [CrossRef]
- Bai, S.-T.; De Smet, G.; Liao, Y.; Sun, R.; Zhou, C.; Beller, M.; Maes, B.U.W.; Sels, B.F. Homogeneous and heterogeneous catalysts for hydrogenation of CO2 to methanol under mild conditions. Chem. Soc. Rev. 2020, 50, 4259–4298. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Ning, P.; He, L.; Guan, Q.; Shi, Y.; Miao, R. Near-room temperature transesterification over bifunctional CunO-Bs/SBA-15 catalyst for biodiesel production. Renew. Energy 2021, 170, 1–11. [Google Scholar] [CrossRef]
- Mukhtar, A.; Saqib, S.; Lin, H.; Shah, M.U.H.; Ullah, S.; Younas, M.; Rezakazemi, M.; Ibrahim, M.; Mahmood, A.; Asif, S.; et al. Current status and challenges in the heterogeneous catalysis for biodiesel production. Renew. Sustain. Energy Rev. 2022, 157, 112012. [Google Scholar] [CrossRef]
- Jan, H.A.; Saqib, N.U.; Khusro, A.; Sahibzada, M.U.K.; Rauf, M.; Alghamdi, S.; Almehmadi, M.; Khandaker, M.U.; Bin Emran, T.; Mohafez, H. Synthesis of biodiesel from Carthamus tinctorius L. oil using TiO2 nanoparticles as a catalyst. J. King Saud Univ.-Sci. 2022, 34, 102317. [Google Scholar] [CrossRef]
- Sajjad, N.; Orfali, R.; Perveen, S.; Rehman, S.; Sultan, A.; Akhtar, T.; Nazir, A.; Muhammad, G.; Mehmood, T.; Ghaffar, S.; et al. Biodiesel production from alkali-catalyzed transesterification of Tamarindus indica seed oil and optimization of process conditions. Molecules 2022, 27, 3230. [Google Scholar] [CrossRef]
- Prabhahar, R.S.S.; Benitha, V.S.; Nagarajan, J. Improved yield of palm oil biodiesel through nano catalytic transesterification. Mater. Today Proc. 2021, 46, 8433–8437. [Google Scholar] [CrossRef]
- Jambhulkar, D.K.; Ugwekar, R.P.; Bhanvase, B.A.; Barai, D.P. A review on solid base heterogeneous catalysts: Preparation, characterization and applications. Chem. Eng. Commun. 2022, 209, 433–484. [Google Scholar] [CrossRef]
- Alipour, Z.; Borugadda, V.B.; Wang, H.; Dalai, A.K. Syngas production through dry reforming: A review on catalysts and their materials, preparation methods and reactor type. Chem. Eng. J. 2023, 452, 139416. [Google Scholar] [CrossRef]
- Zhu, W.; Chen, X.; Li, C.; Liu, Z.; Liang, C. Manipulating morphology and surface engineering of spinel cobalt oxides to attain high catalytic performance for propane oxidation. J. Catal. 2021, 396, 179–191. [Google Scholar] [CrossRef]
- Riaz, S.; Park, S.-J. An overview of TiO2-based photocatalytic membrane reactors for water and wastewater treatments. J. Ind. Eng. Chem. 2020, 84, 23–41. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Zhang, M.; Zhou, Q.; Li, X.; Chen, T.; Wang, S. Preparation of Ag3PO4/TiO2 (B) heterojunction nanobelt with extended light response and enhanced photocatalytic performance. Molecules 2021, 26, 6987. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, Y.; Li, J.; Wei, S.; Gao, X.; Wang, P. Combustion-impregnation preparation of Ni/SiO2 catalyst with improved low-temperature activity for CO2 methanation. Int. J. Hydrogen Energy 2021, 46, 20919–20929. [Google Scholar] [CrossRef]
- Dias, A.P.S.; Ramos, M.; Catarino, M.; Puna, J.; Gomes, J. Solvent assisted biodiesel production by co-processing beef tallow and soybean oil over calcium catalysts. Waste Biomass-Valorization 2020, 11, 6249–6259. [Google Scholar] [CrossRef]
- Fattah, I.M.R.; Ong, H.C.; Mahlia, T.M.I.; Mofijur, M.; Silitonga, A.S.; Rahman, S.M.A.; Ahmad, A. State of the art of catalysts for biodiesel production. Front. Energy Res. 2020, 8, 101. [Google Scholar] [CrossRef]
- Osazuwa, O.U.; Abidin, S.Z. The Functionality of ion exchange resins for esterification, transesterification and hydrogenation reactions. ChemistrySelect 2020, 5, 7658–7670. [Google Scholar] [CrossRef]
- Praveen, B.V.S.; Pradhan, N.C.; Ashok, A.; Guduru, R.K.; Vij, R.K.; Jeeru, L.R. Production of biodiesel: Kinetics and reusability studies of the Mg–Al hydrotalcite catalyst using Jatropha oil. React. Chem. Eng. 2023, 8, 1729–1737. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Z.; Shang, Y.; Yu, S.; Li, L.; Liu, S.; Song, Z. Preparation of Hydrocarbon Rich Biofuel from Cracking of Waste Cooking Oil Catalyzed by Basic Mesoporous Molecular Sieve Me-KIT-6. Catal. Lett. 2023, 153, 3392–3404. [Google Scholar] [CrossRef]
- Fu, J.; Dong, J.; Si, R.; Sun, K.; Zhang, J.; Li, M.; Yu, N.; Zhang, B.; Humphrey, M.G.; Fu, Q.; et al. Synergistic effects for enhanced catalysis in a dual single-atom catalyst. ACS Catal. 2021, 11, 1952–1961. [Google Scholar] [CrossRef]
- Islam, M.W. A review of dolomite catalyst for biomass gasification tar removal. Fuel 2020, 267, 117095. [Google Scholar] [CrossRef]
- Ge, Y.; Qin, X.; Li, A.; Deng, Y.; Lin, L.; Zhang, M.; Ma, D. Maximizing the synergistic effect of CoNi catalyst on α-MoC for robust hydrogen production. J. Am. Chem. Soc. 2020, 143, 628–633. [Google Scholar] [CrossRef] [PubMed]
- Health, Safety and Environmental Department. Safety Data Sheet 2024; Thermo Fisher Scientific Chemicals, Inc.: Ward Hill, MA, USA, 2024. [Google Scholar]
- Jeon, J.P.; Kweon, D.H.; Jang, B.J.; Ju, M.J.; Baek, J.B. Enhancing the photocatalytic activity of TiO2 catalysts. Adv. Sustain. Syst. 2020, 4, 2000197. [Google Scholar] [CrossRef]
- Dar, G.I.; Saeed, M.; Wu, A. Toxicity of TiO2 nanoparticles. In TiO2 Nanoparticles: Applications in Nanobiotechnology and Nanomedicine; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2020; pp. 67–103. [Google Scholar]
- Zhang, Y.; Xu, X. Machine learning band gaps of doped-TiO2 photocatalysts from structural and morphological parameters. ACS Omega 2020, 5, 15344–15352. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, C.; Degennaro, L.; Luisi, R. Titanium dioxide as a catalyst in biodiesel production. Catalysts 2019, 9, 75. [Google Scholar] [CrossRef]
- Uni-Onward Corp., Ltd. TiO2 Property Table; Uni-Onward Corp., Ltd.: New Taipei City, Taiwan, 2020. [Google Scholar]
- Kandathil, V.; Kulkarni, B.; Siddiqa, A.; Kempasiddaiah, M.; Sasidhar, B.S.; Patil, S.A.; Patil, S.A. Immobilized N-heterocyclic carbene-palladium (II) complex on graphene oxide as efficient and recyclable catalyst for Suzuki–Miyaura cross-coupling and reduction of nitroarenes. Catal. Lett. 2020, 150, 384–403. [Google Scholar] [CrossRef]
- Yang, J.; Chen, J.; Bi, H.; Gu, H.; Liu, Z.; Peng, W. Molecules and functions of rosewood: Pterocarpus indicus. Therm. Sci. 2020, 24 Pt A, 1869–1876. [Google Scholar] [CrossRef]
- Thakur, S.; Thakur, V.; Kaur, A.; Singh, L. Synthesis and the study of structural, thermal and optical properties of (100-x) Bi2O3-x (BaO-TiO2) glass system. Optik 2020, 223, 165646. [Google Scholar] [CrossRef]
- Wang, S.J.; Chen, Z.; Hu, X.; Zou, J.; Xie, Z.; Mi, H.Y.; Jing, X. MXene reinforced organohydrogels with ultra-stability, high sensitivity and anti-freezing ability for flexible strain sensors. J. Mater. Chem. C. 2022, 10, 11914–11923. [Google Scholar] [CrossRef]
- Saravanan, S.; Dubey, R.S. Optical and morphological studies of TiO2 nanoparticles prepared by sol–gel method Mater. Today Proc. 2021, 47, 1811–1814. [Google Scholar]
- Lu, L.; Sun, M.; Lu, Q.; Wu, T.; Huang, B. High energy X-ray radiation sensitive scintillating materials for medical imaging, cancer diagnosis and therapy. Nano Energy 2021, 79, 105437. [Google Scholar] [CrossRef]
- Tong, X.; Yang, P.; Wang, Y.; Qin, Y.; Guo, X. Enhanced photoelectrochemical water splitting performance of TiO2 nanotube arrays coated with an ultrathin nitrogen-doped carbon film by molecular layer deposition. Nanoscale 2014, 6, 6692–6700. [Google Scholar] [CrossRef] [PubMed]
- Krishna, D.N.G.; Philip, J. Review on surface-characterization applications of X-ray photoelectron spectroscopy (XPS): Recent developments and challenges. Appl. Surf. Sci. Adv. 2022, 12, 100332. [Google Scholar] [CrossRef]
- Manikandan, B.; Murali, K.R.; John, R. Optical, morphological and microstructural investigation of TiO2 nanoparticles for photocatalytic application. Iran. J. Catal. 2021, 11, 1–11. [Google Scholar]
- Singh, B.; Grewal, J.S.; Kumar, R.; Sharma, S.; Kumar, R.; Singh, H.; Lozanovic, J. Tribomechanical, and Microstructural Morphological analysis of Nitride Ferrous Powder Metallurgy Composites for Enhanced Automotive Valve Guide Performance. J. Mater. Res. Technol. 2024, 31, 6–25. [Google Scholar] [CrossRef]
- Nabi, G.; Ain, Q.U.; Tahir, M.B.; Nadeem Riaz, K.; Iqbal, T.; Rafique, M.; Rizwan, M. Green synthesis of TiO2 nanoparticles using lemon peel extract: Their optical and photocatalytic properties. J. Environ. Anal. Chem. 2022, 102, 434–442. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Z.; Ji, Y.; Yang, J.; Fan, K.; Ma, X.; Chen, Y. Surface engineering induced hierarchical porous Ni12P5-Ni2P polymorphs catalyst for efficient wide pH hydrogen production. Appl. Catal. B Environ. 2021, 282, 119609. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).