Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents
Abstract
:1. Introduction
2. Modelling Approach and Reaction Mechanism
3. Model Validation
4. Result and Discussion
5. Estimation of Diffusion Coefficient and Effect of Co-Solvent on CO2 Diffusion in Various Aqueous and Water-Lean Solvent Systems
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, J.; Qian, J.; He, Y. Water-lean triethylenetetramine/N, N-diethylethanolamine/n-propanol biphasic solvents: Phase-separation performance and mechanism for CO2 capture. Sep. Purif. Technol. 2022, 289, 120740. [Google Scholar] [CrossRef]
- Tan, Y.; Nookuea, W.; Li, H.; Thorin, E.; Yan, J. Property impacts on Carbon Capture and Storage (CCS) processes: A review. Energy Convers. Manag. 2016, 118, 204–222. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Luo, J. Life cycle cost analysis of power generation from underground coal gasification with carbon capture and storage (CCS) to measure the economic feasibility. Resour. Policy 2024, 92, 104996. [Google Scholar] [CrossRef]
- Desai, B.H. United Nations Environment Programme (UNEP). Yearb. Int. Environ. Law 2020, 31, 319–325. [Google Scholar] [CrossRef]
- Fernández, J.; Sotenko, M.; Derevschikov, V.; Lysikov, A.; Rebrov, E.V. A radiofrequency heated reactor system for post-combustion carbon capture. Chem. Eng. Process. Process Intensif. 2016, 108, 17–26. [Google Scholar] [CrossRef]
- Rubin, E.S.; Davison, J.E.; Herzog, H. The cost of CO2 capture and storage. Int. J. Greenh. Gas Control 2015, 40, 378–400. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Pinto, D.D.; Knuutila, H.K. From hybrid solvents to water-lean solvents–A critical and historical review. Sep. Purif. Technol. 2021, 260, 118193. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Koech, P.K.; Glezakou, V.-A.; Rousseau, R.; Malhotra, D.; Cantu, D.C. Water-lean solvents for post-combustion CO2 capture: Fundamentals, uncertainties, opportunities, and outlook. Chem. Rev. 2017, 117, 9594–9624. [Google Scholar] [CrossRef]
- Ping, T.; Dong, Y.; Shen, S. Energy-efficient CO2 capture using nonaqueous absorbents of secondary alkanolamines with a 2-butoxyethanol cosolvent. ACS Sustain. Chem. Eng. 2020, 8, 18071–18082. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Pinto, D.D.; Knuutila, H.K. Investigating opportunities for water-lean solvents in CO2 capture: VLE and heat of absorption in water-lean solvents containing MEA. Sep. Purif. Technol. 2020, 231, 115883. [Google Scholar] [CrossRef]
- Wang, N.; Peng, Z.; Gao, H.; Sema, T.; Shi, J.; Liang, Z. New insight and evaluation of secondary Amine/N-butanol biphasic solutions for CO2 Capture: Equilibrium Solubility, phase separation Behavior, absorption Rate, desorption Rate, energy consumption and ion species. Chem. Eng. J. 2022, 431, 133912. [Google Scholar] [CrossRef]
- Shi, X.; Li, C.; Guo, H.; Shen, S. Density, viscosity, and excess properties of binary mixtures of 2-(methylamino) ethanol with 2-methoxyethanol, 2-ethoxyethanol, and 2-butoxyethanol from 293.15 to 353.15 K. J. Chem. Eng. Data 2019, 64, 3960–3970. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Shi, X.; Li, H.; Shen, S. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Alkhatib, I.I.; Pereira, L.M.; AlHajaj, A.; Vega, L.F. Performance of non-aqueous amine hybrid solvents mixtures for CO2 capture: A study using a molecular-based model. J. CO2 Util. 2020, 35, 126–144. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, P.; He, X.; Yu, Q. Preparation of intercalated MXene by TPAOH and its adsorption characteristics towards U (VI). J. Radioanal. Nucl. Chem. 2024, 333, 1999–2014. [Google Scholar] [CrossRef]
- Garcia, M.; Knuutila, H.K.; Aronu, U.E.; Gu, S. Influence of substitution of water by organic solvents in amine solutions on absorption of CO2. Int. J. Greenh. Gas Control 2018, 78, 286–305. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, J.; Hu, L.; Liu, J.; Zhou, J.; Cen, K. Phase-changing solution PZ/DMF for efficient CO2 capture and low corrosiveness to carbon steel. Fuel 2018, 216, 418–426. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, C.; Chen, H.; Shen, Y.; Zhang, S.; Wang, L.; Chen, J. Novel biphasic solvent with tunable phase separation for CO2 capture: Role of water content in mechanism, kinetics, and energy penalty. Environ. Sci. Technol. 2019, 53, 4470–4479. [Google Scholar] [CrossRef]
- Lin, Y.-J.; Pan, T.-H.; Wong, D.S.-H.; Jang, S.-S.; Chi, Y.-W.; Yeh, C.-H. Plantwide control of CO2 capture by absorption and stripping using monoethanolamine solution. Ind. Eng. Chem. Res. 2011, 50, 1338–1345. [Google Scholar] [CrossRef]
- Park, Y.; Lin, K.-Y.A.; Park, A.-H.A.; Petit, C. Recent advances in anhydrous solvents for CO2 capture: Ionic liquids, switchable solvents, and nanoparticle organic hybrid materials. Front. Energy Res. 2015, 3, 42. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Wang, L.; Li, Q.; Zhang, S.; Chen, B.; Jiang, L.; Zhang, Y. Superior energy-saving splitter in monoethanolamine-based biphasic solvents for CO2 capture from coal-fired flue gas. Appl. Energy 2019, 242, 302–310. [Google Scholar] [CrossRef]
- Li, Q.; Gao, G.; Wang, R.; Zhang, S.; An, S.; Wang, L. Role of 1-methylimidazole in regulating the CO2 capture performance of triethylenetetramine-based biphasic solvents. Int. J. Greenh. Gas Control 2021, 108, 103330. [Google Scholar] [CrossRef]
- Hu, H.; Fang, M.; Liu, F.; Wang, T.; Xia, Z.; Zhang, W.; Ge, C.; Yuan, J. Novel alkanolamine-based biphasic solvent for CO2 capture with low energy consumption and phase change mechanism analysis. Appl. Energy 2022, 324, 119570. [Google Scholar] [CrossRef]
- Chronopoulos, T.; Fernandez-Diez, Y.; Maroto-Valer, M.M.; Ocone, R.; Reay, D.A. CO2 desorption via microwave heating for post-combustion carbon capture. Microporous Mesoporous Mater. 2014, 197, 288–290. [Google Scholar] [CrossRef]
- Qian, J.; Sun, R.; Sun, S.; Gao, J. Computer-Free Group-Addition Method for p K a Prediction of 73 Amines for CO2 Capture. J. Chem. Eng. Data 2017, 62, 111–122. [Google Scholar] [CrossRef]
- El Hadri, N.; Quang, D.V.; Goetheer, E.L.; Zahra, M.R.A. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2017, 185, 1433–1449. [Google Scholar] [CrossRef]
- Barzagli, F.; Mani, F.; Peruzzini, M. A comparative study of the CO2 absorption in some solvent-free alkanolamines and in aqueous monoethanolamine (MEA). Environ. Sci. Technol. 2016, 50, 7239–7246. [Google Scholar] [CrossRef]
- Liu, B.; Luo, X.; Gao, H.; Idem, R.; Tontiwachwuthikul, P.; Olson, W.; Liang, Z. Reaction kinetics of the absorption of carbon dioxide (CO2) in aqueous solutions of sterically hindered secondary alkanolamines using the stopped-flow technique. Chem. Eng. Sci. 2017, 170, 16–25. [Google Scholar] [CrossRef]
- Bihong, L.; Kexuan, Y.; Xiaobin, Z.; Zuoming, Z.; Guohua, J. 2-Amino-2-methyl-1-propanol based non-aqueous absorbent for energy-efficient and non-corrosive carbon dioxide capture. Appl. Energy 2020, 264, 114703. [Google Scholar] [CrossRef]
- Tan, L.; Shariff, A.; Lau, K.; Bustam, M. Impact of high pressure on high concentration carbon dioxide capture from natural gas by monoethanolamine/N-methyl-2-pyrrolidone solvent in absorption packed column. Int. J. Greenh. Gas Control 2015, 34, 25–30. [Google Scholar] [CrossRef]
- Henni, A.; Mather, A.E. Solubility of carbon dioxide in methyldiethanolamine+ methanol+ water. J. Chem. Eng. Data 1995, 40, 493–495. [Google Scholar] [CrossRef]
- Wanderley, R.R.; Evjen, S.; Pinto, D.D.D.; Knuutila, H.K. The salting-out effect in some physical absorbents for CO2 capture. Chem. Eng. Trans. 2018, 69, 97–102. [Google Scholar]
- Schlecht, M.F. Molecular Modeling on the PC; Wiley-VCH: New York, NY, USA, 1998. [Google Scholar]
- Li, Z.; Gan, B.; Li, Z.; Zhang, H.; Wang, D.; Zhang, Y.; Wang, Y. Kinetic mechanisms of methane hydrate replacement and carbon dioxide hydrate reorganization. Chem. Eng. J. 2023, 477, 146973. [Google Scholar] [CrossRef]
- Narimani, M.; Amjad-Iranagh, S.; Modarress, H. Performance of tertiary amines as the absorbents for CO2 capture: Quantum mechanics and molecular dynamics studies. J. Nat. Gas Sci. Eng. 2017, 47, 154–166. [Google Scholar] [CrossRef]
- Song, Z.; Han, D.; Yang, M.; Huang, J.; Shao, X.; Li, H. Formic acid formation via direct hydration reaction (CO+ H2O→ HCOOH) on magnesia-silver composite. Appl. Surf. Sci. 2023, 607, 155067. [Google Scholar] [CrossRef]
- Sharif, M.; Fan, H.; Sultan, S.; Yu, Y.; Zhang, T.; Wu, X.; Zhang, Z. Evaluation of CO2 absorption and stripping process for primary and secondary amines. Mol. Simul. 2023, 49, 565–575. [Google Scholar] [CrossRef]
- Biovia. Material Studio, Biovia: 2019 Version; Available online: https://www.3ds.com/products/biovia/materials-studio (accessed on 24 July 2024).
- Meunier, M. Diffusion coefficients of small gas molecules in amorphous cis-1, 4-polybutadiene estimated by molecular dynamics simulations. J. Chem. Phys. 2005, 123, 134906. [Google Scholar] [CrossRef]
- Allen, M.P.; Tildesley, D.J. Computer Simulation of Liquids; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Zhang, G.; Liu, J.; Qian, J.; Zhang, X.; Liu, Z. Review of Research Progress and Stability Studies of Amine-based Biphasic Absorbents for CO2 Capture. J. Ind. Eng. Chem. 2024, 134, 28–50. [Google Scholar] [CrossRef]
- Shamiri, A.; Shafeeyan, M.; Tee, H.; Leo, C.; Aroua, M.; Aghamohammadi, N. Absorption of CO2 into aqueous mixtures of glycerol and monoethanolamine. J. Nat. Gas Sci. Eng. 2016, 35, 605–613. [Google Scholar] [CrossRef]
- Olyaei, E.; Hafizi, A.; Rahimpour, M. Low energy phase change CO2 absorption using water-lean mixtures of glycine amino acid: Effect of co-solvent. J. Mol. Liq. 2021, 336, 116286. [Google Scholar] [CrossRef]
- Versteeg, G.; van Swaaij, W.P.M. On the kinetics between CO2 and alkanolamines both in aqueous and non-aqueous solutions—II. Tertiary amines. Chem. Eng. Sci. 1988, 43, 587–591. [Google Scholar] [CrossRef]
- Laddha, S.; Danckwerts, P. Reaction of CO2 with ethanolamines: Kinetics from gas-absorption. Chem. Eng. Sci. 1981, 36, 479–482. [Google Scholar] [CrossRef]
- Matsubara, H.; Pichierri, F.; Kurihara, K. Mechanism of diffusion slowdown in confined liquids. Phys. Rev. Lett. 2012, 109, 197801. [Google Scholar] [CrossRef]
- Chemspider. Royal Society of Chemistry Database. 2020. Available online: https://www.chemspider.com/ (accessed on 24 July 2024).
- Yaws, C.L. Yaws’ Thermophysical Properties of Chemicals and Hydrocarbons; Knovel: New York, NY, USA, 2009. [Google Scholar]
- Harun, N.; Masiren, E. Molecular dynamic simulation of amine-CO2 absorption process. Indian J. Sci. Technol. 2017, 10. [Google Scholar] [CrossRef]
- Masiren, E.; Harun, N.; Ibrahim, W.; Adam, F. Intermolecular Interaction of Monoethanolamine, Diethanolamine, Methyl diethanolamine, 2-Amino-2-methyl-1-propanol and Piperazine Amines in Absorption Process to Capture CO2 using Molecular Dynamic Simulation Approach. Universiti Malasiya Pahang. The National Conference for Postgraduate Researc. 2016. Available online: http://umpir.ump.edu.my/id/eprint/15835/1/P053%20pg385-397.pdf (accessed on 24 July 2024).
- Yuan, Y.; Rochelle, G.T. CO2 absorption rate in semi-aqueous monoethanolamine. Chem. Eng. Sci. 2018, 182, 56–66. [Google Scholar] [CrossRef]
- Sharif, M.; Han, T.; Wang, T.; Shi, X.; Fang, M.; Shuming, D.; Meng, R.; Gao, X. Investigation of Rational Design of Amine Solvents for CO2 Capture: A Computational Approach. Chem. Eng. Res. Des. 2024, 204, 524–535. [Google Scholar] [CrossRef]
- Sharif, M.; Wang, T.; Xu, Y.; Fang, M.; Wu, H.; Gao, X. Evaluating solvent efficiency for carbon capture: Comparative analysis of temperature, concentration and diffusivity effects. Geoenergy Sci. Eng. 2024, 237, 212833. [Google Scholar] [CrossRef]
- Al-Ghawas, H.A.; Hagewiesche, D.P.; Ruiz-Ibanez, G.; Sandall, O.C. Physicochemical properties important for carbon dioxide absorption in aqueous methyldiethanolamine. J. Chem. Eng. Data 1989, 34, 385–391. [Google Scholar] [CrossRef]
- Varady, M.J.; Knox, C.K.; Cabalo, J.B.; Bringuier, S.A.; Pearl, T.P.; Lambeth, R.H.; Mantooth, B.A. Molecular dynamics study of competing hydrogen bonding interactions in multicomponent diffusion in polyurethanes. Polymer 2018, 140, 140–149. [Google Scholar] [CrossRef]
- Xu, Y.; Yang, Q.; Puxty, G.; Yu, H.; Conway, W.; Fang, M.; Wang, T.; Mulder, R.J. Diffusivity in Novel Diamine-Based Water-Lean Absorbent Systems for CO2 Capture Applications. Ind. Eng. Chem. Res. 2022, 61, 12493–12503. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.-W.; Choi, B.-S.; Lee, J.-W. Absorption of carbon dioxide into non-aqueous solutions of N-methyldiethanolamine. Korean J. Chem. Eng. 2006, 23, 806–811. [Google Scholar] [CrossRef]
- Xu, H.-J.; Zhang, C.-F.; Zheng, Z.-S. Solubility of hydrogen sulfide and carbon dioxide in a solution of methyldiethanolamine mixed with ethylene glycol. Ind. Eng. Chem. Res. 2002, 41, 6175–6180. [Google Scholar] [CrossRef]



| Generation | Type of Solvents | Energy Consumption (GJ/tCO2) | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|
| 1st Generation 1930s | Amine Solvents 30 wt% MEA PZ aqueous solvents | 3.7–4.0 | Fast reaction rate High capture capacity | High energy penalty solvent regeneration | [21] |
| 2nd Generation 1990s | Blended amine solvent | 2.5–3.2 | Improved capture efficiency Low energy compared to single amine | Possible increased volatility and corrosiveness depending on the blend | [22] |
| 3rd Generation 2000s | Water-lean solvent/Phase-change Absorbent | 1.8–2.4 | Lower overall energy consumption compared to aqueous amine systems Lower water content reduce energy consumption for regeneration Enhanced selectivity and capacity for CO2 capture | Higher viscosity can lead to mass transfer limitations Limited long term operation data available | [23] |
| Name | Molecular Structure | Molecular Weight (g.mol−1) | Density (g.mL−1) | CAS No. |
|---|---|---|---|---|
| MDEA | 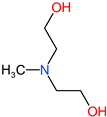 | 119 | 1.1 | 105-59-9 |
| MeOH |  | 32 | 0.80 | 67-56-1 |
| MAE |  | 75 | 0.94 | 109-83-1 |
| EG | 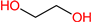 | 64 | 1.1 | 107-21-1 |
| NMP | 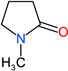 | 99 | 1.03 | 872-50-4 |
| System | Descriptions | MDEA | NMP | CO2 | H2O | |
|---|---|---|---|---|---|---|
| MDEA-NMP (40Wt%H2O) | Density of mixture (g.mL−1) | 1.053 | ||||
| No. of molecules | 12 | 10 | 11 | 111 | ||
| Weight% | 30% | 20% | 10% | 40% | ||
| MDEA-MeOH (40Wt%H2O) | Descriptions | MDEA | MeOH | CO2 | H2O | |
| Density of mixture (g.mL−1) | 1.007 | |||||
| No. of molecules | 120 | 310 | 220 | 2220 | ||
| Weight% | 30% | 20% | 10% | 40% | ||
| MDEA-EG (40Wt%H2O) | Descriptions | MDEA | EG | CO2 | H2O | |
| Density of mixture (g.mL−1) | 1.067 | |||||
| No. of molecules | 44 | 54 | 40 | 80 | ||
| Weight% | 30% | 20% | 10% | 40% | ||
| System | Descriptions | MDEA | MAE | NMP | CO2 | H2O |
| MDEA-MAE-NMP (30%H2O) | Density of mixture (g.mL−1) | 1.044 | ||||
| No. of molecules | 25 | 13 | 20 | 22 | 166 | |
| Weight% | 30% | 10% | 20% | 10% | 30% | |
| MDEA-MAE-MEG (30%H2O) | Descriptions | MDEA | MAE | MEG | CO2 | H2O |
| Density of mixture (g.mL−1) | 1.058 | |||||
| No. of molecules | 252 | 133 | 312 | 222 | 1666 | |
| Weight% | 30% | 10% | 20% | 10% | 30% | |
| MDEA-MAE-MeOH (30%H2O) | Descriptions | MDEA | MAE | MeOH | CO2 | H2O |
| Density of mixture (g.mL−1) | 1.002 | |||||
| No. of molecules | 12 | 6 | 30 | 11 | 83 | |
| Weight% | 30% | 10% | 20% | 10% | 30% | |
| MDEA-MAE-H2O (50%H2O) | Descriptions | MDEA | MAE | CO2 | H2O | |
| Density of mixture (g.mL−1) | 1.04 | |||||
| No. of molecules | 25 | 13 | 22 | 277 | ||
| Weight% | 30% | 10% | 10% | 50% |
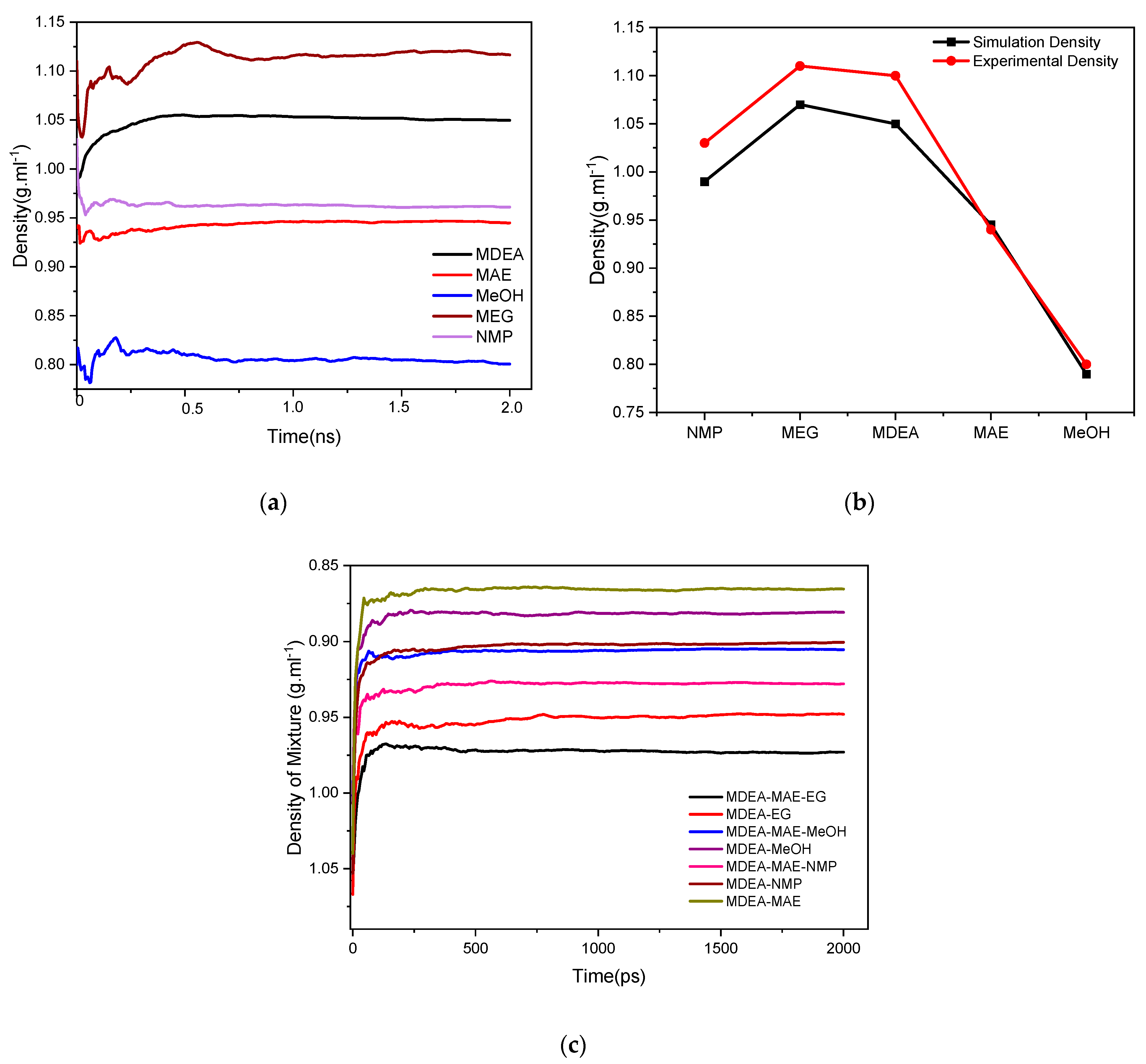
| Name of Solvent | Experimental Density (X1) [47] | Simulation Density (X2) Present Work | Std. Deviation |
|---|---|---|---|
| NMP | 1.03 | 0.99 | (−) 0.02 |
| MEG | 1.11 | 1.07 | (−) 0.02 |
| MDEA | 1.10 | 1.05 | (−) 0.025 |
| MAE | 0.940 | 0.945 | (+) 0.0025 |
| MeOH | 0.80 | 0.79 | (−) 0.005 |
| Solvent System | Initial Density (X1) | Final Density(X2) | Std. Deviation |
| MDEA-MAE-EG | 1.058 | 0.99 | (−) 0.034 |
| MDEA-EG | 1.067 | 0.99 | (−) 0.0385 |
| MDEA-MAE-MeOH | 1.002 | 0.90 | (−) 0.051 |
| MDEA-MeOH | 1.007 | 0.89 | (−) 0.0585 |
| MDEA-MAE-NMP | 1.044 | 0.92 | (−) 0.062 |
| MDEA-NMP | 1.053 | 0.90 | (−) 0.0765 |
| MDEA-MAE | 1.040 | 0.90 | (−) 0.070 |
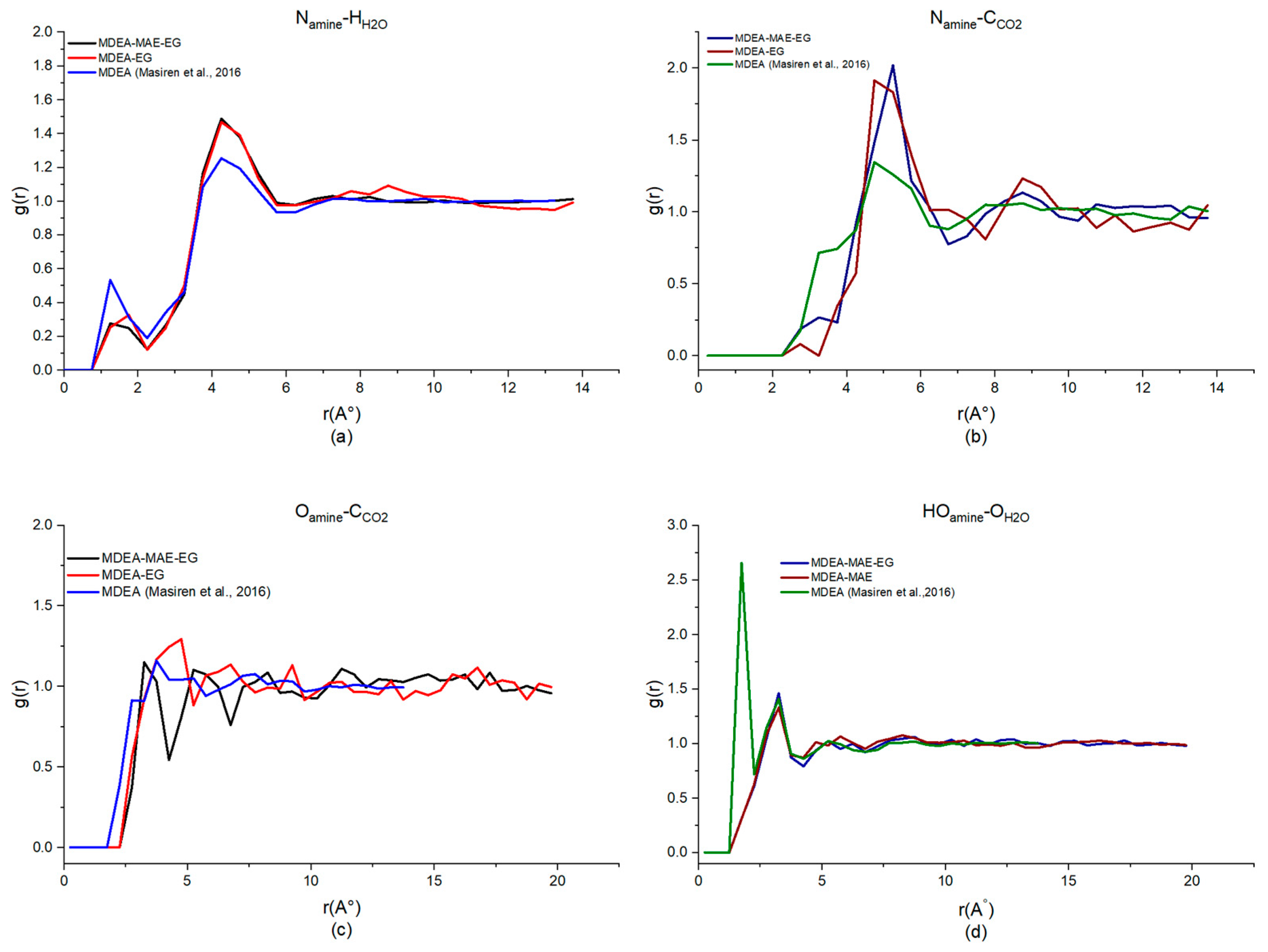
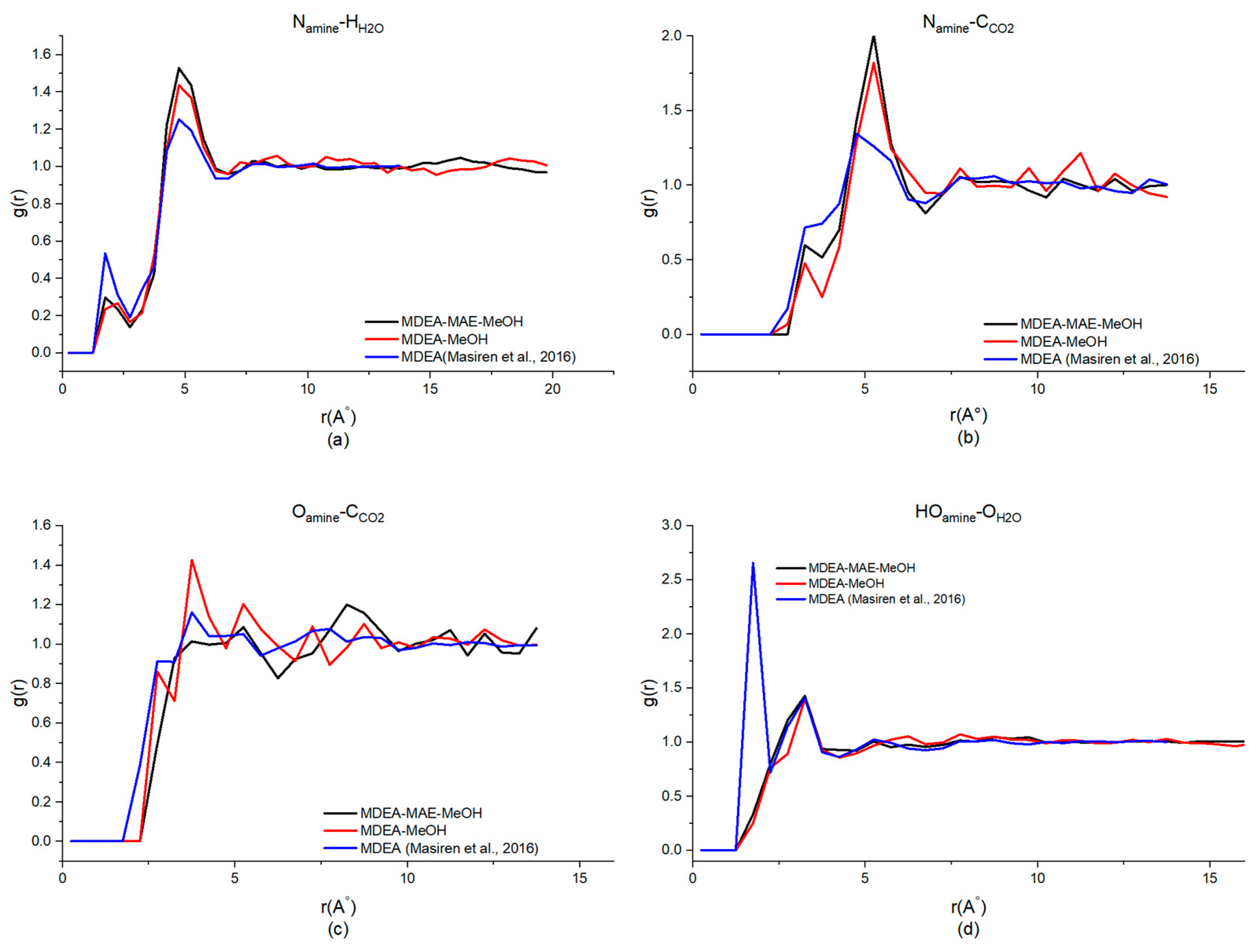
| System | Observed Interactions | |||
|---|---|---|---|---|
| Namine-HH2O | Namine-CCO2 | Oamine-CCO2 | HOamine-OH2O | |
| MDEA-NMP (40%H2O) | 4.75, 1.49 | 5.25, 1.90 | 5.25, 1.20 | 3.25, 1.37 |
| MDEA-MAE-NMP (30%H2O) | 4.75, 1.31 | 5.25, 1.67 | 5.25, 1.21 | 3.25, 1.68 |
| MDEA-EG (40%H2O) | 4.75, 1.47 | 4.75, 1.91 | 4.75, 1.29 | 3.25, 1.33 |
| MDEA-MAE-EG (30%H2O) | 4.75, 1.22 | 5.25, 2.02 | 5.25, 1.10 | 3.25, 1.45 |
| MDEA-MeOH (40%H2O) | 4.75, 1.44 | 5.25, 1.82 | 3.75, 1.43 | 3.25, 1.40 |
| MDEA-MAE-MeOH (30%H2O) | 4.75, 1.53 | 5.25, 2.01 | 3.25, 0.92 | 3.25, 1.43 |
| MDEA-MAE (50%H2O) | 4.75, 1.29 | 5.25, 1.67 | 5.25, 1.18 | 3.25, 1.35 |
| MDEA [49,50] | 4.25, 1.25 | 4.75, 1.34 | 3.75, 1.14 | 1.75, 2.65 |
| Solvent System * | Interactions Results | Advantages | Challenges |
|---|---|---|---|
| Methanol | Strong CO2 interaction, slightly weaker solubility | Low molecular weight High solubility Effective interactions with amine and CO2 Enhances Hydrogen bonding | High Volatility Evaporation loss Handling issue [7,8] |
| N-Methyl-2-Pyrolidone | Improved stability and strong hydrogen bonds | High boiling point Strong solvation properties Improved stability for CO2 capture Higher interaction with CO2 Significantly enhances CO2 capture efficiency | Higher viscosity Affects flow properties Increased energy requirement for solvent circulation [48] |
| Ethylene Glycol | Strongest CO2 interaction, enhanced stability | Hygroscopic Nature Interaction shows High affinity for water Stable interactions Balances strong hydrogen bonding with effective CO2 capture | Higher molecular weight Potential viscosity issues [41] |
| MDEA-MAE-Aqueous Solvent | Moderate CO2 capture and stability | Increased hydrogen bonding with water Enhanced CO2 capture efficiency Stable interactions due to higher water content | Possible handling and processing challenges due to higher water content Higher energy requirement for solvent regeneration [49,51] |
| Co-Solvent | Water Concentration | Generalized Impact |
|---|---|---|
| NMP | 30%H2O and 40%H2O | Exhibits stable CO2 interaction across different water concentrations, making it a versatile co-solvent. |
| EG | 30%H2O and 40%H2O | Shows higher CO2 interaction at 40%H2O, indicating better performance at higher water content but is temperature-sensitive. |
| MeOH | 30%H2O and 40%H2O | Demonstrates higher CO2 interaction at 40%H2O, but has handling challenges that must be managed. |
| MDEA-MAE (No Co-Solvent) | 50%H2O | Provides effective CO2 interactions with a good balance at 50%H2O, but lower than with Co-solvent blends |
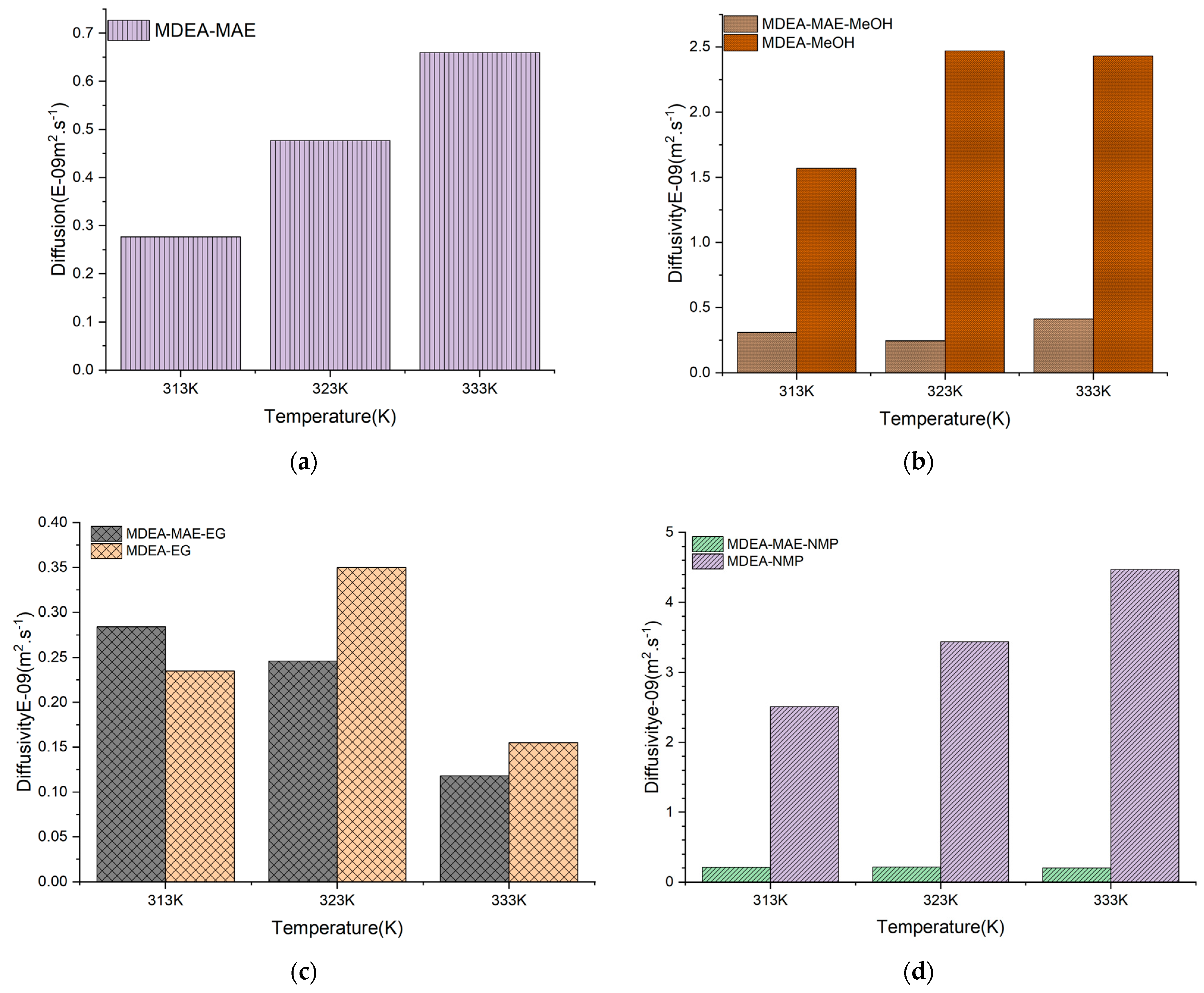
| Solvent System | CO2 Diffusivity (m2.s−1)/Temperature | ||
|---|---|---|---|
| 313 K | 323 K | 333 K | |
| MDEA-NMP (40%H2O) | 2.51 × 10−9 | 3.44 × 10−9 | 4.47 × 10−9 |
| MDEA-MAE-NMP (30%H2O) | 0.210 × 10−9 | 0.213 × 10−9 | 0.202 × 10−9 |
| MDEA-MeOH (40%H2O) | 1.57 × 10−9 | 2.47 × 10−9 | 3.06 × 10−9 |
| MDEA-MAE-MeOH (30%H2O) | 0.308 × 10−9 | 0.246 × 10−9 | 0.413 × 10−9 |
| MDEA-EG (40%H2O) | 0.235 × 10−9 | 0.350 × 10−9 | 0.155 × 10−9 |
| MDEA-MAE-EG (30%H2O) | 0.284 × 10−9 | 0.244 × 10−9 | 0.1180 × 10−9 |
| MDEA-MAE (50%H2O) | 0.276 × 10−9 | 0.477 × 10−9 | 0.660 × 10−9 |
| 50wt%MDEA | 0.62 × 10−9 (298 K) | 1.20 × 10−9 (313 K) | 1.80 × 10−9 (323 K) |
| CO2 in pure H2O | 1.61 × 10−9 (298 K) | 2.66 × 10−9 (313 K) | 3.78 × 10−9 (323 K) |
| System | MDEA-50wt% | Reference | ||
|---|---|---|---|---|
| Temperature (K) | 298 | 313 | 323 | |
| CO2 Diffusivity in MDEA-50wt% (m2.s−1) | 0.622 × 10−9 | 1.214 × 10−9 | 1.68 × 10−9 1.70 × 10−9 | [54] |
| 0.624 × 10−9 | 1.204 × 10−9 | 1.80 × 10−9 | This work | |
| Density (g.mL−1) of MDEA mixture | 1.0427 | 1.0331 | 1.0269 | [54] |
| 1.047 | 1.047 | 1.047 | This work | |
| System | Pure H2O | Reference | ||
| CO2 Diffusivity (m2.s−1) in H2O | 1.93 × 10−9 | 2.71 × 10−9 | 3.34 × 10−9 | [54] |
| 1.61 × 10−9 | 2.66 × 10−9 | 3.78 × 10−9 | This work | |
| Density of mixture (g.mL−1) | 0.9970 | 0.9922 | 0.9880 | [54] |
| 1.00 | 1.00 | 1.00 | This work | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharif, M.; Ge, C.; Wang, T.; Zhang, W.; Fang, M.; Gao, X. Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents. Processes 2024, 12, 1588. https://doi.org/10.3390/pr12081588
Sharif M, Ge C, Wang T, Zhang W, Fang M, Gao X. Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents. Processes. 2024; 12(8):1588. https://doi.org/10.3390/pr12081588
Chicago/Turabian StyleSharif, Maimoona, Chunliang Ge, Tao Wang, Wei Zhang, Mengxiang Fang, and Xiang Gao. 2024. "Computational Investigation of Co-Solvent Influence on CO2 Absorption and Diffusion in Water Lean Solvents" Processes 12, no. 8: 1588. https://doi.org/10.3390/pr12081588





