Abstract
A cobalt-loaded magnetic biochar (Co-MBC) catalyst was synthesized to enhance the removal of metronidazole (MNZ). Study explored the performance and mechanism of MNZ degradation by Co-MBC activated permonosulfate (PMS). Results showed that cobalt oxides were effectively deposited onto the biochar surface, new oxygen functional groups were added to the modified biochar, and the presence of the metallic element Co enhanced the efficiency of PMS activation in the composite. More than 90% of MNZ was removed after 60 min with a catalyst dosage of 0.2 g/L and a PS concentration of 1 mM. After four reuses, Co-MBC still showed excellent catalytic performance to degrade over 75% of MNZ. The reaction system performed well even in the presence of inorganic anions and organic macromolecules. However, the degradation rate was inhibited under alkaline conditions. The quenching experiment indicated that •SO4−, •OH, 1O2, and •O2− synergistically degraded MNZ, and that•SO4− played a dominant role. LC-MS was applied to assess intermediate degradation products, in which CO2, H2O, and NO3− were the final degradation products, and potential degradation pathways were suggested. In conclusion, Co-MBC was an efficient and stable catalytic material, and its ability to activate PMS was improved to effectively degrade antibiotics, a typical priority pollutant.
1. Introduction
Globally, the use of various antibiotic medicines is rapidly increasing, reaching 100,000 to 200,000 tons per year [1]. In aquatic environments, antibiotic resistance can be greatly enhanced by excess antibiotics and their derivatives [2]. Antibiotic contamination has become an issue of concern, presenting significant challenges to public safety and the ecological environment [3,4]. Metronidazole (MNZ) is a widely utilized antibiotic that may cause carcinogenic, mutagenic, and genotoxic effects in organisms. MNZ has great solubility in water, is difficult to biodegrade, has very slow absorption, and can persist in the environment for extended durations [5,6,7]. Hence, it is particularly important to control and treat MNZ contamination in aquatic environments.
Currently, advanced oxidation processes (AOPs) are extensively employed to degrade emerging organic pollutants in the environment, such as antibiotics [8,9,10]. AOPs can produce highly reactive oxygen species (ROS) to oxidize nonbiodegradable pollutants [11,12]. The ROS can be produced by advanced oxidation methods such as Fenton [13], ozone [14], activated persulfate [15], electrochemistry [16], ionizing radiation [17], and photocatalytic [18]. The activated persulfate method has been widely studied because of its high oxidizing power, wide pH range, and excellent water solubility [19,20]. Peroxydisulfate (PDS) and permonosulfate (PMS) are commonly used oxidizing agents in SR-AOPs [21]. Due to the asymmetry in the structure of the PMS molecule itself, PMS is easier to activate using a catalyst in the oxidizing system [22]. However, it is difficult for PMS to be used directly for the oxidative degradation of organic pollutants, and some methods are needed to activate PMS to degrade the pollutants.
UV light, transition metals, heat, and carbon-based materials can activate PMS to generate ROS, which degrade and mineralize organic contaminants. Among them, carbon-based materials have a wide range of applications in removing antibiotic contamination owing to their cost-effectiveness, economic viability, and environmentally benign nature [23]. Carbon materials, including graphene [24], carbon nanotubes [25], activated carbon [26], biochar [27], and aerogel [28], are extensively researched as a result of their low cost of use and strong biocompatibility [20]. Because of its enormous specific surface area, pore structure, and numerous oxygen-containing functional groups, biochar (BC) can efficiently catalyze PMS [4,29]. Magnetic biochar (MBC) materials are obtained by adding magnetic substances such as cobalt and iron to biochar, which retains the excellent properties of biochar and also has the property of being magnetizable and separable [30]. The surface of MBC holds persistent free radicals (PFRs), oxygenated functional groups (OFGs), magnetic compounds, and graphitic frameworks, enhancing its adsorption capabilities and catalytic qualities [31,32,33]. Therefore, MBC can boost PMS activation, contributing to more ROS generation and rapidly degrading organic pollutants.
The loading of metal materials on biochar can significantly modulate the surface properties of biochar, and through the covalent bonding interactions between the biochar carrier and the metal, it can help to improve the stability of the catalyst and further enhance the catalytic activity of the biochar catalysts [34,35]. The redox potential of Co3+/Co2+ (E0 = 1.92 V) is higher than that of Fe3+/Fe2+ (E0 = 0.77 V), Mn3+/Mn2+ (E0 = 1.54 V), and Cu2+/Cu+ (E0 = 0.153 V), which results in higher catalytic activity in cobalt-based catalysts [36]. In addition, the biochar carrier and cobalt active sites can form a synergistic effect by promoting electron transfer, which improves the catalytic activity and stability of the catalyst, effectively activates the PMS, and reduces the cost [36,37]. Therefore, cobalt-modified biochar was selected in this study to degrade an antibiotic. Yi et al. prepared cobalt-loaded water hyacinth biochar (Co-BC) as a catalyst to promote the degradation of norfloxacin (NOR) by effective activation of PS, and found that the Co-BC/PS system could remove 97.66% of NOR within 180 min [38]. In the present study, cobalt-loaded biochar was prepared using a simple method, and PMS was chosen as the oxidizing agent because it is more susceptible to catalyst activation, which leads to the efficient degradation of antibiotics in a shorter period of time.
Thie intention of this study was to prepare cobalt-loaded magnetic biochar (Co-MBC) to activate PMS for degrading MNZ and examine the degradation performance and mechanism. In this study, rapeseed straw biomass was selected for the preparation of Co-MBC by impregnation pyrolysis, which was magnetically modified in order to achieve the recovery and reuse of the catalyst. Meanwhile, the impact of factors such as catalyst dosage, PMS concentration, initial pH, and inorganic anions on degrading MNZ was explored. The active species connected to the degradation process was determined by quenching assays. Additionally, the degradation pathways of MNZ were investigated through employing liquid chromatography-mass spectrometry (LC-MS). This study promotes the recycling of waste biomass resources while degrading the targeted antibiotics.
2. Materials and Methods
2.1. Materials and Chemicals
Cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O) was obtained from Shanghai Epi Chemical Reagent Co., Ltd., Shanghai, China. Metronidazole (MNZ), permonosulfate (PMS), isopropyl alcohol (IPA), p-benzoquinone (BQ), humic acid, sodium hydroxide, sodium bicarbonate, sodium chloride, sodium dihydrogen phosphate, and anhydrous ethanol were obtained from Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China. Methanol (MeOH) was obtained from Wuxi Zhanwang Chemical Reagent Co., Ltd., Wuxi, China. L-Histidine (L-His) and tert-butanol (TBA) were purchased from Shanghai Macklin Biochemical Technology Co., Ltd., Shanghai, China. Nitric acid was obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Nitrogen was provided by Wuxi Shengma Gas Co., Ltd., Wuxi, China. All reagents and chemicals were of analytical reagent grade without purification steps.
2.2. Preparing Rapeseed Straw Biomass
Rapeseed straw was obtained from rapeseed fields in a district of Jiangsu Province, China. The processing method was as follows: firstly, the rape straw was gathered, cleaned with deionized water, and then dried in an oven at 80 °C. Subsequently, the products were crushed by a wall breaker and passed through a 100-mesh sieve. The powdered biomass material obtained was labeled BC and collected for spare use.
2.3. Preparation of Cobalt Nitrate Modified Biochar
This experiment improved the preparation of biocarbon composites based on previous methods [39]. Co-MBC was prepared by the impregnation pyrolysis method. Five grams of rapeseed straw powder was added to 100 mL of cobalt nitrate solution (14 g/L) and stirred magnetically for 12 h to achieve a thorough mixing of rapeseed straw biomass and the cobalt nitrate solution. After mixing, the biomass was placed in a blower drying oven at 80 °C. After being completely dried, the biomass was placed in a mortar and ground into a powder. Subsequently, the cobalt-loaded rape straw powder was transferred to a volatile matter crucible to spread out, and then subjected to pyrolysis through a tube furnace at 700 °C for 2 h. The heating and pyrolysis procedure occurred in an N2 environment with a temperature rise of 5 °C/min. After the furnace temperature dropped to room temperature, the magnetic biocarbon material was taken out, ground, and sieved. The material was repeatedly cleaned with ethanol and deionized water, then dried in the oven. After complete drying, the sample was taken out and labeled as Co-MBC.
2.4. Experimental Procedures
The experiments took place in 250 mL conical flasks containing 100 mL of MNZ solution (20 mg/L). The reactions took place at room temperature with a pH of 7. Appropriate amounts of PMS and Co-MBC were reacted with MNZ solution at 600 rpm for 60 min. A specific volume of the solution was withdrawn at 0, 5, 10, 15, 20, 30, 40, and 60 min. The samples were passed through a 0.45 μm filter and then transferred into the centrifugal tube. Additionally, 0.5 mL of methanol was included to stop the oxidation process. The UV spectrophotometer was applied to determine the concentration of MNZ at 320 nm.
The effectiveness of catalyst activation of PMS for degrading MNZ was studied in the impact factor tests. The effects of catalyst dosage (0.005 g, 0.01 g, 0.02 g, 0.03 g, and 0.04 g), PMS concentration (0.5 mM, 1 mM, 1.5 mM, and 2 mM), and initial pH (3, 5, 7, 9, and 11) on the degradation effectiveness of MNZ were investigated. The pH was modified by 1 M NaOH and 1 M HNO3. The impact of anions (Cl−, NO3−, HCO3−, H2PO4−, and SO42−) on the degrading efficiency of MNZ was investigated in distinct concentrations (1 mM, 5 mM, 10 mM, and 20 mM). Humic acid was added to the solution to investigate whether macromolecular organic matter impacted the entire reaction system. A degradation kinetic model was fitted to evaluate the degradation performance of Co-MBC on MNZ.
The reusability of Co-MBC catalysts for practical applications was explored through cycling experiments. Following the degradation experiment, the catalyst was recovered by the magnetic separation technique. The impurities were removed with deionized water and anhydrous ethanol, followed by drying and recycling for the next degradation experiment, in order to assess the stability and reproducibility of the catalyst.
2.5. Free Radical Identification Experiment
The active substances and their mechanisms of action that significantly contribute to the degrading reaction were identified by adding quenching agents. Tert-butanol (TBA) was employed to quench hydroxyl radicals (•OH), methanol (MeOH) was employed for hydroxyl radicals (•OH) and sulfate radicals (•SO4−), p-benzoquinone (BQ) was employed for superoxide radicals (•O2−), and L-histidine was employed for singlet oxygen (1O2), respectively. In quenching experiments, the catalyst and oxidizer were introduced first, followed by the addition of the bursting agent. The MNZ concentration after the reaction was determined, the impacts of various quenching agents on MNZ removal were investigated, and the key active substances involved were analyzed.
3. Results and Discussions
3.1. Analysis of Morphological and Structural
Scanning electron microscopy (SEM, FEI Quanta FEG 250, FEI Company, Hillsboro, OR, USA) was implemented for analyzing the morphology and structure of Co-MBC in order to determine the existence of magnetic cobalt oxides. Figure 1 shows that Co-MBC retained the lamellar structure of BC, but due to pyrolysis, the structure of the MBC collapsed and fragmented to form faults and new pores. Additionally, a few scattered particles appeared on the original smooth surface, which indicates that cobalt oxides were successfully doped on the surface of Co-MBC. The cobalt oxide was uniformly dispersed throughout the loading process, contributing to the exposure of active sites on the catalyst surface [40].

Figure 1.
SEM of Co-MBC. (a) shows the morphological structure of Co-MBC at a magnification of 2000×, and (b) shows the morphological structure of Co-MBC at a magnification of 5000×.
3.2. Specific Surface Area and Pore Size Analysis
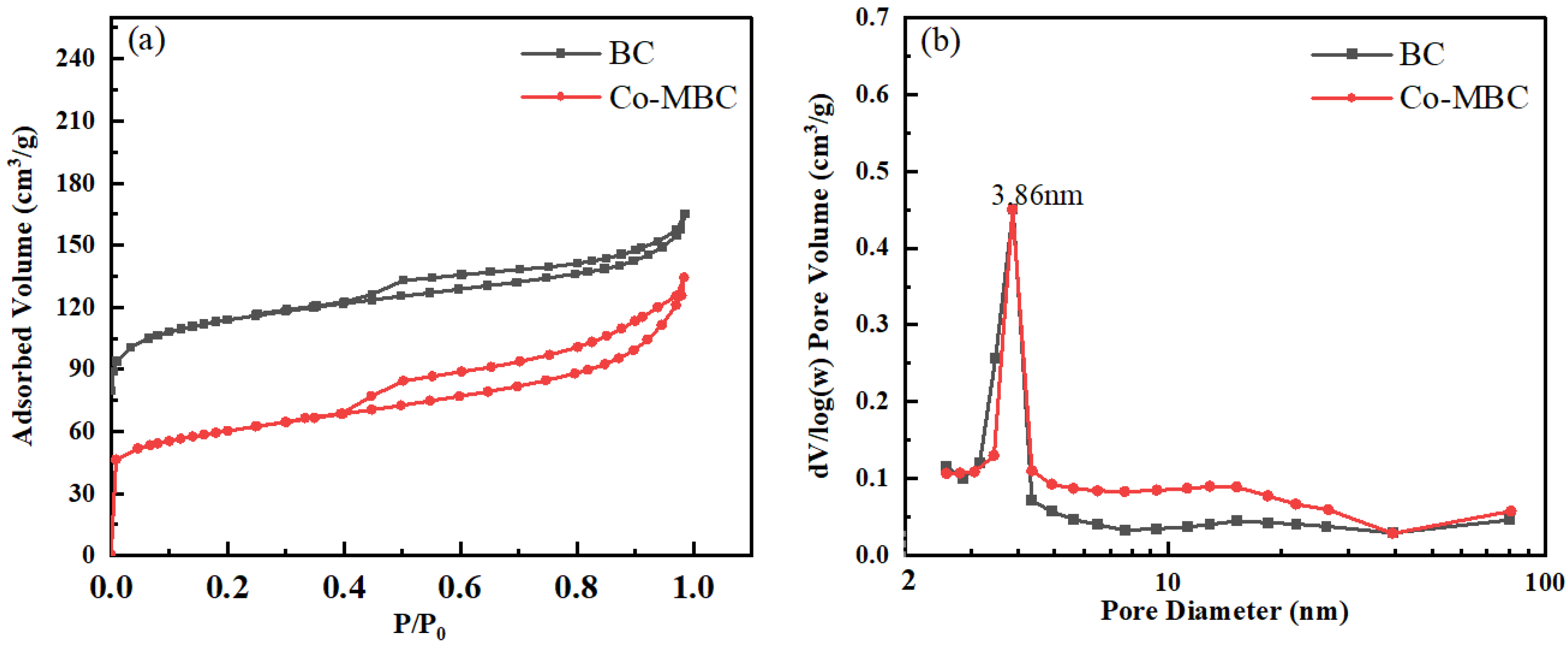
The specific surface area, pore size volume, and pore size distribution of BC and Co-MBC were determined using N2 adsorption-desorption experiments employing a physical adsorption analyzer (BET, Micromeritics ASAP 2020 HD88, Micromeritics Instruments Corp, Atlanta, GA, USA). Figure 2 indicates the N2 adsorption-desorption isotherms (a) and the distribution of pore sizes (b) of BC and Co-MBC. The samples had type IV adsorption isotherm features with distinct hysteresis loops, suggesting that the catalysts possessed micropore and mesopore structures [41].
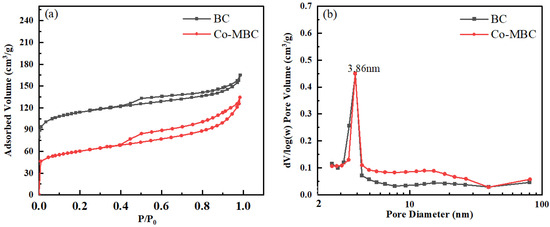
Figure 2.
BET of BC and Co-MBC. (a) shows the nitrogen adsorption and desorption of the material, and (b) shows the pore size distribution of the material, both with corresponding descriptions in the text.
The results show an inflection point at P/P0 = 0.05, followed by a significant rise in adsorption at low pressures, which implies a robust connection between the sample surface and nitrogen molecules, along with a notably high microporous content in the sample.
Table 1 shows that the micropore volume of biochar was three times greater than that of the modified biochar. The nitrogen adsorption isotherms proved that the highest adsorption capacity of Co-MBC at P/P0 (0.98) was notably lower compared to that of BC. The total pore volume declined by 17% after modification, probably due to cobalt oxides filling the pores and occupying many voids.

Table 1.
Specific area and pore size parameters for BC and Co-MBC.
Figure 2b illustrates that the mesoporous pore diameters of the samples, both before and after modification, mostly varied from 2 to 80 nm, with the highest pore volume observed at a mesoporous pore size of 3.86 nm. The mesopore pore volume in the modified samples increased, and the signal of micropore pore volume weakened, indicating that the mesopore porosity increased while the microporous pore volume decreased after modification. This may be due to the fact that the cobalt oxides mainly entered into micropores, and the cobalt oxides adsorbed on the surface of the samples formed more mesoporous pores, resulting in an increase in mesopore pore volume. In conclusion, in the modified biochar, the cobalt oxides mainly entered the microporous pore channels and increased the mesoporous porosity, thus providing more activation sites.
3.3. Crystal Structure of Analysis
The performance of catalysts is influenced by their crystal structure, which in this study, was determined and studied by X-ray diffraction (XRD, Rigaku Smartlab 3kwX, Rigaku Corporation, Tokyo, Japan).
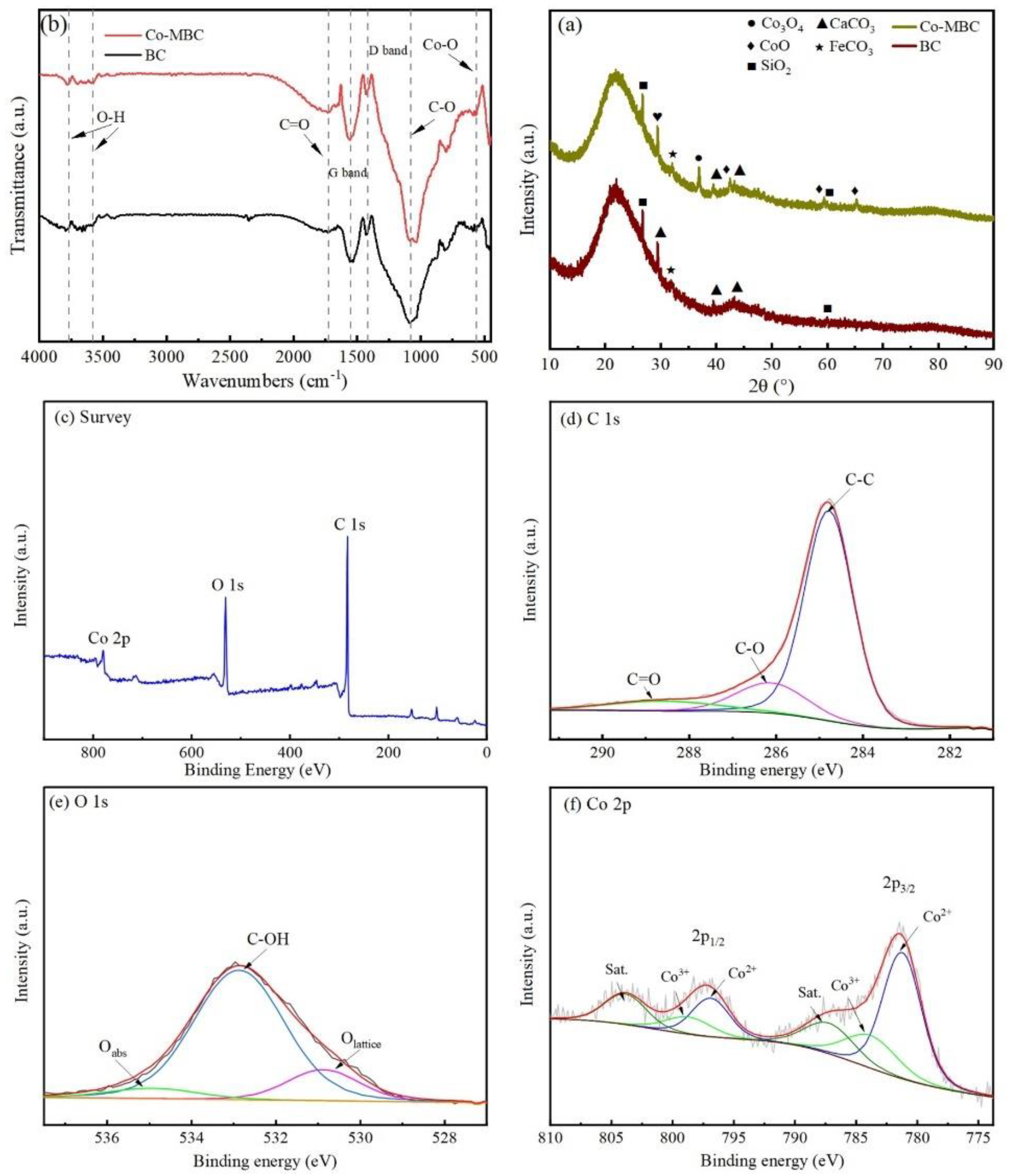
Within a swept range of 10°–90°, the XRD diffractograms from BC and Co-MBC showed broader diffraction peaks at about 2θ = 20–25° (Figure 3a), which indicates the presence of amorphous carbon in the material [42]. The crystallographic peaks occurring at diffraction angles of approximately 29.4°, 39.4°, and 47.6° are the characteristic peaks of the (104), (102), and (018) crystallographic planes of calcite (CaCO3), according to standard chart card PDF#01-083-0578. The crystallographic peaks at diffraction angles of about 31.3°, 36.8°, and 59.4° are characteristic peaks of (220), (311), and (5111) crystallographic planes of Co3O4 in the cubic crystal system and are referenced to the standard atlas with the file number PDF#97-002-4210. The crystallographic peaks seen at a diffraction angle of about 42.4° correspond to the characteristic peaks of the (200) crystallographic plane of the cubic crystal structure of CoO, according to the standard atlas with the file number PDF#97-000-9865. The characteristic peaks at 2θ = 60° are based on the (111) crystallographic plane of the Co metal CoO of the JCPDS#05-0727 card.
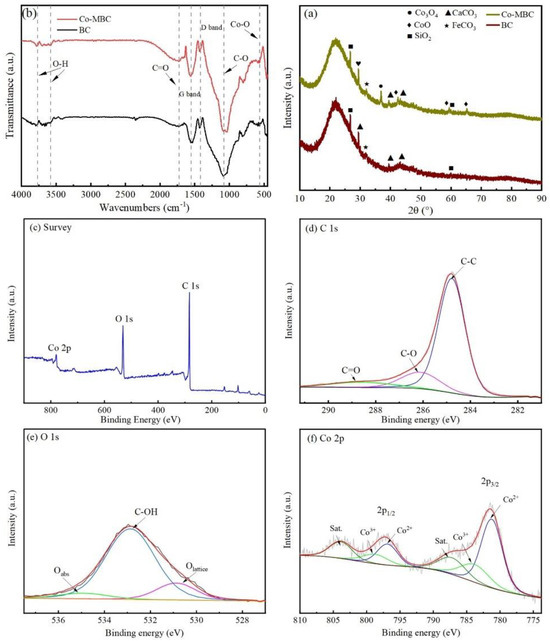
Figure 3.
(a) XRD patterns of BC and Co-MBC. (b) Infrared spectra of BC and Co-MBC modified materials. XPS spectra of Co-MBC: (c) Full spectrum; (d) C 1S; (e) O 1S; and (f) Co 2p.
The XRD analysis indicated that the small width of the characteristic peaks of Co3O4 implied the high crystallinity of the synthesized Co-MBC [43]. The characterization results of Co-MBC proved the successful loading of cobalt oxides and the successful preparation of the material.
3.4. Identifying Functional Groups of a Catalyst
Fourier transform infrared (FT-IR, Thermo Scientific Nicolet iS20, Waltham, MA, USA) spectroscopy was utilized to ascertain the various functional groups of the catalyst. The FTIR spectra of Co-MBC and BC indicated that the primary absorption peaks of the two materials were seen at 1740, 1550, 1420, and 1080 cm−1, respectively (Figure 3b). An absorption peak at 2350 cm−1 vanished as a result of high-temperature pyrolysis, causing the degradation of the structure of BC and the loss of its original functional groups.
A peak at 1725 cm−1 in the Co-MBC spectrum was caused by the C=O stretching vibration, indicating the presence of carboxyl groups within the catalyst, which is essential for PMS activation [44].
Both the BC and Co-MBC samples showed two characteristic peaks near 1420 cm−1 and 1550 cm−1, correlating the D and G bands associated with disordered sp3 and ordered sp2 bonded carbon atoms, respectively. The band of absorption around 1080 cm−1 was caused by the C-O stretching vibration. More importantly, IR absorption peaks at 3580 and 3770 cm−1 were related to the O-H stretching vibration, indicating the existence of intermolecular hydrogen bonding in the samples [45]. Additionally, the peaks at 550–570 cm−1 were associated with the Co-O vibration of Co [46].
3.5. Analysis of Catalyst Bonding State and Elements
The bonding state and valence distribution of various elements in catalysts can be detected using X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, Thermo Fisher Scientific, Waltham, MA, USA).
Figure 3c shows peaks corresponding to C1s, O1s, and Co in the Co-MBC catalyst. Figure 3d shows the high-resolution C1s spectrum with three peaks near 284.86 eV, 286.26 eV, and 288.86 eV, representing C-C, C-O, and C=O, respectively [47]. Figure 3e shows that the O 1s spectrum of Co-MBC demonstrated the presence of three types of oxygen, with peaks located at 530.74 eV, 532.87 eV, and 535.16 eV, which belong to lattice oxygen (Olat), surface hydroxyl group (Osurf), and surface adsorbed oxygen (Oads), respectively [48]. Figure 3 shows the high-resolution XPS spectra of cobalt in Co-MBC. Depending on the valence state and binding energy, four peaks for Co2+ (781.36 eV and 796.96 eV) and Co3+ (784.16 eV and 798.86 eV) were observed by fitting Co 2P1/2 and Co 2P3/2. This correlates with the XRD spectrum analysis, confirming the presence of cobalt oxides in Co-MBC.
The signals of Co3+ corresponded with the XRD spectra analysis, indicating the presence of cobalt oxides in Co-MBC. The XPS results showed that the cobalt species in the crystals were both Co2+ and Co3+, and that the cobalt oxides had been successfully loaded into biochar.
4. Catalytic Evaluation
4.1. Effect of Different Catalytic Systems on MNZ Degradation
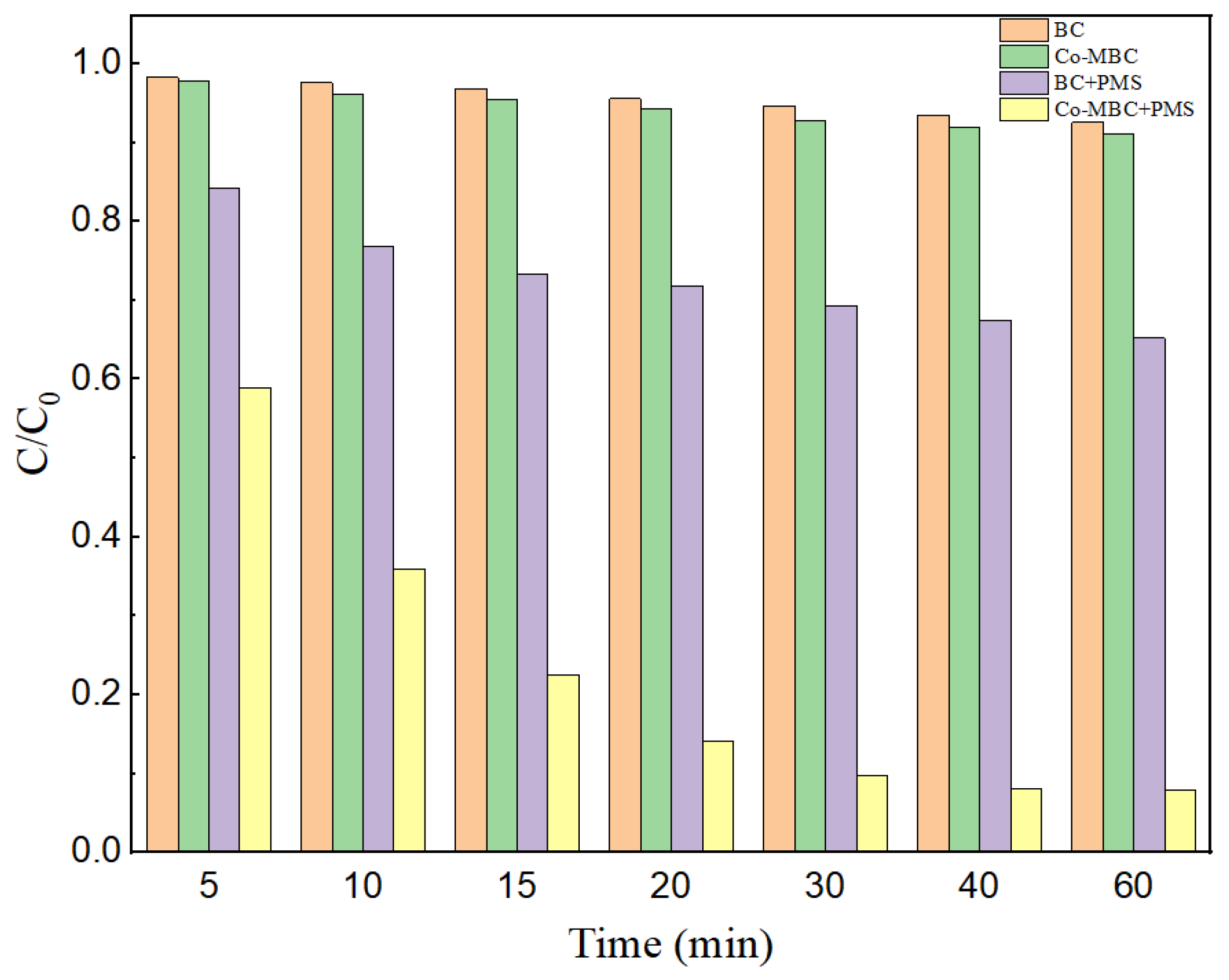
Figure 4 points out the effect of different systems of BC, Co-MBC, BC+PMS, and Co-MBC+PMS on the degradation of MNZ.
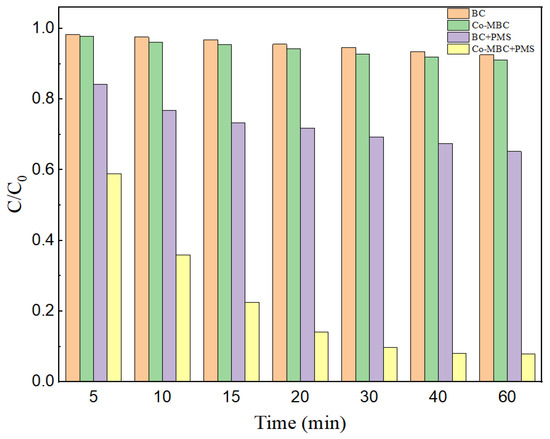
Figure 4.
MNZ removal rate in different catalytic systems. Reaction conditions: [catalysts] = 0.3 g/L; [PMS] = 1 mM; [MNZ] = 20 mg/L; T = 25 ± 3 °C; initial pH = 7.
When only BC was present within the reaction system, the MNZ removal efficiency was only 7.4%, while in the Co-MBC reaction system, the removal rate was elevated to 8.9%. Compared with the BC system, the enhancement of the adsorption effect was not obvious. This is because Co-MBC has a smoother surface and expanded pore structure compared to BC, but incorporating metal elements decreased the specific surface area of Co-MBC, restricting its interior pore structure.
The Co-MBC+PMS system reached a 90.3% removal of MNZ within 60 min, which was much higher than that of other systems. This was due to the fact that more active spots were added to the surface of the modified biochar, and more active substances were generated to take part in the reaction during PMS activation, which enhanced the degradation of MNZ. Therefore, Co-MBC effectively triggered the activation of PMS, leading to the enhancement of MNZ degradation. The Co-MBC+PMS system was chosen for subsequent studies.
4.2. Effectiveness of Catalyst Dosage on MNZ Degradation
The dosage of catalyst applied significantly impacts the efficiency of the catalytic process. A catalyst dosage that is too small will lead to insufficient active sites in the reaction process, thus reducing the catalytic efficiency, while too much catalyst leads to difficulties in catalyst recovery, an increased risk of metal ion leaching, and greater disposal costs.
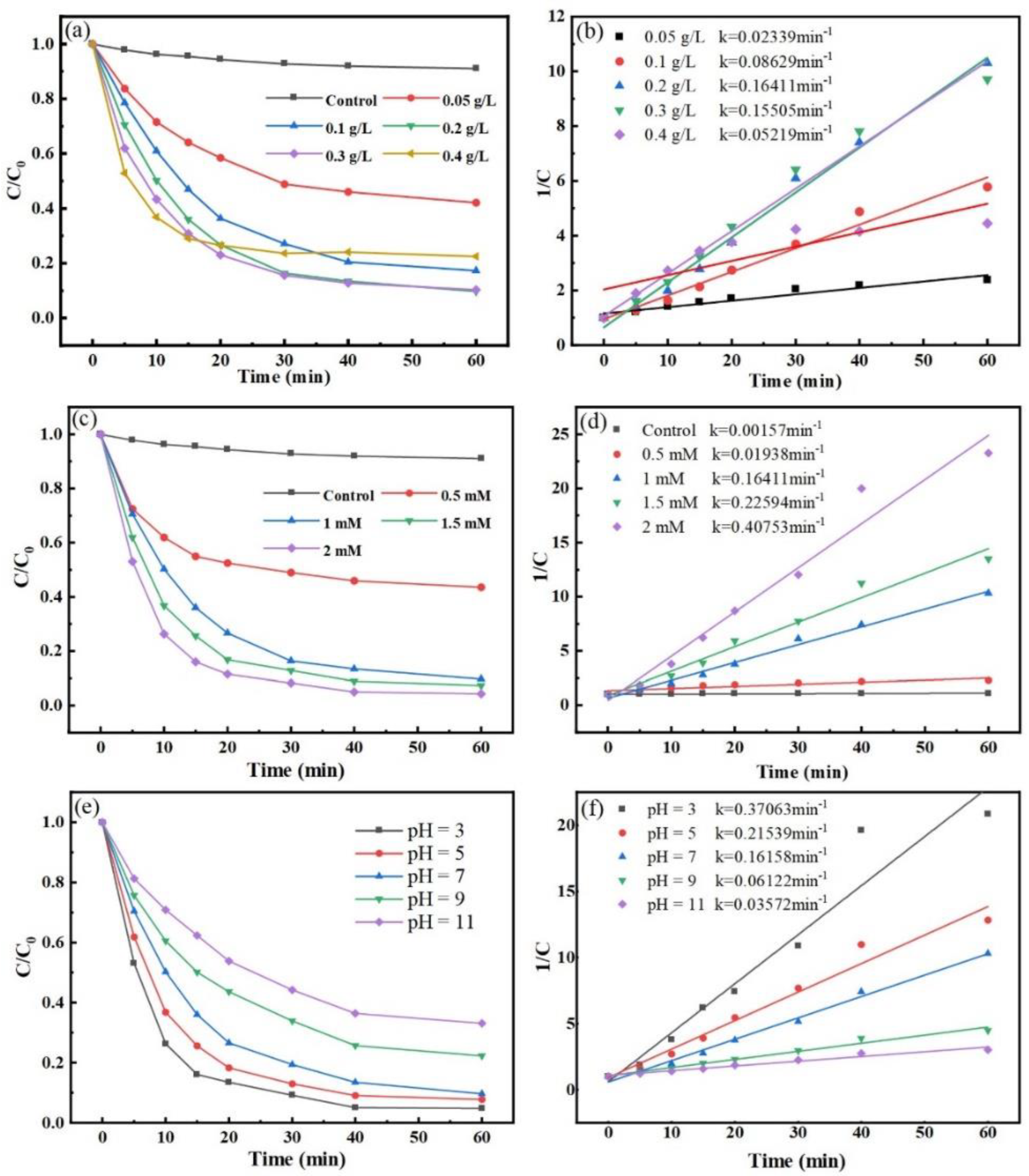
Therefore, the catalytic efficiency of the reaction system for MNZ degradation was studied by changing the degree of catalyst dosage. The degradation effects of different Co-MBC additions on MNZ are shown in Figure 5a. The degradation of MNZ exhibited a tendency of first rising and then decreasing as the addition of catalyst varied from 0 g/L to 0.4 g/L.
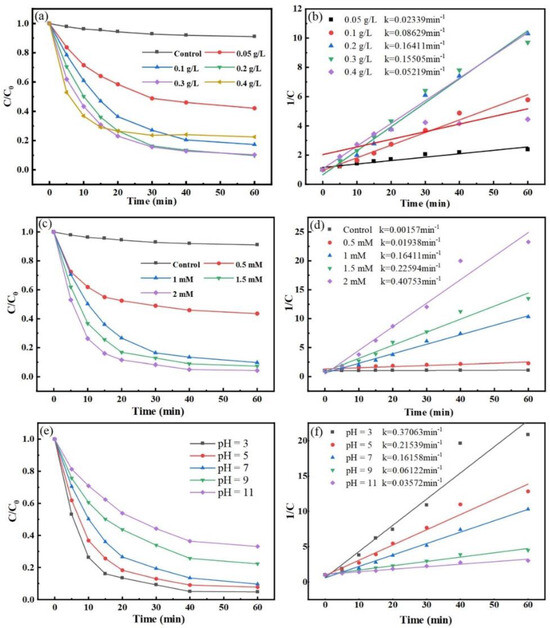
Figure 5.
Influence of catalyst dosage. (a) (Reaction conditions: [PMS] = 1 mM; [MNZ] = 20 mg/L; T = 25 ± 3 °C; initial pH = 7), PS concentration; (c) (Reaction conditions: [Co-MBC] = 0.2 g/L; [MNZ] = 20 mg/L; T = 25 ± 3 °C; initial pH = 7), and pH; (e) (Reaction conditions: [Co-MBC] = 0.2 g/L; [PMS] = 1 mM; [MNZ] = 20 mg/L; T = 25 ± 3 °C). MNZ degradation kinetic curves (b,d,f) correspond to (a,c,e), respectively.
Specifically, when no catalyst was added, only PMS and MNZ were present in the reaction system, resulting in a little degradation of MNZ due to the activation of PMS by light. Expanding the catalyst amount from 0.05 g/L to 0.2 g/L resulted in the degradation rate of MNZ rising from 57.9% to 90.3%. Increasing the catalyst amount caused the quantity of active sites in the reaction process to rise, leading to quicker degradation of MNZ. Figure 5a shows that the degradation effect on MNZ did not increase significantly but instead decreased when the catalyst dosage was increased to 0.3 g/L and 0.4 g/L compared to the 0.2 g/L catalyst dosage. Owing to magnetic forces, excessive catalysts were drawn together and formed clusters, which covered their active sites and hindered their ability to catalyze PMS efficiently [49]. In addition, the excess catalyst may have activated PMS to produce high concentrations of •SO4−, which can lead to quenching reactions with the oxidizer, resulting in poor degradation [50]. Excessive catalyst use not only wastes resources, but also increases economic costs, and the removal effect on pollutants becomes less than ideal. When using catalyst amounts of 0.2 g/L and 0.3 g/L, the resultant reaction rates were 0.16411 min−1 and 0.15505 min−1, respectively (Figure 5b), resulting in little change in reaction and degradation rates. Thus, a catalyst dosage of 0.2 g/L was selected for the next investigation.
4.3. Effectiveness of PMS Concentration on MNZ Degradation
PMS can be activated to produce •SO4−; hence, the concentration of PMS directly influences the overall degradation process [51]. The previous study in this experiment showed that the removal of MNZ by Co-MBC without the addition of PMS was only 8.9% after 60 min. The effects of different concentrations of PMS on the degradation of MNZ were investigated. The degradation rates of MNZ after 60 min were 56.4%, 90.3%, 92.6%, and 95.7% at PMS concentrations of 0.5 mM, 1 mM, 1.5 mM, and 2 mM, respectively (Figure 5c). The result showed that an increased amount of oxidizing agent in the reaction system resulted in a more effective degradation of pollutants.
The possible reason for this is that when the concentration of the oxidizer is low, it cannot produce enough •SO4−, so the degradation effect of the whole reaction process is reduced. Increasing the concentration enhances the catalyst touch possibilities with PMS, contributing to the increased production of •SO4− for the reaction, leading to steady degradation and a higher degradation rate. Once the concentration of PMS is excessive, the excess HSO5− in the solution is absorbed by the catalyst, saturating the reactive sites of Co-MBC and restrictedly generating free radicals. Furthermore, the •SO4− in the reaction system experiences a self-quenching reaction, resulting in the creation of S2O82−, which consumes •SO4− and influences the degradation reaction. Moreover, excessive •SO4− is an environmental pollutant as it causes corrosive effects upon contact with basic surfaces, such as drainage pipes. Therefore, in this study, 1 mM PMS was selected due to the observation of enhanced degradation rates at other concentrations without a substantial enhancement in the final degradation.
4.4. Effectiveness of pH on MNZ Degradation
The initial pH has been shown in earlier research to impact the surface characteristics of catalytic materials and the production of free radicals, thereby influencing the removal of pollutants [52,53]. The pH was adjusted in accordance with the preceding section. Figure 5e reveals that altering the solution pH impacted the degradation of MNZ by Co-MBC+PMS, showing that the degradation efficiency was reduced as the pH increased.
From Figure 5e,f, it can be seen that when the pH = 3, the reaction rate was 0.37063 min−1, and MNZ degradation by the Co-MBC+PMS system was as high as 95.2% after 60 min. When the pH = 11, the constant of reaction rate was 0.03572 min−1; the reaction rate became slower, and the degradation rate decreased to 66.9%. The degradation effect was shown to reach a rate of more than 90% in neutral and acidic systems. In the alkaline system, the degradation rate was slowed down, but still had some effect. The results show that the modified biochar Co-MBC could work under different pH conditions and has good potential for practical applications. This happens because •SO5−, which has lower oxidizing capacity, replaces HSO5− as the predominant form present under alkaline conditions, and OH− will interact with •SO4− to generate less reactive •OH, which is unfavorable for the degradation of MNZ in the reaction system, as shown in Equations (1)–(3) [54]. The experimental findings demonstrated that Co-MBC proved efficient in treating MNZ wastewater throughout an extensive pH range in real environments.
HSO5− + •SO4− → •SO5− + HSO4−
•SO4− + OH− → •OH + SO42−
H2O + •SO4− → HSO4− + •OH
4.5. Influence of Anions on MNZ Degradation
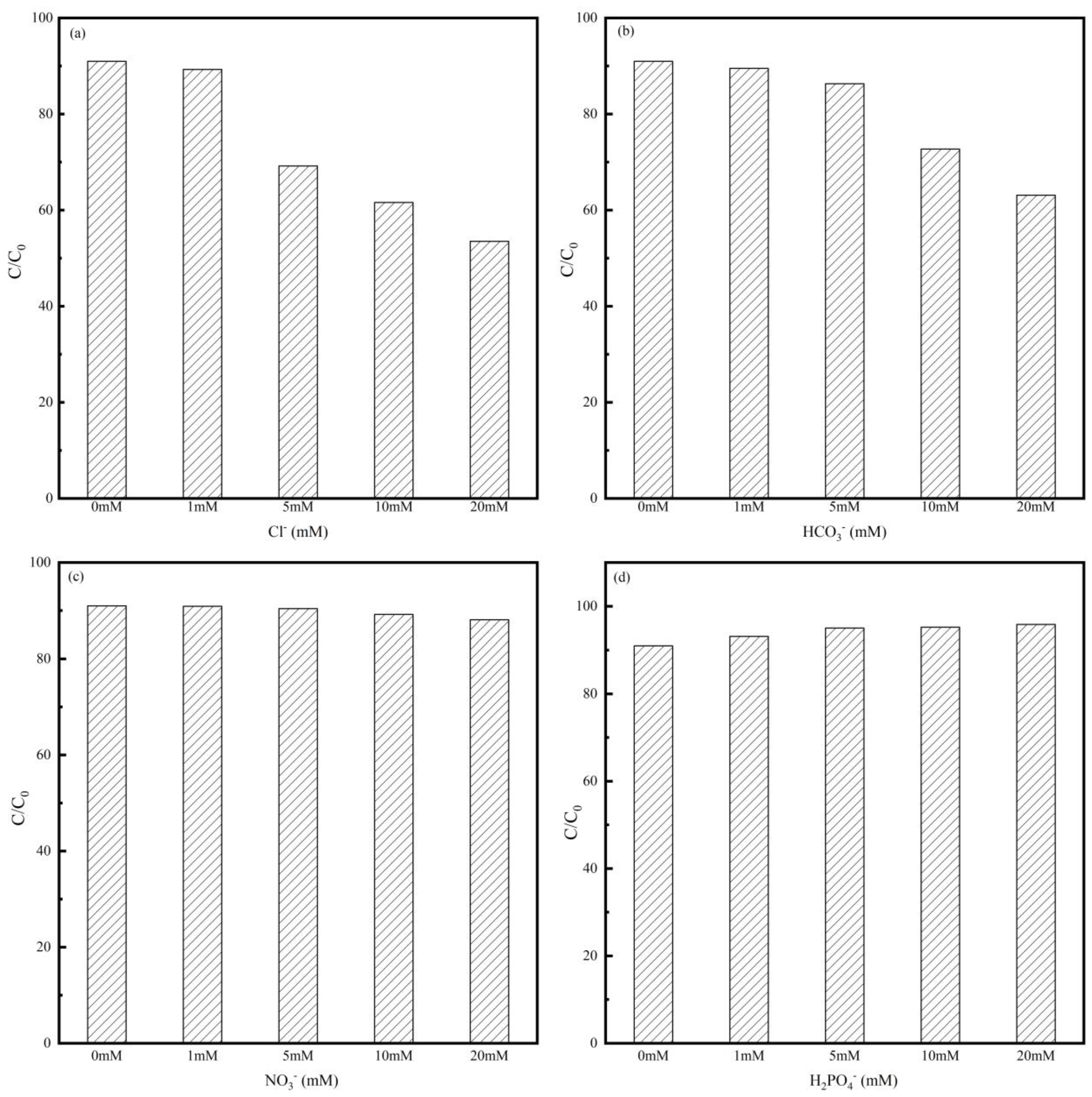
Anions present in natural bodies of water may influence the degradation of MNZ in a Co-MBC system. Degradation experiments were conducted to explore the influence of varying concentrations of inorganic anions (NO3−, HCO3−, H2PO4−, and Cl−) on the degradation process.
As can be seen in Figure 6, the coexisting anions mainly had a restricting impact on MNZ degradation. From Figure 6a,b, it is obvious that Cl− and HCO3− exerted inhibitory effects on the reaction system with increasing anion concentrations. This could be because Cl− and HCO3− reacted with •SO4−, thus quenching •SO4− and •OH and generating minor active radicals •Cl and •O2− (Equations (4) and (5)) [55,56]. In reverse, the addition of H2PO4− ions promoted the reaction system, as shown in Figure 6d, which was due to the fact that the phosphate anion itself had a certain activation effect on PMS [57].
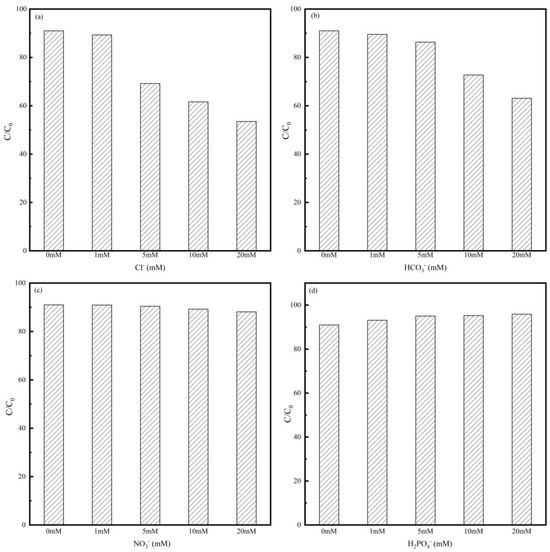
Figure 6.
Effect of inorganic anions on MNZ degradation efficiency: (a) Cl−; (b) HCO3−; (c) NO3−; and (d) H2PO4−. Reaction conditions: [Co-MBC] = 0.2 g/L; [PMS] = 1 mM; [MNZ] = 20 mg/L; T = 25 ± 3 °C; initial pH = 7.
Figure 6c illustrates a 5% reduction in the MNZ degradation rate at the NO3− ionic concentration of 20 mM. The reason for this is that NO3− interacts with the free radicals in the system as shown in Equation (6), quenching a small portion of •SO4− and occupying the active site, and thus having a weak inhibitory effect on the reaction system [58]. Overall, the results showed that in the low concentration range of ionic concentration, interference did not have much of an effect on degradation.
Cl−+ •SO4− → •Cl + SO42−
Cl− +•OH + O2 → HOCl + •O2
NO3− + •SO4− → •NO3− + SO42−
4.6. Effect of Humic Acid on MNZ Degradation
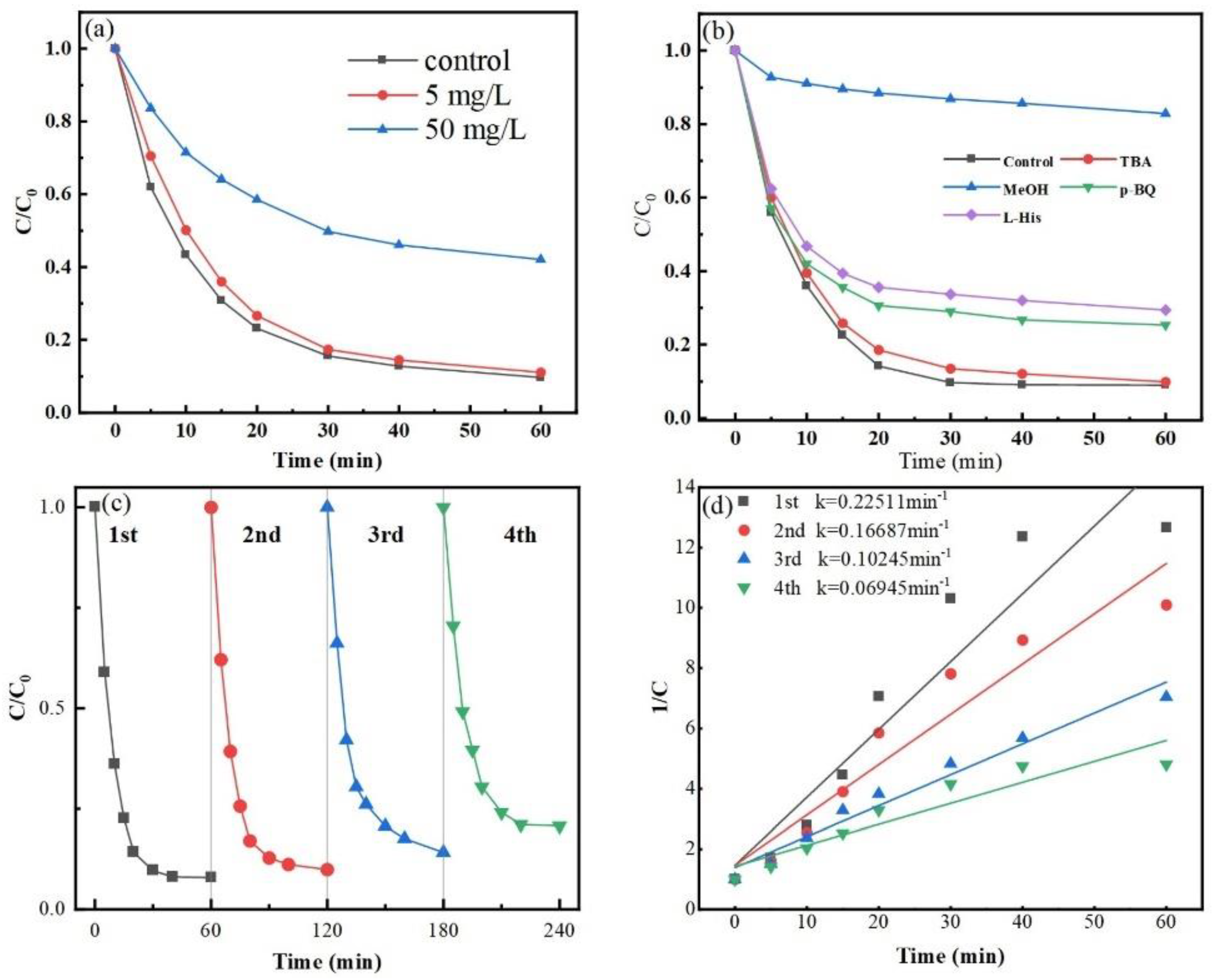
In this work, humic acid was introduced at various doses to examine the impact of macromolecular organic compounds on the Co-MBC/PMS system. Figure 7a suggests that the presence of 5 mg/L of humic acid did not significantly impact the degradation of MNZ. However, at HA levels of 50 mg/L, the degradation rate fell considerably compared to the system without HA.
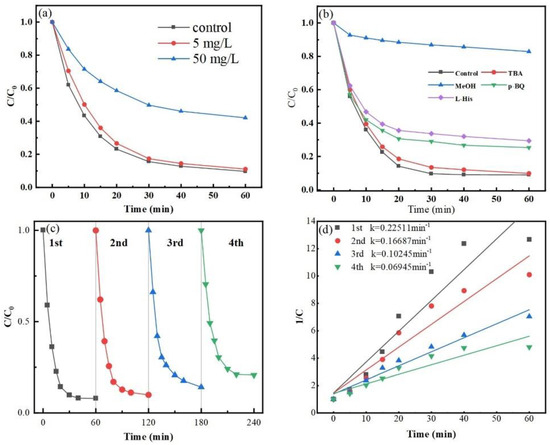
Figure 7.
(a) Effect of organic matter on MNZ degradation efficiency. (b) Impact of various quenching agents on the Co-MBC/PMS system. Recurrent experiments with Co-MBC: (c) degradation efficiency; (d) kinetic curves. (Reaction conditions: [Co-MBC] = 0.2 g/L; [PMS] = 1 mM; [MNZ] = 20 mg/L; T = 25 ± 3 °C; initial pH = 7).
This situation may be caused by the quinone group in HA stimulating PMS to generate slightly activated free radicals in the reaction system while also quenching •SO4−. The low concentration of HA was adsorbed by Co-MBC, and the bursting effect was not obvious, but the excess of the large molecular HA could have acted as a radical cleaner in the system, which reduced the degradation effect [59].
4.7. Free Radical Quenching Experiment
An assessment was performed regarding the impact of various radicals on degrading MNZ in a Co-MBC/PMS system. Methanol was utilized to quench •SO4− and •OH, tert-butanol was used to quench •OH, p-benzoquinone was employed to quench •O2−, and L-histidine was applied to quench 1O2 [60,61]. The concentrations of all of the quenchers employed were 0.05 M.
Figure 7b shows a substantial reduction in the rate of degradation of MNZ after methanol was added to the reaction system during a 60 min period. In contrast, the addition of tert-butanol slightly inhibited MNZ degradation, which suggests that the dominant role is played by •SO4− instead of •OH. At the same time, the degradation rate reduced correspondingly following the addition of p-benzoquinone and L-histidine, indicating that the burst of •O2− and 1O2 also had an inhibiting effect on the reaction system.
4.8. Recurrent Experiment
Whether a catalyst can be reused is one of the necessary prerequisites for determining its potential in future practical applications. Since the cobalt nitrate-modified biochar itself had strong magnetism, the catalyst material could be recovered after use by using an applied magnetic field. The recovered material was washed, dried, and then used in a cyclic experiment.
As shown in Figure 7c, the degradation effect of Co-MBC on MNZ was reduced by 13% after four re-uses, indicating that the material still showed good degradation performance. However, the reaction rate tended to decrease as the experiment was repeated, as shown in Figure 7d. This is because the small molecule intermediates generated by MNZ decomposition bind to the catalyst, diminishing the functionality of the active site and hindering the production of •SO4−, resulting in reduced degradation efficiency.
4.9. Degradation Pathway of MNZ
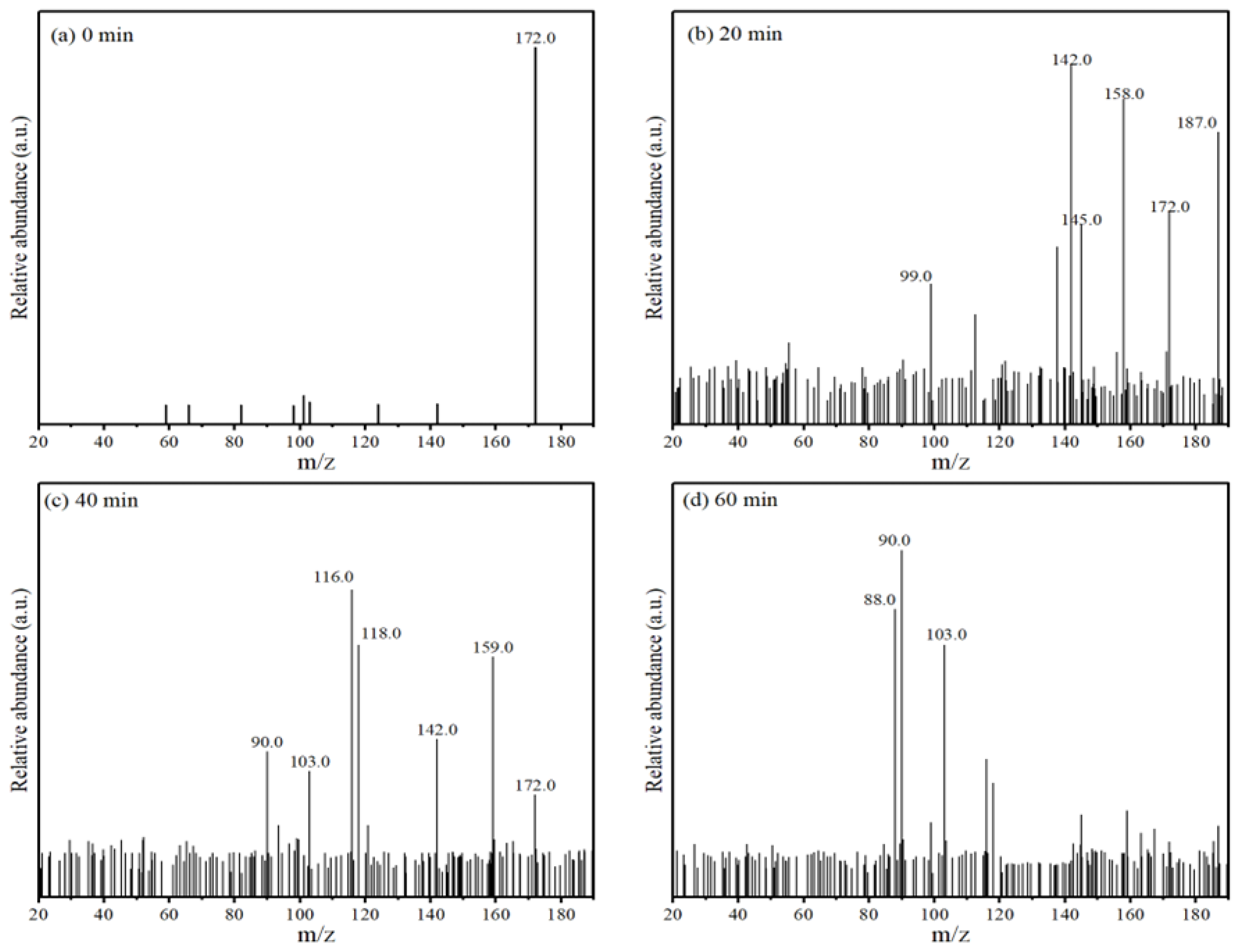
The intermediates of MNZ degradation were analyzed by LC-MS. Figure 8 displays the major mass peaks appearing at m/z = 142 (P2), 145 (P3), 159 (P4), 103 (P5), 187 (P6), 99 (P7), 118 (P8), 88 (P9), 158 (P10), 116 (P11), and 90 (P12), and the information about these intermediates is summarized in Table 2. Among them, P2, P6, and P10 were weighted more heavily, and it was speculated that these three substances may be the main intermediates that formed during the reaction.
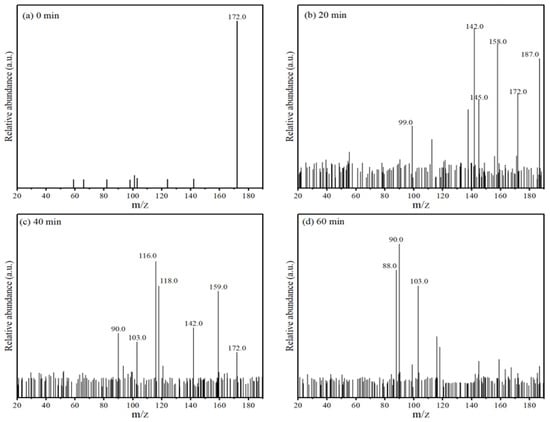
Figure 8.
Mass spectrum of MNZ degraded by Co-MBC/PMS system.

Table 2.
Intermediates of MNZ degradation.
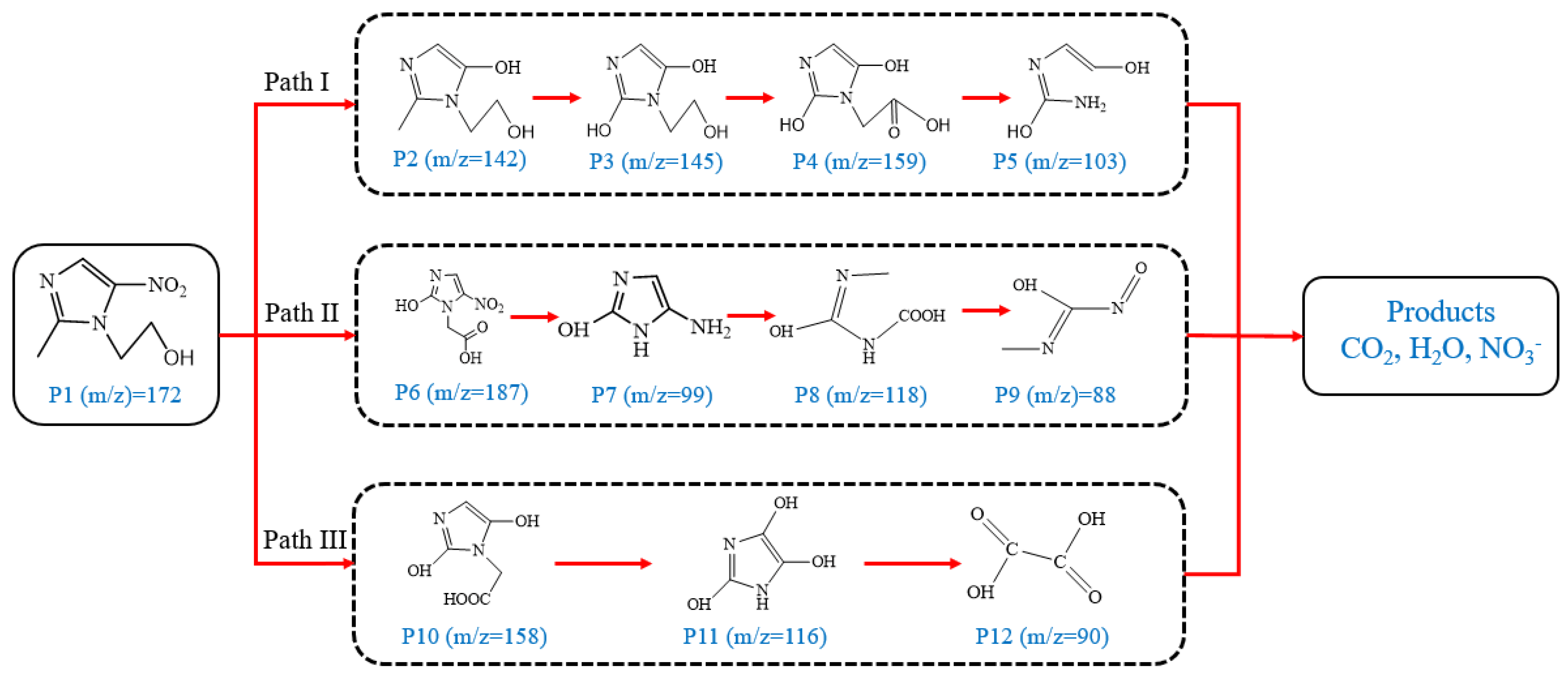
With the increase in degradation time, the MNZ signal gradually disappeared, and the signal of small molecules was enhanced. Combined with the compounds produced by degradation, it can be concluded that ring-opening oxidation, denitrohydroxylation, demethylation, and decarboxylation are the main pathways for the degradation of MNZ. A potential degradation pathway for MNZ was indicated by the identification of intermediates.
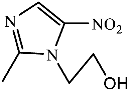
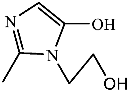
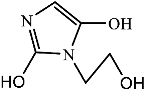
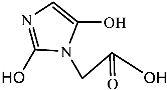
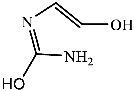
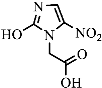
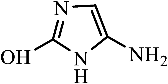
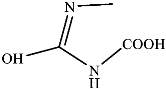
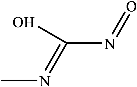
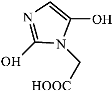
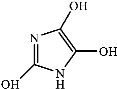
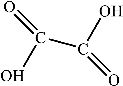
Figure 9 shows the three pathways of MNZ degradation, with an initial m/z of 171 for MNZ. In pathway I, free radicals attacked the C-N bond of MNZ through denitrohydroxylation to generate 1-(2-hydroxyethyl)-2-methyl-1H-imidazol-5-ol (P2, m/z = 142). P2 underwent hydroxylation to produce 1-(2-hydroxyethyl)-1H-imidazole-2,5-diol (P3, m/z = 144) [62]. P3 was oxidized to 2-(2,5-dihydroxy-1H-imidazol-1-yl) acetic acid (P4, m/z = 159) by reactive oxygen species. Finally, P4 was degraded to (E)-N’-((E)-2-hydroxyethenyl) carbamic acid (P5, m/z = 102) through C-N bond cleavage [63]. In pathway II, MNZ underwent direct oxidation by reactive oxygen species to form 2-(2-hydroxy-5-nitro-1H-imidazol-1-yl) acetic acid (P6, m/z = 187), which was subsequently transformed into 5-amino-1H-imidazol-2-ol (P7, m/z = 99) through nitro reduction and cleavage. This was further converted to carbamoylglycine (P8, m/z = 118) by breaking the C=C bond of P7, and then dehydrogenated through the hydroxylamino function to yield (Z)-N-methyl-1-nitrosimidic acid (P9, m/z = 88). In pathway III, MNZ underwent hydroxylation to produce 2-(2,5-dihydroxy-1H-imidazol-1-yl) acetic acid (P10, m/z = 158), followed by decarboxylation to generate 1H-imidazole-2,4,5-triol (P11, m/z = 116), and then experienced a ring-opening process to yield ethanedioic acid (P12, m/z = 90). Finally, P5, P9, and P12 were oxidized to CO2, H2O, and NO3− by the active substances [64,65].
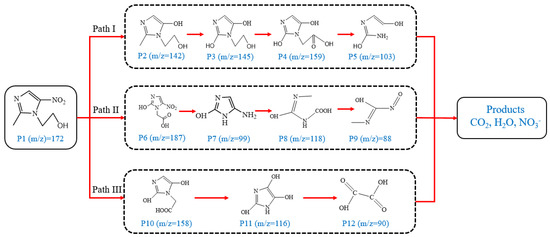
Figure 9.
MNZ degradation pathways.
5. Conclusions
A cobalt-loaded magnetic biochar (Co-MBC) catalyst was successfully synthesized in this study, and it was shown that this material can be used to enhance the removal of the antibiotic pollutant metronidazole (MNZ). The degradation process conformed to the proposed secondary reaction kinetics, resulting in the degradation rate of MNZ reaching 90% after 60 min of reaction in the Co-MBC/PMS system under optimal conditions. The active species, including •OH, •SO4−, •O2−, and 1O2, cooperated together to degrade MNZ, but •SO4− was the main participant throughout the whole reaction process, attacking the MNZ macromolecules through the processes of ring-opening oxidation, dinitro-hydroxylation, demethylation, and decarboxylation. In addition, the Co-MBC/PMS reaction system was shown to be highly resistant to interference, and was able to degrade MNZ well even across an extensive pH range and in the presence of different anions. In conclusion, this study demonstrated that the high redox potential and magnetic properties of cobalt are favorable for expanding the specific surface area and increasing the active sites in cobalt-loaded biochar, which can promote electron transfer. The cobalt-loaded magnetic biochar material was shown to efficiently activate PMS and exhibit good catalytic performance in degrading the antibiotic MNZ while also realizing the effective recycling of biochar materials. The material had high reusability and stability. The resource utilization of waste biomass was realized while effectively removing pollutants. This research offers a reference for preparing effective, reusable, and environmentally friendly catalysts for the degradation of non-biodegradable pollutants.
Author Contributions
Formal analysis, L.S.; Writing—original draft, L.H.; Writing—review & editing, E.H.D.; Supervision, H.L.; Funding acquisition, N.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the National Natural Science Foundation of China (No. 52100071).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ahankar, H.; Ramazani, A.; Ślepokura, K.; Lis, T.; Kinzhybalo, V. Magnetic cobalt ferrite nanoparticles functionalized with citric acid as a green nanocatalyst for one-pot three-component sonochemical synthesis of substituted 3-pyrrolin-2-ones. Res. Chem. Intermed. 2019, 45, 5007–5025. [Google Scholar] [CrossRef]
- Alamgir; Talha, K.; Wang, B.; Liu, J.-H.; Ullah, R.; Feng, F.; Yu, J.; Chen, S.; Li, J.-R. Effective adsorption of metronidazole antibiotic from water with a stable Zr(IV)-MOFs: Insights from DFT, kinetics and thermodynamics studies. J. Environ. Chem. Eng. 2020, 8, 103642. [Google Scholar] [CrossRef]
- Arast, N.; Farhadian, M.; Tangestaninejad, S.; Navarchian, A.H. Efficient photocatalytic performance of BiVO4/ZIF-8/Cu2S/Ag2S incorporated in solar driven-cleaning ABS/MWCNT membrane applied in metronidazole decontamination. Process Saf. Environ. Prot. 2023, 176, 87–100. [Google Scholar] [CrossRef]
- Cao, J.; Li, J.; Chu, W.; Cen, W. Facile synthesis of Mn-doped BiOCl for metronidazole photodegradation: Optimization, degradation pathway, and mechanism. Chem. Eng. J. 2020, 400, 125813. [Google Scholar] [CrossRef]
- Cha, J.S.; Choi, J.-C.; Ko, J.H.; Park, Y.-K.; Park, S.H.; Jeong, K.-E.; Kim, S.-S.; Jeon, J.-K. The low-temperature SCR of NO over rice straw and sewage sludge derived char. Chem. Eng. J. 2010, 156, 321–327. [Google Scholar] [CrossRef]
- Chen, C.; Sun, H.; Zhang, S.; Su, X. Non-metal activated peroxydisulfate by straw biochar for tetracycline hydrochloride oxidative degradation: Catalytic activity and mechanism. Environ. Sci. Pollut. Res. 2023, 30, 50815–50828. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yu, Y.; Liang, L.; Duan, X.; Li, R.; Lu, X.; Yan, B.; Li, N.; Wang, S. Remediation of antibiotic wastewater by coupled photocatalytic and persulfate oxidation system: A critical review. J. Hazard. Mater. 2021, 408, 124461. [Google Scholar] [CrossRef] [PubMed]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Crofts, T.S.; Gasparrini, A.J.; Dantas, G. Next-generation approaches to understand and combat the antibiotic resistome. Nat. Rev. Microbiol. 2017, 15, 422–434. [Google Scholar] [CrossRef]
- Dang, T.T.; Do, V.M.; Trinh, V.T. Nano-Catalysts in Ozone-Based Advanced Oxidation Processes for Wastewater Treatment. Curr. Pollut. Rep. 2020, 6, 217–229. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, Y.; Lu, S.; Zhang, X.; Li, Y.; Zhong, Y.; Zhang, H. A novel strategy using persulfate activated with thiosulfate for strong enhancement of trace 2,2′-dichlorobiphenyl removal: Influencing factors, and mechanisms. Chem. Eng. J. 2021, 415, 128969. [Google Scholar] [CrossRef]
- Ding, Y.; Zhu, L.; Wang, N.; Tang, H. Sulfate radicals induced degradation of tetrabromobisphenol A with nanoscaled magnetic CuFe2O4 as a heterogeneous catalyst of peroxymonosulfate. Appl. Catal. B Environ. 2013, 129, 153–162. [Google Scholar] [CrossRef]
- Dong, C.; Fang, W.; Yi, Q.; Zhang, J. A comprehensive review on reactive oxygen species (ROS) in advanced oxidation processes (AOPs). Chemosphere 2022, 308, 136205. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Gu, L.; Wu, D.; Liu, Z. Mackinawite (FeS) activation of persulfate for the degradation of p-chloroaniline: Surface reaction mechanism and sulfur-mediated cycling of iron species. Chem. Eng. J. 2018, 333, 657–664. [Google Scholar] [CrossRef]
- Feng, Y.; Song, Q.; Lv, W.; Liu, G. Degradation of ketoprofen by sulfate radical-based advanced oxidation processes: Kinetics, mechanisms, and effects of natural water matrices. Chemosphere 2017, 189, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yuan, R.; Wang, F.; Chen, Z.; Zhou, B.; Chen, H. Preparation of magnetic biochar and its application in catalytic degradation of organic pollutants: A review. Sci. Total Environ. 2021, 765, 142673. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.-T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. J. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Gao, J.; Han, D.; Xu, Y.; Liu, Y.; Shang, J. Persulfate activation by sulfide-modified nanoscale iron supported by biochar (S-nZVI/BC) for degradation of ciprofloxacin. Sep. Purif. Technol. 2020, 235, 116202. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A. Degradation of antibiotic pollutants by persulfate activated with various carbon materials. Chem. Eng. J. 2022, 429, 132387. [Google Scholar] [CrossRef]
- Hou, J.; He, X.; Zhang, S.; Yu, J.; Feng, M.; Li, X. Recent advances in cobalt-activated sulfate radical-based advanced oxidation processes for water remediation: A review. Sci. Total Environ. 2021, 770, 145311. [Google Scholar] [CrossRef]
- Huang, Y.; Tian, X.; Nie, Y.; Yang, C.; Wang, Y. Enhanced peroxymonosulfate activation for phenol degradation over MnO2 at pH 3.5-9.0 via Cu(II) substitution. J. Hazard. Mater. 2018, 360, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.; Liu, W.; Dong, Y.; Lu, Y.; Yang, C.; Lin, H. Single atom catalysts for degradation of antibiotics from aqueous environments by advanced oxidation processes: A review. J. Clean. Prod. 2023, 423, 138688. [Google Scholar] [CrossRef]
- Karkman, A.; Do, T.T.; Walsh, F.; Virta, M.P.J. Antibiotic-Resistance Genes in Waste Water. Trends Microbiol. 2018, 26, 220–228. [Google Scholar] [CrossRef]
- Kumar, A.; Pal, D. Antibiotic resistance and wastewater: Correlation, impact and critical human health challenges. J. Environ. Chem. Eng. 2018, 6, 52–58. [Google Scholar] [CrossRef]
- Li, M.-F.; Liu, Y.-G.; Zeng, G.-M.; Liu, N.; Liu, S.-B. Graphene and graphene-based nanocomposites used for antibiotics removal in water treatment: A review. Chemosphere 2019, 226, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, J.; Wang, M.; Kang, J.; Zhang, J.; Liu, S.; Tang, Y.; Li, S. Peroxymonosulfate activation by cobalt particles embedded into biochar for levofloxacin degradation: Efficiency, stability, and mechanism. Sep. Purif. Technol. 2022, 294, 121082. [Google Scholar] [CrossRef]
- Liu, S.; Zhao, C.; Wang, Z.; Ding, H.; Deng, H.; Yang, G.; Li, J.; Zheng, H. Urea-assisted one-step fabrication of a novel nitrogen-doped carbon fiber aerogel from cotton as metal-free catalyst in peroxymonosulfate activation for efficient degradation of carbamazepine. Chem. Eng. J. 2020, 386, 124015. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, M.; Yongmei, H. Study on the adsorption of Cu(II) by EDTA functionalized Fe3O4 magnetic nano-particles. Chem. Eng. J. 2013, 218, 46–54. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Yang, Y.; Feng, Y.; Wu, D.; Mao, S. Activation of persulfate with metal–organic framework-derived nitrogen-doped porous Co@C nanoboxes for highly efficient p-Chloroaniline removal. Chem. Eng. J. 2019, 358, 408–418. [Google Scholar] [CrossRef]
- Liu, Z.; Shi, X.; Yan, Z.; Sun, Z. Activation of peroxymonosulfate by biochar in-situ enriched with cobalt tungstate and cobalt: Insights into the role of rich oxygen vacancies and catalytic mechanism. Chem. Eng. J. 2023, 475, 146124. [Google Scholar] [CrossRef]
- Lou, X.; Wu, L.; Guo, Y.; Chen, C.; Wang, Z.; Xiao, D.; Fang, C.; Liu, J.; Zhao, J.; Lu, S. Peroxymonosulfate activation by phosphate anion for organics degradation in water. Chemosphere 2014, 117, 582–585. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yi, Y.; Ying, G.; Fang, Z.; Zhang, Y. Activation of persulfate for highly efficient degradation of metronidazole using Fe(II)-rich potassium doped magnetic biochar. Sci. Total Environ. 2022, 819, 152089. [Google Scholar] [CrossRef]
- Martins, P.M.; Salazar, H.; Aoudjit, L.; Gonçalves, R.; Zioui, D.; Fidalgo-Marijuan, A.; Costa, C.M.; Ferdov, S.; Lanceros-Mendez, S. Crystal morphology control of synthetic giniite for enhanced photo-Fenton activity against the emerging pollutant metronidazole. Chemosphere 2021, 262, 128300. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Song, M.; Wei, Y.; Wang, Y. The contribution of oxygen-containing functional groups to the gas-phase adsorption of volatile organic compounds with different polarities onto lignin-derived activated carbon fibers. Environ. Sci. Pollut. Res. 2019, 26, 7195–7204. [Google Scholar] [CrossRef]
- Mirzaee, R.; Darvishi Cheshmeh Soltani, R.; Khataee, A.; Boczkaj, G. Combination of air-dispersion cathode with sacrificial iron anode generating Fe2+Fe3+2O4 nanostructures to degrade paracetamol under ultrasonic irradiation. J. Mol. Liq. 2019, 284, 536–546. [Google Scholar] [CrossRef]
- Muttakin, M.; Mitra, S.; Thu, K.; Ito, K.; Saha, B.B. Theoretical framework to evaluate minimum desorption temperature for IUPAC classified adsorption isotherms. Int. J. Heat Mass Transf. 2018, 122, 795–805. [Google Scholar] [CrossRef]
- Peng, L.; Shang, Y.; Gao, B.; Xu, X. Co3O4 anchored in N, S heteroatom co-doped porous carbons for degradation of organic contaminant: Role of pyridinic N-Co binding and high tolerance of chloride. Appl. Catal. B Environ. 2021, 282, 119484. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Das, I.; Ghangrekar, M.M.; Blaney, L. Advanced oxidation processes: Performance, advantages, and scale-up of emerging technologies. J. Environ. Manag. 2022, 316, 115295. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ge, B.; Zhang, Y.; Jiang, B.; Wang, C.; Akram, M.; Xu, X. Three-dimensional porous graphene-like biochar derived from Enteromorpha as a persulfate activator for sulfamethoxazole degradation: Role of graphitic N and radicals transformation. J. Hazard. Mater. 2020, 399, 123039. [Google Scholar] [CrossRef]
- Ren, F.; Zhu, W.; Zhao, J.; Liu, H.; Zhang, X.; Zhang, H.; Zhu, H.; Peng, Y.; Wang, B. Nitrogen-doped graphene oxide aerogel anchored with spinel CoFe2O4 nanoparticles for rapid degradation of tetracycline. Sep. Purif. Technol. 2020, 241, 116690. [Google Scholar] [CrossRef]
- Sinha, R.; Kumar, R.; Sharma, P.; Kant, N.; Shang, J.; Aminabhavi, T.M. Removal of hexavalent chromium via biochar-based adsorbents: State-of-the-art, challenges, and future perspectives. J. Environ. Manag. 2022, 317, 115356. [Google Scholar] [CrossRef]
- Thomas, N.; Dionysiou, D.D.; Pillai, S.C. Heterogeneous Fenton catalysts: A review of recent advances. J. Hazard. Mater. 2021, 404, 124082. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wei, L.; Hu, J.; Lu, J. Boosting reactive oxygen species generation over Bi3O4Br/CuBi2O4 by activating peroxymonosulfate under visible light irradiation. Sep. Purif. Technol. 2022, 289, 120794. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. A comprehensive review on persulfate activation treatment of wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef]
- Wang, C.; Liu, H.; Wang, G.; Huang, W.; Wei, Z.; Fang, H.; Shen, F. Visible light driven S-scheme heterojunction Zn3In2S6/Bi2MoO6 for efficient degradation of metronidazole. J. Alloys Compd. 2022, 917, 165507. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Wang, J.; Zhuan, R.; Chu, L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: An overview. Sci. Total Environ. 2019, 646, 1385–1397. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, M. Catalytic degradation of sulfamethoxazole by peroxymonosulfate activation system composed of nitrogen-doped biochar from pomelo peel: Important roles of defects and nitrogen, and detoxification of intermediates. J. Colloid Interface Sci. 2022, 613, 57–70. [Google Scholar] [CrossRef]
- Wang, W.; Chen, M.; Wang, D.; Yan, M.; Liu, Z. Different activation methods in sulfate radical-based oxidation for organic pollutants degradation: Catalytic mechanism and toxicity assessment of degradation intermediates. Sci. Total Environ. 2021, 772, 145522. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Shangguan, W. A review on photocatalysis in antibiotic wastewater: Pollutant degradation and hydrogen production. Chin. J. Catal. 2020, 41, 1440–1450. [Google Scholar] [CrossRef]
- Wu, D.; Song, W.; Chen, L.; Duan, X.; Xia, Q.; Fan, X.; Li, Y.; Zhang, F.; Peng, W.; Wang, S. High-performance porous graphene from synergetic nitrogen doping and physical activation for advanced nonradical oxidation. J. Hazard. Mater. 2020, 381, 121010. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Hu, Y.H. A comprehensive review on catalysts for electrocatalytic and photoelectrocatalytic degradation of antibiotics. Chem. Eng. J. 2021, 409, 127739. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, H.; Wang, W.; Li, W.; Ren, Y.; Li, X. Synthesis of Fe0/Fe3O4@porous carbon through a facile heat treatment of iron-containing candle soots for peroxymonosulfate activation and efficient degradation of sulfamethoxazole. J. Hazard. Mater. 2021, 411, 124952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Chen, Y.; Duan, X.; Zhou, S.; Niu, Y.; Sun, H.; Zhi, L.; Wang, S. Unzipping carbon nanotubes to nanoribbons for revealing the mechanism of nonradical oxidation by carbocatalysis. Appl. Catal. B Environ. 2020, 276, 119146. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, Y.; Du, M.; Du, X.; Huang, S. Insights into the mechanism of non-radical activation of persulfate via activated carbon for the degradation of p-chloroaniline. Chem. Eng. J. 2019, 362, 262–268. [Google Scholar] [CrossRef]
- Yi, Y.; Fu, Y.; Wang, Y.; Cai, Y.; Liu, Y.; Xu, Z.; Diao, Z. Persulfate oxidation of norfloxacin by cobalt doped water hyacinth biochar composite: The key role of cobalt and singlet oxygen. J. Water Process Eng. 2024, 59, 104967. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Shi, Z.; Li, Y.; Zhao, Z.; He, B.; Cheng, X. Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Sep. Purif. Technol. 2021, 272, 118889. [Google Scholar] [CrossRef]
- Zeng, H.; Yang, B.; Shi, W.; Huang, K.; Ye, C.; Ma, X.; Wang, Z.; Huang, F.; Li, X.; Deng, J. Peroxymonosulfate activation by sulfur doped CoFe2O4 rod for arsanilic acid removal: Performance and arsenic enrichment. J. Environ. Chem. Eng. 2023, 11, 111044. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, Y.; Nutakki, T.U.K.; Alghassab, M.A.; Alkhalaf, S.; Islam, S.; Elmasry, Y. Preparation of CeO2-WO3 binary heterojunction photocatalyst for sustainable tetracycline degradation: Optimization of synthesis and degradation conditions, characterization, transformation pathway, and dominant reactive species. Surf. Interfaces 2024, 44, 103793. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, J.; Wang, C.; Wang, L.; Guo, Z.; Song, Y. Recent advance of Fe-based bimetallic persulfate activation catalysts for antibiotics removal: Performance, mechanism, contribution of the key ROSs and degradation pathways. Chem. Eng. J. 2024, 487, 150514. [Google Scholar] [CrossRef]
- Zhong, Q.; Lin, Q.; Huang, R.; Fu, H.; Zhang, X.; Luo, H.; Xiao, R. Oxidative degradation of tetracycline using persulfate activated by N and Cu codoped biochar. Chem. Eng. J. 2020, 380, 122608. [Google Scholar] [CrossRef]
- Zhu, K.; Wang, X.; Geng, M.; Chen, D.; Lin, H.; Zhang, H. Catalytic oxidation of clofibric acid by peroxydisulfate activated with wood-based biochar: Effect of biochar pyrolysis temperature, performance and mechanism. Chem. Eng. J. 2019, 374, 1253–1263. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, F.; Lin, X.; Zhang, D.; Duan, X.; Shi, J.; Sun, Z. Highly efficient catalysts of polyoxometalates supported on biochar for antibiotic wastewater treatment: Performance and mechanism. Process Saf. Environ. Prot. 2023, 172, 425–436. [Google Scholar] [CrossRef]
- Zhu, M.-P.; Yang, J.-C.E.; Duan, X.; Zhang, D.-D.; Wang, S.; Yuan, B.; Fu, M.-L. Interfacial CoAl2O4 from ZIF-67@γ-Al2O3 pellets toward catalytic activation of peroxymonosulfate for metronidazole removal. Chem. Eng. J. 2020, 397, 125339. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).