Marine Mannitol: Extraction, Structures, Properties, and Applications
Abstract
:1. Introduction
1.1. Primary Sources of Mannitol
1.2. Marine Algae as a Source of Mannitol
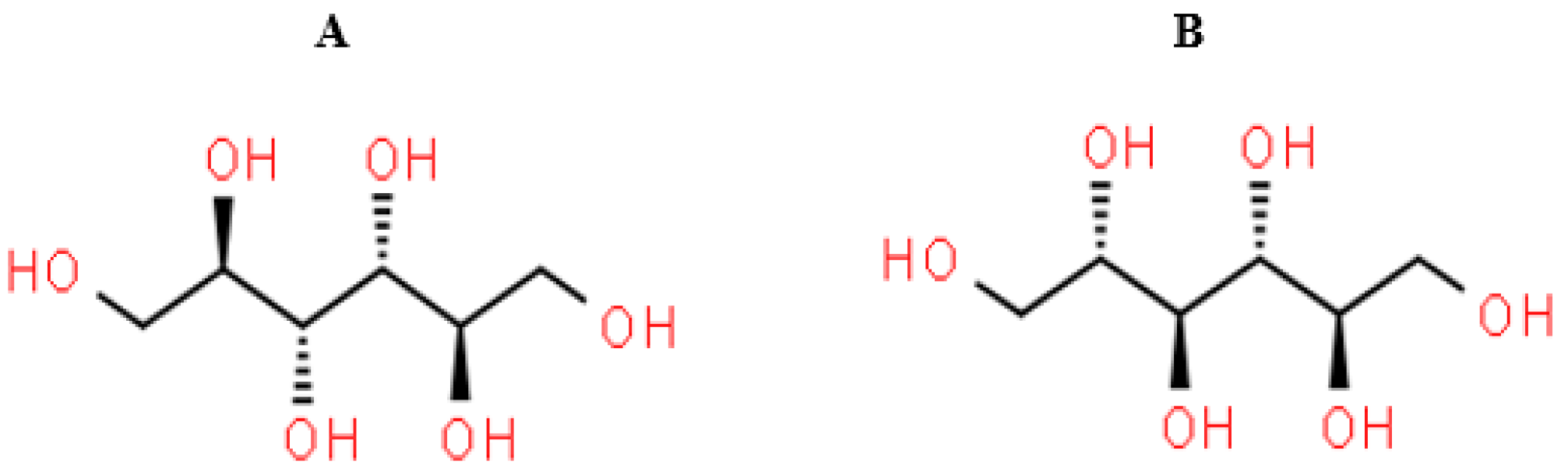
1.3. Chemical Properties of Mannitol
2. Mannitol Synthesis Methods
2.1. Chemical Synthesis of Mannitol
2.2. Enzymatic Method for Mannitol Production
2.3. Stages of Biorefinery for Mannitol Extraction
2.3.1. Mannitol Production by Heterofermentative LAB
2.3.2. Production of Mannitol from Agro-Industrial Waste or Inexpensive Substrate
3. Physiological Functions
4. Other Applications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Godswill, A.C. Sugar alcohols: Chemistry, production, health concerns and nutritional importance of mannitol, sorbitol, xylitol, and erythritol. Int. J. Adv. Acad. Sci. Res. 2017, 3, 31–66. [Google Scholar]
- Marcus, J.B. Carbohydrate basics: Sugars, starches and fibers in foods and health. Culin. Nutr. 2013, 149, 187. [Google Scholar]
- Bonin, P.; Groisillier, A.; Raimbault, A.; Guibert, A.; Boyen, C. Molecular and biochemical characterization of mannitol-1-phosphate dehydrogenase from the model brown alga Ectocarpus sp. Phytochemistry 2015, 117, 509–520. [Google Scholar] [PubMed]
- Saha, B.C.; Racine, F.M. Biotechnological production of mannitol and its applications. Appl. Microbiol. Biotechnol. 2011, 89, 879–891. [Google Scholar]
- Bhatt, S.M.; Mohan, A.; Srivastava, S.K. Challenges in enzymatic route of mannitol production. Int. Sch. Res. Not. 2013, 2013, 914187. [Google Scholar]
- Cock, J.M.; Peters, A.F.; Coelho, S.M. Brown algae. Curr. Biol. 2011, 21, R573–R575. [Google Scholar] [PubMed]
- Stiller, J.W.; Schreiber, J.; Yue, J.; Guo, H.; Ding, Q.; Huang, J. The evolution of photosynthesis in chromist algae through serial endosymbioses. Nat. Commun. 2014, 5, 5764. [Google Scholar]
- Black, W. Seasonal variation in chemical constitution of some common British Laminariales. Nature 1948, 161, 174. [Google Scholar]
- Hatcher, B.; Chapman, A.O.; Mann, K. An annual carbon budget for the kelp Laminaria longicruris. Mar. Biol. 1977, 44, 85–96. [Google Scholar]
- Honya, M.; Kinoshita, T.; Ishikawa, M.; Mori, H.; Nisizawa, K. Monthly determination of alginate, M/G ratio, mannitol, and minerals in cultivated Laminaria japonica. Bull. Jpn. Soc. Sci. Fish. 1993, 59, 295–299. [Google Scholar]
- Rosell, K.-G.; Srivastava, L.M. Seasonal variation in the chemical constituents of the brown algae Macrocystis integrifolia and Nereocystis luetkeana. Can. J. Bot. 1984, 62, 2229–2236. [Google Scholar]
- Rao, M.U. Seasonal variations in growth, alginic acid and mannitol contents of Sargassum wightii and Turbinaria conoides from the Gulf of Mannar, India. Proc. Int. Seaweed Symp. 1969, 6, 579–584. [Google Scholar]
- Zubia, M.; Payri, C.; Deslandes, E. Alginate, mannitol, phenolic compounds and biological activities of two range-extending brown algae, Sargassum mangarevense and Turbinaria ornata (Phaeophyta: Fucales), from Tahiti (French Polynesia). J. Appl. Psychol. 2008, 20, 1033–1043. [Google Scholar]
- Gómez, I.; Wiencke, C. Seasonal changes in C, N and major organic compounds and their significance to morpho-functional processes in the endemic Antarctic brown alga Ascoseira mirabilis. Pol. Biol. 1998, 19, 115–124. [Google Scholar]
- Kaliaperumal, N.; Kalimuthu, S. Changes in growth, reproduction, alginic acid and mannitol contents of Turbinaria decurrens Bory. Bot. Mar. 1976, 19, 161–178. [Google Scholar]
- Wong, S.Y.; Ang, P., Jr. Mannitol as a resource for the growth and reproduction of Sargassum siliquastrum (Mertens ex Turner) C. Agardh. J. Appl. Psychol. 2024, 36, 995–1008. [Google Scholar]
- Premarathna, A.D.; Tuvikene, R.; Somasiri, M.; De Silva, M.; Adhikari, R.; Ranahewa, T.H.; Wijesundara, R.; Wijesekera, S.K.; Dissanayake, I.; Wangchuk, P.; et al. A novel therapeutic effect of mannitol-rich extract from the brown seaweed Sargassum ilicifolium using in vitro and in vivo models. BMC Complement. Med. Ther. 2023, 23, 26. [Google Scholar]
- Iwamoto, K.; Shiraiwa, Y. Salt-regulated mannitol metabolism in algae. Mar. Biotechnol. 2005, 7, 407–415. [Google Scholar]
- Montaño, N. Mass Production of Mannitol from Sargassum Seaweeds; PCAMRD Book Series (Philippines); Philippine Council for Aquatic and Marine Research and Development: Los Banos, Philippines, 1999. [Google Scholar]
- Soetaert, W. Production of mannitol with Leuconostoc mesenteroides. Meded. Fac. Landbouwwet. Rijksuniv. Gent 1990, 55, 1549–1552. [Google Scholar]
- Wisselink, H.; Weusthuis, R.; Eggink, G.; Hugenholtz, J.; Grobben, G.J. Mannitol production by lactic acid bacteria: A review. Int. Dairy J. 2002, 12, 151–161. [Google Scholar]
- del Mar Contreras, M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar]
- Gómez-Cruz, I.; Contreras, M.D.M.; Romero, I.; Castro, E. Sequential extraction of hydroxytyrosol, mannitol and triterpenic acids using a green optimized procedure based on ultrasound. Antioxidants 2021, 10, 1781. [Google Scholar] [CrossRef] [PubMed]
- Horn, S.; Aasen, I.; Østgaard, K. Ethanol production from seaweed extract. J. Ind. Microbiol. Biotechnol. 2000, 25, 249–254. [Google Scholar]
- Carvalheiro, F.; Moniz, P.; Duarte, L.C.; Esteves, M.P.; Gírio, F.M. Mannitol production by lactic acid bacteria grown in supplemented carob syrup. J. Ind. Microbiol. Biotechnol. 2011, 38, 221–227. [Google Scholar] [PubMed]
- Gomaa, E.Z.; Rushdy, A.A. Improvement of Lactobacillus brevis NM101-1 grown on sugarcane molasses for mannitol, lactic and acetic acid production. Ann. Microbiol. 2014, 64, 983–990. [Google Scholar]
- Cao, H.; Yue, M.; Liu, G.; Du, Y.; Yin, H. Microbial production of mannitol by Lactobacillus brevis 3-A5 from concentrated extract of Jerusalem artichoke tubers. Biotechnol. Appl. Biochem. 2018, 65, 484–489. [Google Scholar]
- Chi, Z.-M.; Zhang, T.; Cao, T.-S.; Liu, X.-Y.; Cui, W.; Zhao, C.-H. Biotechnological potential of inulin for bioprocesses. Bioresour. Technol. 2011, 102, 4295–4303. [Google Scholar]
- Goharrizi, M.A.S.B.; Ghodsi, S.; Mokhtari, M.; Moravveji, S.S. Non-invasive STEMI-related biomarkers based on meta-analysis and gene prioritization. Comput. Biol. Med. 2023, 161, 106997. [Google Scholar]
- Yang, F.; Liu, Z.; Dong, W.; Zhu, L.; Chen, X.; Li, X. Ethanol production using a newly isolated S accharomyces cerevisiae strain directly assimilating intact inulin with a high degree of polymerization. Biotechnol. Appl. Biochem. 2014, 61, 418–425. [Google Scholar]
- Saha, B.C. Production of mannitol from inulin by simultaneous enzymatic saccharification and fermentation with Lactobacillus intermedius NRRL B-3693. Enzyme Microb. Technol. 2006, 39, 991–995. [Google Scholar]
- Wei, N.; Quarterman, J.; Jin, Y.-S. Marine macroalgae: An untapped resource for producing fuels and chemicals. Trends Biotechnol. 2013, 31, 70–77. [Google Scholar] [PubMed]
- Jacobsen, J.H.; Frigaard, N.-U. Engineering of photosynthetic mannitol biosynthesis from CO2 in a cyanobacterium. Metab. Eng. 2014, 21, 60–70. [Google Scholar] [PubMed]
- Moravveji, S.S.; Khoshbakht, S.; Mokhtari, M.; Salimi, M.; Lanjanian, H.; Nematzadeh, S.; Torkamanian-Afshar, M.; Masoudi-Nejad, A. Impact of 5HydroxyMethylCytosine (5hmC) on reverse/direct association of cell-cycle, apoptosis, and extracellular matrix pathways in gastrointestinal cancers. BMC Genom. Data 2022, 23, 49. [Google Scholar]
- Xia, A.; Jacob, A.; Herrmann, C.; Tabassum, M.R.; Murphy, J.D. Production of hydrogen, ethanol and volatile fatty acids from the seaweed carbohydrate mannitol. Bioresour. Technol. 2015, 193, 488–497. [Google Scholar] [PubMed]
- Adams, J.; Ross, A.; Anastasakis, K.; Hodgson, E.; Gallagher, J.A.; Jones, J.M.; Donnison, I.S. Seasonal variation in the chemical composition of the bioenergy feedstock Laminaria digitata for thermochemical conversion. Bioresour. Technol. 2011, 102, 226–234. [Google Scholar] [PubMed]
- Ge, L.; Wang, P.; Mou, H. Study on saccharification techniques of seaweed wastes for the transformation of ethanol. Renew. Energy 2011, 36, 84–89. [Google Scholar]
- Jung, K.-H.; Ji-Hyeon, Y.; Lee, S.; Choi, W.; Kang, D.H.; Lee, H.Y.; Jung, K.H. Repeated-batch operation of surface-aerated fermentor for bioethanol production from the hydrolysate of seaweed Sargassum sagamianum. J. Microbiol. Biotechnol. 2011, 21, 323–331. [Google Scholar]
- Quiñones, V. Parametric Study on Pretreatment and Saccharification of Turbinaria ornata (Phaeophyta) for Hydrolysate Production. Undergraduate Thesis, University of the Philippines, Los Baños, Philippines, 2010. [Google Scholar]
- Alquiros, A. Parametric Study on Fermentation of Cellulose and Mannitol from Macroalgae (Sargassum spp.) for Bioethanol Production. Undergraduate Thesis, University of the Philippines, Los Baños, Philippines, 2013. [Google Scholar]
- Atienza, G. A Parametric Study on the Fermentation of Mannitol from Sargassum sp. Forbioethanol Production Using Saccharomyces cerevisiae 2055. Undergraduate Thesis, University of the Philippines, Los Baños, Philippines, 2013. [Google Scholar]
- Gatdula, K.M. Parametric Study on Ethanol Fermentation of Cellulose and Alginic Acid of Macroalgae (Sargassum spp.) Using Saccharomyces cerevisiae 2055. Undergraduate Thesis, University of the Philippines, Los Baños, Philippines, 2013. [Google Scholar]
- Isa, A.; Mishima, Y.; Takimura, O.; Minowa, T. Preliminary study on ethanol production by using macro green algae. J. Jpn. Inst. Energy 2009, 88, 912–917. [Google Scholar]
- Yoon, J.J.; Kim, Y.J.; Kim, S.H.; Ryu, H.J.; Choi, J.Y.; Kim, G.S.; Shin, M.K. Production of polysaccharides and corresponding sugars from red seaweed. Adv. Mater. Res. 2010, 93, 463–466. [Google Scholar]
- Meinita, M.D.N.; Hong, Y.-K.; Jeong, G.-T. Comparison of sulfuric and hydrochloric acids as catalysts in hydrolysis of Kappaphycus alvarezii (cottonii). Bioprocess Biosyst. Eng. 2012, 35, 123–128. [Google Scholar]
- Khambhaty, Y.; Mody, K.; Gandhi, M.R.; Thampy, S.; Maiti, P.; Brahmbhatt, H.; Eswaran, K.; Ghosh, P.K. Kappaphycus alvarezii as a source of bioethanol. Bioresour. Technol. 2012, 103, 180–185. [Google Scholar] [PubMed]
- Daroch, M.; Geng, S.; Wang, G. Recent advances in liquid biofuel production from algal feedstocks. Appl. Energy 2013, 102, 1371–1381. [Google Scholar]
- Kawai, S.; Murata, K. Biofuel production based on carbohydrates from both brown and red macroalgae: Recent developments in key biotechnologies. Int. J. Mol. Sci. 2016, 17, 145. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.; Carrascosa, C.; Raheem, D.; Ramos, F.; Raposo, A. Natural sweeteners: The relevance of food naturalness for consumers, food security aspects, sustainability and health impacts. Int. J. Environ. Res. Public Health 2020, 17, 6285. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R. Carbohydrate metabolism. Surgery 2009, 27, 6–10. [Google Scholar]
- Chen, M.; Zhang, W.; Wu, H.; Guang, C.; Mu, W. Mannitol: Physiological functionalities, determination methods, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 2020, 104, 6941–6951. [Google Scholar] [PubMed]
- Zhang, M.; Gu, L.; Cheng, C.; Ma, J.; Xin, F.; Liu, J.; Wu, H.; Jiang, M. Recent advances in microbial production of mannitol: Utilization of low-cost substrates, strain development and regulation strategies. World J. Microbiol. Biotechnol. 2018, 34, 1–7. [Google Scholar]
- Tanghe, A.; Prior, B.; Thevelein, J.M. Yeast responses to stresses. In Biodiversity and Ecophysiology of Yeasts; Springer: Berlin, Germany, 2006; pp. 175–195. [Google Scholar]
- Song, S.H.; Vieille, C. Recent advances in the biological production of mannitol. Appl. Microbiol. Biotechnol. 2009, 84, 55–62. [Google Scholar]
- Dai, Y.; Meng, Q.; Mu, W.; Zhang, T. Recent advances in the applications and biotechnological production of mannitol. J. Funct. Foods 2017, 36, 404–409. [Google Scholar]
- Ohrem, H.L.; Schornick, E.; Kalivoda, A.; Ognibene, R. Why is mannitol becoming more and more popular as a pharmaceutical excipient in solid dosage forms? Pharm. Dev. Technol. 2014, 19, 257–262. [Google Scholar]
- Black, W.; Dewar, E.; Woodward, F. Manufacture of algal chemicals. II. Laboratory-scale isolation of mannitol from brown marine algae. J. Appl. Chem. 1951, 1, 414–424. [Google Scholar]
- Chapman, V.J. Seaweeds and Their Uses; Methuen & Co. Ltd.: New York, NY, USA, 1970. [Google Scholar]
- Bambase, M.; Demafelis, R.B.; Borines, M.G.; Gatdula, K.M.; Joy, A.; Alquiros, A.; Atienza, E.A. Ethanol production from Mannitol of Sargassum using Saccharomyces cerevisiae 2055. Philipp. J. Crop. Sci. 2015, 40, 76–85. [Google Scholar]
- Chades, T.; Scully, S.M.; Ingvadottir, E.M.; Orlygsson, J. Fermentation of mannitol extracts from brown macro algae by Thermophilic Clostridia. Front. Microbiol. 2018, 9, 1931. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuen, A.K.; de Nys, R.; Masters, A.F.; Maschmeyer, T. Step by step extraction of bio-actives from the brown seaweeds, Carpophyllum flexuosum, Carpophyllum plumosum, Ecklonia radiata and Undaria pinnatifida. Algal Res. 2020, 52, 102092. [Google Scholar]
- Wichmann, R.; Wandrey, C.; Bückmann, A.F.; Kula, M.R. Continuous enzymatic transformation in an enzyme membrane reactor with simultaneous NAD (H) regeneration. Biotechnol. Bioeng. 2000, 67, 791–804. [Google Scholar] [CrossRef]
- Park, Y.-C.; Oh, E.J.; Jo, J.-H.; Jin, Y.-S.; Seo, J.-H. Recent advances in biological production of sugar alcohols. Curr. Opin. Biotechnol. 2016, 37, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.E.; Bleckwedel, J.; Raya, R.R.; Mozzi, F. Biotechnological and in situ food production of polyols by lactic acid bacteria. Appl. Microbiol. Biotechnol. 2013, 97, 4713–4726. [Google Scholar] [PubMed]
- Ortiz, M.E.; Fornaguera, M.J.; Raya, R.R.; Mozzi, F. Lactobacillus reuteri CRL 1101 highly produces mannitol from sugarcane molasses as carbon source. Appl. Microbiol. Biotechnol. 2012, 95, 991–999. [Google Scholar] [PubMed]
- Soetaert, W.; Vanhooren, P.T.; Vandamme, E.J. The production of mannitol by fermentation. Carbohydr. Biotechnol. Protocols 1999, 862, 261–275. [Google Scholar]
- Yun, J.W.; Kim, D.H. A comparative study of mannitol production by two lactic acid bacteria. J. Ferment. Bioeng. 1998, 85, 203–208. [Google Scholar] [CrossRef]
- Martínez-Miranda, J.G.; Chairez, I.; Durán-Páramo, E. Mannitol production by heterofermentative lactic acid bacteria: A review. Appl. Biochem. Biotechnol. 2022, 194, 2762–2795. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, M.E.; Raya, R.R.; Mozzi, F. Efficient mannitol production by wild-type Lactobacillus reuteri CRL 1101 is attained at constant pH using a simplified culture medium. Appl. Microbiol. Biotechnol. 2015, 99, 8717–8729. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. A low-cost medium for mannitol production by Lactobacillus intermedius NRRL B-3693. Appl. Microbiol. Biotechnol. 2006, 72, 676–680. [Google Scholar] [CrossRef]
- Saha, B.C.; Nakamura, L.K. Production of mannitol and lactic acid by fermentation with Lactobacillus intermedius NRRL B-3693. Biotechnol. Bioeng. 2003, 82, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.C. Effect of salt nutrients on mannitol production by Lactobacillus intermedius NRRL B-3693. J. Ind. Microbiol. Biotechnol. 2006, 33, 887–890. [Google Scholar] [CrossRef] [PubMed]
- Jennings, D. Polyol metabolism in fungi. Adv. Microb. Physiol. 1985, 25, 149–193. [Google Scholar]
- Kets, E.P. Compatible Solutes in Lactic acid Bacteria Subjected to Water Stress Wageningen University and Research. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 1997. [Google Scholar]
- Leslie, S.B.; Israeli, E.; Lighthart, B.; Crowe, J.H.; Crowe, L.M. Trehalose and sucrose protect both membranes and proteins in intact bacteria during drying. Appl. Environ. Microbiol. 1995, 61, 3592–3597. [Google Scholar] [CrossRef] [PubMed]
- Chibbar, R.; Båga, M. Genetic modification of primary metabolism: Carbohydrates. In Encyclopedia of Applied Plant Sciences; Elsevier: Amsterdam, The Netherlands, 2003; pp. 449–459. [Google Scholar]
- Ribeiro, T.B.; Oliveira, A.L.; Costa, C.; Nunes, J.; Vicente, A.; Pintado, M. Total and sustainable valorisation of olive pomace using a fractionation approach. Appl. Sci. 2020, 10, 6785. [Google Scholar] [CrossRef]
- Chiang, S.-S.; Liang, Z.-C.; Wang, Y.-C.; Liang, C.-H. Effect of light-emitting diodes on the production of cordycepin, mannitol and adenosine in solid-state fermented rice by Cordyceps militaris. J. Food Compos. Anal. 2017, 60, 51–56. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, X.; Biswas, D. Polyphenols and tri-terpenoids from Olea europaea L. in alleviation of enteric pathogen infections through limiting bacterial virulence and attenuating inflammation. J. Funct. Foods 2017, 36, 132–143. [Google Scholar]
- Medina, E.; Romero, C.; Brenes, M. Residual olive paste as a source of phenolic compounds and triterpenic acids. Eur. J. Lipid Sci. Technol. 2018, 120, 1700368. [Google Scholar] [CrossRef]
- Blanco-Cabra, N.; Vega-Granados, K.; Moya-Andérico, L.; Vukomanovic, M.; Parra, A.; de Cienfuegos, L.Á.; Torrents, E. Novel oleanolic and maslinic acid derivatives as a promising treatment against bacterial biofilm in nosocomial infections: An in vitro and in vivo study. ACS Infect. Dis. 2019, 5, 1581–1589. [Google Scholar] [CrossRef] [PubMed]
- Daneshmehr, M.A.; Tafazoli, A. Providing evidence for use of Echinacea supplements in Hajj pilgrims for management of respiratory tract infections. Complement. Ther. Clin. Pract. 2016, 23, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, M.; Khoshbakht, S.; Akbari, M.E.; Moravveji, S.S. BMC3PM: Bioinformatics multidrug combination protocol for personalized precision medicine and its application in cancer treatment. BMC Med. Genet. 2023, 16, 328. [Google Scholar] [CrossRef]
- Shawkat, H.; Westwood, M.-M.; Mortimer, A. Mannitol: A review of its clinical uses. Contin. Educ. Anaesth. Crit. Care Pain 2012, 12, 82–85. [Google Scholar] [CrossRef]
- Burger, A.; Henck, J.-O.; Hetz, S.; Rollinger, J.M.; Weissnicht, A.A.; Stöttner, H. Energy/temperature diagram and compression behavior of the polymorphs of D-mannitol. J. Pharm. Sci. 2000, 89, 457–468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, S.V.; Dastgerdi, H.E.; Tahergorabi, R. Marine Mannitol: Extraction, Structures, Properties, and Applications. Processes 2024, 12, 1613. https://doi.org/10.3390/pr12081613
Hosseini SV, Dastgerdi HE, Tahergorabi R. Marine Mannitol: Extraction, Structures, Properties, and Applications. Processes. 2024; 12(8):1613. https://doi.org/10.3390/pr12081613
Chicago/Turabian StyleHosseini, Seyed Vali, Hajar Ebrahimi Dastgerdi, and Reza Tahergorabi. 2024. "Marine Mannitol: Extraction, Structures, Properties, and Applications" Processes 12, no. 8: 1613. https://doi.org/10.3390/pr12081613




