Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Gluten-Free Flour
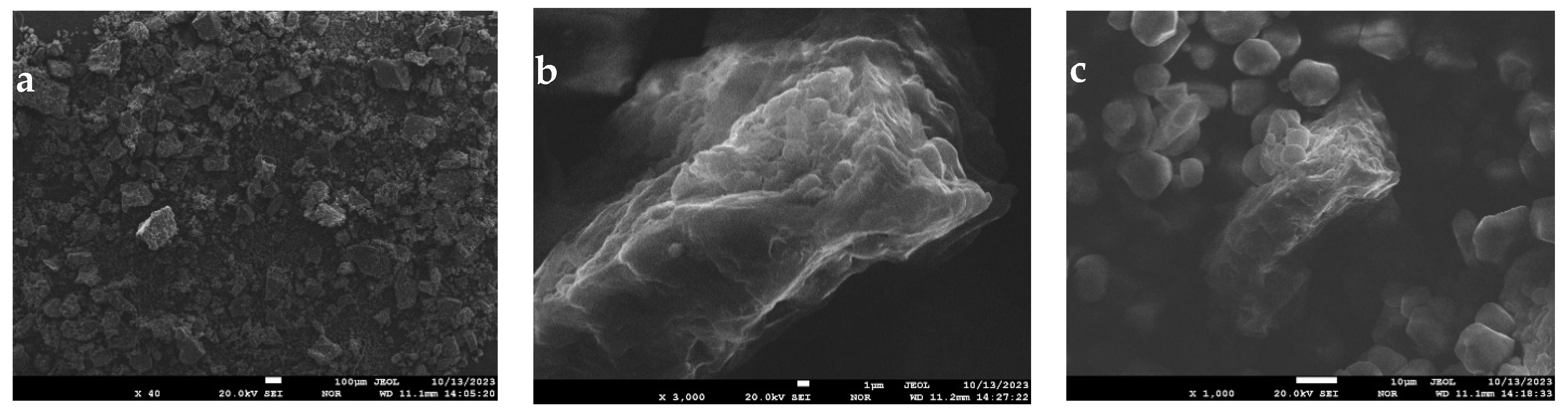
2.3. Study of Oil Cake Microstructure
2.4. Electron Microscopy Sub-Micrometer Range
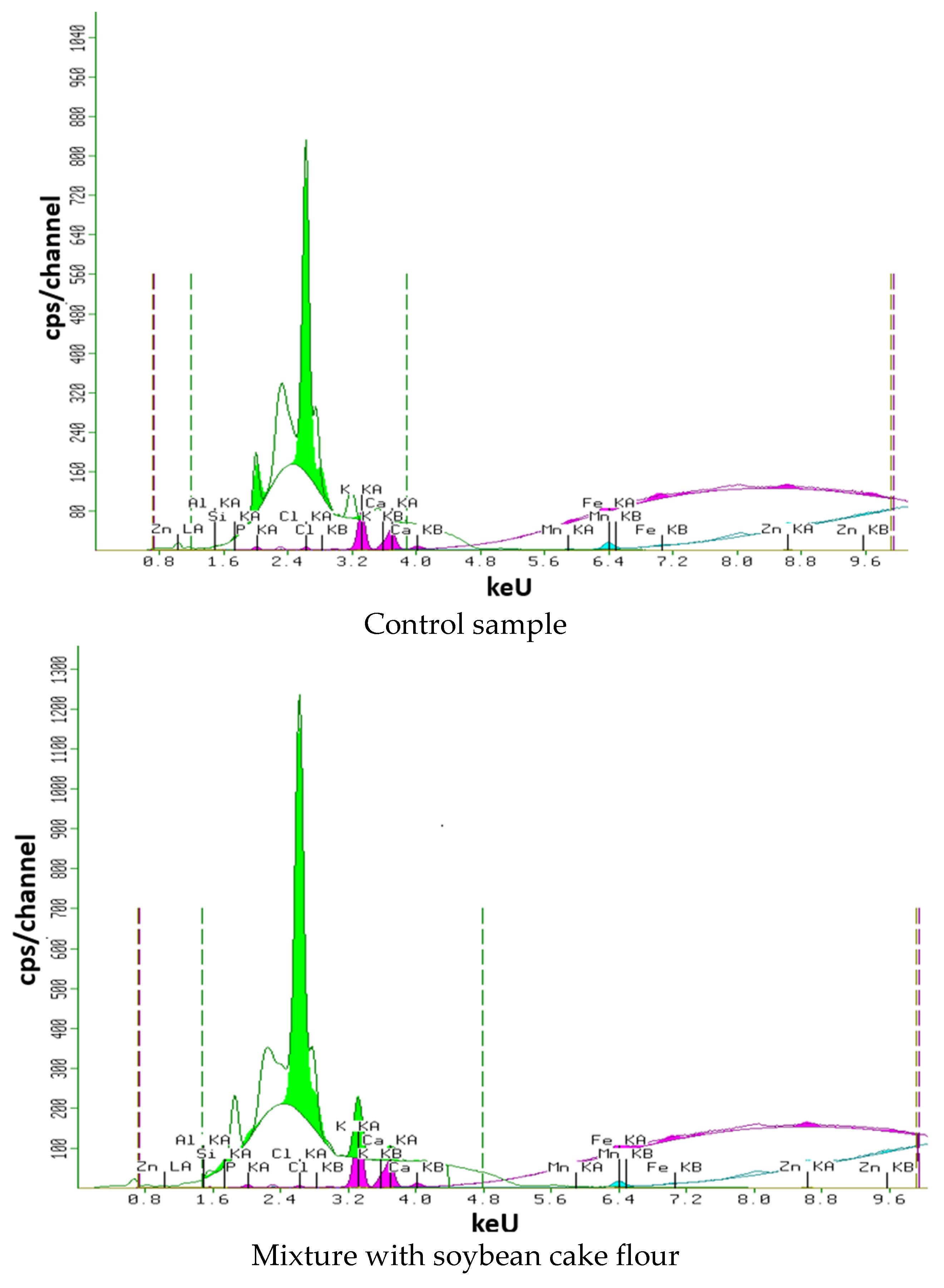
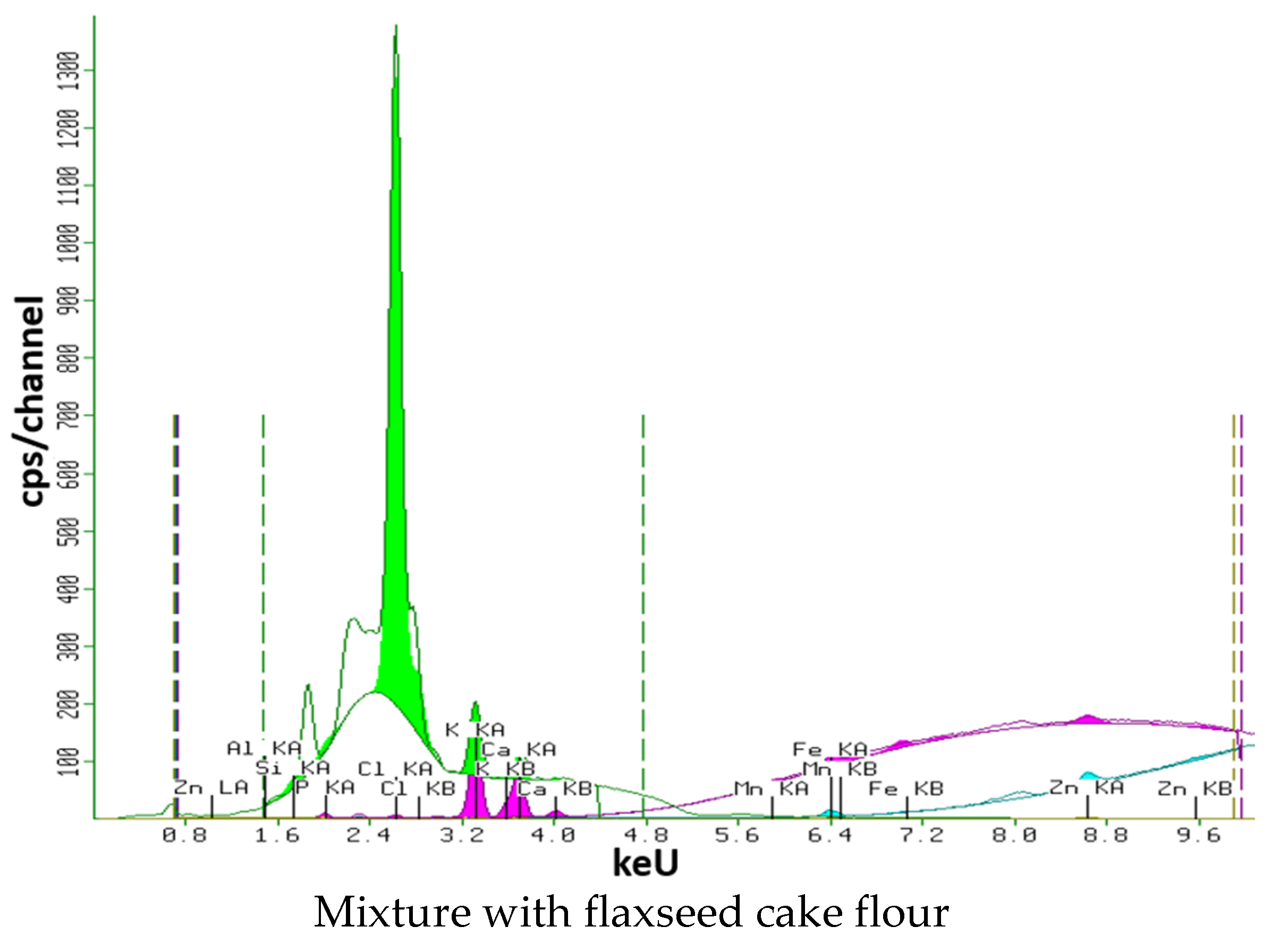
2.5. X-ray Fluorescence Semi-Qualitative Structural Analysis
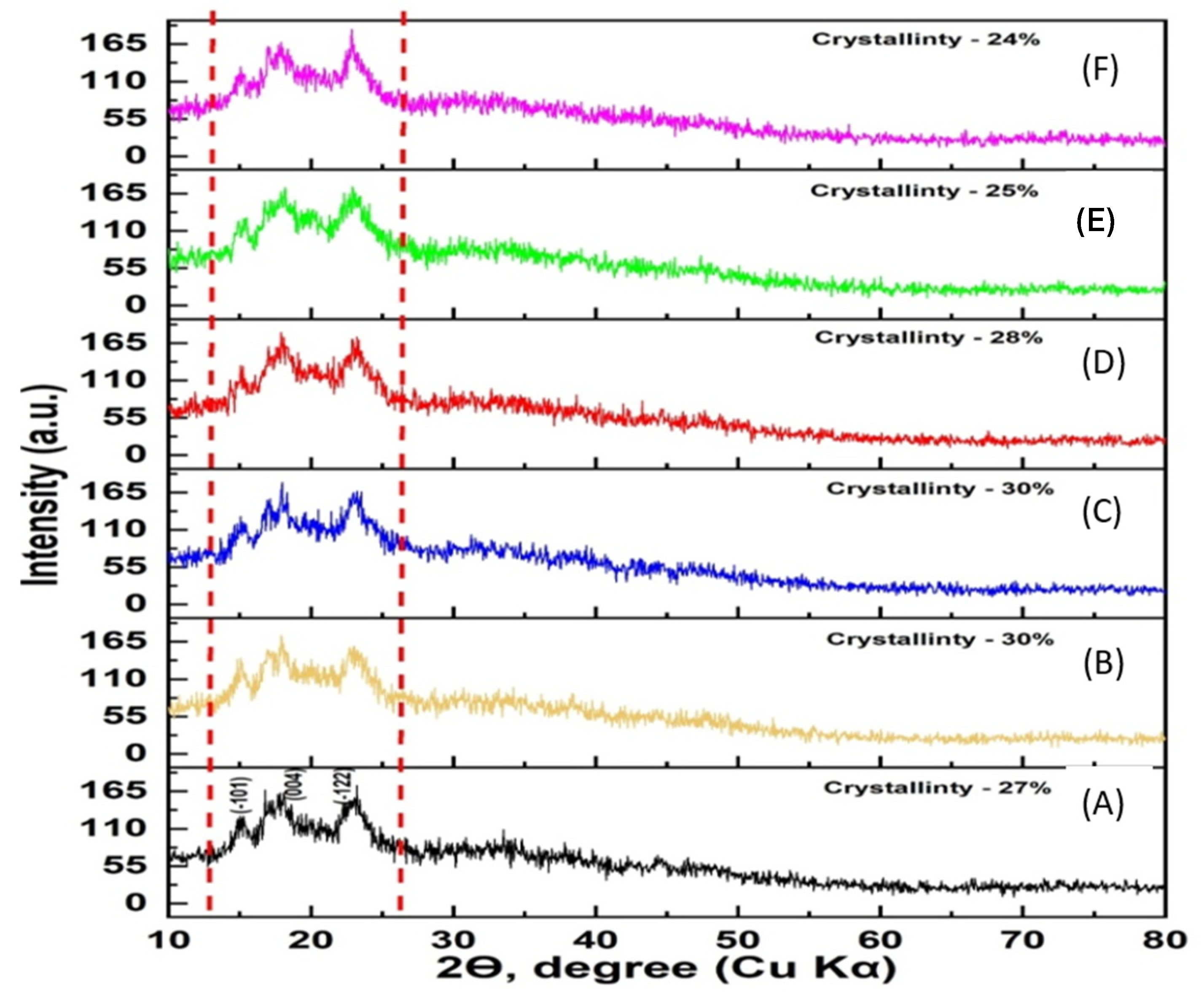
2.6. X-ray Phase Analysis
2.7. Determination of Mineral Content
2.8. Determination of Amino Acid Content
2.9. Determination of Fatty Acid Content
2.10. Determination of Chemical Composition
2.11. Statistics
3. Results and Discussion
3.1. Organoleptic Analysis of Oil Cakes
3.2. Nutritional Profile of Oil Cake
3.3. Amino Acid Composition of Oil Cake
3.4. Fatty Acid Composition of Oil Cake
3.5. Mineral Composition of Oil Cake
3.6. X-ray Fluorescence Analysis
3.7. Electron Microscopy
3.7.1. Gluten-Free Mixture (Control Sample)
3.7.2. Soy Cake Flour Mixture
3.7.3. Pumpkin Cake Flour Mixture
3.7.4. Sunflower Cake Flour Mixture
3.7.5. Peanut Cake Flour Mixture
3.7.6. Flaxseed Cake Flour Mixture
3.8. X-ray Phase Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- USDA Forecasts 2M Tonnes Rise in Global 2022/23 Oilseed Production to 657M Tonnes. Published 24 October 2022. Available online: https://www.ofimagazine.com/news/usda-forecasts-2m-tonnes-rise-in-global-2022-23-oilseed-production-to-657m-tonnes (accessed on 18 July 2024).
- Sarwar, F.; Qadri, N.A.; Moghal, S. The role of oilseeds nutrition in human health: A critical review. J. Cereals Oilseeds 2013, 4, 97–100. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, R.; Sharma, S.; Singh, B. Oilseed as potential functional food Ingredient. In Trends & Prospects in Food Technology, Processing and Preservation, 1st ed.; Prodyut Kumar, P., Mahawar, M.K., Abobatta, W., Panja, P., Eds.; Today and Tomorrow’s Printers and Publishers: New Delhi, India, 2018; pp. 25–58. [Google Scholar]
- Rakita, S.; Kokić, B.; Manoni, M.; Mazzoleni, S.; Lin, P.; Luciano, A.; Ottoboni, M.; Cheli, F.; Pinotti, L. Cold-Pressed Oilseed Cakes as Alternative and Sustainable Feed Ingredients: A Review. Foods 2023, 12, 432. [Google Scholar] [CrossRef] [PubMed]
- Bárta, J.; Bártová, V.; Jarošová, M.; Švajner, J.; Smetana, P.; Kadlec, J.; Filip, V.; Kyselka, J.; Berčíková, M.; Zdráhal, Z.; et al. Oilseed Cake Flour Composition, Functional Properties and Antioxidant Potential as Effects of Sieving and Species Differences. Foods 2021, 10, 2766. [Google Scholar] [CrossRef]
- Teh, S.-S.; Morlock, G.E. Analysis of Bioactive Components of Oilseed Cakes by High-Performance Thin-Layer Chromatography-(Bio)assay Combined with Mass Spectrometry. Chromatography 2015, 2, 125–140. [Google Scholar] [CrossRef]
- Terpinc, P.; Čeh, B.; Ulrih, N.; Abramovič, H. Studies of the correlation between antioxidant properties and the total phenolic content of different oil cake extracts. Ind. Crops Prod. 2012, 39, 210–217. [Google Scholar] [CrossRef]
- The, S.S.; Bekhit, A.E. Utilization of oilseed cakes for human nutrition and health benefits. In Agricultural Biomass Based Potential Materials; Hakeem, K.R., Jawaid, M., Alothman, O.Y., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 191–229. [Google Scholar] [CrossRef]
- Zając, M.; Guzik, P.; Kulawik, P.; Tkaczewska, J.; Florkiewicz, A.; Migdal, W. The quality of pork loaves with the addition of hemp seeds, de-hulled hemp seeds, hemp protein and hemp flour. LWT-Food Sci. Technol. 2019, 105, 190–199. [Google Scholar] [CrossRef]
- Muhammad Anjum, F.; Nadeem, M.; Issa Khan, M.; Hussain, S. Nutritional and therapeutic potential of sunflower seeds: A review. Br. Food J. 2012, 114, 544–552. [Google Scholar] [CrossRef]
- Ancuţa, P.; Sonia, A. Oil press-cakes and meals valorization through circular economy approaches. Appl. Sci. 2020, 10, 7432. [Google Scholar] [CrossRef]
- Kuijsten, A.; Arts, I.C.W.; Van’t Veer, P.; Hollman, P.C.H. The relative bioavailability of enterolignans in humans is enhanced by milling and crushing of flaxseed. J. Nutr. 2005, 135, 2812–2816. [Google Scholar] [CrossRef]
- Edel, A.L.; Aliani, M.; Pierce, G.N. Stability of bioactives in flaxseed and flaxseed-fortified foods. Food Res. Int. 2015, 77, 140–155. [Google Scholar] [CrossRef]
- Kaur, S.; Kumar, K.; Singh, L.; Sharanagat, V.S.; Nema, P.K.; Mishra, V.; Bhushan, B. Gluten-free grains: Importance, processing and its effect on quality of gluten-free products. Crit. Rev. Food Sci. Nutr. 2022, 64, 1988–2015. [Google Scholar] [CrossRef] [PubMed]
- Bouasla, A.; Wójtowicz, A.; Zidoune, M.N. Gluten-free precooked rice pasta enriched with legumes flours: Physical properties, texture, sensory attributes and microstructure. LWT 2017, 75, 569–577. [Google Scholar] [CrossRef]
- Sajid Mushtaq, B.; Zhang, W.; Al-Ansi, W.; Ul Haq, F.; Rehman, A.; Omer, R.; Mahmood Khan, I.; Niazi, S.; Ahmad, A.; Ali Mahdi, A.; et al. A Critical Review on the Development, Physicochemical Variations and Technical Concerns of Gluten Free Extrudates in Food Systems. Food Rev. Int. 2021, 39, 2806–2834. [Google Scholar] [CrossRef]
- Pashchenko, L.P.; Zharkova, I.M. Rational Use of Vegetable Protein-Containing Raw Materials in Bread Technology; FGUP IPF “Voronezh”: Voronezh, Russia, 2003. [Google Scholar]
- Murphy, D.B.; Davidson, M.W. Fundamentals of Light Microscopy and Electronic Imaging; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Tang, Y.; Gotor, F.J.; Sayagués, M.J. Development by Mechanochemistry of La0.8Sr0.2Ga0.8Mg0.2O2.8 Electrolyte for SOFCs. Materials 2020, 13, 1366. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.G.; Chow, J.C.; Frazier, C.A. Chapter 2: X-ray Fluorescence Analysis of Ambient Air Samples. In Elemental Analysis of Airborne Particles; Landsberger, S., Creatchman, M., Eds.; Gordon and Breach Science: Amsterdam, The Netherlands, 1999; Volume 1. [Google Scholar]
- Yessimbekov, Z.; Kakimov, A.; Caporaso, N.; Suychinov, A.; Kabdylzhar, B.; Shariati, M.A.; Baikadamova, A.; Domínguez, R.; Lorenzo, J.M. Use of Meat-Bone Paste to Develop Calcium-Enriched Liver Pâté. Foods 2021, 10, 2042. [Google Scholar] [CrossRef] [PubMed]
- GOST R 55569-2013; Fodders, Mixed Fodders, Mixed Fodder Raw Materials. Determination of Proteinogenic Amino Acids by Capillary Electrophoresis. Standardinform: Moscow, Russia, 2014; 18p.
- GOST R 55483-2013; Meat and Meat Products. Determination of Fatty Acid Composition by Gas Chromatography: Introduced for the First Time: Date of Introduction 1 July 2014; Developed by V.M. Gorbatov VNIIMP of Rosselkhozakademia. Standardinform: Moscow, Russia, 2014; 16p.
- Zauer, E.A.; Ershov, A.B. Modern analyzers for the determination of nitrogen by the Kjeldahl method. Anal. Control 2019, 23, 168–192. [Google Scholar] [CrossRef]
- GOST R 54705-2011; Cakes, Meal and Mustard Powder. Method for Determining the Mass Fraction of Moisture and Volatile Substances. Izd vo Standards: Moscow, Russia, 2011; 8p.
- GOST 13979.6-69; Cakes, Meal and Mustard Powder. Method for Determination of Ash. Izd vo Standards: Moscow, Russia, 1969; 3p.
- Ogunronbi, O.; Jooste, P.J.; Abu, J.O.; Van der Merwe, B. Chemical composition, storage stability and effect of cold-pressed flaxseed oil cake inclusion on bread quality. J. Food Process. Preserv. 2011, 35, 64–79. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, B.; Kaur, A.; Singh, N. Proximate, mineral, amino acid composition, phenolic profile, antioxidant and functional properties of oilseed cakes. Int. J. Food Sci. Technol. 2021, 56, 6732–6741. [Google Scholar] [CrossRef]
- Mannucci, A.; Castagna, A.; Santin, M.; Serra, A.; Mele, M.; Ranieri, A. Quality of flaxseed oil cake under different storage conditions. LWT 2019, 104, 84–90. [Google Scholar] [CrossRef]
- Aydin, E.; Turgut, S.S.; Aydin, S.; Cevik, S.; Ozcelik, A.; Aksu, M.; Ozcelik, M.M.; Ozkan, G. A New Approach for the Development and Optimization of Gluten-Free Noodles Using Flours from Byproducts of Cold-Pressed Okra and Pumpkin Seeds. Foods 2023, 12, 2018. [Google Scholar] [CrossRef]
- Budžaki, S.; Strelec, I.; Krnić, M.; Alilović, K.; Tišma, M.; Zelić, B. Proximate analysis of cold-press oil cakes after biological treatment with Trametes versicolor and Humicola grisea. Eng. Life Sci. 2018, 18, 924–931. [Google Scholar] [CrossRef] [PubMed]
- Vershinina, O.; Derevenko, V.; Milovanova, E. Production of bread of increased nutritional value enriched with pumpkin cake. Khleboprodukty 2010, 11, 42–43. (In Russian) [Google Scholar]
- Khramova, V.N.; Proskurina, O.Y.; Dolgova, V.A. Development of products of functional purpose using regional raw materials. Izv. Nizhnevolzhskogo Agrouniversitetskogo Kompleks. Nauka Vyss. Vocat. Obraz. 2013, 2, 164–168. [Google Scholar]
- Nourmohammadi, E.; SadeghiMahoonak, A.; Alami, M.; Ghorbani, M. Amino acid composition and antioxidative properties of hydrolysed pumpkin (Cucurbita pepo L.) oil cake protein. Int. J. Food Prop. 2017, 20, 3244–3255. [Google Scholar] [CrossRef]
- Murzyn, A.; Krasowska, A.; Stefanowicz, P.; Dziadkowiec, D.; Łukaszewicz, M. Capric Acid Secreted by S. boulardii Inhibits C. albicans Filamentous Growth, Adhesion and Biofilm Formation. PLoS ONE 2010, 5, e12050. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.B.; Alimova, Y.; Myers, T.M.; Ebersole, J.L. Short- and medium-chain fatty acids exhibit antimicrobial activity for oral microorganisms. Arch. Oral Biol. 2011, 56, 650–654. [Google Scholar] [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. In Advances in Immunology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 91–119. [Google Scholar] [CrossRef]
- Balta, I.; Stef, L.; Pet, I.; Iancu, T.; Stef, D.; Corcionivoschi, N. Essential Fatty Acids as Biomedicines in Cardiac Health. Biomedicines 2021, 9, 1466. [Google Scholar] [CrossRef]
- Dighriri, I.M.; Alsubaie, A.M.; Hakami, F.M.; Hamithi, D.M.; Alshekh, M.M.; Khobrani, F.A.; Dalak, F.E.; Hakami, A.A.; Alsueaadi, E.H.; Alsaawi, L.S.; et al. Effects of Omega-3 Polyunsaturated Fatty Acids on Brain Functions: A Systematic Review. Cureus 2022, 14, e30091. [Google Scholar] [CrossRef]
- Ali, A.A.H. Overview of the vital roles of macro minerals in the human body. J. Trace Elem. Miner. 2023, 4, 100076. [Google Scholar] [CrossRef]
- Karppanen, H.; Karppanen, P.; Mervaala, E. Why and how to implement sodium, potassium, calcium, and magnesium changes in food items and diets? J. Hum. Hypertens. 2005, 19 (Suppl. S3), S10–S19. [Google Scholar] [CrossRef]
- Huang, L.; Drake, V.J.; Ho, E. Zinc. Adv. Nutr. 2015, 6, 224–226. [Google Scholar] [CrossRef]
- Petraru, A.; Ursachi, F.; Amariei, S. Nutritional Characteristics Assessment of Sunflower Seeds, Oil and Cake. Perspective of Using Sunflower Oil cakes as a Functional Ingredient. Plants 2021, 10, 2487. [Google Scholar] [CrossRef]
- Kolláthová, R.; Varga, B.; Ivanišová, E.; Bíro, D.; Rolinec, M.; Juráček, M.; Šimko, M.; Gálik, B. Mineral Profile Analysis of Oilseeds and Their By-Products As Feeding Sources for Animal Nutrition. Slovak J. Anim. Sci 2019, 52, 9–15. [Google Scholar]
- Ali, M.E.; Das, R.; Eaqub, A.; Bee, S.; Hamid, A. Current Applications of X-ray Powder. Rev. Adv. Mater. Sci 2014, 38, 95–109. [Google Scholar]
- Fatimah, S.; Ragadhita, R.; Husaeni, D.F.A.; Nandiyanto, A.B.D. How to Calculate Crystallite Size from X-ray Diffraction (XRD) using Scherrer Method. ASEAN J. Sci. Eng. 2021, 2, 65–76. [Google Scholar] [CrossRef]
- Otaka, A.; Hokura, A.; Nakai, I. Determination of trace elements in soybean by X-ray fluorescence analysis and its application to identification of their production areas. Food Chem. 2014, 147, 318–326. [Google Scholar] [CrossRef]
- de Oliveira, M.L.; Oliveira, A.C.S.; Arthur, V.; Almeida, E. Evaluation of brown and golden flaxseed (Linum usitatissimum L.) by EDXRF. In Proceedings of the International Nuclear Atlantic Conference—INAC, Belo Horizonte, Brazil, 24–28 October 2011; Available online: https://repositorio-api.ipen.br/server/api/core/bitstreams/68e383fb-abaf-403e-b7cc-83650f9e7164/content (accessed on 18 July 2024).
- Akintade, A.O.; Awolu, O.O.; Ifesan, B.O. Nutritional Evaluation of Fermented, Germinated and Roasted Pumpkin (Cucurbita maxima) Seed Flour. Acta Univ. Cibiniensis Ser. E Food Technol. 2019, 23, 179–186. [Google Scholar] [CrossRef]







| Ingredient | Control | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|---|
| Rice flour | 50 | 50 | 50 | 50 | 50 | 50 |
| Corn flour | 10 | 10 | 10 | 10 | 10 | 10 |
| Corn starch | 40 | 30 | 30 | 30 | 30 | 30 |
| Pumpkin cake flour | - | 10 | - | - | - | - |
| Soybean cake flour | - | - | 10 | - | - | - |
| Flax cake flour | - | - | - | 10 | - | - |
| Sunflower cake flour | - | - | - | - | 10 | - |
| Peanut cake flour | - | - | - | - | - | 10 |
| Name of Indicators | Oil Cake Type | ||||
|---|---|---|---|---|---|
| Flaxseed | Soybean | Pumpkin | Peanut | Sunflower | |
| Appearance | Free of foreign inclusions and impurities that are visually visible | ||||
| Color | Powder of dark brown color | Yellow colored powder | Yellow-green colored powder | Powder of light brown color with red specks | Powder of gray color |
| Odor | Proper for the respective type of oilseed raw material without extraneous odor (musty, moldy, burning, etc.) | ||||
| Taste | Proper for the respective type of oilseed raw material, without rancid and other foreign flavors | ||||
| Metallomagnetic impurity, mg per 1 kg of oil cake, not more than | <0.01 mg | ||||
| Consistency | Bulk, homogeneous | ||||
| Other foreign impurities | <0.01 mg | ||||
| Pest (barn weevil, rice weevil, grain weevil, grain borer, pinworm, mealybug, mite, and others) infestation or evidence of infestation | <1.5–2.0 mm | ||||
| Oil Cake Type | Content, % | Calories, Kcal | ||
|---|---|---|---|---|
| Protein | Fat | Carbohydrates | ||
| Flaxseed | 34.4 ± 0.67 b | 25.4 ± 0.42 c | 12.0 ± 0.21 a | 464.2 |
| Soybean | 32.6 ± 0.48 b | 18.8 ± 0.28 a | 35.9 ± 0.64 e | 443.2 |
| Sunflower | 32.5 ± 0.31 b | 20.2 ± 0.33 b | 30.6 ± 0.38 d | 434.2 |
| Peanut | 29.6 ± 0.39 a | 25.6 ± 0.35 c | 18.0 ± 0.32 b | 420.8 |
| Pumpkin | 37.5 ± 0.42 c | 25.5 ± 0.44 c | 23.0 ± 0.21 c | 471.5 |
| Amino Acid | Oil Cake Samples | |||
|---|---|---|---|---|
| Flaxseed | Soybean | Peanut | Pumpkin | |
| Content, in g per 100 g of Product | ||||
| Arginine | 4.765 ± 1.906 d | 1.786 ± 0.714 a | 3.644 ± 1.457 b | 4.409 ± 1.764 c |
| Lysine | 1.400 ± 0.476 b | 2.017 ± 0.686 c | 1.358 ± 0.462 b | 1.146 ± 0.390 a |
| Tyrosine | 0.834 ± 0.250 a | 1.152 ± 0.346 b | 1.789 ± 0.537 c | 1.764 ± 0.529 c |
| Phenylalanine | 1.787 ± 0.536 a | 1.729 ± 0.519 a | 2.418 ± 0.725 b | 2.763 ± 0.829 c |
| Histidine | 0.476 ± 0.238 a | 0.778 ± 0.389 b | 0.894 ± 0.447 c | 0.882 ± 0.441 c |
| Leucine+isoleucine | 2.144 ± 0.557 a | 2.132 ± 0.554 a | 2.318 ± 0.603 b | 2.675 ± 0.695 c |
| Methionine | 0.506 ± 0.172 c | 0.248 ± 0.084 a | 0.321 ± 0.109 b | 0.882 ± 0.300 d |
| Valine | 2.412 ± 0.965 d | 1.441 ± 0.576 a | 1.954 ± 0.782 b | 2.087 ± 0.835 c |
| Proline | 1.817 ± 0.472 a | 2.132 ± 0.554 b | 2.451 ± 0.637 c | 2.352 ± 0.611 c |
| Threonine | 1.459 ± 0.584 c | 1.008 ± 0.403 b | 0.861 ± 0.344 a | 1.029 ± 0.412 b |
| Serine | 1.817 ± 0.472 c | 1.268 ± 0.330 a | 1.722 ± 0.448 c | 1.529 ± 0.397 b |
| Alanine | 1.995 ± 0.519 c | 1.210 ± 0.315 a | 1.590 ± 0.413 b | 1.617 ± 0.420 b |
| Glycine | 2.233 ± 0.759 c | 1.152 ± 0.392 a | 2.484 ± 0.845 d | 1.940 ± 0.660 b |
| # | Fatty Acid | The Chemical Formula of Fatty Acids | Oil Cake Samples | |||
|---|---|---|---|---|---|---|
| Flaxseed | Soybean | Peanut | Pumpkin | |||
| Saturated fatty acids | ||||||
| 1 | Butyric acid | C4H8O2 | 2.22 ± 0.04 a | 2.20 ± 0.02 a | 2.25 ± 0.03 a | 2.15 ± 0.02 a |
| 2 | Caprylic acid | C6H12O2 | 97.5 ± 1.42 a | 97.65 ± 1.86 a | 97.51 ± 1.06 a | 97.73 ± 1.55 a |
| 3 | Capric acid | C10H20O2 | 0.001 ± 0.000 a | nd | 0.006 ± 0.000 c | 0.0023 ± 0.000 c |
| 4 | Myristic acid | C14H28O2 | 0.011 ± 0.000 c | 0.006 ± 0.000 a | nd | 0.009 ± 0.000 b |
| 5 | Palmitic acid | C16H32O2 | 0.001 ± 0.000 a | 0.001 ± 0.000 a | 0.002 ± 0.000 b | 0.005 ± 0.000 c |
| 6 | Stearic acid | C18H36O2 | 0.240 ± 0.005 b | 0.004 ± 0.000 a | 0.004 ± 0.000 a | nd |
| 7 | Behenic acid | C22H44O2 | nd | nd | 0.004 ± 0.000 | nd |
| Total SFA | 99.9728 | 99.8616 | 99.776 | 99.89649 | ||
| Monounsaturated fatty acids | ||||||
| 1 | Palmitoleic acid | C16H30O2 | 0.0003 ± 0.000 a | 0.0004 ± 0.000 b | 0.0008 ± 0.000 c | nd |
| 2 | Oleic acid | C18H34O2 | 0.0024 ± 0.000 b | 0.0024 ± 0.000 b | 0.0025 ± 0.000 b | 0.0007 ± 0.000 a |
| 3 | Nervonic acid | C24H46O2 | 0.0038 ± 0.000 c | 0.0030 ± 0.000 a | 0.0039 ± 0.000 c | 0.0032 ± 0.000 b |
| Total MUFA | 0.00648 | 0.0058 | 0.0072 | 0.0039 | ||
| Polyunsaturated fatty acids | ||||||
| 1 | Eicosapentaenoic acid (Timnodonic acid, Omega-3) | C20H30O2 | 0.0011 ± 0.000 a | 0.128 ± 0.002 c | 0.210 ± 0.003 d | 0.095 ± 0.001 b |
| Total PUFA | 0.0011 | 0.128 | 0.21 | 0.095 | ||
| Mineral | Oil Cake Samples | |||
|---|---|---|---|---|
| Flaxseed | Soybean | Peanut | Pumpkin | |
| Potassium, K | 831.43 ± 16.63 a | 2549.0 ± 5.1 d | 982.17 ± 19.64 c | 897.55 ± 17.95 b |
| Magnesium, Mg | 347.29 ± 6.95 c | 286.30 ± 5.73 b | 215.42 ± 4.31 a | 472.63 ± 9.45 d |
| Calcium, Ca | 225.92 ± 4.50 c | 214.71 ± 4.29 c | 101.16 ± 2.02 b | 67.81 ± 1.36 a |
| Sodium, Na | 41.30 ± 0.83 b | 7.82 ± 0.16 a | 48.14 ± 0.96 c | n/d |
| Phosphorus, P | 672.31 ± 13.44 c | 638.97 ± 12.78 b | 428.27 ± 8.56 a | 893.69 ± 17.87 d |
| Iron, Fe | 5.37 ± 0.11 a | 9.13 ± 0.18 c | 9.12 ± 0.18 c | 7.43 ± 0.15 b |
| Copper, Cu | 1.01 ± 0.02 a | 2.03 ± 0.04 d | 1.38 ± 0.03 b | 1.85 ± 0.04 c |
| Zinc, Zn | 5.63 ± 0.11 c | 3.01 ± 0.06 a | 4.39 ± 0.09 b | 6.74 ± 0.13 d |
| Sample | P | Cl | K | Ca | Fe | Si | Mn | Zn | Al |
|---|---|---|---|---|---|---|---|---|---|
| Control sample | 0.384 ± 0.004 c | 0.095 ± 0.002 c | 0.527 ± 0.008 a | 0.281 ± 0.004 a | 0.029 ± 0.001 c | 0.028 ± 0.001 a | 0.004 ± 0.000 a | 0.006 ± 0.000 a | 0.013 ± 0.000 a |
| Mixture with soybean cake flour | 0.088 ± 0.001 b | 0.078 ± 0.001 b | 1.017 ± 0.011 d | 0.458 ± 0.008 c | 0.033 ± 0.001 d | 0.178 ± 0.003 c | 0.007 ± 0.000 b | 0.011 ± 0.000 b | 0.107 ± 0.002 d |
| Mixture with pumpkin cake flour | 0.073 ± 0.001 a | 0.071 ± 0.001 a | 0.701 ± 0.012 b | 0.373 ± 0.006 b | 0.027 ± 0.001 b | n/d | 0.010 ± 0.001 d | 0.019 ± 0.001 e | 0.052 ± 0.000 c |
| Mixture with sunflower cake flour | 0.090 ± 0.002 b | 0.081 ± 0.001 b | 0.807 ± 0.016 c | 0.400 ± 0.008 b | 0.025 ± 0.001 a | 0.176 ± 0.003 c | 0.007 ± 0.000 b | 0.015 ± 0.000 c | 0.102 ± 0.001 d |
| Mixture with peanut cake flour | 0.080 ± 0.001 b | 0.080 ± 0.001 b | 0.845 ± 0.009 c | 0.380 ± 0.005 b | 0.030 ± 0.001 c | 0.153 ± 0.003 b | 0.008 ± 0.000 c | 0.018 ± 0.000 d | 0.112 ± 0.001 de |
| Mixture with flaxseed cake flour | 0.080 ± 0.001 b | 0.074 ± 0.001 a | 0.837 ± 0.009 c | 0.530 ± 0.009 d | 0.025 ± 0.001 a | 0.160 ± 0.003 b | 0.008 ± 0.000 c | 0.017 ± 0.000 d | 0.040 ± 0.000 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mashanova, N.; Satayeva, Z.; Smagulova, M.; Kundyzbayeva, N.; Karimova, G. Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes. Processes 2024, 12, 1616. https://doi.org/10.3390/pr12081616
Mashanova N, Satayeva Z, Smagulova M, Kundyzbayeva N, Karimova G. Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes. Processes. 2024; 12(8):1616. https://doi.org/10.3390/pr12081616
Chicago/Turabian StyleMashanova, Nurbibi, Zhuldyz Satayeva, Mirgul Smagulova, Nazigul Kundyzbayeva, and Gulmaida Karimova. 2024. "Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes" Processes 12, no. 8: 1616. https://doi.org/10.3390/pr12081616
APA StyleMashanova, N., Satayeva, Z., Smagulova, M., Kundyzbayeva, N., & Karimova, G. (2024). Nutritional and Structural Evaluation of Gluten-Free Flour Mixtures Incorporating Various Oilseed Cakes. Processes, 12(8), 1616. https://doi.org/10.3390/pr12081616










