Experimental and Numerical Simulation Study on Enhancing Gas Recovery with Impure CO2 in Gas Reservoirs
Abstract
:1. Introduction
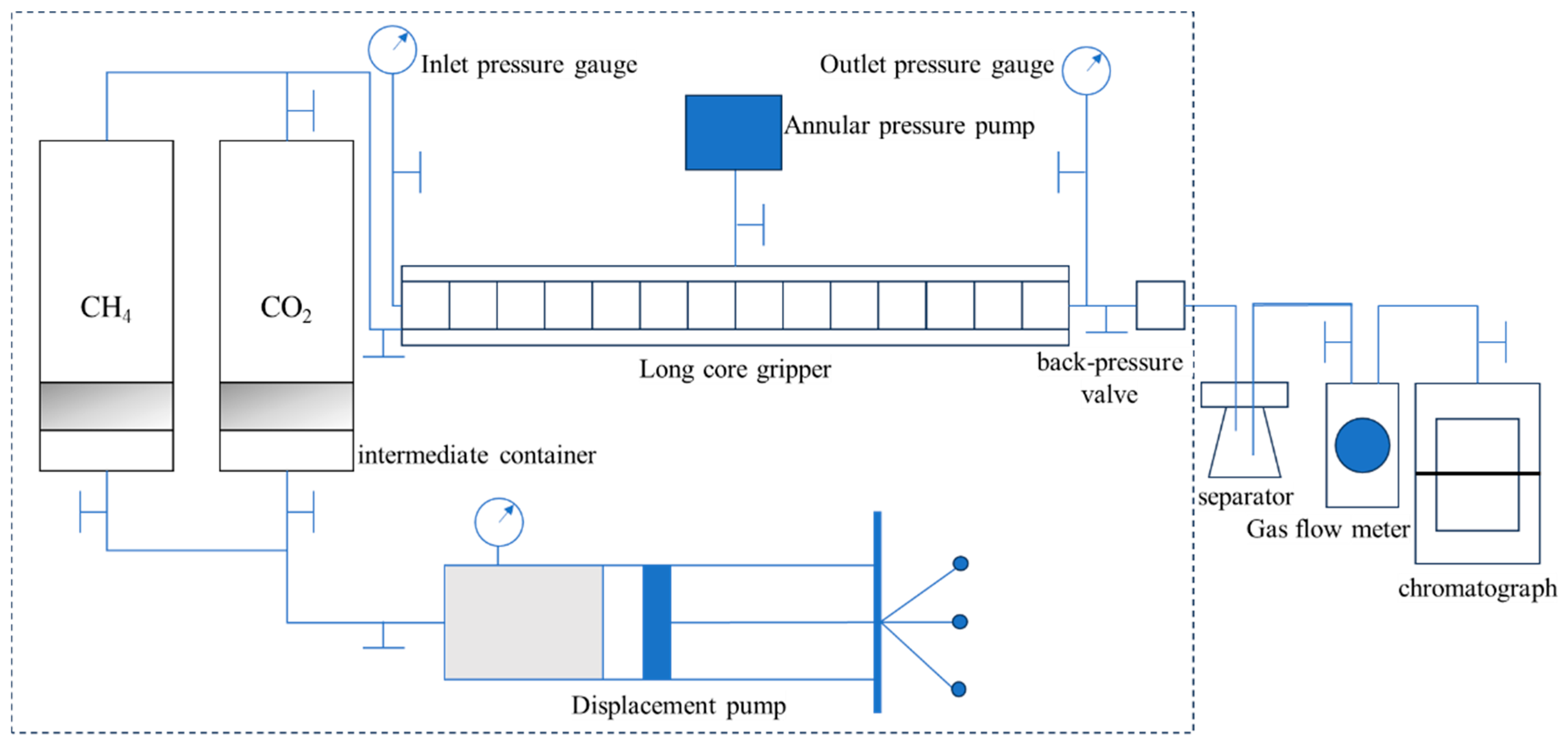
2. High-Temperature and High-Pressure CO2-EGR Displacement Experiments
2.1. Experimental Materials and Content
2.2. Experimental Results and Analysis
2.2.1. Depletion Experiment
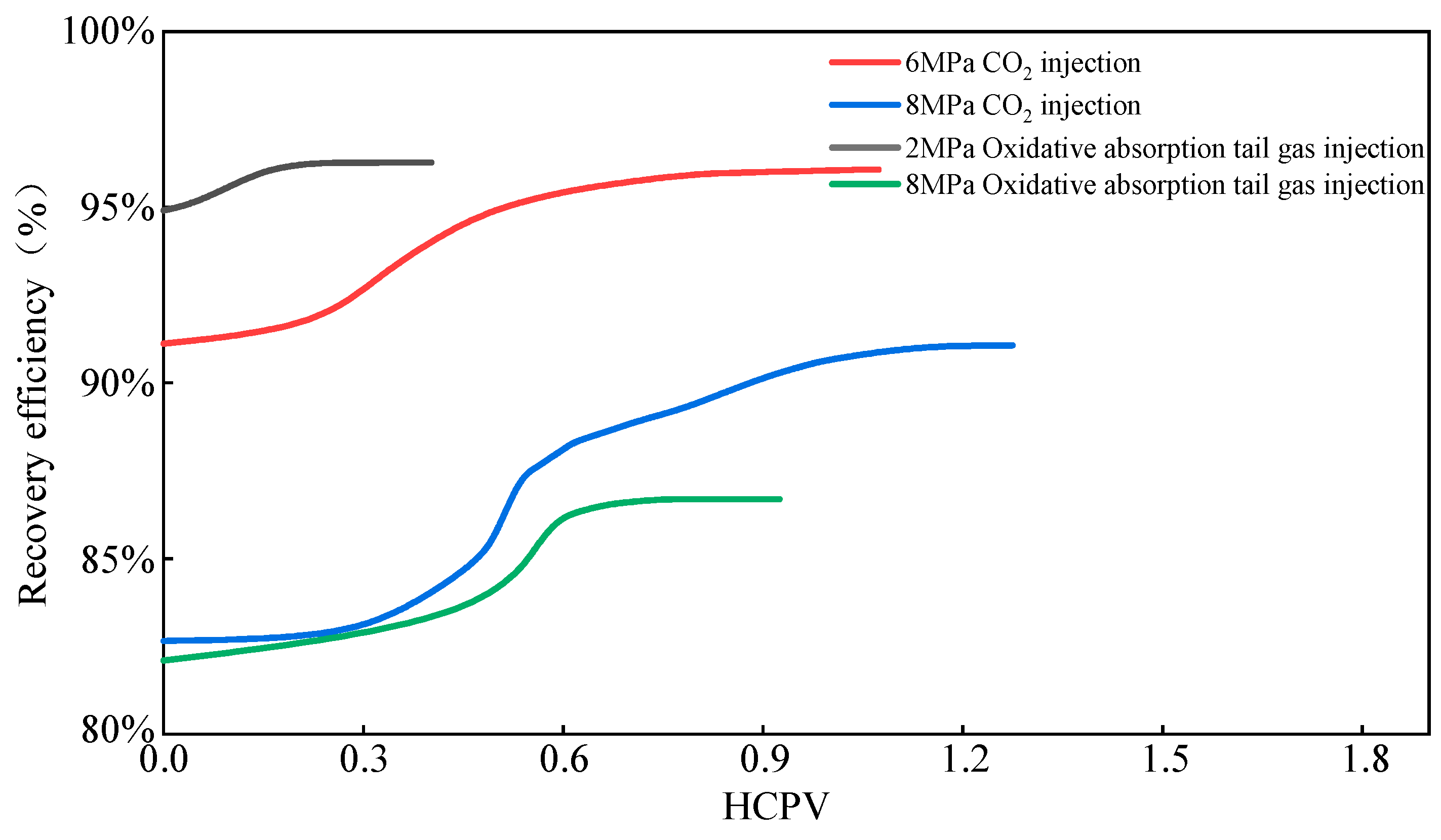
2.2.2. CO2 Injection for Gas Displacement—Ultimate Recovery
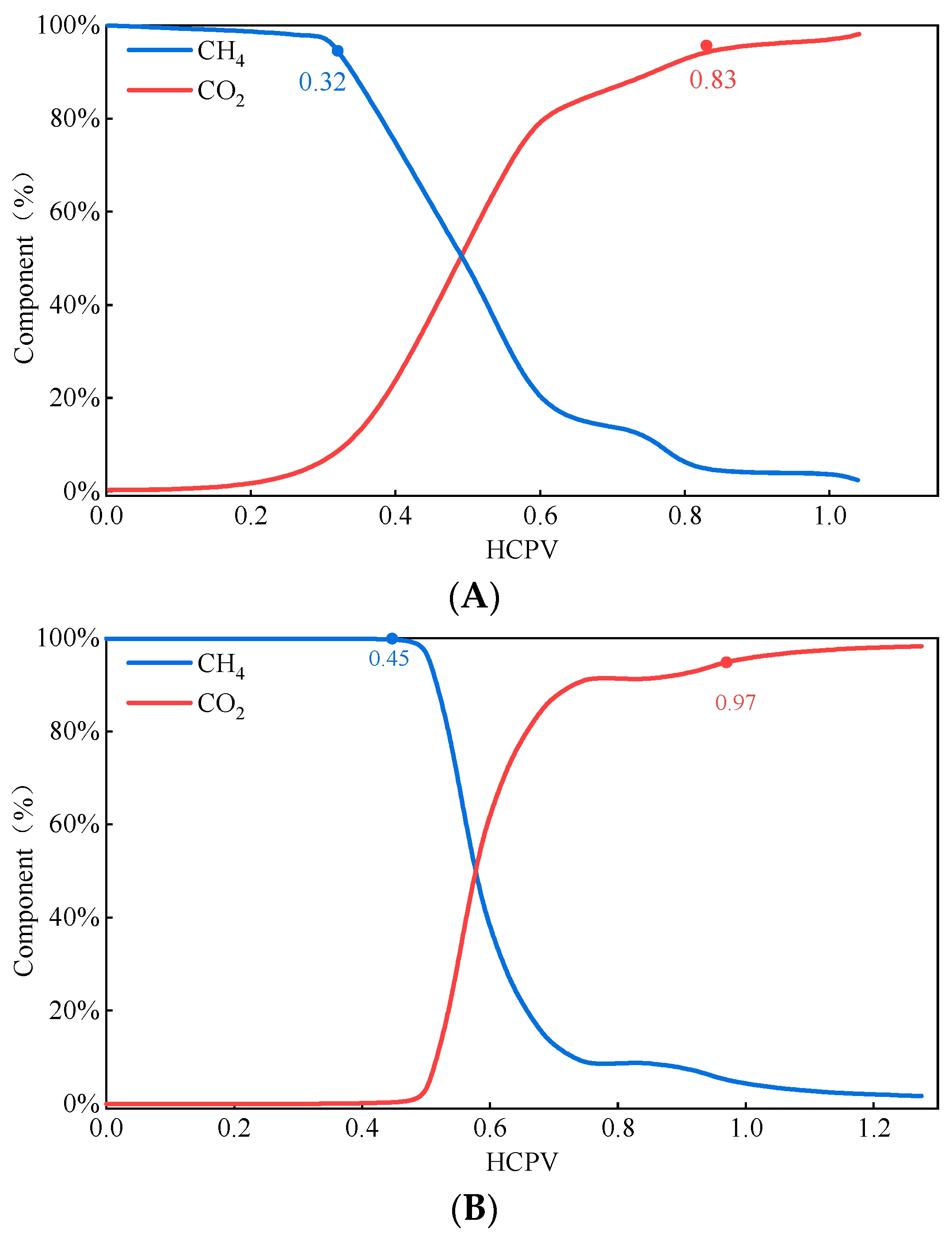
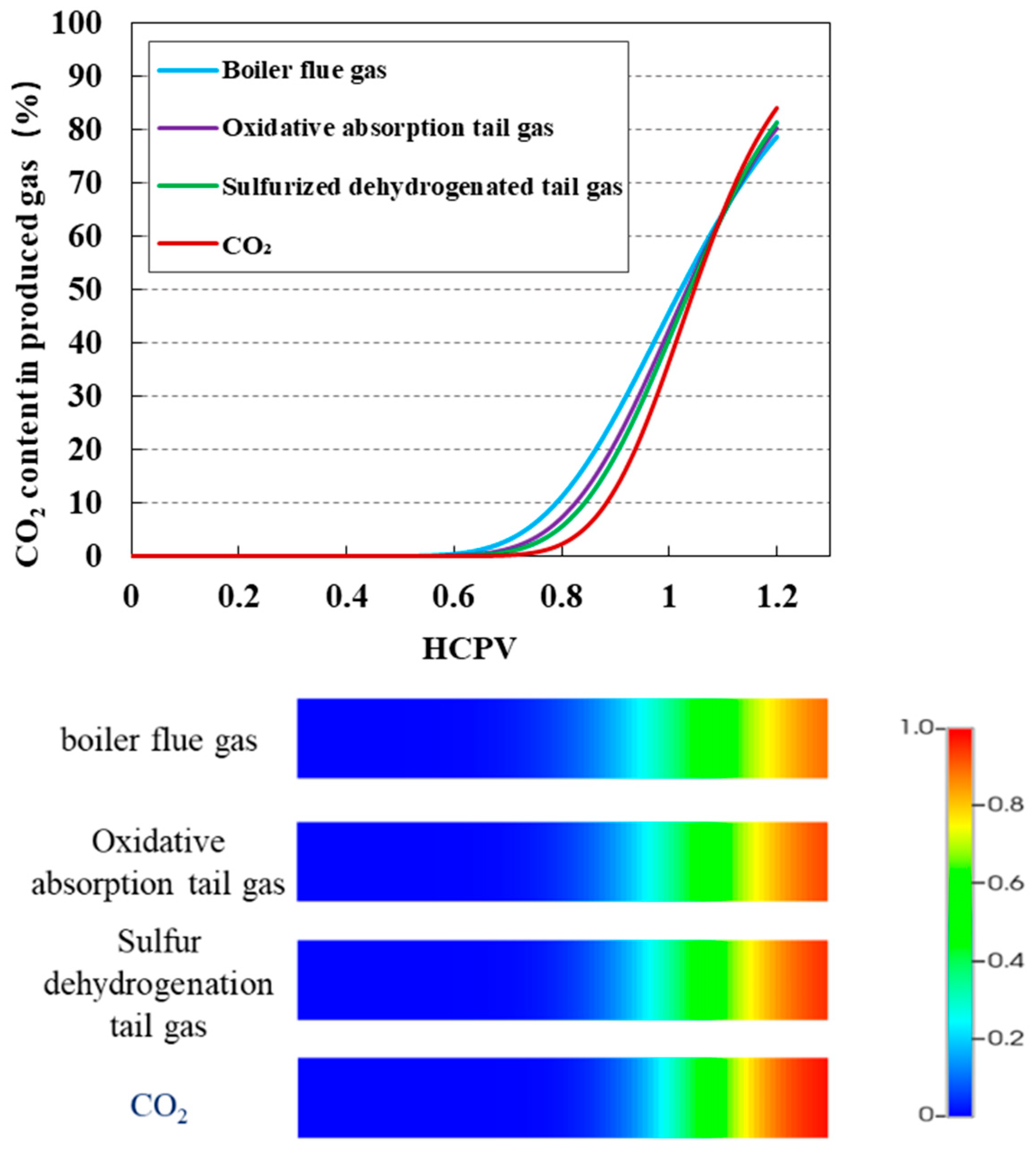
2.2.3. CO2 Injection for Gas Displacement—Gas Components
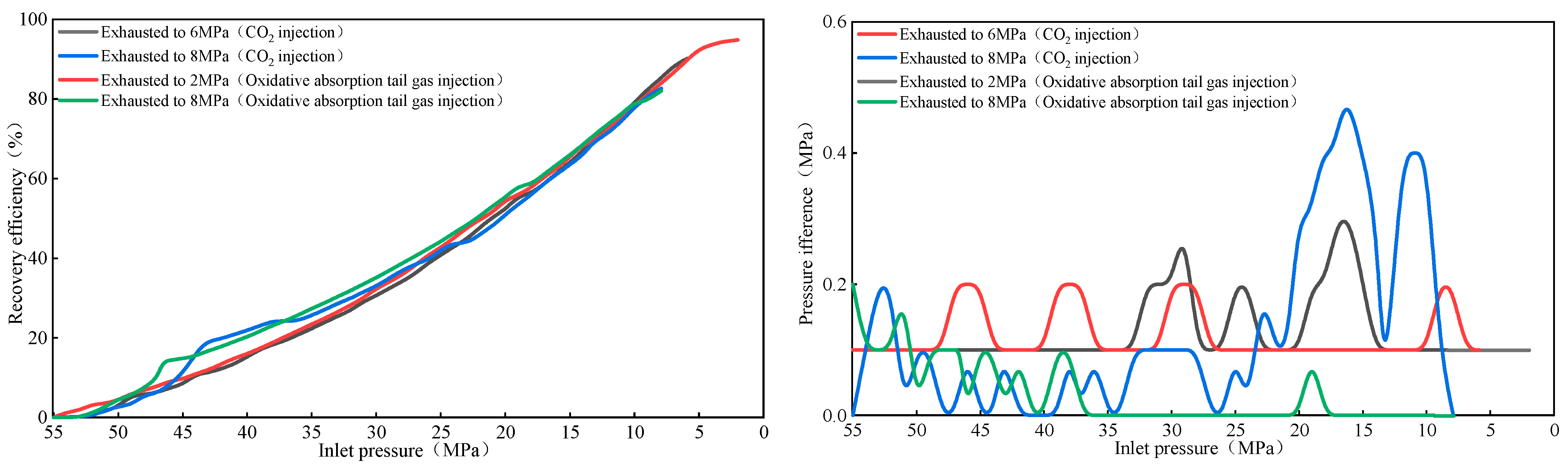
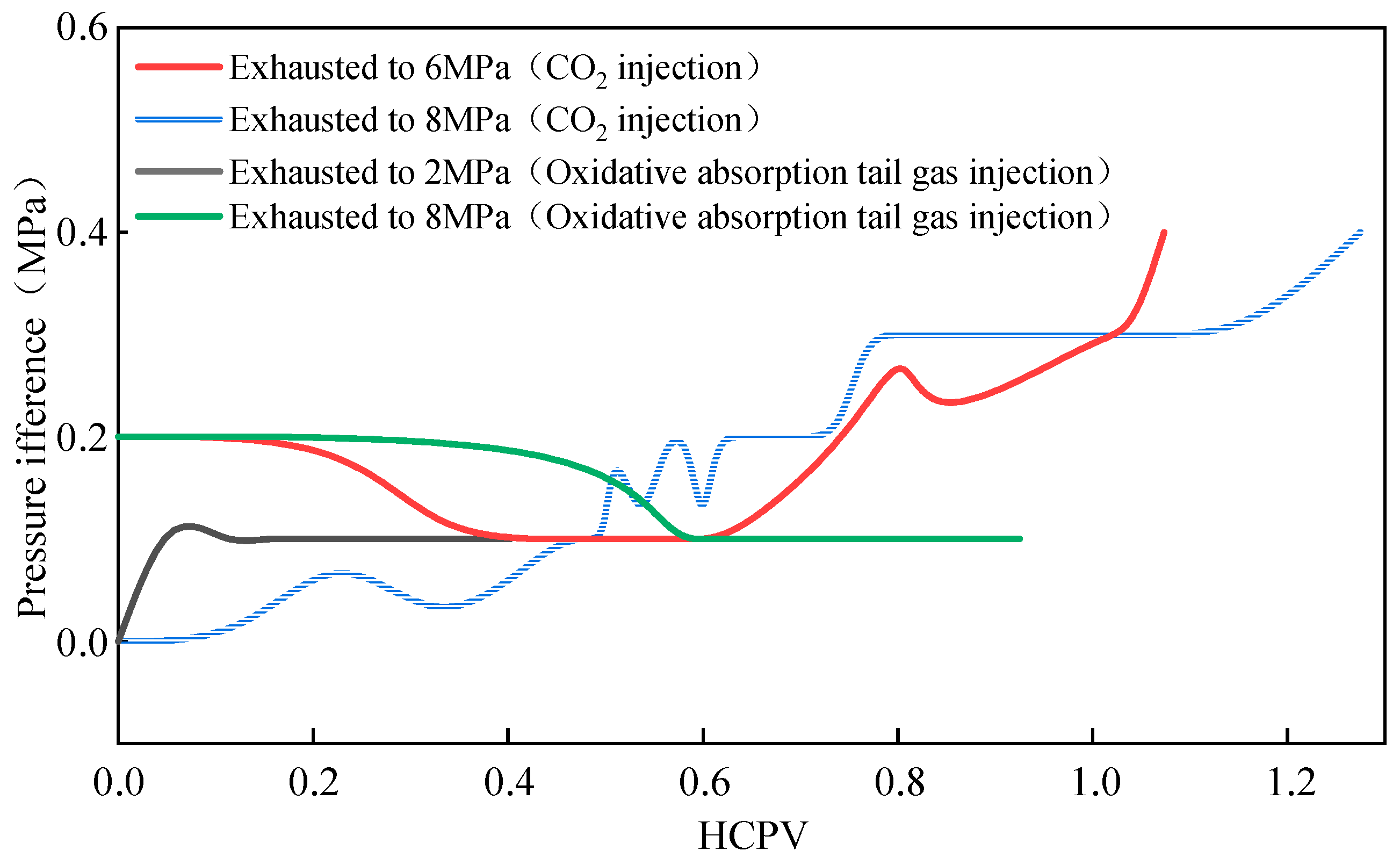
2.2.4. CO2 Injection for Gas Displacement—Pressure Variations
3. CO2 Injection in Long-Core Models for Enhanced Gas Recovery and Sequestration Simulation
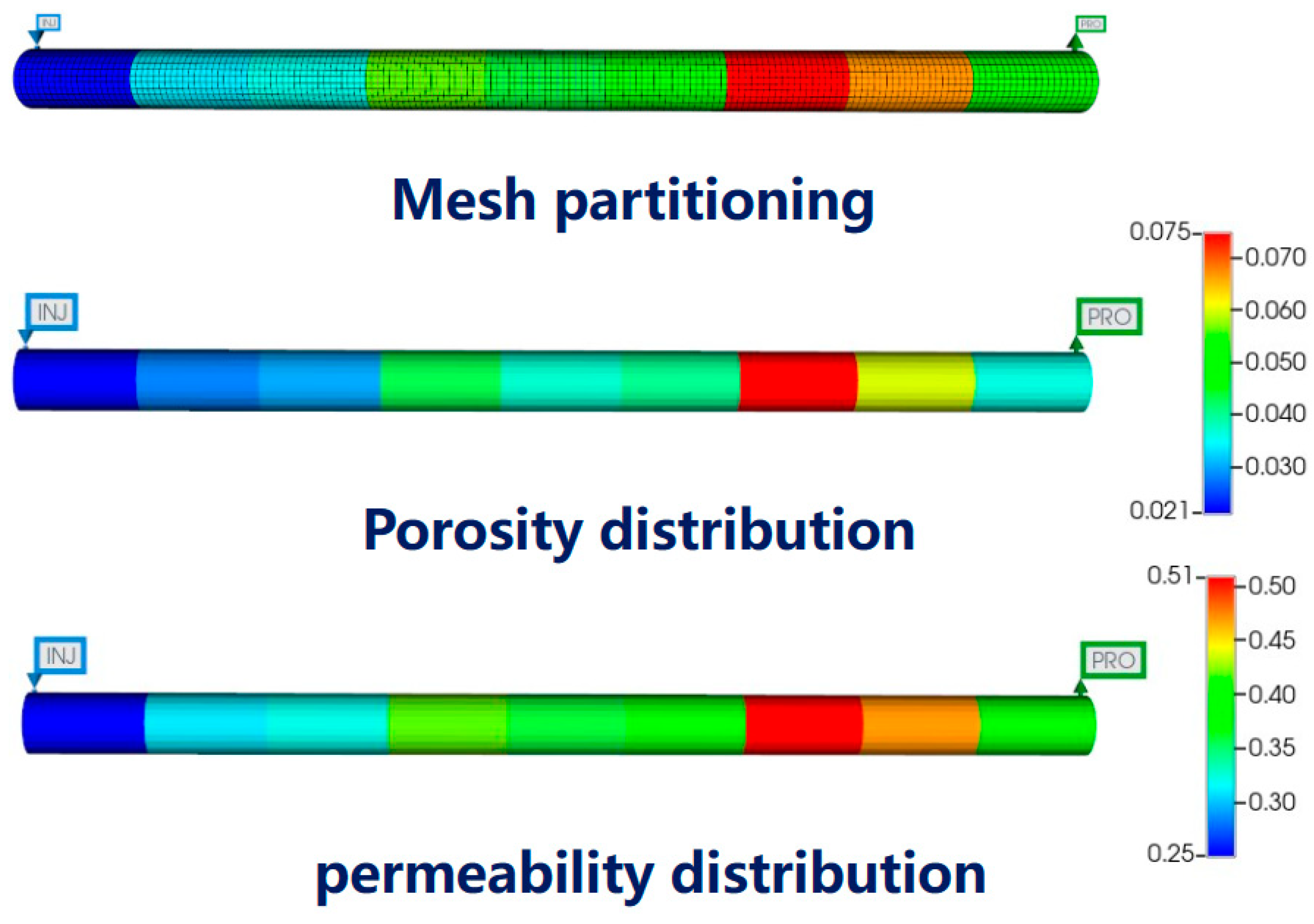
3.1. Core Model Parameters
3.2. Mass-Conservation Equation
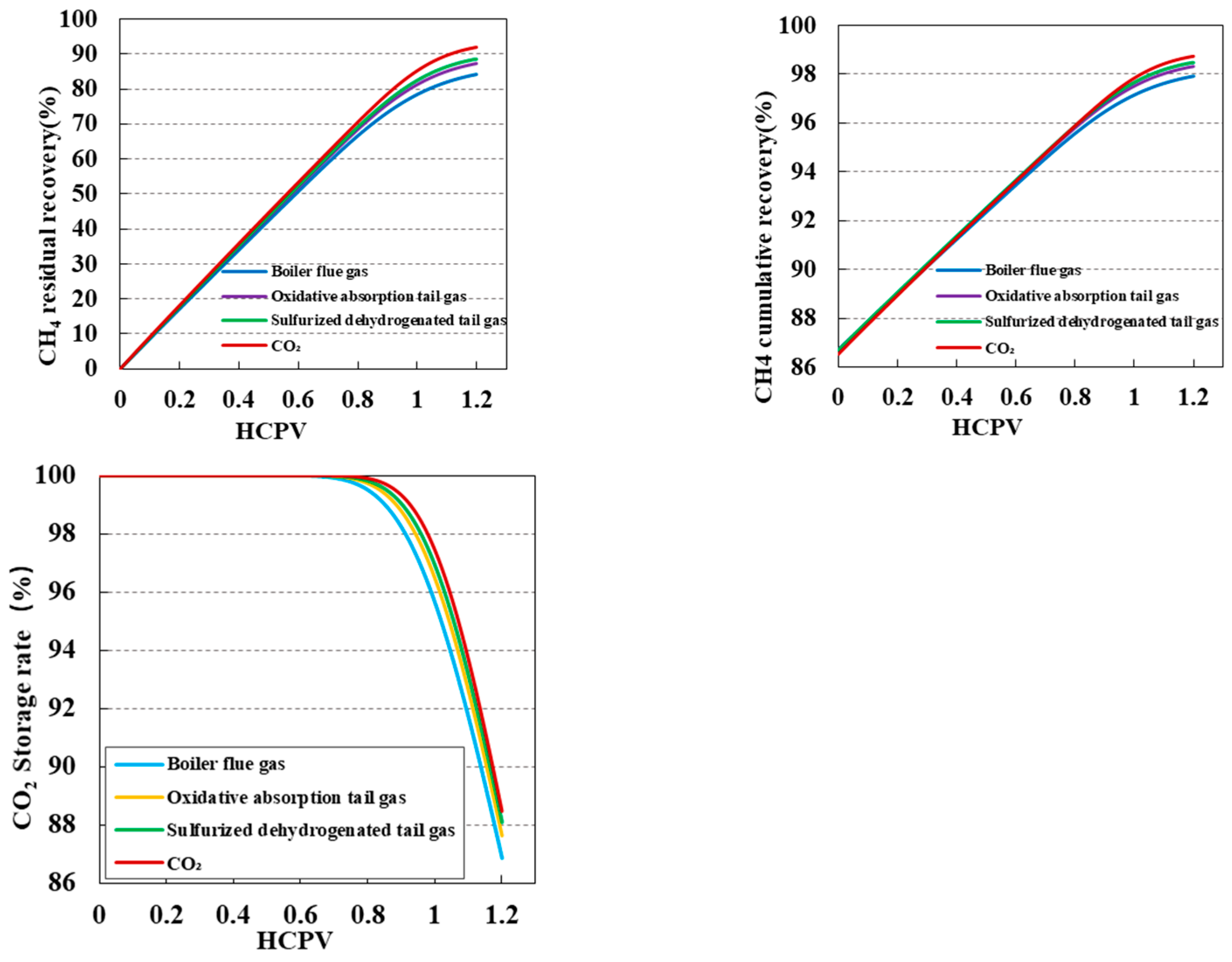
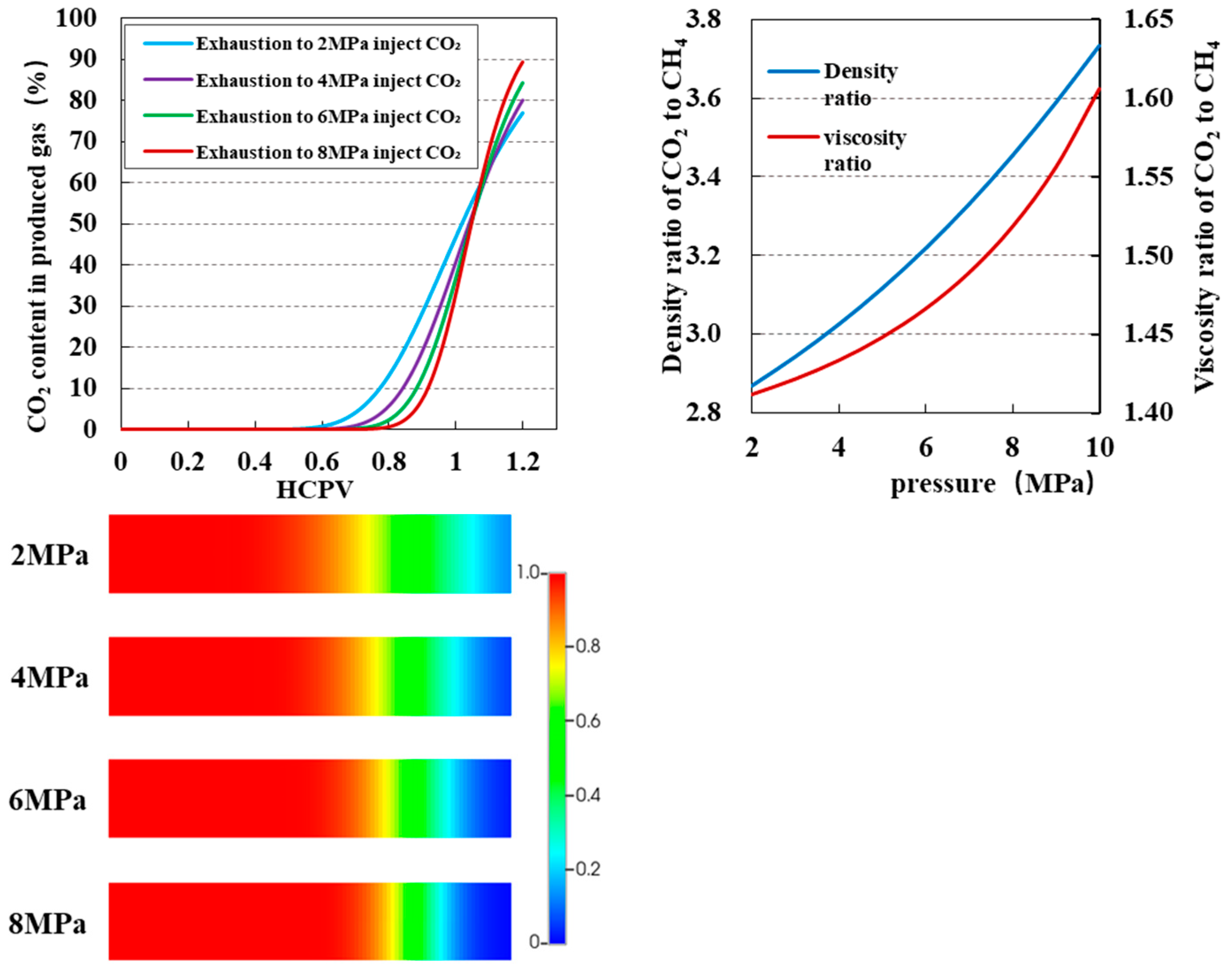
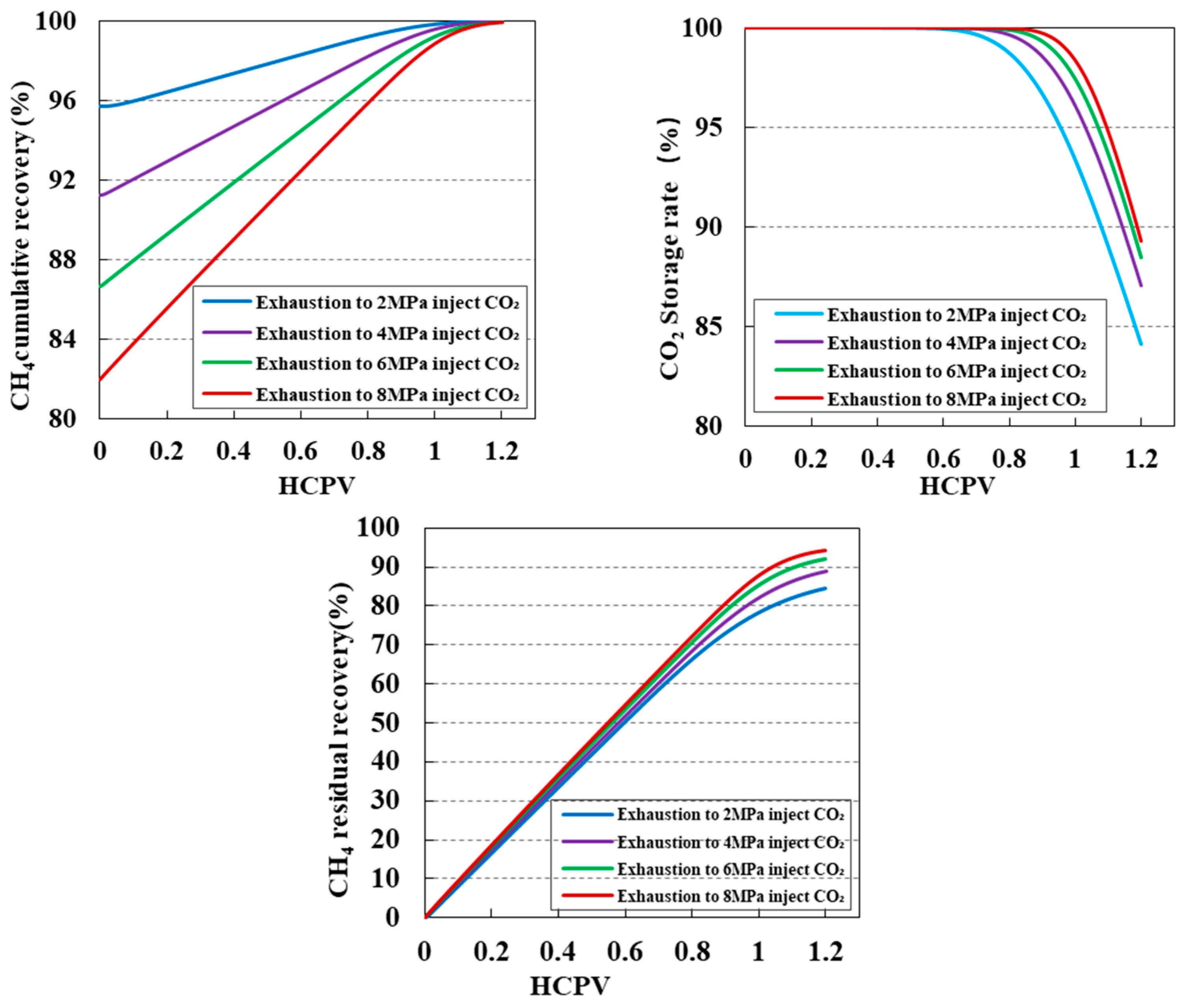
3.3. Impact of Injection Purity on CO2 Displacement Efficiency and Sequestration
3.4. Impact of Injection Timing on CO2 Displacement Efficiency and Sequestration
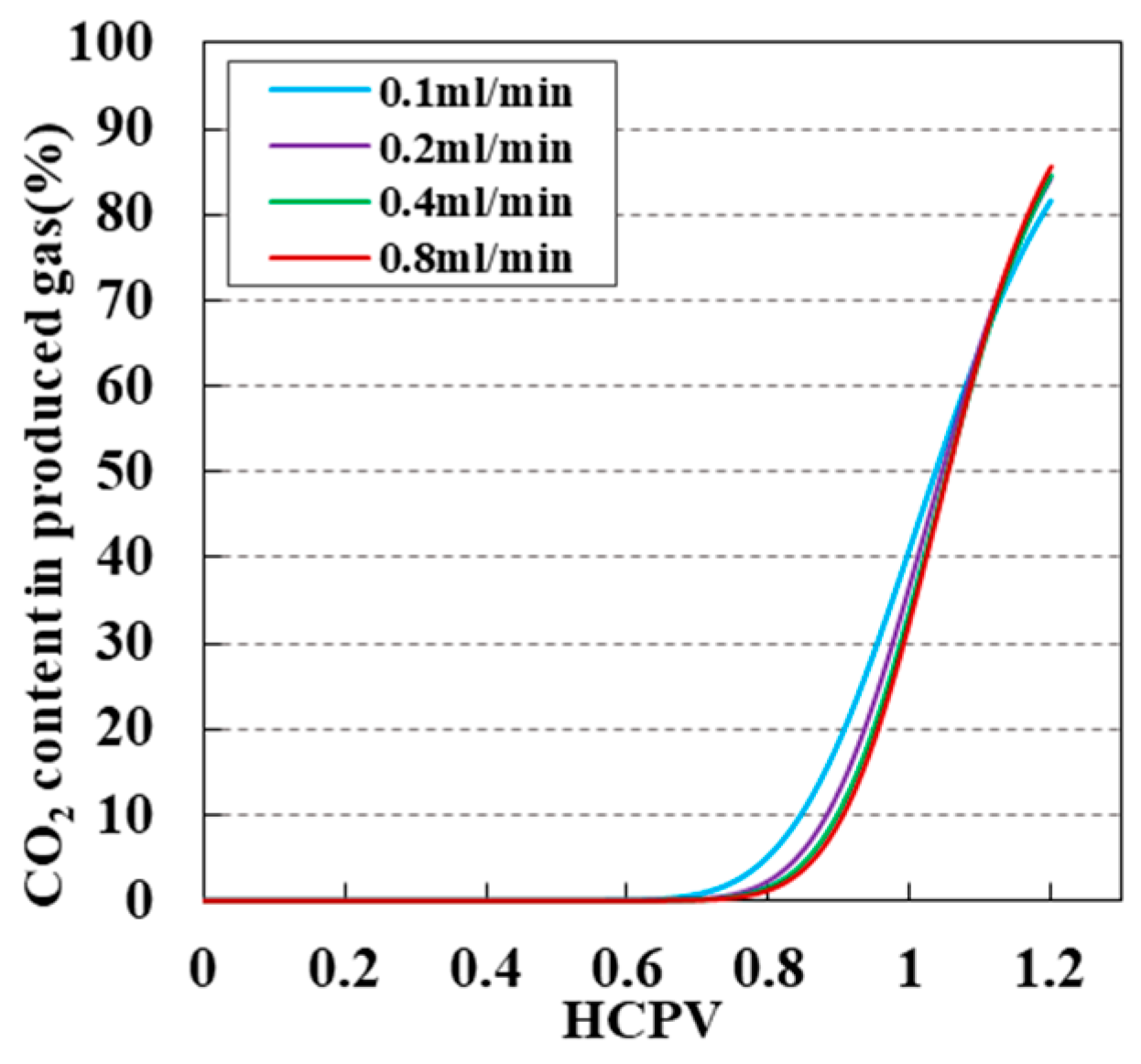
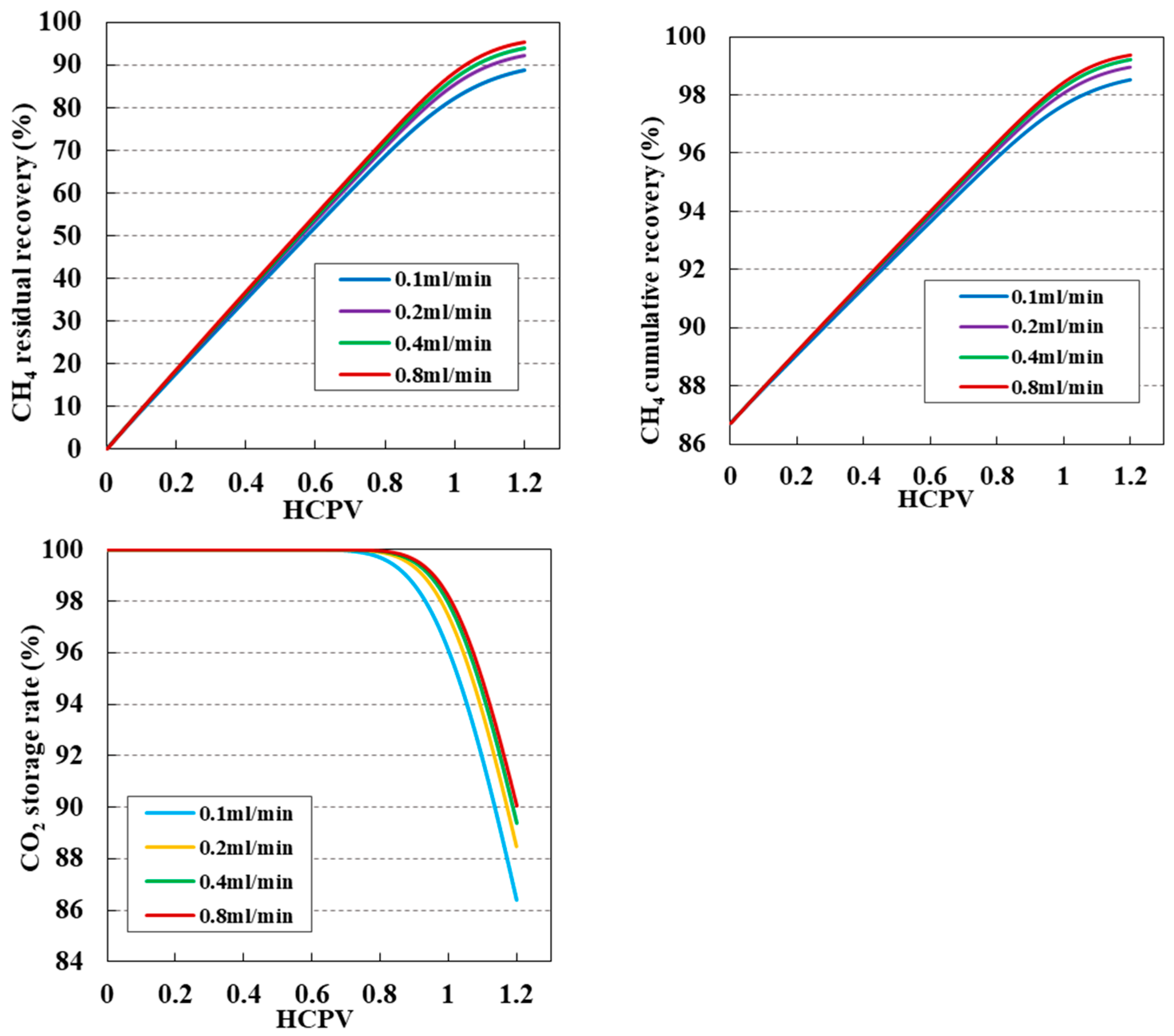
3.5. Impact of Injection Speed on CO2 Displacement Efficiency and Sequestration
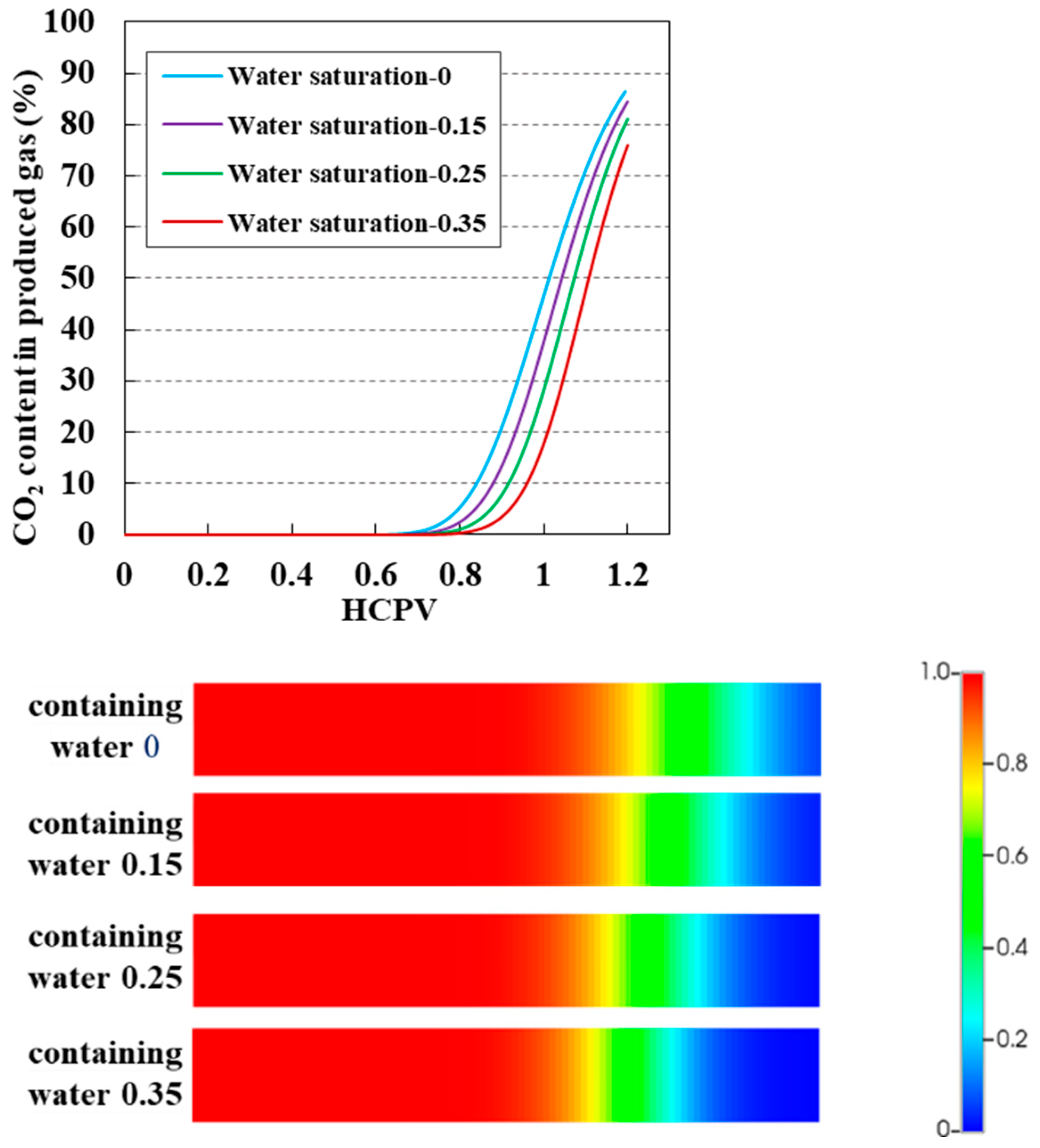
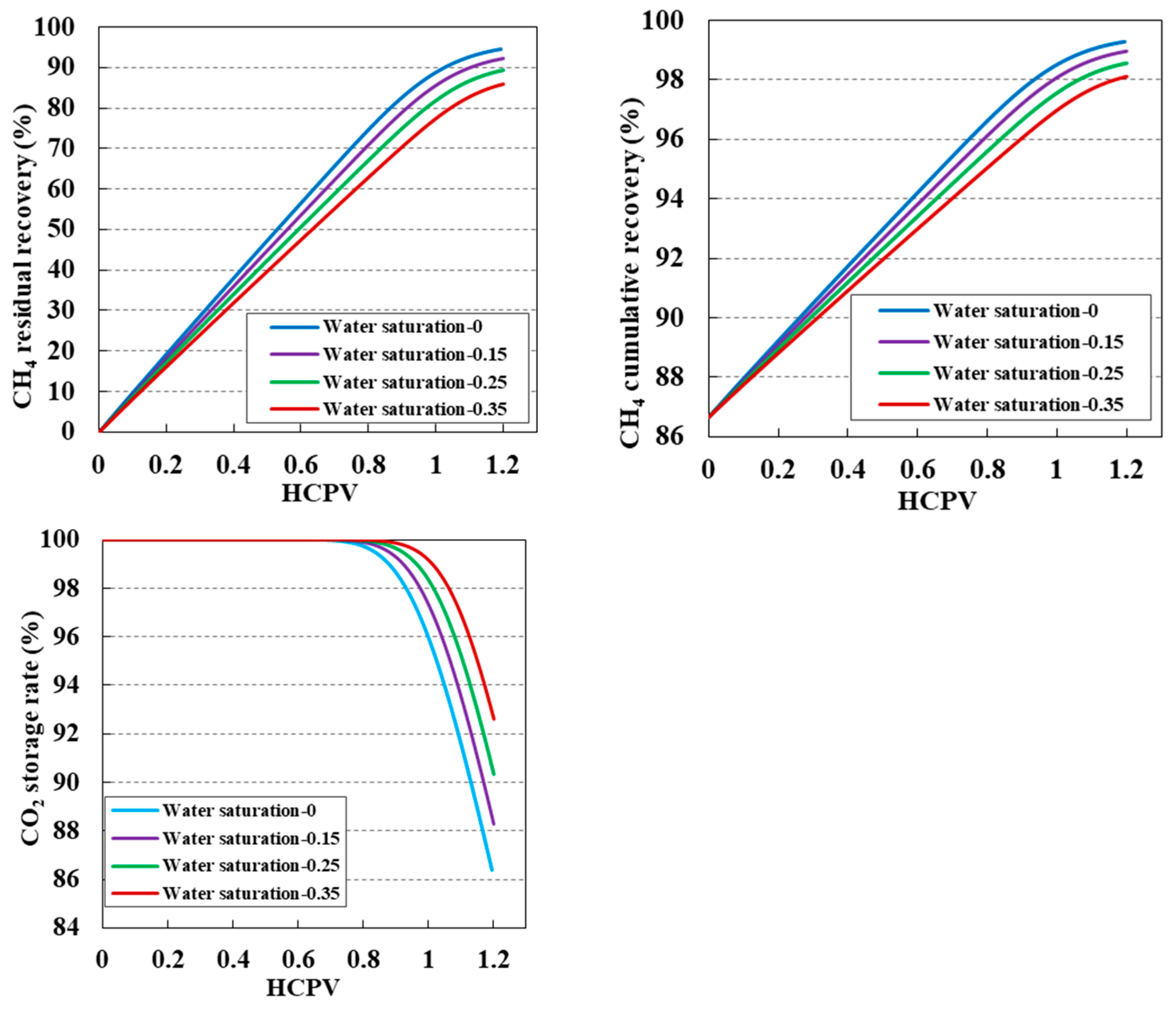
3.6. Impact of Reservoir Bound Water Saturation on CO2 Displacement Efficiency and Sequestration
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- EI. Statistical Review of World Energy. 2023. Available online: https://www.energyinst.org/__data/assets/pdf_file/0004/1055542/EI_Stat_Review_PDF_single_3.pdf (accessed on 26 June 2023).
- Wang, Z.; Kong, Y.; Li, W. Review on the development of China’s natural gas industry in the background of “carbon neutrality”. Nat. Gas Ind. 2021, 41, 194–202. [Google Scholar] [CrossRef]
- Guo, P.; Jing, S.; Peng, C. Technology and countermeasures for gas recovery enhancement. Nat. Gas Ind. 2014, 34, 48–55. [Google Scholar]
- Van der Burgt, M.; Cantle, J.; Boutkan, V.J.E.C. Carbon dioxide disposal from coal-based IGCC’s in depleted gas fields. Energy Convers. Manag. 1992, 33, 603–610. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, C.; Wen, S.; Zhao, Y.; Peng, X.; Wu, J. Thoughts on the developmengt of CO2-EGR under the background of carbon peak and carbon neutrality. Nat. Gas Ind. 2023, 43, 13–22. [Google Scholar]
- Kubus, P. CCS and CO2-storage possibilities in Hungary. In Proceedings of the SPE International Conference on CO2 Capture, Storage, and Utilization, New Orleans, Louisiana, USA, 10 November 2010; OnePetro: Richardson, TX, USA, 2010. [Google Scholar]
- Pooladi-Darvish, M.; Hong, H.; Theys, S.S.R.; Bachu, S.; Dashtgard, S. CO2 injection for enhanced gas recovery and geological storage of CO2 in the Long Coulee Glauconite F Pool, Alberta. In Proceedings of the SPE Annual Technical Conference and Exhibition? Denver, CO, USA, 21 September 2008; SPE: Lumpur, Malaysia, 2008. [Google Scholar]
- Jenkins, C.R.; Cook, P.J.; Ennis-King, J.; Urosevic, M. Safe storage and effective monitoring of CO2 in depleted gas fields. Proc. Natl. Acad. Sci. USA 2012, 109, E35–E41. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, L.; Kreft, E.; Geel, C.; Hartman, J. K12-B a test site for CO2 storage and enhanced gas recovery. In Proceedings of the SPE Europec Featured at EAGE Conference and Exhibition? Madrid, Spain, 13–16 June 2005; SPE: Lumpur, Malaysia, 2005. [Google Scholar]
- Ye, J.; Feng, S.; Fan, Z.; William, D.G.; Wang, S.; Robinson, J.R. Micro-pilot test for enhanced coalbed methane recovery by injecting carbon dioxide in south part of Qinshui Basin. Acta Pet. Sin. 2007, 28, 77–80. [Google Scholar]
- Shi, Y.; Jia, Y.; Pan, W.; Yan, J.; Huang, L. Mechanism of supercritical CO2 flooding in low-permeability tight gas reservoirs. Oil Gas Geol. 2017, 38, 610–616. [Google Scholar]
- Oldenburg, C.; Pruess, K.; Benson, S.M. Process modeling of CO2 injection into natural gas reservoirs for carbon sequestration and enhanced gas recovery. Energy Fuels 2001, 15, 293–298. [Google Scholar] [CrossRef]
- Gao, Y. Evaluation of the Effect of Replacing Shale Gas with Supercritical CO2 in Shale. Master’s Thesis, Southwest Petroleum University, Chengdu, China, 2016. [Google Scholar]
- Ding, J.; Yan, C.; Wang, G.; He, Y.; Zhao, R. Competitive adsorption between CO2 and CH4 in tight sandstone and its influence on CO2-injection enhanced gas recovery (EGR). Int. J. Greenh. Gas Control 2022, 113, 103530. [Google Scholar] [CrossRef]
- Al-Hashami, A.; Ren, S.; Tohidi, B. CO2 injection for enhanced gas recovery and geo-storage: Reservoir simulation and economics. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid Spain, 13–16 June 2005; OnePetro: Richardson, TX, USA, 2005. [Google Scholar]
- Nogueira de Mago, M.C. Effect of Flue Gas Impurities on the Process of Injection and Storage of Carbon Dioxide in Depleted Gas Reservoirs. Master’s Thesis, Texas A&M University, College Station, TX, USA, 2005. [Google Scholar]
- Cao, C.; Hou, Z.; Li, Z.; Pu, X.; Liao, J.; Wang, G. Numerical modeling for CO2 storage with impurities associated with enhanced gas recovery in depleted gas reservoirs. J. Nat. Gas Sci. Eng. 2022, 102, 104554. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, R.; Liu, X.; Li, S. Mechanism of enhanced gas recovery in shale gas reservoirs based on competitive adsorption. Xinjiang Pet. Geol. 2021, 42, 224–231. [Google Scholar]
- Du, J.; Zhao, Y.; Guo, P.; Zhao, Q. Experimental research on recovery rfficiency improvement by CO2 injection in tight gas reservoirs in northern hubei. Sci. Technol. Eng. 2020, 20, 6459–6465. [Google Scholar]
- Turta, A.T.; Sim, S.S.; Singhal, A.K.; Hawkins, B.F. Basic investigations on enhanced gas recovery by gas-gas displacement. J. Can. Pet. Technol. 2008, 47, PETSOC-08-10-39. [Google Scholar] [CrossRef]


| SN | Core Number | Length (cm) | Permeability (mD) | Porosity (%) |
|---|---|---|---|---|
| 1 | 3-22-42 | 4.374 | 2.79 | 2.18 |
| 2 | 3-19-42 | 4.734 | 1.01 | 1.67 |
| 3 | 3-19-42-b | 4.504 | 1.16 | 2.24 |
| 4 | 3-17-42 | 4.342 | 0.981 | 1.85 |
| 5 | 4-5-127-a | 3.99 | 0.683 | 2.36 |
| 6 | 3-19-42-a | 4.852 | 2.76 | 3.02 |
| Experimental Sequence | Influencing Factors | Experimental Content |
|---|---|---|
| 1 | Injection timing | Depletion from 55 MPa to 6 MPa, CO2 injection at 0.05 mL/min |
| 2 | Injection timing and injection components | Depletion from 55 MPa to 8 MPa, CO2 injection at 0.05 mL/min |
| 3 | Injection timing | Depletion from 55 MPa to 2 MPa, oxidation-absorption tail gas injection at 0.05 mL/min |
| 4 | Injection timing and injection components | Depletion from 55 MPa to 8 MPa, oxidation-absorption tail gas injection at 0.05 mL/min |
| Experiment Name | Depletion Recovery | Ultimate Recovery | Increment |
|---|---|---|---|
| Depletion from 55 MPa to 6 MPa, CO2 injection at 0.05 mL/min | 91.11% | 96.07% | 4.96% |
| Depletion from 55 MPa to 8 MPa, CO2 injection at 0.05 mL/min | 82.66% | 91.07% | 8.41% |
| Depletion from 55 MPa to 2 MPa, oxidation-absorption tail gas injection at 0.05 mL/min | 94.91% | 96.27% | 1.36% |
| Depletion from 55 MPa to 8 MPa, oxidation-absorption tail gas injection at 0.05 mL/min | 82.10% | 86.69% | 4.59% |
| Core Number | Porosity | Permeability |
|---|---|---|
| 1 | 2.1 | 0.25 |
| 2 | 2.8 | 0.31 |
| 3 | 3.2 | 0.32 |
| 4 | 4.3 | 0.42 |
| 5 | 3.7 | 0.36 |
| 6 | 4.0 | 0.39 |
| 7 | 7.5 | 0.51 |
| 8 | 6.0 | 0.47 |
| 9 | 3.6 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Wen, S.; Wang, M.; Zhang, L.; Cao, C.; Yang, C.; Li, L. Experimental and Numerical Simulation Study on Enhancing Gas Recovery with Impure CO2 in Gas Reservoirs. Processes 2024, 12, 1663. https://doi.org/10.3390/pr12081663
Zhao Z, Wen S, Wang M, Zhang L, Cao C, Yang C, Li L. Experimental and Numerical Simulation Study on Enhancing Gas Recovery with Impure CO2 in Gas Reservoirs. Processes. 2024; 12(8):1663. https://doi.org/10.3390/pr12081663
Chicago/Turabian StyleZhao, Zihan, Shaomu Wen, Mengyu Wang, Lianjin Zhang, Cheng Cao, Changcheng Yang, and Longxin Li. 2024. "Experimental and Numerical Simulation Study on Enhancing Gas Recovery with Impure CO2 in Gas Reservoirs" Processes 12, no. 8: 1663. https://doi.org/10.3390/pr12081663





