Effects of Biochar-Amended Composts on Selected Enzyme Activities in Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Sample Collection and Soil Enzyme Assays
2.3. Statistical Analysis
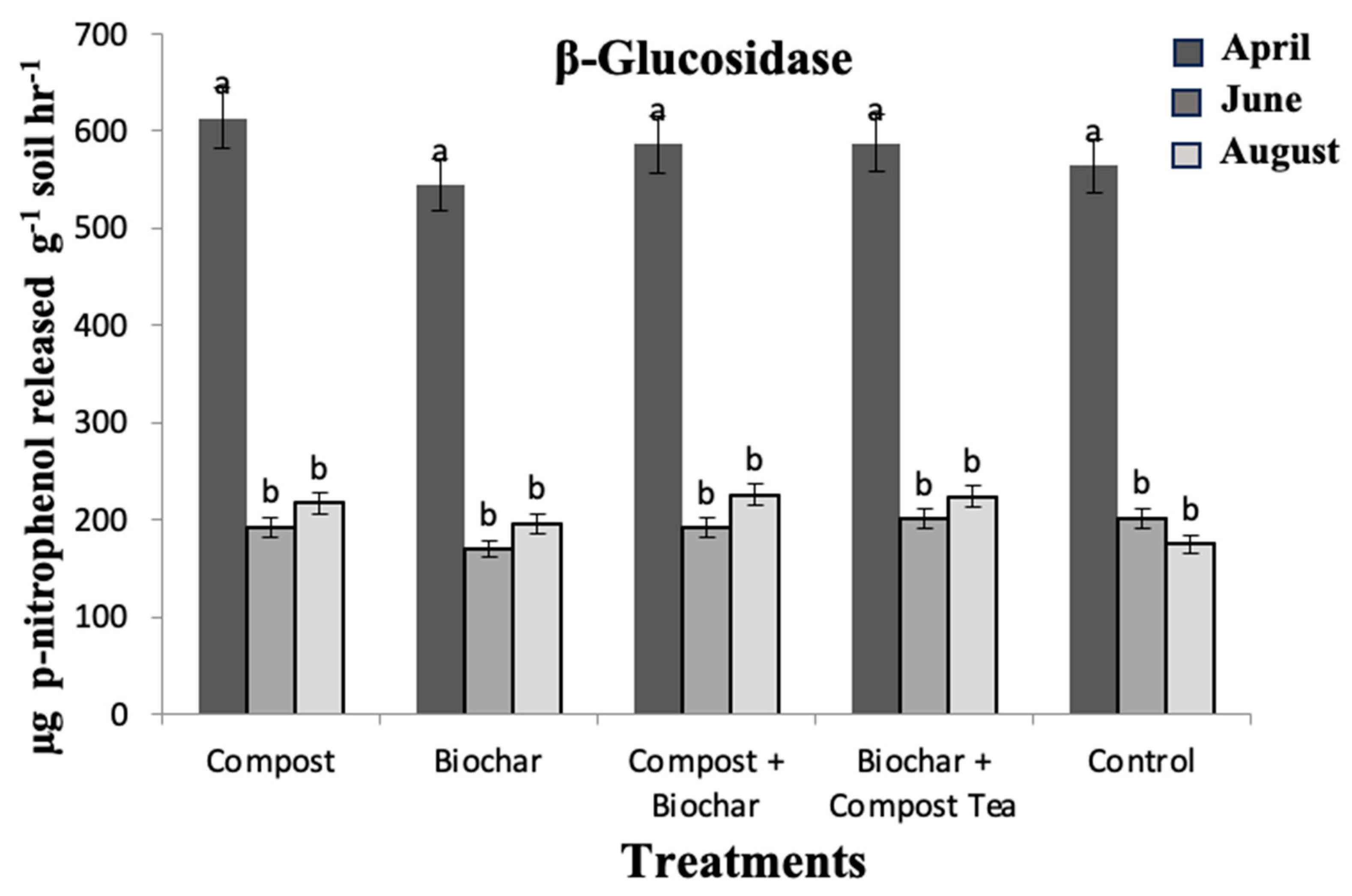
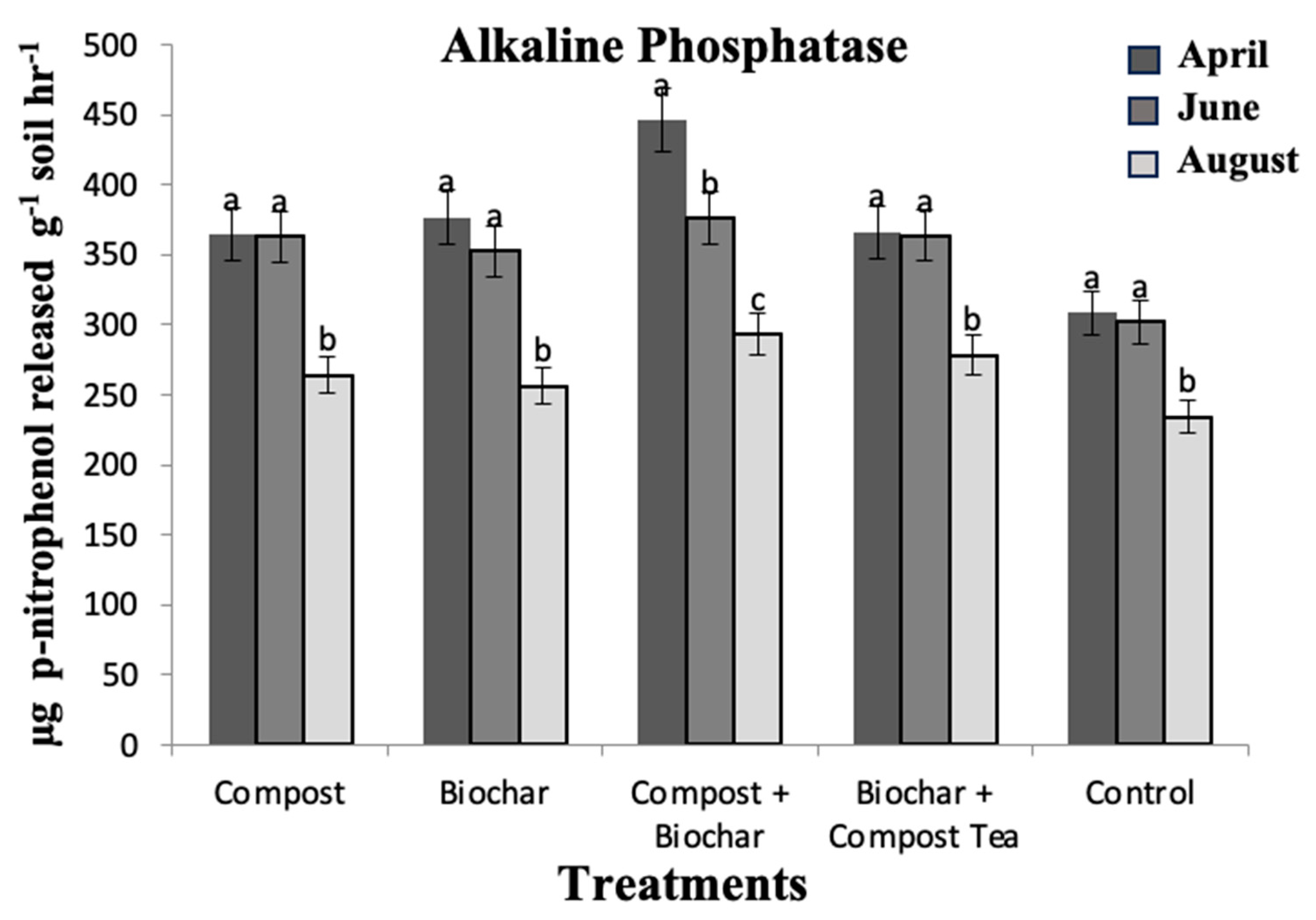
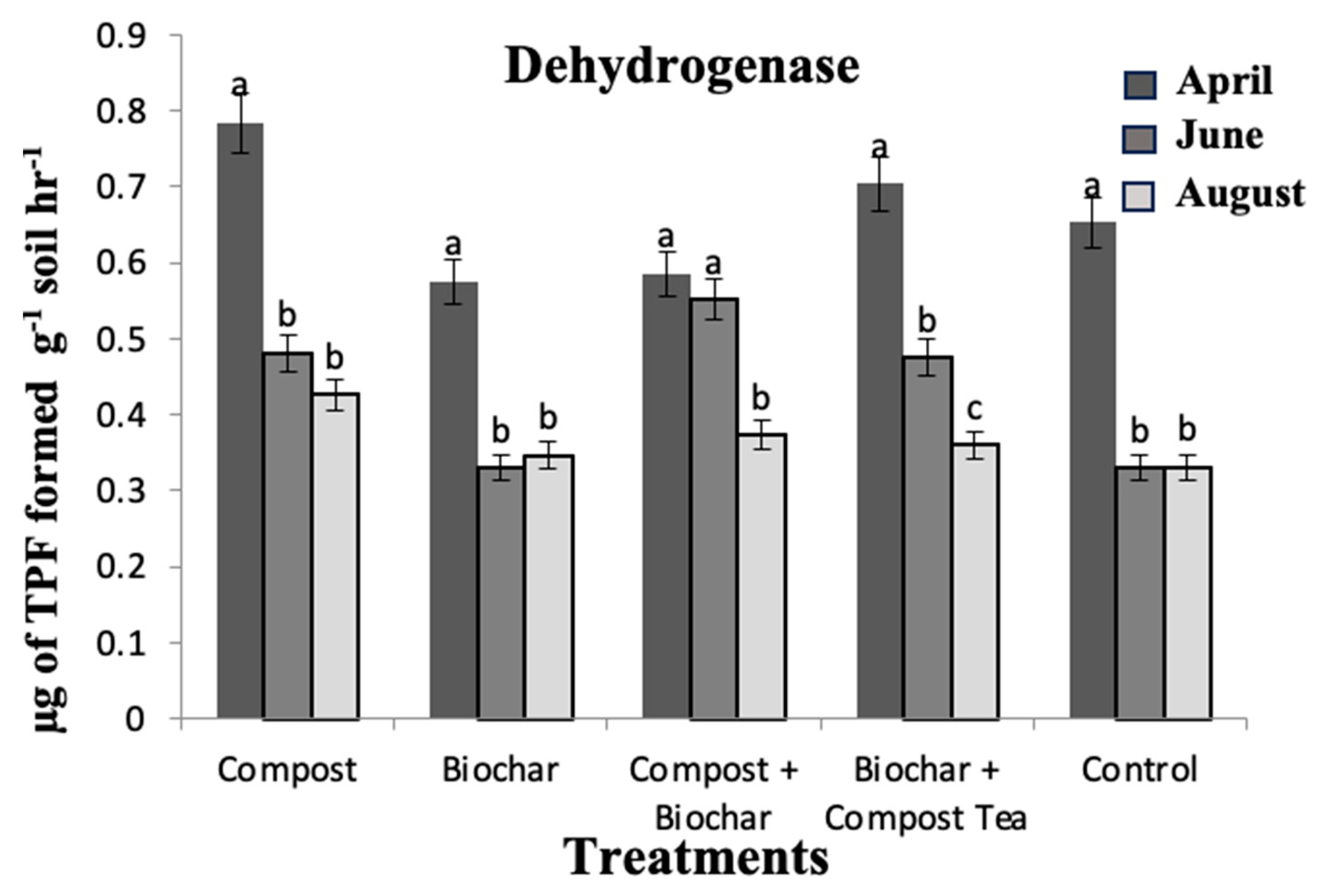
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thapa, R.B.; Coupal, R.H.; Dangi, M.B.; Stahl, P.D. An Assessment of Plant Growth and Soil Properties Using Coal Char and Biochar as a Soil Amendment. Agronomy 2024, 14, 320. [Google Scholar] [CrossRef]
- Thapa, R.B.; Budhathoki, S.; Shilpakar, C.; Panday, D.; Alsunuse, B.T.B.; Tang, S.; Stahl, P.D. Enhancing Corn Yield and Soil Quality in Irrigated Semiarid Region with Coal Char and Biochar Amendments. Soil Syst. 2024, 8, 82. [Google Scholar] [CrossRef]
- Kapoor, A.; Sharma, R.; Kumar, A.; Sepehya, S. Biochar as a means to improve soil fertility and crop productivity: A review. J. Plant Nutr. 2022, 45, 2380–2388. [Google Scholar] [CrossRef]
- Fischer, D.; Glaser, B. Synergisms between compost and biochar for sustainable soil amelioration. Manag. Org. Waste 2012, 1, 167–198. [Google Scholar]
- Blanco-Canqui, H. Biochar and soil physical properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Ghorbani, M.; Amirahmadi, E.; Bernas, J.; Konvalina, P. Testing biochar’s ability to moderate extremely acidic soils in tea-growing areas. Agronomy 2024, 14, 533. [Google Scholar] [CrossRef]
- Domingues, R.R.; Sánchez-Monedero, M.A.; Spokas, K.A.; Melo, L.C.; Trugilho, P.F.; Valenciano, M.N.; Silva, C.A. Enhancing cation exchange capacity of weathered soils using biochar: Feedstock, pyrolysis conditions and addition rate. Agronomy 2020, 10, 824. [Google Scholar] [CrossRef]
- Zheng, H.; Wang, Z.; Deng, X.; Herbert, S.; Xing, B. Impacts of adding biochar on nitrogen retention and bioavailability in agricultural soil. Geoderma 2013, 206, 32–39. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of pesticides on microorganisms, enzymatic activity and plant in biochar-amended soil. Geoderma 2014, 214, 10–18. [Google Scholar] [CrossRef]
- Bailey, V.L.; Fansler, S.J.; Smith, J.L.; Bolton, H., Jr. Reconciling apparent variability in effects of biochar amendment on soil enzyme activities by assay optimization. Soil Biol. Biochem. 2011, 43, 296–301. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Charcoal effects on soil solution chemistry and growth of Koeleria macrantha in the ponderosa pine/Douglas-fir ecosystem. Biol. Fertil. Soils 2007, 43, 303–311. [Google Scholar] [CrossRef]
- Lima, I.M.; Marshall, W.E. Adsorption of selected environmentally important metals by poultry manure-based granular activated carbons. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2005, 80, 1054–1061. [Google Scholar] [CrossRef]
- Singh, B.; Cowie, A. A novel approach, using 13C natural abundance, for measuring decomposition of biochars in soil. In Carbon and Nutrient Management in Agriculture; Massey University, Fertilizer and Lime Research Centre: Palmerston North, New Zealand, 2008. [Google Scholar]
- Sun, Z.; Bruun, E.W.; Arthur, E.; de Jonge, L.W.; Moldrup, P.; Hauggaard-Nielsen, H.; Elsgaard, L. Effect of biochar on aerobic processes, enzyme activity, and crop yields in two sandy loam soils. Biol. Fertil. Soils 2014, 50, 1087–1097. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef]
- Demisie, W.; Liu, Z.; Zhang, M. Effect of biochar on carbon fractions and enzyme activity of red soil. Catena 2014, 121, 214–221. [Google Scholar] [CrossRef]
- Steinbeiss, S.; Gleixner, G.; Antonietti, M. Effect of biochar amendment on soil carbon balance and soil microbial activity. Soil Biol. Biochem. 2009, 41, 1301–1310. [Google Scholar] [CrossRef]
- Keech, O.; Dizengremel, P.; Gardeström, P. Preparation of leaf mitochondria from Arabidopsis thaliana. Physiol. Plant. 2005, 124, 403–409. [Google Scholar] [CrossRef]
- Baldock, J.A.; Smernik, R.J. Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org. Geochem. 2002, 33, 1093–1109. [Google Scholar] [CrossRef]
- Krull, E.S.; Baldock, J.A.; Skjemstad, J.O. Importance of mechanisms and processes of the stabilisation of soil organic matter for modelling carbon turnover. Funct. Plant Biol. 2003, 30, 207–222. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Kimber, S.; Morris, S.; Chan, K.Y.; Downie, A.; Rust, J.; Joseph, S.; Cowie, A. Effects of biochar from slow pyrolysis of papermill waste on agronomic performance and soil fertility. Plant Soil 2010, 327, 235–246. [Google Scholar] [CrossRef]
- Rondon, M.A.; Lehmann, J.; Ramírez, J.; Hurtado, M. Biological nitrogen fixation by common beans (Phaseolus vulgaris L.) increases with bio-char additions. Biol. Fertil. Soils 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Joseph, S.; Peacocke, C.; Lehmann, J.; Munroe, P. Developing a biochar classification and test methods. In Biochar for Environmental Management; Routledge: London, UK, 2012; pp. 139–158. [Google Scholar]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Liang, Y.S.; Choi, Y.H.; Kim, H.K.; Linthorst, H.J.; Verpoorte, R. Metabolomic analysis of methyl jasmonate treated Brassica rapa leaves by 2-dimensional NMR spectroscopy. Phytochemistry 2006, 672, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Palansooriya, K.N.; Wong, J.T.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; ACSESS: Sydney, Australia, 1994; Volume 5, pp. 775–833. [Google Scholar]
- Alsunuse, B.T.B. Effects of Cover Crops and Crop Rotation on Soil Health in Northwestern Wyoming. Ph.D. Thesis, University of Wyoming, Laramie, WY, USA, December 2023. [Google Scholar]
- Longo, M.A.; Combes, D. Analysis of the thermal deactivation kinetics of α-chymotrypsin modified by chemoenzymatic glycosylation. In Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 15, pp. 135–140. [Google Scholar]
- Sohi, S.; Lopez-Capel, E.; Krull, E.; Bol, R. Biochar, climate change and soil: A review to guide future research. CSIRO Land Water Sci. Rep. 2009, 5, 17–31. [Google Scholar]
- Rahman, G.M.; Rahman, M.M.; Alam, M.S.; Kamal, M.Z.; Mashuk, H.A.; Datta, R.; Meena, R.S. Biochar and organic amendments for sustainable soil carbon and soil health. In Carbon and Nitrogen Cycling in Soil; Springer: Singapore, 2020; pp. 45–85. [Google Scholar]
- Available online: https://casoilresource.lawr.ucdavis.edu/gmap/ (accessed on 12 January 2023).
- Ansari, J.; Eivazi, F.; Anderson, S.H.; Bardhan, S. Selected Enzyme Activities Under Different Land Use Management in Lower Missouri River Floodplain Soils. Commun. Soil Sci. Plant Anal. 2024, 55, 27–39. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Acosta-Martinez, V.; Tabatabai, M.A. Enzyme activities in a limed agricultural soil. Biol. Fertil. Soils 2000, 31, 85–91. [Google Scholar] [CrossRef]
- Deng, S.P.; Tabatabai, M.A. Cellulase activity of soils. Soil Biol. Biochem. 1994, 26, 1347–1354. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Part 3 Chemical Methods; John Wiley & Sons: Hoboken, NJ, USA, 1996; Volume 5, pp. 961–1010. [Google Scholar]
- Ejaz, U.; Sohail, M.; Ghanemi, A. Cellulases: From bioactivity to a variety of industrial applications. Biomimetics 2021, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Sinsabaugh, R.L.; Gallo, M.E.; Lauber, C.; Waldrop, M.P.; Zak, D.R. Extracellular enzyme activities and soil organic matter dynamics for northern hardwood forests receiving simulated nitrogen deposition. Biogeochemistry 2005, 75, 201–215. [Google Scholar] [CrossRef]
- Garcia, C.; Hernandez, T.; Barahona, A.; Costa, F. Organic matter characteristics and nutrient content in eroded soils. Environ. Manag. 1996, 20, 133–141. [Google Scholar] [CrossRef]
- de Mora, A.P.; Ortega-Calvo, J.J.; Cabrera, F.; Madejón, E. Changes in enzyme activities and microbial biomass after “in situ” remediation of a heavy metal-contaminated soil. Appl. Soil Ecol. 2005, 28, 125–137. [Google Scholar] [CrossRef]
- Bandick, A.K.; Dick, R.P. Field management effects on soil enzyme activities. Soil Biol. Biochem. 1999, 31, 1471–1479. [Google Scholar] [CrossRef]
- Acosta-Martínez, V.; Tabatabai, M.A. Arylamidase activity in soils: Effect of trace elements and relationships to soil properties and activities of amidohydrolases. Soil Biol. Biochem. 2001, 33, 17–23. [Google Scholar] [CrossRef]
- Zang, X.; Liu, M.; Fan, Y.; Xu, J.; Xu, X.; Li, H. The structural and functional contributions of β-glucosidase-producing microbial communities to cellulose degradation in composting. Biotechnol. Biofuels 2018, 11, 51. [Google Scholar] [CrossRef]
- Rachwał, K.; Gustaw, K.; Kazimierczak, W.; Waśko, A. Is soil management system really important? comparison of microbial community diversity and structure in soils managed under organic and conventional regimes with some view on soil properties. PLoS ONE 2021, 16, e0256969. [Google Scholar] [CrossRef]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Goll, D.; Richter, A.; Obersteiner, M.; Asensio, D.; et al. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Solhtalab, M.; Thongsomboon, W.; Aristilde, L. Strategies of organic phosphorus recycling by soil bacteria: Acquisition, metabolism, and regulation. Environ. Microbiol. Rep. 2022, 14, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for using phosphate-solubilizing microorganisms as natural fertilizers in agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Basta, N.T.; Busalacchi, D.M.; Hundal, L.S.; Kumar, K.; Dick, R.P.; Lanno, R.P.; Carlson, J.; Cox, A.E.; Granato, T.C. Restoring ecosystem function in degraded urban soil using biosolids, biosolids blend, and compost. J. Environ. Qual. 2016, 45, 74–83. [Google Scholar] [CrossRef]
- Romillac, N.; Slezack-Deschaumes, S.; Amiaud, B.; Piutti, S. Soil Microbial Communities Involved in Proteolysis and Sulfate-Ester Hydrolysis Are More Influenced by Interannual Variability than by Crop Sequence. Agronomy 2023, 13, 180. [Google Scholar] [CrossRef]
- Tejada, M.; Hernandez, M.T.; Garcia, C. Application of two organic amendments on soil restoration: Effects on the soil biological properties. J. Environ. Qual. 2006, 35, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Ndiaye, E.L.; Sandeno, J.M.; McGrath, D.; Dick, R.P. Integrative biological indicators for detecting change in soil quality. Am. J. Altern. Agric. 2000, 15, 26–36. [Google Scholar] [CrossRef]
- Knight, T.R.; Dick, R.P. Differentiating microbial and stabilized β-glucosidase activity relative to soil quality. Soil Biol. Biochem. 2004, 36, 2089–2096. [Google Scholar] [CrossRef]
- Wolińska, A.; Stępniewska, Z. Dehydrogenase activity in the soil environment. Dehydrogenases 2012, 10, 183–210. [Google Scholar]
- Sánchez-Monedero, M.A.; Mondini, C.; Cayuela, M.L.; Roig, A.; Contin, M.; De Nobili, M. Fluorescein diacetate hydrolysis, respiration and microbial biomass in freshly amended soils. Biol. Fertil. Soils 2008, 44, 885–890. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, P.; Kong, C.H. Urease, invertase, dehydrogenase and polyphenoloxidase activities in paddy soil influenced by allelopathic rice variety. Eur. J. Soil Biol. 2009, 45, 436–441. [Google Scholar] [CrossRef]
- Ekenler, M.; Tabatabai, M.A. Arylamidase and amidohydrolases in soils as affected by liming and tillage systems. Soil Tillage Res. 2004, 77, 157–168. [Google Scholar] [CrossRef]
- Jayasekara, S.; Ratnayake, R. Microbial cellulases: An overview and applications. Cellulose 2019, 22, 10–5772. [Google Scholar]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms—a review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Eriksson, K.E.; Blanchette, R.A.; Ander, P. Microbial and Enzymatic Degradation of Wood and Wood Components; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Cordero, I.; Snell, H.; Bardgett, R.D. High throughput method for measuring urease activity in soil. Soil Biol. Biochem. 2019, 134, 72–77. [Google Scholar] [CrossRef]
- Vitolo, M. Notes on urea hydrolysis by urease. J. Pharm. Pharm. Sci. 2022, 11, 96–135. [Google Scholar]





| Treatments | pHs | EC dS m−1 | %OM | P Bray I mgkg−1 | Ca mgkg−1 | Mg mgkg−1 | K mgkg−1 | CEC cmolc kg−1 |
|---|---|---|---|---|---|---|---|---|
| Compost | 6.9 | 10.3 | 4 | 80.6 | 2872.0 | 764.6 | 527.3 | 12.2 |
| Biochar | 6.8 | 9.23 | 4.2 | 88.8 | 2914.8 | 814.2 | 593.4 | 11.8 |
| Compost + Biochar | 6.9 | 12.6 | 4.85 | 197.5 | 4998.4 | 1120.8 | 1120.8 | 12.4 |
| Biochar + Compost Tea | 6.9 | 13.4 | 4.3 | 85.1 | 4013.0 | 695.6 | 695.6 | 11.7 |
| Control | 6.65 | 1.7 | 2.75 | 27.7 | 5712.2 | 588.5 | 588.5 | 11.3 |
| Fescue | 7.1 | 3.3 | 3.15 | 2.7 | 3830.2 | 263.1 | 263.1 | 11.3 |
| Treatments | April | June | August |
|---|---|---|---|
| Compost | 6.18 | 6.24 | 6.61 |
| Biochar | 6.64 | 6.61 | 6.71 |
| Compost + Biochar | 6.75 | 6.78 | 6.77 |
| Biochar + Compost Tea | 6.51 | 6.69 | 6.76 |
| Control | 6.36 | 6.56 | 6.74 |
| Fescue | 6.55 | 6.66 | 6.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaid, F.; Al-Awwal, N.; Yang, J.; Anderson, S.H.; Alsunuse, B.T.B. Effects of Biochar-Amended Composts on Selected Enzyme Activities in Soils. Processes 2024, 12, 1678. https://doi.org/10.3390/pr12081678
Zaid F, Al-Awwal N, Yang J, Anderson SH, Alsunuse BTB. Effects of Biochar-Amended Composts on Selected Enzyme Activities in Soils. Processes. 2024; 12(8):1678. https://doi.org/10.3390/pr12081678
Chicago/Turabian StyleZaid, Faraj, Nasruddeen Al-Awwal, John Yang, Stephen H. Anderson, and Bouzeriba T. B. Alsunuse. 2024. "Effects of Biochar-Amended Composts on Selected Enzyme Activities in Soils" Processes 12, no. 8: 1678. https://doi.org/10.3390/pr12081678
APA StyleZaid, F., Al-Awwal, N., Yang, J., Anderson, S. H., & Alsunuse, B. T. B. (2024). Effects of Biochar-Amended Composts on Selected Enzyme Activities in Soils. Processes, 12(8), 1678. https://doi.org/10.3390/pr12081678






