Green Synthesis of Yttrium Derivatives Nanoparticles Using Pine Needle Leaf Extract: Characterization, Docking, Antibacterial, and Antioxidant Potencies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the PNLE and Synthesis of the YNPs
2.2. Characterization Techniques of the YNPs
2.2.1. X-ray Diffraction (XRD)
2.2.2. Scanning Electron Microscopy (SEM) and Energy-Dispersive X-ray (EDX)
2.2.3. Transmission Electron Microscopy (TEM)
2.2.4. X-ray Photoelectron Spectroscopy (XPS)
2.2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.6. UV–Visible Spectroscopy (UV–Vis)
2.2.7. Photoluminescence (PL)
2.2.8. Vibrating Sample Magnetometry (VSM)
2.3. Antibacterial Activity of the YNPs
2.3.1. Preparation of the Bacterial Isolates
2.3.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) Broth Microdilution Assay
2.3.3. Agar Well Diffusion Assay
2.3.4. Anti-Biofilm Assay
2.4. 1,1, Diphenyl 1-2 Picrylhydrazyl (DPPH) Free Radical Scavenging Assay
2.5. Molecular Docking Simulation
2.6. Statistical Analysis
3. Results
3.1. Characterization of the YNPs
3.1.1. X-ray Diffraction (XRD)
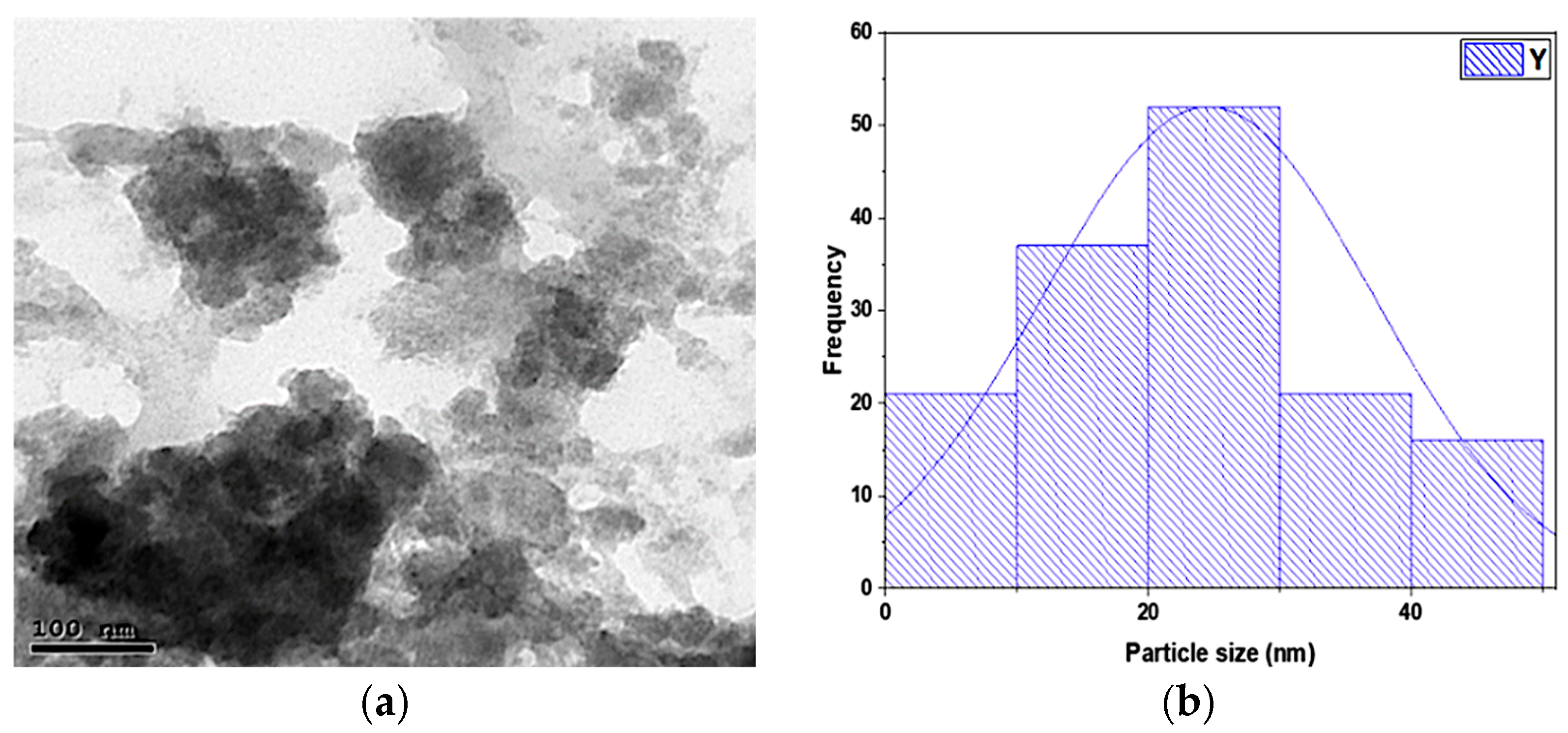
3.1.2. Transmission Electron Microscopic (TEM) Analysis
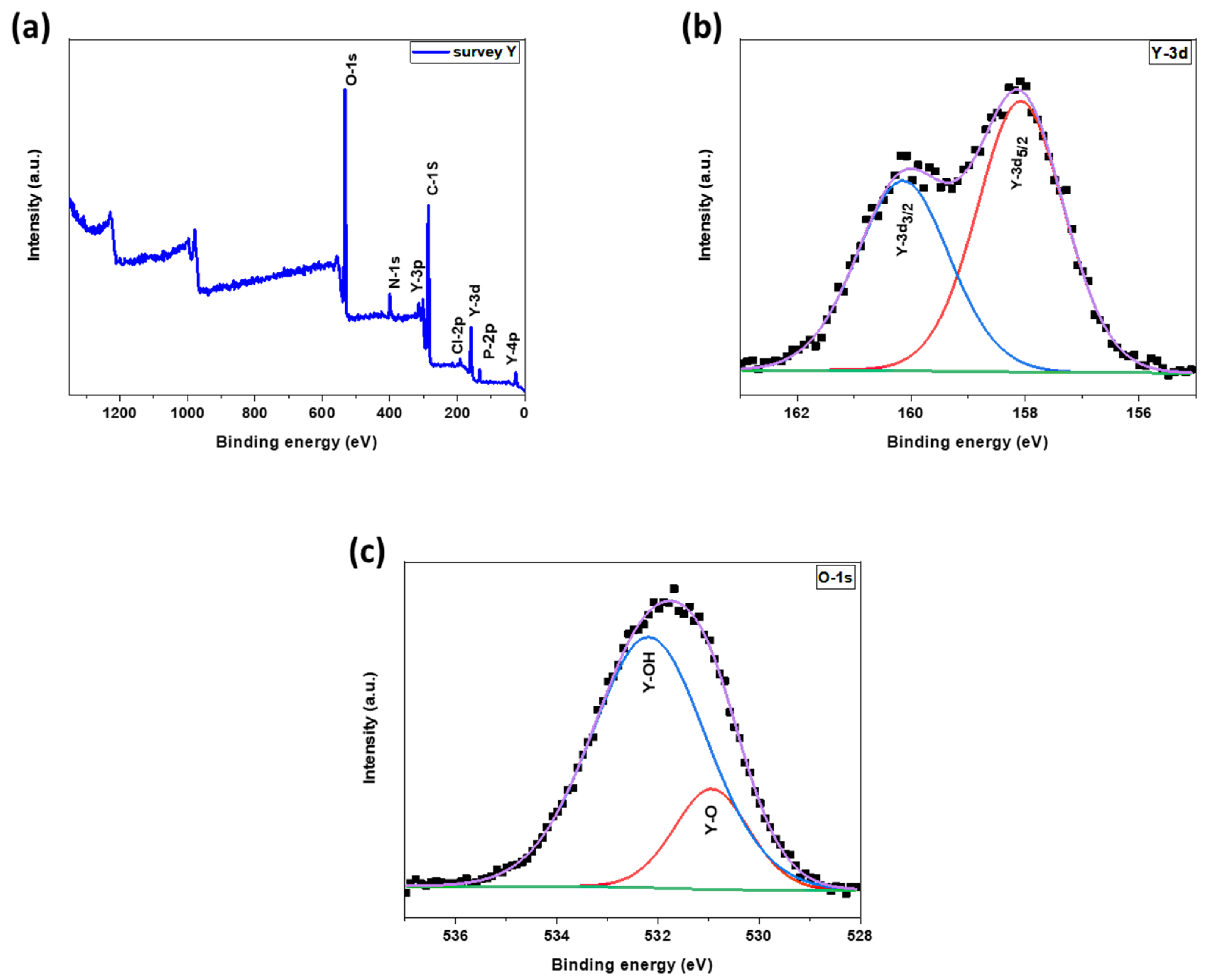
3.1.3. X-ray Photoelectron Spectroscopy (XPS)
3.1.4. Scanning Electron Microscopy (SEM) with Energy-Dispersive X-ray (EDX) Spectroscopy Analysis
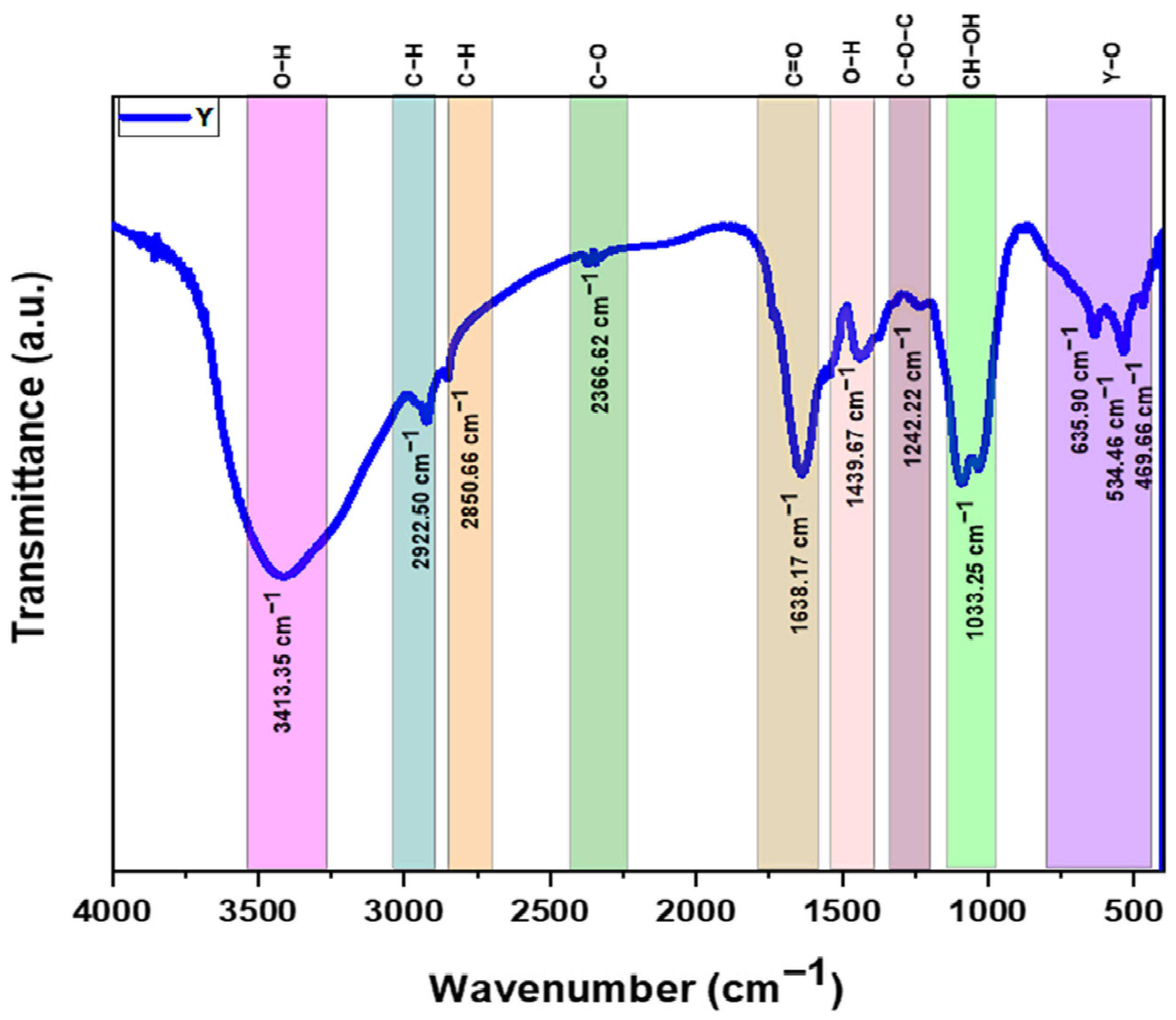
3.1.5. Fourier Transform Infrared (FTIR) Analysis
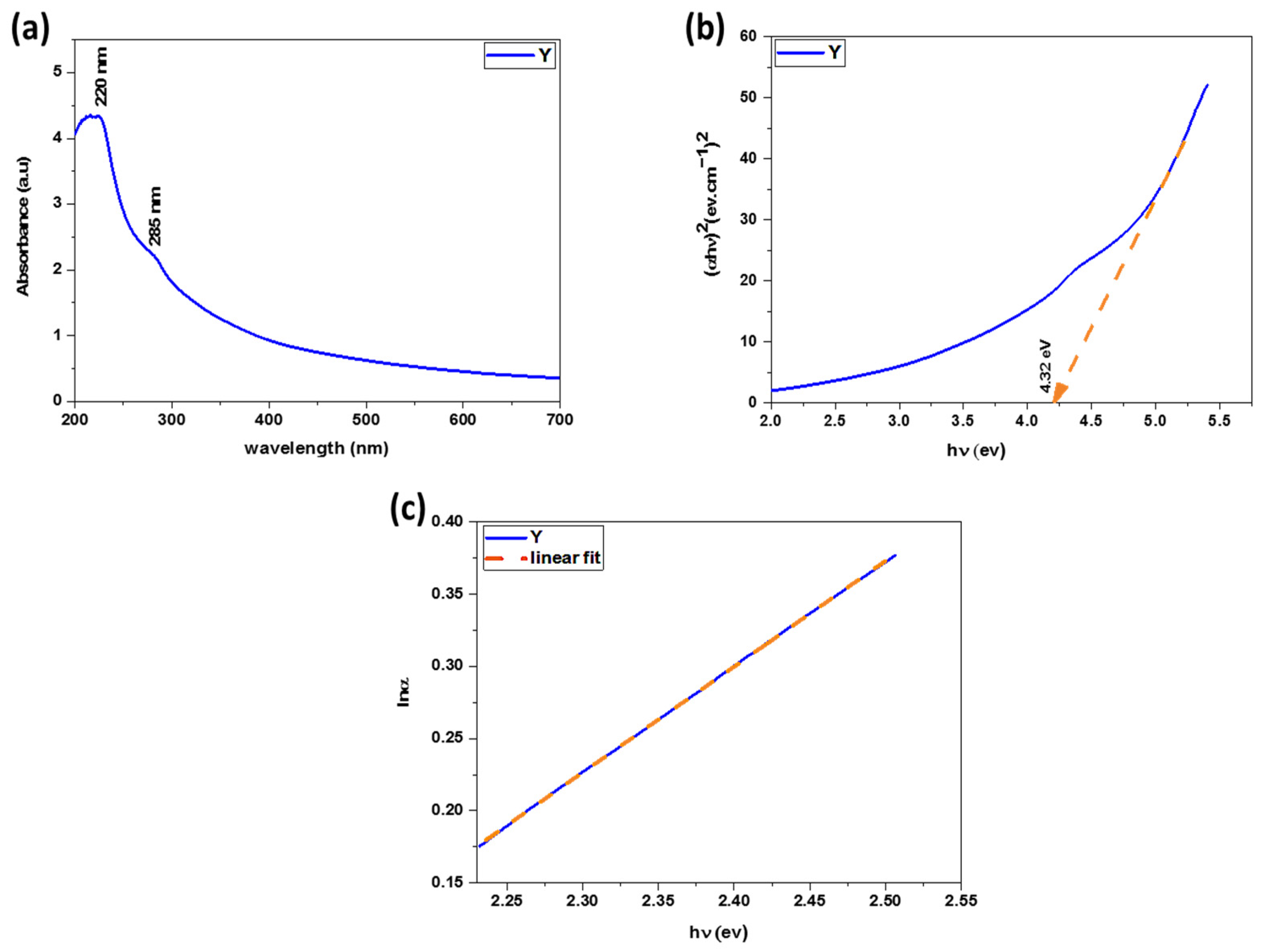
3.1.6. Visual Observation and UV–Vis Spectroscopy Analysis
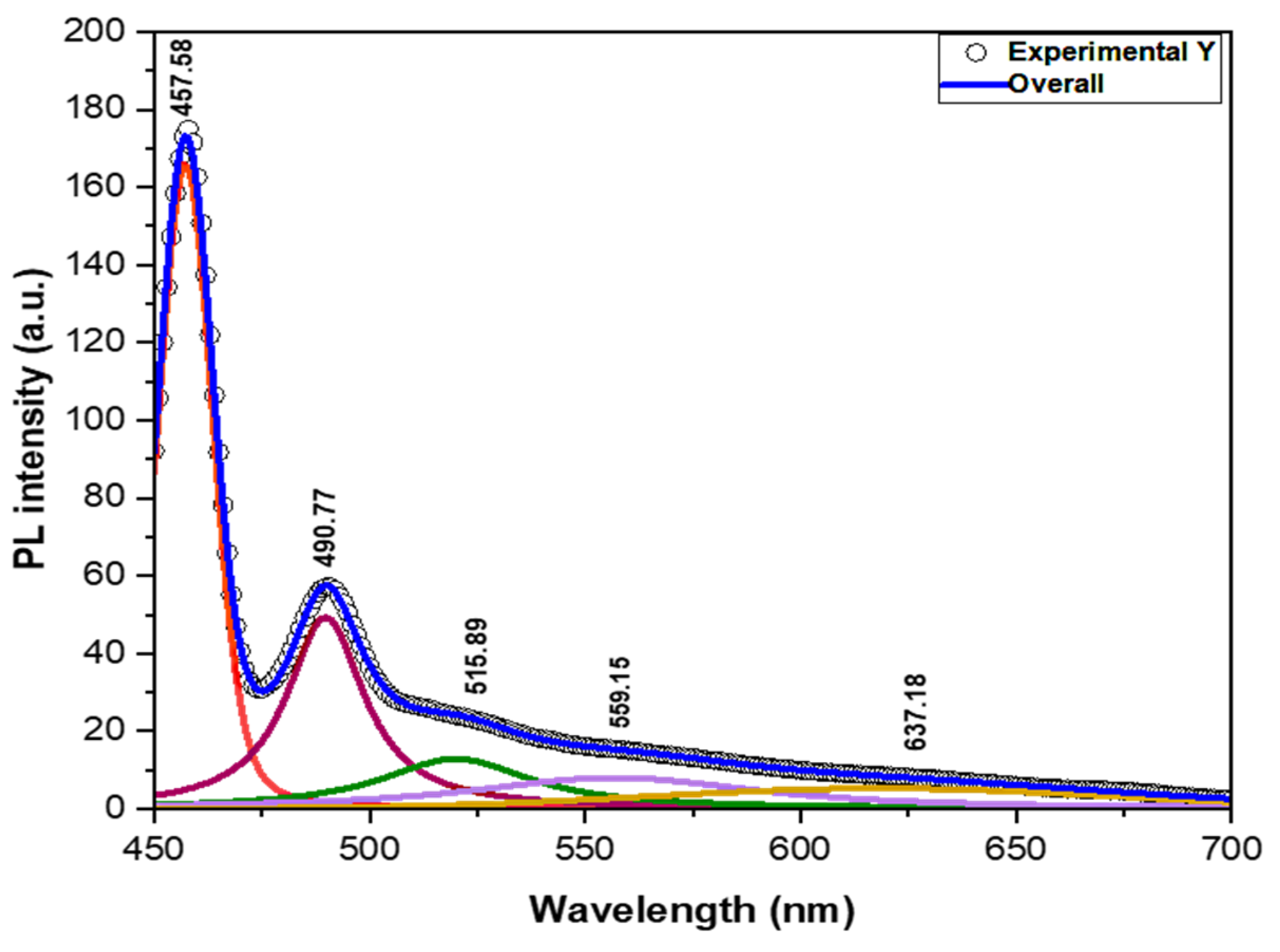
3.1.7. Photoluminescence (PL) Studies
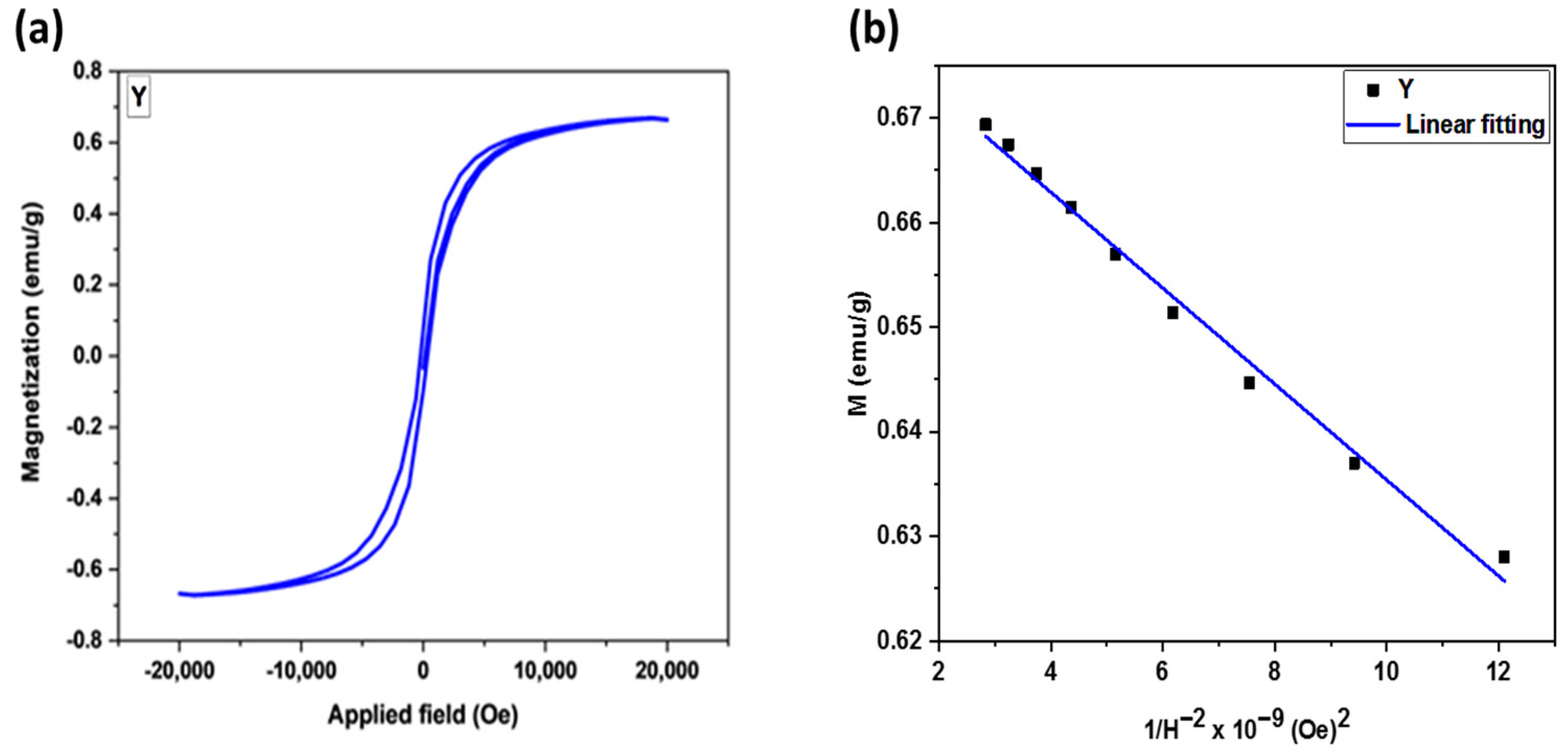
3.1.8. Vibrating Sample Magnetometer (VSM)
3.2. Antibacterial Activity of the YNPs
3.2.1. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) of the YNPs against the Bacterial Isolates
3.2.2. Agar Well Diffusion Results of the Yttrium Nanoparticles against the Bacterial Isolates
3.2.3. Inhibition of Bacterial Biofilm Formation and Destruction of Pre-Formed Bacterial Biofilms by the YNPs
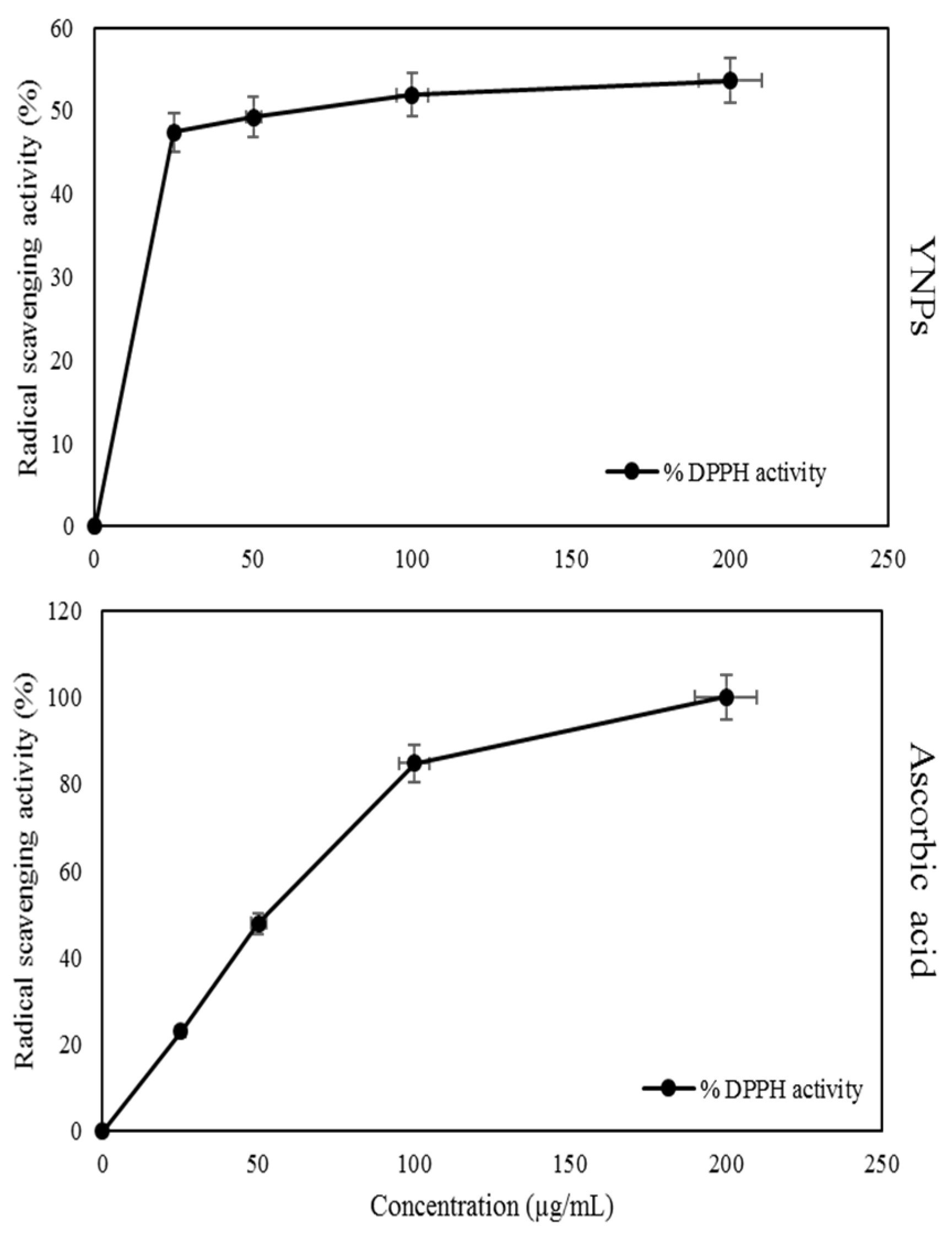
3.3. Antioxidant Radical Scavenging Activity Results of the YNPs against DPPH
3.4. Molecular Docking of the YNPs with Antibacterial Target Proteins
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Makowski, M.; Silva, Í.C.; Pais Do Amaral, C.; Gonçalves, S.; Santos, N.C. Advances in Lipid and Metal Nanoparticles for Antimicrobial Peptide Delivery. Pharmaceutics 2019, 11, 588. [Google Scholar] [CrossRef]
- Gupta, A.; Mumtaz, S.; Li, C.-H.; Hussain, I.; Rotello, V.M. Combatting antibiotic-resistant bacteria using nanomaterials. Chem. Soc. Rev. 2019, 48, 415–427. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Chakra, C.S.; Rajendar, V.; Rao, K.V.; Kumar, M. Enhanced antimicrobial and anticancer properties of ZnO and TiO2 nanocomposites. 3 Biotech 2017, 7, 89. [Google Scholar] [CrossRef]
- Perveen, A.; Molardi, C.; Fornaini, C. Applications of Laser Welding in Dentistry: A State-of-the-Art Review. Micromachines 2018, 9, 209. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef]
- Bahrulolum, H.; Nooraei, S.; Javanshir, N.; Tarrahimofrad, H.; Mirbagheri, V.S.; Easton, A.J.; Ahmadian, G. Green synthesis of metal nanoparticles using microorganisms and their application in the agrifood sector. J. Nanobiotechnol. 2021, 19, 86. [Google Scholar] [CrossRef]
- Andelman, T.; Gordonov, S.; Busto, G.; Moghe, P.V.; Riman, R.E. Synthesis and Cytotoxicity of Y2O3 Nanoparticles of Various Morphologies. Nanoscale Res. Lett. 2010, 5, 263. [Google Scholar] [CrossRef]
- Chang, M.; Tie, S. Fabrication of novel luminor Y2O3: Eu3+@SiO2@YVO4 Eu3+ with core/shell heteronanostructure. Nanotechnology 2008, 19, 075711. [Google Scholar] [CrossRef] [PubMed]
- Prabitha, V.G.; Sahadevan, J.; Madhavan, M.; Muthu, S.E.; Kim, I.; Sudheer, T.K.; Sivaprakash, P. Effect of Yttrium doping on antibacterial and antioxidant property of LaTiO3. Discov. Nano 2023, 18, 155. [Google Scholar] [CrossRef]
- Mezher, M.; Hajj, R.E.; Khalil, M. Investigating the antimicrobial activity of essential oils against pathogens isolated from sewage sludge of southern Lebanese villages. GERMS 2022, 12, 488–506. [Google Scholar] [CrossRef]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Govindasamy, R.; Govindarasu, M.; Alharthi, S.S.; Mani, P.; Bernaurdshaw, N.; Gomathi, T.; Ansari, M.A.; Alomary, M.N.; Atwah, B.; Malik, M.S.; et al. Sustainable Green Synthesis of Yttrium Oxide (Y2O3) Nanoparticles Using Lantana camara Leaf Extracts: Physicochemical Characterization, Photocatalytic Degradation, Antibacterial, and Anticancer Potency. Nanomaterials 2022, 12, 2393. [Google Scholar] [CrossRef]
- Shivaramu, N.J.; Lakshminarasappa, B.N.; Nagabhushana, K.R.; Singh, F. Synthesis characterization and luminescence studies of gamma irradiated nanocrystalline yttrium oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 154, 220–231. [Google Scholar] [CrossRef]
- Kannan, S.K.; Sundrarajan, M. Biosynthesis of Yttrium oxide nanoparticles using Acalypha indica leaf extract. Bull. Mater. Sci. 2015, 38, 945–950. [Google Scholar] [CrossRef]
- Amanzadeh, E.; Esmaeili, A.; Abadi, R.E.N.; Kazemipour, N.; Pahlevanneshan, Z.; Beheshti, S. Quercetin conjugated with superparamagnetic iron oxide nanoparticles improves learning and memory better than free quercetin via interacting with proteins involved in LTP. Sci. Rep. 2019, 9, 6876. [Google Scholar] [CrossRef]
- Emamzadeh Yazdi, S.; Heyman, H.M.; Prinsloo, G.; Klimkait, T.; Meyer, J.J.M. Identification of Anti-HIV Biomarkers of Helichrysum Species by NMR-Based Metabolomic Analysis. Front. Pharmacol. 2022, 13, 904231. [Google Scholar] [CrossRef]
- Aghazadeh, M.; Ghaemi, M.; Nozad Golikand, A.; Yousefi, T.; Jangju, E. Yttrium Oxide Nanoparticles Prepared by Heat Treatment of Cathodically Grown Yttrium Hydroxide. ISRN Ceram. 2011, 2011, 542104. [Google Scholar] [CrossRef]
- Wörner, H.J.; Arrell, C.A.; Banerji, N.; Cannizzo, A.; Chergui, M.; Das, A.K.; Hamm, P.; Keller, U.; Kraus, P.M.; Liberatore, E.; et al. Charge migration and charge transfer in molecular systems. Struct. Dyn. 2017, 4, 061508. [Google Scholar] [CrossRef]
- Luis Aleixandre-Tudo, J.; Du Toit, W. The Role of UV-Visible Spectroscopy for Phenolic Compounds Quantification in Winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Lidia Solís-Oviedo, R., De La Cruz Pech-Canul, Á., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Sisa, M.; Bonnet, S.L.; Ferreira, D.; Van Der Westhuizen, J.H. Photochemistry of Flavonoids. Molecules 2010, 15, 5196–5245. [Google Scholar] [CrossRef]
- Verma, A.; Mehata, M.S. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J. Radiat. Res. Appl. Sci. 2016, 9, 109–115. [Google Scholar] [CrossRef]
- Al-Otibi, F.; Perveen, K.; Al-Saif, N.A.; Alharbi, R.I.; Bokhari, N.A.; Albasher, G.; Al-Otaibi, R.M.; Al-Mosa, M.A. Biosynthesis of silver nanoparticles using Malva parviflora and their antifungal activity. Saudi J. Biol. Sci. 2021, 28, 2229–2235. [Google Scholar] [CrossRef]
- Bashir, B.; Khalid, M.U.; Aadil, M.; Zulfiqar, S.; Warsi, M.F.; Agboola, P.O.; Shakir, I. CuxNi1−xO nanostructures and their nanocomposites with reduced graphene oxide: Synthesis, characterization, and photocatalytic applications. Ceram. Int. 2021, 47, 3603–3613. [Google Scholar] [CrossRef]
- Cursaru, L.M.; Piticescu, R.M.; Dragut, D.V.; Tudor, I.A.; Kuncser, V.; Iacob, N.; Stoiciu, F. The Influence of Synthesis Parameters on Structural and Magnetic Properties of Iron Oxide Nanomaterials. Nanomaterials 2020, 10, 85. [Google Scholar] [CrossRef]
- El-Said Bakeer, D. Investigation on Optical, Dielectric, and Magnetic Properties of CoAl2−xFexO4 Nanoparticles. J. Supercond. Nov. Magn. 2020, 33, 1789–1801. [Google Scholar] [CrossRef]
- Adnan, R.; Abdallah, A.M.; Mezher, M.; Noun, M.; Khalil, M.; Awad, R. Impact of Mg-doping on the structural, optical, and magnetic properties of CuO nanoparticles and their antibiofilm activity. Phys. Scr. 2023, 98, 055935. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, C.; Zhou, J.; Huo, M. Somatostatin Receptor-Mediated Tumor-Targeting Nanocarriers Based on Octreotide-PEG Conjugated Nanographene Oxide for Combined Chemo and Photothermal Therapy. Small 2016, 12, 3578–3590. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Salvadori, M.R.; Rafatullah, M.; Siddiqui, M.R.; Khan, M.A.; Alshareef, S.A. Exploration of Microbial Factories for Synthesis of Nanoparticles—A Sustainable Approach for Bioremediation of Environmental Contaminants. Front. Microbiol. 2021, 12, 658294. [Google Scholar] [CrossRef]
- Khan, A.; Fayyaz, O.; Shakoor, R.A.; Mansoor, B. Recent Trends in Applications of X-ray Photoelectron Spectroscopy (XPS) Technique in Coatings for Corrosion Protection. In Recent Developments in Analytical Techniques for Corrosion Research; Toor, I.U., Ed.; Springer: Cham, Switzerland, 2022; pp. 167–186. [Google Scholar] [CrossRef]
- Porosnicu, I.; Butnaru, C.M.; Tiseanu, I.; Stancu, E.; Munteanu, C.V.A.; Bita, B.I.; Duliu, O.G.; Sima, F. Y2O3 Nanoparticles and X-ray Radiation-Induced Effects in Melanoma Cells. Molecules 2021, 26, 3403. [Google Scholar] [CrossRef]
- Phatak, R.S.; Hendre, A.S. Sunlight induced green synthesis of silver nanoparticles using sundried leaves extract of Kalanchoe pinnata and evaluation of its photocatalytic potential. Der Pharm. Lett. 2015, 7, 313–324. [Google Scholar]
- Khalifa, K.S.; Hamouda, R.A.; Hamza, D.H.A. In vitro antitumor activity of silver nanoparticles biosynthesized by marine algae. Dig. J. Nanomater. Biostruct. 2016, 11, 213–221. [Google Scholar]
- Palithya, S.; Gaddam, S.A.; Kotakadi, V.S.; Penchalaneni, J.; Golla, N.; Krishna, S.B.N.; Naidu, C.V. Green synthesis of silver nanoparticles using flower extracts of Aerva lanata and their biomedical applications. Part. Sci. Technol. 2022, 40, 84–96. [Google Scholar] [CrossRef]
- Parangusan, K.; Subramanium, V.; Sundarabharathi, L.; Kannan, K.; Radhika, D. Influence of pH on Structural, Morphological, Optical, Photocatalytic, and Antibacterial Properties of Yttrium Oxide Nanoparticles via Co-Precipitation Method. Res. Sq. 2021. [Google Scholar] [CrossRef]
- Khalid, A.; Ahmad, P.; Alharthi, A.I.; Muhammad, S.; Khandaker, M.U.; Rehman, M.; Faruque, M.R.I.; Din, I.U.; Alotaibi, M.A.; Alzimami, K.; et al. Structural, Optical, and Antibacterial Efficacy of Pure and Zinc-Doped Copper Oxide Against Pathogenic Bacteria. Nanomaterials 2021, 11, 451. [Google Scholar] [CrossRef]
- Xing, Y.; Liao, X.; Liu, X.; Li, W.; Huang, R.; Tang, J.; Xu, Q.; Li, X.; Yu, J. Characterization and Antimicrobial Activity of Silver Nanoparticles Synthesized with the Peel Extract of Mango. Materials 2021, 14, 5878. [Google Scholar] [CrossRef]
- Kanwal, Z.; Raza, M.A.; Riaz, S.; Manzoor, S.; Tayyeb, A.; Sajid, I.; Naseem, S. Synthesis and characterization of silver nanoparticle-decorated cobalt nanocomposites (Co@AgNPs) and their density-dependent antibacterial activity. R. Soc. Open Sci. 2019, 6, 182135. [Google Scholar] [CrossRef]
- Adnan, R.M.; Mezher, M.; Abdallah, A.M.; Awad, R.; Khalil, M.I. Synthesis, Characterization, and Antibacterial Activity of Mg-Doped CuO Nanoparticles. Molecules 2022, 28, 103. [Google Scholar] [CrossRef]
- Rabaa, M.; Mezher, M.; Aridi, A.; Naoufal, D.; Khalil, M.I.; Awad, R.; Abdeen, W. Influence of Lanthanum Doping on the Photocatalytic and Antibacterial Capacities of Mg0.33Ni0.33Co0.33Fe2O4 Nanoparticles. Catalysts 2023, 13, 693. [Google Scholar] [CrossRef]
- Rabaa, M.; Mezher, M.; Aridi, A.; Naoufal, D.; Khalil, M.; Awad, R. Improved Photocatalytic and Antibacterial Activity of Mg0.33Ni0.33Co0.33GdxFe2−xO4 Nanoparticles Synthesized via the Co-precipitation Method. ChemistrySelect 2023, 8, e202301951. [Google Scholar] [CrossRef]
- Famuyide, I.M.; Aro, A.O.; Fasina, F.O.; Eloff, J.N.; McGaw, L.J. Antibacterial and antibiofilm activity of acetone leaf extracts of nine under-investigated south African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complement. Altern. Med. 2019, 19, 141. [Google Scholar] [CrossRef]
- Iseppi, R.; Tardugno, R.; Brighenti, V.; Benvenuti, S.; Sabia, C.; Pellati, F.; Messi, P. Phytochemical Composition and In Vitro Antimicrobial Activity of Essential Oils from the Lamiaceae Family against Streptococcus agalactiae and Candida albicans Biofilms. Antibiotics 2020, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Bedlovičová, Z.; Strapáč, I.; Baláž, M.; Salayová, A. A Brief Overview on Antioxidant Activity Determination of Silver Nanoparticles. Molecules 2020, 25, 3191. [Google Scholar] [CrossRef]
- Siddique, M.A.B.; Imran, M.; Haider, A.; Shahzadi, A.; Ul-Hamid, A.; Nabgan, W.; Mahmood, A. Enhancing catalytic and antibacterial activity with size-controlled yttrium and graphene quantum dots doped MgO nanostructures: A molecular docking analysis. Mater. Today Sustain. 2024, 25, 100690. [Google Scholar] [CrossRef]
- Ayub, A.; Ikram, M.; Haider, A.; Shahzadi, I.; Ul-Hamid, A.; Shahzadi, A.; Algaradah, M.M.; Fouda, A.M.; Nabgan, W.; Imran, M. Enhanced Industrial Dye Degradation and Antibacterial Activity Supported by the Molecular Docking Study of Yttrium and Carbon Sphere-Doped Lanthanum Oxide Nanostructures. ACS Omega 2023, 8, 37564–37572. [Google Scholar] [CrossRef]
- Ikram, M. Multiple phases of yttrium-doped molybdenum trioxide nanorods as efficient dye degrader and bactericidal agents with molecular docking analysis. Chemosphere 2023, 340, 139855. [Google Scholar] [CrossRef]




| NPs 1 | Presented Elements | Atomic % |
|---|---|---|
| YNPs 2 | O-1s | 71.44 |
| Y–3d | 7.41 | |
| N-1s | 13.4 | |
| P-2p | 7.75 |
| Sample | M20kOe (emu/g) 2 | Ms (emu/g) 3 | Mr × 10−3 (emu/g) 4 | Hci (Oe) 5 | S 6 |
|---|---|---|---|---|---|
| YNPs 1 | 0.664 | 0.681 | 85.540 | 289.24 | 0.127 |
| Bacterial Isolates | Concentration (mg/mL) | |
|---|---|---|
| MIC 1 | MBC 2 | |
| Gram-positive bacteria | ||
| S. aureus | 1 | - 3 |
| S. haemolyticus | 0.5 | - |
| E. faecalis | 0.5 | - |
| Gram-negative bacteria | ||
| E. coli | 1 | - |
| K. pneumonia | 0.5 | - |
| P. aeruginosa | 0.5 | - |
| YNPs Concentration (mg/mL) | Bacterial Isolates | |||||
|---|---|---|---|---|---|---|
| Gram-Positive | Gram-Negative | |||||
| S. aureus | S. haemolyticus | E. faecalis | E. coli | K. pneumonia | P. aeruginosa | |
| ZOI ± SEM (mm)2 | ||||||
| 0.0625 p-value Significance | 7.00 ± 0.00 0.001 *** | 7.00 ± 0.00 0.001 *** | 7.33 ± 0.27 ˂0.001 *** | 0.00 ± 0.00 ˂0.001 *** | 0.00 ± 0.00 ˂0.001 *** | 0.00 ± 0.00 ˂0.001 *** |
| 0.125 p-value Significance | 7.00 ± 0.00 0.001 *** | 7.00 ± 0.00 0.001 *** | 9.33 ± 0.27 0.001 *** | 0.0 ± 0.00 ˂0.001 *** | 0.0 ± 0.00 ˂0.001 *** | 0.00 ± 0.00 ˂0.001 *** |
| 0.25 p-value Significance | 7.33 ± 0.27 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** | 9.33 ± 0.27 ˂0.001 *** | 0.00 ± 0.00 ˂0.001 *** | 0.0 ± 0.00 ˂0.001 *** | 0.00 ± 0.00 ˂0.001 *** |
| 0.5 p-value Significance | 7.33 ± 0.27 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** | 10.00 ± 0.00 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** |
| 1 p-value Significance | 8.33 ± 0.7 ˂0.001 *** | 7.33 ± 0.27 ˂0.002 ** | 10.00 ± 0.00 ˂0.001 *** | 10.33 ± 0.27 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** | 7.33 ± 0.27 ˂0.001 *** |
| Bacteria | Proteins 3D Structure | Hydrophilic Interactions | Hydrophobic Contacts | No. of H-Bonds | No. of Total Bonds | Affinity kcal mol-1 | |||
|---|---|---|---|---|---|---|---|---|---|
| Residue (H-Bond) | Length | Residue (Bond Type) | Length | ||||||
| 1 | E. coli |  | Asn46 (H-Bond) Val120 (H-Bond) Ser121 (H-Bond) Glu42(H-Bond) Asn46(H-Bond) Asn46(H-Bond) Gly119(H-Bond) | 2.31 2.10 2.73 3.17 2.72 2.64 2.54 | Gly119 (C–H Bond) Asn46 (Metal–Acceptor) | 2.54 2.88 | 7 | 9 | –12.54 |
| 2 | E. faecalis |  | Ser367 (H-Bond) Thr418 (H-Bond) Tyr420 (H-Bond) Ile550(H-Bond) Gln552(H-Bond) Met654(H-Bond) Ile547(H-Bond) Phe416(H-Bond) | 2.11 2.95 3.03 3.66 2.23 2.85 2.54 2.14 | Phe416 (C–H Bond) Tyr420 (Pi–Donor H Bond) | 2.56 3.51 | 8 | 10 | –8.90 |
| 3 | K. pneumonia |  | Ser71 (H-Bond) Arg161 (H-Bond) Ser181 (H-Bond) Ser182(H-Bond) Val186(H-Bond) Tyr247(H-Bond) Tyr264(H-Bond) Thr180(H-Bond) Pro67(H-Bond) Phe66(H-Bond) | 1.94 2.86 2.98 2.68 2.85 3.06 2.41 3.23 2.90 3.25 | Leu68 (C–H Bond) Phe72 (C–H Bond) Val186 (C–H Bond) Tyr247 (Metal–Acceptor) Tyr264 (Metal–Acceptor) | 2.66 2.71 3.40 3.29 3.09 | 10 | 15 | –10.22 |
| 4 | P. aeruginosa |  | Gly38, (H-Bond) Arg61, (H-Bond) Ala127, (H-Bond) Asp73, (H-Bond) Tyr47, (H-Bond) Tyr64, (H-Bond) | 2.37 2.59 2.41 3.07 2.47 2.51 | Tyr64, (Pi–Lone Pair) Tyr47 (Pi–Donor Hydrogen) | 4.07 2.51 | 6 | 8 | –8.90 |
| 5 | S. aureus |  | Asn11, (H-Bond) Arg52, (H-Bond) Lys203, (H-Bond) Gln105, (H-Bond) Arg239, (H-Bond) | 2.68 1.75 2.84 2.95 1.80 | - | - | 5 | 5 | –6.87 |
| 6 | S. haemolyticus | 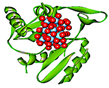 | Arg78, (H-Bond) His92, (H-Bond) Asp48, (H-Bond) Phe140, (H-Bond) Asp28, (H-Bond) Gly29, (H-Bond) | 2.17 2.08 2.65 2.93 2.93 2.35 | Gly29, (C–H bond) His92, (C–H bond) | 3.56 3.05 | 6 | 8 | –9.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Darwich, N.A.; Mezher, M.; Abdallah, A.M.; El-Sayed, A.F.; El Hajj, R.; Hamdalla, T.A.; Khalil, M.I. Green Synthesis of Yttrium Derivatives Nanoparticles Using Pine Needle Leaf Extract: Characterization, Docking, Antibacterial, and Antioxidant Potencies. Processes 2024, 12, 1713. https://doi.org/10.3390/pr12081713
Darwich NA, Mezher M, Abdallah AM, El-Sayed AF, El Hajj R, Hamdalla TA, Khalil MI. Green Synthesis of Yttrium Derivatives Nanoparticles Using Pine Needle Leaf Extract: Characterization, Docking, Antibacterial, and Antioxidant Potencies. Processes. 2024; 12(8):1713. https://doi.org/10.3390/pr12081713
Chicago/Turabian StyleDarwich, Nourhane A., Malak Mezher, Alaa M. Abdallah, Ahmed F. El-Sayed, Rana El Hajj, Taymour A. Hamdalla, and Mahmoud I. Khalil. 2024. "Green Synthesis of Yttrium Derivatives Nanoparticles Using Pine Needle Leaf Extract: Characterization, Docking, Antibacterial, and Antioxidant Potencies" Processes 12, no. 8: 1713. https://doi.org/10.3390/pr12081713







