Future Prospects of MeOH and EtOH Blending in Gasoline: A Comparative Study on Fossil, Biomass, and Renewable Energy Sources Considering Economic and Environmental Factors
Abstract
1. Introduction
- LP models have been developed for typical refining plants, incorporating alcohols sourced from both fossil energy and renewable energy sources into gasoline blending.
- An ELM-based model for gasoline blending has been introduced to enhance accuracy in describing and predicting blend compositions.
- The LP-ELM framework has been developed to integrate linear programming models with the ELM-based gasoline blending model, enabling refinery-wide linear programming and comprehensive carbon footprint tracking for gasoline products.
- Economic and environmental performance analysis models have been established to thoroughly evaluate the benefits associated with blended-alcohol gasoline. This includes assessing the potential market viability of alcohol blends produced from renewable energy sources, especially under future carbon pricing scenarios.
2. Methodology
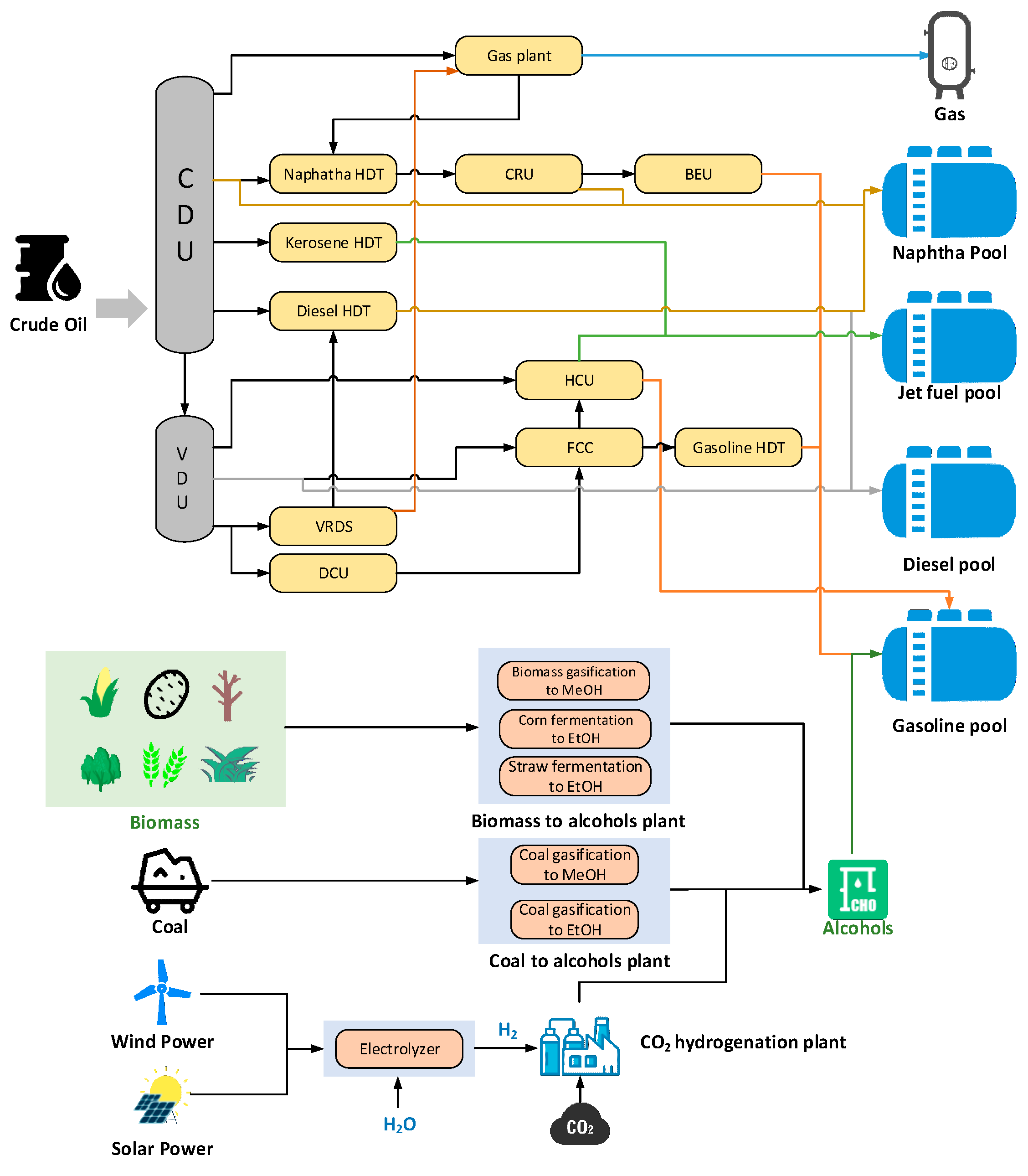
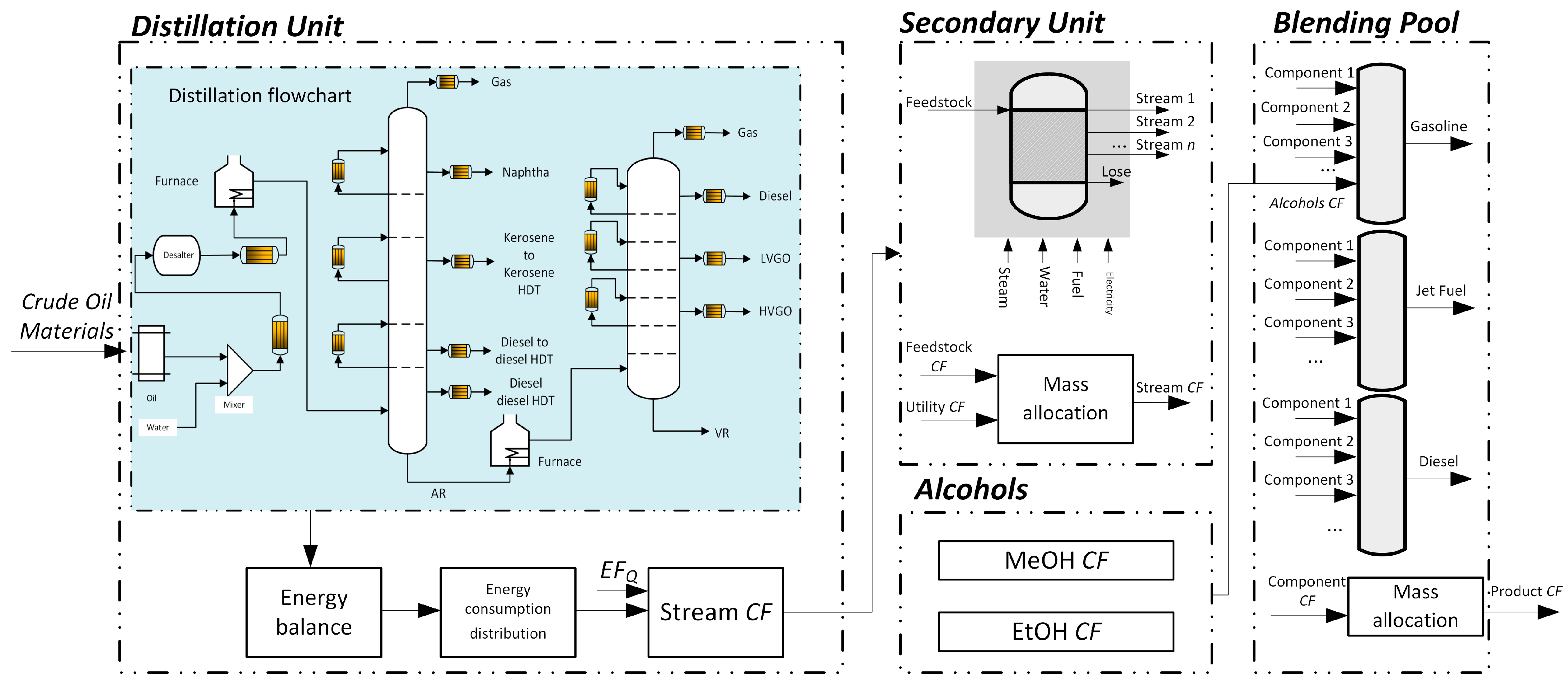
2.1. LP Modeling
2.2. Blending Model
2.2.1. Property Blending
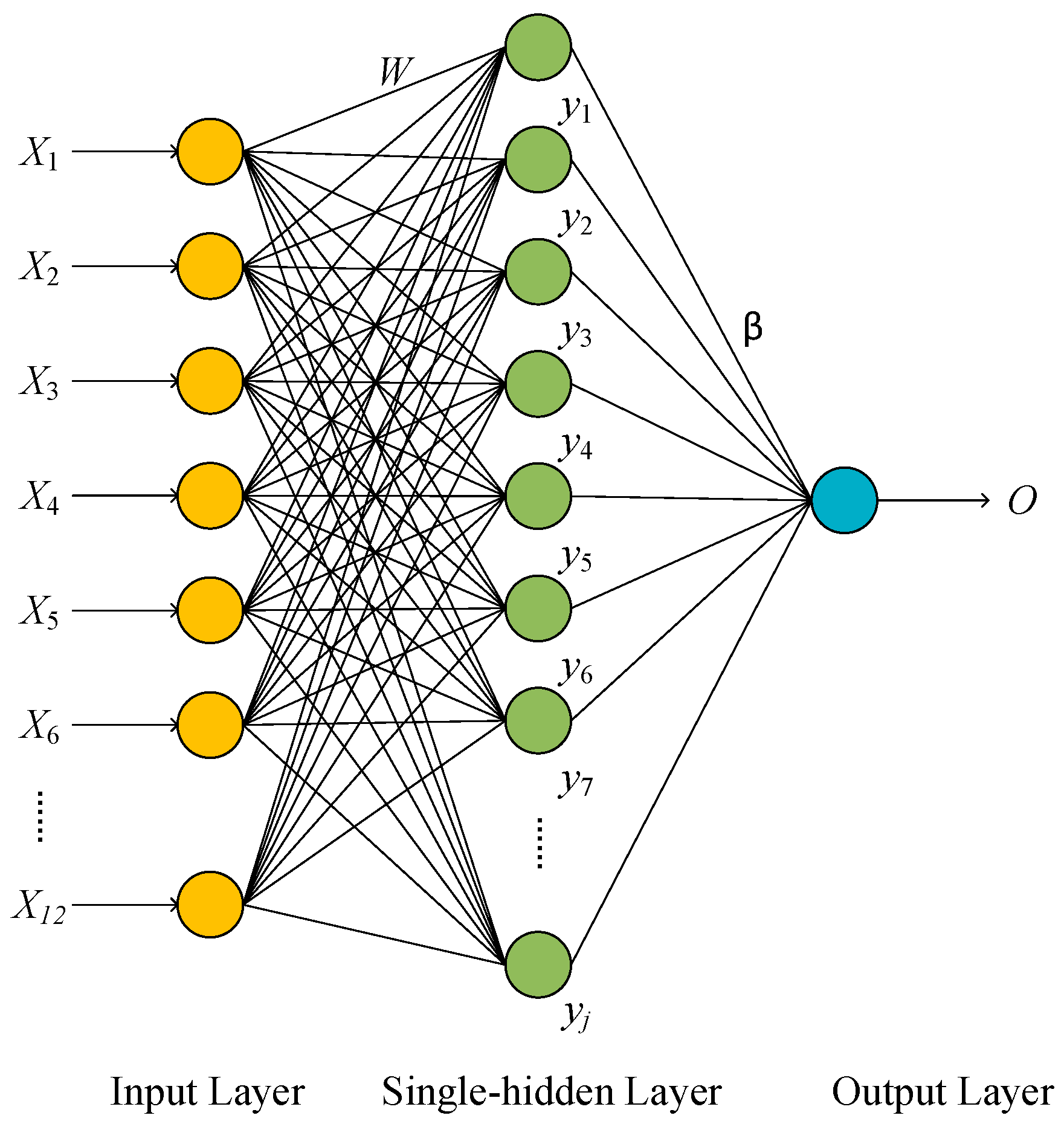
2.2.2. RON Blending Prediction Based on ELM
2.3. Carbon Footprint Tracking
2.4. LP-ELM Framework
| Algorithm 1 Integration of LP and ELM for Octane Blending Optimization |
| Input: LP input (raw material constraints, device capacity, yield of device side line, stream properties, property constraints, product constraints, utility consumption, price system), ELM input (blending component octane number data) Output: Blending scheme, carbon footprint of products, GM, global flow balance |
| 1. Start 2. Collect data and preprocess data 3. Build and train ELM neural network model 4. Build LP model 5. Define objective function: Maximize gross margin 6. Set initial optimization parameters 7. while not Convergence do 8. Solve LP model by SciPy 9. Obtain optimal blending solution: Blending component quantities 10. ELMPredictRON ← ELM_predict(BlendingComponentQuantities) 11. if ELMPredictRON > OctaneConstrainLowerBound then 12. RONLowerBound(LP model) = RONLowerBound(LP model) + δ 13. else if ELMPredictRON > RONConstrainLowerBound then 14. RONLowerBound(LP model) = RONLowerBound(LP model) − δ 15. end if 16. if TerminationConditionMet then 17. Output results 18. break 19. end if 20. end while 21. Output final optimization solution 22. end |
2.5. Alcohol-Based Blendstock Break-Even Value Characterization
2.6. Price System Establishment
2.7. Case Studies
3. Results and Discussion
3.1. RON Prediction
3.1.1. Parameter Settings
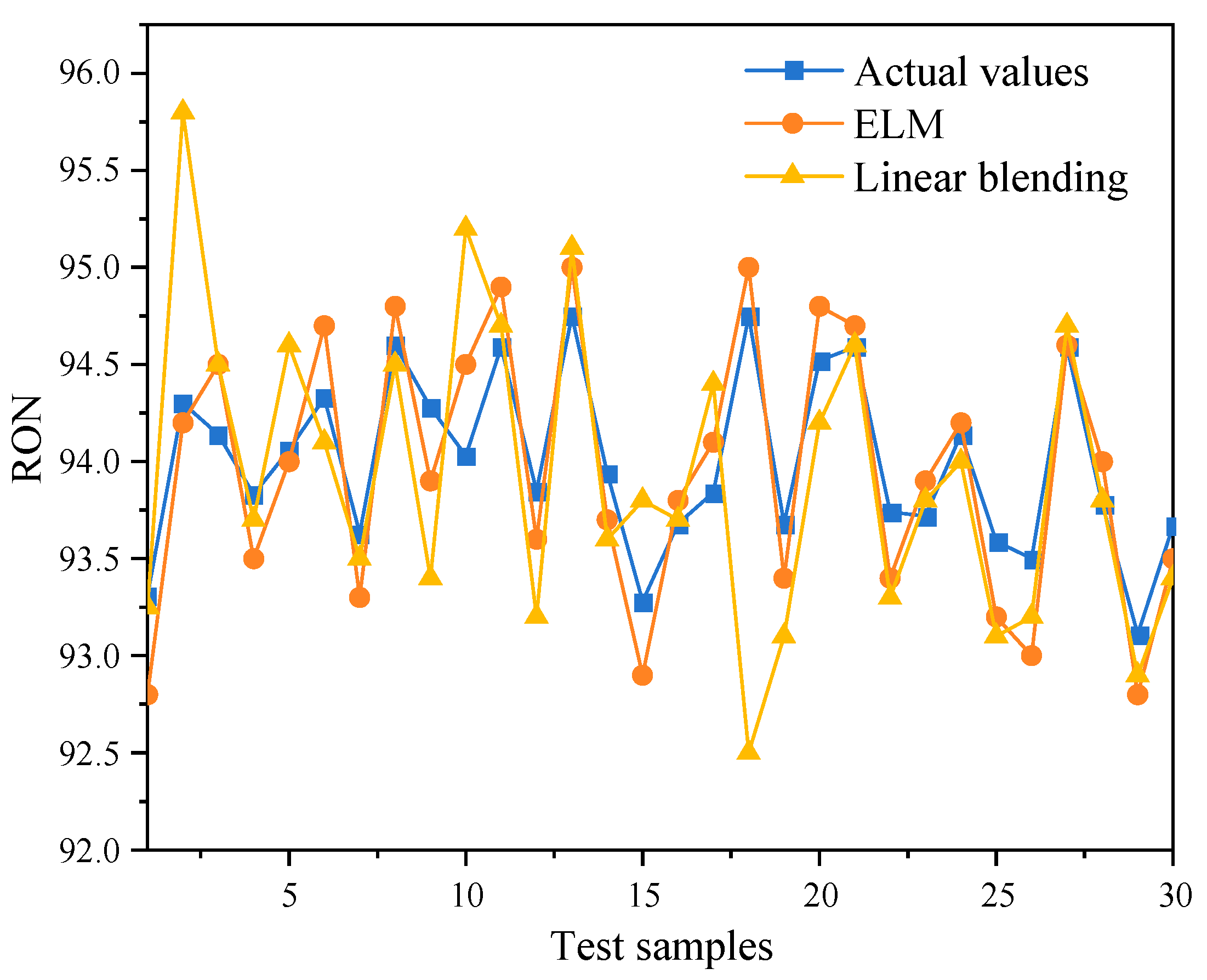
3.1.2. RON Prediction Results
3.2. Economic Performance Comparison
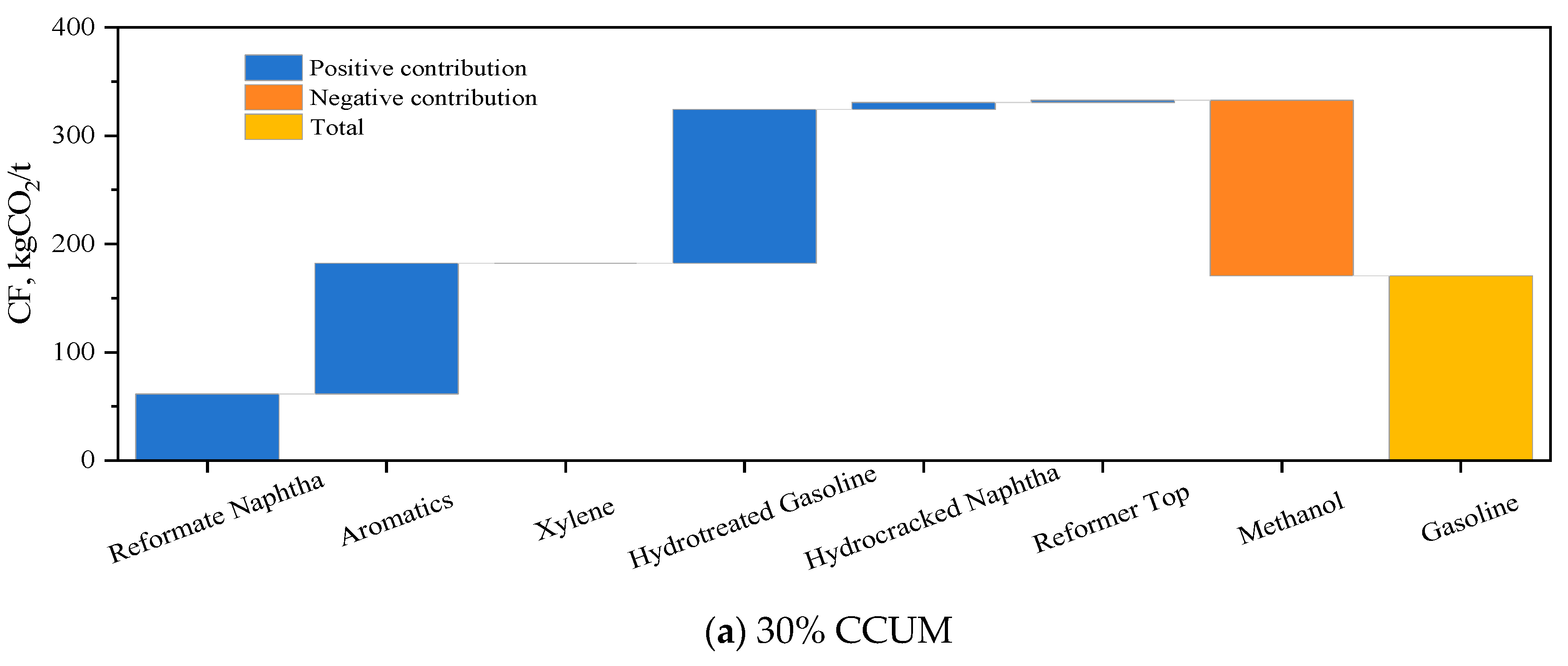
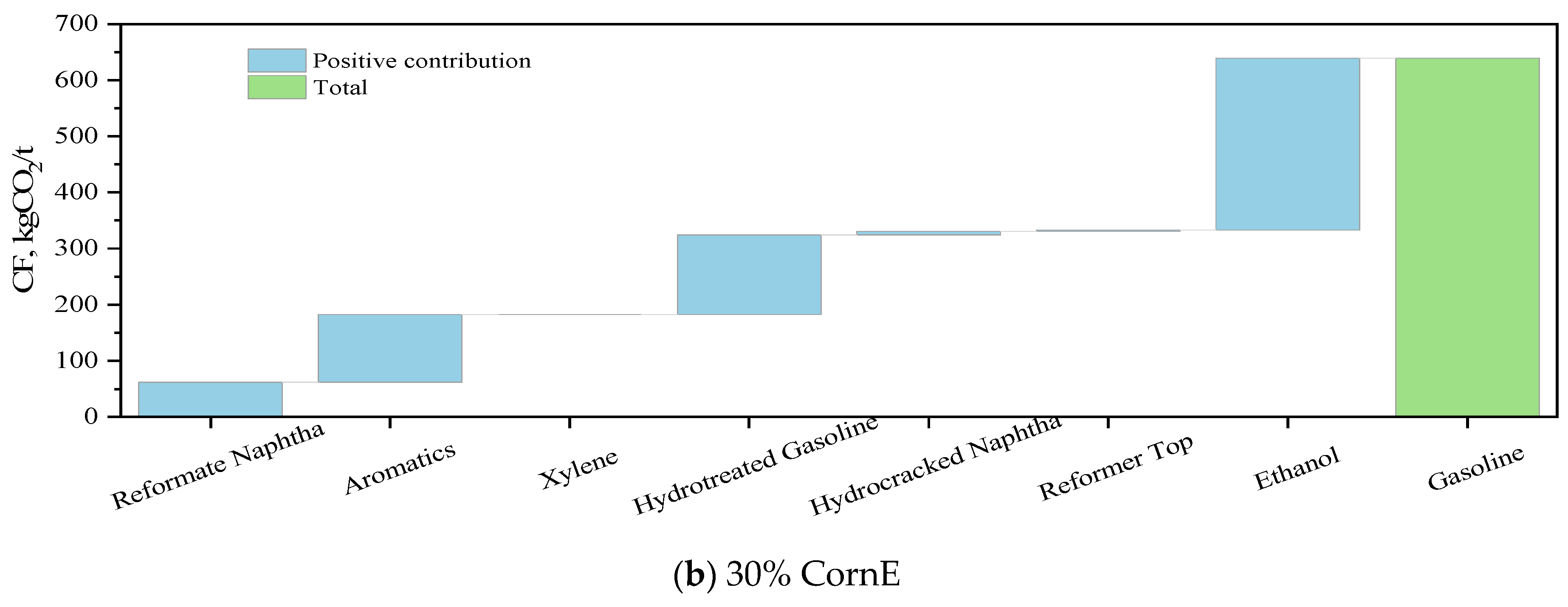
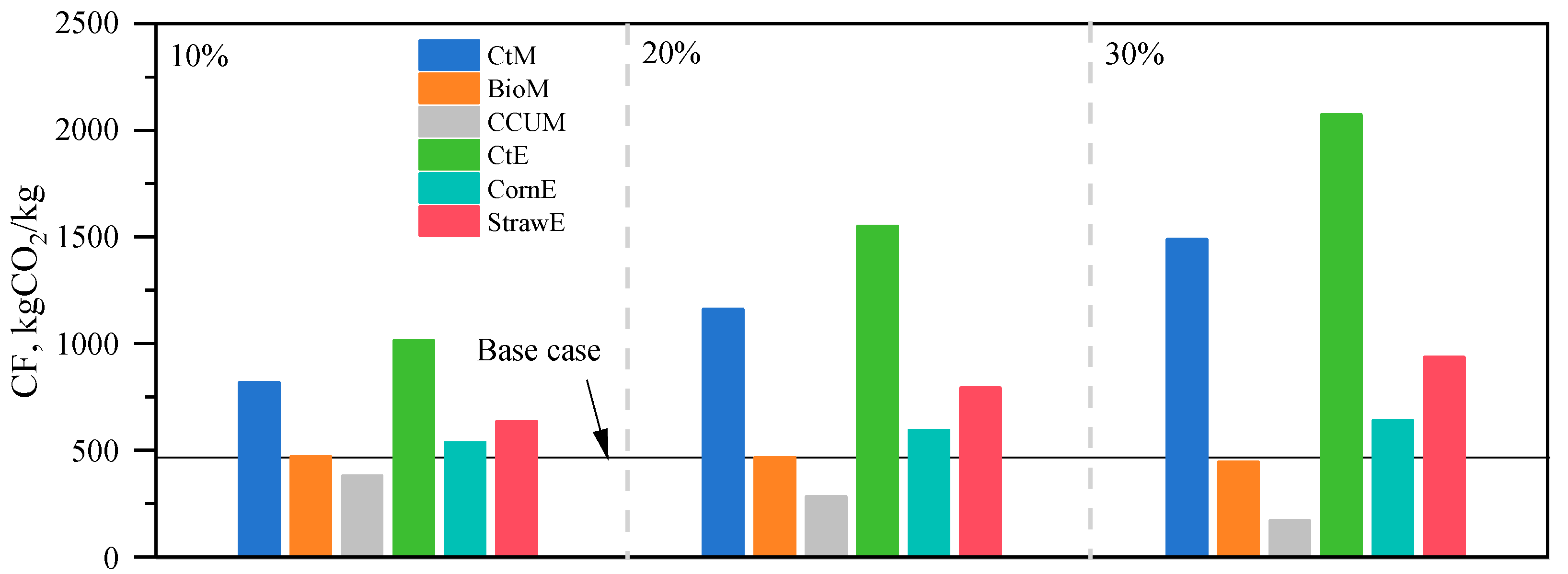
3.3. Carbon Footprint
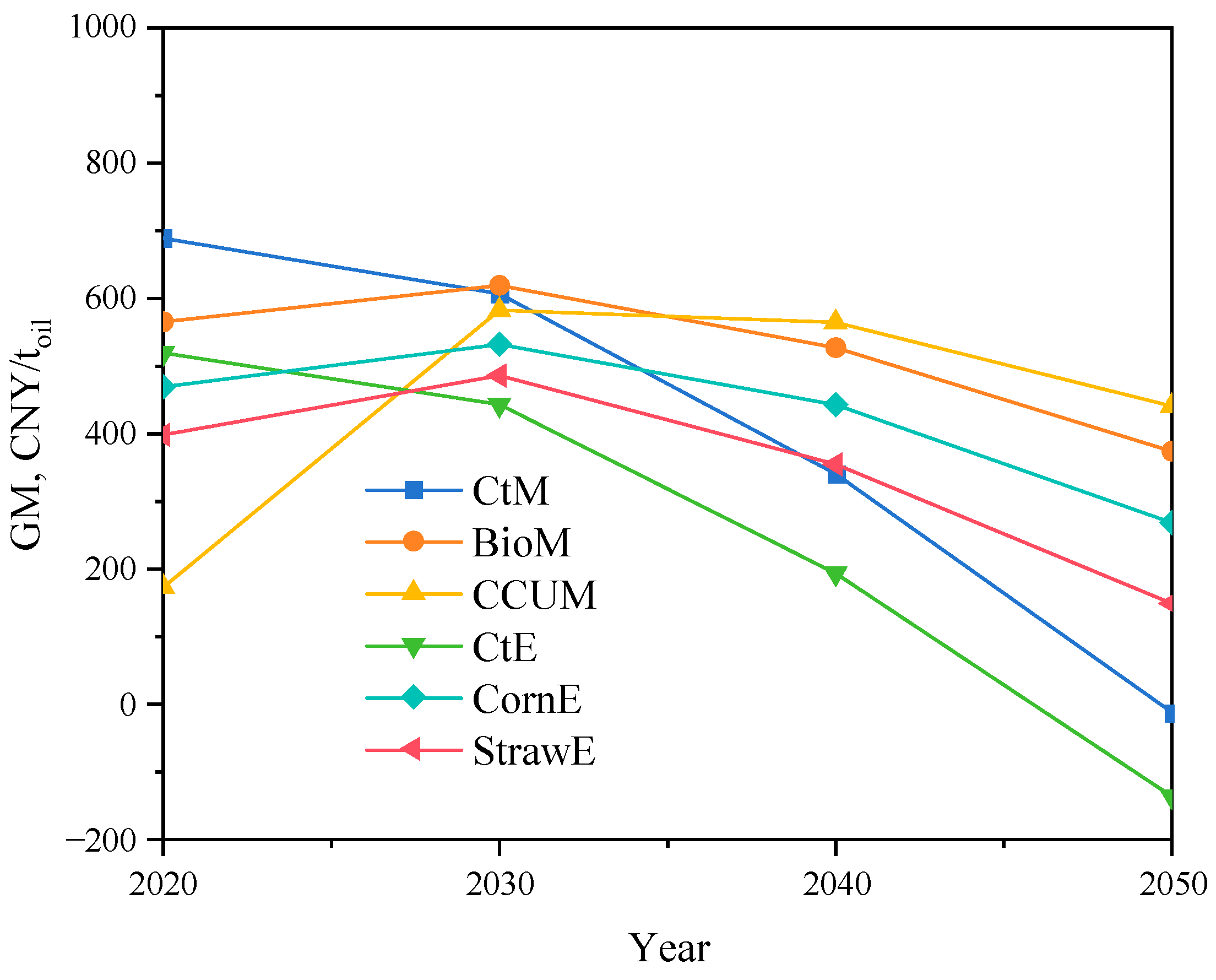
3.4. Future Trend Analysis
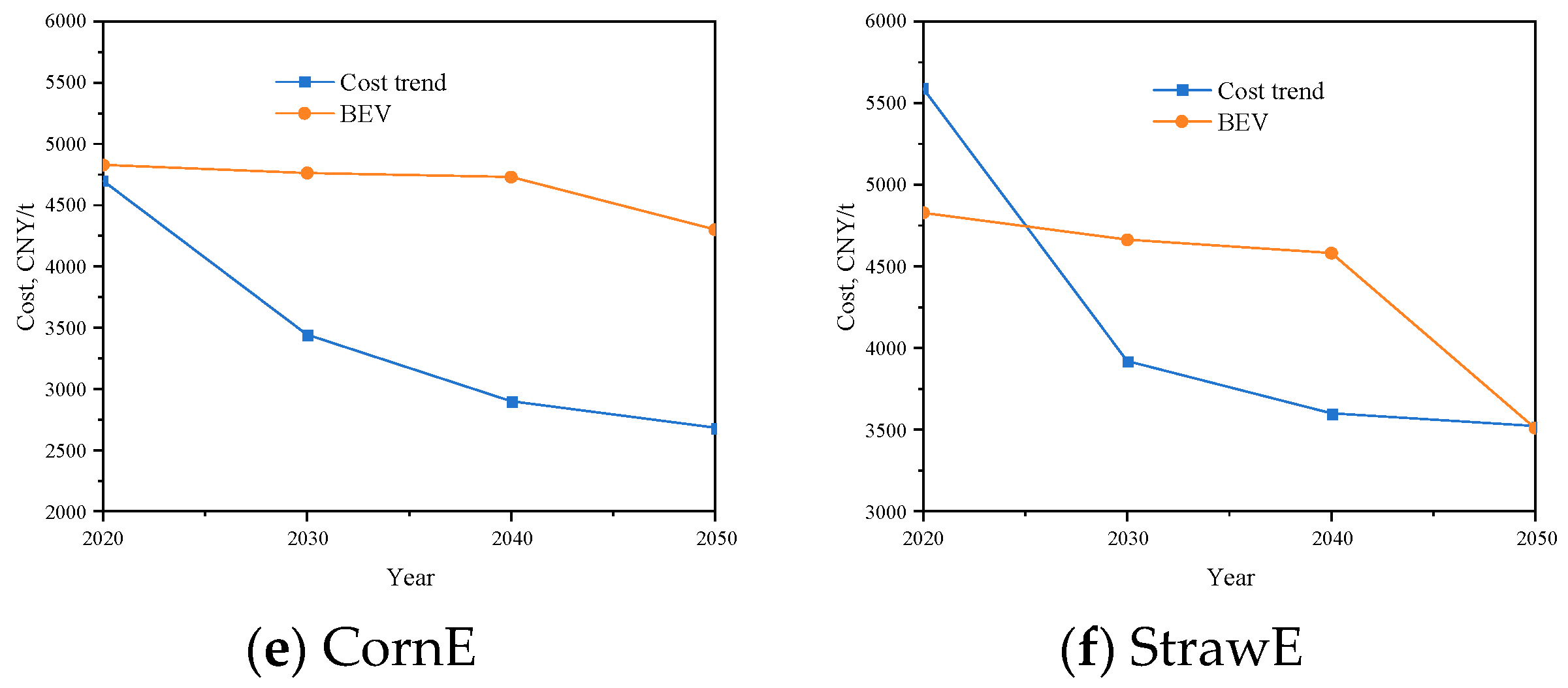
3.4.1. Cost Trend of Alcohols
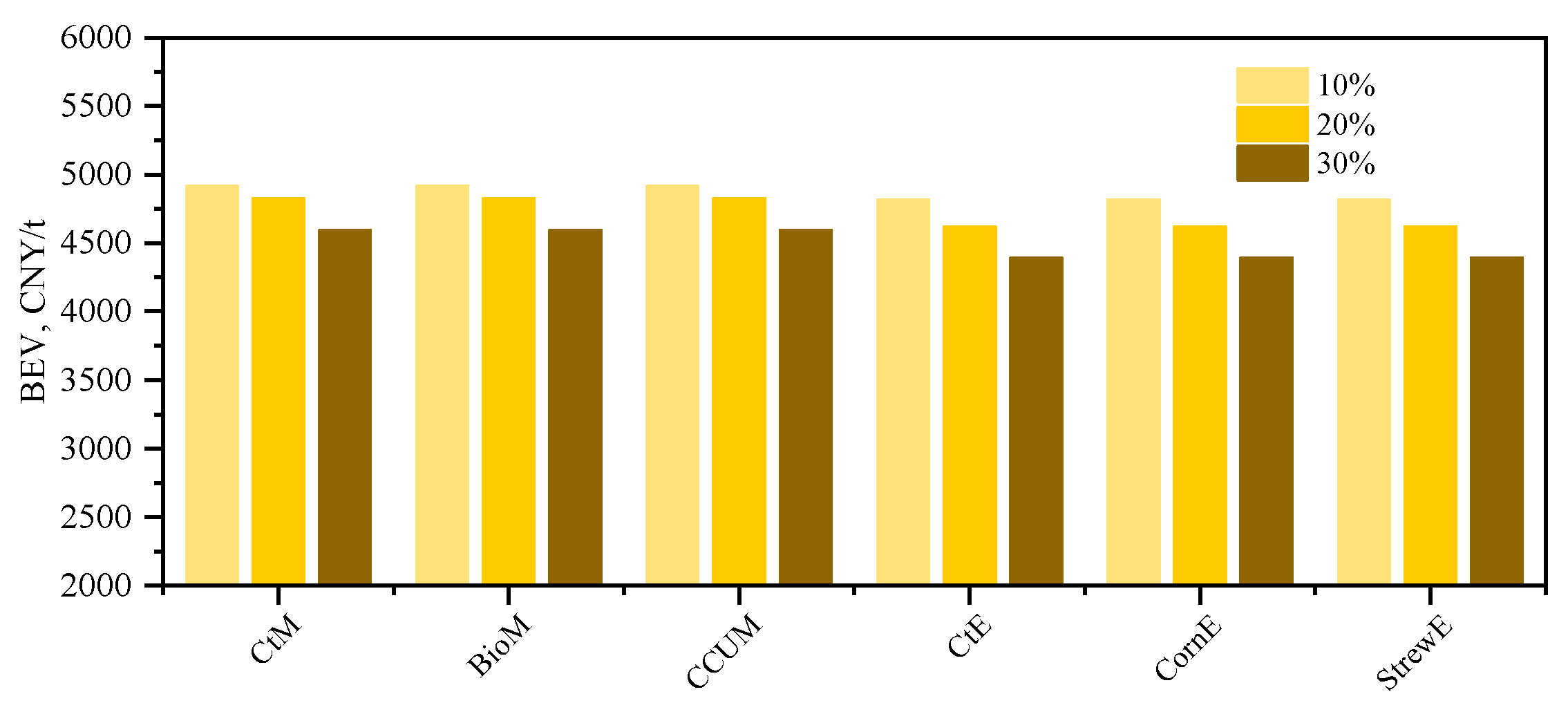
3.4.2. Estimating the Break-Even Value
3.5. Outlook
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| AI | Artificial intelligence |
| ANNs | Artificial neural networks |
| BioM | BioMeOH |
| CBFE | Coal-based fuel EtOH |
| CBFE | Coal-based fuel EtOH |
| CCUM | MeOH from carbon capture and usage |
| CF | Carbon footprint |
| CornE | Corn to EtOH |
| CtE | Coal-based EtOH |
| CtM | Coal-based MeOH |
| E15 | 15% EtOH |
| EIA | Energy information administration |
| ELM | Extreme Learning Machine |
| FGFB | First-generation fuel bioEtOH |
| GA | Genetic algorithms |
| GCM | Group contribution method |
| GM | Gross margin |
| LP-ELM | A model framework integrating LP model and ELM model |
| M15 | 15% MeOH |
| MAE | Mean absolute error |
| MeOH | MeOH |
| MON | Motor octane number |
| MSE | Mean squared error |
| MTBE | Methyl tert-butyl ether |
| R2 | Coefficient of determination |
| RF | Random forest |
| RMSE | Root mean squared error |
| RON | Research octane number |
| SGFB | Second-generation fuel bioEtOH |
| SLFN | Single-layer feedforward network |
| StrawE | Straw to EtOH |
| TAN | Total acid number |
| WTI | West Texas intermediate |
References
- Kloth, M. Transport Demand Set to Triple, But Sector Faces Potential Disruptions. Available online: https://www.itf-oecd.org/transport-demand-set-triple-sector-faces-potential-disruptions (accessed on 2 July 2024).
- Liu, J.; Li, S.; Ji, Q. Regional differences and driving factors analysis of carbon emission intensity from transport sector in China. Energy 2021, 224, 120178. [Google Scholar] [CrossRef]
- Agency, I.E. Global EV Outlook 2024; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Oh, J.J. The 500-Million-Vehicle Question: What Will It Take for China to Decarbonize Transport? Available online: https://blogs.worldbank.org/en/transport/500-million-vehicle-question-what-will-it-take-china-decarbonize-transport#_ftn1 (accessed on 2 July 2024).
- Yu, X.; Sandhu, N.S.; Yang, Z.; Zheng, M. Suitability of energy sources for automotive application–A review. Appl. Energy 2020, 271, 115169. [Google Scholar] [CrossRef]
- Liu, Y.; Cruz-Morales, P.; Zargar, A.; Belcher, M.S.; Pang, B.; Englund, E.; Dan, Q.; Yin, K.; Keasling, J.D. Biofuels for a sustainable future. Cell 2021, 184, 1636–1647. [Google Scholar] [CrossRef]
- Kenkel, P.; Wassermann, T.; Zondervan, E. Renewable Fuels from Integrated Power- and Biomass-to-X Processes: A Superstructure Optimization Study. Processes 2022, 10, 1298. [Google Scholar] [CrossRef]
- Montoya Sánchez, N.; Link, F.; Chauhan, G.; Halmenschlager, C.; El-Sayed, H.E.; Sehdev, R.; Lehoux, R.; de Klerk, A. Conversion of waste to sustainable aviation fuel via Fischer–Tropsch synthesis: Front-end design decisions. Energy Sci. Eng. 2022, 10, 1763–1789. [Google Scholar] [CrossRef]
- Ampah, J.D.; Liu, X.; Sun, X.; Pan, X.; Xu, L.; Jin, C.; Sun, T.; Geng, Z.; Afrane, S.; Liu, H. Study on characteristics of marine heavy fuel oil and low carbon alcohol blended fuels at different temperatures. Fuel 2022, 310, 122307. [Google Scholar] [CrossRef]
- Li, C.; Negnevitsky, M.; Wang, X. Prospective assessment of methanol vehicles in China using FANP-SWOT analysis. Transp. Policy 2020, 96, 60–75. [Google Scholar] [CrossRef]
- Iliev, S. A Comparison of Ethanol, Methanol, and Butanol Blending with Gasoline and Its Effect on Engine Performance and Emissions Using Engine Simulation. Processes 2021, 9, 1322. [Google Scholar] [CrossRef]
- Liu, W.; Shadloo, M.S.; Tlili, I.; Maleki, A.; Bach, Q.-V. The effect of alcohol–gasoline fuel blends on the engines’ performances and emissions. Fuel 2020, 276, 117977. [Google Scholar] [CrossRef]
- Fan, Q.; Liu, S.; Qi, Y.; Cai, K.; Wang, Z. Investigation into ethanol effects on combustion and particle number emissions in a spark-ignition to compression-ignition (SICI) engine. Energy 2021, 233, 121170. [Google Scholar] [CrossRef]
- Likhanov, V.; Lopatin, O. Study of toxicity of diesel engine on alcohol fuel. IOP Conf. Ser. Earth Environ. Sci. 2020, 421, 072018. [Google Scholar] [CrossRef]
- Çelebi, Y.; Aydın, H. An overview on the light alcohol fuels in diesel engines. Fuel 2019, 236, 890–911. [Google Scholar] [CrossRef]
- Balki, M.K.; Sayin, C.; Canakci, M. The effect of different alcohol fuels on the performance, emission and combustion characteristics of a gasoline engine. Fuel 2014, 115, 901–906. [Google Scholar] [CrossRef]
- Eyidogan, M.; Ozsezen, A.N.; Canakci, M.; Turkcan, A. Impact of alcohol–gasoline fuel blends on the performance and combustion characteristics of an SI engine. Fuel 2010, 89, 2713–2720. [Google Scholar] [CrossRef]
- Göktaş, M.; Balki, M.K.; Sayin, C.; Canakci, M. An evaluation of the use of alcohol fuels in SI engines in terms of performance, emission and combustion characteristics: A review. Fuel 2021, 286, 119425. [Google Scholar] [CrossRef]
- Li, C.; Bai, H.; Lu, Y.; Bian, J.; Dong, Y.; Xu, H. Life-cycle assessment for coal-based methanol production in China. J. Clean. Prod. 2018, 188, 1004–1017. [Google Scholar] [CrossRef]
- Blumberg, T.; Tsatsaronis, G.; Morosuk, T. On the economics of methanol production from natural gas. Fuel 2019, 256, 115824. [Google Scholar] [CrossRef]
- Collodi, G.; Azzaro, G.; Ferrari, N.; Santos, S. Demonstrating large scale industrial CCS through CCU–a case study for methanol production. Energy Procedia 2017, 114, 122–138. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Rumayor, M.; Dominguez-Ramos, A.; Irabien, A. Innovative alternatives to methanol manufacture: Carbon footprint assessment. J. Clean. Prod. 2019, 225, 426–434. [Google Scholar] [CrossRef]
- Esmaeili, S.A.H.; Sobhani, A.; Szmerekovsky, J.; Dybing, A.; Pourhashem, G. First-generation vs. second-generation: A market incentives analysis for bioethanol supply chains with carbon policies. Appl. Energy 2020, 277, 115606. [Google Scholar] [CrossRef]
- Geddes, C.C.; Nieves, I.U.; Ingram, L.O. Advances in ethanol production. Curr. Opin. Biotechnol. 2011, 22, 312–319. [Google Scholar] [CrossRef][Green Version]
- Cardona, C.A.; Sánchez, Ó.J. Fuel ethanol production: Process design trends and integration opportunities. Bioresour. Technol. 2007, 98, 2415–2457. [Google Scholar] [CrossRef]
- Mcgrath, C. Biofuels Annual. Beijing. 2020. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/DownloadReportByFileName?fileName=Biofuels%20Annual_Beijing_China%20-%20People%27s%20Republic%20of_08-16-2021.pdf (accessed on 1 May 2024).
- Pereira, L.G.; Cavalett, O.; Bonomi, A.; Zhang, Y.; Warner, E.; Chum, H.L. Comparison of biofuel life-cycle GHG emissions assessment tools: The case studies of ethanol produced from sugarcane, corn, and wheat. Renew. Sustain. Energy Rev. 2019, 110, 1–12. [Google Scholar] [CrossRef]
- Wang, X.; Guo, L.; Lv, J.; Li, M.; Huang, S.; Wang, Y.; Ma, X. Process design, modeling and life cycle analysis of energy consumption and GHG emission for jet fuel production from bioethanol in China. J. Clean. Prod. 2023, 389, 136027. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W. Comparison of life-cycle energy consumption, carbon emissions and economic costs of coal to ethanol and bioethanol. Appl. Energy 2020, 277, 115574. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Soleymani Angili, T.; Grzesik, K.; Rödl, A.; Kaltschmitt, M. Life Cycle Assessment of Bioethanol Production: A Review of Feedstock, Technology and Methodology. Energies 2021, 14, 2939. [Google Scholar] [CrossRef]
- Barcelos, C.A.; Maeda, R.N.; Santa Anna, L.M.M.; Pereira, N., Jr. Sweet sorghum as a whole-crop feedstock for ethanol production. Biomass Bioenergy 2016, 94, 46–56. [Google Scholar] [CrossRef]
- Ali, S.S.; Ali, S.S.; Tabassum, N. A review on CO2 hydrogenation to ethanol: Reaction mechanism and experimental studies. J. Environ. Chem. Eng. 2022, 10, 106962. [Google Scholar] [CrossRef]
- Kusama, H.; Okabe, K.; Sayama, K.; Arakawa, H. CO2 hydrogenation to ethanol over promoted Rh/SiO2 catalysts. Catal. Today 1996, 28, 261–266. [Google Scholar] [CrossRef]
- Zhen, X.; Wang, Y. An overview of methanol as an internal combustion engine fuel. Renew. Sustain. Energy Rev. 2015, 52, 477–493. [Google Scholar] [CrossRef]
- Schrader, J.; Schilling, M.; Holtmann, D.; Sell, D.; Villela Filho, M.; Marx, A.; Vorholt, J.A. Methanol-based industrial biotechnology: Current status and future perspectives of methylotrophic bacteria. Trends Biotechnol. 2009, 27, 107–115. [Google Scholar] [CrossRef]
- Assaf, J.C.; Mortada, Z.; Rezzoug, S.-A.; Maache-Rezzoug, Z.; Debs, E.; Louka, N. Comparative Review on the Production and Purification of Bioethanol from Biomass: A Focus on Corn. Processes 2024, 12, 1001. [Google Scholar] [CrossRef]
- Elfasakhany, A. Comparative Analysis of the Engine Performance and Emissions Characteristics Powered by Various Ethanol–Butanol–Gasoline Blends. Processes 2023, 11, 1264. [Google Scholar] [CrossRef]
- Li, S.H.; Wen, Z.; Hou, J.; Xi, S.; Fang, P.; Guo, X.; Li, Y.; Wang, Z.; Li, S. Effects of Ethanol and Methanol on the Combustion Characteristics of Gasoline with the Revised Variation Disturbance Method. ACS Omega 2022, 7, 17797–17810. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Shukla, P.C.; Gupta, J.G.; Patel, C.; Prasad, R.K.; Sharma, N. Unregulated emissions from a gasohol (E5, E15, M5, and M15) fuelled spark ignition engine. Appl. Energy 2015, 154, 732–741. [Google Scholar] [CrossRef]
- Khor, C.S.; Varvarezos, D. Petroleum refinery optimization. Optim. Eng. 2016, 18, 943–989. [Google Scholar] [CrossRef]
- Carlson, N.A.; Singh, A.; Talmadge, M.S.; Jiang, Y.; Zaimes, G.G.; Li, S.; Hawkins, T.R.; Sittler, L.; Brooker, A.; Gaspar, D.J.; et al. Economic analysis of the benefits to petroleum refiners for low carbon boosted spark ignition biofuels. Fuel 2023, 334, 126183. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, F.; Liu, J.; Duan, X. Prediction of gasoline research octane number using multiple feature machine learning models. Fuel 2023, 333, 126510. [Google Scholar] [CrossRef]
- Al Ibrahim, E.; Farooq, A. Octane Prediction from Infrared Spectroscopic Data. Energy Fuels 2019, 34, 817–826. [Google Scholar] [CrossRef]
- Wang, X.; Yang, K.; Kalivas, J.H. Comparison of extreme learning machine models for gasoline octane number forecasting by near-infrared spectra analysis. Optik 2020, 200, 163325. [Google Scholar] [CrossRef]
- Li, R.; Herreros, J.M.; Tsolakis, A.; Yang, W. Machine learning regression based group contribution method for cetane and octane numbers prediction of pure fuel compounds and mixtures. Fuel 2020, 280, 118589. [Google Scholar] [CrossRef]
- Zhang, F.; Su, X.; Tan, A.; Yao, J.; Li, H. Prediction of research octane number loss and sulfur content in gasoline refining using machine learning. Energy 2022, 261, 124823. [Google Scholar] [CrossRef]
- Alboqami, F.; van Oudenhoven, V.C.O.; Ahmed, U.; Zahid, U.; Emwas, A.-H.; Sarathy, S.M.; Abdul Jameel, A.G. A Methodology for Designing Octane Number of Fuels Using Genetic Algorithms and Artificial Neural Networks. Energy Fuels 2022, 36, 3867–3880. [Google Scholar] [CrossRef]
- Wang, H.; Chu, X.; Chen, P.; Li, J.; Liu, D.; Xu, Y. Partial least squares regression residual extreme learning machine (PLSRR-ELM) calibration algorithm applied in fast determination of gasoline octane number with near-infrared spectroscopy. Fuel 2022, 309, 122224. [Google Scholar] [CrossRef]
- Shi, X.; Wang, G.; Wang, X.; Chen, B. A Study on the Promoting Role of Renewable Hydrogen in the Transformation of Petroleum Refining Pathways. Processes 2024, 12, 1317. [Google Scholar] [CrossRef]
- Jiang, Y.; Zaimes, G.G.; Li, S.; Hawkins, T.R.; Singh, A.; Carlson, N.; Talmadge, M.; Gaspar, D.J.; Ramirez-Corredores, M.M.; Beck, A.W.; et al. Economic and environmental analysis to evaluate the potential value of co-optima diesel bioblendstocks to petroleum refiners. Fuel 2023, 333, 126233. [Google Scholar] [CrossRef]
- GB 17930-2016; Gasoline for Motor Vehicles. Standardization Administration of China: Beijing, China, 2017.
- GB 6537-2018; No. 3 Jet Fuel. Standardization Administration of China: Beijing, China, 2018.
- GB 19147-2016; Automobile Diesel Fuels. Standardization Administration of China: Beijing, China, 2016.
- EIA. Petroleum &Others liquids spot price. Available online: https://www.eia.gov/dnav/pet/PET_PRI_SPT_S1_D.htm (accessed on 28 May 2024).
- Khani, L.; Mohammadpourfard, M. Investigation of a New Methanol, Hydrogen, and Electricity Production System Based on Carbon Capture and Utilization. In Energy Systems Transition: Digitalization, Decarbonization, Decentralization and Democratization; Vahidinasab, V., Mohammadi-Ivatloo, B., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 87–129. [Google Scholar]
- EIA. Refiner petroleum product prices by sales type. Available online: https://www.eia.gov/dnav/pet/pet_pri_refoth_dcu_nus_m.htm (accessed on 27 May 2024).
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol fuel production, utilization, and techno-economy: A review. Environ. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Yang, Y.; Zhang, X.; Wang, N.; Zheng, Y.; Tian, Y.; Xie, K. Life cycle assessment and techno-economic analysis of ethanol production via coal and its competitors: A comparative study. Appl. Energy 2022, 312, 118791. [Google Scholar] [CrossRef]
- Duarte, A.; Uribe, J.C.; Sarache, W.; Calderón, A. Economic, environmental, and social assessment of bioethanol production using multiple coffee crop residues. Energy 2021, 216, 119170. [Google Scholar] [CrossRef]
- Han, Y.; Shi, K.; Qian, Y.; Yang, S. Design and operational optimization of a methanol-integrated wind-solar power generation system. J. Environ. Chem. Eng. 2023, 11, 109992. [Google Scholar] [CrossRef]
- Li, X.; Zhou, B.; Jin, W.; Deng, H. A Comprehensive Assessment of the Carbon Footprint of the Coal-to-Methanol Process Coupled with Carbon Capture-, Utilization-, and Storage-Enhanced Oil Recovery Technology. Sustainability 2024, 16, 3573. [Google Scholar] [CrossRef]
- Mousazadeh, F.; Naeem, M.H.T.; Daneshfar, R.; Soulgani, B.S.; Naseri, M. Predicting the condensate viscosity near the wellbore by ELM and ANFIS-PSO strategies. J. Pet. Sci. Eng. 2021, 204, 108708. [Google Scholar] [CrossRef]
- Chen, H.; Xu, M.-l.; Guo, Q.; Yang, L.; Ma, Y. A review on present situation and development of biofuels in China. J. Energy Inst. 2016, 89, 248–255. [Google Scholar] [CrossRef]
- Dahmen, N.; Abeln, J.; Eberhard, M.; Kolb, T.; Leibold, H.; Sauer, J.; Stapf, D.; Zimmerlin, B. The bioliq process for producing synthetic transportation fuels. Wiley Interdiscip. Rev. Energy Environ. 2017, 6, e236. [Google Scholar] [CrossRef]
- IRENA. Innovation Outlook: Renewable Methanol. Abu Dhabi. 2021. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2021/Jan/IRENA_Innovation_Renewable_Methanol_2021.pdf (accessed on 1 May 2024).
- Wei, J.; Zhao, D.; Liang, L. Estimating the growth models of news stories on disasters. J. Am. Soc. Inf. Sci. Technol. 2009, 60, 1741–1755. [Google Scholar] [CrossRef]
- Zhen, Z.; Wang, Y.; Wang, Y.; Wang, X.; Ou, X.; Zhou, S. Hydrogen production paths in China based on learning curve and discrete choice model. J. Clean. Prod. 2023, 415, 137848. [Google Scholar] [CrossRef]
- Burnes, D.; Camou, A. Impact of fuel composition on gas turbine engine performance. J. Eng. Gas Turbines Power 2019, 141, 101006. [Google Scholar] [CrossRef]
- Niven, R.K. Ethanol in gasoline: Environmental impacts and sustainability review article. Renew. Sustain. Energy Rev. 2005, 9, 535–555. [Google Scholar] [CrossRef]
- Jiao, J.; Li, J.; Bai, Y. Ethanol as a vehicle fuel in China: A review from the perspectives of raw material resource, vehicle, and infrastructure. J. Clean. Prod. 2018, 180, 832–845. [Google Scholar] [CrossRef]
- Li, C.; Jia, T.; Wang, S.; Wang, X.; Negnevitsky, M.; Wang, H.; Hu, Y.; Xu, W.; Zhou, N.; Zhao, G. Methanol Vehicles in China: A Review from a Policy Perspective. Sustainability 2023, 15, 9201. [Google Scholar] [CrossRef]





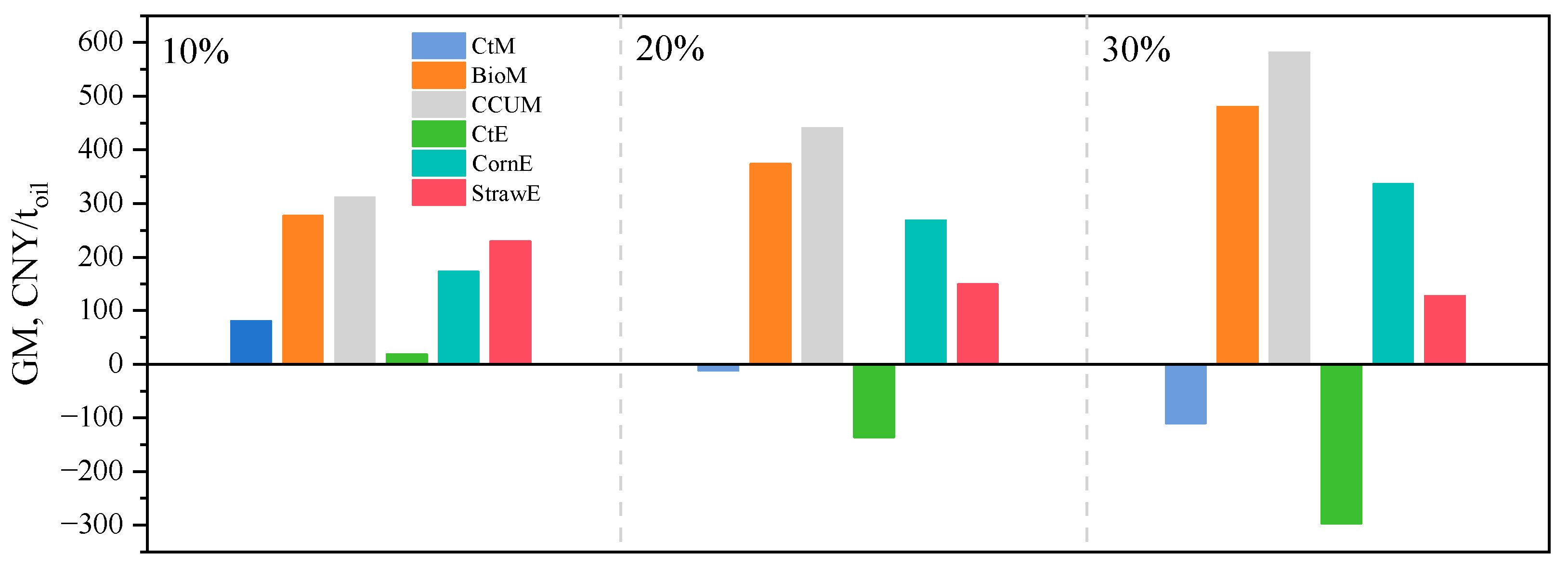
| Study | Method(s) Used | Key Results |
|---|---|---|
| Sun et al. [44] | Random Forest | Achieved a maximum prediction error of 0.46 for Research Octane Number (RON), closely aligning with experimental uncertainty. |
| Ibrahim et al. [45] | Artificial Neural Network (ANN) | Demonstrated effective modeling of hydrocarbon mixtures and gasoline–EtOH blends, capturing nonlinear octane rating relationships using infrared spectroscopy data. |
| Li et al. [47] | Group Contribution Method (GCM) | Successfully predicted RON for both pure fuel compounds and blends |
| Zhang et al. [48] | Robust machine learning | Predicted RON loss and sulfur content during refining with high accuracy and strong generalization. |
| Alboqami et al. [49] | Genetic Algorithms (GA); ANN | Developed an optimized method for predicting fuel octane ratings, effectively minimizing the use of high-octane components. |
| Wang et al. [50] | Extreme Learning Machine | Compared the performance of various ELM approaches, highlighting their effectiveness in different scenarios. |
| Materials | Cost and Price, CNY/t | Carbon Footprint, kgCO2/t |
|---|---|---|
| CtM | 1953 | 6580 |
| BioM | 3500 | 370 |
| CCUM | 8400 | −540 |
| CornE | 4700 | 1020 |
| StrawE | 5588 | 2010 |
| CtE | 4071 | 5880 |
| Saudi Medium Crude Oil | 3480 | —— |
| Gasoline | 4775 | —— |
| Jet fuel | 4767 | —— |
| Diesel | 4281 | —— |
| Aromatics | 6075 | —— |
| LPG | 2787 | —— |
| Petroleum Coke | 1383 | —— |
| Methods | MSE | MAE | RMSE | R2 |
|---|---|---|---|---|
| ELM | 0.0316 | 0.1700 | 0.1783 | 0.8423 |
| Linear blending | 0.0796 | 0.2360 | 0.2821 | 0.6054 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Yu, Z.; Lin, T.; Wu, S.; Fu, Y.; Chen, B. Future Prospects of MeOH and EtOH Blending in Gasoline: A Comparative Study on Fossil, Biomass, and Renewable Energy Sources Considering Economic and Environmental Factors. Processes 2024, 12, 1751. https://doi.org/10.3390/pr12081751
Shi X, Yu Z, Lin T, Wu S, Fu Y, Chen B. Future Prospects of MeOH and EtOH Blending in Gasoline: A Comparative Study on Fossil, Biomass, and Renewable Energy Sources Considering Economic and Environmental Factors. Processes. 2024; 12(8):1751. https://doi.org/10.3390/pr12081751
Chicago/Turabian StyleShi, Xiaofei, Zihao Yu, Tangmao Lin, Sikan Wu, Yujiang Fu, and Bo Chen. 2024. "Future Prospects of MeOH and EtOH Blending in Gasoline: A Comparative Study on Fossil, Biomass, and Renewable Energy Sources Considering Economic and Environmental Factors" Processes 12, no. 8: 1751. https://doi.org/10.3390/pr12081751
APA StyleShi, X., Yu, Z., Lin, T., Wu, S., Fu, Y., & Chen, B. (2024). Future Prospects of MeOH and EtOH Blending in Gasoline: A Comparative Study on Fossil, Biomass, and Renewable Energy Sources Considering Economic and Environmental Factors. Processes, 12(8), 1751. https://doi.org/10.3390/pr12081751







