Hydrogen-Rich Syngas Production from Waste Textile Gasification Coupling with Catalytic Reforming under Steam Atmosphere
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
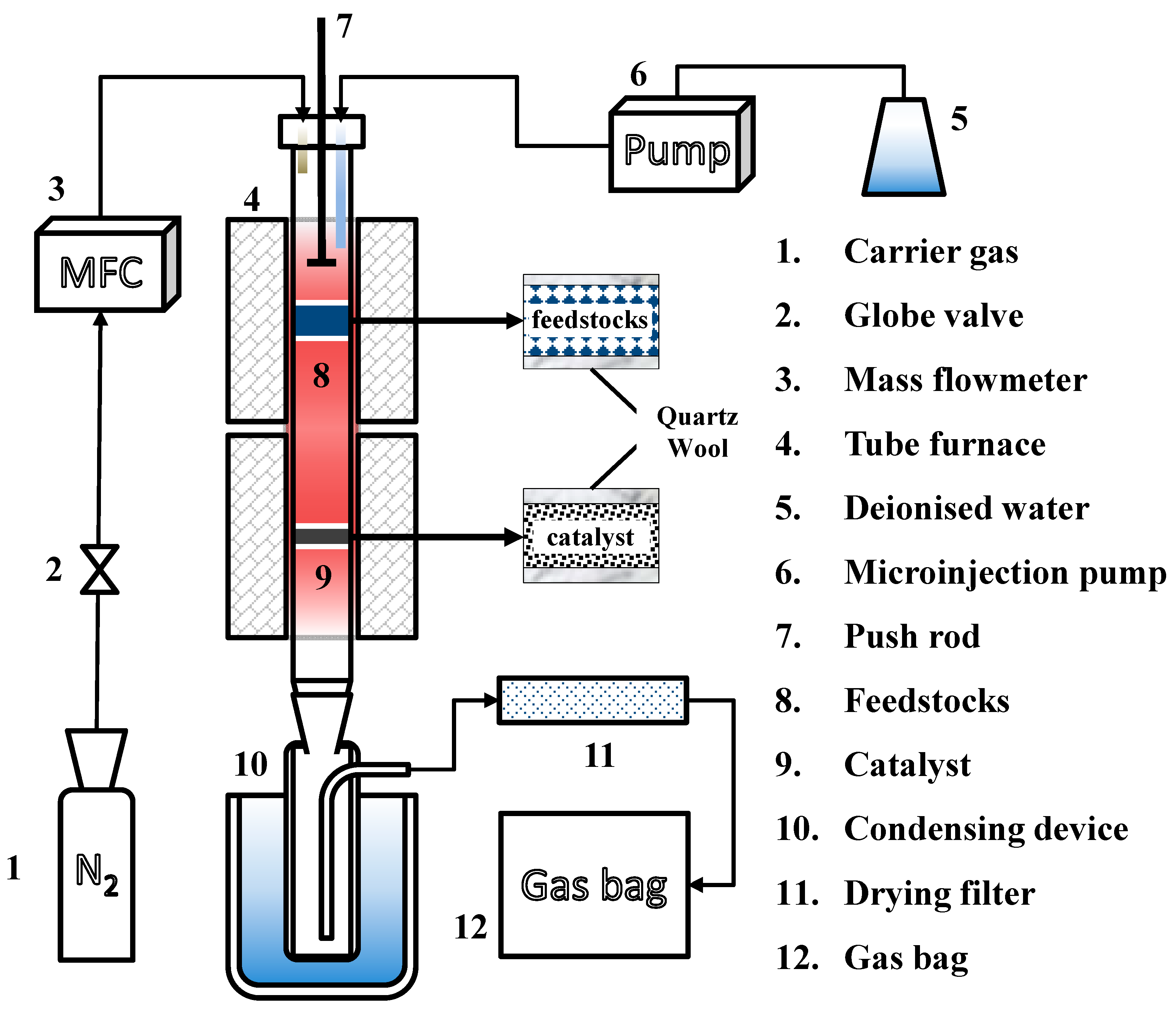
2.3. Experimental Setup and Procedure
2.4. Characterization
2.5. Data Analysis
3. Results and Discussion
3.1. Characterization of Fresh Catalyst
3.2. Performance of Waste Textile Gasification
3.2.1. Performance of Gasification under Steam Atmosphere
3.2.2. Performance of Catalytic Gasification under Steam Atmosphere
3.3. Mechanism
3.3.1. Mechanism of Gasification under Steam Atmosphere
3.3.2. Mechanism of Gasification Coupling with Catalytic Reforming under Steam Atmosphere
- (1)
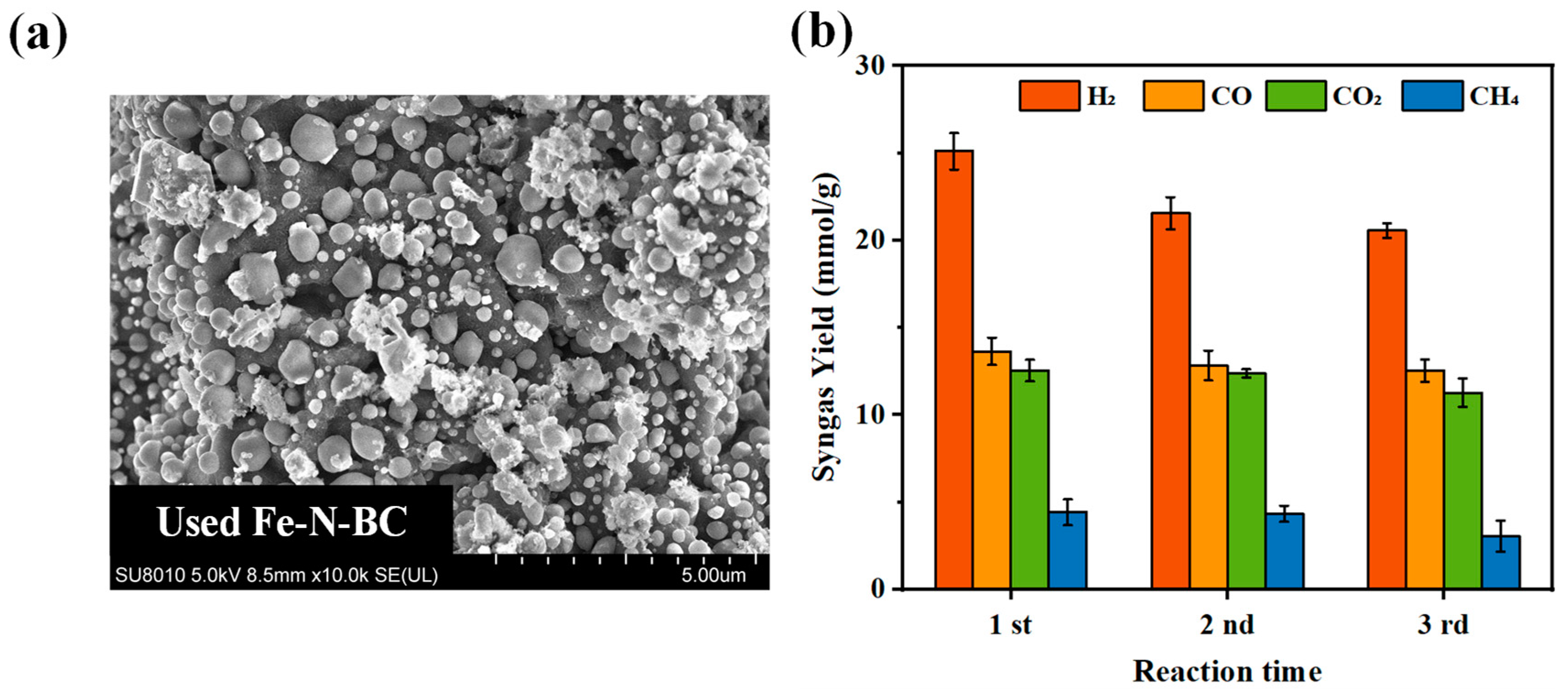
- Characterization and performance of used Fe-N-BC
- (2)
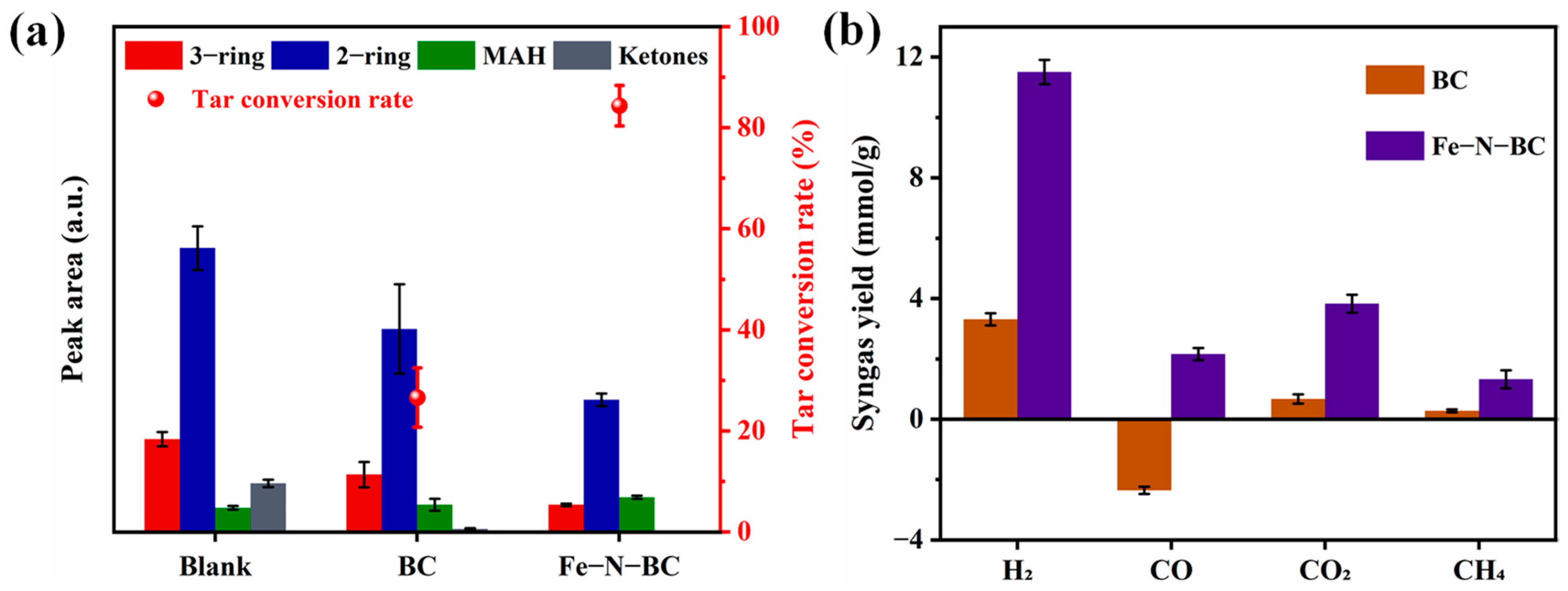
- Tar production and actual catalytic effect
- (3)
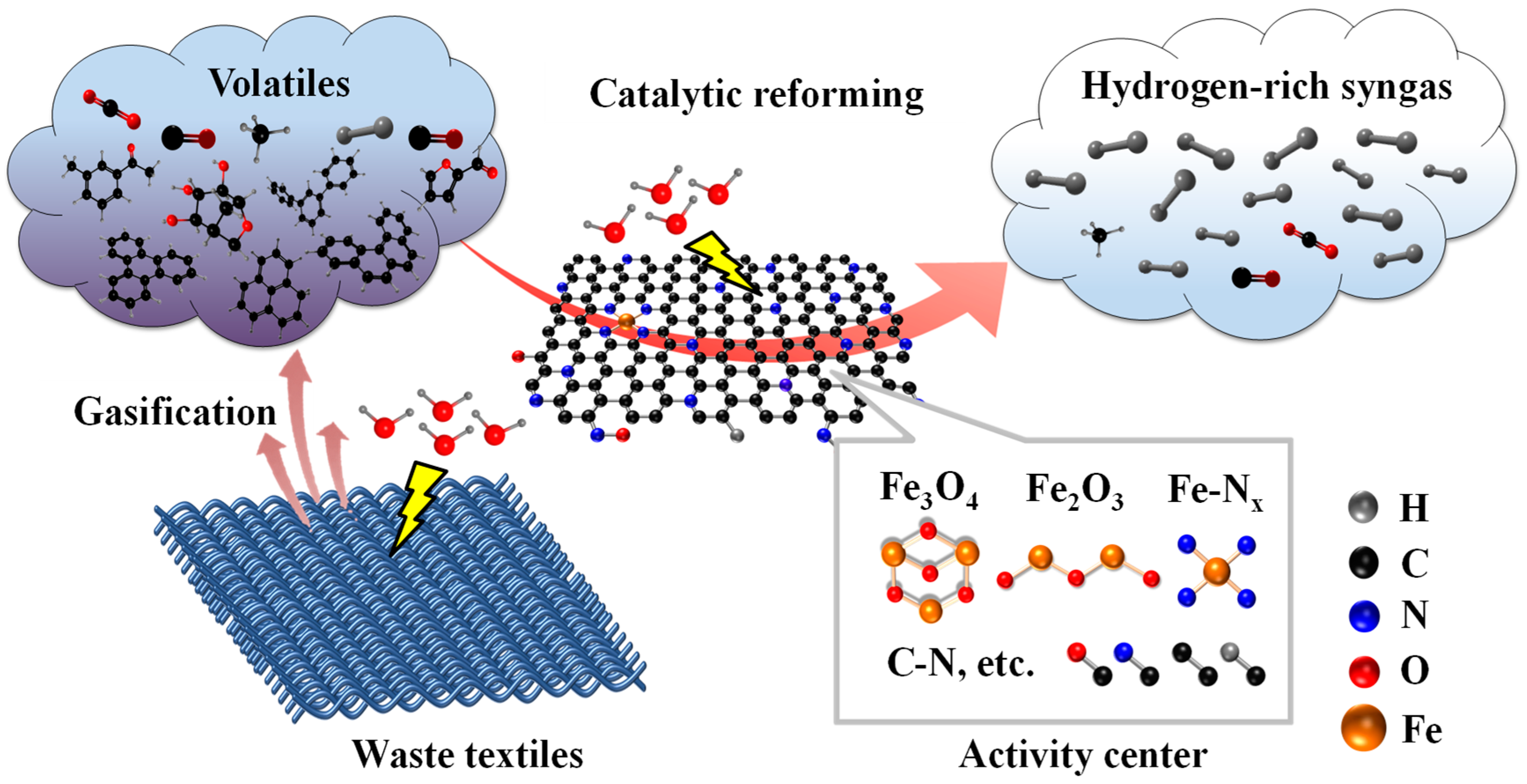
- Mechanism of gasification coupling with catalytic reforming under steam atmosphere
3.4. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef]
- Andini, E.; Bhalode, P.; Gantert, E.; Sadula, S.; Vlachos, D.G. Chemical recycling of mixed textile waste. Sci. Adv. 2024, 10, eado6827. [Google Scholar] [CrossRef]
- Fan, W.; Wang, Y.; Liu, R.; Zou, J.; Yu, X.; Liu, Y.; Zhi, C.; Meng, J. Textile production by additive manufacturing and textile waste recycling: A review. Environ. Chem. Lett. 2024, 22, 1929–1987. [Google Scholar] [CrossRef]
- Franco, M.A. Circular economy at the micro level: A dynamic view of incumbents’ struggles and challenges in the textile industry. J. Clean. Prod. 2017, 168, 833–845. [Google Scholar] [CrossRef]
- Haslinger, S.; Hietala, S.; Hummel, M.; Maunu, S.L.; Sixta, H. Solid-state NMR method for the quantification of cellulose and polyester in textile blends. Carbohydr. Polym. 2019, 207, 11–16. [Google Scholar] [CrossRef]
- Wang, S.; Salmon, S. Progress toward Circularity of Polyester and Cotton Textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Subramanian, K.; Sarkar, M.K.; Wang, H.; Qin, Z.-H.; Chopra, S.S.; Jin, M.; Kumar, V.; Chen, C.; Tsang, C.-W.; Lin, C.S.K. An overview of cotton and polyester, and their blended waste textile valorisation to value-added products: A circular economy approach—Research trends, opportunities and challenges. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3921–3942. [Google Scholar] [CrossRef]
- Ribul, M.; Lanot, A.; Tommencioni Pisapia, C.; Purnell, P.; McQueen-Mason, S.J.; Baurley, S. Mechanical, chemical, biological: Moving towards closed-loop bio-based recycling in a circular economy of sustainable textiles. J. Clean. Prod. 2021, 326, 129325. [Google Scholar] [CrossRef]
- Kalfas, G.A. Mathematical Modeling of the Depolymerization of Polyamide Mixtures—Part I: Kinetic Mechanism and Parametric Studies in Batch Reactors. Polym. React. Eng. 1998, 6, 41–67. [Google Scholar] [CrossRef]
- Sert, E.; Yılmaz, E.; Atalay, F.S. Chemical Recycling of Polyethlylene Terephthalate by Glycolysis Using Deep Eutectic Solvents. J. Polym. Environ. 2019, 27, 2956–2962. [Google Scholar] [CrossRef]
- Lee, N.; Joo, J.; Lin, K.-Y.A.; Lee, J. Thermochemical conversion of mulching film waste via pyrolysis with the addition of cattle excreta. J. Environ. Chem. Eng. 2021, 9, 106362. [Google Scholar] [CrossRef]
- Athanasopoulos, P.; Zabaniotou, A. Post-consumer textile thermochemical recycling to fuels and biocarbon: A critical review. Sci. Total Environ. 2022, 834, 155387. [Google Scholar] [CrossRef]
- Zhong, D.; Chang, Z.; Zeng, K.; Li, J.; Qiu, Y.; Lu, Q.; Flamant, G.; Yang, H.; Chen, H. Solar pyrolysis of biomass—Part II: The physicochemical structure evolution of char. Fuel 2023, 333, 126474. [Google Scholar] [CrossRef]
- Hu, J.; Shao, J.; Yang, H.; Lin, G.; Chen, Y.; Wang, X.; Zhang, W.; Chen, H. Co-gasification of coal and biomass: Synergy, characterization and reactivity of the residual char. Bioresour. Technol. 2017, 244, 1–7. [Google Scholar] [CrossRef]
- Yasin, S.; Curti, M.; Rovero, G.; Hussain, M.; Sun, D. Spouted-Bed Gasification of Flame Retardant Textiles as a Potential Non-Conventional Biomass. Appl. Sci. 2020, 10, 946. [Google Scholar] [CrossRef]
- Yek, P.N.Y.; Chan, Y.H.; Foong, S.Y.; Mahari, W.A.W.; Chen, X.; Liew, R.K.; Ma, N.L.; Tsang, Y.F.; Sonne, C.; Cheng, Y.W.; et al. Co-processing plastics waste and biomass by pyrolysis–gasification: A review. Environ. Chem. Lett. 2024, 22, 171–188. [Google Scholar] [CrossRef]
- Zhong, M.; Li, J.; Zhou, L.; Wang, T.; Liu, J.; Mei, M.; Chen, S. Co-pyrolysis of cellulose and polyethylene terephthalate by TG-MS: Pyrolysis behavior, conventional gas and solid phase product characteristics. J. Anal. Appl. Pyrolysis 2023, 172, 106002. [Google Scholar] [CrossRef]
- Zou, X.; Zhai, M.; Liu, G.; Guo, L.; Zhang, Y.; Wang, X. Microdynamics of biomass steam gasification: A review. Energy Convers. Manag. 2024, 306, 118274. [Google Scholar] [CrossRef]
- Feng, L.; Dong, M.; Qin, B.; Pang, J.; Babaee, S. H2 production enhancement in underground coal gasification with steam addition: Effect of injection conditions. Energy 2024, 291, 130379. [Google Scholar] [CrossRef]
- Wu, Y.; Wen, C.; Chen, X.; Jiang, G.; Liu, G.; Liu, D. Catalytic pyrolysis and gasification of waste textile under carbon dioxide atmosphere with composite Zn-Fe catalyst. Fuel Process. Technol. 2017, 166, 115–123. [Google Scholar] [CrossRef]
- Wen, C.; Wu, Y.; Chen, X.; Jiang, G.; Liu, D. The pyrolysis and gasification performances of waste textile under carbon dioxide atmosphere. J. Therm. Anal. Calorim. 2017, 128, 581–591. [Google Scholar] [CrossRef]
- Vela, I.C.; Maric, J.; Seemann, M. Valorisation of textile waste via steam gasification in a fluidized bed reactor. In Proceedings of the HERAKLION 2019 7th International Conference on Sustainable Solid Waste Management, Crete, Greece, 26 June 2019. [Google Scholar]
- Song, H.; Yang, G.; Xue, P.; Li, Y.; Zou, J.; Wang, S.; Yang, H.; Chen, H. Recent development of biomass gasification for H2 rich gas production. Appl. Energy Combust. Sci. 2022, 10, 100059. [Google Scholar] [CrossRef]
- Chan, F.L.; Tanksale, A. Catalytic steam gasification of pinewood and eucalyptus sawdust using reactive flash volatilization. Appl. Catal. B Environ. 2016, 187, 310–327. [Google Scholar] [CrossRef]
- Qin, T.; Yuan, S. Research progress of catalysts for catalytic steam reforming of high temperature tar:A review. Fuel 2023, 331, 125790. [Google Scholar] [CrossRef]
- Xue, Q.; Li, Z.; Jiang, Z.; Chen, M.; Yan, B.; Wang, Y.; Luo, G.J.F. Effect of characteristic component on diesel steam reforming to hydrogen over highly dispersed Ni–Rh-and Ni-based catalysts: Experiment and DFT calculation study. Fuel 2021, 303, 121306. [Google Scholar] [CrossRef]
- Liu, Q.; Xiong, Z.; Syed-Hassan, S.S.A.; Deng, Z.; Zhao, X.; Su, S.; Xiang, J.; Wang, Y.; Hu, S. Effect of the pre-reforming by Fe/bio-char catalyst on a two-stage catalytic steam reforming of bio-oil. Fuel 2019, 239, 282–289. [Google Scholar] [CrossRef]
- Zhang, Z.; Ou, Z.; Qin, C.; Ran, J.; Wu, C.J.F. Roles of alkali/alkaline earth metals in steam reforming of biomass tar for hydrogen production over perovskite supported Ni catalysts. Fuel 2019, 257, 116032. [Google Scholar] [CrossRef]
- Claude, V.; Mahy, J.; Geens, J.; Lambert, S.J.M.T.C. Ni-doped γ-Al2O3 as secondary catalyst for bio-syngas purification: Influence of Ni loading, catalyst preparation, and gas composition on catalytic activity. Mater. Today Chem. 2019, 13, 98–109. [Google Scholar] [CrossRef]
- Xu, H.; Shen, Z.; Zhang, S.; Chen, G.; Pan, H.; Ge, Z.; Zheng, Z.; Wang, Y.; Wang, Y.; Li, X.J.J.o.C.; et al. Arming wood carbon with carbon-coated mesoporous nickel-silica nanolayer as monolithic composite catalyst for steam reforming of toluene. J. Colloid Interface Sci. 2021, 599, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, L.; Liu, F.; Sun, Y.; Shao, Y.; Sun, K.; Zhang, S.; Liu, Q.; Hu, G.; Hu, X. Tailoring the surface properties of Ni/SiO2 catalyst with sulfuric acid for enhancing the catalytic efficiency for steam reforming of guaiacol. Renew. Energy 2020, 156, 423–439. [Google Scholar] [CrossRef]
- Xu, C.; Du, Z.; Yang, S.; Ma, H.; Feng, J. Effects of inherent potassium on the catalytic performance of Ni/biochar for steam reforming of toluene as a tar model compound. Chin. J. Chem. Eng. 2021, 35, 189–195. [Google Scholar] [CrossRef]
- Farooq, A.; Rhee, G.H.; Lee, I.-H.; Khan, M.A.; Lee, S.H.; Jung, S.-C.; Jeon, B.-H.; Chen, W.-H.; Park, Y.-K. Waste furniture gasification using rice husk based char catalysts for enhanced hydrogen generation. Bioresour. Technol. 2021, 341, 125813. [Google Scholar] [CrossRef]
- Li, Y.; Nahil, M.A.; Williams, P.T. Hydrogen/Syngas Production from Different Types of Waste Plastics Using a Sacrificial Tire Char Catalyst via Pyrolysis–Catalytic Steam Reforming. Energy Fuels 2023, 37, 6661–6673. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.-P.; Zhao, X.-Y.; Liu, Y.-L. Fundamentals and applications of char in biomass tar reforming. Fuel Process. Technol. 2021, 216, 106782. [Google Scholar] [CrossRef]
- Tahir, M.H.; Chen, D. Integrated approach for H2-Rich syngas production from wastes using carbon-based catalysts and subsequent CO2 adsorption by carbon-based adsorbents: A review. Int. J. Hydrogen Energy 2024, 59, 679–696. [Google Scholar] [CrossRef]
- Irfan, M.; Li, A.; Zhang, L.; Ji, G.; Gao, Y.; Khushk, S. Hydrogen-rich syngas from wet municipal solid waste gasification using Ni/Waste marble powder catalyst promoted by transition metals. Waste Manag. 2021, 132, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nejati, B.; Adami, P.; Bozorg, A.; Tavasoli, A.; Mirzahosseini, A.H. Catalytic pyrolysis and bio-products upgrading derived from Chlorella vulgaris over its biochar and activated biochar-supported Fe catalysts. J. Anal. Appl. Pyrolysis 2020, 152, 104799. [Google Scholar] [CrossRef]
- Anto, S.; Sudhakar, M.P.; Shan Ahamed, T.; Samuel, M.S.; Mathimani, T.; Brindhadevi, K.; Pugazhendhi, A. Activation strategies for biochar to use as an efficient catalyst in various applications. Fuel 2021, 285, 119205. [Google Scholar] [CrossRef]
- Yang, G.; Hu, Q.; Hu, J.; Yang, H.; Yan, S.; Chen, Y.; Wang, X.; Chen, H. Hydrogen-rich syngas production from biomass gasification using biochar-based nanocatalysts. Bioresour. Technol. 2023, 379, 129005. [Google Scholar] [CrossRef]
- Hou, C.; Zhao, J.; Yang, L.; Chen, J.; Xia, X.; Zhou, X.; Zhang, Y. A single-atom Fe-N-C catalyst with superior Fenton-like reaction performance prepared facilely using microalgae: Key roles of oxygen and interactions between Fe-Nx and Fe/Fe compounds. Appl. Catal. B Environ. 2023, 339, 123135. [Google Scholar] [CrossRef]
- Fredriksson, H.O.A.; Lancee, R.J.; Thüne, P.C.; Veringa, H.J.; Niemantsverdriet, J.W. Olivine as tar removal catalyst in biomass gasification: Catalyst dynamics under model conditions. Appl. Catal. B Environ. 2013, 130–131, 168–177. [Google Scholar] [CrossRef]
- Nordgreen, T.; Liliedahl, T.; Sjöström, K. Metallic iron as a tar breakdown catalyst related to atmospheric, fluidised bed gasification of biomass. Fuel 2006, 85, 689–694. [Google Scholar] [CrossRef]
- Nordgreen, T.; Liliedahl, T.; Sjöström, K. Elemental Iron as a Tar Breakdown Catalyst in Conjunction with Atmospheric Fluidized Bed Gasification of Biomass: A Thermodynamic Study. Energy Fuels 2006, 20, 890–895. [Google Scholar] [CrossRef]
- Han, J.; Liu, X.; Yue, J.; Xi, B.; Gao, S.; Xu, G. Catalytic Upgrading of in Situ Coal Pyrolysis Tar over Ni-Char Catalyst with Different Additives. Energy Fuels 2014, 28, 4934–4941. [Google Scholar] [CrossRef]
- Xue, N.; Xue, X.; Aihemaiti, A.; Zhu, H.; Yin, J. Atomically Dispersed Ce Sites Augmenting Activity and Durability of Fe-Based Oxygen Reduction Catalyst in PEMFC. Small 2024, 20, e2311034. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Enhancement of persulfate activation by Fe-biochar composites: Synergism of Fe and N-doped biochar. Appl. Catal. B Environ. 2022, 303, 120926. [Google Scholar] [CrossRef]
- Qu, S.; Yuan, Y.; Yang, X.; Xu, H.; Mohamed, A.K.; Zhang, J.; Zhao, C.; Liu, L.; Wang, B.; Wang, X.; et al. Carbon defects in biochar facilitated nitrogen doping: The significant role of pyridinic nitrogen in peroxymonosulfate activation and ciprofloxacin degradation. Chem. Eng. J. 2022, 441, 135864. [Google Scholar] [CrossRef]
- Chang, B.; Shi, W.; Yin, H.; Zhang, S.; Yang, B. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture. Chem. Eng. J. 2019, 358, 1507–1518. [Google Scholar] [CrossRef]
- Wang, R.; Yang, Y.; Zhao, Y.; Yang, L.; Yin, P.; Mao, J.; Ling, T. Multiscale structural engineering of atomically dispersed FeN4 electrocatalyst for proton exchange membrane fuel cells. J. Energy Chem. 2021, 58, 629–635. [Google Scholar] [CrossRef]
- Sa, Y.J.; Kim, J.H.; Joo, S.H. Recent Progress in the Identification of Active Sites in Pyrolyzed Fe−N/C Catalysts and Insights into Their Role in Oxygen Reduction Reaction. J. Electrochem. Sci. Technol 2017, 8, 169–182. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental investigation of tar formation and producer gas composition in biomass steam gasification in a 100 kW dual fluidised bed gasifier. Renew. Energy 2019, 132, 416–424. [Google Scholar] [CrossRef]
- Zhang, Z.; Pang, S. Experimental investigation of biomass devolatilization in steam gasification in a dual fluidised bed gasifier. Fuel 2017, 188, 628–635. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; He, M. Hydrogen-rich gas from catalytic steam gasification of biomass in a fixed bed reactor: Influence of temperature and steam on gasification performance. Int. J. Hydrogen Energy 2009, 34, 2191–2194. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Wang, S.; Li, X. Hydrogen production via steam reforming of acetic acid over biochar-supported nickel catalysts. Int. J. Hydrogen Energy 2018, 43, 18160–18168. [Google Scholar] [CrossRef]
- Hu, J.; Jia, Z.; Zhao, S.; Wang, W.; Zhang, Q.; Liu, R.; Huang, Z. Activated char supported Fe-Ni catalyst for syngas production from catalytic gasification of pine wood. Bioresour. Technol. 2021, 340, 125600. [Google Scholar] [CrossRef]
- Xin, Y.; Cao, H.; Yuan, Q.; Wang, D. Two-step gasification of cattle manure for hydrogen-rich gas production: Effect of biochar preparation temperature and gasification temperature. Waste Manag. 2017, 68, 618–625. [Google Scholar] [CrossRef]
- Hu, M.; Cui, B.; Xiao, B.; Luo, S.; Guo, D. Insight into the Ex Situ Catalytic Pyrolysis of Biomass over Char Supported Metals Catalyst: Syngas Production and Tar Decomposition. Nanomaterials 2020, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, M.; Mitsakis, P.; Meng, X.; de Jong, W.; Spliethoff, H.; Gaderer, M. Influence of pressure, temperature and steam on tar and gas in allothermal fluidized bed gasification. Fuel 2012, 99, 204–209. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Müller, H.S.; Zhao, T. Influence of freeze-thaw cycles on capillary absorption and chloride penetration into concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, P.; Zhao, K.; Du, Z.; Zhao, T. Application of Ag/AgCl Sensor for Chloride Monitoring of Mortar under Dry-Wet Cycles. Sensors 2020, 20, 1394. [Google Scholar] [CrossRef]
- Zhao, H.; Song, Q.; Liu, S.; Li, Y.; Wang, X.; Shu, X. Study on catalytic co-pyrolysis of physical mixture/staged pyrolysis characteristics of lignite and straw over an catalytic beds of char and its mechanism. Energy Convers. Manag. 2018, 161, 13–26. [Google Scholar] [CrossRef]
- Xu, D.; Xiong, Y.; Zhang, S.; Su, Y. The synergistic mechanism between coke depositions and gas for H2 production from co-pyrolysis of biomass and plastic wastes via char supported catalyst. Waste Manag. 2021, 121, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Zhang, T.; Wang, Q. Hydrogen-rich syngas production from biomass pyrolysis and catalytic reforming using biochar-based catalysts. Fuel 2022, 313, 123006. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Y.; Ma, Q.; Zhou, H.; Luo, X.; Liu, X.; Wang, S. Evolution of the chemical composition, functional group, pore structure and crystallographic structure of bio-char from palm kernel shell pyrolysis under different temperatures. J. Anal. Appl. Pyrolysis 2017, 127, 350–359. [Google Scholar] [CrossRef]
- Hu, X.; Sun, Z.; Sun, Z. Synthesis of a novel macromolecular carbon-nitrogen-phosphorous intumescent flame retardant. Adv. Powder Technol. 2021, 32, 1341–1349. [Google Scholar] [CrossRef]
- Liu, Y.; Paskevicius, M.; Wang, H.; Parkinson, G.; Veder, J.-P.; Hu, X.; Li, C.-Z. Role of O-containing functional groups in biochar during the catalytic steam reforming of tar using the biochar as a catalyst. Fuel 2019, 253, 441–448. [Google Scholar] [CrossRef]
- Li, S.; Cañete Vela, I.; Järvinen, M.; Seemann, M. Polyethylene terephthalate (PET) recycling via steam gasification—The effect of operating conditions on gas and tar composition. Waste Manag. 2021, 130, 117–126. [Google Scholar] [CrossRef]
- Guo, F.; Jia, X.; Liang, S.; Zhou, N.; Chen, P.; Ruan, R. Development of biochar-based nanocatalysts for tar cracking/reforming during biomass pyrolysis and gasification. Bioresour. Technol. 2020, 298, 122263. [Google Scholar] [CrossRef]
- Li, P.; Wan, K.; Chen, H.; Zheng, F.; Zhang, Z.; Niu, B.; Zhang, Y.; Long, D. Value-Added Products from Catalytic Pyrolysis of Lignocellulosic Biomass and Waste Plastics over Biochar-Based Catalyst: A State-of-the-Art Review. Catalysts 2022, 12, 1067. [Google Scholar] [CrossRef]
- Li, S.; Inayat, M.; Järvinen, M. Steam gasification of polyethylene terephthalate (PET) with CaO in a bubbling fluidized bed gasifier for enriching H2 in syngas with Response Surface Methodology (RSM). Appl. Energy 2023, 348, 121536. [Google Scholar] [CrossRef]
- Bernardo, G.; Araújo, T.; da Silva Lopes, T.; Sousa, J.; Mendes, A. Recent advances in membrane technologies for hydrogen purification. Int. J. Hydrogen Energy 2020, 45, 7313–7338. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Mohideen, M.M.; Subramanian, B.; Sun, J.; Ge, J.; Guo, H.; Radhamani, A.V.; Ramakrishna, S.; Liu, Y. Techno-economic analysis of different shades of renewable and non-renewable energy-based hydrogen for fuel cell electric vehicles. Renew. Sustain. Energy Rev. 2023, 174, 113153. [Google Scholar] [CrossRef]
- Rehfeldt, M.; Worrell, E.; Eichhammer, W.; Fleiter, T. A review of the emission reduction potential of fuel switch towards biomass and electricity in European basic materials industry until 2030. Renew. Sustain. Energy Rev. 2020, 120, 109672. [Google Scholar] [CrossRef]
- Santos, R.G.d.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Sollai, S.; Porcu, A.; Tola, V.; Ferrara, F.; Pettinau, A. Renewable methanol production from green hydrogen and captured CO2: A techno-economic assessment. J. CO2 Util. 2023, 68, 102345. [Google Scholar] [CrossRef]
- Sun, W.; Harrison, G.P.; Dodds, P.E. A multi-model method to assess the value of power-to-gas using excess renewable. Int. J. Hydrogen Energy 2022, 47, 9103–9114. [Google Scholar] [CrossRef]
- Mayyas, A.; Wei, M.; Levis, G. Hydrogen as a long-term, large-scale energy storage solution when coupled with renewable energy sources or grids with dynamic electricity pricing schemes. Int. J. Hydrogen Energy 2020, 45, 16311–16325. [Google Scholar] [CrossRef]
- Luo, M.; Zhang, H.; Wang, S.; Cai, J.; Qin, Y.; Zhou, L. Syngas production by chemical looping co-gasification of rice husk and coal using an iron-based oxygen carrier. Fuel 2022, 309, 122100. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, L.; Hu, Z.; Cheng, G.; Jiang, C.; Zhang, Y.; Xu, T.; He, P.; Luo, S.; Xiao, B. Hydrogen-rich gas production by steam gasification of char from biomass fast pyrolysis in a fixed-bed reactor: Influence of temperature and steam on hydrogen yield and syngas composition. Bioresour. Technol. 2010, 101, 5633–5637. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Liao, Y.; Wu, Y.; Ma, X.; Chen, L. Characteristics of microalgae gasification through chemical looping in the presence of steam. Int. J. Hydrogen Energy 2017, 42, 22730–22742. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, X.; Zhu, N.; Li, F.; Lin, H.; Liang, C.; Dang, Z.; Zou, Y. Hydrogen-Rich Syngas Production from Waste Textile Gasification Coupling with Catalytic Reforming under Steam Atmosphere. Processes 2024, 12, 1790. https://doi.org/10.3390/pr12091790
Zhuang X, Zhu N, Li F, Lin H, Liang C, Dang Z, Zou Y. Hydrogen-Rich Syngas Production from Waste Textile Gasification Coupling with Catalytic Reforming under Steam Atmosphere. Processes. 2024; 12(9):1790. https://doi.org/10.3390/pr12091790
Chicago/Turabian StyleZhuang, Xinchao, Nengwu Zhu, Fei Li, Haisheng Lin, Chao Liang, Zhi Dang, and Yuquan Zou. 2024. "Hydrogen-Rich Syngas Production from Waste Textile Gasification Coupling with Catalytic Reforming under Steam Atmosphere" Processes 12, no. 9: 1790. https://doi.org/10.3390/pr12091790




