Bio-Recovery of Metals through Biomining within Circularity-Based Solutions
Abstract
:1. Introduction
2. Sources of Metals in the Environment and Their Toxicity
3. Raw Critical Material Recovery
3.1. Biomining as a New Aspect of Circularity in Waste Management
3.2. Current Status of Metal Recycling and Circularity
4. Biotechnologies Applied for Metal Recovery from Solid Matrices
4.1. Remediation versus Recovery
4.2. Microbial Consortia Involved in Biomining Process
4.2.1. Bioleaching by Bacteria
4.2.2. Bioleaching by Fungi
4.2.3. Bioleaching by Cyanogenic Microorganisms
5. Case Studies of Successful Microbial Applications in Metals Recovery
5.1. Reusing Bio-Recovered Metals in Circular Economy
5.2. Application of Biomining Process in Metal-Contaminated Soil/Sediment
5.3. Industrial-Scale Biomining Techniques
6. Multi-Objective Decision-Making Methods Exploited to Select Sustainable Biomining/Remediation
6.1. Sustainable Remediation
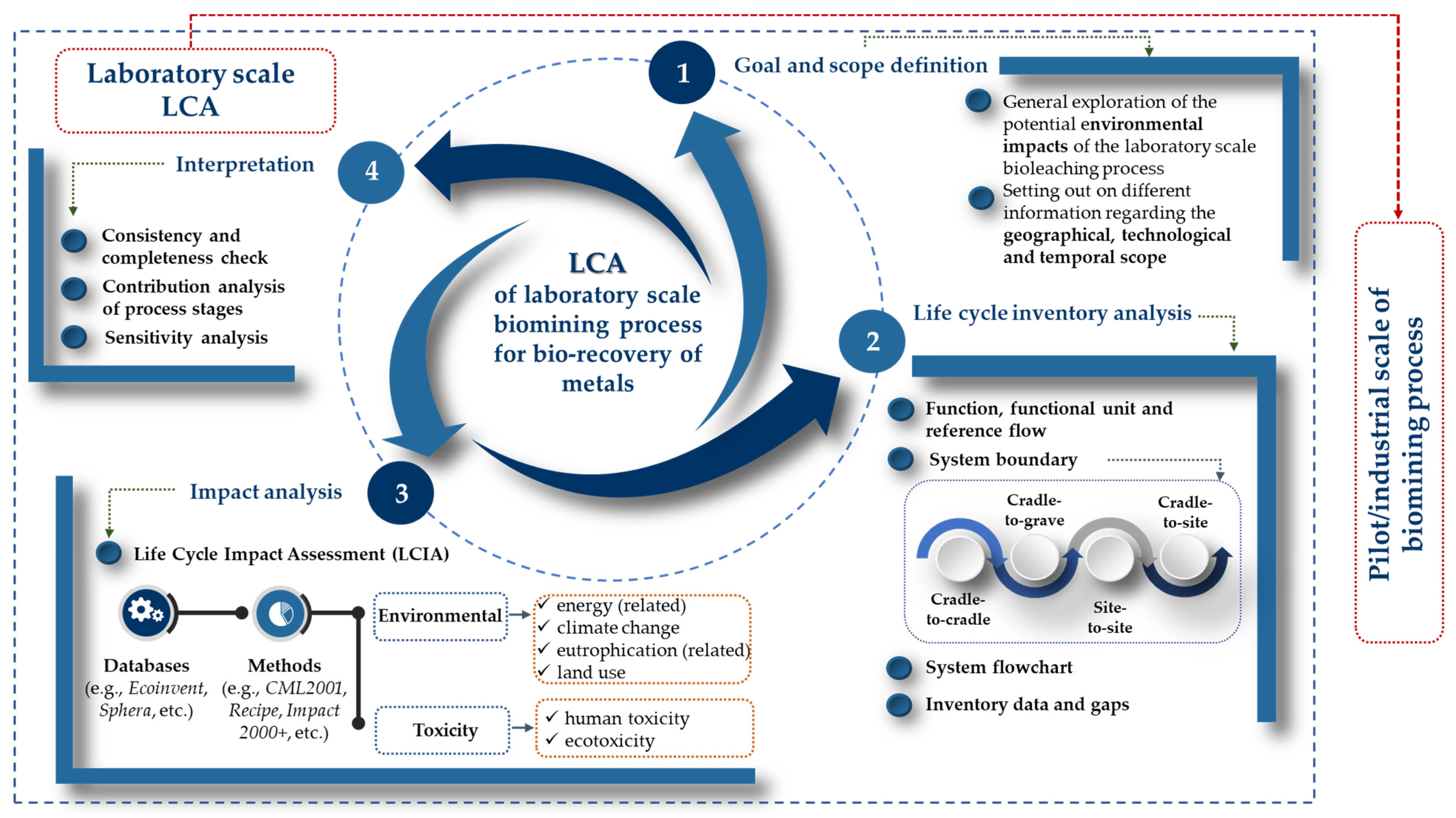
6.2. Application of Sustainability Assessment Tools in Biomining
7. Final Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- UN Take Action for the Sustainable Development Goals. Available online: https://www.un.org/sustainabledevelopment/sustainable-consumption-production/ (accessed on 11 July 2024).
- European Environment Agency; United Nations Environment Programme. Down to Earth: Soil Degradation and Sustainable Development in Europe: A Challenge for the 21st Century; European Environment Agency: Copenhaga, Denmark, 2000.
- Dror, I.; Yaron, B.; Berkowitz, B. The Human Impact on All Soil-Forming Factors during the Anthropocene. ACS Environ. Au 2022, 2, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Telo da Gama, J. The Role of Soils in Sustainability, Climate Change, and Ecosystem Services: Challenges and Opportunities. Ecologies 2023, 4, 552–567. [Google Scholar] [CrossRef]
- Ballabio, C.; Jones, A.; Panagos, P. Cadmium in Topsoils of the European Union—An Analysis Based on LUCAS Topsoil Database. Sci. Total Environ. 2024, 912, 168710. [Google Scholar] [CrossRef] [PubMed]
- Pirastu, R.; Zona, A.; Ancona, C.; Bruno, C.; Fano, V.; Fazzo, L.; Iavarone, I.; Minichilli, F.; Mitis, F.; Pasetto, R.; et al. Mortality Results in SENTIERI Project (Risultati Dell’analisi Della Mortalità Nel Progetto SENTIERI). Epidemiol. Prev. 2011, 35, 29–152. [Google Scholar]
- Panagos, P.; Van Liedekerke, M.; Yigini, Y.; Montanarella, L. Contaminated Sites in Europe: Review of the Current Situation Based on Data Collected through a European Network. J. Environ. Public. Health 2013, 2013, 158764. [Google Scholar] [CrossRef]
- Tóth, G.; Hermann, T.; Szatmári, G.; Pásztor, L. Maps of Heavy Metals in the Soils of the European Union and Proposed Priority Areas for Detailed Assessment. Sci. Total Environ. 2016, 565, 1054–1062. [Google Scholar] [CrossRef] [PubMed]
- European Environment Agency. Progress in the Management of Contaminated Sites (Indicator). Available online: https://www.eea.europa.eu/en/european-zero-pollution-dashboards/indicators/progress-in-the-management-of-contaminated-sites (accessed on 11 July 2024).
- FAO; United Nations Environment Programme. Global Assessment of Soil Pollution—Summary for Policy Makers; FAO: Rome, Italy, 2021. [Google Scholar]
- ur Rehman, Z.; Junaid, M.F.; Ijaz, N.; Khalid, U.; Ijaz, Z. Remediation Methods of Heavy Metal Contaminated Soils from Environmental and Geotechnical Standpoints. Sci. Total Environ. 2023, 867, 161468. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Rinklebe, J.; Tack, F.M.G.; Hou, D. A Review of Green Remediation Strategies for Heavy Metal Contaminated Soil. Soil. Use Manag. 2021, 37, 936–963. [Google Scholar] [CrossRef]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent Developments and Prospects of Sustainable Remediation Treatments for Major Contaminants in Soil: A Review. Sci. Total Environ. 2024, 912, 168769. [Google Scholar] [CrossRef]
- European Commission. Towards an EU Research and Innovation Policy Agenda for Nature-Based Solutions & Re-Naturing Cities, Final. Report. of the Horizon 2020 Expert Group on ‘Nature-Based Solutions and Re-Naturing Cities’; Publications Office of the EU: Brussels, Belgium, 2015. [Google Scholar]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Gomes, H.I.; Funari, V.; Ferrari, R. Bioleaching for Resource Recovery from Low-Grade Wastes like Fly and Bottom Ashes from Municipal Incinerators: A SWOT Analysis. Sci. Total Environ. 2020, 715, 136945. [Google Scholar] [CrossRef] [PubMed]
- Tezyapar Kara, I.; Kremser, K.; Wagland, S.T.; Coulon, F. Bioleaching Metal-Bearing Wastes and by-Products for Resource Recovery: A Review. Environ. Chem. Lett. 2023, 21, 3329–3350. [Google Scholar] [CrossRef]
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B. Copper Demand, Supply, and Associated Energy Use to 2050. Glob. Environ. Chang. 2016, 39, 305–315. [Google Scholar] [CrossRef]
- MINING.COM Copper, the Most Critical Metal. Available online: https://www.mining.com/web/copper-the-most-critical-metal/ (accessed on 18 July 2024).
- Garg, R.; Dang, S.; Gauba, P. E-Waste: A Concern. VSRD Int. J. Bio-Technol. Pharm. Sci. 2022, XI, 37–44. [Google Scholar]
- Ghiga, S.C.; Simion, I.M.; Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Gavrilescu, M. Sources, Composition and Management Strategies of Waste Electrical and Electronic Equipment: A Review. Environ. Eng. Manag. J. 2023, 22, 509–526. [Google Scholar] [CrossRef]
- Rendón-Castrillón, L.; Ramírez-Carmona, M.; Ocampo-López, C.; Gómez-Arroyave, L. Bioleaching Techniques for Sustainable Recovery of Metals from Solid Matrices. Sustainability 2023, 15, 10222. [Google Scholar] [CrossRef]
- Van Yken, J.; Boxall, N.J.; Cheng, K.Y.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. E-Waste Recycling and Resource Recovery: A Review on Technologies, Barriers and Enablers with a Focus on Oceania. Metals 2021, 11, 1313. [Google Scholar] [CrossRef]
- Castro, C.; Donati, E.R. Innovative Biomining. In Heavy Metals in the Environment; CRC Press: Boca Raton, FL, USA, 2019; pp. 160–172. [Google Scholar]
- Gavrilescu, M. Microbial Recovery of Critical Metals from Secondary Sources. Bioresour. Technol. 2022, 344, 126208. [Google Scholar] [CrossRef]
- Johnson, D.B. Biomining-Biotechnologies for Extracting and Recovering Metals from Ores and Waste Materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef]
- Adeeyo, A.O.; Bello, O.S.; Agboola, O.S.; Adeeyo, R.O.; Oyetade, J.A.; Alabi, M.A.; Edokpayi, J.N.; Makungo, R. Recovery of Precious Metals from Processed Wastewater: Conventional Techniques Nexus Advanced and Pragmatic Alternatives. Water Reuse 2023, 13, 134–161. [Google Scholar] [CrossRef]
- Temple, K.L.; Colmer, A.R. The Autotrophic Oxidation of Iron by a New Bacterium: Thiobacillus Ferrooxidans. J. Bacteriol. 1951, 62, 605–611. [Google Scholar] [CrossRef]
- Cozma, P.; Hlihor, R.; Roșca, M.; Minuț, M.; Diaconu, M.; Gavrilescu, M. Coupling Phytoremediation with Plant Biomass Valorisation and Metal Recovery: An Overview. In Proceedings of the 9th IEEE International Conference on E-Health and Bioengineering—EHB 2021, Iasi, Romania, 18–19 November 2021; pp. 1–4. [Google Scholar]
- Shahzad, L.; Hayyat, M.U.; Sharif, F.; Ameer, R.; Amir, M.; Khalil, M.; Saleem, M. Health risk assessment of heavy metals in various brands of sausages and salami in local markets of lahore, pakistan. Environ. Eng. Manag. J. 2023, 22, 167–176. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Ramesh, B.; Srinivasan, S. Removal of Toxic Heavy Metals Using Genetically Engineered Microbes: Molecular Tools, Risk Assessment and Management Strategies. Chemosphere 2022, 298, 134341. [Google Scholar] [CrossRef] [PubMed]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Brown, R.M.; Mirkouei, A.; Reed, D.; Thompson, V. Current Nature-Based Biological Practices for Rare Earth Elements Extraction and Recovery: Bioleaching and Biosorption. Renew. Sustain. Energy Rev. 2023, 173, 113099. [Google Scholar] [CrossRef]
- Jeyakumar, P.; Debnath, C.; Vijayaraghavan, R.; Muthuraj, M. Trends in Bioremediation of Heavy Metal Contaminations. Environ. Eng. Res. 2023, 28, 220631. [Google Scholar] [CrossRef]
- Newsome, L.; Falagán, C. The Microbiology of Metal Mine Waste: Bioremediation Applications and Implications for Planetary Health. Geohealth 2021, 5, e2020GH000380. [Google Scholar] [CrossRef]
- Filote, C.; Roșca, M.; Hlihor, R.M.; Cozma, P.; Simion, I.M.; Apostol, M.; Gavrilescu, M. Sustainable Application of Biosorption and Bioaccumulation of Persistent Pollutants in Wastewater Treatment: Current Practice. Processes 2021, 9, 1696. [Google Scholar] [CrossRef]
- Rusu, M.; Cara, I.G.; Filip, M.; Calistru, A.-E.; Topa, D.; Jitareanu, G. Transfer of Heavy Metals in Soil In-Plum Cultivation: A Field Study in Adamachi Iasi, Romania. J. Appl. Life Sci. Environ. 2023, 56, 59–74. [Google Scholar] [CrossRef]
- Azzam, M.A.; Rizwan Khan, M.; Moustafa Youssef, H. Drinking Water as a Substantial Source of Toxic Alkali, Alkaline and Heavy Metals: Toxicity and Their Implications on Human Health. J. King Saud. Univ. Sci. 2023, 35, 102761. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud. Univ. Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A Review on Heavy Metal Pollution, Toxicity and Remedial Measures: Current Trends and Future Perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Nag, R.; O’Rourke, S.M.; Cummins, E. Risk Factors and Assessment Strategies for the Evaluation of Human or Environmental Risk from Metal(Loid)s—A Focus on Ireland. Sci. Total Environ. 2022, 802, 149839. [Google Scholar] [CrossRef] [PubMed]
- Nag, R.; Cummins, E. Human Health Risk Assessment of Lead (Pb) through the Environmental-Food Pathway. Sci. Total Environ. 2022, 810, 151168. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Lin, C.J.; Bi, X.; Liu, J.; Feng, X.; Zhang, H.; Chen, J.; Wu, T. Health Risks of Heavy Metal Exposure through Vegetable Consumption near a Large-Scale Pb/Zn Smelter in Central China. Ecotoxicol. Environ. Saf. 2018, 161, 99–110. [Google Scholar] [CrossRef]
- Sarker, A.; Al Masud, M.A.; Deepo, D.M.; Das, K.; Nandi, R.; Ansary, M.W.R.; Islam, A.R.M.T.; Islam, T. Biological and Green Remediation of Heavy Metal Contaminated Water and Soils: A State-of-the-Art Review. Chemosphere 2023, 332, 138861. [Google Scholar] [CrossRef] [PubMed]
- Nedjimi, B. Phytoremediation: A Sustainable Environmental Technology for Heavy Metals Decontamination. SN Appl. Sci. 2021, 3, 286. [Google Scholar] [CrossRef]
- Tahir, I.; Ali Alkheraije, K. A Review of Important Heavy Metals Toxicity with Special Emphasis on Nephrotoxicity and Its Management in Cattle. Front. Vet. Sci. 2023, 10, 1149720. [Google Scholar] [CrossRef]
- Carabulea, V.; Motelica, D.-M.; Vrinceanu, N.O.; Plopeanu, G.; Costea, M.; Oprea, B.S.; Tanase, V. Bioaccumulation of Heavy Metals in Garlic Bulbs (Allium Sativum, L.) in Correlation with Soil from Private Gardens in the Copșa Mică Area, Romania. J. Appl. Life Sci. Environ. 2023, 55, 245–255. [Google Scholar] [CrossRef]
- Nowak, J.; Faure, N.; Glorieux, C.; Vile, D.; Pauwels, M.; Frérot, H. Sublethal Effects of Metal Toxicity and the Measure of Plant Fitness in Ecotoxicological Experiments. Environ. Pollut. 2022, 304, 119138. [Google Scholar] [CrossRef]
- Freepik. Available online: www.freepik.com (accessed on 5 July 2024).
- PNG ALL. Available online: www.pngall.com (accessed on 5 July 2024).
- Karali, N.; Shah, N. Bolstering Supplies of Critical Raw Materials for Low-Carbon Technologies through Circular Economy Strategies. Energy Res. Soc. Sci. 2022, 88, 102534. [Google Scholar] [CrossRef]
- Mancini, S.; Casale, M.; Tazzini, A.; Dino, G.A. Use and Recovery of Extractive Waste and Tailings for Sustainable Raw Materials Supply. Mining 2024, 4, 149–167. [Google Scholar] [CrossRef]
- OECD. Global Material Resources Outlook to 2060: Economic Drivers an Environmental Consequences; OECD: Paris, France, 2019. [Google Scholar]
- European Commission. Proposal for a REGULATION OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) 168/2013, (EU) 2018/858, 2018/1724 and (EU) 2019/1020; European Commission: Brussels, Belgium, 2023. [Google Scholar]
- European Commission RMIS—Raw Materials Information System. Available online: https://rmis.jrc.ec.europa.eu/eu-critical-raw-materials (accessed on 16 July 2024).
- Regulation (EU) 2024/1252 of the European Parliament and of the Council of 11 April 2024 Establishing a Framework for Ensuring a Secure and Sustainable Supply of Critical Raw Materials and Amending Regulations (EU) No 168/2013, (EU) 2018/858, (EU) 2018/1724 and (EU) 2019/1020. Off. J. Eur. Union 2024, EN L series 3.5.2024. 1–67.
- Hool, A.; Helbig, C.; Wierink, G. Challenges and Opportunities of the European Critical Raw Materials Act. Miner. Econ. 2023, 2023, 1–8. [Google Scholar] [CrossRef]
- Giese, E.C. Biomining in the Post-COVID-19 Circular Bioeconomy: A “Green Dispute” for Critical Metals. Int. Res. J. Multidiscip. Technovation 2021, 3, 35–38. [Google Scholar] [CrossRef]
- European Environment Agency. Circular Material Use Rate in Europe; European Environment Agency: Copenhagen, Denmark, 2024.
- Charles, R.G.; Douglas, P.; Dowling, M.; Liversage, G.; Davies, M.L. Towards Increased Recovery of Critical Raw Materials from WEEE– Evaluation of CRMs at a Component Level and Pre-Processing Methods for Interface Optimisation with Recovery Processes. Resour. Conserv. Recycl. 2020, 161, 104923. [Google Scholar] [CrossRef]
- Agrawal, R.; Bhagia, S.; Satlewal, A.; Ragauskas, A.J. Urban Mining from Biomass, Brine, Sewage Sludge, Phosphogypsum and e-Waste for Reducing the Environmental Pollution: Current Status of Availability, Potential, and Technologies with a Focus on LCA and TEA. Environ. Res. 2023, 224, 115523. [Google Scholar] [CrossRef]
- Kucuker, M.A.; Kuchta, K. Biomining—Biotechnological Systems for the Extraction and Recovery of Metals from Secondary Sources. Glob. Nest J. 2018, 20, 737–742. [Google Scholar] [CrossRef]
- Srivastava, P. Recent Trends in Biomining Microorganisms for Solid Waste Management. In Microbial Technology for Sustainable E-waste Management; Springer International Publishing: Berlin/Heidelberg, Germany, 2023; pp. 273–286. ISBN 9783031256783. [Google Scholar]
- Vo, P.H.N.; Danaee, S.; Hai, H.T.N.; Huy, L.N.; Nguyen, T.A.H.; Nguyen, H.T.M.; Kuzhiumparambil, U.; Kim, M.; Nghiem, L.D.; Ralph, P.J. Biomining for Sustainable Recovery of Rare Earth Elements from Mining Waste: A Comprehensive Review. Sci. Total Environ. 2024, 908, 168210. [Google Scholar] [CrossRef]
- Johnson, D.B. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals 2018, 8, 343. [Google Scholar] [CrossRef]
- Sánchez-García, E.; Martínez-Falcó, J.; Marco-Lajara, B.; Manresa-Marhuenda, E. Revolutionizing the Circular Economy through New Technologies: A New Era of Sustainable Progress. Environ. Technol. Innov. 2024, 33, 103509. [Google Scholar] [CrossRef]
- Babbitt, C.W.; Althaf, S.; Cruz Rios, F.; Bilec, M.M.; Graedel, T.E. The Role of Design in Circular Economy Solutions for Critical Materials. One Earth 2021, 4, 353–362. [Google Scholar] [CrossRef]
- Kinnunen, P.; Karhu, M.; Yli-Rantala, E.; Kivikytö-Reponen, P.; Mäkinen, J. A Review of Circular Economy Strategies for Mine Tailings. Clean. Eng. Technol. 2022, 8, 100499. [Google Scholar] [CrossRef]
- Kinnunen, P.; Hedrich, S. Biotechnological Strategies to Recover Value from Waste. Hydrometallurgy 2023, 222, 106182. [Google Scholar] [CrossRef]
- Watari, T.; Nansai, K.; Nakajima, K. Major Metals Demand, Supply, and Environmental Impacts to 2100: A Critical Review. Resour. Conserv. Recycl 2021, 164, 105107. [Google Scholar] [CrossRef]
- Born, K.; Ciftci, M.M. The Limitations of End-of-Life Copper Recycling and Its Implications for the Circular Economy of Metals. Resour. Conserv. Recycl. 2024, 200, 107318. [Google Scholar] [CrossRef]
- Korf, N.; Løvik, A.N.; Figi, R.; Schreiner, C.; Kuntz, C.; Mählitz, P.M.; Rösslein, M.; Wäger, P.; Rotter, V.S. Multi-Element Chemical Analysis of Printed Circuit Boards—Challenges and Pitfalls. Waste Manag. 2019, 92, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Saidani, M.; Kendall, A.; Yannou, B.; Leroy, Y.; Cluzel, F. Closing the Loop on Platinum from Catalytic Converters: Contributions from Material Flow Analysis and Circularity Indicators. J. Ind. Ecol. 2019, 23, 1143–1158. [Google Scholar] [CrossRef]
- Hagelüken, C.; Goldmann, D. Recycling and Circular Economy—Towards a Closed Loop for Metals in Emerging Clean Technologies. Miner. Econ. 2022, 35, 539–562. [Google Scholar] [CrossRef]
- Godoy León, M.F.; Blengini, G.A.; Dewulf, J. Cobalt in End-of-Life Products in the EU, Where Does It End up?—The MaTrace Approach. Resour. Conserv. Recycl. 2020, 158, 104842. [Google Scholar] [CrossRef]
- Pauliuk, S.; Kondo, Y.; Nakamura, S.; Nakajima, K. Regional Distribution and Losses of End-of-Life Steel throughout Multiple Product Life Cycles—Insights from the Global Multiregional MaTrace Model. Resour. Conserv. Recycl. 2017, 116, 84–93. [Google Scholar] [CrossRef] [PubMed]
- EuRIC AISBL. Recycling: Bridging Circular Economy & Climate Policy; EuRIC AISBL: Schaerbeek, Belgium, 2020. [Google Scholar]
- International Energy Agency. End-of-Life Recycling Rates for Selected Metals; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Pathak, A.; Al-Sheeha, H.; Navvamani, R.; Kothari, R.; Marafi, M.; Rana, M.S. Recycling of Platinum Group Metals from Exhausted Petroleum and Automobile Catalysts Using Bioleaching Approach: A Critical Review on Potential, Challenges, and Outlook. Rev. Environ. Sci. Biotechnol. 2022, 21, 1035–1059. [Google Scholar] [CrossRef]
- Ryan, M. Nassar Anis. In Proceedings of the World Economic Forum, Davos, Switzerland, 15–19 January 2024. [Google Scholar]
- Eurostat. Circular Economy-Material Flows; Eurostat: Luxembourg, 2023.
- Espinoza, L.T.; Rostek, L.; Loibl, A.; Stijepic, D. The Promise and Limits of Urban Mining; Fraunhofer Society: Karlsruhe, Germany, 2020. [Google Scholar]
- Roberto, F.F.; Schippers, A. Progress in Bioleaching: Part B, Applications of Microbial Processes by the Minerals Industries. Appl. Microbiol. Biotechnol. 2022, 106, 5913–5928. [Google Scholar] [CrossRef] [PubMed]
- Colmer, A.R.; Hinkle, M.E. The Role of Microorganisms in Acid Mine Drainage: A Preliminary Report. Science 1947, 106, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, H.L. Beginnings of Rational Bioleaching and Highlights in the Development of Biohydrometallurgy: A Brief History. Eur. J. Miner. Process. Environ. Prot. 2004, 4, 102–112. [Google Scholar]
- Jerez, C.A. Biomining Microorganisms: Molecular Aspects and Applications in Biotechnology and Bioremediation. In Advances in Applied Bioremediation. Soil Biology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 239–256. [Google Scholar]
- Gulliani, S.; Volpe, M.; Messineo, A.; Volpe, R. Recovery of Metals and Valuable Chemicals from Waste Electric and Electronic Materials: A Critical Review of Existing Technologies. RSC Sustain. 2023, 1, 1085–1108. [Google Scholar] [CrossRef]
- Dong, Y.; Zan, J.; Lin, H. Bioleaching of Heavy Metals from Metal Tailings Utilizing Bacteria and Fungi: Mechanisms, Strengthen Measures, and Development Prospect. J. Environ. Manag. 2023, 344, 118511. [Google Scholar] [CrossRef]
- Brune, K.D.; Bayer, T.S. Engineering Microbial Consortia to Enhance Biomining and Bioremediation. Front. Microbiol. 2012, 3, 203. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.P. Microbial Leaching of Heavy Metals Using Escherichia Coli and Evaluation of Bioleaching Mechanism. Bioresour. Technol. Rep. 2020, 9, 100368. [Google Scholar] [CrossRef]
- Kaur, P.; Sharma, S.; Albarakaty, F.M.; Kalia, A.; Hassan, M.M.; Abd-Elsalam, K.A. Biosorption and Bioleaching of Heavy Metals from Electronic Waste Varied with Microbial Genera. Sustainability 2022, 14, 935. [Google Scholar] [CrossRef]
- Vera, M.; Schippers, A.; Hedrich, S.; Sand, W. Progress in Bioleaching: Fundamentals and Mechanisms of Microbial Metal Sulfide Oxidation—Part A. Appl. Microbiol. Biotechnol. 2022, 106, 6933–6952. [Google Scholar] [CrossRef] [PubMed]
- Kutschke, S.; Guézennec, A.G.; Hedrich, S.; Schippers, A.; Borg, G.; Kamradt, A.; Gouin, J.; Giebner, F.; Schopf, S.; Schlömann, M.; et al. Bioleaching of Kupferschiefer Blackshale—A Review Including Perspectives of the Ecometals Project. Min. Eng. 2015, 75, 116–125. [Google Scholar] [CrossRef]
- Romo, E.; Weinacker, D.F.; Zepeda, A.B.; Figueroa, C.A.; Chavez-Crooker, P.; Farias, J.G. Bacterial Consortium for Copper Extraction from Sulphide Ore Consisting Mainly of Chalcopyrite. Braz. J. Microbiol. 2013, 44, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.R.; Van Bronswijk, W. An X-Ray Photoelectron Spectroscopy Study of the Mechanism of Oxidative Dissolution of Chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Zhang, L.; Cheng, H.; Wang, Y.; Tang, R.; Zhou, H. Bioleaching of Copper-Containing Electroplating Sludge. J. Environ. Manag. 2021, 285, 112133. [Google Scholar] [CrossRef] [PubMed]
- Bayat, B.; Sari, B. Comparative Evaluation of Microbial and Chemical Leaching Processes for Heavy Metal Removal from Dewatered Metal Plating Sludge. J. Hazard. Mater. 2010, 174, 763–769. [Google Scholar] [CrossRef]
- Marchioretto, M.M.; Binning, H.; Hien, P.; Rulkens, W.H. Bioleaching and Chemical Leaching of Heavy Metals from Anaerobically Digested Sludge. In Wastewater Sludge as a Resource; Odegaard, H., Ed.; IWA Publishing: London, UK, 2003; pp. 457–464. [Google Scholar]
- Dolker, T.; Pant, D. Chemical-Biological Hybrid Systems for the Metal Recovery from Waste Lithium Ion Battery. J. Environ. Manag. 2019, 248, 109270. [Google Scholar] [CrossRef]
- Wu, W.; Liu, X.; Zhang, X.; Zhu, M.; Tan, W. Bioleaching of Copper from Waste Printed Circuit Boards by Bacteria-Free Cultural Supernatant of Iron–Sulfur-Oxidizing Bacteria. Bioresour. Bioprocess. 2018, 5, 10. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Li, B.; Sun, C.; Deng, F.; Song, C.; Wang, S. Isolation of Thiobacillus Spp. and Its Application in the Removal of Heavy Metals from Activated Sludge. Afr. J. Biotechnol. 2012, 11, 16336–16341. [Google Scholar] [CrossRef]
- Rakhshani, Y.; Rahpeyma, S.S.; Tabandeh, F.; Arabnezhad, M.; Azimi, A.; Raheb, J. Multi-Objective Optimization of Copper Bioleaching: Comparative Study of Pure and Co-Cultured Cultivation. Iran. J. Biotechnol. 2023, 21, 27–37. [Google Scholar] [CrossRef]
- Lewis, G.; Gaydardzhiev, S.; Bastin, D.; Bareel, P.F. Bio Hydrometallurgical Recovery of Metals from Fine Shredder Residues. Min. Eng. 2011, 24, 1166–1171. [Google Scholar] [CrossRef]
- Valix, M. Bioleaching of Electronic Waste: Milestones and Challenges. In Current Developments in Biotechnology and Bioengineering: Solid Waste Management; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 407–442. ISBN 9780444636751. [Google Scholar]
- Hubau, A.; Guezennec, A.G.; Joulian, C.; Falagán, C.; Dew, D.; Hudson-Edwards, K.A. Bioleaching to Reprocess Sulfidic Polymetallic Primary Mining Residues: Determination of Metal Leaching Mechanisms. Hydrometallurgy 2020, 197, 105484. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Yang, W.; Chen, J.; Wang, X.; Xing, D.; Dong, W.; Wang, H.; Wang, J. The Combination of Aerobic Digestion and Bioleaching for Heavy Metal Removal from Excess Sludge. Chemosphere 2022, 290, 133231. [Google Scholar] [CrossRef]
- Abraham, J.; Chatterjee, A.; Sharma, J. Isolation and Characterization of a New Bacillus Licheniformis Strain for Bioleaching Heavy Metals. J. Appl. Biotechnol. Rep. 2020, 7, 139–144. [Google Scholar] [CrossRef]
- Chen, C.; Li, H.; Cui, F.; Wang, Z.; Liu, X.; Jiang, G.; Cheng, T.; Bai, R.; Song, L. Novel Combination of Bioleaching and Persulfate for the Removal of Heavy Metals from Metallurgical Industry Sludge. Environ. Sci. Pollut. Res. 2022, 29, 33751–33763. [Google Scholar] [CrossRef]
- Dusengemungu, L.; Kasali, G.; Gwanama, C.; Mubemba, B. Overview of Fungal Bioleaching of Metals. Environ. Adv. 2021, 5, 100083. [Google Scholar] [CrossRef]
- Sedlakova-Kadukova, J.; Marcincakova, R.; Luptakova, A.; Vojtko, M.; Fujda, M.; Pristas, P. Comparison of Three Different Bioleaching Systems for Li Recovery from Lepidolite. Sci. Rep. 2020, 10, 14594. [Google Scholar] [CrossRef]
- García-Balboa, C.; Martínez-Alesón García, P.; López-Rodas, V.; Costas, E.; Baselga-Cervera, B. Microbial Biominers: Sequential Bioleaching and Biouptake of Metals from Electronic Scraps. Microbiologyopen 2022, 11, e1265. [Google Scholar] [CrossRef]
- Kumari, D.; Pan, X.; Achal, V.; Zhang, D.; Al-Misned, F.A.; Golam Mortuza, M. Multiple Metal-Resistant Bacteria and Fungi from Acidic Copper Mine Tailings of Xinjiang, China. Environ. Earth Sci. 2015, 74, 3113–3121. [Google Scholar] [CrossRef]
- Khan, I.; Ali, M.; Aftab, M.; Shakir, S.; Qayyum, S.; Haleem, K.S.; Tauseef, I. Mycoremediation: A Treatment for Heavy Metal-Polluted Soil Using Indigenous Metallotolerant Fungi. Environ. Monit. Assess. 2019, 191, 622. [Google Scholar] [CrossRef]
- Pathak, A.; Kothari, R.; Vinoba, M.; Habibi, N.; Tyagi, V.V. Fungal Bioleaching of Metals from Refinery Spent Catalysts: A Critical Review of Current Research, Challenges, and Future Directions. J. Environ. Manag. 2021, 280, 111789. [Google Scholar] [CrossRef] [PubMed]
- Liaquat, F.; Munis, M.F.H.; Haroon, U.; Arif, S.; Saqib, S.; Zaman, W.; Khan, A.R.; Shi, J.; Che, S.; Liu, Q. Evaluation of Metal Tolerance of Fungal Strains Isolated from Contaminated Mining Soil of Nanjing, China. Biology 2020, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- Amiri, F.; Yaghmaei, S.; Mousavi, S.M. Bioleaching of Tungsten-Rich Spent Hydrocracking Catalyst Using Penicillium Simplicissimum. Bioresour. Technol. 2011, 102, 1567–1573. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.; Bao, P.; Liu, A.; Wang, M.; Shen, L.; Yu, R.; Liu, Y.; Chen, M.; Li, J.; Wu, X.; et al. Bioleaching of Low-Grade Waste Printed Circuit Boards by Mixed Fungal Culture and Its Community Structure Analysis. Resour. Conserv. Recycl. 2018, 136, 267–275. [Google Scholar] [CrossRef]
- Alavi, N.; Partovi, K.; Majlessi, M.; Rashidi, M.; Alimohammadi, M. Bioleaching of Metals from Cellphones Batteries by a Co-Fungus Medium in Presence of Carbon Materials. Bioresour. Technol. Rep. 2021, 15, 100768. [Google Scholar] [CrossRef]
- Sinha, R.; Chauhan, G.; Singh, A.; Kumar, A.; Acharya, S. A Novel Eco-Friendly Hybrid Approach for Recovery and Reuse of Copper from Electronic Waste. J. Environ. Chem. Eng. 2018, 6, 1053–1061. [Google Scholar] [CrossRef]
- Vakilchap, F.; Mousavi, S.M.; Baniasadi, M.; Farnaud, S. Development and Evolution of Biocyanidation in Metal Recovery from Solid Waste: A Review. Rev. Environ. Sci. Biotechnol. 2020, 19, 509–530. [Google Scholar] [CrossRef]
- Aminian-Dehkordi, J.; Mousavi, S.M.; Marashi, S.A.; Jafari, A.; Mijakovic, I. A Systems-Based Approach for Cyanide Overproduction by Bacillus Megaterium for Gold Bioleaching Enhancement. Front. Bioeng. Biotechnol. 2020, 8, 528. [Google Scholar] [CrossRef]
- Kumar, A.; Saini, H.S.; Şengör, S.; Sani, R.K.; Kumar, S. Bioleaching of Metals from Waste Printed Circuit Boards Using Bacterial Isolates Native to Abandoned Gold Mine. BioMetals 2021, 34, 1043–1058. [Google Scholar] [CrossRef]
- Diaz, M.A.; De Ranson, I.U.; Dorta, B.; Banat, I.M.; Blazquez, M.L.; Gonzalez, F.; Muñoz, J.A.; Ballester, A. Metal Removal from Contaminated Soils Through Bioleaching with Oxidizing Bacteria and Rhamnolipid Biosurfactants. Soil. Sediment. Contam. 2015, 24, 16–29. [Google Scholar] [CrossRef]
- Pourhossein, F.; Mousavi, S.M. Enhancement of Copper, Nickel, and Gallium Recovery from LED Waste by Adaptation of Acidithiobacillus Ferrooxidans. Waste Manag. 2018, 79, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Ilyas, S.; Anwar, M.A.; Niazi, S.B.; Afzal Ghauri, M. Bioleaching of Metals from Electronic Scrap by Moderately Thermophilic Acidophilic Bacteria. Hydrometallurgy 2007, 88, 180–188. [Google Scholar] [CrossRef]
- Seh-Bardan, B.J.; Othman, R.; Wahid, S.A.; Husin, A.; Sadegh-Zadeh, F. Bioleaching of Heavy Metals from Mine Tailings by Aspergillus fumigatus. Bioremediat. J. 2012, 16, 57–65. [Google Scholar] [CrossRef]
- Rasoulnia, P.; Mousavi, S.M. Maximization of Organic Acids Production by Aspergillus Niger in a Bubble Column Bioreactor for V and Ni Recovery Enhancement from Power Plant Residual Ash in Spent-Medium Bioleaching Experiments. Bioresour. Technol. 2016, 216, 729–736. [Google Scholar] [CrossRef]
- Gumulya, Y.; Boxall, N.J.; Khaleque, H.N.; Santala, V.; Carlson, R.P.; Kaksonen, A.H. In a Quest for Engineering Acidophiles for Biomining Applications: Challenges and Opportunities. Genes 2018, 9, 116. [Google Scholar] [CrossRef]
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.A.; Contamine, F.; D’Hugues, P. A Review of Sulfide Minerals Microbially Assisted Leaching in Stirred Tank Reactors. Int. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Biswal, B.K.; Balasubramanian, R. Recovery of Valuable Metals from Spent Lithium-Ion Batteries Using Microbial Agents for Bioleaching: A Review. Front. Microbiol. 2023, 14, 1197081. [Google Scholar] [CrossRef] [PubMed]
- das Neves Vasconcellos Brandão, I.Y.; Munakata, A.A.; Lourenço, L.A.; Maass, D. How Biomining Has Been Used to Recover Metals from Ores and Waste? A Review. Int. J. Earth Environ. Sci. 2021, 6, 24–31. [Google Scholar] [CrossRef]
- Adetunji, A.I.; Oberholster, P.J.; Erasmus, M. Bioleaching of Metals from E-Waste Using Microorganisms: A Review. Minerals 2023, 13, 828. [Google Scholar] [CrossRef]
- Tapia, J.; Dueñas, A.; Cheje, N.; Soclle, G.; Patiño, N.; Ancalla, W.; Tenorio, S.; Denos, J.; Taco, H.; Cao, W.; et al. Bioleaching of Heavy Metals from Printed Circuit Boards with an Acidophilic Iron-Oxidizing Microbial Consortium in Stirred Tank Reactors. Bioengineering 2022, 9, 79. [Google Scholar] [CrossRef]
- Benzal, E.; Solé, M.; Lao, C.; Gamisans, X.; Dorado, A.D. Elemental Copper Recovery from E-Wastes Mediated with a Two-Step Bioleaching Process. Waste Biomass Valorization 2020, 11, 5457–5465. [Google Scholar] [CrossRef]
- Chen, J.; Tang, D.; Zhong, S.; Zhong, W.; Li, B. The Influence of Micro-Cracks on Copper Extraction by Bioleaching. Hydrometallurgy 2020, 191, 105243. [Google Scholar] [CrossRef]
- Mäkinen, J.; Salo, M.; Khoshkhoo, M.; Sundkvist, J.E.; Kinnunen, P. Bioleaching of Cobalt from Sulfide Mining Tailings; a Mini-Pilot Study. Hydrometallurgy 2020, 196, 105418. [Google Scholar] [CrossRef]
- Bhatti, T.M. Bioleaching of Organic Carbon Rich Polymetallic Black Shale. Hydrometallurgy 2015, 157, 246–255. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Won, S.; Ha, M.-G.; Nguyen, D.D.; Kang, H.Y. Bioleaching for Environmental Remediation of Toxic Metals and Metalloids: A Review on Soils, Sediments, and Mine Tailings. Chemosphere 2021, 282, 131108. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Yang, Z.; Chen, R. Study of Characteristics on Metabolism of Penicillium Chrysogenum F1 during Bioleaching of Heavy Metals from Contaminated Soil. Can. J. Microbiol. 2019, 65, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, Z.; Chai, L.; Wang, Y.; Liu, Y.; Xiao, R. Bioleaching Remediation of Heavy Metal-Contaminated Soils Using Burkholderia Sp. Z-90. J. Hazard. Mater. 2016, 301, 145–152. [Google Scholar] [CrossRef]
- Xinhui, D.; Runhua, C.; Yan, S.; Shengnan, Z. Preliminary Bioleaching of Heavy Metals from Contaminated Soil Applying Aspergillus niger F2. Am. J. Environ. Sci. Eng. 2018, 2, 72–78. [Google Scholar] [CrossRef]
- Gilstrap, Z. Bioleaching of Arsenic from Agricultural Soils; Clemson University: Clemson, SC, USA, 2022. [Google Scholar]
- Sur, I.M.; Micle, V.; Hegyi, A.; Lăzărescu, A.-V. Extraction of Metals from Polluted Soils by Bioleaching in Relation to Environmental Risk Assessment. Materials 2022, 15, 3973. [Google Scholar] [CrossRef]
- Qayyum, S.; Meng, K.; Pervez, S.; Nawaz, F.; Peng, C. Optimization of PH, Temperature and Carbon Source for Bioleaching of Heavy Metals by Aspergillus Flavus Isolated from Contaminated Soil. Main. Group. Met. Chem. 2019, 42, 1–7. [Google Scholar] [CrossRef]
- Tang, A.; Lu, Y.; Li, Q.; Zhang, X.; Cheng, N.; Liu, H.; Liu, Y. Simultaneous Leaching of Multiple Heavy Metals from a Soil Column by Extracellular Polymeric Substances of Aspergillus Tubingensis F12. Chemosphere 2021, 263, 127883. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, R.; Ding, Z.; Ruan, X.; Luo, J.; Chen, J.; Zheng, J.; Tang, J. Discovery of a Novel Native Bacterium of Providencia Sp. with High Biosorption and Oxidation Ability of Manganese for Bioleaching of Heavy Metal Contaminated Soils. Chemosphere 2020, 241, 125039. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Q.; Yang, Y.; Sun, J.; Li, G.; Liu, X.; Shu, S.; Li, X.; Liao, H. Uranium Removal from a Radioactive Contaminated Soil by Defined Bioleaching Bacteria. J. Radioanal. Nucl. Chem. 2022, 331, 439–449. [Google Scholar] [CrossRef]
- Sabra, N.; Dubourguier, H.; Duval, M.; Hamieh, T. Study of Canal Sediments Contaminated with Heavy Metals: Fungal versus Bacterial Bioleaching Techniques. Environ. Technol. 2011, 32, 1307–1324. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Jiang, M.; Hsieh, L.; Cai, Y.; Shen, Y.; Wang, H.; Lin, Q.; Shen, C.; Hu, B.; Lou, L. Feasibility of Bioleaching of Heavy Metals from Sediment with Indigenous Bacteria Using Agricultural Sulfur Soil Conditioners. Sci. Total Environ. 2020, 703, 134812. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Y.; Wu, J.Q.; Sung, S. Effects of Sulfur Dosage on Continuous Bioleaching of Heavy Metals from Contaminated Sediment. J. Hazard. Mater. 2022, 424, 127257. [Google Scholar] [CrossRef]
- Rouchalova, D.; Rouchalova, K.; Janakova, I.; Cablik, V.; Janstova, S. Bioleaching of Iron, Copper, Lead, and Zinc from the Sludge Mining Sediment at Different Particle Sizes, PH, and Pulp Density Using Acidithiobacillus Ferrooxidans. Minerals 2020, 10, 1013. [Google Scholar] [CrossRef]
- Tran, T.M.; Han, H.-J.; Ko, J.-I.; Lee, J.-U. Effect of Indigenous Microbial Consortium on Bioleaching of Arsenic from Contaminated Soil by Shewanella Putrefaciens. Sustainability 2020, 12, 3286. [Google Scholar] [CrossRef]
- Andrzejewska-Górecka, D.A.; Macherzynski, B.; Wszelaka-Rylik, M. Bioleaching of Cadmium(Cd) and Zinc(Zn) from the Contaminated Soil Using Bacteria from Wastewater Sludge. Desalination Water Treat. 2021, 232, 372–379. [Google Scholar] [CrossRef]
- Zhang, Q.; Zou, D.; Zeng, X.; Yang, Y.; Zeng, C.; Li, M.; Fu, Z.; Zeng, Q. Bioleaching of Cd from Contaminated Helianthus Annuus, L. Stalk and the Safe Utilization of Its Byproducts by Aspergillus Niger. Environ. Res. 2024, 251, 118714. [Google Scholar] [CrossRef]
- Fonti, V.; Dell’Anno, A.; Beolchini, F. Does Bioleaching Represent a Biotechnological Strategy for Remediation of Contaminated Sediments? Sci. Total Environ. 2016, 563–564, 302–319. [Google Scholar] [CrossRef] [PubMed]
- Faraji, F.; Golmohammadzadeh, R.; Pickles, C.A. Potential and Current Practices of Recycling Waste Printed Circuit Boards: A Review of the Recent Progress in Pyrometallurgy. J. Environ. Manag. 2022, 316, 115242. [Google Scholar] [CrossRef]
- Pradhan, D.; Patra, A.K.; Kim, D.J.; Chung, H.S.; Lee, S.W. A Novel Sequential Process of Bioleaching and Chemical Leaching for Dissolving Ni, V, and Mo from Spent Petroleum Refinery Catalyst. Hydrometallurgy 2013, 131–132, 114–119. [Google Scholar] [CrossRef]
- Srichandan, H.; Pathak, A.; Singh, S.; Blight, K.; Kim, D.J.; Lee, S.W. Sequential Leaching of Metals from Spent Refinery Catalyst in Bioleaching–Bioleaching and Bioleaching–Chemical Leaching Reactor: Comparative Study. Hydrometallurgy 2014, 150, 130–143. [Google Scholar] [CrossRef]
- Naseri, T.; Beiki, V.; Mousavi, S.M.; Farnaud, S. A Comprehensive Review of Bioleaching Optimization by Statistical Approaches: Recycling Mechanisms, Factors Affecting, Challenges, and Sustainability. RSC Adv. 2023, 13, 23570–23589. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M. A Comparison Study of Heap Bioleaching Sites in Chile and Finland for Further Development of Biotechnology for Mining. Evergreen 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Lazenby, H. DNI Adds Scandium to Alberta Project’s Product Basket. Creamer Media Mining Weekly, 9 April 2014. [Google Scholar]
- d’Hugues, P.; Norris, P.R.; Hallberg, K.B.; Sánchez, F.; Langwaldt, J.; Grotowski, A.; Chmielewski, T.; Groudev, S. Bioshale FP6 European Project: Exploiting Black Shale Ores Using Biotechnologies? Min. Eng. 2008, 21, 111–120. [Google Scholar] [CrossRef]
- Mining Technology Talvivaara Bioheapleach Nickel Mine, Finland. Available online: https://www.mining-technology.com/projects/talvivaara/ (accessed on 18 July 2024).
- Abdel Azim, A.; Bellini, R.; Vizzarro, A.; Bassani, I.; Pirri, C.F.; Menin, B. Highlighting the Role of Archaea in Urban Mine Waste Exploitation and Valorisation. Recycling 2023, 8, 20. [Google Scholar] [CrossRef]
- European Commission Critical Raw Materials: Their Role in Nanotechnology-Based Value Chains. Nanotechnology as a Vehicle for Substitution. Available online: https://single-market-economy.ec.europa.eu/sectors/raw-materials/eip/raw-materials-commitment/critical-raw-materials-their-role-nanotechnology-based-value-chains-nanotechnology-vehicle_en (accessed on 10 July 2024).
- NEMO Project Near-Zero-Waste Recycling of Low-Grade Sulphidic Mining Waste for Critical-Metal, Mineral and Construction Raw-Material Production in a Circular Economy. Available online: https://h2020-nemo.eu/ (accessed on 12 July 2024).
- Guezennec, A.-G.; Hubau, A.; Pino-Herrera, D.; Heikkinen, V.; Dew, D.; Falagan, C.; Hudson-Edwards, K.; Khoshkhoo, M.; Sand, A.; Makinen, J.; et al. Pilot-Scale Demonstrations of Innovative Biohydrometallurgy for Sustainable Valorisation of Mining Waste: Main Outcomes from H2020-NEMO Project. In Proceedings of the Biomining 2023, Cornwall, UK, 5–6 June 2023. [Google Scholar]
- Piña, R.; Gervilla, F.; Barnes, S.J.; Ortega, L.; Lunar, R. Distribution of Platinum-Group and Chalcophile Elements in the Aguablanca Ni-Cu Sulfide Deposit (SW Spain): Evidence from a LA-ICP-MS Study. Chem. Geol. 2012, 302–303, 61–75. [Google Scholar] [CrossRef]
- BioProLat project BioProLat—Reductive Bioprocessing for Cobalt and Nickel Recovery from Laterites in Brazil. Available online: https://www.bgr.bund.de/EN/Themen/Min_rohstoffe/Projekte/Lagerstaettenforschung_laufend_en/BioProLat/BioProLat_en.html;jsessionid=74CF4E262A253E113813F4905FBDAC7B.internet971?nn=5658028 (accessed on 18 July 2024).
- Metso BIOX® Process. Available online: https://www.metso.com/portfolio/biox-process/ (accessed on 18 July 2024).
- Zeng, W.M.; Peng, Y.P.; Peng, T.J.; Nan, M.H.; Chen, M.; Qiu, G.Z.; Shen, L. Electrochemical Studies on Dissolution and Passivation Behavior of Low Temperature Bioleaching of Chalcopyrite by Acidithiobacillus Ferrivorans YL15. Min. Eng. 2020, 155, 106416. [Google Scholar] [CrossRef]
- Langdahl, B.R.; Ingvorsen, K. Temperature Characteristics of Bacterial Iron Solubilisation and 14C Assimilation in Naturally Exposed Sulfide Ore Material at Citronen Fjord, North Greenland (83 °N). FEMS Microbiol. Ecol. 2006, 23, 275–283. [Google Scholar] [CrossRef]
- Credence Research Biomining Market by Type of Metal Extracted (Copper, Iron, Gold, Zinc, Cobalt, Others), by Type of Process (Slope, Heap, In-Situ), by Application (Mining, Metal Pollution Control): Growth, Size, Future Prospects, and Competitive Analysis, 2015–2017. Available online: https://www.credenceresearch.com/report/biomining-market (accessed on 15 July 2024).
- Braun, A.B.; da Silva Trentin, A.W.; Visentin, C.; Thomé, A. Proposal for an Optimized Method for Sustainable Remediation Evaluation and Application: Implementation of a Multi-Criteria Process. Environ. Sci. Pollut. Res. 2019, 26, 35996–36006. [Google Scholar] [CrossRef] [PubMed]
- Braun, A.B.; Visentin, C.; Thomé, A. Design of a Central Tool for Sustainability Evaluation and Weighting to Decision-Making Support of Contaminated Soils Remediation. J. Environ. Manag. 2024, 351, 119624. [Google Scholar] [CrossRef]
- Li, X.; Cundy, A.B.; Chen, W.; Lyu, S. Systematic and Bibliographic Review of Sustainability Indicators for Contaminated Site Remediation: Comparison between China and Western Nations. Environ. Res. 2021, 200, 111490. [Google Scholar] [CrossRef] [PubMed]
- Bardos, R.P.; Bone, B.D.; Boyle, R.; Evans, F.; Harries, N.D.; Howard, T.; Smith, J.W.N. The Rationale for Simple Approaches for Sustainability Assessment and Management in Contaminated Land Practice. Sci. Total Environ. 2016, 563–564, 755–768. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Green Remediation: Incorporating Sustainable Environmental Practices into Remediation of Contaminated Sites; United States Environmental Protection Agency: Washington, DC, USA, 2008.
- The Interstante Technology & Regulatory Council (ITRC). Sustainable Resilient Remediation (SRR); The Interstante Technology & Regulatory Council (ITRC): Washington, DC, USA, 2021. [Google Scholar]
- Kumar, G.; Reddy, K.R. Addressing Climate Change Impacts and Resiliency in Contaminated Site Remediation. J. Hazard. Toxic. Radioact. Waste 2020, 24, 04020026. [Google Scholar] [CrossRef]
- Grifoni, M.; Franchi, E.; Fusini, D.; Vocciante, M.; Barbafieri, M.; Pedron, F.; Rosellini, I.; Petruzzelli, G. Soil Remediation: Towards a Resilient and Adaptive Approach to Deal with the Ever-Changing Environmental Challenges. Environments 2022, 9, 18. [Google Scholar] [CrossRef]
- Panagiotakis, I.; Dermatas, D. New European Union Soil Strategy: A Potential Worldwide Tool for Sustainable Waste Management and Circular Economy. Waste Manag. Res. 2022, 40, 245–247. [Google Scholar] [CrossRef]
- Hou, D.; Qi, S.; Zhao, B.; Rigby, M.; O’Connor, D. Incorporating Life Cycle Assessment with Health Risk Assessment to Select the ‘greenest’ Cleanup Level for Pb Contaminated Soil. J. Clean. Prod. 2017, 162, 1157–1168. [Google Scholar] [CrossRef]
- Kim, D.H.; Hwang, B.R.; Moon, D.H.; Kim, Y.S.; Baek, K. Environmental Assessment on a Soil Washing Process of a Pb-Contaminated Shooting Range Site: A Case Study. Environ. Sci. Pollut. Res. 2013, 20, 8417–8424. [Google Scholar] [CrossRef]
- Huysegoms, L.; Cappuyns, V. Critical Review of Decision Support Tools for Sustainability Assessment of Site Remediation Options. J. Environ. Manag. 2017, 196, 278–296. [Google Scholar] [CrossRef] [PubMed]
- Careghini, A.; Mastorgio, A.; Sala, A.; Saponaro, S.; Sezenna, E. Sustainable Remediation: Which Approach Should I Use? In Bioremediation and Sustainable Environmental Technologies—2015, Proceedings of the Third International Symposium on Bioremediation and Sustainable Environmental Technologies; Darlington, R., Barton, A.C., Eds.; Battelle Memorial Institute: Miami, FL, USA, 2015. [Google Scholar]
- Xiao, M.; Li, X.; Seuntjens, P.; Sharifi, M.; Mao, D.; Dong, J.; Yang, X.; Zhang, H. Qualitative and Quantitative Simulation of Best Management Practices (BMPs) for Contaminated Megasite Remediation Using the SiteWiseTM Tool. J. Env. Manag. 2024, 360, 121098. [Google Scholar] [CrossRef] [PubMed]
- Favara, P.; Raymond, D.; Ambrusch, M.; Libera, A.; Wolf, G.; Simon, J.A.; Maco, B.; Collins, E.R.; Harclerode, M.A.; McNally, A.D.; et al. Ten Years Later: The Progress and Future of Integrating Sustainable Principles, Practices, and Metrics into Remediation Projects. Remediation 2019, 29, 5–30. [Google Scholar] [CrossRef]
- Smith, J.W.N. Debunking Myths about Sustainable Remediation. Remediation 2019, 29, 7–15. [Google Scholar] [CrossRef]
- Cappuyns, V. Inclusion of Social Indicators in Decision Support Tools for the Selection of Sustainable Site Remediation Options. J. Environ. Manag. 2016, 184, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, R.; Harries, N.; Bardos, P. Case Studies and Analysis of Sustainable Remediation Techniques and Technologies; Cincawe: Brussels, Belgium, 2023. [Google Scholar]
- Reddy, K.R.; Chirakkara, R.A. Green and Sustainable Remedial Strategy for Contaminated Site: Case Study. Geotech. Geol. Eng. 2013, 31, 1653–1661. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Qadir, Z.; Asad, M.; Kouzani, A.Z.; Parvez Mahmud, M.A. Environmental Footprint Assessment of a Cleanup at Hypothetical Contaminated Site. Appl. Sci. 2021, 11, 4907. [Google Scholar] [CrossRef]
- Pagano, M.; Mosangini, C.; Avantaggiato, A.; Midence, R. A Low Impact Technology Chemical Oxidation, Bioremediation and Groundwater Reinjection Analysed with SiteWise TM and SEFA. In Proceedings of the Ecomondo, Rimini, Italy, 6–9 November 2018; pp. 1–5. [Google Scholar]
- Søndergaard, G.L.; Binning, P.J.; Bondgaard, M.; Bjerg, P.L. Multi-Criteria Assessment Tool for Sustainability Appraisal of Remediation Alternatives for a Contaminated Site. J. Soils Sediments 2018, 18, 3334–3348. [Google Scholar] [CrossRef]
- Filote, C.; Roșca, M.; Simion, I.M.; Hlihor, R.M. Continuous Systems Bioremediation of Wastewaters Loaded with Heavy Metals Using Microorganisms. Processes 2022, 10, 1758. [Google Scholar] [CrossRef]
- Villares, M.; Işildar, A.; Mendoza Beltran, A.; Guinee, J. Applying an Ex-Ante Life Cycle Perspective to Metal Recovery from e-Waste Using Bioleaching. J. Clean. Prod. 2016, 129, 315–328. [Google Scholar] [CrossRef]
- Ding, N.; Meng, X.; Zhang, Z.; Ma, J.; Shan, Y.; Zhong, Z.; Yu, H.; Li, M.; Jiao, W. A Review of Life Cycle Assessment of Soil Remediation Technology: Method Applications and Technological Characteristics. Rev. Environ. Contam. Toxicol. 2024, 262, 4. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, M. Providing Efficient Decision Support for Green Operations Management: An Integrated Perspective. In Efficient Decision Support Systems—Practice and Challenges in Multidisciplinary Domains; Jao, C., Ed.; IntechOpen: Rijeka, Croatia, 2011; pp. 247–270. [Google Scholar]
- Magrini, C.; Jagodzińska, K. Can Bioleaching of NIB Magnets Be an Answer to the Criticality of Rare Earths? An Ex-Ante Life Cycle Assessment and Material Flow Cost Accounting. J. Clean. Prod. 2022, 365, 132672. [Google Scholar] [CrossRef]
- Di Maria, A.; Khoshkhoo, M.; Sand, A.; Van Acker, K. Towards Sustainable Resource Valorization: A Life Cycle Sustainability Assessment of Metals Recovery from Sulfidic Mining Residues in Sweden. Resour. Conserv. Recycl. 2024, 204, 107513. [Google Scholar] [CrossRef]
- Diwan, H.; Mandy, J. Technological Assessment of Waste Value Change: Implementation of CE Concepts for Implementing Loop Economy. Technol. Anal. Strat. Manag. 2024, 1–18. [Google Scholar] [CrossRef]
- Broadhurst, J.L.; Kunene, M.C.; Von Blottnitz, H.; Franzidis, J.P. Life Cycle Assessment of the Desulfurisation Flotation Process to Prevent Acid Rock Drainage: A Base Metal Case Study. Min. Eng. 2015, 76, 126–134. [Google Scholar] [CrossRef]
- Sun, M.; Wang, Y.; Hong, J.; Dai, J.; Wang, R.; Niu, Z.; Xin, B. Life Cycle Assessment of a Bio-Hydrometallurgical Treatment of Spent Zn-Mn Batteries. J. Clean. Prod. 2016, 129, 350–358. [Google Scholar] [CrossRef]
- Zhang, C.L.; Wang, J.W.; Bai, J.F.; Wu, W.J. Life Cycle Assessment of the Bio- Hydrometallurgical Process of Recycling Copper from Printed Circuit Boards Scraps. Adv. Manuf. Technol. 2011, 156–157, 929–932. [Google Scholar] [CrossRef]
- Stamp, A.; Althaus, H.J.; Wäger, P.A. Limitations of Applying Life Cycle Assessment to Complex Co-Product Systems: The Case of an Integrated Precious Metals Smelter-Refinery. Resour. Conserv. Recycl. 2013, 80, 85–96. [Google Scholar] [CrossRef]
- Falke, A.; Höck, M. Ex-Ante Life Cycle Assessment of Bioleaching in Indium Recovery from LCD-Waste. MRS Adv. 2019, 4, 1949–1955. [Google Scholar] [CrossRef]
- Konaré, Z.M.; Ajayi, D.D.; Ba, S.; Aremu, A.K. Application of Life Cycle Sustainability Assessment (LCSA) in the Gold Mining Sector: A Systematic Review. Int. J. Life Cycle Assess. 2023, 28, 684–703. [Google Scholar] [CrossRef]
- Villares, M.; Işıldar, A.; van der Giesen, C.; Guinée, J. Does Ex Ante Application Enhance the Usefulness of LCA? A Case Study on an Emerging Technology for Metal Recovery from e-Waste. Int. J. Life Cycle Assess. 2017, 22, 1618–1633. [Google Scholar] [CrossRef]
- Agusdinata, D.B.; Liu, W.; Sulistyo, S.; LeBillon, P.; Wegner, J. Evaluating Sustainability Impacts of Critical Mineral Extractions: Integration of Life Cycle Sustainability Assessment and SDGs Frameworks. J. Ind. Ecol. 2023, 27, 746–759. [Google Scholar] [CrossRef]


| Microbial Group | Solid Wastes | Metal Recovery (%) | Operating Conditions | References |
|---|---|---|---|---|
| Bacteria | ||||
| Mixed A. ferrooxidans with A. thiooxidans Mixed L. ferrooxidans with A. thiooxidans | Copper- Sulfide ore | Cu: 70% Cu: 35% | - 250 mL Erlenmeyer flasks containing 5 g copper sulfide ore+ 90 mL medium + 10 mL bacterial inoculum; - Agitation: 170 rpm; - Temperature: 30 °C; - Time (days): 35; - pH = 2.3. | [94] |
| Mixed acidophilic microorganisms Leptospirillum ferriphilum, Acidithiobacillus caldus, and Sulfobacillus acidophilus | Copper-containing electroplating sludge (CCES) | Cu: 84.3% | - 500 mL shake flasks containing 250 mL basalt medium + 50 mL of fresh mixed consortium with 15 g CCES; - Agitation: 180 rpm; - Temperature: 45 °C; - Time (days): 10; - pH = 1.8–2.38. | [96] |
| Microbial consortium of Leptospirillum ferriphilum and Sulfobacillus thermosulfidooxidans | Printed circuit boards (PCBs) | Cu: 93.4% | - 250 mL Erlenmeyer flask with 10% inoculum, cultivated at the initial pH of 0.9, 42 °C, and 200 rpm with 5 g/L PCBs; - Time (days): 9; - Pulp density: 100 g/L. | [100] |
| Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans isolated from native excess activated sludge (individual and mixed consortium) | Activated sludge | Mixed culture: 98.32% Cu and 98.60% Zn A. ferrooxidans: 95.98% of Cu and 96.49% of Zn A. thiooxidans: 95.87% of Cu and 96.83% of Zn. | - 500 mL Erlenmeyer flasks containing 200 mL distilled water with 5% (w/v) sludge and culture medium; - pH = 4 to 2 after 9 days; - Agitation: 150 rpm; - Time: 9 days; - Temperature: 30 °C; - A. ferrooxidans bioleaching system: 8.8 g FeSO4·7H2O and 10% (v/v) A. ferrooxidans; - A. thiooxidans bioleaching system, 2.0 g sulfur and 10% (v/v) A. thiooxidans; - Mixed culture of A. ferrooxidans and A. thiooxidans: 8.8 g FeSO4∙7H2O and 2.0 g S, together with 5% (v/v) A. ferrooxidans and 5% (v/v) A. thiooxidans. | [101] |
| Mixed culture of Thiobacillus ferrooxidans, Thiobacillus thiooxidans, and Leptospirillum ferrooxidans | Polymetallic concentrates | In all tested conditions, copper and zinc maximum extractions were above 95% within 48 h; Optimum leaching time: 50 h. | - Two stage bioleaching process: fermentor filled with ceramic rings as biofilm carriers, operated in batch mode, and inoculated with the substrate-adapted cultures; - Pulp solids density: 5–20%; - Initial iron concentration: 0–15 g/L; - pH = 1.9; - Temperatures: 25, 35, and 50 °C; - Agitation: 300 and 700 rpm; - Time: 0–190 h. | [103] |
| Mixed bacterial culture of Acidithiobacillus thiooxidans and Acidithiobacillus ferrooxidans | Waste from metal ore-mining processes/soil contaminated with metals | 50% Zn and 19% Fe | - 250 mL Erlenmeyer flasks containing 10 g of sterilized contaminated soil, 90 mL of 0K medium (pH 1.7), and 10 mL of cell suspension; - Temperature: 30 °C; - Agitation: 150 rpm; - Time: 42 days. | [123] |
| Acidithiobacillus ferrooxidans | Light emitting diode (LED) waste | Adapted A. ferrooxidans had higher metal bioleaching rates than the non-adapted strain: 84% Cu, 96% Ni, and 60% Ga. The highest amount of LED powder tolerated by A. ferrooxidans was 20 g/L. | - 250 mL Erlenmeyer flask; - The adaptation of cell culture was carried out in five steps of 5, 10, 15, 20, and 25 g/L LED powder, respectively - Temperature: 29 °C; - pH = 2; - Agitation: 140 rpm; - Time: 30 days. | [124] |
| Pure and mixed cultures of moderately thermophilic bacteria Sulfobacillus thermosulfidooxidans and acidophilic heterotroph A1TSB | Electronic scrap/printed circuit boards (PCBs) | Maximum bioleachability: washed electronic scrap with mixed consortium of metal-adapted culture: 89% Cu, 81% Ni, 79% Al, and 83% Zn | - 250 mL Erlenmeyer flasks with 10% scrap concentration under different experimental conditions; - Temperature: 45 °C; - pH = 1.2–2; - Time: 18 days. | [125] |
| Consortium of autotrophic bacteria Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans | Abundant Li ore (lepidolite) | Bioleaching yield of almost 9% Li | - 250 mL Erlenmeyer flasks containing 190 mL of nutrient rich or poor medium + 10 mL of adapted bacterial consortium + 10 g/L crushed lepidolite; - Temperature: 30 °C; - pH =1.5; - Agitation: 160 rpm; - Time: 366 days. | [110] |
| Fungi | ||||
| Aspergillus niger | Abundant Li ore (lepidolite) | Bioaccumulation of Li into the biomass was observed: 77% of the total solubilized Li Lowest bioleaching yield of 0.2% Li | - 250 mL Erlenmeyer flasks containing 200 mL of standard liquid bioleaching media + 10 g/L crushed lepidolite; - Prior to leaching, the medium and mineral were sterilized; - Temperature: 21 °C; - pH = 5.1; - Agitation: 160 rpm; - Time: 366 days. | [110] |
| Aspergillus niger (M1DGR), Aspergillus fumigatus (M3Ai), and Penicillium rubens (M2Aii) | Industrial contaminated soil | All isolates (A. fumigatus, Penicillium rubens, and A. niger) showed higher efficiency for Cd removal with 79%, 98%, and 98% in Sabouraud dextrose broth (SDB) medium A. niger and A. fumigatus showed higher efficiency for Cr (43% and 69%) in SDB medium | - A two-stage bioleaching process: 250 mL flask containing three different media (yeast peptone glucose (YPG), Sabouraud dextrose broth (SDB), and CM) + 2.5 g of sterilized contaminated soil sample; - Agitation: 130 rpm; - Temperature: 30 °C; - Time: 72 h. | [113] |
| Aspergillus sclerotiorum (A1), Aspergillus aculeatus (E1), Aspergillus niger (G03), Komagataella phaffii (WS), and Trichoderma harzianum (Y1) | Contaminated mining soil | The better bioaccumulation capacity was exhibited by K. phaffii: Cd (25.23 mg/g); Cu (21.63 mg/g); Pb (20.63 mg/g), | - About 0.8 g of fresh biomass was transferred to 20 mL PDB, supplemented with different metal concentrations (50, 100, 150, and 200 ppm); - pH = 5; - Temperature: 25 °C; - Agitation: 150 rpm; - Time: 7 days. | [115] |
| Mixed fungal culture of Purpureocillium lilacinum and Aspergillus niger | Waste PCBs | Extraction efficiency: 56.1% (Cu), 15.7 % (Al), 20.5% (Pb), 49.5% (Zn), and 8.1% (Sn) | - 3 L stirred tank reactor with 8% (w/v) pulp density of sterile waste PCBs; - Temperature: 30 °C; - Agitation: 300 rpm; - Air flow rate = 500 mL/min; - pH = 5. | [117] |
| Aspergillus fumigatus | Soil mine tailings | One-step process at 1% (w/v): Pb (56%), As (62%), Fe (58%), Mn (100%), Zn (54%) Two-step process at 1% (w/v): Pb (88%), As (32%), Fe (45%), Mn (58.4%), Zn (31.3%) | - One-step process: sterilized 250 mL Erlenmeyer flasks containing 1 mL spore suspension inoculated + 100 mL sucrose medium + sterilized tailings samples at 1%, 2%, 4%, or 8% (w/v); - Two-step process: 250 mL Erlenmeyer flask containing 100 mL sterilized sucrose medium inoculated + 1 mL of fungal spore suspension +, after 15 days, sterilized tailings samples at 1%, 2%, 4%, or 8% (w/v) were added; - Temperature: 30 °C; - Agitation: 150 rpm; - pH = 2.75 to 8.16; - Time: 40 days. | [126] |
| Aspergillus niger | Power plant residual (PPR) ash | At maximum pulp density of 9 (%w/v): V (83%) and Ni (30%) | - Spent-medium bioleaching: bubble column bioreactor fermentation experiments followed by leaching tests in Erlenmeyer flasks with various pulp densities of PPR ash (1, 2, 3, 5, 7, and 9 (%w/v)); - Time: 7 days; - Temperature: 60 °C; - Agitation: 130 rpm; - Optimum conditions in bubble column: Aeration rate of 762.5 (mL/min), sucrose concentration of 101.9 (g/L), and inoculum size of 40 (mL/L). | [127] |
| Soil Type | Pollutant Type | Bioleaching Strain | Operating Conditions | Efficiency | References |
|---|---|---|---|---|---|
| Industrial soil sites (Pakistan) | Pb, Hg | Indigenous fungi isolated from soil (Aspergillus niger (M1), Aspergillus fumigatus (M3), Aspergillus terreus (M6), and Aspergillus flavus (M7)) | - Lab scale: 250 mL pre-sterilized conical flasks inoculated with types of media (YPG, CYE, and SDB); - Two-step bioleaching; - Temperature: 30 °C; - Shaking at 120 rpm for 120 h. | - Pb removal rate at 99.20% and 99.30% using A. fumigatus and A. flavus, respectively; - Hg removal rate of 96% and 95.50% in the YPG medium using A. niger and A. terreus, respectively; - The highest Pb uptake efficiency: 8.52 mg/g in the YPG medium; - The highest Hg uptake efficiency: 0.41 mg/g in the CYE medium. | [113] |
| Industrial soil site (smeltery) (China) | Cd, Cu, Pb, Zn | Penicillium chrysogenum strain F1 | - Lab-scale: 250 mL flask; - Two-step bioleaching; - Temperature: 28 °C; - Shaking at 120 rpm for 8 days. | - Cd: 152 mg/kg, 30.8%; - Cu: 564 mg/kg, 97.5%; - Pb: 3160 mg/kg, 32.8%; - Zn: 7812 mg/kg, 80.4%. | [139] |
| Industrial soil site (smeltery) (China) | Zn, Pb, Mn, Cd, Cu | Microorganism isolated from a vegetable oil sample: Burkholderia sp. Z-90 | - Lab-scale: 500 mL flask; - Two-step bioleaching; - Temperature: 35 °C; - Shaking at 180 rpm for 5 days. | - 44.0% for Zn, 32.5% for Pb, 52.2% for Mn, 37.7% for Cd, 24.1% for Cu, and 31.6% for As. | [140] |
| Contaminated soil from a smelting plant (China) | Pb, Zn, Cd, Cu | Aspergillus niger F2 | - Lab-scale: 250 mL flask; - Two-step bioleaching; - Temperatures: 25 °C, 30 °C, and 35 °C; - pH = 5, 7, and 9; - Shaking at 120 rpm for 7 days. | - At 30 °C, pH = 5, bioleaching by A. niger with sucrose, glucose, maltose, lactose, and starch: 69.86% for Cd, 66.57% for Cu, 64.59% for Pb, and 69.01% for Zn. | [141] |
| Agricultural soil (Clemson University, South Carolina) | As | Aspergillus niger | - Lab-scale: 250 mL flask containing 180 mL growth medium + 15 g soil + 1 mL of A. niger inoculum (with or without glucose as a carbon source); - One-step bioleaching; - Temperature: 30 °C; - Shaking at 200 rpm for 28 days. | - 7.9% removal of As in case of glucose/ A. niger treatment. | [142] |
| Industrial soil sites (Romania) | Cu, Pb, Cr, Ni | Thiobacillus ferrooxidans (TF) | - Lab-scale: 250 mL flask: TF inoculated in 9K medium * (20 mL or and 40 mL) +10 g of soil; - One-step bioleaching; - Temperature: 27 °C; - Shaking at 200 rpm for 12 h. | Cu: 29–76%; Pb: 10–32%; Cr: 39–72%; Ni: 44–68%. | [143] |
| Soil of smelting industry site (China) | Cd, Pb, Zn | Aspergillus flavus | - One-step bioleaching: fungus incubated with the medium and sterile soil in a rotary shaking incubator for 15 days; - Two-step bioleaching: pure culture of the fungus was run for 6 days, and then sterile soil was added; bioleaching experiment: rotary shaker for 9 days. | - 130 mg/L sucrose for A. flavus in bioleaching for 15 days (16.38% for Pb, 30.55% for Cd and 52.66% for Zn); - Cd and Zn were higher in two-step bioleaching (49.66% for Cd and 65.73% for Zn); - Optimum conditions: sucrose as carbon source, pH 7, and 30 °C. | [144] |
| Agricultural soil (China) | Pb, Zn, Cr, Cu, Ni, Cd | Aspergillus tubingensis F12 | - Soil column: length of 2 cm, inner diameter of 1 cm with 1 g of soil + 20 mL of EPS solution. | - EPS adsorbed metals at a significantly higher rate than the F12 pellets. | [145] |
| Soil of smelting industry site (China) | Pb, Cr, Cd, Cu, Mn, Zn | Soil-originated Mn(II)-oxidizing bacteria (Providencia sp. LLDRA6)+ BioMnOx (oxidation of Mn(II) into BioMnOx on the cell surface) | - 500 mL of leachate in 1 L Erlenmeyer flask, continuously shaking at 35 °C at 180 rpm; - Solid ratio: 1:5, 1:10, 1:15 and 1:20 (w/v). | Pb: 81.72%; Cr: 88.29%; Cd: 90.34%; Cu: 91.25%; Mn: 56.13%; Zn: 59.83%. | [146] |
| Soil sample collected from uranium tailings (China) | U | Acidithiobacillus ferrooxidans ATCC 23,270, Leptospirillum ferriphilum YSK, Acidithiobacillus thiooxidans A01, and Acidithiobacillus ferrivorans YL15 | - Lab-scale: 250 mL flask: 15 g of sterilized soil + 150 mL culture medium + 5 g/L iron +5 g/L elemental sulfur inoculated with the four bacteria in a volume ratio of 1:1:1:1 | - 85.81% removal of U, in case of mixed consortium. | [147] |
| Sediment samples (Deûle Canal, France) | Mn, Zn, Cu, Cd, Pb | - Fungi (Aspergillus niger and Penicillium chrysogenum); - Indigenous microflora. | - Semi-pilot scale: 45 L air-lift bioreactors; - Temperature: 30 °C; - Fungal leaching experiment: inoculum ratio of 10% (w/w); - Bacterial leaching experiment: inoculum ratio of 5% (w/w) sulfur enrichment. | - Fungi under saccharose treatment after 45 days: 77% for Mn, 44% for Zn, 12% for Cu, 1.6% for Cd, and <2% for Pb; - Bacteria under sulfur treatment after 30 days: 72% of Cu, 85% of Mn, 91% of Zn, and 93% of Cd. | [148] |
| Sediment samples (Puti Lake, China) | Cu, Ni, Zn | Indigenous sludge bacteria obtained from the Hangzhou Qige Wastewater Treatment Plant | - Lab-scale: 250 mL flask (150 mL working volume); - Inoculum: 1% (v/v); - Solid ratio: 1% (w/v); - Sulfur: 3 g/L; - Initial pH: 8.0; - Temperature: 28 °C; - Shaking at 180 rpm for 9 days. | - Cu: 284 mg/kg, 74.27%; - Ni: 84 mg/kg, 35.35%; - Zn: 394 mg/kg, 69.92%. | [149] |
| Contaminated sediment samples from Ell Ren River (southern Taiwan) | Zn, Cu, Ni, Cr | Indigenous sulfur-oxidizing microorganisms from sediment | - Continuous bioleaching experiments: 50 L (working volume) continuous stirred-tank reactor (CSTR) agitated at 200 rpm and 30 °C; - 10% (v/v) of acclimated sulfur-oxidizing microorganisms; - Hydraulic retention time: 10 days; - Aeration rate: 12 L-air/min; - Operation time: 30 days. | -At 3–5% of sulfur dosage: Zn: 47–81%; Ni: 60–93%; Cu: 41–91%; Cr: 13–72%. | [150] |
| Sludge mine sediment (Czech Republic) | Fe, Zn, Cu, Pb | Acidithiobacillus ferrooxidans | - Bioleaching of samples in pilot plant conditions in a Bioflo/CelliGen 310 bioreactor; - Temperature: 30 °C; - pH = 2.0; - 2.5% and 4.2% (w/v) pulp densities; - Agitation: 150 rpm; - Particle size < 40–200 µm; - Time: 42 days. | - At 2.5% (w/v): Zn: 97.08%; Fe: 58.75%; Cu: 79.11%; Pb: 89.35%. - At 4.2% (w/v): Zn: 95.03%; Fe: 47.08%; Cu: 73.03%; Pb: 80.69%. | [151] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cozma, P.; Bețianu, C.; Hlihor, R.-M.; Simion, I.M.; Gavrilescu, M. Bio-Recovery of Metals through Biomining within Circularity-Based Solutions. Processes 2024, 12, 1793. https://doi.org/10.3390/pr12091793
Cozma P, Bețianu C, Hlihor R-M, Simion IM, Gavrilescu M. Bio-Recovery of Metals through Biomining within Circularity-Based Solutions. Processes. 2024; 12(9):1793. https://doi.org/10.3390/pr12091793
Chicago/Turabian StyleCozma, Petronela, Camelia Bețianu, Raluca-Maria Hlihor, Isabela Maria Simion, and Maria Gavrilescu. 2024. "Bio-Recovery of Metals through Biomining within Circularity-Based Solutions" Processes 12, no. 9: 1793. https://doi.org/10.3390/pr12091793









