Metal-Nitrate-Catalyzed Levulinic Acid Esterification with Alkyl Alcohols: A Simple Route to Produce Bioadditives
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Catalytic Runs
2.3. Reaction Monitoring and Identification of Main Reaction Products
3. Results
3.1. Catalytic Tests
3.1.1. Effect of Metal Nitrate Catalyst
3.1.2. Effect of Iron Catalyst Nature
3.1.3. Effect of Fe(NO3)3 Catalyst Concentration
3.1.4. Effect of Reaction Temperature
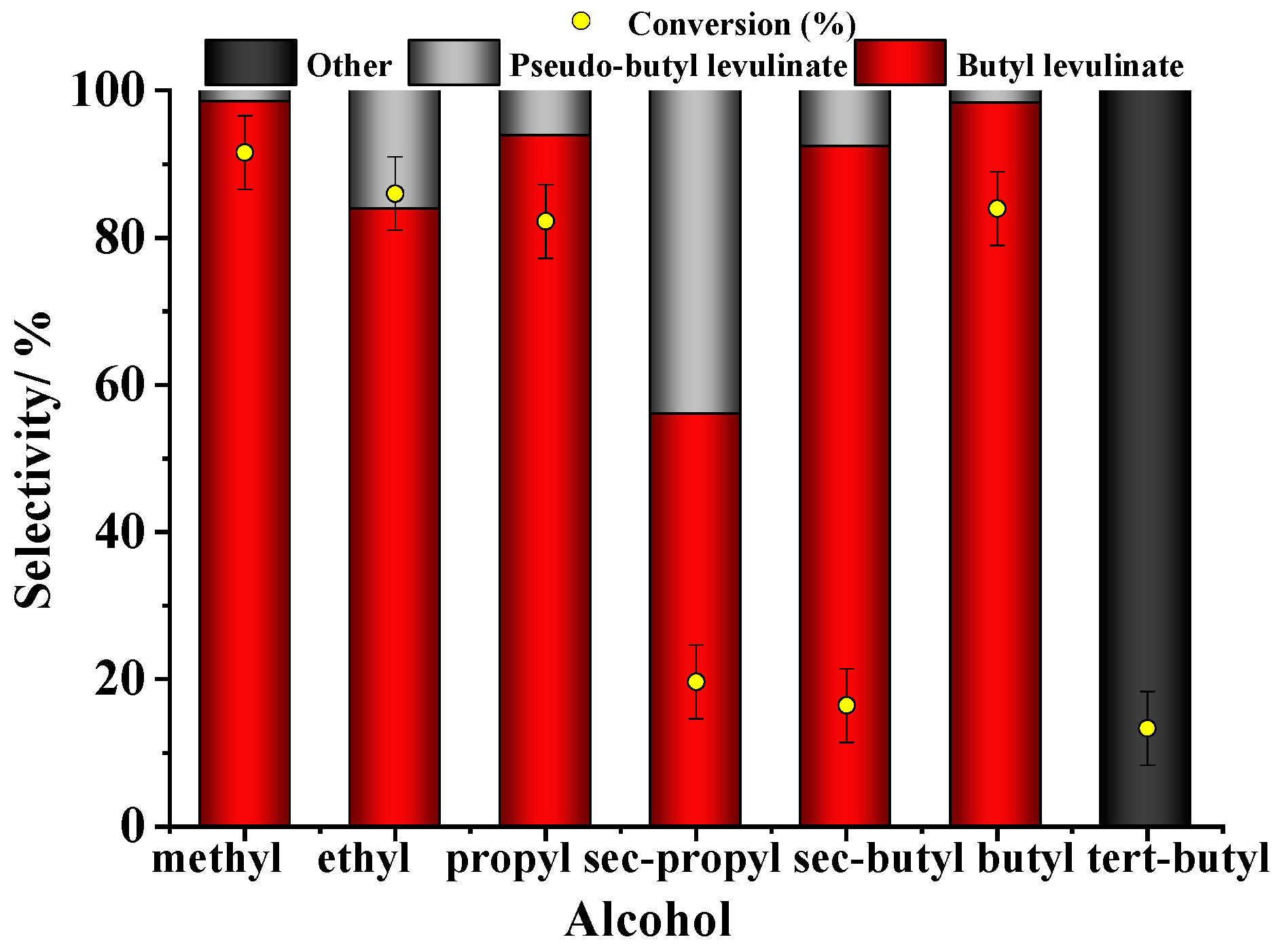
3.1.5. Effect of Alcohol in Levulinic Acid Esterification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lange, J.-P.; van der Heide, E.; van Buijtenen, J.; Price, R. Furfural, A promising platform for lignocellulosic biofuels. ChemSusChem 2012, 5, 150–166. [Google Scholar] [CrossRef] [PubMed]
- Brodin, M.; Vallejos, M.; Opedal, M.T.; Cristina Area, M.; Chinga-Carrasco, G. Lignocellulosics as sustainable resources for production of bioplastics-A Review. J. Clean. Prod. 2017, 162, 646–664. [Google Scholar] [CrossRef]
- Foroutan, R.; Jamaleddin, S.; Peighambardoust, R.M.; Peighambardoust, S.H.; Ramavandi, B. Application of walnut shell ash/ZnO/K2CO3 as a new composite catalyst for biodiesel generation from Moringa oleifera oil. Fuel 2022, 311, 122624–122633. [Google Scholar] [CrossRef]
- Foroutan, R.; Peighambardoust, S.J.; Mohammadi, R.; Peighambardoust, S.H.; Ramavandi, B. Application of waste chalk/CoFe2O4/K2CO3 composite as a reclaimable catalyst for biodiesel generation from sunflower oil. Chemosphere 2022, 289, 133226–133237. [Google Scholar] [CrossRef]
- Di Bucchianico, D.D.M.; Wang, Y.; Buvat, J.-C.; Pan, Y.; Moreno, V.C.; Leveneur, S. Production of levulinic acid and alkyl levulinates: A process insight. Green Chem. 2022, 24, 614–646. [Google Scholar] [CrossRef]
- Khan, M.A.; Dharmalingam, B.; Chuetor, S.; Cheng, Y.-S.; Sriariyanun, M. Comprehensive review on effective conversion of lignocellulosic biomass to levulinic acid. Biomass Convers. Biorefinery 2023. [Google Scholar] [CrossRef]
- Galletti, A.M.R.; Antonetti, C.; Fulignati, S.; Licursi, D. Direct Alcoholysis of carbohydrate precursors and real cellulosic Biomasses to Alkyl Levulinates: A Critical Review. Catalysts 2020, 10, 1221. [Google Scholar] [CrossRef]
- Christensen, E.; Williams, A.; Paul, S.; Burton, S.; McCormick, R.L. Properties and performance of levulinate esters as diesel blend components. Energy Fuel 2011, 25, 5422–5428. [Google Scholar] [CrossRef]
- Werpy, T.; Petersen, G. Top Value-Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; Sugars, Candidates: Golden, CO, USA, 2004. [Google Scholar]
- Lei, T.; Wang, Z.; Chang, X.; Lin, L.; Yan, X.; Sun, Y.; Shi, X.; He, X.; Zhu, J. Performance and emission characteristics of a diesel engine running on optimized ethyl levulinate biodiesel diesel blends. Energy 2016, 95, 29–40. [Google Scholar] [CrossRef]
- Windom, B.C.; Lovestead, T.M.; Mascal, M.; Nikitin, E.B.; Bruno, T.J. Advanced distillation curve analysis on ethyl levulinate as a diesel fuel oxygenate and a hybrid biodiesel fuel. Energy Fuel 2011, 25, 1878–1890. [Google Scholar] [CrossRef]
- Joshi, H.J.; Moser, B.R.; Toler, J.; Smith, W.F.; Walker, T. Ethyl levulinate: A potential bio-based diluent for biodiesel which improves cold flow properties. Biomass Bioenergy 2011, 35, 3266. [Google Scholar] [CrossRef]
- Xu, W.-P.; Chen, X.-F.; Guo, H.-J.; Li, H.-L.; Zhang, H.-R.; Xiong, L.; Chen, X.-D. Conversion of levulinic acid to valuable chemicals: A review. J. Chem. Technol. Biotechnol. 2021, 96, 3009–3024. [Google Scholar] [CrossRef]
- Badgujar, K.C.; Badgujar, V.C.; Bhanage, B.M. Lipase as a green and sustainable material for production of levulinate compounds: State of the art. J. Mater. Sci. Energy Technol. 2022, 5, 232–242. [Google Scholar] [CrossRef]
- Liu, X.; Yang, W.; Zhang, Q.; Li, C.; Wu, H. Current approaches to alkyl levulinates via efficient valorization of biomass derivatives. Front. Chem. 2020, 8, 794–807. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Abdullah, F. Study of density, surface tension, and refractive index of binary mixtures containing alkyl levulinate and n-alcohol from 298 to 323. J. Chem. Eng. Data 2021, 66, 1856–1876. [Google Scholar] [CrossRef]
- Demolis, A.; Essayem, N.; Rataboul, F. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng. 2014, 2, 1338–1352. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Niphadkar, P.S.; Deshpande, S.S.; Bokade, V.V. Esterification of renewable levulinic acid to ethyl levulinate biodiesel catalyzed by highly active and reusable desilicated H-ZSM-5. J. Chem. Technol. Biotechnol. 2014, 89, 1507–1515. [Google Scholar] [CrossRef]
- Bringue, R.; Ramirez, E.; Iborra, M.; Tejero, J.; Cunill, F. Esterification of furfuryl alcohol to butyl levulinate over ion-exchange resins. Fuel 2019, 257, 116010. [Google Scholar] [CrossRef]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: Progress, challenges, and prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Cirujano, F.G.; Corma, A.; i Xamena, F.L. Conversion of levulinic acid into chemicals: Synthesis of biomass derived levulinate esters over Zr-containing MOFs. Chem. Eng. Sci. 2015, 124, 52–60. [Google Scholar] [CrossRef]
- Fernandes, D.R.; Rocha, A.S.; Mai, E.F.; Mota, C.J.A.; Teixeira da Silva, V. Levulinic acid esterification with ethanol to ethyl levulinate production over solid acid catalysts. Appl. Catal. A Gen. 2012, 425, 199–204. [Google Scholar] [CrossRef]
- Jia, B.; Liu, C.; Qi, X. Selective production of ethyl levulinate from levulinic acid by lipase-immobilized mesoporous silica nanoflowers composite. Fuel Process. Technol. 2020, 210, 106578. [Google Scholar] [CrossRef]
- da Silva, M.J.; Ayala, D.A.M. Unravelling transition metal-catalyzed terpenic alcohol esterification: A straightforward process for the synthesis of fragrances. Catal. Sci. Technol. 2016, 6, 3197–3207. [Google Scholar] [CrossRef]
- Leonard, N.; Oswald, M.; Freiberg, D.; Nattier, B.; Smith, R.; Mohan, R. A Simple and versatile method for the synthesis of acetals from aldehydes and ketones using bismuth triflate. J. Org. Chem. 2002, 67, 5202–5207. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.J.; Teixeira, M.G. Assessment on the double role of the transition metal salts on the acetalization of furfural: Lewis and Bronsted acid catalysts. Mol. Catal. 2018, 461, 40–47. [Google Scholar] [CrossRef]
- Smith, B.M.; Kubczyk, T.M.; Graham, A.E. Indium(III) triflate catalysed transacetalisation reactions of diols and triols under solvent-free conditions. Tetrahedron 2012, 68, 7775–7781. [Google Scholar] [CrossRef]
- da Silva, M.J.; de Oliveira, C.M. Metal nitrate-catalyzed one-pot oxidative esterification of benzaldehyde with hydrogen peroxide in alcoholic solutions at room temperature. New J. Chem. 2021, 45, 3683–3691. [Google Scholar] [CrossRef]
- da Silva, M.J.; Julio, A.A.; Mosqueira Ayala, D.A.; de Miranda, L.M.P. Fe(SO4)3-catalyzed synthesis of terpenic alcohols esters: A simple and bifunctional reusable solid catalyst. ChemistrySelect 2018, 3, 5742–5748. [Google Scholar] [CrossRef]
- Martins, F.P.; Rodrigues, F.A.; Silva, M.J. Fe2(SO4)3-catalyzed levulinic acid esterification: Production of fuel bioadditives. Energies 2018, 11, 1263. [Google Scholar] [CrossRef]
- Yu, B.-Y.; Tseng, T.-Y.; Yang, Z.-Y.; Shen, S.-J. Evaluation on the solketal production processes: Rigorous design, optimization, environmental analysis, and control. Process Saf. Environ. Prot. 2022, 157, 140–155. [Google Scholar] [CrossRef]
- Huang, Y.B.; Yang, T.; Cai, B.; Chang, X.; Pan, H. Highly Efficient Metal Salt Catalyst for the Esterification of Biomass Derived Levulinic Acid under Microwave Irradiation. RSC Adv. 2016, 6, 2106–2111. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; Pastore, C. Effective Upgrading of Levulinic Acid into Hexyl Levulinate Using AlCl3 6H2O as a Catalyst. Biomass 2023, 3, 266–278. [Google Scholar] [CrossRef]
- Jia, S.; Ma, J.; Wang, D.; Wang, K.; Zheng, Q.; Song, C.; Guo, X. Fast and Efficient Upgrading of Levulinic Acid into Long-Chain Alkyl Levulinate Fuel Additives with a Tungsten Salt Catalyst at Low Temperature. Sustain. Energy Fuels 2020, 4, 2018–2025. [Google Scholar] [CrossRef]
- Pearson, R. Absolute electronegativity and hardness: Application to inorganic chemistry. Inorg. Chem. 1988, 27, 734–740. [Google Scholar] [CrossRef]
- Zhao, W.; Ding, H.; Zhu, J.; Liu, X.; Xu, Q.; Yin, D. Esterification of levulinic acid into N-butyl levulinate catalyzed by sulfonic acid-functionalized lignin-montmorillonite complex. J. Bioresour. Bioprod. 2020, 5, 291–299. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Neves, S.C.G.; da Silva, M.J. Kinetic study of alcoholysis of the fatty acids catalyzed by tin chloride(II): An alternative catalyst for biodiesel production. Energy Fuels 2009, 23, 1718–1722. [Google Scholar] [CrossRef]
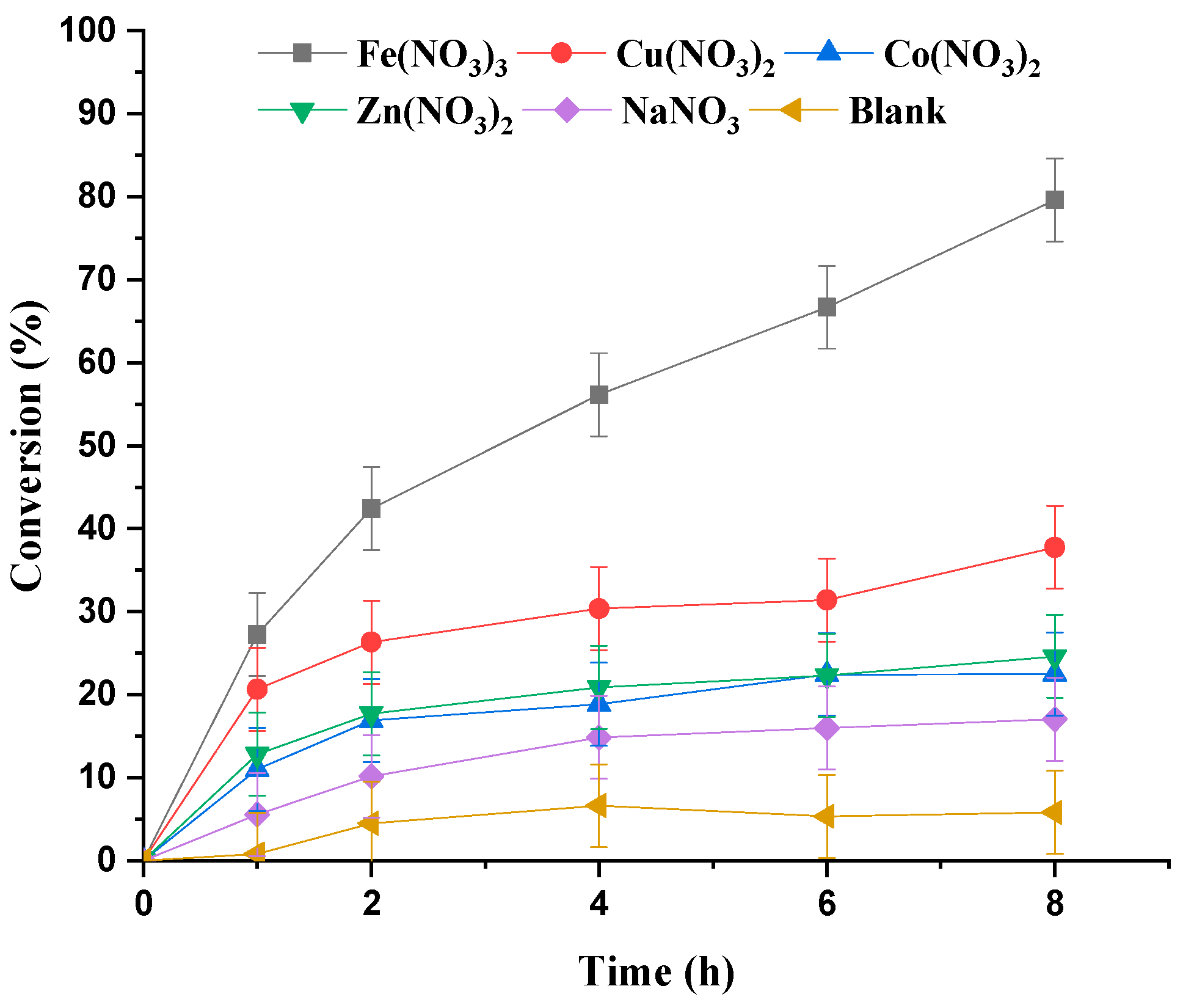



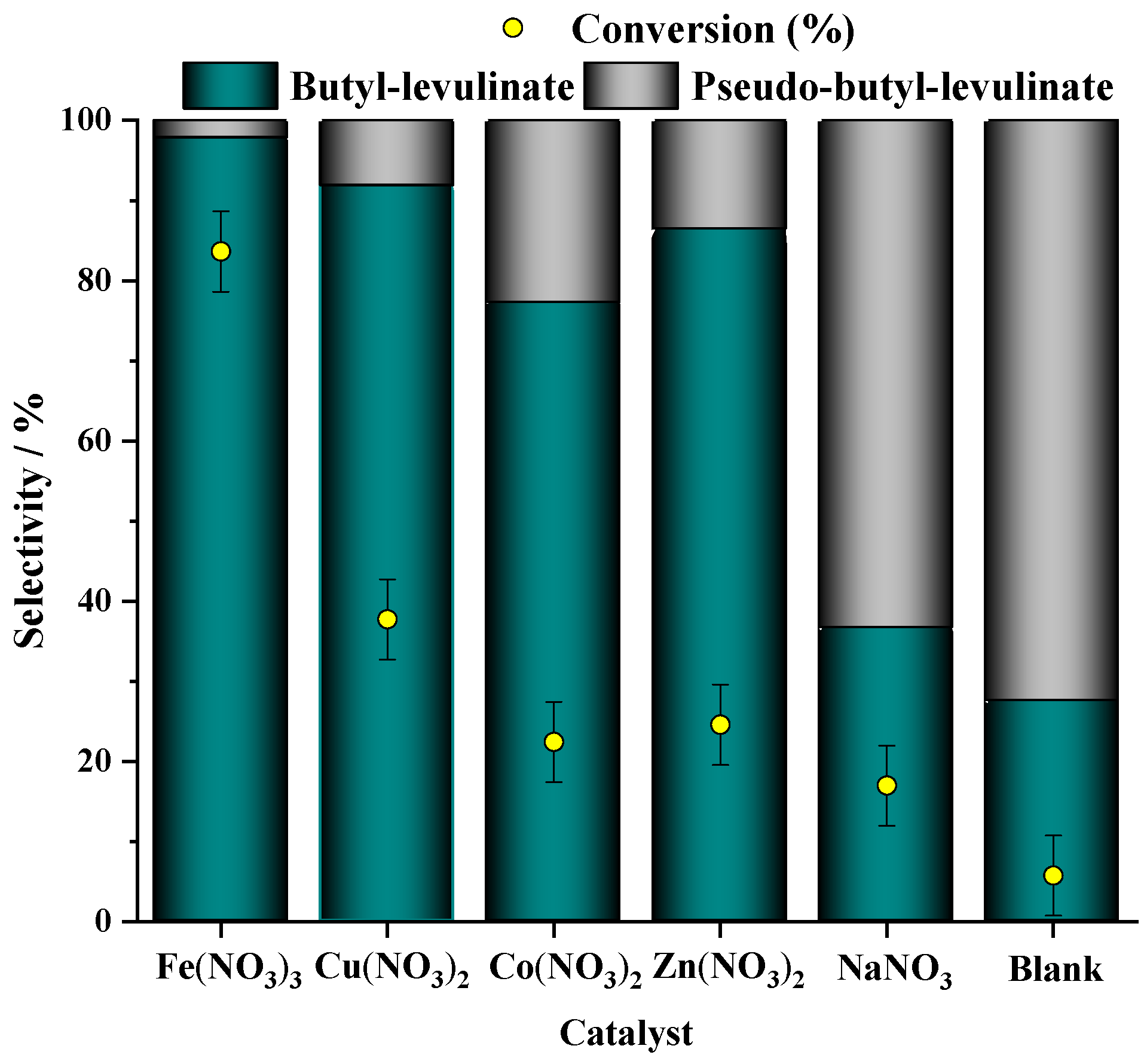
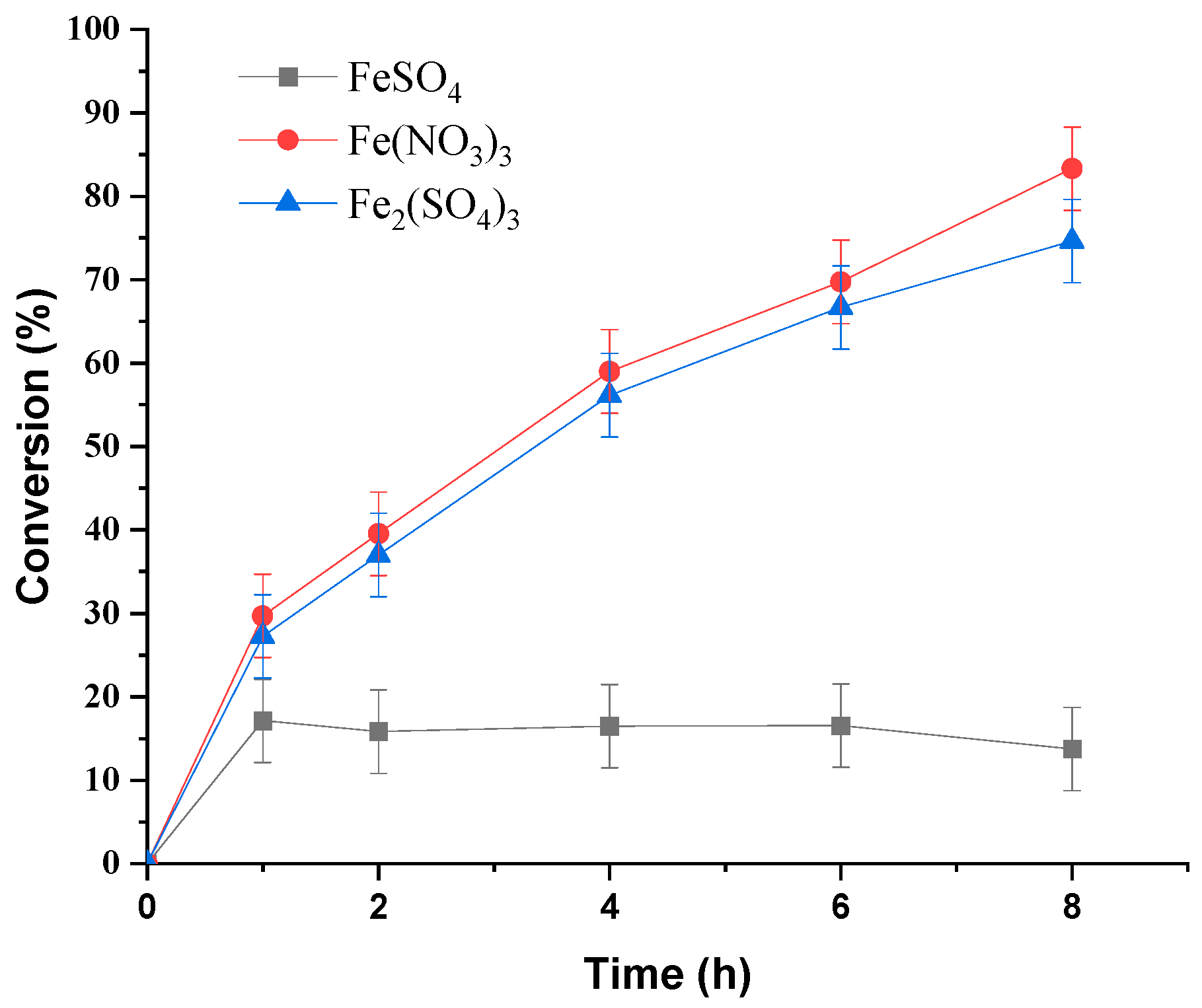
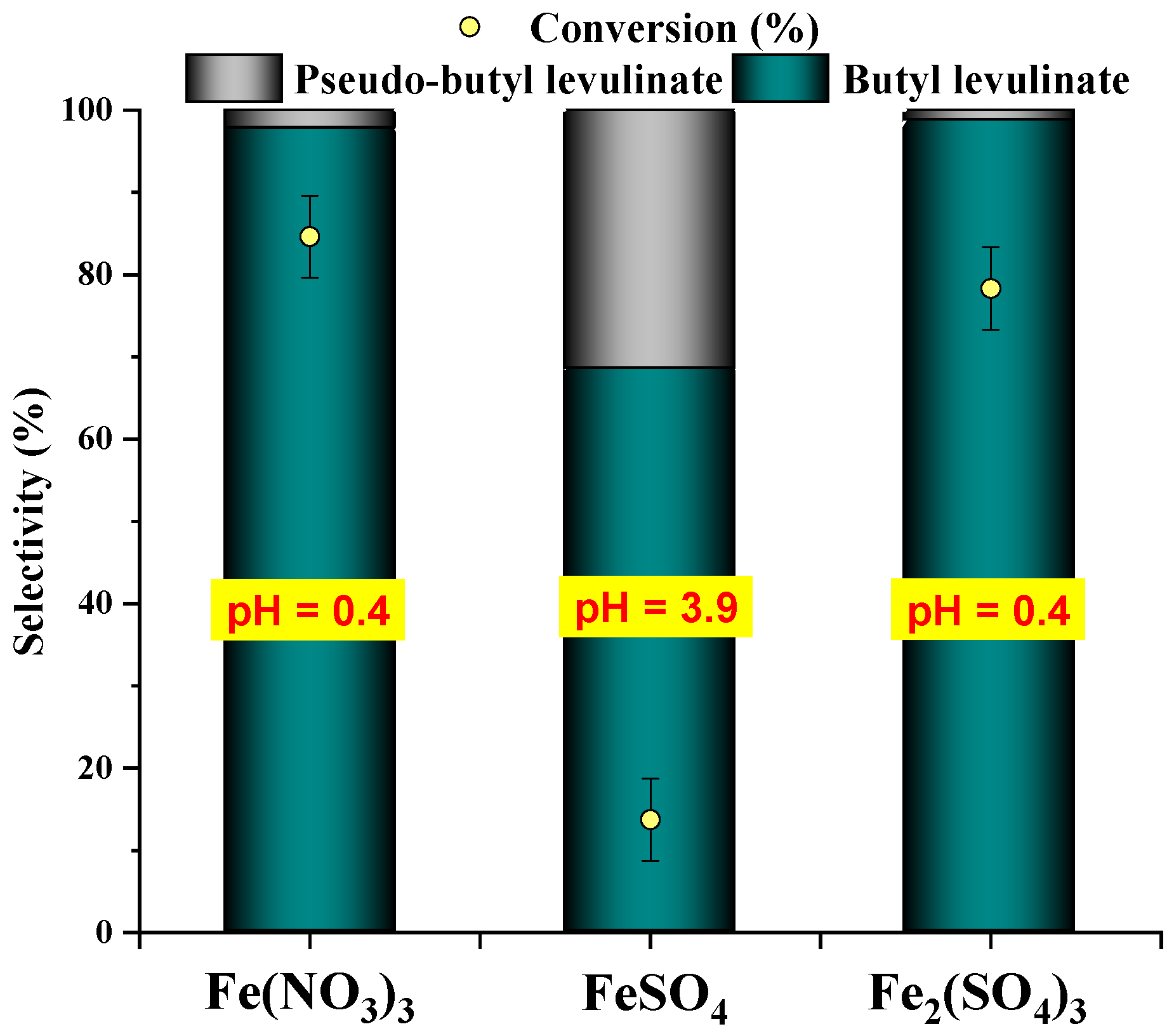

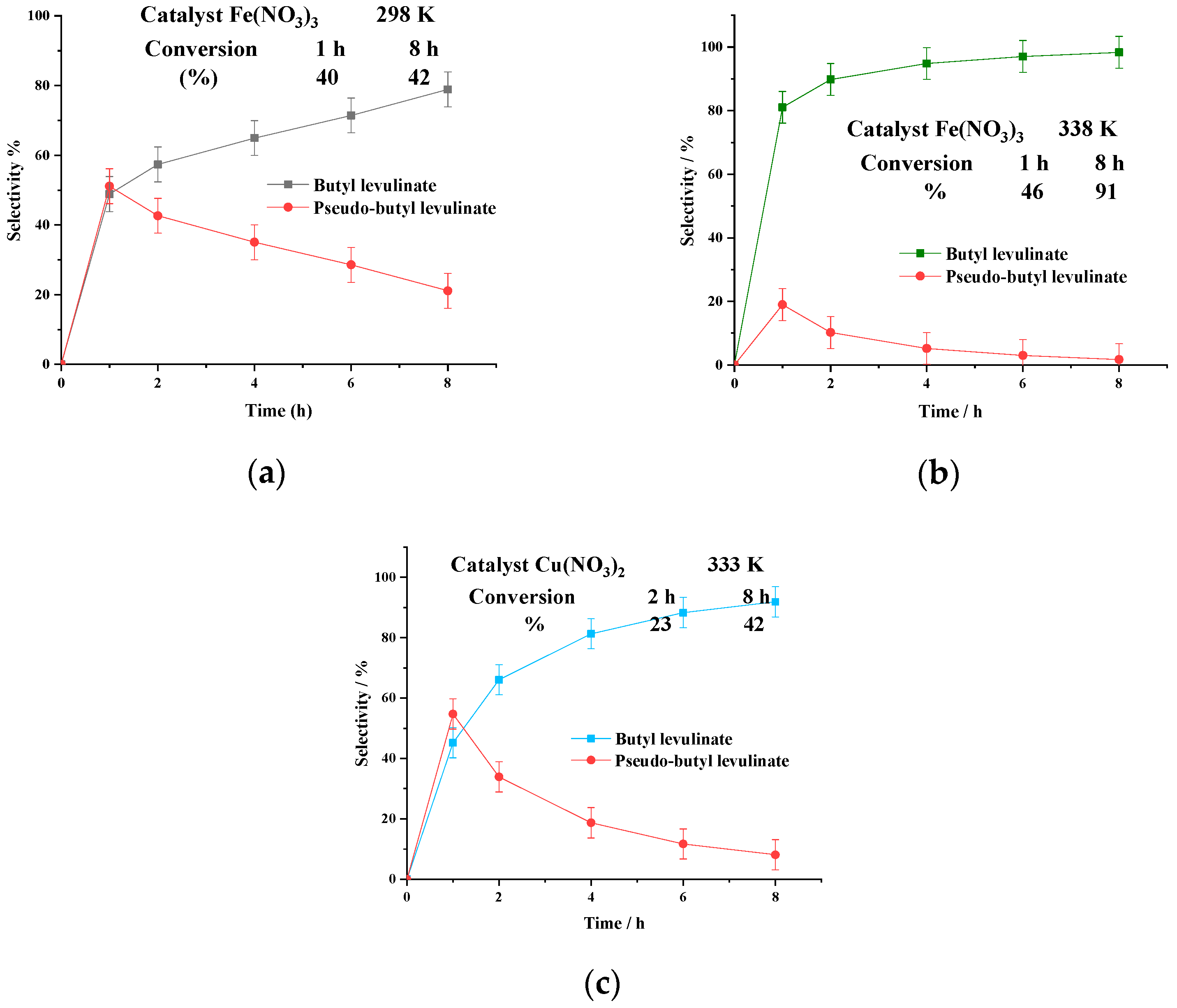
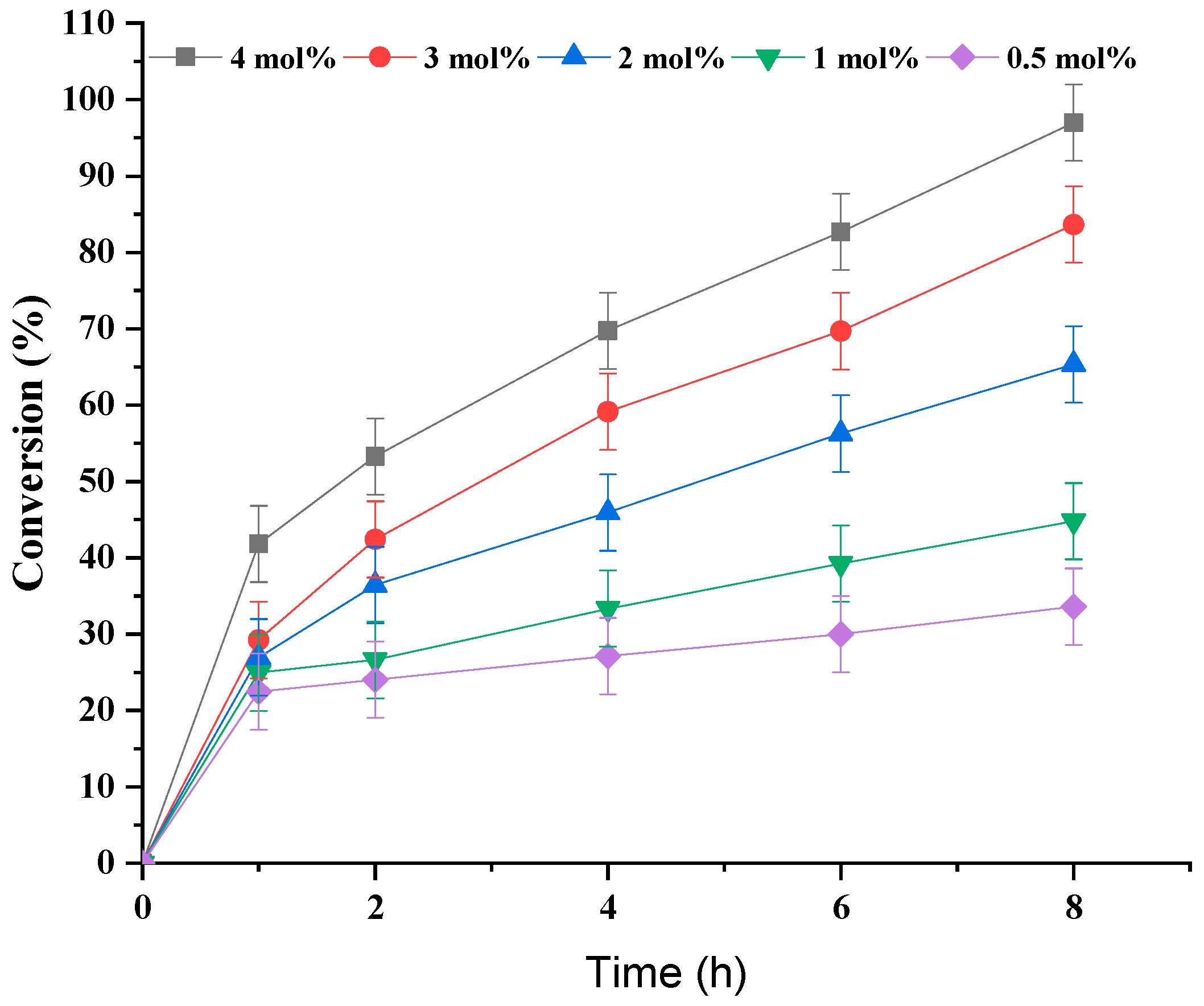
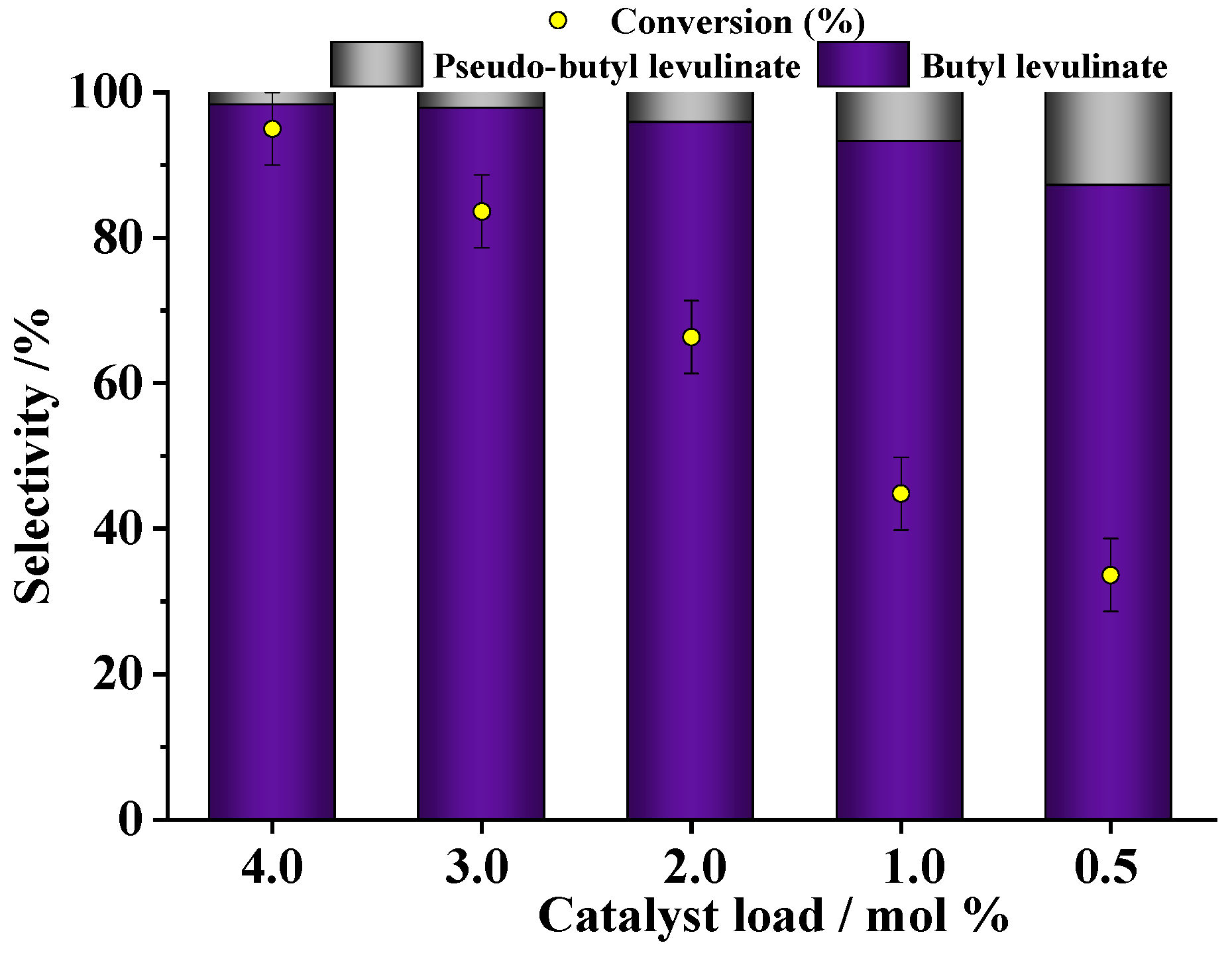
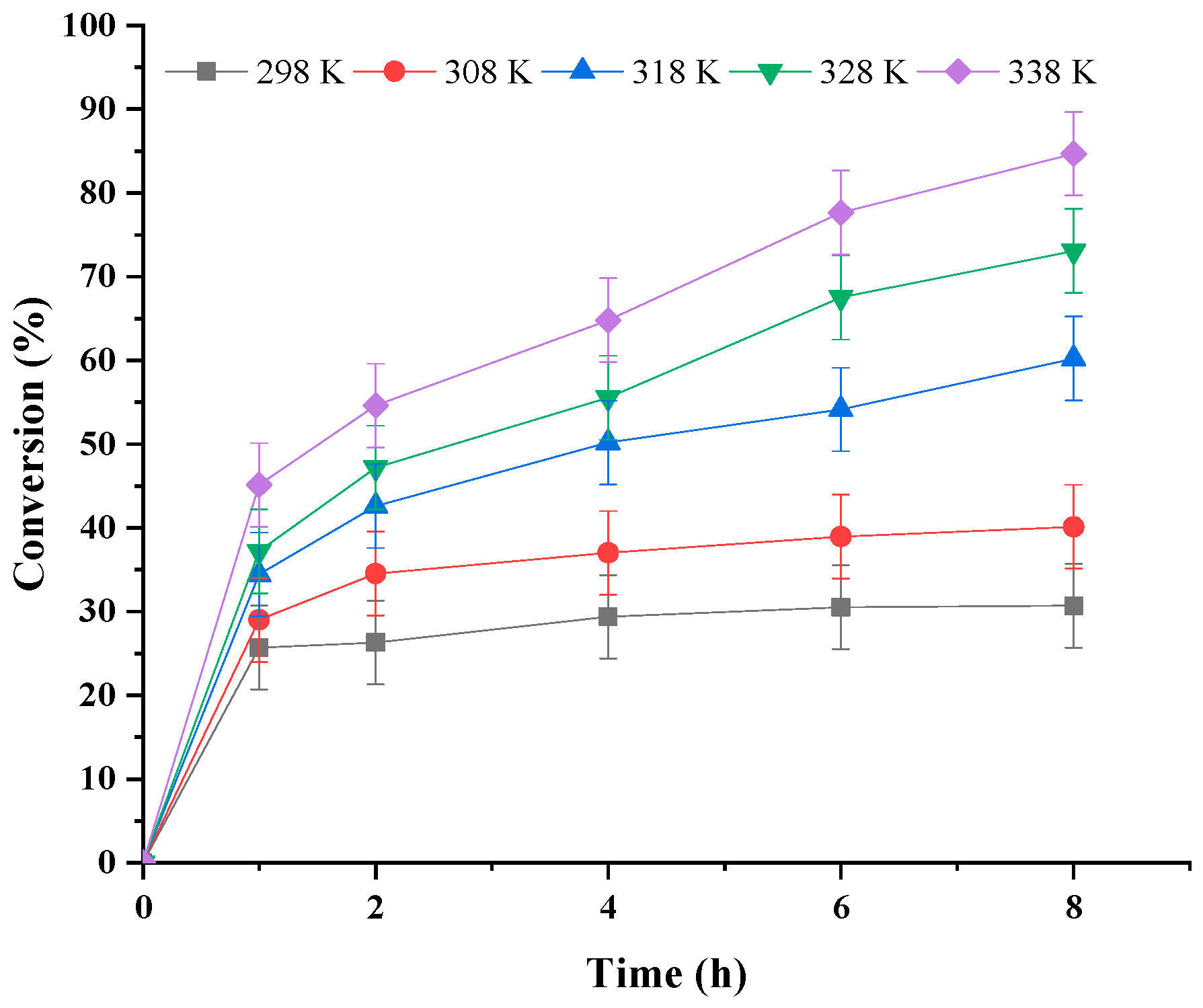


| Run | Catalyst | pH | Conversion/% |
|---|---|---|---|
| 1 | - | 6.0 | 5.0 |
| 2 | Fe(NO3)3 9 H2O | 0.4 | 83.0 |
| 3 | Cu(NO3)2 3 H2O | 1.7 | 40.0 |
| 4 | Co(NO3)2 6 H2O | 2.3 | 24.0 |
| 5 | Zn(NO3)2 6 H2O | 2.7 | 26.0 |
| 6 | NaNO3 | 3.6 | 18.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, M.J.d.; Cordeiro, M.T. Metal-Nitrate-Catalyzed Levulinic Acid Esterification with Alkyl Alcohols: A Simple Route to Produce Bioadditives. Processes 2024, 12, 1802. https://doi.org/10.3390/pr12091802
Silva MJd, Cordeiro MT. Metal-Nitrate-Catalyzed Levulinic Acid Esterification with Alkyl Alcohols: A Simple Route to Produce Bioadditives. Processes. 2024; 12(9):1802. https://doi.org/10.3390/pr12091802
Chicago/Turabian StyleSilva, Márcio José da, and Mariana Teixeira Cordeiro. 2024. "Metal-Nitrate-Catalyzed Levulinic Acid Esterification with Alkyl Alcohols: A Simple Route to Produce Bioadditives" Processes 12, no. 9: 1802. https://doi.org/10.3390/pr12091802






