Biodecolorization and Biodegradation of Sulfur Black by the Strain Aspergillus sp. DS-28
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Enrichment and Isolation of Sulfur Black Degrading Fungi
2.3. Evaluation of the Capabilities of Strain DS-28 for Degrading Sulfur Black
2.4. Topographical, Physical, and Chemical Characterizations of Sulfur Black Surfaces by SEM, XRD, and FTIR
3. Results and Discussion
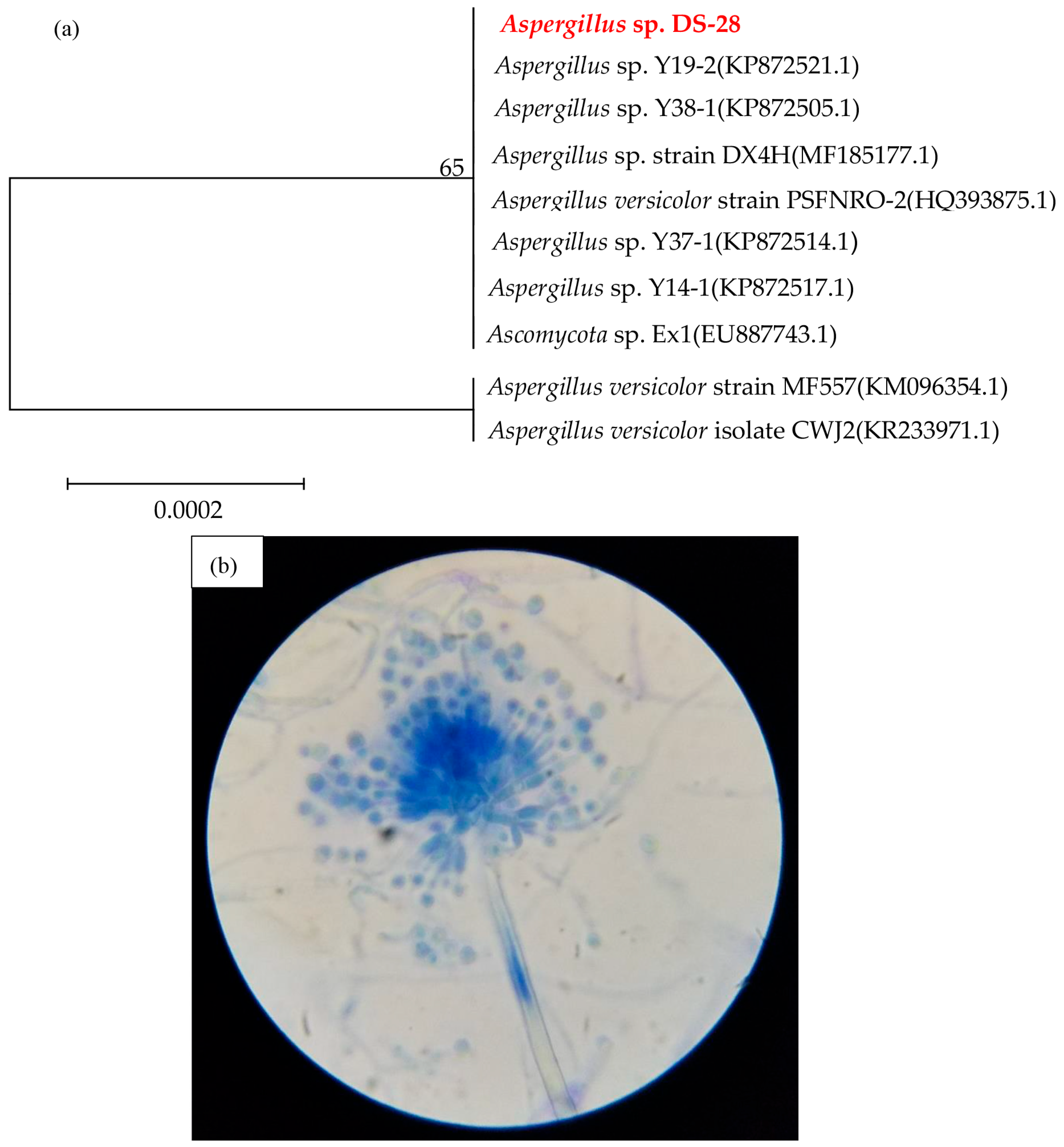
3.1. Isolation and Identification of Sulfur Black Degraders
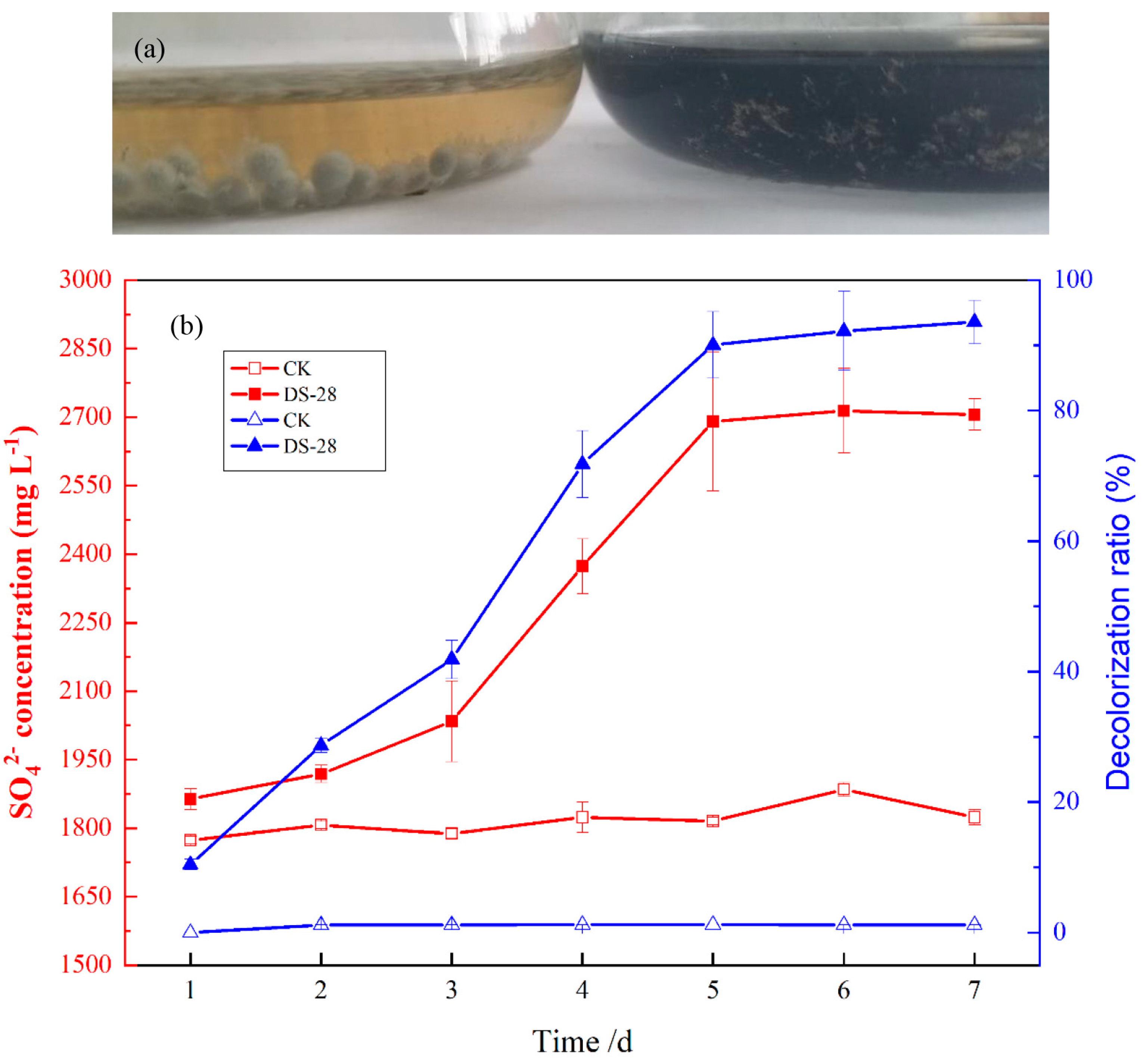
3.2. Sulfur Black Dye Removal Efficiency
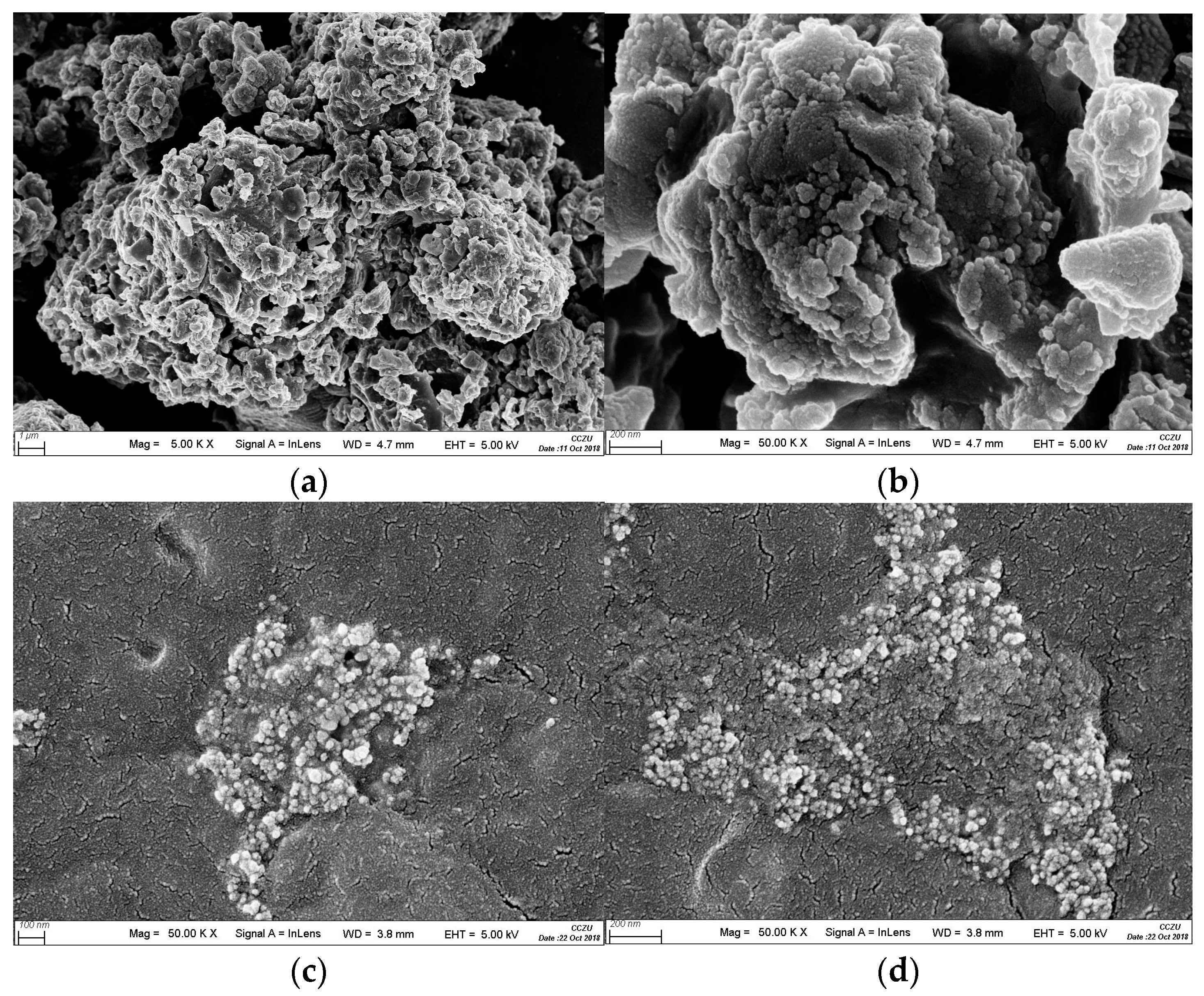
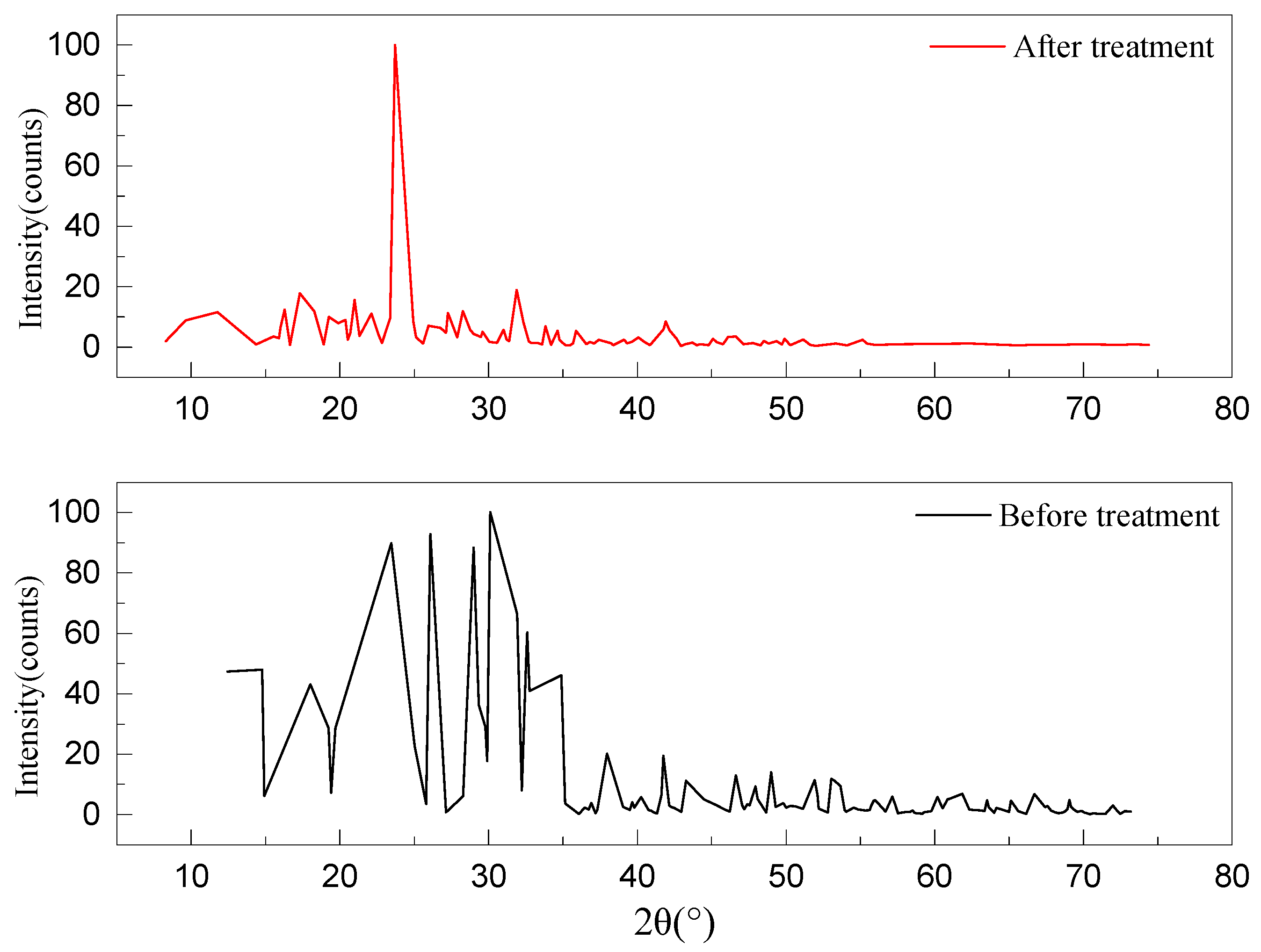
3.3. Changes in Physical, Chemical, and Topographical Properties of Sulfur Black Surface after Strain DS-28 Treatment
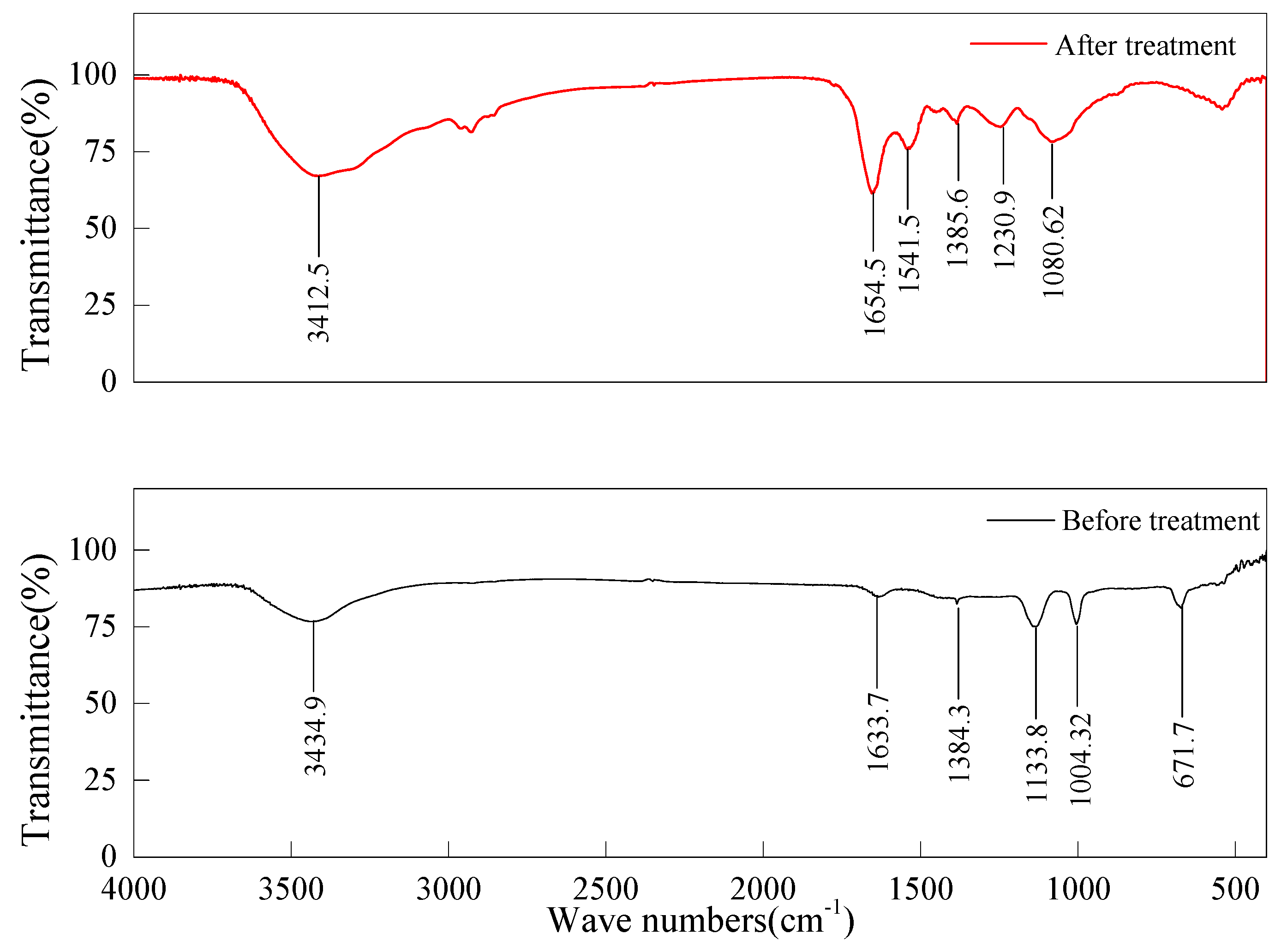
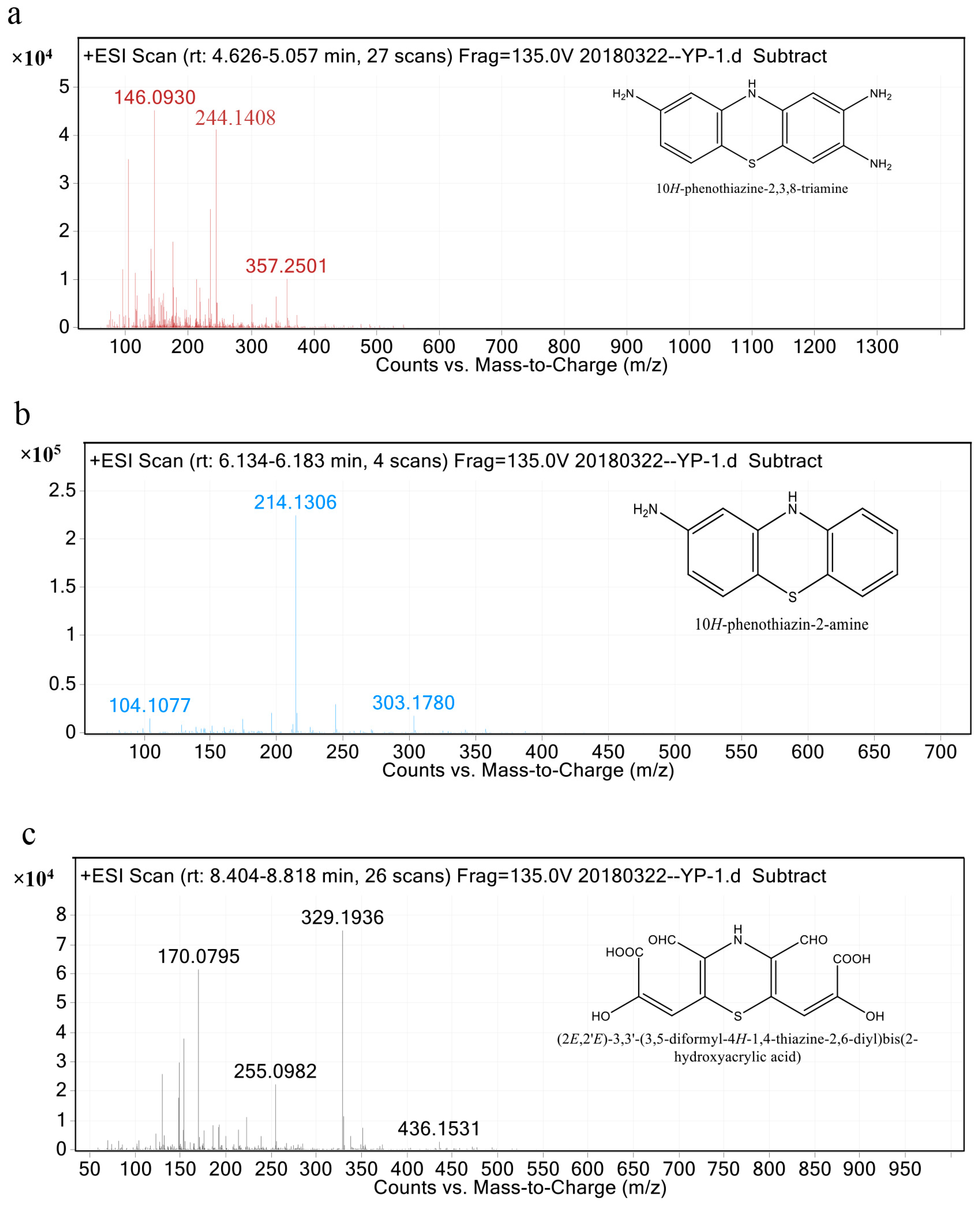
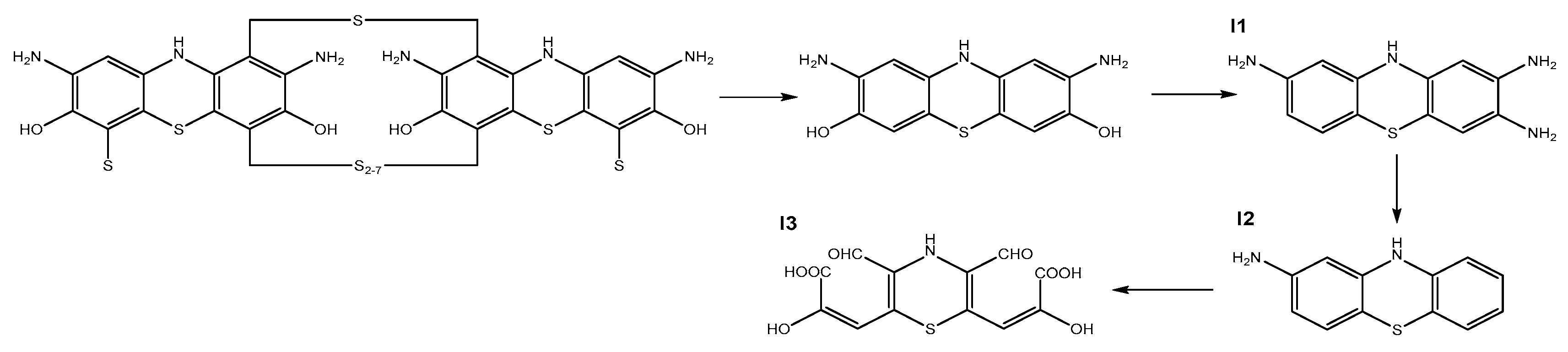
3.4. FTIR Analysis and Metabolite Identification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nguyen, T.A.; Juang, R.-S. Treatment of waters and wastewaters containing sulfur dyes: A review. Chem. Eng. J. 2013, 219, 109–117. [Google Scholar] [CrossRef]
- Lenninger, M.; Bechtold, T.; Pham, T. Improvement of wet rub fastness in continuous dyeing with c.i. Sulphur Black 1 by ultrasonic treatment. Ultrason. Sonochem. 2023, 99, 106558. [Google Scholar] [CrossRef]
- Bechtold, T.; Berktold, F.; Turcanu, A. The redox behaviour of CI Sulphur Black 1-a′basis for improved understanding of sulphur dyeing. Color. Technol. 2006, 116, 215–221. [Google Scholar] [CrossRef]
- Brown, K.A. Sulphur in the environment: A review. Environ. Pollut. Ser. B Chem. Phys. 1982, 3, 47–80. [Google Scholar] [CrossRef]
- Han, R.; Zhang, S.; Zhao, W.; Li, X.; Jian, X. Treating sulfur black dye wastewater with quaternized poly (phthalazinone ether sulfone ketone) nanofiltration membranes. Sep. Purif. Technol. 2009, 67, 26–30. [Google Scholar] [CrossRef]
- Ogunlaja, O.O.; Aemere, O. Evaluating the efficiency of a textile wastewater treatment plant located in Oshodi, Lagos. Afr. J. Pure Appl. Chem. 2009, 3, 189–196. [Google Scholar] [CrossRef]
- Andleeb, S.; Atiq, N.; Ali, M.; Razi-Ul-Hussnain, R.; Shafique, M.; Ahmad, B.; Ghumro, P.B.; Hussain, M.; Hameed, A.; Ahmad, S. Biological treatment of textile effluent in stirred tank bioreactor. Int. J. Agric. Biol. 2010, 12, 256–260. [Google Scholar] [CrossRef]
- Marmagne, O.; Coste, C.U. Color removal from textile plant effluents. Am. Dyest. Report. 1996, 85, 6. [Google Scholar] [CrossRef]
- Al-Hemiri, A.; Al-Anbari, H.; Shakir, I.K. Dye Removal from Wastewater Using Iron Salts. Iraqi J. Chem. Pet. Eng. 2008, 9, 1–7. [Google Scholar] [CrossRef]
- Blackburn, R.S. Natural Polysaccharides and Their Interactions with Dye Molecules: Applications in Effluent Treatment. Environ. Sci. Technol. 2004, 38, 4905–4909. [Google Scholar] [CrossRef]
- Dasgupta, J.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Remediation of textile effluents by membrane based treatment techniques: A state of the art review. J. Environ. Manag. 2015, 147, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Keung, M.K. Bacterium Useful in the Removal of Sulphur Black Dye from a Substrate. U.S. Patent 5,610,064, 1997. [Google Scholar]
- Gu, Y.; Zhu, X.; Yu, W.; Zhong, W.; Guo, J.; Cai, Z. Oxidization and biodegradation of sulfur black by a newly isolated strain Acinetobacter sp. DS-9. Desalination Water Treat. 2021, 227, 321–329. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumar, R.; Kumar, M.; Tewari, L. Eco-friendly detoxification of hazardous Congo red dye using novel fungal strain Trametes flavida WTFP2: Deduced enzymatic biomineralization process through combinatorial in-silico and in-vitro studies. J. Hazard. Mater. 2023, 455, 131503. [Google Scholar] [CrossRef]
- Coelho, G.D.; Silva, M.A.; de Melo Pinheiro, M.A.; Nadvorny, D.; Costa Amador, V.; Maia, R.T. In silico and in vitro assays suggests Congo red dye degradation by a Lentinus sp. laccase enzyme. J. Biomol. Struct. Dyn. 2024, 42, 3802–3813. [Google Scholar] [CrossRef]
- Alexander, D.G.S.; Thatheyus, A.J. Fungal bioremediation of toxic textile dye effluents. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 353–380. [Google Scholar]
- Akansha, K.; Kaur, T.; Yadav, A.; Kour, D.; Kumar Rai, A.; Singh, S.; Mishra, S.; Kumar, L.; Miglani, K.; Singh, K.; et al. Microbe-mediated remediation of dyes: Current status and future challenges. J. Appl. Biol. Biotechnol. 2022, 11, 1–23. [Google Scholar] [CrossRef]
- Polman, J.; Breckenridge, C.R. Biomass-mediated binding and recovery of textile dyes from waste effluents. Text. Chem. Color. 1996, 28, 31–35. [Google Scholar]
- White, T.J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols a Guide to Methods and Applications; Academic Press: Cambridge, MA, USA, 1990. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, W.; Ma, J.; Cai, J.; Li, S.; Zhu, X.; Yang, G.; Zhao, X. Biodegradation of Azo Dye Disperse Orange S-RL by a Newly Isolated Strain Acinetobacter sp. SRL8. Water Environ. Res. 2015, 87, 516–523. [Google Scholar] [CrossRef]
- Xu, H.; Wang, K.; Jin, L.; Yang, L.; Yuan, J.; Zhang, W.; He, G.; Chen, H. Synergistically engineering of vacancy and doping in thiospinel to boost electrocatalytic oxygen evolution in alkaline water and seawater. J. Colloid. Interface Sci. 2023, 650, 1500–1508. [Google Scholar] [CrossRef]
- Klose, S.; Bilen, S.; Tabatabai, M.A.; Dick, W.A. Sulfur Cycle Enzymes. In Methods of Soil Enzymology; Soil Science Society of America: Madison, WI, USA, 2011; Volume 9, pp. 125–159. [Google Scholar] [CrossRef]
- Waring, D.R.; Hallas, G. The Chemistry and Application of Dyes; Springer: New York, NY, USA, 1990. [Google Scholar] [CrossRef]
- Chen, J.; Garcia, E.S.; Zimmerman, S.C. Intramolecularly Cross-Linked Polymers: From Structure to Function with Applications as Artificial Antibodies and Artificial Enzymes. Acc. Chem. Res. 2020, 53, 1244–1256. [Google Scholar] [CrossRef]
- Chen, S.H.; Yien Ting, A.S. Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. isolated from compost. J. Environ. Manag. 2015, 150, 274–280. [Google Scholar] [CrossRef]
- Li, R.; Wang, W.; Wei, B.; Zhou, C.; Zhang, S. Sulfur black dyeing process with environment friendly reducing agent. J. Text. Res. 2020, 41, 50–54. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, P.; Xie, C.; Zhang, W.; Sun, J.; Qian, W.-J.; Yang, B. Biodegradation of alkaline lignin by Bacillus ligniniphilus L1. Biotechnol. Biofuels 2017, 10, 44. [Google Scholar] [CrossRef] [PubMed]
- Arentshorst, M.; Falco, M.D.; Moisan, M.-C.; Reid, I.D.; Spaapen, T.O.M.; van Dam, J.; Demirci, E.; Powlowski, J.; Punt, P.J.; Tsang, A.; et al. Identification of a Conserved Transcriptional Activator-Repressor Module Controlling the Expression of Genes Involved in Tannic Acid Degradation and Gallic Acid Utilization in Aspergillus niger. Front. Fungal Biol. 2021, 2, 681631. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fu, C. Manganese-oxidizing microbes and biogenic manganese oxides: Characterization, Mn(II) oxidation mechanism and environmental relevance. Rev. Environ. Sci. Bio/Technol. 2020, 19, 489–507. [Google Scholar] [CrossRef]
- Bilal, M.; Zdarta, J.; Jesionowski, T.; Iqbal, H.M.N. Manganese peroxidases as robust biocatalytic tool—An overview of sources, immobilization, and biotechnological applications. Int. J. Biol. Macromol. 2023, 234, 123531. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Abad-Sojos, S.; Torres-Constante, O.; Ruiz-Rubio, F.; Bucio, E. Lignin-Based Membrane for Dye Removal. In Membrane Based Methods for Dye Containing Wastewater; Springer: Berlin/Heidelberg, Germany, 2021; pp. 181–213. [Google Scholar] [CrossRef]
- Raychaudhuri, A.; Behera, M. Chapter 9-Microbial degradation of lignin: Conversion, application, and challenges. In Development in Wastewater Treatment Research and Processes; Shah, M.P., Rodriguez-Couto, S., Kapoor, R.T., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–219. [Google Scholar] [CrossRef]
- Dhir, B. Degradation of Dyes Using Filamentous Fungi. In Dye Biodegradation, Mechanisms and Techniques; Springer: Berlin/Heidelberg, Germany, 2021; pp. 51–66. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Z.; Wang, Y.; Chen, W.; Li, Y.; Yue, W.; Cai, Z. Biodecolorization and Biodegradation of Sulfur Black by the Strain Aspergillus sp. DS-28. Processes 2024, 12, 1818. https://doi.org/10.3390/pr12091818
Guan Z, Wang Y, Chen W, Li Y, Yue W, Cai Z. Biodecolorization and Biodegradation of Sulfur Black by the Strain Aspergillus sp. DS-28. Processes. 2024; 12(9):1818. https://doi.org/10.3390/pr12091818
Chicago/Turabian StyleGuan, Zhipeng, Yating Wang, Wentao Chen, Yanchen Li, Wenlong Yue, and Zhiqiang Cai. 2024. "Biodecolorization and Biodegradation of Sulfur Black by the Strain Aspergillus sp. DS-28" Processes 12, no. 9: 1818. https://doi.org/10.3390/pr12091818
APA StyleGuan, Z., Wang, Y., Chen, W., Li, Y., Yue, W., & Cai, Z. (2024). Biodecolorization and Biodegradation of Sulfur Black by the Strain Aspergillus sp. DS-28. Processes, 12(9), 1818. https://doi.org/10.3390/pr12091818





