The Green Synthesis of Nanostructured Silicon Carbides (SiCs) from Sugarcane Bagasse Ash (SCBA) as Anodes in Lithium-Ion (Li-Ion) Batteries: A Review Paper
Abstract
1. Introduction
2. Biomass
3. Sugarcane Bagasse Ash: Pretreatment and Characteristics
3.1. The Chemistry of Sugarcane Bagasse
3.2. Pretreatment
3.2.1. Chemical Activation
3.2.2. Thermal Activation (Pyrolysis)
4. Silicon Carbides
4.1. Research Progress in the Synthesis of Silicon Carbides
4.2. Carbothermic Reduction
4.2.1. Acheson Process
4.2.2. Physical Vapor Deposition (PVD)
4.2.3. Carbon Vapour Deposition
4.2.4. Sol–Gel Method
4.3. Advancements in Green Synthesis of Silicon Carbides
5. Synthesis of Silicon Carbide Using Sugarcane Bagasse
5.1. Characterisation of Silicon Carbides Synthesised from Sugarcane Bagasse: Techniques That Can Possibly Be Used for Analysis
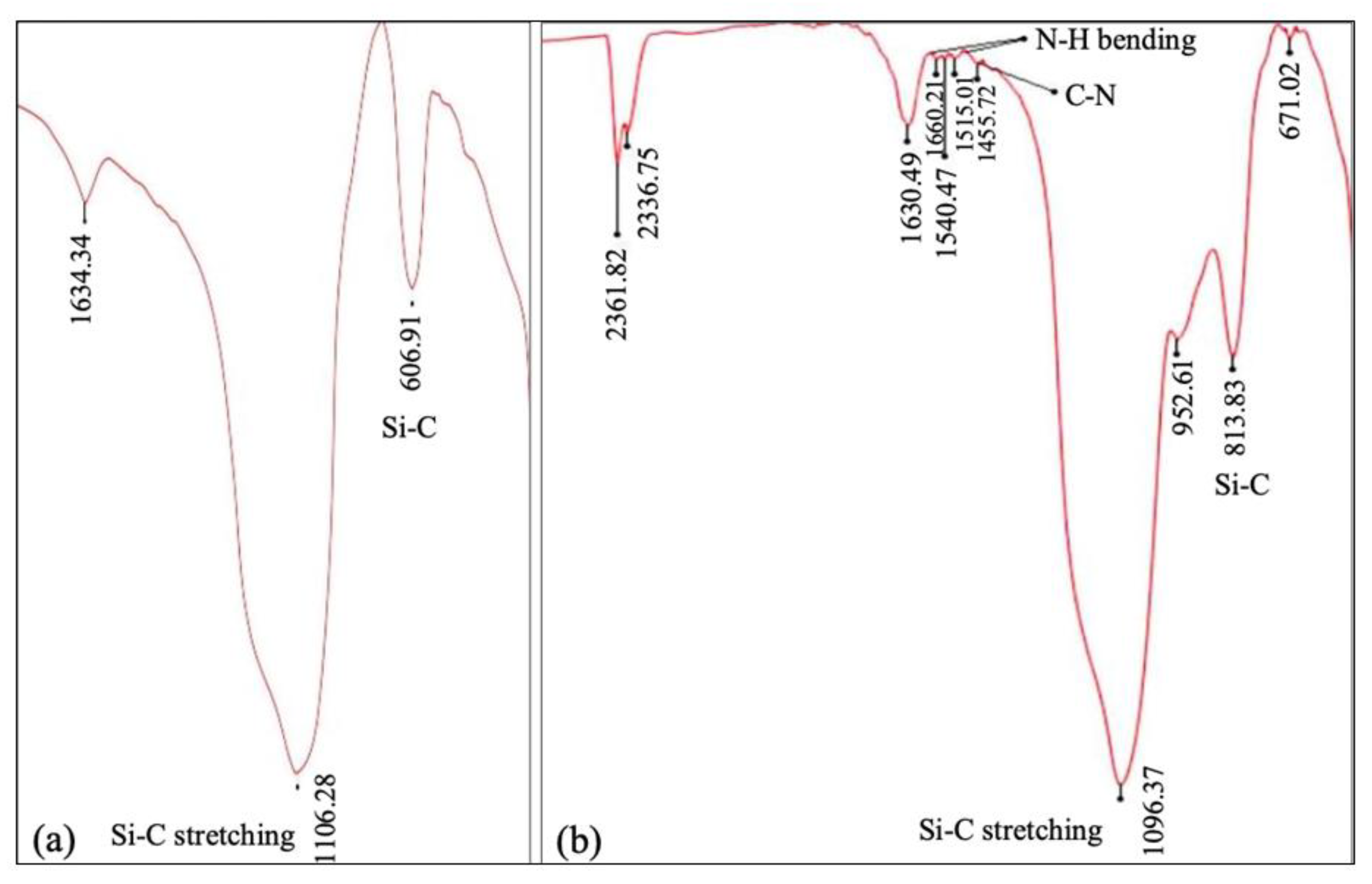
5.1.1. Fourier-Transform Infrared Spectroscopy
5.1.2. XRD and Raman Spectroscopy
5.2. Performance of Silicon Carbide in Lithium-Ion Batteries
6. Challenges and Prospects
7. Conclusions

Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hashmi, S.A. Hard carbon anode derived from pre-treated bio-waste sugarcane bagasse for high capacity sodium-ion battery fabricated with bio-deFgradable porous polymer electrolyte. J. Energy Storage 2024, 83, 110694. [Google Scholar]
- Zhang, X.Q.; Zhao, C.Z.; Huang, J.Q.; Zhang, Q. Recent advances in energy chemical engineering of next-generation lithium batteries. Engineering 2018, 4, 831–847. [Google Scholar] [CrossRef]
- Jin, Y.; Zhu, B.; Lu, Z.; Liu, N.; Zhu, J. Challenges and recent progress in the development of Si anodes for lithium-ion battery. Adv. Energy Mater. 2017, 7, 1700715. [Google Scholar] [CrossRef]
- Li, M.; Lu, J.; Chen, Z.; Amine, K. 30 years of lithium-ion batteries. Adv. Mater. 2018, 30, 1800561. [Google Scholar] [CrossRef] [PubMed]
- Yanamandra, K.; Pinisetty, D.; Gupta, N. Impact of carbon additives on lead-acid battery electrodes: A review. Renew. Sustain. Energy Rev. 2023, 173, 113078. [Google Scholar] [CrossRef]
- Blumbergs, E.; Serga, V.; Platacis, E.; Maiorov, M.; Shishkin, A. Cadmium recovery from spent Ni-Cd batteries: A brief review. Metals 2021, 11, 1714. [Google Scholar] [CrossRef]
- Smdani, G.; Islam, M.R.; Ahmad Yahaya, A.N.; Bin Safie, S.I. Performance evaluation of advanced energy storage systems: A review. Energy Environ. 2023, 34, 1094–1141. [Google Scholar] [CrossRef]
- Pradhan, S.K.; Chakraborty, B. Substrate materials and novel designs for bipolar lead-acid batteries: A review. J. Energy Storage 2020, 32, 101764. [Google Scholar] [CrossRef]
- Kumar, M.; Nagaiah, T.C. High energy density aqueous rechargeable sodium-ion/sulfur batteries in “water in salt” electrolyte. Energy Storage Mater. 2022, 49, 390–400. [Google Scholar] [CrossRef]
- Senthil, C.; Lee, C.W. Biomass-derived biochar materials as sustainable energy sources for electrochemical energy storage devices. Renew. Sustain. Energy Rev. 2021, 137, 110464. [Google Scholar] [CrossRef]
- Xie, L.; Tang, C.; Bi, Z.; Song, M.; Fan, Y.; Yan, C.; Li, X.; Su, F.; Zhang, Q.; Chen, C. Hard carbon anodes for next-generation Li-ion batteries: Review and perspective. Adv. Energy Mater. 2021, 11, 2101650. [Google Scholar] [CrossRef]
- Kebede, A.A.; Coosemans, T.; Messagie, M.; Jemal, T.; Behabtu, H.A.; Van Mierlo, J.; Berecibar, M. Techno-economic analysis of lithium-ion and lead-acid batteries in stationary energy storage application. J. Energy Storage 2021, 40, 102748. [Google Scholar] [CrossRef]
- Falk, G.; Shinhe, G.P.; Teixeira, L.B.; Moraes, E.G.; de Oliveira, A.N. Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions. Ceram. Int. 2019, 45, 21618–21624. [Google Scholar] [CrossRef]
- Arun, V.; Kannan, R.; Ramesh, S.; Vijayakumar, M.; Raghavendran, P.S.; Siva Ramkumar, M.; Anbarasu, P.; Sundramurthy, V.P. Review on li-ion battery vs nickel metal hydride battery in EV. Adv. Mater. Sci. Eng. 2022, 2022, 7910072. [Google Scholar] [CrossRef]
- Galos, J.; Pattarakunnan, K.; Best, A.S.; Kyratzis, I.L.; Wang, C.H.; Mouritz, A.P. Energy storage structural composites with integrated lithium-ion batteries: A review. Adv. Mater. Technol. 2021, 6, 2001059. [Google Scholar] [CrossRef]
- Diouf, B.; Pode, R. Potential of lithium-ion batteries in renewable energy. Renew. Energy 2015, 76, 375–380. [Google Scholar] [CrossRef]
- Poullikkas, A. A comparative overview of large-scale battery systems for electricity storage. Renew. Sustain. Energy Rev. 2013, 27, 778–788. [Google Scholar] [CrossRef]
- Yudhistira, R.; Khatiwada, D.; Sanchez, F. A comparative life cycle assessment of lithium-ion and lead-acid batteries for grid energy storage. J. Clean. Prod. 2022, 358, 131999. [Google Scholar] [CrossRef]
- Deng, D. Li-ion batteries: Basics, progress, and challenges. Energy Sci. Eng. 2015, 3, 385–418. [Google Scholar] [CrossRef]
- Liu, B.; Jia, Y.; Yuan, C.; Wang, L.; Gao, X.; Yin, S.; Xu, J. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: A review. Energy Storage Mater. 2020, 24, 85–112. [Google Scholar] [CrossRef]
- Molaiyan, P.; Dos Reis, G.S.; Karuppiah, D.; Subramaniyam, C.M.; Garcia-Alvarado, F.; Lassi, U. Recent progress in biomass-derived carbon materials for Li-ion and Na-ion batteries—A review. Batteries 2023, 9, 116. [Google Scholar] [CrossRef]
- Chen, T.; Jin, Y.; Lv, H.; Yang, A.; Liu, M.; Chen, B.; Xie, Y.; Chen, Q. Applications of lithium-ion batteries in grid-scale energy storage systems. Trans. Tianjin Univ. 2020, 26, 208–217. [Google Scholar] [CrossRef]
- Zhao, L.; Ding, B.; Qin, X.Y.; Wang, Z.; Lv, W.; He, Y.B.; Yang, Q.H.; Kang, F. Revisiting the roles of natural graphite in ongoing lithium-ion batteries. Adv. Mater. 2022, 34, 2106704. [Google Scholar] [CrossRef]
- Lian, P.; Zhu, X.; Liang, S.; Li, Z.; Yang, W.; Wang, H. Large reversible capacity of high quality graphene sheets as an anode material for lithium-ion batteries. Electrochim. Acta 2010, 55, 3909–3914. [Google Scholar] [CrossRef]
- Shi, M.; Song, C.; Tai, Z.; Zou, K.; Duan, Y.; Dai, X.; Sun, J.; Chen, Y.; Liu, Y. Coal-derived synthetic graphite with high specific capacity and excellent cyclic stability as anode material for lithium-ion batteries. Fuel 2021, 292, 120250. [Google Scholar] [CrossRef]
- Bai, L.Z.; Zhao, D.L.; Zhang, T.M.; Xie, W.G.; Zhang, J.M.; Shen, Z.M. A comparative study of electrochemical performance of graphene sheets, expanded graphite and natural graphite as anode materials for lithium-ion batteries. Electrochim. Acta 2013, 107, 555–561. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, challenge and perspective of graphite-based anode materials for lithium batteries: A review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Salah, M.; Murphy, P.; Hall, C.; Francis, C.; Kerr, R.; Fabretto, M. Pure silicon thin-film anodes for lithium-ion batteries: A review. J. Power Sources 2019, 414, 48–67. [Google Scholar] [CrossRef]
- Xu, W.; Welty, C.; Peterson, M.R.; Read, J.A.; Stadie, N.P. Exploring the limits of the rapid-charging performance of graphite as the anode in lithium-ion batteries. J. Electrochem. Soc. 2022, 169, 010531. [Google Scholar] [CrossRef]
- Kong, L.; Cui, J.; Sun, H.; Zhuang, Y.; Prakash, B.A.; Zhang, C. Autoregressive diffusion model for graph generation. In Proceedings of the International Conference on Machine Learning, Honolulu, HI, USA, 23–29 July 2023; PMLR. pp. 17391–17408. [Google Scholar]
- Weng, S.; Yang, G.; Zhang, S.; Liu, X.; Zhang, X.; Liu, Z.; Cao, M.; Ateş, M.N.; Li, Y.; Chen, L.; et al. Kinetic limits of graphite anode for fast-charging lithium-ion batteries. Nano-Micro Lett. 2023, 15, 215. [Google Scholar] [CrossRef]
- Buqa, H.; Goers, D.; Holzapfel, M.; Spahr, M.E.; Novák, P. High rate capability of graphite negative electrodes for lithium-ion batteries. J. Electrochem. Soc. 2005, 152, A474. [Google Scholar] [CrossRef]
- Moradi, B.; Botte, G.G. Recycling of graphite anodes for the next generation of lithium ion batteries. J. Appl. Electrochem. 2016, 46, 123–148. [Google Scholar] [CrossRef]
- Fitzhugh, W.; Li, X. Modulation of Ionic Current Limitations by Doping Graphite Anodes. J. Electrochem. Soc. 2018, 165, A2233. [Google Scholar] [CrossRef]
- Dimov, N.; Kugino, S.; Yoshio, M. Carbon-coated silicon as anode material for lithium ion batteries: Advantages and limitations. Electrochim. Acta 2003, 48, 1579–1587. [Google Scholar] [CrossRef]
- Zhao, H.; Li, J.; Zhao, Q.; Huang, X.; Jia, S.; Ma, J.; Ren, Y. Si-Based Anodes: Advances and Challenges in Li-Ion Batteries for Enhanced Stability. Electrochem. Energy Rev. 2024, 7, 11. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, F.; Han, J.; Bai, S.; Tan, J.; Liu, J.; Li, F. Challenges and recent progress on silicon-based anode materials for next-generation lithium-ion batteries. Small Struct. 2021, 2, 2100009. [Google Scholar] [CrossRef]
- Ozanam, F.; Rosso, M. Silicon as anode material for Li-ion batteries. Mater. Sci. Eng. B 2016, 213, 2–11. [Google Scholar] [CrossRef]
- Luo, W.; Chen, X.; Xia, Y.; Chen, M.; Wang, L.; Wang, Q.; Li, W.; Yang, J. Surface and interface engineering of silicon-based anode materials for lithium-ion batteries. Adv. Energy Mater. 2017, 7, 1701083. [Google Scholar] [CrossRef]
- Rajarao, R.; Ferreira, R.; Sadi, S.H.F.; Khanna, R.; Sahajwalla, V. Synthesis of silicon carbide nanoparticles by using electronic waste as a carbon source. Mater. Lett. 2014, 120, 65–68. [Google Scholar] [CrossRef]
- Cheng, T.W.; Hsu, C.W. A study of silicon carbide synthesis from waste serpentine. Chemosphere 2006, 64, 510–514. [Google Scholar] [CrossRef]
- Mohlala, L.M.; Bodunrin, M.O.; Awosusi, A.A.; Daramola, M.O.; Cele, N.P.; Olubambi, P.A. Beneficiation of corncob and sugarcane bagasse for energy generation and materials development in Nigeria and South Africa: A short overview. Alex. Eng. J. 2016, 55, 3025–3036. [Google Scholar] [CrossRef]
- Parameswaran, B. Sugarcane bagasse. In Biotechnology for Agro-Industrial Residues Utilisation: Utilisation of Agro-Residues; Springer: Berlin/Heidelberg, Germany, 2009; pp. 239–252. [Google Scholar]
- Kishore, S.C.; Perumal, S.; Atchudan, R.; Edison, T.N.J.I.; Sundramoorthy, A.K.; Alagan, M.; Sangaraju, S.; Lee, Y.R. Eco-friendly synthesis of functionalized carbon nanodots from cashew nut skin waste for bioimaging. Catalysts 2023, 13, 547. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Zeng, H.; Zhong, L.; Liu, K.; Cao, H.; Li, W.; Yan, H. Silicon carbide recovered from photovoltaic industry waste as photocatalysts for hydrogen production. J. Hazard. Mater. 2017, 329, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tun, M.M.; Juchelkova, D.; Win, M.M.; Thu, A.M.; Puchor, T. Biomass energy: An overview of biomass sources, energy potential, and management in Southeast Asian countries. Resources 2019, 8, 81. [Google Scholar] [CrossRef]
- Ciubota-Rosie, C.; Gavrilescu, M.; Macoveanu, M. BIOMASS—AN IMPORTANT RENEWABLE SOURCE OF ENERGY IN ROMANIA. Environ. Eng. Manag. J. (EEMJ) 2008, 7, 559–568. [Google Scholar] [CrossRef]
- Silva, D.A.L.; Delai, I.; Montes, M.L.D.; Ometto, A.R. Life cycle assessment of the sugarcane bagasse electricity generation in Brazil. Renew. Sustain. Energy Rev. 2014, 32, 532–547. [Google Scholar] [CrossRef]
- Du, Y.; Ma, S.; Dai, J.; Lin, J.; Zhou, X.; Chen, T.; Gu, X. Biomass Carbon Materials Contribute Better Alkali-Metal–Selenium Batteries: A Mini-Review. Batteries 2022, 8, 123. [Google Scholar] [CrossRef]
- Kane, S.; Storer, A.; Xu, W.; Ryan, C.; Stadie, N.P. Biochar as a renewable substitute for carbon black in lithium-ion battery electrodes. ACS Sustain. Chem. Eng. 2022, 10, 12226–12233. [Google Scholar] [CrossRef]
- Abed Hussein, B.; Mahdi, A.B.; Emad Izzat, S.; Acwin Dwijendra, N.K.; Romero Parra, R.M.; Barboza Arenas, L.A.; Mustafa, Y.F.; Yasin, G.; Thaeer Hammid, A. Production, structural properties nano biochar and effects nano biochar in soil: A review. Egypt. J. Chem. 2022, 65, 607–618. [Google Scholar]
- Salimi, P.; Tieuli, S.; Taghavi, S.; Venezia, E.; Fugattini, S.; Lauciello, S.; Prato, M.; Marras, S.; Li, T.; Signoretto, M.; et al. Sustainable lithium-ion batteries based on metal-free tannery waste biochar. Green Chem. 2022, 24, 4119–4129. [Google Scholar] [CrossRef]
- Lee, Y.; Ryu, C.; Park, Y.K.; Jung, J.H.; Hyun, S. Characteristics of biochar produced from slow pyrolysis of Geodae-Uksae 1. Bioresour. Technol. 2013, 130, 345–350. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Dodla, S.K.; Wang, J.J. Fundamental and molecular composition characteristics of biochars produced from sugarcane and rice crop. Chemosphere 2016, 142, 4–13. [Google Scholar] [CrossRef]
- Xiao, Y.; Raheem, A.; Ding, L.; Chen, W.H.; Chen, X.; Wang, F.; Lin, S.L. Pretreatment, modification and applications of sewage sludge-derived biochar for resource recovery—A review. Chemosphere 2022, 287, 131969. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef]
- Das, R.; Panda, S.N. Preparation and applications of biochar based nanocomposite: A review. J. Anal. Appl. Pyrolysis 2022, 167, 105691. [Google Scholar] [CrossRef]
- Iwuozor, K.O.; Emenike, E.C.; Abdulkadir, M.; Samuel, O.; Adeniyi, A.G. Effect of salt modification on biochar obtained from the thermochemical conversion of sugarcane bagasse. Sugar Tech 2023, 25, 223–233. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, H.; Ma, T.; Deng, H.; Zha, Z. Effect of cotton stalk particle size on the structure of biochar and the performance of anode for lithium-ion battery. J. Phys. Chem. Solids 2022, 169, 110845. [Google Scholar] [CrossRef]
- Li, R.; Miao, C.; Zhang, M.; Xiao, W. Novel hierarchical structural SnS 2 composite supported by biochar carbonized from chewed sugarcane as enhanced anodes for lithium ion batteries. Ionics 2020, 26, 1239–1247. [Google Scholar] [CrossRef]
- Chen, X.; Li, F.; Su, S.; Chen, H.; Zhang, J.; Cai, D. Efficient honeycomb–shaped biochar anodes for lithium-ion batteries from Eichhornia crassipes biomass. Environ. Chem. Lett. 2021, 19, 3505–3510. [Google Scholar] [CrossRef]
- Xia, G.; Li, X.; He, J.; Wang, Y.; Gu, Y.; Liu, L.; Huang, J.; Dong, P.; Duan, J.; Wang, D.; et al. A biomass-derived biochar-supported NiS/C anode material for lithium-ion batteries. Ceram. Int. 2021, 47, 20948–20955. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Feng, N.; Qiao, L.; Li, X.; He, D. A new carbonaceous material derived from biomass source peels as an improved anode for lithium ion batteries. J. Anal. Appl. Pyrolysis 2013, 100, 181–185. [Google Scholar] [CrossRef]
- Wan, H.; Hu, X. Nitrogen doped biomass-derived porous carbon as anode materials of lithiumion batteries. Solid State Ion. 2019, 341, 115030. [Google Scholar] [CrossRef]
- Ma, Y.; Chen, K.; Ma, J.; Xu, G.; Dong, S.; Chen, B.; Li, J.; Chen, Z.; Zhou, X.; Cui, G. A biomass based free radical scavenger binder endowing a compatible cathode interface for 5 V lithium-ion batteries. Energy Environ. Sci. 2019, 12, 273–280. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Yang, L.; Wang, Y.; Liu, Y.; Chen, Y.; Guo, X.; Wu, Z.; Zhong, B. N, O co-doped chlorella-based biomass carbon modified separator for lithium-sulfur battery with high capacity and long cycle performance. J. Colloid Interface Sci. 2021, 585, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Zhang, Y.; Sun, Y.; Xu, Y.; Zhang, H.; Ye, P.; Zheng, M.; Zhou, N.; Wang, D. Template-free synthesis of biomass-derived carbon coated Li4Ti5O12 microspheres as high performance anodes for lithium-ion batteries. Appl. Surf. Sci. 2018, 459, 572–582. [Google Scholar] [CrossRef]
- Nastasi, B.; Markovska, N.; Puksec, T.; Duić, N.; Foley, A. Renewable and sustainable energy challenges to face for the achievement of Sustainable Development Goals. Renew. Sustain. Energy Rev. 2022, 157, 112071. [Google Scholar] [CrossRef]
- Afgan, N.H.; Al Gobaisi, D.; Carvalho, M.G.; Cumo, M. Sustainable energy development. Renew. Sustain. Energy Rev. 1998, 2, 235–286. [Google Scholar] [CrossRef]
- Haghighi Mood, S.; Pelaez-Samaniego, M.R.; Garcia-Perez, M. Perspectives of engineered biochar for environmental applications: A review. Energy Fuels 2022, 36, 7940–7986. [Google Scholar] [CrossRef]
- Mian, M.M.; Alam, N.; Ahommed, M.S.; He, Z.; Ni, Y. Emerging applications of sludge biochar-based catalysts for environmental remediation and energy storage: A review. J. Clean. Prod. 2022, 360, 132131. [Google Scholar] [CrossRef]
- Uday, V.; Harikrishnan, P.S.; Deoli, K.; Zitouni, F.; Mahlknecht, J.; Kumar, M. Current trends in production, morphology, and real-world environmental applications of biochar for the promotion of sustainability. Bioresour. Technol. 2022, 359, 127467. [Google Scholar] [CrossRef]
- Batool, F.; Masood, A.; Ali, M. Characterization of sugarcane bagasse ash as pozzolan and influence on concrete properties. Arab. J. Sci. Eng. 2020, 45, 3891–3900. [Google Scholar] [CrossRef]
- Carrier, M.; Hardie, A.G.; Uras, Ü.; Görgens, J.; Knoetze, J.H. Production of char from vacuum pyrolysis of South-African sugar cane bagasse and its characterization as activated carbon and biochar. J. Anal. Appl. Pyrolysis 2012, 96, 24–32. [Google Scholar] [CrossRef]
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20. [Google Scholar] [CrossRef]
- Subramanian, S.; Arunachalam, B.; Nallasivam, K.; Pramanik, A. Investigations on tribo-mechanical behaviour of Al-Si10-Mg/sugarcane bagasse ash/SiC hybrid composites. China Foundry 2019, 16, 277–284. [Google Scholar] [CrossRef]
- Almeida, R.P.A.; Cordeiro, G.C. Sustainable approach to simultaneously improve the pozzolanic activity of sugarcane bagasse ash and the vinasse fertilization potential. Clean. Eng. Technol. 2023, 13, 100617. [Google Scholar] [CrossRef]
- Inyang, M.; Gao, B.; Pullammanappallil, P.; Ding, W.; Zimmerman, A.R. Biochar from anaerobically digested sugarcane bagasse. Bioresour. Technol. 2010, 101, 8868–8872. [Google Scholar] [CrossRef]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane bagasse: A biomass sufficiently applied for improving global energy, environment and economic sustainability. Bioresour. Bioprocess. 2021, 8, 1–25. [Google Scholar] [CrossRef]
- Karp, S.G.; Woiciechowski, A.L.; Soccol, V.T.; Soccol, C.R. Pretreatment strategies for delignification of sugarcane bagasse: A review. Braz. Arch. Biol. Technol. 2013, 56, 679–689. [Google Scholar] [CrossRef]
- Konde, K.S.; Nagarajan, S.; Kumar, V.; Patil, S.V.; Ranade, V.V. Sugarcane bagasse based biorefineries in India: Potential and challenges. Sustain. Energy Fuels 2021, 5, 52–78. [Google Scholar] [CrossRef]
- Ni’mah, Y.L.; Muhaiminah, Z.H.; Suprapto, S. Synthesis of silica nanoparticles from sugarcane bagasse by sol-gel method. In AIP Conference Proceedings; AIP Publishing: Melville, NY, USA, 2023; Volume 2540. [Google Scholar]
- Ahn, J.; Kim, H.S.; Pyo, J.; Lee, J.K.; Yoo, W.C. Variation in crystalline phases: Controlling the selectivity between silicon and silicon carbide via magnesiothermic reduction using silica/carbon composites. Chem. Mater. 2016, 28, 1526–1536. [Google Scholar] [CrossRef]
- Chew, T.W.; H’Ng, P.S.; Luqman Chuah Abdullah, B.C.T.G.; Chin, K.L.; Lee, C.L.; Mohd Nor Hafizuddin, B.M.S.; TaungMai, L. A Review of Bio-Based Activated Carbon Properties Produced from Different Activating Chemicals during Chemicals Activation Process on Biomass and Its Potential for Malaysia. Materials 2023, 16, 7365. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Remón, J.; Jiang, Z.; Ding, W. Recent Advances in the Preparation and Application of Biochar Derived from Lignocellulosic Biomass: A Mini Review. Polymers 2024, 16, 851. [Google Scholar] [CrossRef] [PubMed]
- Panwar, N.L.; Pawar, A. Influence of activation conditions on the physicochemical properties of activated biochar: A review. Biomass Convers. Biorefinery 2020, 12, 925–947. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Rattanasak, U. Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci. Rep. 2020, 10, 9890. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, H.; Cai, C.; Zhang, Z.; Hu, W.; Dong, H.; Ding, S. Embedding silicon nanoparticle in porous carbon fiber for highly stable lithium-ion battery anode. Mater. Lett. 2024, 361, 136015. [Google Scholar] [CrossRef]
- Chiew, Y.L.; Cheong, K.Y. A review on the synthesis of SiC from plant-based biomasses. Mater. Sci. Eng. B 2011, 176, 951–964. [Google Scholar] [CrossRef]
- Soodesh, C.Y.; Seriyala, A.K.; Chattopadhyay, P.; Rozhkova, N.; Michalkiewicz, B.; Chatterjee, S.; Roy, B. Carbonaceous catalysts (biochar and activated carbon) from agricultural residues and their application in production of biodiesel: A review. Chem. Eng. Res. Des. 2024, 203, 759–788. [Google Scholar] [CrossRef]
- Sztymela, K.; Rossignol, F.; Bienia, M.; Zapp, N.; Nikolowski, K.; Cerbelaud, M. Fabrication of 3D silicon anode by inkjet printing: Opportunities and challenges. J. Energy Storage 2024, 75, 109567. [Google Scholar] [CrossRef]
- Chabi, S.; Guler, Z.; Brearley, A.J.; Benavidez, A.D.; Luk, T.S. The creation of true two-dimensional silicon carbide. Nanomaterials 2021, 11, 1799. [Google Scholar] [CrossRef]
- Seroka, N.S.; Luo, H.; Khotseng, L. Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review. Batteries 2024, 10, 144. [Google Scholar] [CrossRef]
- Thulasiraman, A.V.; Ganesapillai, M. A systematic review on the synthesis of silicon carbide: An alternative approach to valorisation of residual municipal solid waste. Processes 2023, 11, 283. [Google Scholar] [CrossRef]
- Soltys, L.M.; Mironyuk, I.F.; Mykytyn, I.M.; Hnylytsia, I.D.; Turovska, L.V. Synthesis and Properties of Silicon Carbide. Phys. Chem. Solid State 2023, 24, 5–16. [Google Scholar] [CrossRef]
- Zafeer, M.K.; Menezes, R.A.; Venkatachalam, H.; Bhat, K.S. Sugarcane bagasse-based biochar and its potential applications: A review. Emergent Mater. 2024, 7, 133–161. [Google Scholar] [CrossRef]
- Xu, M.; Girish, Y.R.; Rakesh, K.P.; Wu, P.; Manukumar, H.M.; Byrappa, S.M.; Byrappa, K. Recent advances and challenges in silicon carbide (SiC) ceramic nanoarchitectures and their applications. Mater. Today Commun. 2021, 28, 102533. [Google Scholar] [CrossRef]
- Oliveros, A.; Guiseppi-Elie, A.; Saddow, S.E. Silicon carbide: A versatile material for biosensor applications. Biomed. Microdevices 2013, 15, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Shields, V.B. Applications of Silicon Carbide for High Temperature Electronics and Sensors; Tech Briefs; NASA Jet Propulsion Laboratory: Pasadena, CA, USA, 1995.
- Teng, Y.; Liu, D.; Li, Q.; Bai, X.; Song, Y. Research progress on application in energy conversion of silicon carbide-based catalyst carriers. Catalysts 2023, 13, 236. [Google Scholar] [CrossRef]
- Martin, H.P.; Ecke, R.; Müller, E. Synthesis of nanocrystalline silicon carbide powder by carbothermal reduction. J. Eur. Ceram. Soc. 1998, 18, 1737–1742. [Google Scholar] [CrossRef]
- Abderrazak, H.; Hmida, E.S.B.H. Silicon carbide: Synthesis and properties. In Properties and Applications of Silicon Carbide; IntechOpen: London, UK, 2011; pp. 361–388. [Google Scholar]
- Karelin, V.A.; Strashko, A.N.; Sazonov, A.V.; Dubrovin, A.V. Obtaining the fine-grained silicon carbide, used in the synthesis of construction ceramics. Resour.-Effic. Technol. 2016, 2, 50–60. [Google Scholar]
- Raj, P.; Gupta, G.S. Temperature measurements in a laboratory scale furnace for manufacturing of silicon carbide through Acheson process. Measurement 2020, 151, 107131. [Google Scholar] [CrossRef]
- El Omari, G.; Elkindoussy, K.; Aqil, M.; Dahbi, M.; Alami, J.; Makha, M. Advances in Physical Vapor Deposited Silicon/Carbon based anode materials for Li-ion batteries. Heliyon 2024, 10, E30431. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Cheng, L.; Wang, Y. Chemical vapour deposition of zirconium carbide and silicon carbide hybrid whiskers. Mater. Lett. 2010, 64, 552–554. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Bi, Y.; Li, H.; Chen, Y.; Jia, Q. Synthesis of SiC whiskers via catalytic reaction method in self-bonded SiC composites. Ceram. Int. 2020, 46, 12975–12985. [Google Scholar] [CrossRef]
- Du, J.; Zhu, R.; Chen, Q.; Xie, J.; Xian, H.; Zhang, J.; Zhu, J. In situ synthesis of stable silicon carbide-reinforced silicon nanosheets from organoclay for high-performance lithium-ion battery anodes. Appl. Surf. Sci. 2023, 617, 156566. [Google Scholar] [CrossRef]
- Najafi, A.; Golestani-Fard, F.; Rezaie, H.R.; Ehsani, N. SYNTHESIS AND CHARACTERIZATION OFSILICON CARBIDE NANO POWDER BY SOL GEL PROCESSING. Iran. J. Mater. Sci. Eng. 2011, 8, 41–47. [Google Scholar]
- Li, B.; Song, Y.C.; Zhang, C.R.; Yu, J.S. Synthesis and characterization of nanostructured silicon carbide crystal whiskers by sol–gel process and carbothermal reduction. Ceram. Int. 2014, 40, 12613–12616. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhao, H. Synthesis of highly dispersed silicon carbide powders by a solvothermal-assisted sol–gel process. Appl. Phys. A 2018, 124, 1–5. [Google Scholar] [CrossRef]
- Najafi, A.; Golestani-Fard, F.; Rezaie, H.R.; Saeb, S.P. Sol-Gel synthesis and characterization of SiC–B4C nano powder. Ceram. Int. 2021, 47, 6376–6387. [Google Scholar] [CrossRef]
- Rajarao, R.; Sahajwalla, V. A cleaner, sustainable approach for synthesising high purity silicon carbide and silicon nitride nanopowders using macadamia shell waste. J. Clean. Prod. 2016, 133, 1277–1282. [Google Scholar] [CrossRef]
- Afsharpour, M.; Khomand, E. Synthesis of bio-inspired porous silicon carbides using Cortaderia selloana and Equisetum arvense grasses as remarkable sulfur adsorbents. Int. J. Environ. Sci. Technol. 2019, 16, 3125–3134. [Google Scholar] [CrossRef]
- Khomand, E.; Afsharpour, M. Green synthesis of nanostructured SiCs by using natural biopolymers (guar, tragacanth, Arabic, and xanthan gums) for oxidative desulfurization of model fuel. Int. J. Environ. Sci. Technol. 2019, 16, 2359–2372. [Google Scholar] [CrossRef]
- Sun, K.; Wang, T.; Chen, Z.; Lu, W.; He, X.; Gong, W.; Tang, M.; Liu, F.; Huang, Z.; Tang, J.; et al. Clean and low-cost synthesis of high purity beta-silicon carbide with carbon fiber production residual and a sandstone. J. Clean. Prod. 2019, 238, 117875. [Google Scholar] [CrossRef]
- Hossain, S.T.; Johra, F.T.; Jung, W.G. Fabrication of silicon carbide from recycled silicon wafer cutting sludge and its purification. Appl. Sci. 2018, 8, 1841. [Google Scholar] [CrossRef]
- Zeraati, M.; Tahmasebi, K.; Irannejad, A. Formation of SiC nanocrystals prepared by sol-gel processing of green carbon sources and DFT calculations. J. Nanostruct. 2020, 10, 660–670. [Google Scholar]
- Shipley, L.W. Process for Production of High Purity Amorphous Silica from Biogenic Material. U.S. Patent 6 2002,406,678, 18 June 2002. [Google Scholar]
- Sharma, A.; Kumar, D.; Kumar, V.; Singh, S.P.; Sharma, A.R.; Sharma, S.K. Electronic structure and chemical states of green synthesized silica nanoparticles from biomasses. Hybrid Adv. 2024, 5, 100133. [Google Scholar] [CrossRef]
- Wang, M.S.; Song, W.L.; Wang, J.; Fan, L.Z. Highly uniform silicon nanoparticle/porous carbon nanofiber hybrids towards free-standing high-performance anodes for lithium-ion batteries. Carbon 2015, 82, 337–345. [Google Scholar] [CrossRef]
- Gupta, S.; Yadav, A.; Singh, S.; Verma, N. Synthesis of silicon carbide-derived carbon as an electrode of a microbial fuel cell and an adsorbent of aqueous Cr (VI). Ind. Eng. Chem. Res. 2017, 56, 1233–1244. [Google Scholar] [CrossRef]
- Shen, Y. Rice husk silica derived nanomaterials for sustainable applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Thakur, A.K.; Kaviti, A.K.; Siddiqi, M.T.; Aseer, J.R.; Singh, R.; Gehlot, A. Taguchi optimization of fracture toughness of silicon carbide extracted from agricultural wastes. Silicon 2022, 14, 8021–8029. [Google Scholar] [CrossRef]
- Sekar, S.; Aqueel Ahmed, A.T.; Kim, D.Y.; Lee, S. One-pot synthesized biomass C-Si nanocomposites as an anodic material for high-performance sodium-ion battery. Nanomaterials 2020, 10, 1728. [Google Scholar] [CrossRef]
- Nangir, M.; Massoudi, A.; Tayebifard, S.A. Investigation of the lithium-ion depletion in the silicon-silicon carbide anode/electrolyte interface in lithium-ion battery via electrochemical impedance spectroscopy. J. Electroanal. Chem. 2020, 873, 114385. [Google Scholar] [CrossRef]
- Yu, M.; Temeche, E.; Indris, S.; Lai, W.; Laine, R.M. Silicon carbide (SiC) derived from agricultural waste potentially competitive with silicon anodes. Green Chem. 2022, 24, 4061–4070. [Google Scholar] [CrossRef]
- Prakash, J.; Venugopalan, R.; Tripathi, B.M.; Ghosh, S.K.; Chakravartty, J.K.; Tyagi, A.K. Chemistry of one dimensional silicon carbide materials: Principle, production, application and future prospects. Prog. Solid State Chem. 2015, 43, 98–122. [Google Scholar] [CrossRef]
- Singh, K.; Kumar, R.; Chaudhary, V.; Arya, A.M.; Sharma, S. Sugarcane bagasse: Foreseeable biomass of bio†products and biofuel: An overview. J. Pharmacogn. Phytochem. 2019, 8, 2356–2360. [Google Scholar]
- Ma, J.; Zheng, L.; Yu, F. Current status and future prospects of biochar application in electrochemical energy storage devices: A bibliometric review. Desalination 2024, 581, 117597. [Google Scholar] [CrossRef]
- Lee, H.W.; Kim, Y.M.; Kim, S.; Ryu, C.; Park, S.H.; Park, Y.K. Review of the use of activated biochar for energy and environmental applications. Carbon Lett. 2018, 26, 1–10. [Google Scholar]
- Krstic, V.D. Production of fine, high-purity beta silicon carbide powders. J. Am. Ceram. Soc. 1992, 75, 170–174. [Google Scholar] [CrossRef]
- Cheng, D.J.; Shyy, W.J.; Kuo, D.H.; Hon, M.H. Growth characteristics of CVD beta-silicon carbide. J. Electrochem. Soc. 1987, 134, 3145. [Google Scholar] [CrossRef]
- Davis, R.F.; Kelner, G.; Shur, M.; Palmour, J.W.; Edmond, J.A. Thin film deposition and microelectronic and optoelectronic device fabrication and characterization in monocrystalline alpha and beta silicon carbide. Proc. IEEE 1991, 79, 677–701. [Google Scholar] [CrossRef]
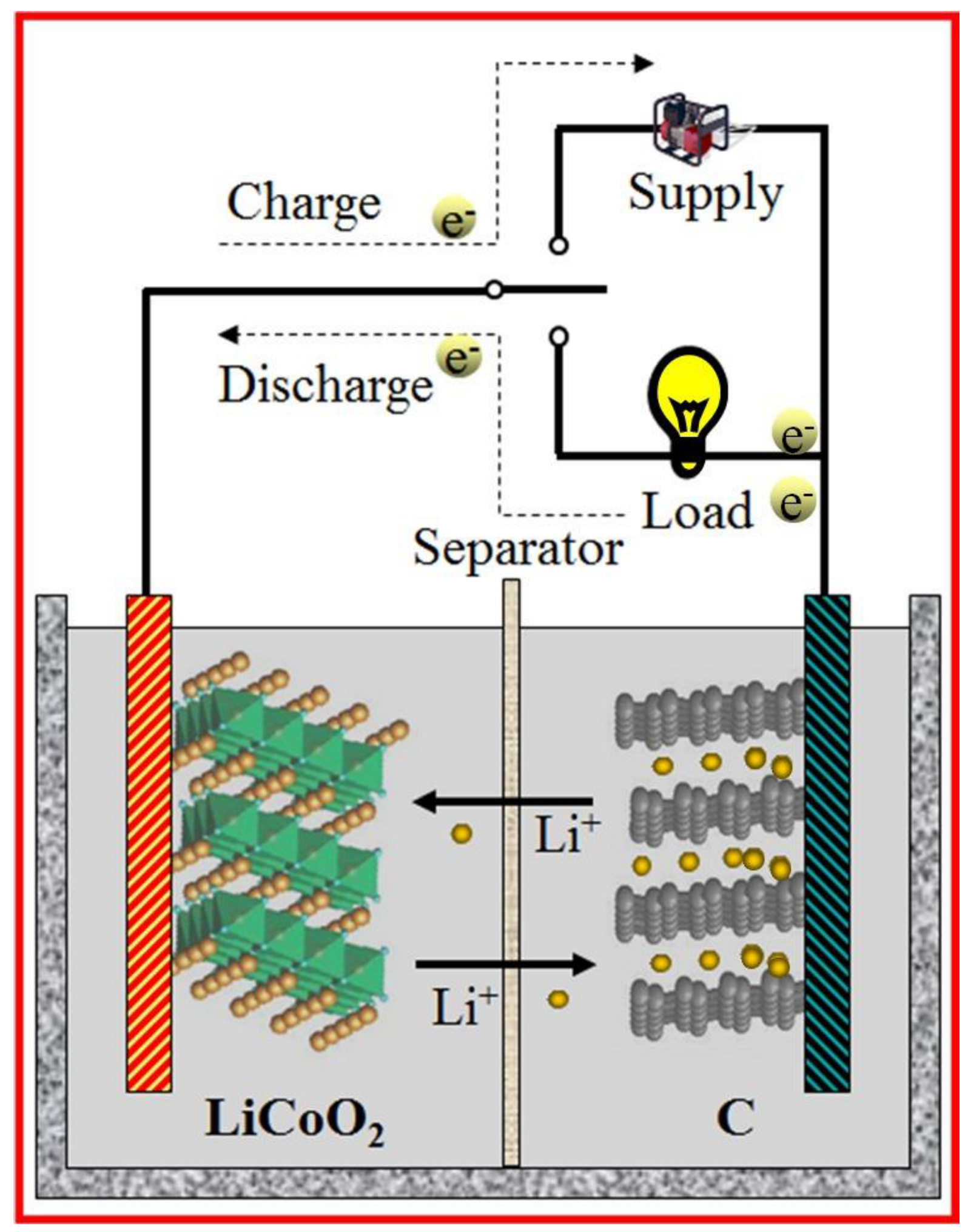

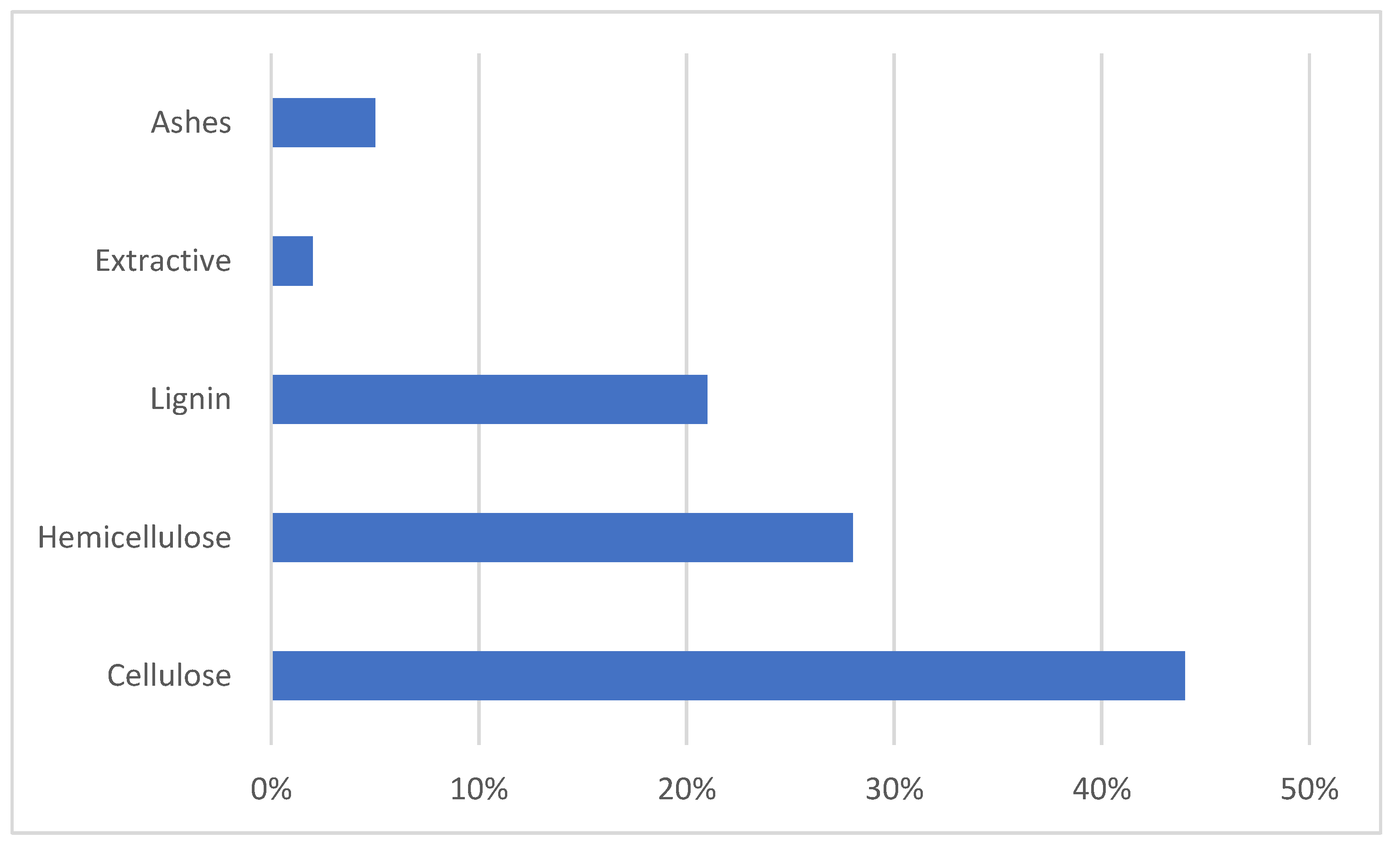
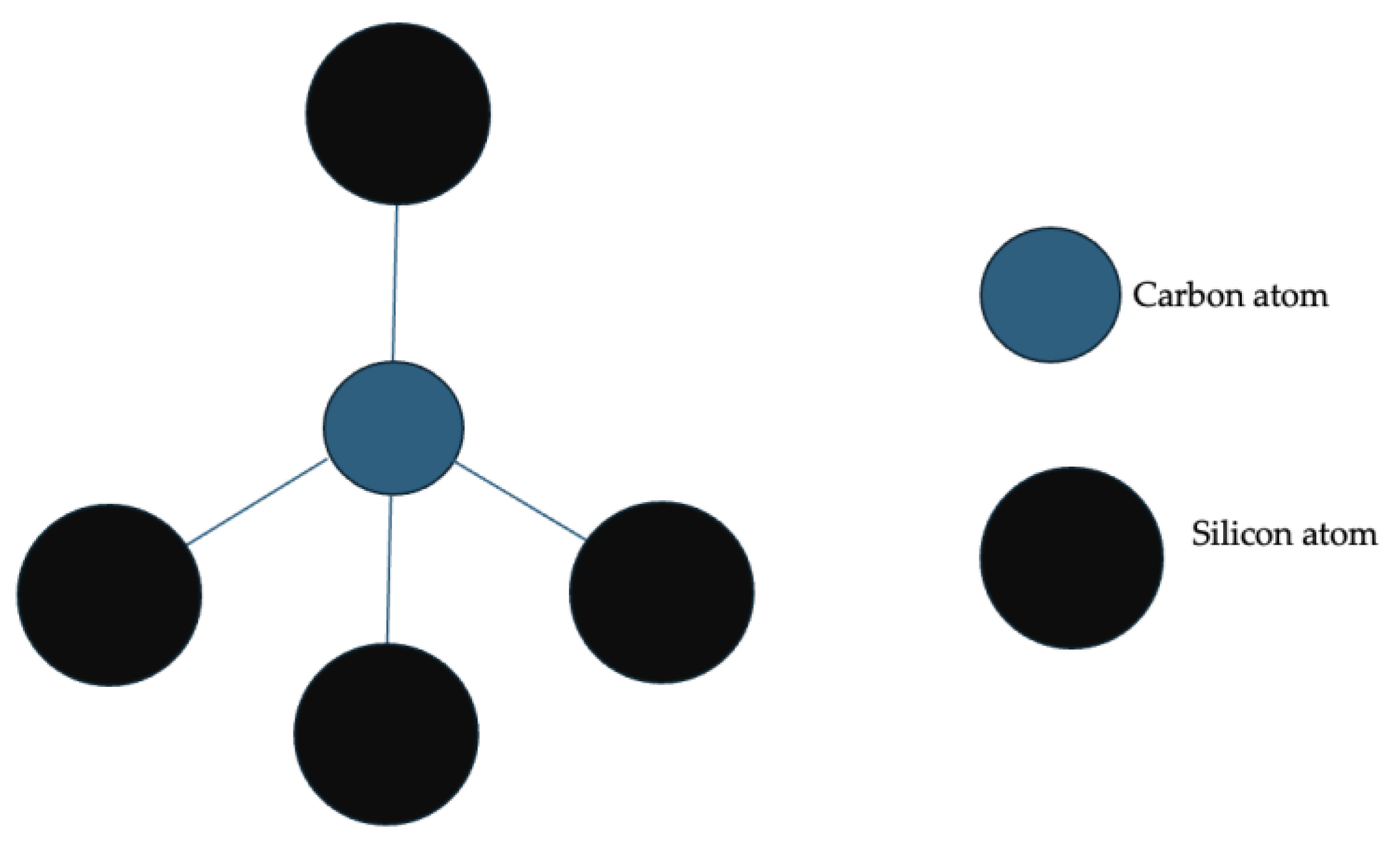

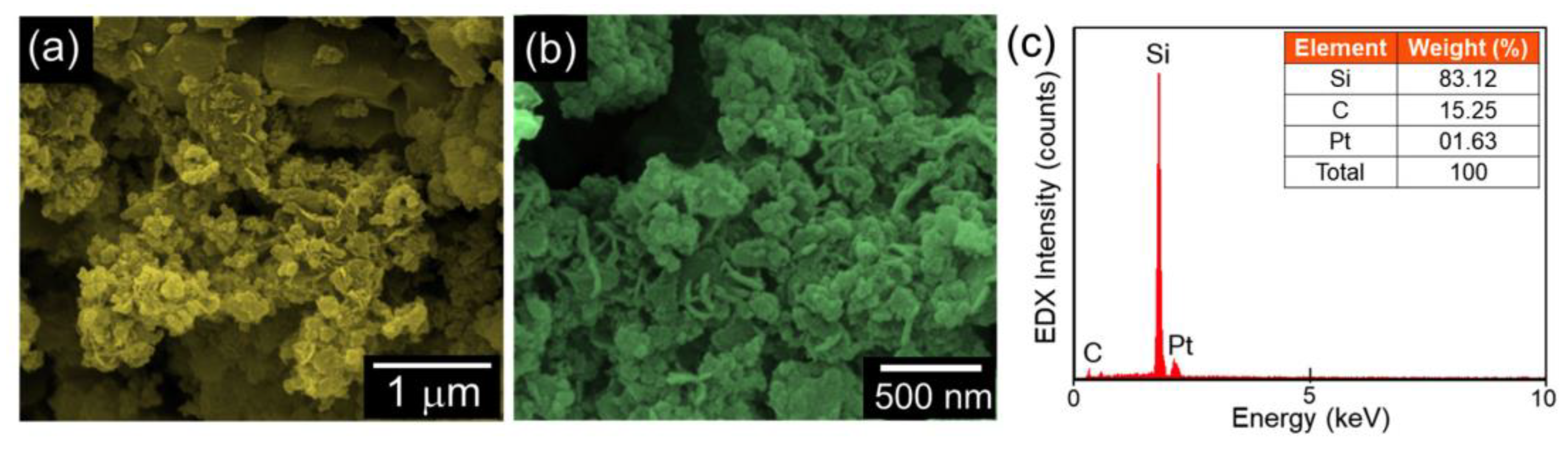

| Energy Storage System (ESS) | Cycle Life | Energy Density (W·kg−1) | Energy Efficiency (%) |
|---|---|---|---|
| Lithium-ion batteries (LIBs) | 4000–5000 [5] | 110–160 [6] | 85–90 [7] |
| Pb acid battery | 500–1000 [5] | 33 [8] | 75–80 [8] |
| Ni-Cd battery | 1500 [6] | 45–80 [6] | 78–85 [6] |
| Na-S battery | 300 [9] | 110.6 [9] | 80–90 [7] |
| Roles of Biomass-Derived Carbon in LIBs? | Biochar Types | Properties of the Biomass | Max Specific Capacity (mAh/g) |
|---|---|---|---|
| Anode material | Hard/soft carbon, activated carbon | Conductivity, defect sites, porous, surface area | 452 [63] |
| Dopant | Chitosan | Heteroatoms like N and O dopants | 780 [64] |
| Binder | Chitin, chitosan | Polymeric nature, chemical stability | 110.8 [65] |
| Separator | Cellulose | Insulator, flexibility, mechanical strength, chemical stability, wettability | 1098 [66] |
| Current collector | Carbon cloth | Conducting, thin film, chemical stability, mechanical strength | 212 [67] |
| Material | C | Si | O | Ca | Mg |
|---|---|---|---|---|---|
| Sugarcane bagasse | 46.08–33.5 | 53.69–41.19 | 46.30–41.90 | 11.69–9.08 | 7.83–0.35 |
| Type of Activating Agent Used | Effect on Biochar |
|---|---|
| Phosphoric acid (H3PO4) |
|
| Zinc chloride (ZnCl2) |
|
| Potassium hydroxide (KOH) |
|
| Sulfuric acid (H2SO4) |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesulo, S.U.; September, L.A.; Kheswa, N.; Seroka, N.S.; Khotseng, L. The Green Synthesis of Nanostructured Silicon Carbides (SiCs) from Sugarcane Bagasse Ash (SCBA) as Anodes in Lithium-Ion (Li-Ion) Batteries: A Review Paper. Processes 2024, 12, 1817. https://doi.org/10.3390/pr12091817
Pesulo SU, September LA, Kheswa N, Seroka NS, Khotseng L. The Green Synthesis of Nanostructured Silicon Carbides (SiCs) from Sugarcane Bagasse Ash (SCBA) as Anodes in Lithium-Ion (Li-Ion) Batteries: A Review Paper. Processes. 2024; 12(9):1817. https://doi.org/10.3390/pr12091817
Chicago/Turabian StylePesulo, Sandy U., Lyle A. September, Ntombizonke Kheswa, Ntalane S. Seroka, and Lindiwe Khotseng. 2024. "The Green Synthesis of Nanostructured Silicon Carbides (SiCs) from Sugarcane Bagasse Ash (SCBA) as Anodes in Lithium-Ion (Li-Ion) Batteries: A Review Paper" Processes 12, no. 9: 1817. https://doi.org/10.3390/pr12091817
APA StylePesulo, S. U., September, L. A., Kheswa, N., Seroka, N. S., & Khotseng, L. (2024). The Green Synthesis of Nanostructured Silicon Carbides (SiCs) from Sugarcane Bagasse Ash (SCBA) as Anodes in Lithium-Ion (Li-Ion) Batteries: A Review Paper. Processes, 12(9), 1817. https://doi.org/10.3390/pr12091817









