Effects of Compositions and Fractal Pores on CO2 Adsorption in Lacustrine Shale
Abstract
:1. Introduction
2. Samples and Methodology
2.1. Geological Backgrounds and Samples
2.2. Analytical Experiments
2.2.1. Fundamental Experiments for Shale Properties
2.2.2. Low-Temperature N2 Gas Adsorption
2.2.3. CO2 Isothermal Adsorption Experiments at 50 °C
- (a)
- Sample preparation: the original shale was crushed to a 60–80 mesh size (0.18–0.25 mm) by a ball mill, and 100–150 g pulverized powder samples were prepared for each experiment.
- (b)
- Sample drying and degassing: powder samples underwent a dry treatment at 110 °C for 72 h in an oven to remove impurity gas and moisture.
- (c)
- Experiment preparation: the powder samples were placed into the sample cell, then a vacuum pump was used to perform vacuum treatment. The target temperature (50 °C) was set through the thermostat bath system.
- (d)
- Free volume calibration: High-pressure helium was injected to detect the tightness of the device. Helium was used as the probe to calibrate the free volume by comparing the mass balance parameters before and after its injection, and the average value was calculated through multiple measurements to reduce the error.
- (e)
- Adsorption test: CO2 with a purity of 99.99% was injected into the reference cell to reach the designed pressure, and then the air valve between the reference cell and sample cell was opened for CO2 adsorption. Gradually, CO2 was adsorbed onto the shale samples until a dynamic equilibrium was reached. The adsorption equilibrium time was maintained for more than 12 h at each equilibrium pressure point. In the present study, eight pressure points were set, ranging from 0 to 7 MPa.
- (f)
- Data collection: The free volume, temperature, and pressure parameters before/after adsorption equilibrium were collected in a computer connected to the apparatus. Finally, the adsorption capacities at each equilibrium pressure were calculated according to the data acquisition system.
2.3. Fractal Theory
3. Results
3.1. Organic Geochemistry and Mineralogy of Lacustrine Shales
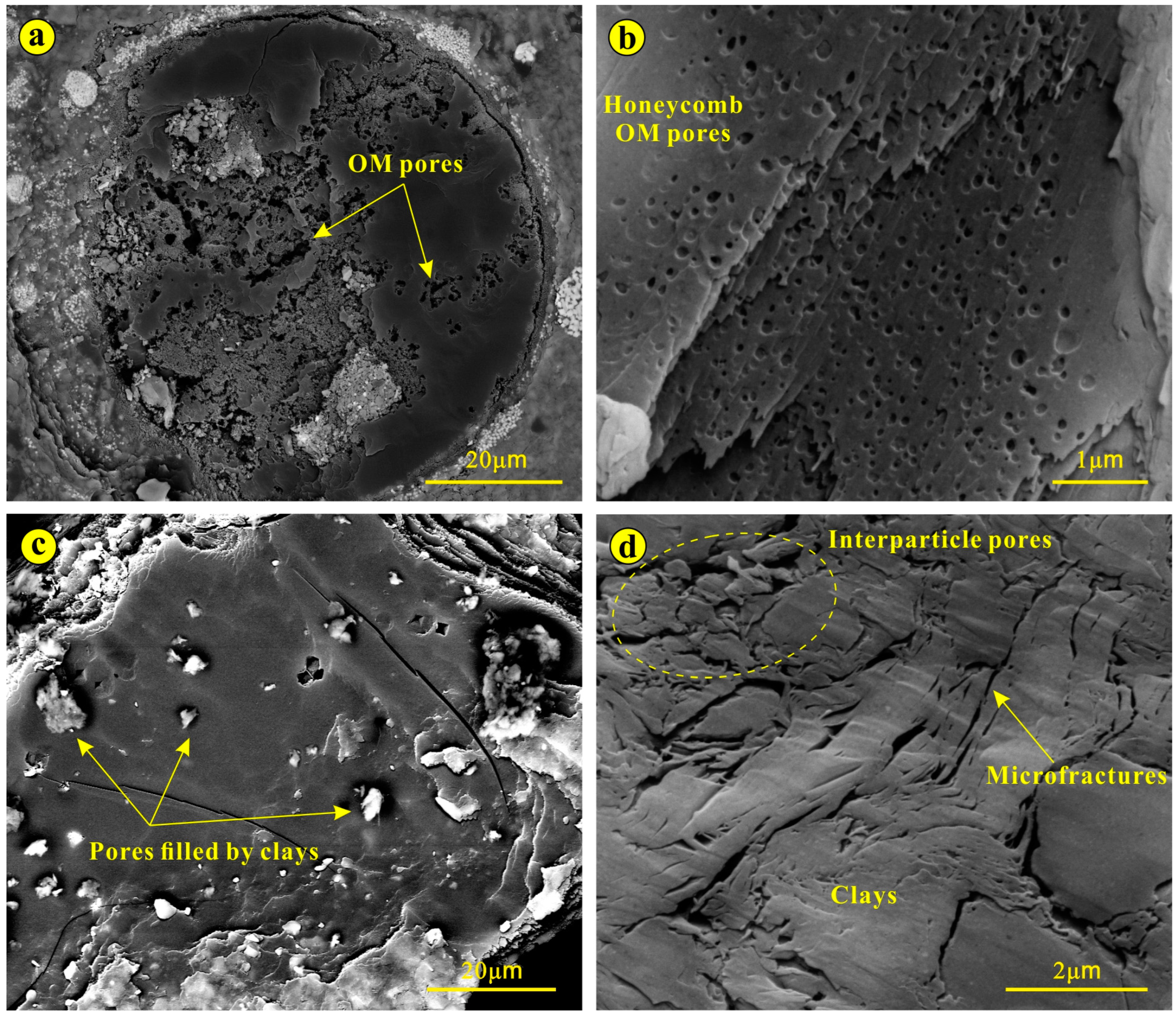
3.2. Pore Characteristics of Lacustrine Shales
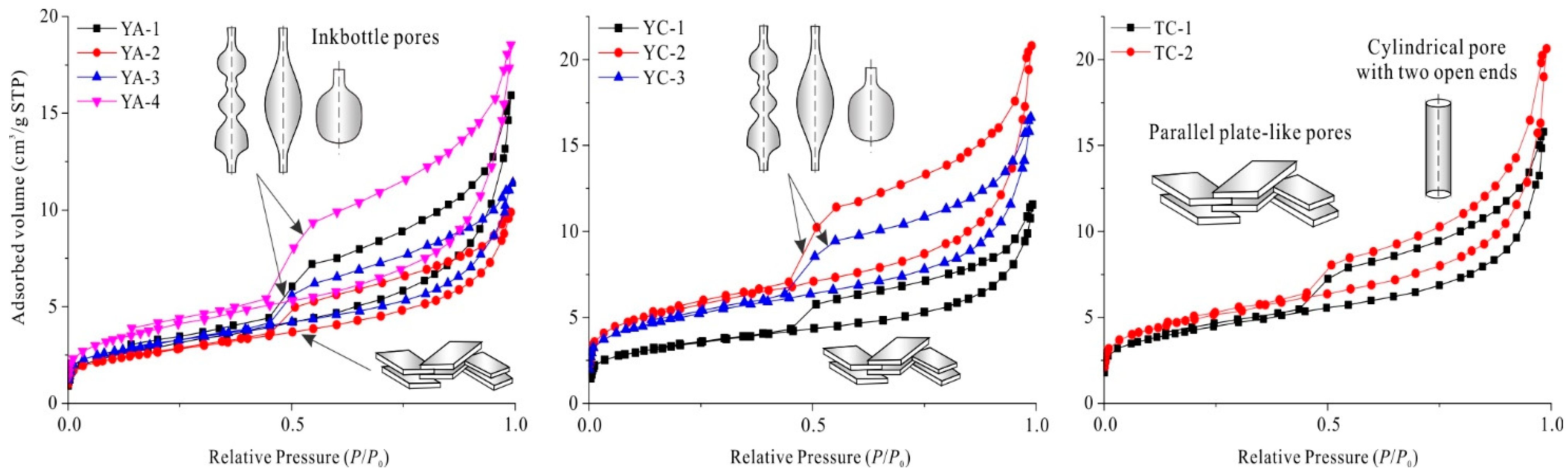
3.2.1. N2 Adsorption/Desorption Isotherms and Pore Shape
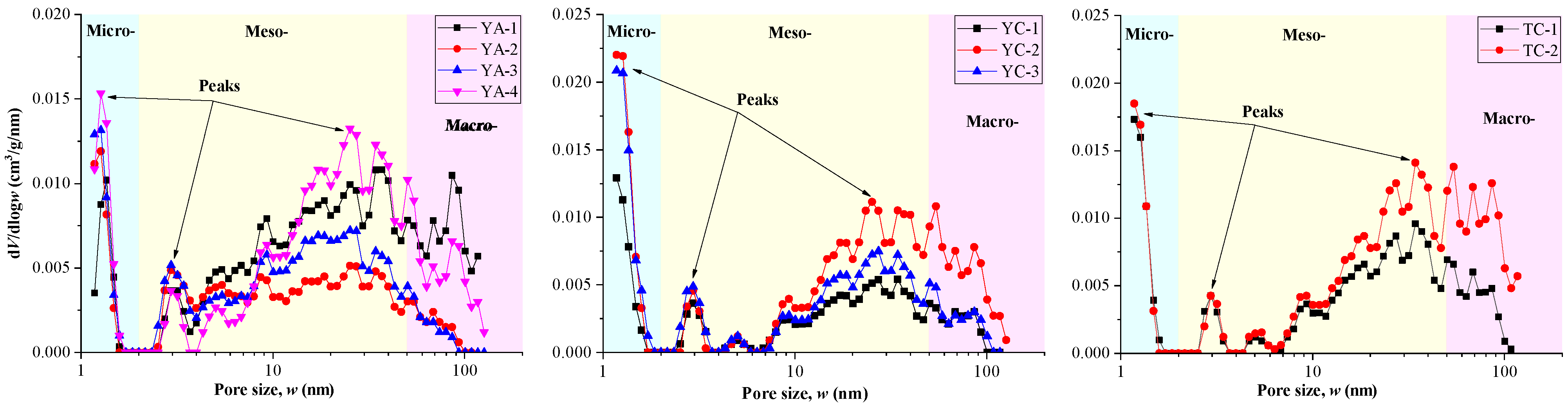
3.2.2. Pore Size Distribution and Pore Structure Parameters
3.3. Fractal Heterogeneity of Pore Structure
3.4. CO2 Adsorption Isotherms of Lacustrine Shales
4. Discussion
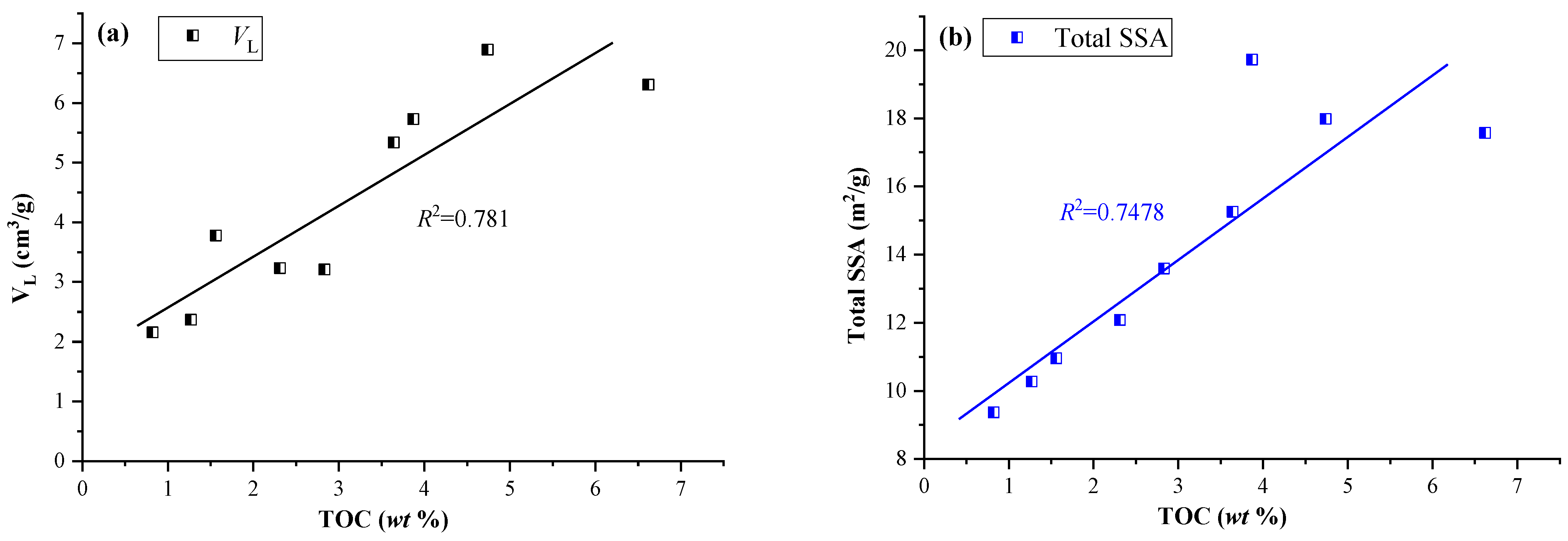
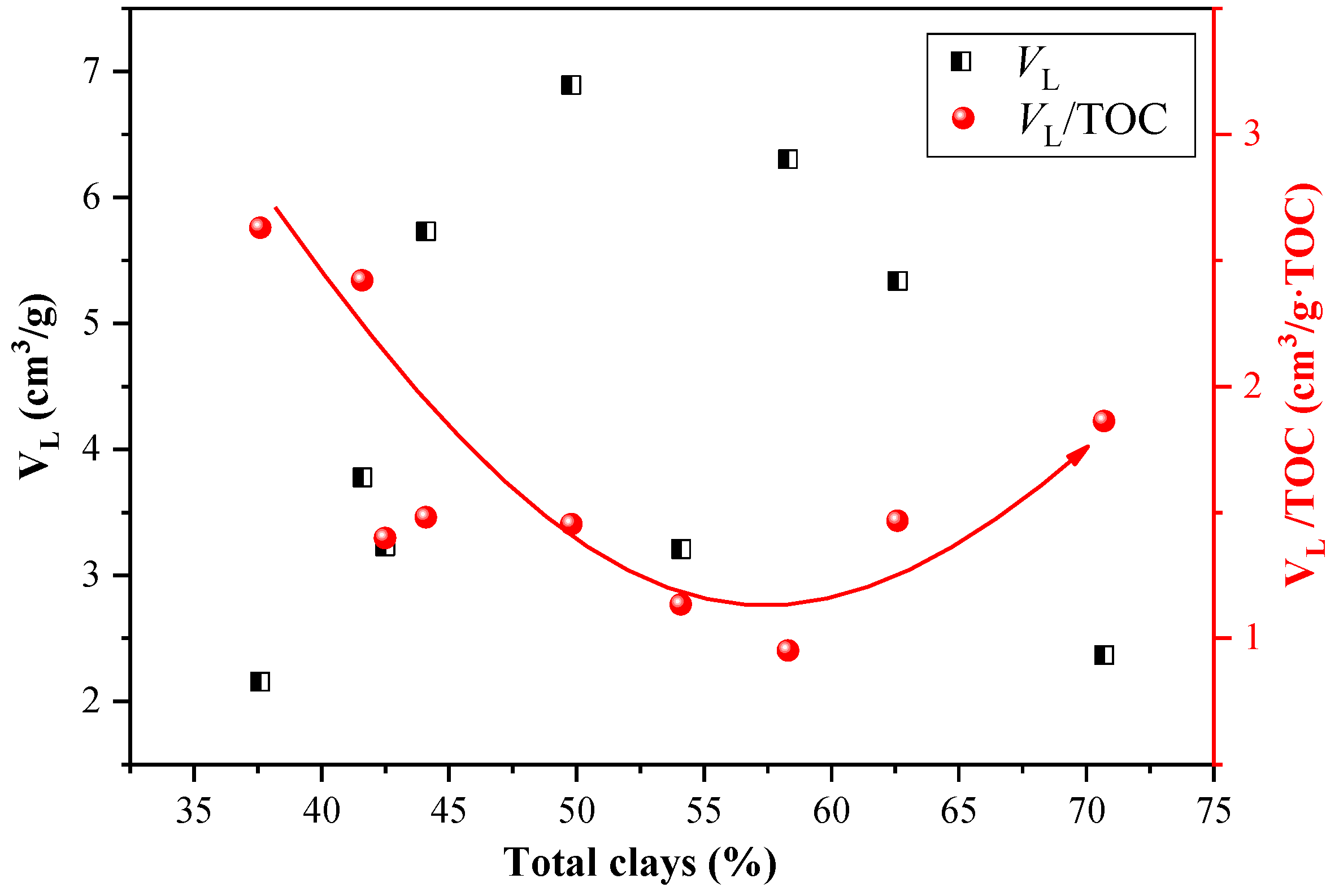
4.1. Effect of Lacustrine Shale Compositions on CO2 Adsorption Capacity
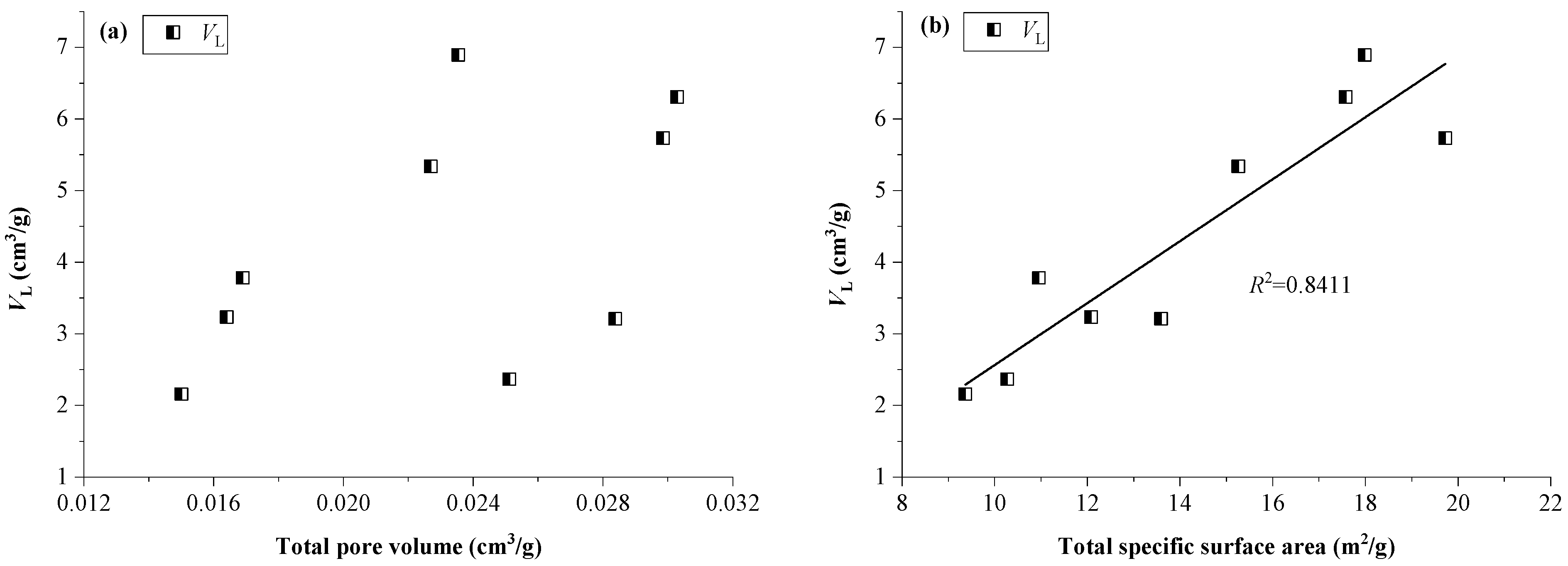
4.2. Effect of Pore Size Distributions on CO2 Adsorption Capacity
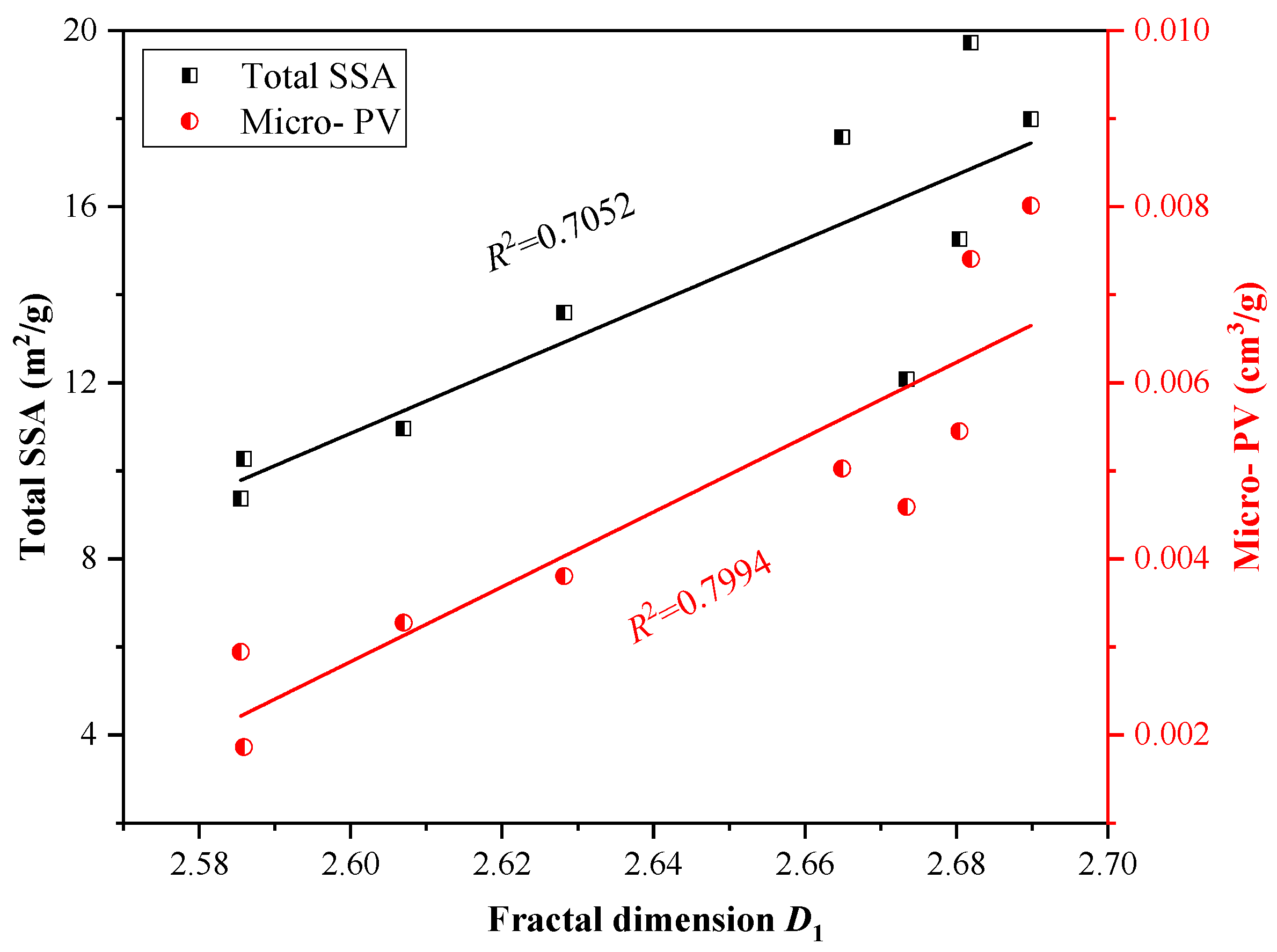
4.3. Effect of Fractal Dimensions on CO2 Adsorption Capacity
5. Conclusions
- (1)
- Distinguished from the mineralogy in typical marine shales, the Ch7 lacustrine shales have a lower quartz content but a higher clay content. The CO2 adsorption isotherms of the Ch7 lacustrine shales show a quick uptake at lower injection pressures, which then levels off gradually as the pressures increase, with the Langmuir volume ranging from 2.16–6.89 cm3/g at 50 °C.
- (2)
- The PSDs are multi-modal with a main peak and two secondary peaks in the Ch7 lacustrine shales. Two-regime fractal characteristics were identified in the pore structure of Ch7 lacustrine shales, and fractal dimensions were quantitatively calculated with the pore surface fractal dimension (D1) and spatial structure fractal dimension (D2) varying at 2.586–2.690 and 2.756–2.855, respectively.
- (3)
- The TOC significantly enhances the capacity for CO2 adsorption in the Ch7 lacustrine shales due to the rich nanopores and specific surface area provided by organic matter. However, the effect of clay minerals on CO2 adsorption is multi-faceted in Ch7 lacustrine shales. As the total clay content increases, the CO2 adsorption capacity initially decreases because the illite–smectite mixed layers fill the pores spaces, limiting the CO2 adsorption capacity, and then increase as the clay-related pores gradually dominate the pore spaces and adsorption sites.
- (4)
- Among the pore size distributions in lacustrine shales, micropores dominate CO2 adsorption as they contribute to a larger specific surface area per unit and possess greater adsorption potentials compared to macropores and mesopores. Moreover, the pore surface fractal dimension (D1) is positively related to CO2 adsorption capacity because of a larger D1 along with a more heterogeneous pore surface and more available locations for CO2 adsorption, while the spatial structure fractal dimension (D2) has a less noticeable impact on the CO2 adsorption capacity.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chronopoulos, T.; Fernandez-Diez, Y.; Maroto-Valer, M.M.; Ocone, R.; Reay, D.A. CO2 desorption via microwave heating for post-combustion carbon capture. Microporous Mesoporous Mat. 2014, 197, 288–290. [Google Scholar] [CrossRef]
- Fernández, J.; Sotenko, M.; Derevschikov, V.; Lysikov, A.; Rebrov, E.V. A radiofrequency heated reactor system for post-combustion carbon capture. Chem. Eng. Process. Process Intensif. 2016, 108, 17–26. [Google Scholar] [CrossRef]
- Du, X.; Gu, M.; Hou, Z.; Liu, Z.; Wu, T. Experimental study on the kinetics of adsorption of CO2 and CH4 in gas-bearing shale reservoirs. Energy Fuels 2019, 33, 12587–12600. [Google Scholar] [CrossRef]
- Fakher, S.; Imqam, A. High pressure-high temperature carbon dioxide adsorption to shale rocks using a volumetric method. Int. J. Greenh. Gas. Control 2020, 95, 102998. [Google Scholar] [CrossRef]
- Hu, K.; Herdegen, V.; Mischo, H. Carbon dioxide adsorption to 40 mpa on extracted shale from Sichuan basin, southwestern China. Fuel 2022, 318, 123666. [Google Scholar] [CrossRef]
- Jeong, S.R.; Park, J.H.; Lee, J.H.; Jeon, P.R.; Lee, C. Review of the adsorption equilibria of CO2, CH4, and their mixture on coals and shales at high pressures for enhanced CH4 recovery and CO2 sequestration. Fluid Phase Equilib. 2023, 564, 113591. [Google Scholar] [CrossRef]
- Khormali, A.; Ahmadi, S. Experimental and modeling analysis on the performance of 2-mercaptobenzimidazole corrosion inhibitor in hydrochloric acid solution during acidizing in the petroleum industry. J. Pet. Explor. Prod. Technol. 2023, 13, 2217–2235. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Sun, X.; Tian, S.; Tang, J.; Shen, F.; Wu, J. The influences of composition and pore structure on the adsorption behavior of CH4 and CO2 on shale. Front. Earth Sci. 2021, 15, 283–300. [Google Scholar] [CrossRef]
- Ambrose, R.J.; Hartman, R.C.; Diaz-Campos, M.; Akkutlu, I.Y.; Sondergeld, C.H. Shale gas-in-place calculations part I: New pore-scale considerations. SPE J. 2012, 17, 219–229. [Google Scholar] [CrossRef]
- Feng, G.; Zhu, Y.; Chen, S.; Wang, Y.; Ju, W.; Hu, Y.; You, Z.; Wang, G.G.X. Supercritical methane adsorption on shale over wide pressure and temperature ranges: Implications for gas-in-place estimation. Energy Fuels 2020, 34, 3121–3134. [Google Scholar] [CrossRef]
- Tang, X.; Ripepi, N.; Luxbacher, K.; Pitcher, E. Adsorption models for methane in shales: Review, comparison, and application. Energy Fuels 2017, 31, 10787–10801. [Google Scholar] [CrossRef]
- Curtis, J. Fractured shale-gas systems. Am. Assoc. Pet. Geol. Bull. 2002, 86, 1921–1938. [Google Scholar] [CrossRef]
- Jarvie, D.M.; Hill, R.J.; Ruble, T.E.; Pollastro, R.M. Unconventional shale-gas systems: The Mississippian Barnett shale of north-central Texas as one model for thermogenic shale-gas assessment. Am. Assoc. Pet. Geol. Bull. 2007, 91, 475–499. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Z.; Zhang, Y.; Sun, Y.; Liu, S.; Li, Z.; Liu, Y.; Yang, S.; Zhao, S. Influence of shale mineral composition and proppant filling patterns on stress sensitivity in shale reservoirs. Processes 2024, 12, 789. [Google Scholar] [CrossRef]
- Feng, G.; Zhu, Y.; Wang, G.G.X.; Chen, S.; Wang, Y.; Ju, W. Supercritical methane adsorption on overmature shale: Effect of pore structure and fractal characteristics. Energy Fuels 2019, 33, 8323–8337. [Google Scholar] [CrossRef]
- Chalmers, G.R.; Bustin, R.M.; Power, I.M. Characterization of gas shale pore systems by porosimetry, pycnometry, surface area, and field emission scanning electron microscopy/transmission electron microscopy image analyses: Examples from the barnett, woodford, haynesville, marcellus, and doig units. Am. Assoc. Pet. Geol. Bull. 2012, 96, 1099–1119. [Google Scholar] [CrossRef]
- Wang, J.J.; Lu, S.F.; Zhang, P.F.; Zhi, Q.; Huang, H.S. Pore distribution characteristics of different lithofacies shales: Evidence from scanning electron microscopy. Processes 2023, 11, 1120. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Li, W.; Xiang, J.; Wang, Y.; Li, J.; Zeng, F. Molecular simulation of methane adsorption in shale based on grand canonical monte carlo method and pore size distribution. J. Nat. Gas Sci. Eng. 2016, 30, 119–126. [Google Scholar] [CrossRef]
- Mosher, K.; He, J.; Liu, Y.; Rupp, E.; Wilcox, J. Molecular simulation of methane adsorption in micro- and mesoporous carbons with applications to coal and gas shale systems. Int. J. Coal Geol. 2013, 109–110, 36–44. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (iupac technical report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Chen, F.; Yang, D.; Liu, D.; Ding, X.; Lu, S. Correction and application of the nuclear magnetic resonance T2 spectra of shale reservoir samples in a centrifugal state: A case study of the Longmaxi Shale in Southeast Chongqing, China. Energy Fuels 2022, 36, 14913–14925. [Google Scholar] [CrossRef]
- Zheng, S.; Yao, Y.; Liu, D.; Cai, Y.; Liu, Y. Characterizations of full-scale pore size distribution, porosity and permeability of coals: A novel methodology by nuclear magnetic resonance and fractal analysis theory. Int. J. Coal Geol. 2018, 196, 148–158. [Google Scholar] [CrossRef]
- Mastalerz, M.; He, L.; Melnichenko, Y.B.; Rupp, J.A. Porosity of coal and shale: Insights from gas adsorption and sans/usans techniques. Energy Fuels 2012, 26, 5109–5120. [Google Scholar] [CrossRef]
- Radlinski, A.; Mastalerz, M.; Hinde, A.; Hainbuchner, M.; Rauch, H.; Baron, M.; Lin, J.; Fan, L.; Thiyagarajan, P. Application of saxs and sans in evaluation of porosity, pore size distribution and surface area of coal. Int. J. Coal Geol. 2004, 59, 245–271. [Google Scholar] [CrossRef]
- Garum, M.; Glover, P.W.J.; Lorinczi, P.; Drummond-Brydson, R.; Hassanpour, A. Micro- and nano-scale pore structure in gas shale using XµU-CT and FIB-SEM techniques. Energy Fuels 2020, 34, 12340–12353. [Google Scholar] [CrossRef]
- Huang, X.; Yang, D.; Kang, Z. Three-phase segmentation method for organic matter recognition in source rocks via CT images: A case study on oil shale pyrolyzed by steam. Energy Fuels 2021, 35, 10075–10085. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Hammes, U. Spectrum of pore types and networks in mudrocks and a descriptive classification for matrix-related mudrock pores. Am. Assoc. Pet. Geol. Bull. 2012, 96, 1071–1098. [Google Scholar] [CrossRef]
- Loucks, R.G.; Reed, R.M.; Ruppel, S.C.; Jarvie, D.M. Morphology, genesis, and distribution of nanometer-scale pores in siliceous mudstones of the Mississippian Barnett shale. J. Sediment. Res. 2009, 79, 848–861. [Google Scholar] [CrossRef]
- Feng, G.; Zhao, X.; Wang, M.; Song, Y.; Zheng, S.; He, Y.; You, Z. Fractal pore and its impact on gas adsorption capacity of outburst coal: Geological significance to coalbed gas occurrence and outburst. Adsorp. Sci. Technol. 2022, 2022, 4273900. [Google Scholar] [CrossRef]
- Huang, Z.; Liu, G.; Li, T.; Li, Y.; Yin, Y.; Wang, L. Characterization and control of mesopore structural heterogeneity for low thermal maturity shale: A case study of Yanchang formation shale, ordos basin. Energy Fuels 2017, 31, 11569–11586. [Google Scholar] [CrossRef]
- Li, Y.; Song, D.; Liu, S.; Ji, X.; Hao, H. Evaluation of pore properties in coal through compressibility correction based on mercury intrusion porosimetry: A practical approach. Fuel 2021, 291, 120130. [Google Scholar] [CrossRef]
- Yang, Q.; Xue, J.; Li, W.; Du, X.; Ma, Q.; Zhan, K.; Chen, Z. Comprehensive evaluation and interpretation of mercury intrusion porosimetry data of coals based on fractal theory, tait equation and matrix compressibility. Fuel 2021, 298, 120823. [Google Scholar] [CrossRef]
- Yu, Y.; Luo, X.; Wang, Z.; Cheng, M.; Lei, Y.; Zhang, L.; Yin, J. A new correction method for mercury injection capillary pressure (micp) to characterize the pore structure of shale. J. Nat. Gas Sci. Eng. 2019, 68, 102896. [Google Scholar] [CrossRef]
- Mu, G.; Hou, H.; Zhang, J.; Tang, Y.; Li, Y.-N.; Sun, B.; Li, Y.; Jones, T.; Yuan, Y.; Shao, L. Fractal characterization of pore structure and its influence on CH4 adsorption and seepage capacity of low-rank coals. Front. Earth Sci. 2022, 16, 916–933. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Tang, D.; Tang, S.; Huang, W.; Liu, Z.; Che, Y. Fractal characterization of seepage-pores of coals from China: An investigation on permeability of coals. Comput. Geosci. 2009, 35, 1159–1166. [Google Scholar] [CrossRef]
- Feng, G.; Li, W.; Zhu, Y.; Wang, X.; Wang, Y.; Song, Y.; Zheng, S.; Shang, F. Matrix compressibility and multifractal nature of nanoporous shale. Energy Fuels 2024, 38, 4057–4075. [Google Scholar] [CrossRef]
- Feng, G.; Li, W.; Zhu, Y.; Wang, Y.; Song, Y.; Zheng, S.; Shang, F. Scale-dependent fractal properties and geological factors for the pore structure in shale: Insights from field emission scanning electron microscopy and fluid intrusion. Energy Fuels 2023, 37, 16519–16535. [Google Scholar] [CrossRef]
- Li, Y.H.; Lu, G.Q.; Rudolph, V. Compressibility and fractal dimension of fine coal particles in relation to pore structure characterisation using mercury porosimetry. Part. Part. Syst. Charact. 1999, 16, 25–31. [Google Scholar] [CrossRef]
- Zhang, M.; Fu, X.; Duan, C.; Li, Y. Influencing factor analysis of the coal matrix compressibility of middle-high rank coals. J. Nat. Gas Sci. Eng. 2020, 81, 103462. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Pan, Z.; Yao, Y.; Li, J.; Qiu, Y. Pore structure and its impact on CH4 adsorption capacity and flow capability of bituminous and subbituminous coals from northeast China. Fuel 2013, 103, 258–268. [Google Scholar] [CrossRef]
- Qi, L.; Tang, X.; Wang, Z.; Peng, X. Pore characterization of different types of coal from coal and gas outburst disaster sites using low temperature nitrogen adsorption approach. Int. J. Min. Sci. Technol. 2017, 27, 371–377. [Google Scholar] [CrossRef]
- Yao, Y.; Liu, D.; Tang, D.; Tang, S.; Huang, W. Fractal characterization of adsorption-pores of coals from north China: An investigation on CH4 adsorption capacity of coals. Int. J. Coal Geol. 2008, 73, 27–42. [Google Scholar] [CrossRef]
- Haki, A.; El Hadi, M.A.; Bouhafid, A. Assessment of the pyrolysis, combustion and fractal dimension of fragmented oil shale particles. Powder Technol. 2017, 318, 569–588. [Google Scholar] [CrossRef]
- Peta, K.; Stemp, W.J.; Chen, R.; Love, G.; Brown, C.A. Multiscale characterizations of topographic measurements on lithic materials and microwear using a gelsight max: Investigating potential archaeological applications. J. Archaeol. Sci. Rep. 2024, 57, 104637. [Google Scholar] [CrossRef]
- Rexer, T.F.; Mathia, E.J.; Aplin, A.C.; Thomas, K.M. High-pressure methane adsorption and characterization of pores in posidonia shales and isolated kerogens. Energy Fuels 2014, 28, 2886–2901. [Google Scholar] [CrossRef]
- Ross, D.J.K.; Bustin, R.M. The importance of shale composition and pore structure upon gas storage potential of shale gas reservoirs. Mar. Pet. Geol. 2009, 26, 916–927. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Liu, S.; Zhang, R. Pore characterization and its impact on methane adsorption capacity for organic-rich marine shales. Fuel 2016, 181, 227–237. [Google Scholar] [CrossRef]
- Crosdale, P.J.; Moore, T.A.; Mares, T.E. Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank, biogenically-sourced gas reservoir. Int. J. Coal Geol. 2008, 76, 166–174. [Google Scholar] [CrossRef]
- Dang, W.; Zhang, J.; Wei, X.; Tang, X.; Chen, Q.; Li, Z.; Zhang, M.; Liu, J. Geological controls on methane adsorption capacity of lower permian transitional black shales in the southern north China basin, central China: Experimental results and geological implications. J. Pet. Sci. Eng. 2017, 152, 456–470. [Google Scholar] [CrossRef]
- Gasparik, M.; Bertier, P.; Gensterblum, Y.; Ghanizadeh, A.; Krooss, B.M.; Littke, R. Geological controls on the methane storage capacity in organic-rich shales. Int. J. Coal Geol. 2014, 123, 34–51. [Google Scholar] [CrossRef]
- Zou, J.; Rezaee, R.; Xie, Q.; You, L. Characterization of the combined effect of high temperature and moisture on methane adsorption in shale gas reservoirs. J. Pet. Sci. Eng. 2019, 182, 106353. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, T.; Milliken, K.L.; Qu, J.; Zhang, X. Experimental investigation of main controls to methane adsorption in clay-rich rocks. Appl. Geochem. 2012, 27, 2533–2545. [Google Scholar] [CrossRef]
- Zhang, T.; Ellis, G.S.; Ruppel, S.C.; Milliken, K.; Yang, R. Effect of organic-matter type and thermal maturity on methane adsorption in shale-gas systems. Org. Geochem. 2012, 47, 120–131. [Google Scholar] [CrossRef]
- Kang, S.M.; Fathi, E.; Ambrose, R.J.; Akkutlu, I.Y.; Sigal, R.F. Carbon dioxide storage capacity of organic-rich shales. SPE J. 2011, 16, 842–855. [Google Scholar] [CrossRef]
- Dai, J.; Li, J.; Luo, X.; Zhang, W.; Hu, G.; Ma, C.; Guo, J.; Ge, S. Stable carbon isotope compositions and source rock geochemistry of the giant gas accumulations in the ordos basin, China. Org. Geochem. 2005, 36, 1617–1635. [Google Scholar] [CrossRef]
- Duan, Y.; Wang, C.Y.; Zheng, C.Y.; Wu, B.X.; Zheng, G.D. Geochemical study of crude oils from the Xifeng oilfield of the ordos basin, China. J. Asian Earth Sci. 2008, 31, 341–356. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, X.; Li, Y.; Xu, L.; Niu, X.; Zhu, S.; Liang, X.; Xue, M.; He, J. Sedimentary characteristics and facies model of gravity flow deposits of late triassic Yanchang formation in southwestern ordos basin, nw China. Pet. Explor. Dev. 2015, 42, 633–645. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Mandelbrot, B.B.; Passoja, D.; Paullay, A.J. Fractal character of fracture surfaces of metals. Nature 1984, 308, 721–722. [Google Scholar] [CrossRef]
- Avnir, D.; Jaroniec, M. An isotherm equation for adsorption on fractal surfaces of heterogeneous porous materials. Langmuir 1989, 5, 1431–1433. [Google Scholar] [CrossRef]
- Yang, F.; Ning, Z.; Liu, H. Fractal characteristics of shales from a shale gas reservoir in the Sichuan basin, China. Fuel 2014, 115, 378–384. [Google Scholar] [CrossRef]
- Zheng, S.; Sang, S.; Wang, M.; Liu, S.; Huang, K.; Feng, G.; Song, Y. Experimental investigations of CO2 adsorption behavior in shales: Implication for CO2 geological storage. Front. Earth Sci. 2023, 10, 1098035. [Google Scholar] [CrossRef]
- Pfeiferper, P.; Avnir, D. Chemistry nonintegral dimensions between two and three. J. Chem. Phys. 1983, 79, 3369–3558. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Wang, H.; Zhou, S.; Zhang, J.; Feng, Z.; Jiao, P.; Zhang, L.; Zhang, Q. Clarifying the effect of clay minerals on methane adsorption capacity of marine shales in Sichuan Basin, China. Energies 2021, 14, 6836. [Google Scholar] [CrossRef]








| Sample ID | Sampling Location | TOC (wt%) | Ro a (%) | Kerogen Type b | Mineralogical Composition (wt%) | Clay Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quartz | Feldspar | Carbonate | Pyrite | Total Clay c | I/S d | Illite | Kaolinite | Chlorite | |||||
| YA-1 | Yan’an region | 1.27 | 1.01 | II1 | 19.3 | 8.2 | 0.8 | 1.0 | 70.7 | 49.5 | 6.4 | 9.9 | 4.9 |
| YA-2 | 0.82 | n/a e | n/a | 25.8 | 29.5 | 2.5 | 4.6 | 37.6 | 22.1 | 9.0 | 2.0 | 4.6 | |
| YA-3 | 1.56 | 0.95 | II2 | 25.0 | 26.7 | 3.3 | 3.4 | 41.6 | 19.8 | 7.7 | 3.6 | 10.5 | |
| YA-4 | 2.83 | 1.12 | II1 | 28.7 | 17.2 | 0 | 0 | 54.1 | 35.0 | 4.8 | 6.2 | 8.2 | |
| YC-1 | Yichuan region | 2.31 | 0.79 | I | 29.1 | 26.6 | 1.8 | 0 | 42.5 | 16.2 | 7.8 | 4.7 | 13.8 |
| YC-2 | 3.87 | n/a | n/a | 30.2 | 22.6 | 0 | 3.1 | 44.1 | 25.6 | 6.6 | 4.0 | 7.9 | |
| YC-3 | 4.74 | 0.75 | II1 | 31.9 | 17.1 | 1.2 | 0 | 49.8 | 29.9 | 6.3 | 7.1 | 6.4 | |
| TC-1 | Tongchuan region | 3.64 | 0.83 | I | 19.8 | 16.7 | 0.9 | 0 | 62.6 | 28.2 | 15.5 | 8.5 | 10.4 |
| TC-2 | 6.62 | n/a | n/a | 22.3 | 16.8 | 1.5 | 1.1 | 58.3 | 34.3 | 12.9 | 5.3 | 5.8 | |
| Sample ID | Average Pore Diameter (nm) | BJH Pore Volume (cm3/g) | BET Specific Surface Area (m2/g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | Micropores | Mesopores | Macropores | Total | Micropores | Mesopores | Macropores | ||
| YA-1 | 10.14 | 0.0251 | 0.0019 | 0.0178 | 0.0054 | 10.271 | 4.774 | 5.259 | 0.238 |
| YA-2 | 6.78 | 0.0150 | 0.0029 | 0.0110 | 0.0011 | 9.371 | 6.014 | 3.317 | 0.039 |
| YA-3 | 6.65 | 0.0169 | 0.0033 | 0.0128 | 0.0008 | 10.958 | 7.202 | 3.720 | 0.036 |
| YA-4 | 8.85 | 0.0284 | 0.0038 | 0.0201 | 0.0045 | 13.592 | 9.069 | 4.343 | 0.181 |
| YC-1 | 6.10 | 0.0164 | 0.0046 | 0.0098 | 0.0020 | 12.080 | 9.768 | 2.238 | 0.075 |
| YC-2 | 6.74 | 0.0299 | 0.0074 | 0.0166 | 0.0059 | 19.724 | 16.438 | 3.063 | 0.223 |
| YC-3 | 5.93 | 0.0235 | 0.0080 | 0.0133 | 0.0023 | 17.988 | 15.149 | 2.761 | 0.078 |
| TC-1 | 6.59 | 0.0227 | 0.0055 | 0.0138 | 0.0034 | 15.261 | 12.318 | 2.809 | 0.134 |
| TC-2 | 7.51 | 0.0303 | 0.0050 | 0.0172 | 0.0081 | 17.577 | 13.545 | 3.674 | 0.357 |
| Sample ID | P/P0 = 0–0.5 | P/P0 = 0.5–1.0 | ||||
|---|---|---|---|---|---|---|
| S1 | D1 = 3 + S1 | R12 | S2 | D2 = 3 + S2 | R22 | |
| YA-1 | −0.4141 | 2.586 | 0.9971 | −0.2091 | 2.791 | 0.9501 |
| YA-2 | −0.4145 | 2.586 | 0.9986 | −0.1624 | 2.838 | 0.9363 |
| YA-3 | −0.393 | 2.607 | 0.9948 | −0.1447 | 2.855 | 0.8794 |
| YA-4 | −0.3718 | 2.628 | 0.9986 | −0.1806 | 2.819 | 0.9346 |
| YC-1 | −0.3266 | 2.673 | 0.9990 | −0.1584 | 2.842 | 0.9788 |
| YC-2 | −0.3181 | 2.682 | 0.9995 | −0.1633 | 2.837 | 0.9713 |
| YC-3 | −0.3102 | 2.690 | 0.9964 | −0.1565 | 2.844 | 0.9785 |
| TC-1 | −0.3196 | 2.680 | 0.9987 | −0.2044 | 2.796 | 0.9853 |
| TC-2 | −0.3351 | 2.665 | 0.9971 | −0.2438 | 2.756 | 0.9864 |
| Sample ID | VL (cm3/g) | PL (MPa) | R2 |
|---|---|---|---|
| YA-1 | 2.37 | 1.63 | 0.9939 |
| YA-2 | 2.16 | 3.07 | 0.9971 |
| YA-3 | 3.78 | 1.50 | 0.9953 |
| YA-4 | 3.21 | 1.58 | 0.9968 |
| YC-1 | 3.23 | 2.74 | 0.9941 |
| YC-2 | 5.73 | 1.73 | 0.9942 |
| YC-3 | 6.89 | 1.21 | 0.9965 |
| TC-1 | 5.34 | 2.58 | 0.9937 |
| TC-2 | 6.30 | 1.74 | 0.9989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, G.; Wang, M.; Zhu, Y.; Song, Y.; Zheng, S.; Dai, X.; Wang, X. Effects of Compositions and Fractal Pores on CO2 Adsorption in Lacustrine Shale. Processes 2024, 12, 1842. https://doi.org/10.3390/pr12091842
Feng G, Wang M, Zhu Y, Song Y, Zheng S, Dai X, Wang X. Effects of Compositions and Fractal Pores on CO2 Adsorption in Lacustrine Shale. Processes. 2024; 12(9):1842. https://doi.org/10.3390/pr12091842
Chicago/Turabian StyleFeng, Guangjun, Meng Wang, Yanming Zhu, Yu Song, Sijian Zheng, Xuguang Dai, and Xuheng Wang. 2024. "Effects of Compositions and Fractal Pores on CO2 Adsorption in Lacustrine Shale" Processes 12, no. 9: 1842. https://doi.org/10.3390/pr12091842





