Abstract
In this study, we report the development of an electro-Fenton (EF) process at a semi-pilot scale plant using an open undivided electrochemical reactor design. To do so, a series of three-dimensional (3D) cathodes constituted of packed and fixed beds of glassy carbon pellets and dimensionally stable anodes (DSAs) were employed. To highlight the treatment efficiency of the EF process, bisphenol A (BPA), which is known to be a persistent molecule, was used as the model molecule. First, the applied current intensity was studied and optimized to determine the limiting current of the O2 reduction under hydrodynamic conditions of 0.6 m3·h−1. The limiting current intensity under hydrodynamic conditions corresponding to 10 L·min−1 (600 L/h) was determined to be near 17.5 A (0.51 A/100 g of glassy carbon pellets). Then, the effect of the number of cathodes on the removal efficiency of BPA versus the time of the electro-Fenton treatment was investigated. The value of Kapp in the typical reactor configuration was found to be 0.076 min−1. Many parameters were carried out using the EF reactor, i.e., the effect of the initial pollutant concentration as well as the effect of the treatment flow rate. The obtained results demonstrate that the degradation efficiency of BPA increases as the number of cathodes increases and the pollution charge decreases. Only a few seconds of treatment by EF process were needed to eliminate BPA from the dilute solutions (≤10 mg·L−1). The biodegradability of the treated solution and its mineralization were also investigated by referring to the measurements of COD, TOC, and BOD5. Finally, strategy of scaling-up the reactor design to an industrial pilot plant is discussed.
1. Introduction
Industrialization in developed countries has led to the emergence of persistent pollutants in the environment [1,2]. These pollutants, known as persistent pollutants, are continuously released into aquatic environments, causing harm to both aquatic and terrestrial organisms, as well as to human health [3,4]. To avoid the entry of this pollution into the environmental cycle and limit its negative impacts, it is crucial to develop new technologies for water remediation.
So far, biological treatment is certainly the process of choice for the remediation of waters; however, the presence of toxic and persistent molecules may limit its application. For this reason, advanced oxidation processes (AOPs) have received great interest in recent years [5,6,7]. These processes consist of producing highly reactive derivatives for the destruction of the target pollutants, mainly hydroxyl radicals (•OH) [8]. These radicals are nonselective oxidant species that typically lead to the degradation of organic matter and enhance the effluent’s biodegradability [9,10,11].
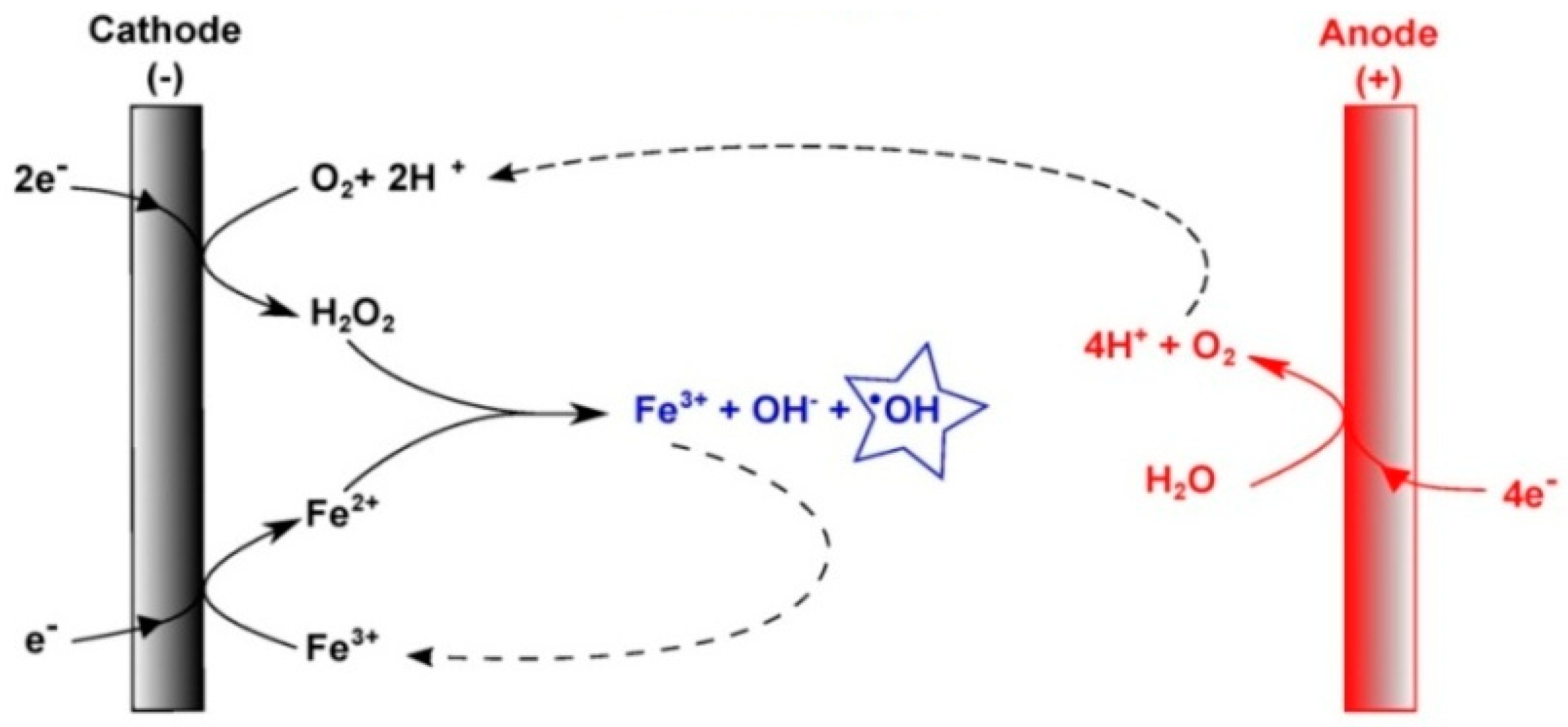
Indeed, the electro-Fenton (EF) process is a powerful advanced oxidation process that has been shown to effectively degrade organic matter at the laboratory scale [12,13]. The process involves reducing dissolved oxygen (O2) at the cathode, which generates hydrogen peroxide (H2O2), also known as Fenton’s reagent [14,15]. This electrochemical reaction occurs in an acidic medium (pH~3) and requires the addition of iron (Fe2+) in low quantities. The electro-generated H2O2 species react with the Fe2+ ions to produce •OH in the homogeneous medium [16] (Figure 1).
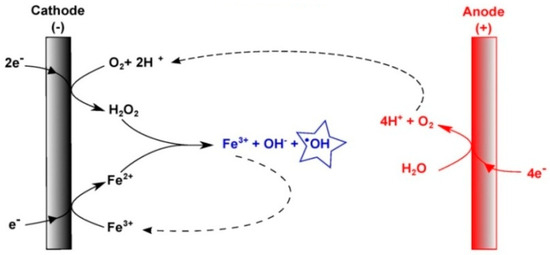
Figure 1.
Schematic representation of the electrochemical production of hydroxyl radicals (●OH) by an electro-Fenton process in a homogeneous medium.
Of course, many studies have reported the effectiveness of the electro-Fenton process in degrading various chemical pollutants, including dyes [17,18,19], pesticides [20,21], pharmaceutical residues [22,23], and other industrial pollutants such as phenols and phenolic compounds, anilines, cresols, and so forth [24,25,26]. This highlights the versatility and potential of the electro-Fenton process as a powerful tool for the remediation of contaminated water; however, these studies have been conducted using various types of electrochemical reactors, which vary in design and the material of the cathode which must reduce oxygen in a two-electron process and the material of the anode (BDD, PbO2, Pt, etc.) [27,28]. These variations in the design and materials used can have an impact on the efficiency and effectiveness of the electro-Fenton process. Therefore, choosing an appropriate electrochemical reactor design and materials is crucial for achieving optimal results in pollutant degradation. Despite the high efficiency of electrochemical reactors at the laboratory scale, it is important to design an appropriate reactor that allows for easy scaling-up of the EF process. This should take into consideration factors such as large electrode surface areas, low-cost electrode materials, and a design that supports high flow rates [29]. Furthermore, it is important to consider the energy consumption of the EF process, as it can have an impact on the final treatment cost. To that end, more energy-efficient processes should be prioritized in order to keep costs low [30].
In our previous works [31,32,33], a new design of an electrochemical reactor for the application of the electro-Fenton process at the laboratory scale was reported (Figure S1). This reactor (PRIAM’s series) is an open undivided electrochemical reactor with a three-dimensional cathode of a fixed bed of glassy carbon pellets. The approach used in the reactor design provides high geometric surface area, which is beneficial for the electro-Fenton process. Additionally, the porous structure of the cathode may also increase the real contact between the solution and the electrode, which can enhance the efficiency of the process [34].
One relevant pollutant is bisphenol A (BPA), which is known to not be effectively degraded by biological treatment and ultimately ends up polluting the environment, including surface water [35,36,37,38]. It is reported that wastewater contains from 1 ng/L to 50 µg/L of bisphenol A. Different processes have been examined to remove BPA from natural water or wastewater as has already been reported elsewhere [39,40,41,42,43,44,45,46,47,48,49,50,51,52]. While these processes have demonstrated high efficiency in treating BPA in small volumes, none of them have been scaled up. Therefore, it is of great importance to test the ability of the electro-Fenton process to treat BPA on a semi-pilot scale.
In this study, we report the design of a new electrochemical reactor at a semi-pilot scale with a fixed bed of packed cathodes made of glassy carbon pellets. This electrochemical reactor is optimized for the application of electro-Fenton process to treat persistent pollutants. Herein, bisphenol A serves as the model molecule for our investigation. We carried out various parameters to scale-up the developed process to an industrial treatment plant. We are also proposing different strategies for combining EF process with the biological treatment (such as using it as a post-treatment step). Additionally, we investigated the mineralization of BPA during treatment and the biodegradability of the treated solutions at different treatment times and for different initial concentrations of the pollutant.
2. Materials and Methods
2.1. Chemicals and Materials
Glassy carbon pellet material (SIGRADUR® K, high purity) was obtained from HTW (Berlin, Germany) and has an appropriate average diameter of 3 mm. The Bisphenol A used to the preparation of polluted water (C15H16O2, 2.2-Bis(4-hydroxyphenyl)propane—purity > 99%) and sodium sulfate used as supporting electrolyte (Na2SO4—purity > 99%) were purchased from Sigma-Aldrich (Burlington, MA, USA). Iron (II) sulfate heptahydrate (Fe2SO4.7H2O—purity 98%) was obtained from Scharlau chemicals (Hamburg, Germany). For mobile phase preparation, acetonitrile (of a grade for high-performance liquid chromatography) was also purchased from Sigma-Aldrich. Demineralized water was obtained in our laboratory by an appropriate production system (Millipore, Burlington, MA, USA). All the measurements and experiments were carried out at laboratory temperature.
2.2. Solution Preparation
Synthetic solutions of bisphenol A were prepared in demineralized water without any organic solvents being added in order to limit the introduction of organic matter only to BPA. The pH of the solution was adjusted to 2.9 ± 0.1 using H2SO4. The Na2SO4 was added as supporting electrolyte at a concentration of 0.05 M. The Fe (II) was added to the solution at a concentration of 0.5 mM.
2.3. Design of Electro-Fenton Process
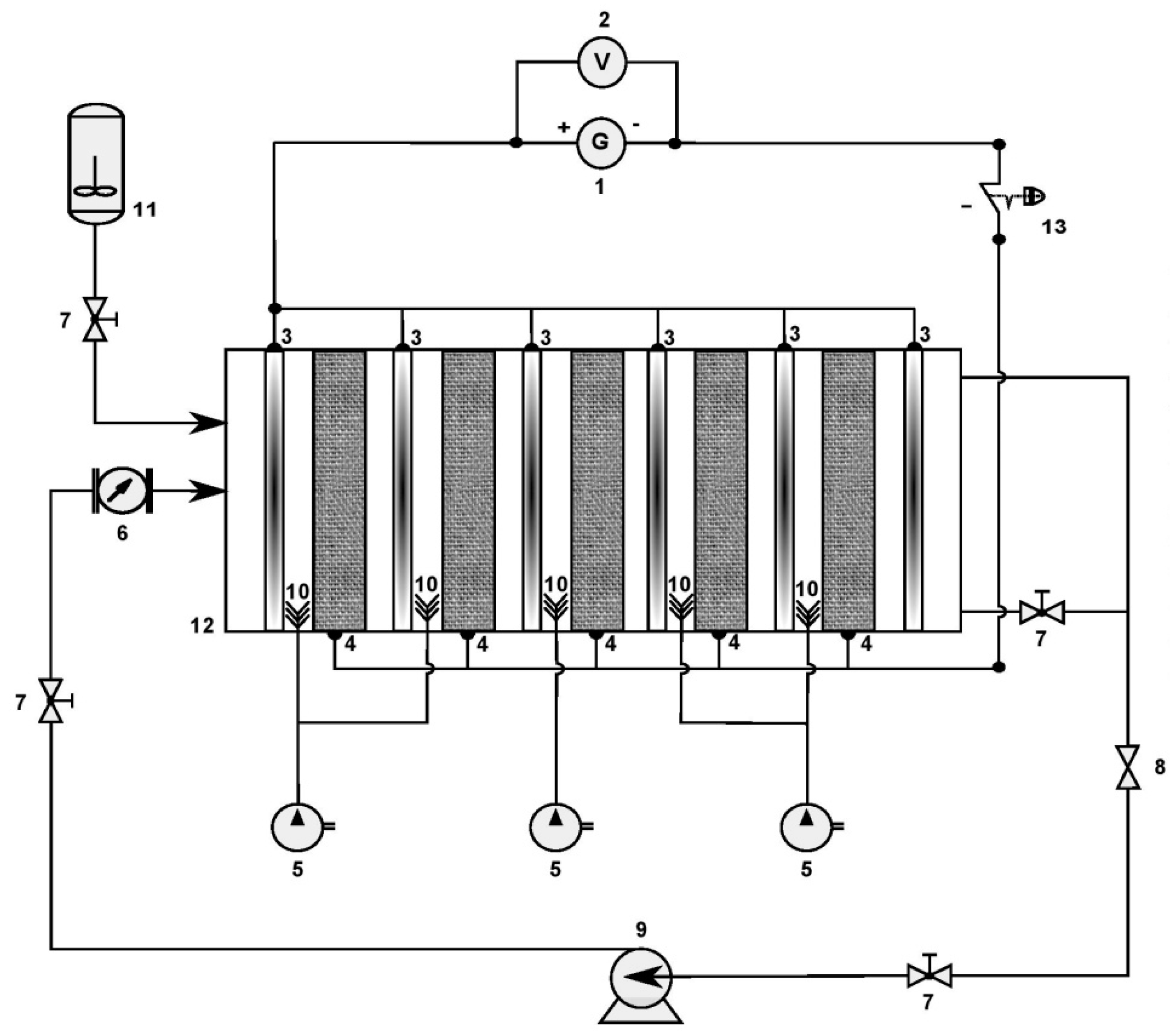
The electrochemical reactor at the semi-pilot scale is designed as an open (one-compartment) reactor made of polyvinyl chloride (PVC) and has rectangular geometric dimensions of 41.5 cm (L) × 19 cm (l) × 32 cm (H). The volume capacity of the reactor is 15 L. The electrochemical reactor at the semi-pilot scale contains up to five three-dimensional (3D) cathodes made up of a compact and fixed bed of glassy carbon pellets. These cathodes were surrounded by 6 dimensionally stable anodes (DSA) made up of titanium mesh and covered with a thin layer of ruthenium oxide (Ti/RuO2). However, the electrodes are removable, allowing the reactor to contain more or fewer electrodes (Figure 2 and Figure 3).
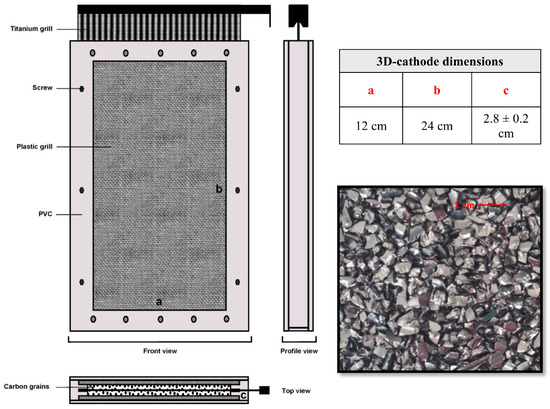
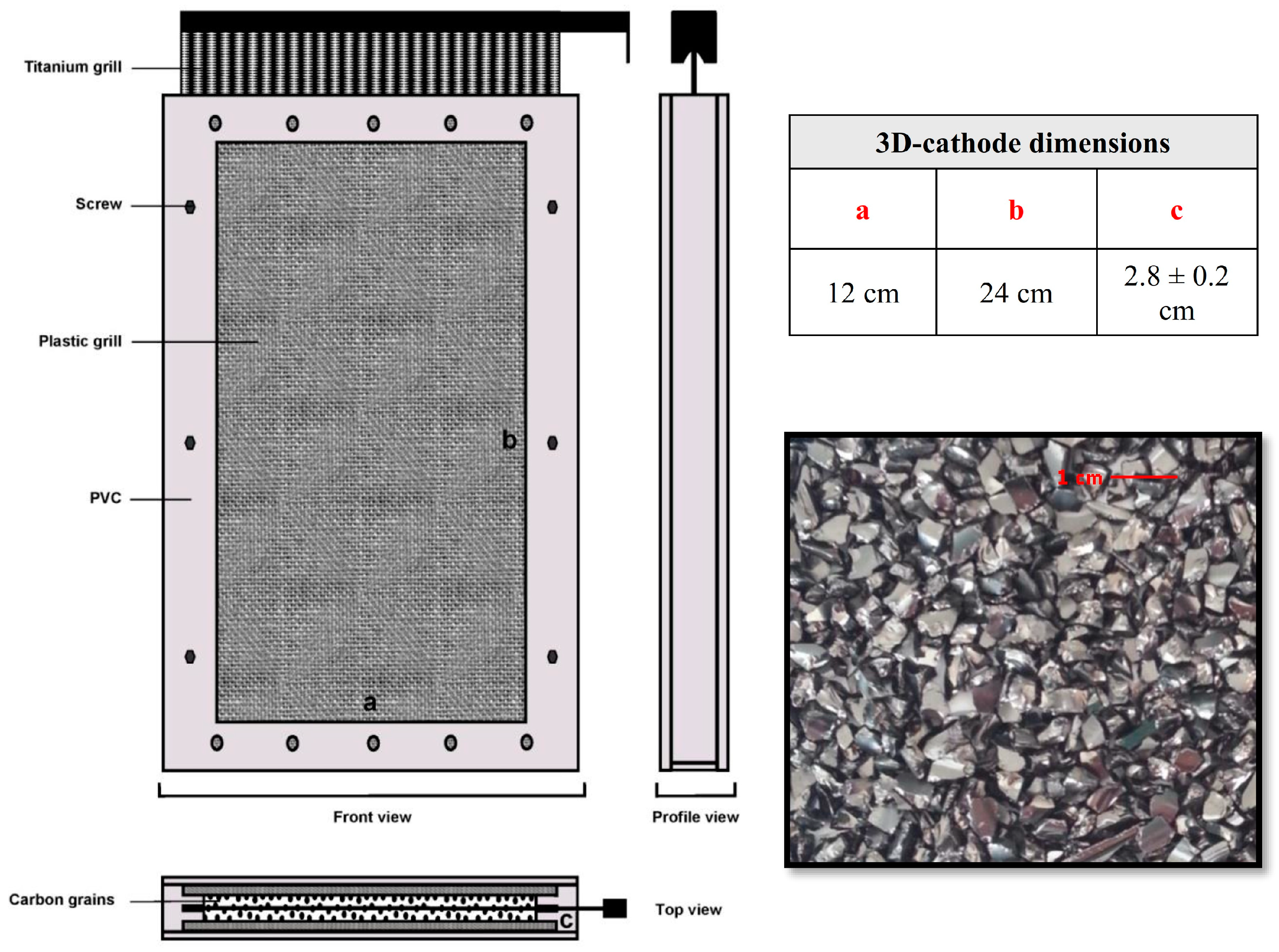
Figure 2.
Three-dimensional cathode of a fixed bed of glassy carbon pellets used in the electro-Fenton process: (a) Length, (b), Height and (c) Width.
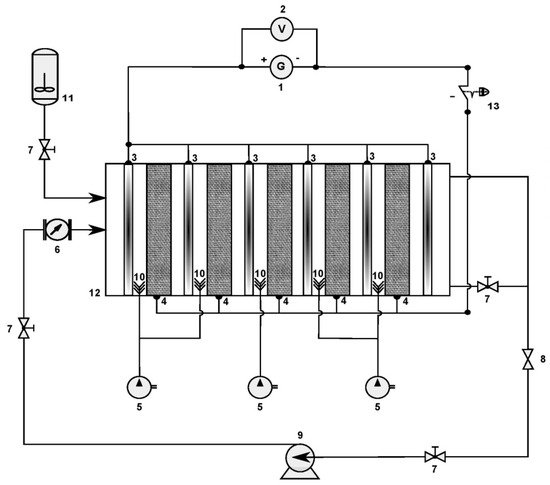
Figure 3.
Electro-Fenton process at semi-pilot scale: (1) current supplier, (2) voltmeter, (3) DSA anode, (4) three-dimensional fixed-bed cathode, (5) air pump, (6) flow meter, (7) valve, (8) sampling valve, (9) centrifugal pump, (10) air diffuser, (11) tank, (12) electrochemical reactor, (13) emergency stop button.
The three-dimensional cathodes have internal geometric dimensions of 24 cm × 12 cm × 2.8 ± 0.2 cm (L, W, and H) and were filled with glassy carbon pellets (≈680 g per cathode) (Figure 2). A metal mesh is placed near to the fixed bed (in the center) as a current collector by the high side. The electrodes are geometrically set up in series but were electrically connected in parallel. The reactor was designed to treat the contaminated solution in batch mode. A centrifugal pump was used for the experimental setup to feed the electrochemical reactor with a constant flow rate that was fixed using a flow meter (typically 10 L·min−1 (0.6 m3·h−1) for the semi-pilot-scale reactor).
Concerning the flow direction, the solution flows through the width of the fixed bed of electrodes (perpendicularly to the electrode section) during the treatment. Also, assuming a plug flow at the level of the cathode, the liquid flow velocity through the fixed bed is estimated to be 0.0058 m·s−1. Air pumps and air diffusers were used in the experimental setup to continually supply the solution with air (oxygen) (Figure 3). Sampling at different times was carried out at the outlet side of the cathode. The main physicochemical proprieties of the glassy carbon pellets used for packing the fixed-bed three-dimensional cathodes are presented in the Supplementary Information (Table S1).
2.4. Analytical Procedures and Equipment
Many analytical techniques were used in this study for characterization during the treatment of the effluent, as reported elsewhere [32]. Briefly, high-performance liquid chromatography (HPLC) was used to monitor the BPA concentration. The total organic carbon (TOC) content was also investigated to determine the remaining organic charge using an automated analyzer. The measurements of the chemical oxygen demand (COD) were carried out using spectrophotometric analysis after digestion using specific kits from HACH. Biochemical oxygen demand (BOD5) determination was carried out using oximeter measurements for 5 days, as already reported [53].
The performance of the treatment was evaluated by monitoring the BPA removal efficiency using the following equation:
where Ct is the bisphenol A concentration after treatment time (t) and C0 is the initial bisphenol A concentration before the treatment [53].
[(C0 − Ct) × 100]/Ct
3. Results and Discussions
In our previous work, we studied and optimized an electrochemical reactor for the treatment of bisphenol A (BPA) using the electro-Fenton process at a laboratory scale [32].
We investigated the effect of the applied current intensity on the removal efficiency of BPA during the treatment process. Our findings revealed that the limiting current intensity under the hydrodynamic conditions of 6 L·min−1 was found to be approximately 0.8 A (0.51 A/100 g of glassy carbon pellets). Furthermore, the limiting current density was determined to be 0.012 A/cm2, taking into account the section of the fixed bed that is exposed to the flow.
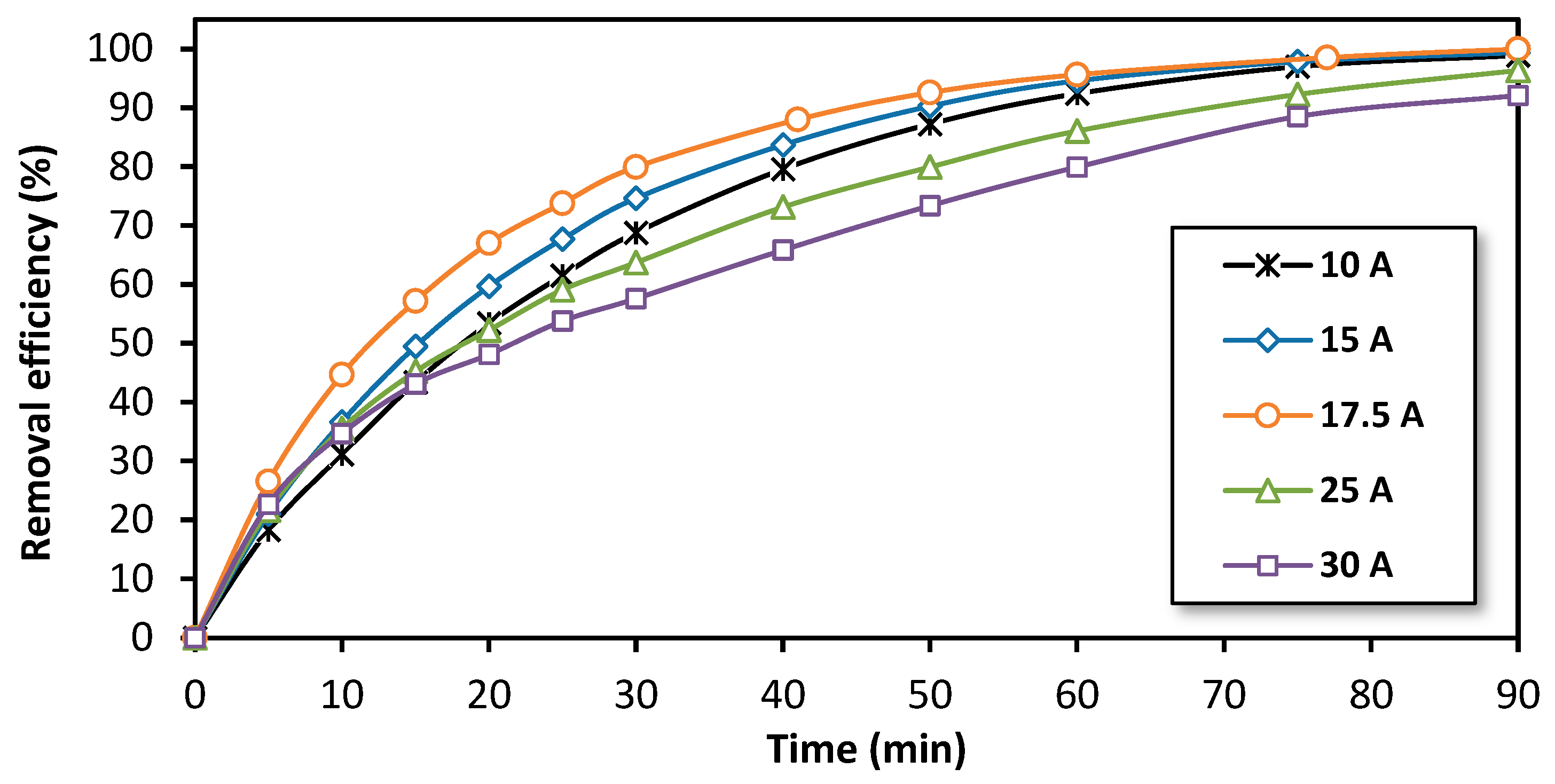
In this work, the focus was on optimizing the current intensity for the treatment of bisphenol A (BPA) using the electro-Fenton process at a semi-pilot scale. To achieve this, the variation of the removal efficiency over time for different applied current intensities ranging from 10 A to 30 A was studied. The results, illustrated in Figure 4, show that the removal efficiency improves with an increase in the applied current intensity, up to the range of 15 to 17.5 A. However, for higher intensities, the treatment appears to be less efficient. This suggests that the applied current intensity exceeds the limiting current for the O2 reduction and that a portion of the produced H2O2 is being reduced to H2O [54,55]. Therefore, the limiting current intensity under the hydrodynamic conditions corresponding to 10 L·min−1 (600 L/h) was determined to be near 17.5 A (0.51 A/100 g of glassy carbon pellets). Theoretically, this current should be divided equally among five cathodes and, therefore, each cathode should be supplied with 3.5 A as the cathodes were connected electrically in parallel.
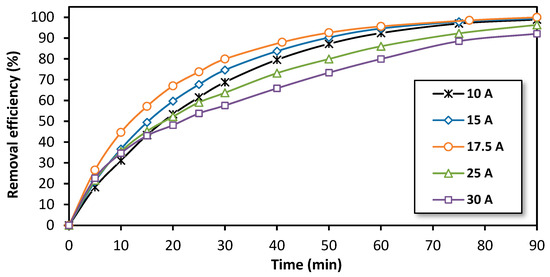
Figure 4.
The effect of the applied current intensity on the removal efficiency of BPA during treatment by the electro-Fenton process at a semi-pilot scale. Experimental conditions: [BPA]i = 150 mg/L, [FeSO4] = 0.5 mM, [Na2SO4] = 0.05 M, pH = 3.0, flow rate = 600 L/h.
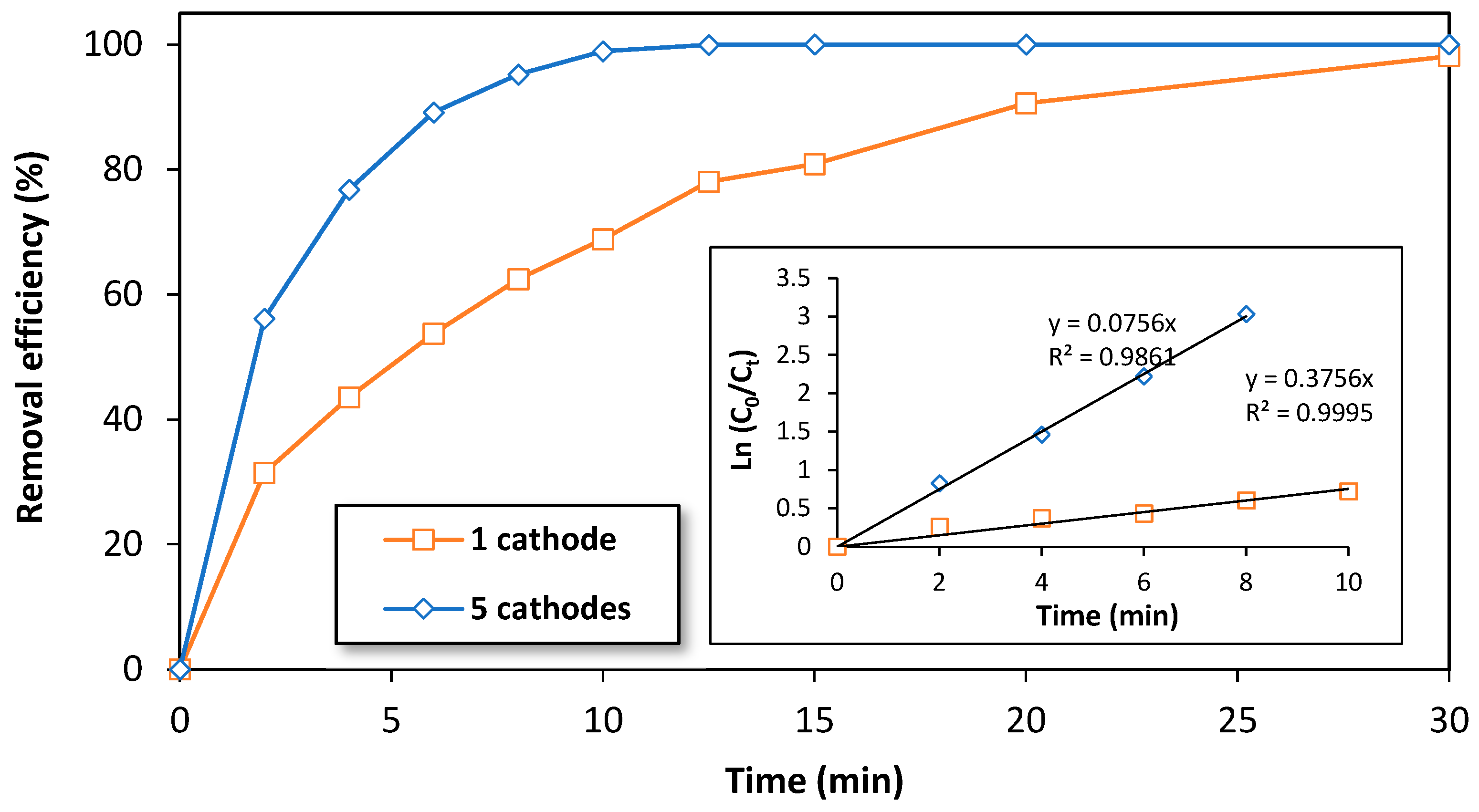
To verify the hypothesis that the number of electrodes affects the removal efficiency of BPA during the treatment with an electro-Fenton process, we investigated the effect of the number of electrodes using the semi-pilot-scale reactor. To do this, two experiments were carried out using an initial concentration of 10 mg/L of BPA. The first experiment used the typical reactor configuration of five cathodes and six anodes, and the second experiment used a configuration of one 3D-cathode and two DSA anodes (configuration 2). Figure 5 shows the variation of the removal efficiency of BPA over time for both cases. As expected, the typical reactor configuration (five cathodes and six anodes) was found to be more efficient in degrading BPA compared to the configuration with only one cathode (configuration 2). This is likely due to the increased efficiency of oxygen reduction into H2O2 and •OH production as the number of cathodes in the electrochemical reactor increases [56].
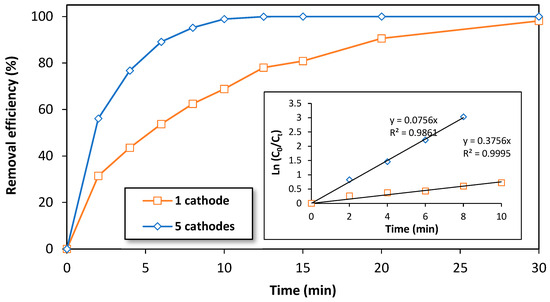
Figure 5.
The effect of the number of cathodes on the removal efficiency of BPA by electro-Fenton process at a semi-pilot scale. Experimental conditions: i= 3.5 A/cathode, [BPA]i = 10 mg/L, [FeSO4] = 0.5 mM, [Na2SO4] = 0.05 M, pH = 3.0, flow rate = 1000 L/h.
Furthermore, it is widely accepted that the reaction of •OH with phenolic compounds is primarily considered as a pseudo-first-order reaction, suggesting a direct attack of hydroxyl radicals on BPA [11]:
O2 + 2H+ + 2e− → H2O2 (rxn. 1)
Fe3+ + e− → Fe2+ (rxn. 2)
Fe2+ + H2O2 → Fe3+ + OH− + •OH (rxn. 3)
In light of this hypothesis, the apparent constant rate (Kapp) of BPA could be calculated using the theoretical equation of a pseudo-first-order reaction. Figure 5 (inset) shows the variation of Ln (C0/Ct) over time during the first 10 min of treatment of BPA (10 mg/L) by electro-Fenton process at the semi-pilot-scale reactor. As expected, a linear relation is observed with a slope of 0.076 min−1 corresponding to the value of Kapp in the typical reactor configuration (five cathodes) and 0.376 min−1 corresponding to the value of Kapp in the configuration containing only one cathode. These results indicate that the typical reactor configuration (five cathodes) is more efficient in degrading BPA compared to the configuration of only one cathode.
The ratio of the obtained slope values is approximately 5, which confirms that the configuration of the EF reactor with five cathodes is five times more efficient than the reactor configuration that contains only one cathode. This confirms that the applied current intensity in the electrochemical reactor was equally distributed among the five cathodes, as the cathodes were electrically connected in parallel, resulting in a current density of 0.012 A/cm2 (Figure S2). This value is comparable to the current density found in the laboratory scale reactor of the electro-Fenton process [33]. In summary, these results confirm that our reactor design could be a good candidate for the scaling-up of the electro-Fenton process for industrial treatment applications.
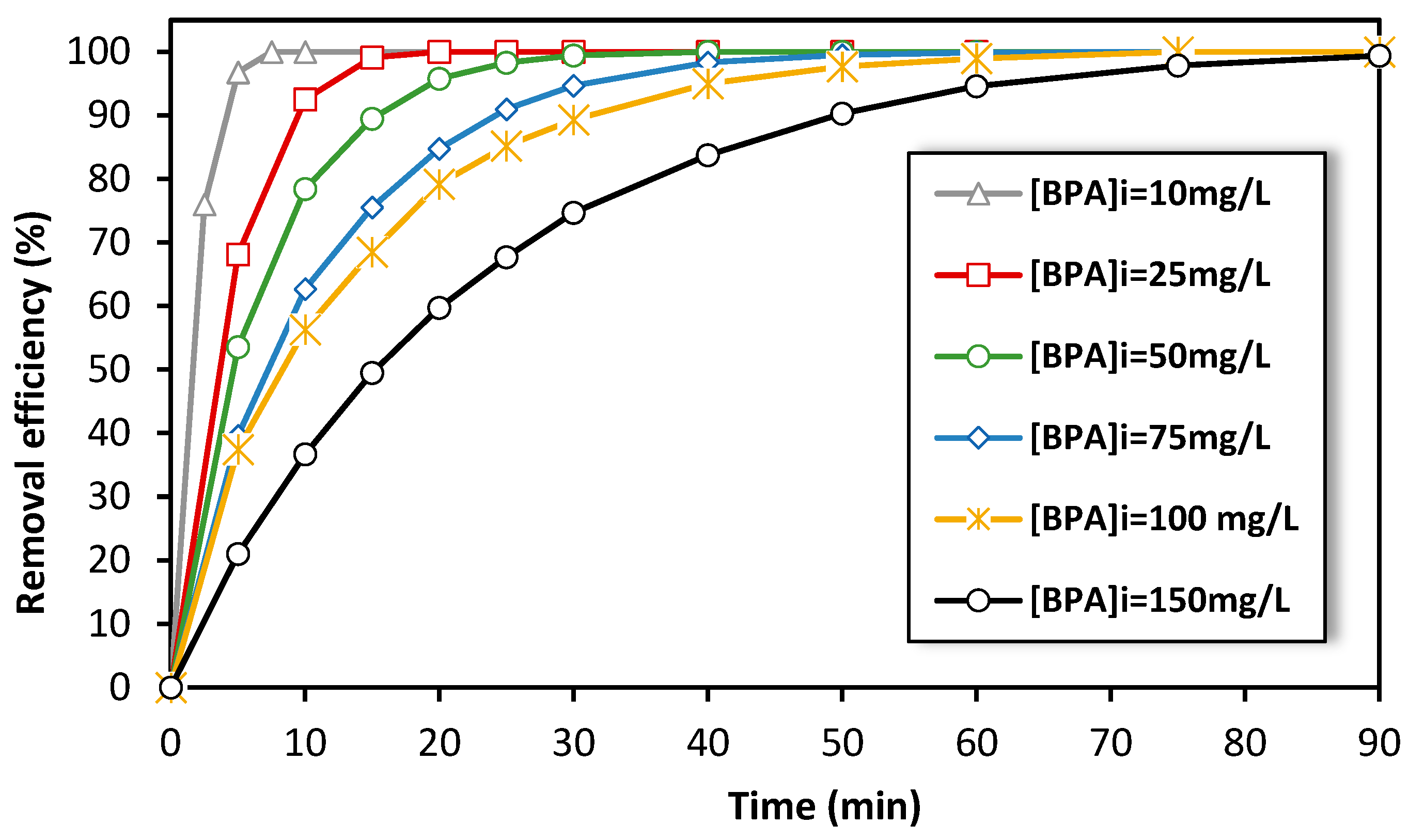
On the other hand, the electro-Fenton experiments at the semi-pilot-scale reactor were initially conducted using high concentrations of BPA solutions in order to monitor the concentration of BPA during treatment and study the degradation kinetics as a function of treatment time. We investigated the impact of initial BPA concentration on the removal efficiency of BPA during treatment using the electro-Fenton process (from 10 to 150 mg·L−1). We carried out experiments for 90 min at 15 A and found that as the initial concentration of BPA increased, the time required to achieve a certain level of removal efficiency also increased (Figure 6). For instance, at a BPA concentration of 150 mg·L−1, it took 50 min to achieve 90% removal efficiency. However, as the initial concentration decreased (i.e., for 10 mg·L−1), the time required to achieve the same removal efficiency decreased as well (less than 5 min). This suggests that this semi-pilot-scale reactor configuration is more efficient in treating low concentrations of persistent pollutants by a single pass of the solution through the fixed-bed cathode.
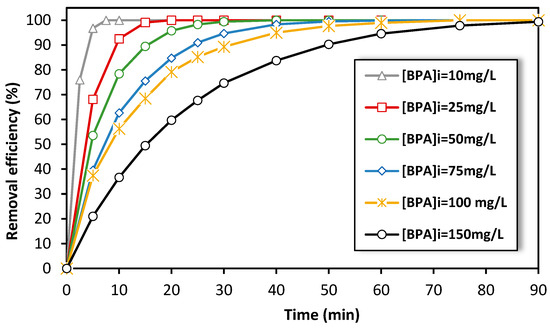
Figure 6.
Effect of the initial concentration of BPA on the removal efficiency by electro-Fenton process at semi-pilot-scale reactor. Experimental conditions: i = 15 A, [FeSO4] = 0.5 mM, [Na2SO4] = 0.05 M, pH = 3.0, flow rate = 600 L/h.
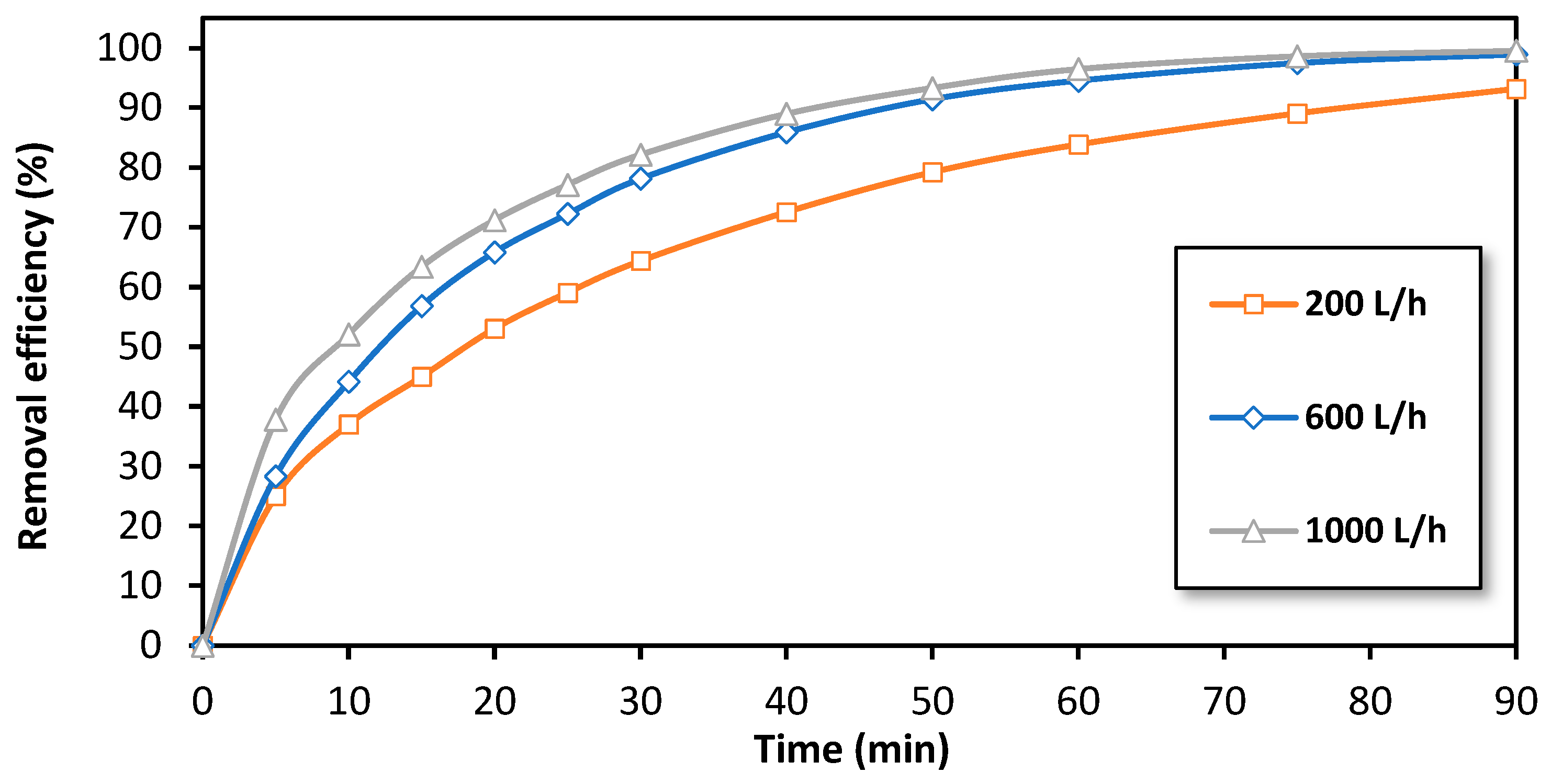
The effect of flow rate on the efficiency of BPA removal by the EF process at the semi-pilot scale was also investigated. Figure 7 illustrates the variation of the removal efficiency over time at 15 A for three different flow rates: 200 L/h, 600 L/h, and 1000 L/h (the maximum flow rate at semi-pilot-scale reactor). As shown, an improvement in removal efficiency was observed as the flow rate increases. For example, after 15 min of treatment, a removal efficiency of 45% was observed at 200 L/h, while the removal efficiency increased to 65% at a flow rate of 1000 L/h. This observation can be attributed to the increase in liquid flow velocity (v) through the fixed bed, which can be calculated as 0.0019 m·s−1 at 200 L/h and as 0.0097 m·s−1 at 1000 L/h, ensuring more favorable hydrodynamic conditions. This results in an increase in the Reynolds number (Re, Equation (1)) and subsequently the mass transfer coefficient (km, Equation (2)), which leads to an improvement in the degradation efficiency of BPA.
where: dp is the particle diameter, n the kinematic viscosity, and A and b are constants [57,58]. Therefore, it is of high importance to provide a reactor design that support high liquid flow rates that make it possible to apply high current intensities in optimized mode and as a result to reach a high removal efficiency of BPA in a short treatment time (a few m3·h−1).
Re = v × dp/n
km = A × vb
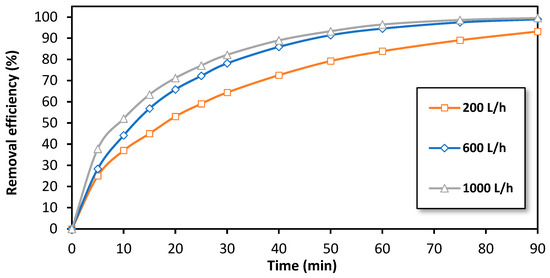
Figure 7.
The effect of the flow rate on the removal efficiency of BPA by the electro-Fenton process at a semi-pilot scale. Experimental conditions: i= 15 A, [BPA]i = 150 mg/L, [FeSO4] = 0.5 mM, [Na2SO4] = 0.05 M, pH = 3.0.
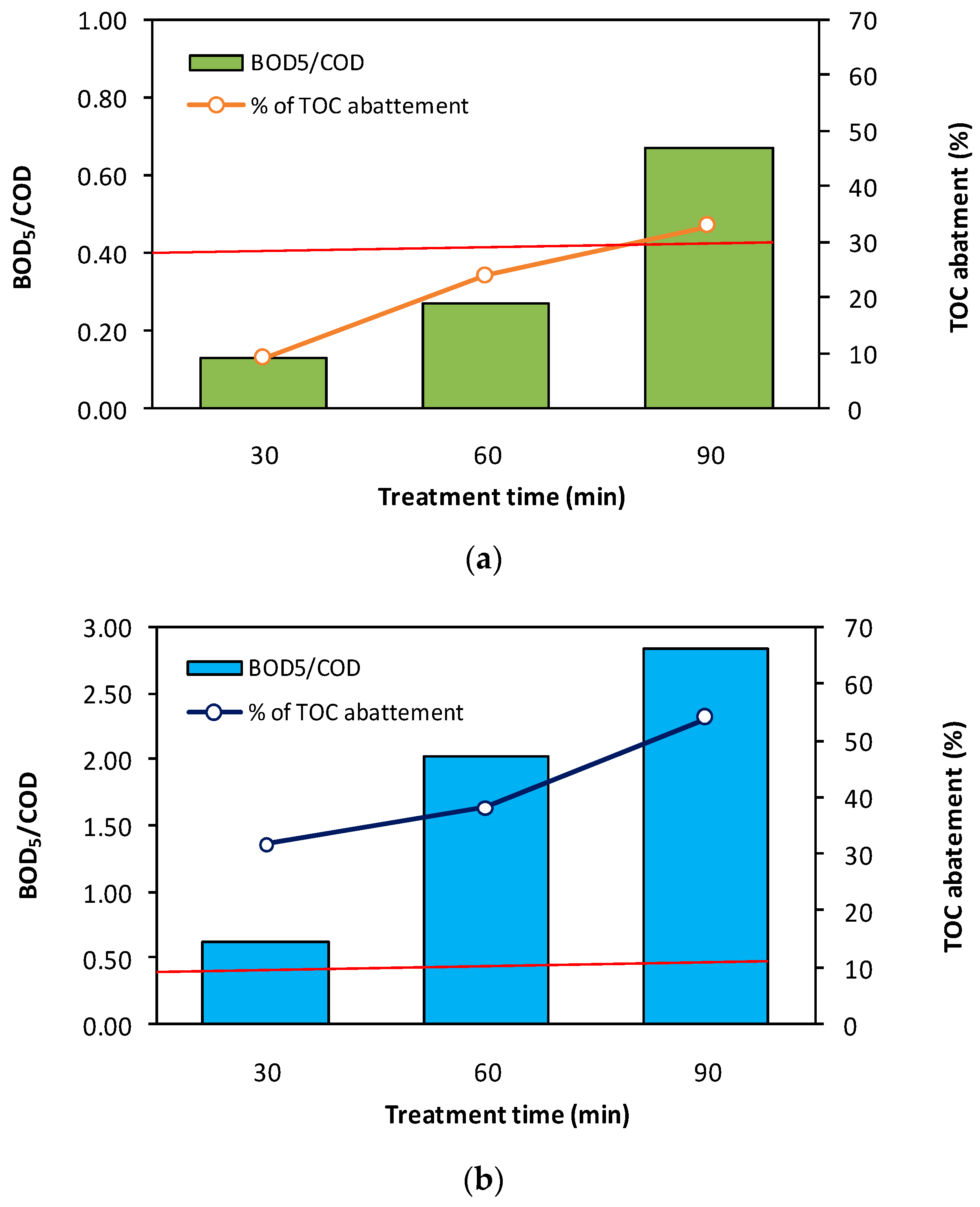
Moreover, the electro-Fenton process typically involves a biodegradability change [59]. Recent studies show that deploying the EF process prior to biological treatment could be a particularly interesting solution for water remediation [60,61,62,63]. For this reason we evaluated the biodegradability change during treatment of BPA by EF process in a semi-pilot-scale reactor. To proceed, we used the BOD5 (biochemical oxygen demand)-to-COD (chemical oxygen demand) ratio, which is a standardized measure of the biodegradability of a solution, and considered a ratio higher than 0.4 to indicate that the solution is biodegradable [64]. We investigated the biodegradability of BPA for different treatment times (30, 60, and 90 min) and for two initial BPA concentrations of 10 and 50 mg·L−1. Additionally, we studied the mineralization of BPA by monitoring the TOC (total organic carbon) versus the time of EF treatment (30, 60, and 90 min).
As seen in Figure 8, a significant improvement in biodegradability (BOD5/COD > 0.6) is observed after 90 min of treatment, with a 33% reduction in TOC at an initial BPA concentration of 50 mg·L−1. Additionally, good results were obtained for low concentrations of BPA (≤10 mg·L−1), where only a few minutes of treatment were needed to improve biodegradability. This enhancement is also reflected in a high TOC abatement (>70%) at the end of the treatment (90 min). As shown, the red line for BOD5/COD indicates that effluents with a lower value than this line are difficulty biodegradable. In contract, solutions with a value higher than the red line are considered to be biodegradable. It can also be observed that COD and TOC abatement increases with treatment time and as the initial BPA concentration decreases. Here, the residual TOC is mainly composed of small organic acids which are difficult to oxidize, as reported elsewhere [33], and typically require many hours of treatment to achieve total mineralization [65].
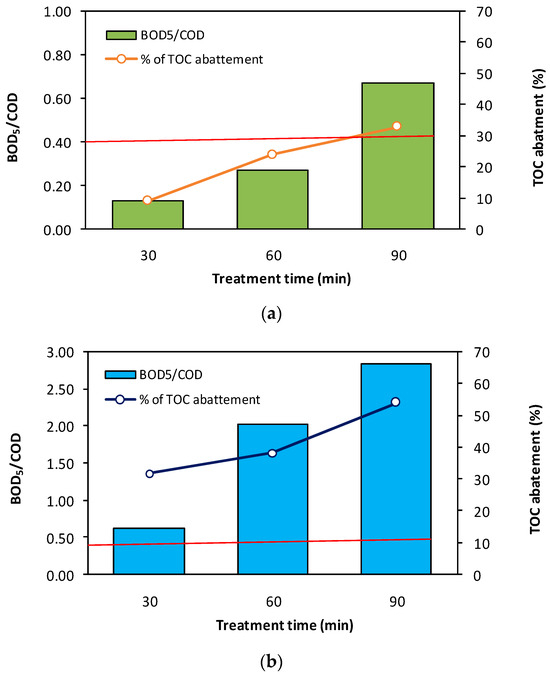
Figure 8.
Variation of the (BOD5/COD) ratio and TOC abatement (%) after treatment by the electro-Fenton process in a semi-pilot-scale reactor, respectively, at 30, 60, and 90 min for: (a) [BPA]i= 50 mg/L (in green) and (b) [BPA]i= 10 mg/L (in blue). The red line for BOD5/COD indicates that effluents with a value lower than this line are difficult to biodegrade. In contrast, solutions with a higher value than the red line are considered to be biodegradable.
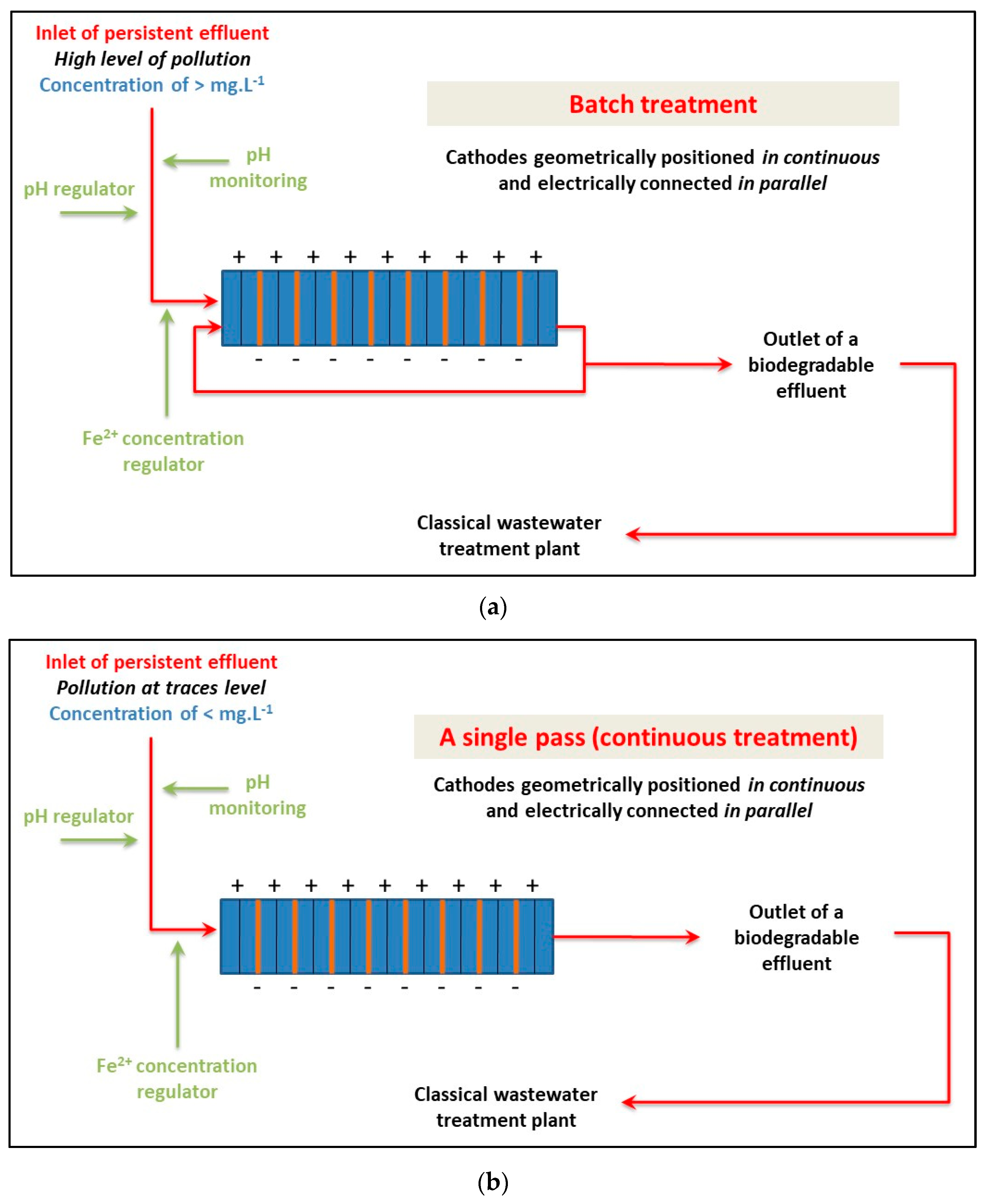
For the scaling up of an EF reactor to an industrial pilot plant, two cases should be considered based on the source and the concentration level of the effluent (Figure 9). For the treatment of industrial discharge with high contamination levels (>mg·L−1), a batch reactor should be designed with multiple fixed-bed cathodes in series as a pre-treatment process. The treatment duration will depend on the pollution charge and biodegradability level. For low concentration levels of contamination (<mg·L−1), treatment can be directly applied through a single pass of the solution in the electrochemical reactor. A few seconds of treatment should be sufficient, and biodegradability can be achieved through the electro-Fenton reactor. The current intensity should be set at 0.51 A per 100 g of glassy carbon pellets (0.012 A·cm−2 for the section of the fixed bed exposed to the flow). Scaling up is limited by the flow rate of the solution, which should be kept within acceptable values (a few m3·h−1). To make scaling up easier, it is recommended to keep the thickness of the 3D cathode bed constant as used in the laboratory and semi-pilot-scale reactor (2 cm).
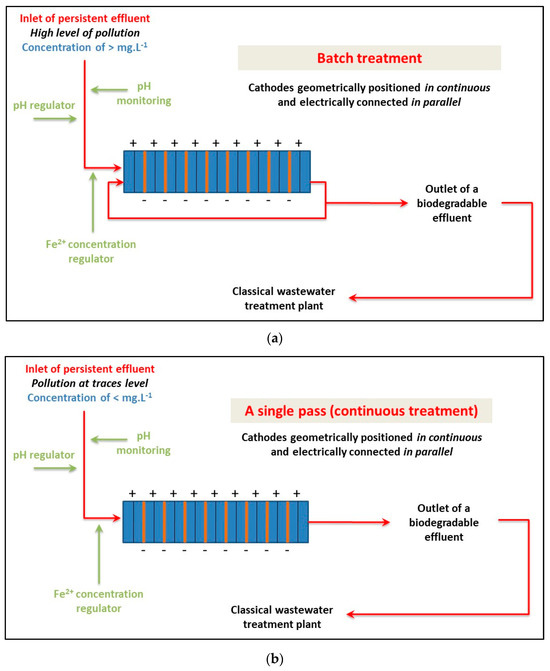
Figure 9.
Scheme for the scaling up of the electro-Fenton process to an industrial pilot plant as a function of the concentration level of the pollutant.: strategy for the treatment of (a) high concentration of pollution (>mg·L−1) and (b) low concentration of pollution (<mg·L−1).
4. Conclusions
In conclusion, the design of an open undivided electrochemical reactor with three-dimensional cathodes made up of a fixed bed of glassy carbon pellets is as an ideal configuration for the scaling-up of the EF process. The semi-pilot-scale plant demonstrated high removal efficiency during the treatment of micropollution by bisphenol A. The short treatment time needed by the electro-Fenton process makes this design of reactor an appropriate option for enhancing the biodegradability of effluents. Here, two strategies were proposed depending on the contamination source and level. For industrial effluents (such as cooling water), the EF process is recommended as a pre-treatment step at the contamination source before biological treatment. This approach reduces the pollution charge and prevents high concentrations of persistent contamination from entering the treatment plant. This allows batch treatment of smaller volumes of highly contaminated effluents. In contrast, the second strategy consists using the EF process as a post-treatment step which is suitable for treating large volumes of effluents with low pollutant concentrations (via a single-pass treatment). Indeed, the most limiting factor in scaling up the EF process may be related to the supply of current at the industrial level (i.e., the generation and use of DC current). However, recent advances in power supply technology have mitigated this limitation and contributed to reducing the cost of treatment by EF process. Finally, although the cost of treatment by electro-Fenton is higher compared to biological treatment (estimated between 0.72 and 1.68 $/m3 for energy consumption, Table S2), the EF is now a completive technology with significant potential for scalability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pr12091850/s1, Figure S1: Electro-Fenton process at the lab scale; Table S1: Main physicochemical proprieties of the glassy carbon pellets as per the provider information used for packing the fixed bed of three-dimensional cathode in the electro-Fenton process; Figure S2: Scheme for the current intensity distribution between the 3D-cathodes in electro-Fenton process reactor at the semi-pilot scale; Table S2: Energy consumption for the semi-pilot scale of electro-Fenton process for 1-h, 10 h and 24-h turnover at the optimized current and flow.
Author Contributions
Conceptualization, A.C. and D.H.; methodology, A.C. and D.H.; software, A.C.; validation, A.C. and G.A.; formal analysis, A.C.; investigation, A.C.; resources, A.C.; data curation, A.C.; writing—original draft preparation, A.C.; writing—review and editing, A.C. and D.H.; visualization, A.C. and D.H.; supervision, S.T. and D.H.; project administration, D.H.; funding acquisition, D.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data and materials that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chmayssem, A.; Hauchard, D. New Detection Method for Alkylphenol Traces in Water Based on an Integrated Electrochemical Cell Sensor. Rev. Des Sci. L’eau 2015, 28, 35–40. [Google Scholar] [CrossRef]
- Chmayssem, A.; Hauchard, D. Direct Ultra-Trace Detection of Alkylphenols in Water Using a Cavity Carbon-Paste Microelectrode Sensor. Desalination Water Treat. 2017, 83, 321–326. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as Emerging Contaminants and Their Removal from Water. A Review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.-Q.; Zhou, Z.; Sharma, V.K. Occurrence, Transportation, Monitoring and Treatment of Emerging Micro-Pollutants in Waste Water—A Review from Global Views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sànchez-Pérez, J.A. Combination of Advanced Oxidation Processes and Biological Treatments for Wastewater Decontamination—A Review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Bouafia, S.C.; Alloune, R. Procédé d’oxydation Avancée Pour Le Traitement Des Eaux Usées: Principe et Applications. Rev. Energ. Renouvelables 2007, 7, 163–170. [Google Scholar]
- Qutob, M.; Shakeel, F.; Alam, P.; Alshehri, S.; Ghoneim, M.M.; Rafatullah, M. A Review of Radical and Non-Radical Degradation of Amoxicillin by Using Different Oxidation Process Systems. Environ. Res 2022, 214, 113833. [Google Scholar] [CrossRef] [PubMed]
- Vinayagam, V.; Palani, K.N.; Ganesh, S.; Rajesh, S.; Akula, V.V.; Avoodaiappan, R.; Kushwaha, O.S.; Pugazhendhi, A. Recent Developments on Advanced Oxidation Processes for Degradation of Pollutants from Wastewater with Focus on Antibiotics and Organic Dyes. Environ. Res. 2024, 240, 117500. [Google Scholar] [CrossRef]
- Zaviska, F.; Drogui, P.; Mercier, G.; Blais, J.-F. Procédés d’oxydation Avancée Dans Le Traitement Des Eaux et Des Effluents Industriels: Application à La Dégradation Des Polluants Réfractaires. Rev. Des Sci. L’eau 2009, 22, 535–563. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Oturan, M.A. Electro-Fenton Process and Related Electrochemical Technologies Based on Fenton’s Reaction Chemistry. Chem. Rev. 2009, 109, 6570–6631. [Google Scholar] [CrossRef] [PubMed]
- Mohmmad, A.; Hamed Mosavian, M.T.; Haddad Khodaparast, M.H. Electro-Fenton Technology for Dairy Wastewater Treatment. Int. J. Environ. Sci. Technol. 2024, 21, 35–42. [Google Scholar] [CrossRef]
- Poza-Nogueiras, V.; Rosales, E.; Pazos, M.; Sanromán, M.Á. Current Advances and Trends in Electro-Fenton Process Using Heterogeneous Catalysts—A Review. Chemosphere 2018, 201, 399–416. [Google Scholar] [CrossRef]
- Jiang, C.; Zhang, J. Progress and Prospect in Electro-Fenton Process for Wastewater Treatment. J. Zhejiang Univ. Sci. A 2007, 8, 1118–1125. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Gandhimathi, R. Trends in Electro-Fenton Process for Water and Wastewater Treatment: An Overview. Desalination 2012, 299, 1–15. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, F.; Bai, H.; Zeng, L.; Zhang, J.; Liu, M.; Zhu, W. A Carbon Felt Cathode Modified by Acidic Oxidised Carbon Nanotubes for the High H2O2 Generation and Its Application in Electro-Fenton. Environ. Technol. 2024, 45, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Rosales, E.; Pazos, M.; Longo, M.A.; Sanromán, M.A. Electro-Fenton Decoloration of Dyes in a Continuous Reactor: A Promising Technology in Colored Wastewater Treatment. Chem. Eng. J. 2009, 155, 62–67. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, Q.; Lei, L.; Barton, G. Electro-Fenton Method for the Removal of Methyl Red in an Efficient Electrochemical System. Sep. Purif. Technol. 2007, 57, 380–387. [Google Scholar] [CrossRef]
- Zahedi, S.; Asadipour, A.; Dolatabadi, M.; Ahmadzadeh, S. Response Surface Modeling for the Treatment of Methylene Blue from Aqueous Media Using Electro-Fenton Process before Determination by UV-Vis Spectrometer: Kinetic and Degradation Mechanism. Anal. Methods Environ. Chem. J. 2022, 5, 39–50. [Google Scholar] [CrossRef]
- Iberache, N.; Titchou, F.E.; Errami, M.; Ben-Aazza, S.; Driouiche, A.; Akbour, R.A.; Hamdani, M.; Hadfi, A. Removal of the Insecticide Imidacloprid from Water in Commercial Formulation Using Electro-Fenton and Photo-Electro-Fenton: Optimization of COD Removal through Response Surface Methodology RSM-CCD. Chem. Eng. Process.-Process Intensif. 2024, 196, 109633. [Google Scholar] [CrossRef]
- Ozcan, A.; Sahin, Y.; Oturan, M.A. Complete Removal of the Insecticide Azinphos-Methyl from Water by the Electro-Fenton Method-A Kinetic and Mechanistic Study. Water Res. 2013, 47, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Fourcade, F.; Soutrel, I.; Hauchard, D.; Bellakhal, N.; Amrane, A. Mineralization of Synthetic and Industrial Pharmaceutical Effluent Containing Trimethoprim by Combining Electro-Fenton and Activated Sludge Treatment. J. Taiwan Inst. Chem. Eng. 2015, 53, 58–67. [Google Scholar] [CrossRef]
- Long, Y.; Feng, Y.; Li, X.; Suo, N.; Chen, H.; Wang, Z.; Yu, Y. Removal of Diclofenac by Three-Dimensional Electro-Fenton-Persulfate (3D Electro-Fenton-PS). Chemosphere 2019, 219, 1024–1031. [Google Scholar] [CrossRef]
- Yuan, S.; Gou, N.; Alshawabkeh, A.N.; Gu, A.Z. Efficient Degradation of Contaminants of Emerging Concerns by a New Electro-Fenton Process with Ti/MMO Cathode. Chemosphere 2013, 93, 2796–2804. [Google Scholar] [CrossRef] [PubMed]
- Asghar, A.; Raman, A.A.A.; Daud, W.M.A.W. Advanced Oxidation Processes for In-Situ Production of Hydrogen Peroxide/Hydroxyl Radical for Textile Wastewater Treatment: A Review. J. Clean. Prod. 2015, 87, 826–838. [Google Scholar] [CrossRef]
- Malinović, B.N.; Markelj, J.; Žgajnar Gotvajn, A.; Kralj Cigić, I.; Prosen, H. Electrochemical Treatment of Wastewater to Remove Contaminants from the Production and Disposal of Plastics: A Review. Environ. Chem. Lett. 2022, 20, 3765–3787. [Google Scholar] [CrossRef]
- Feng, L.; Song, W.; Oturan, N.; Karbasi, M.; van Hullebusch, E.D.; Esposito, G.; Giannakis, S.; Oturan, M.A. Electrochemical Oxidation of Naproxen in Aqueous Matrices: Elucidating the Intermediates’ Eco-Toxicity, by Assessing Its Degradation Pathways via Experimental and Density Functional Theory (DFT) Approaches. Chem. Eng. J. 2023, 451, 138483. [Google Scholar] [CrossRef]
- Yang, L.; Chen, C.; Bao, R.; Huang, Z.; Wang, W.; Zhang, C.; Xia, J.; Geng, J.; Li, H. Effective Green Electro-Fenton Process Induced by Atomic Hydrogen for Rapid Oxidation of Organic Pollutants over a Highly Active and Reusable Carbon Based Palladium Nanocatalyst. Appl. Surf. Sci. 2022, 602, 154325. [Google Scholar] [CrossRef]
- Amarzadeh, M.; Salehizadeh, S.; Damavandi, S.; Mubarak, N.M.; Ghahrchi, M.; Ramavandi, B.; Shahamat, Y.D.; Nasseh, N. Statistical Modeling Optimization for Antibiotics Decomposition by Ultrasound/Electro-Fenton Integrated Process: Non-Carcinogenic Risk Assessment of Drinking Water. J. Environ. Manag. 2022, 324, 116333. [Google Scholar] [CrossRef]
- Wang, S.; Adekunle, A.; Raghavan, V. Bioelectrochemical Systems-Based Metal Removal and Recovery from Wastewater and Polluted Soil: Key Factors, Development, and Perspective. J. Environ. Manag. 2022, 317, 115333. [Google Scholar] [CrossRef] [PubMed]
- Bakhti, H.; Ben Hamida, N.; Hauchard, D. Degradation and Mineralization of Pesticide Isoprocarb by Electro Fenton Process. J. Pure Appl. Chem. Res. 2020, 9, 40–56. [Google Scholar] [CrossRef]
- Chmayssem, A.; Taha, S.; Hauchard, D. Scaled-up Electrochemical Reactor with a Fixed Bed Three-Dimensional Cathode for Electro-Fenton Process: Application to the Treatment of Bisphenol A. Electrochim. Acta 2017, 225, 435–442. [Google Scholar] [CrossRef]
- Chmayssem, A. Développement d’un Procédé Électrochimique et de Capteurs Associés Pour Le Traitement de Perturbateurs Endocriniens Phénoliques Dans Les Eaux. Ph.D. Thesis, École Nationale Supérieure de Chimie de Rennes, Rennes, France, 2023. [Google Scholar]
- Rodrigo Quejigo, J.; Rosa, L.F.M.; Harnisch, F. Electrochemical Characterization of Bed Electrodes Using Voltammetry of Single Granules. Electrochem. Commun. 2018, 90, 78–82. [Google Scholar] [CrossRef]
- Gözmen, B.; Oturan, M.A.; Oturan, N.; Erbatur, O. Indirect Electrochemical Treatment of Bisphenol A in Water via Electrochemically Generated Fenton’s Reagent. Environ. Sci. Technol. 2003, 37, 3716–3723. [Google Scholar] [CrossRef]
- Okon, O.E.; Inam, E.J.; Offiong, N.-A.O.; Akpabio, U.D. Aqueous Adsorptive Removal of Bisphenol A Using Tripartite Magnetic Montmorillonite Composites. Pollutants 2022, 2, 363–387. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Lu, G.; Jiang, R.; Yan, Z.; Li, Y. Occurrence, Toxicity and Ecological Risk of Bisphenol A Analogues in Aquatic Environment—A Review. Ecotoxicol. Environ. Saf. 2021, 208, 111481. [Google Scholar] [CrossRef]
- Niu, L.; Zhang, S.; Wang, S.; An, L.; Manoli, K.; Sharma, V.K.; Yu, X.; Feng, M. Overlooked Environmental Risks Deriving from Aqueous Transformation of Bisphenol Alternatives: Integration of Chemical and Toxicological Insights. J. Hazard. Mater. 2022, 427, 128208. [Google Scholar] [CrossRef]
- Serpone, N.; Horikoshi, S.; Emeline, A.V. Microwaves in Advanced Oxidation Processes for Environmental Applications. A Brief Review. J. Photochem. Photobiol. C Photochem. Rev. 2010, 11, 114–131. [Google Scholar] [CrossRef]
- Chen, P.-J.; Rosenfeldt, E.J.; Kullman, S.W.; Hinton, D.E.; Linden, K.G. Biological Assessments of a Mixture of Endocrine Disruptors at Environmentally Relevant Concentrations in Water Following UV/H2O2 Oxidation. Sci. Total Environ. 2007, 376, 18–26. [Google Scholar] [CrossRef]
- Katsumata, H.; Kawabe, S.; Kaneco, S.; Suzuki, T.; Ohta, K. Degradation of Bisphenol A in Water by the Photo-Fenton Reaction. J. Photochem. Photobiol. A Chem. 2004, 162, 297–305. [Google Scholar] [CrossRef]
- Seibert, D.; Henrique Borba, F.; Bueno, F.; Inticher, J.J.; Módenes, A.N.; Espinoza-Quiñones, F.R.; Bergamasco, R. Two-Stage Integrated System Photo-Electro-Fenton and Biological Oxidation Process Assessment of Sanitary Landfill Leachate Treatment: An Intermediate Products Study. Chem. Eng. J. 2019, 372, 471–482. [Google Scholar] [CrossRef]
- Tay, K.S.; Rahman, N.A.; Radzi, M.; Abas, B. Degradation of Bisphenol A by Ozonation: Rate Constants, Influence of Inorganic Anions, and by-Products. J. Sci. Technol. 2012, 6, 77–94. [Google Scholar]
- Qiang, Z.; Nie, Y.; Ben, W.; Qu, J.; Zhang, H. Degradation of Endocrine-Disrupting Chemicals during Activated Sludge Reduction by Ozone. Chemosphere 2013, 91, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Kuramitz, H.; Matsushita, M.; Tanaka, S. Electrochemical Removal of Bisphenol A Based on the Anodic Polymerization Using a Column Type Carbon Fiber Electrode. Water Res. 2004, 38, 2330–2337. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, C.; Lu, J.; Hu, C.; Peng, S.; Chen, T. Electro-Catalytic Degradation of Bisphenol A with Modified Co3O4/β-PbO2/Ti Electrode. Electrochim. Acta 2014, 118, 169–175. [Google Scholar] [CrossRef]
- Lin, H.; Wu, J.; Zhang, H. Degradation of Bisphenol A in Aqueous Solution by a Novel Electro/Fe3+/Peroxydisulfate Process. Sep. Purif. Technol. 2013, 117, 18–23. [Google Scholar] [CrossRef]
- Brugnera, M.F.; Rajeshwar, K.; Cardoso, J.C.; Zanoni, M.V.B. Bisphenol A Removal from Wastewater Using Self-Organized TiO2 Nanotubular Array Electrodes. Chemosphere 2010, 78, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Li, X.; Chen, G. Electrochemical Degradation of Bisphenol A on Different Anodes. Water Res. 2009, 43, 1968–1976. [Google Scholar] [CrossRef]
- Dolatabadi, M.; Malekahmadi, R.; Ghorbanian, A.; Ahmadzadeh, S. Investigation of Electrocoagulation Process for Efficient Removal of Bisphenol A from the Aqueous Environment: Promising Treatment Strategy. J. Environ. Health Sustain. Dev. 2021, 6, 1275–1283. [Google Scholar] [CrossRef]
- Rivero, M.J.; Alonso, E.; Dominguez, S.; Ribao, P.; Ibañez, R.; Ortiz, I.; Irabien, A. Kinetic Analysis and Biodegradability of the Fenton Mineralization of Bisphenol A. J. Chem. Technol. Biotechnol. 2014, 89, 1228–1234. [Google Scholar] [CrossRef]
- Sajiki, J.; Yonekubo, J. Inhibition of Seawater on Bisphenol A (BPA) Degradation by Fenton Reagents. Environ. Int. 2004, 30, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; Fourcade, F.; Bellakhal, N.; Dachraoui, M.; Hauchard, D.; Amrane, A. Biodegradability Improvement of Sulfamethazine Solutions by Means of an Electro-Fenton Process. Water Air Soil Pollut. 2011, 223, 2023–2034. [Google Scholar] [CrossRef]
- Babić, R.; Metikoš-Huković, M. Oxygen Reduction on Stainless Steel. J. Appl. Electrochem. 1993, 23, 352–357. [Google Scholar] [CrossRef]
- Noe, J.; Latus, A.; Lagrost, C.; Volanschi, E.; Hapiot, P. Evidence for OH Radical Production during Electrocatalysis of Oxygen Reduction on Pt Surfaces: Consequences and Application. J. Am. Chem. Soc. 2012, 131, 2835–2841. [Google Scholar]
- Ni, Y.; Zhou, C.; Xing, M.; Zhou, Y. Oxidation of Emerging Organic Contaminants by In-Situ H2O2 Fenton System. Green Energy Environ. 2024, 9, 417–434. [Google Scholar] [CrossRef]
- Sedahmed, G.H. Mass Transfer Behaviour of a Fixed Bed Electrochemical Reactor with a Gas Evolving Upstream Counter Electrode. Can. J. Chem. Eng. 1996, 74, 487–492. [Google Scholar] [CrossRef]
- M. Benzina. S.; Gabsi, G.L. Liquid-Solid Transfer Coefficient by an Electrochemical Technique. J. Tunis. Chem. Soc. 1991, 3, 125–140. [Google Scholar]
- Hou, C.-H.; Huang, S.-C.; Chou, P.-H.; Den, W. Removal of Bisphenol A from Aqueous Solutions by Electrochemical Polymerization on a Carbon Aerogel Electrode. J. Taiwan Inst. Chem. Eng. 2015, 51, 103–108. [Google Scholar] [CrossRef]
- Olvera-Vargas, H.; Cocerva, T.; Oturan, N.; Buisson, D.; Oturan, M.A. Bioelectro-Fenton: A Sustainable Integrated Process for Removal of Organic Pollutants from Water: Application to Mineralization of Metoprolol. J. Hazard. Mater. 2016, 319, 13–23. [Google Scholar] [CrossRef]
- Mansour, D.; Fourcade, F.; Huguet, S.; Soutrel, I.; Bellakhal, N.; Dachraoui, M.; Hauchard, D.; Amrane, A. Improvement of the Activated Sludge Treatment by Its Combination with Electro Fenton for the Mineralization of Sulfamethazine. Int. Biodeterior. Biodegrad. 2014, 88, 29–36. [Google Scholar] [CrossRef]
- Arellano, M.; Oturan, N.; Pazos, M.; Ángeles Sanromán, M.; Oturan, M.A. Coupling Electro-Fenton Process to a Biological Treatment, a New Methodology for the Removal of Ionic Liquids? Sep. Purif. Technol. 2020, 233, 115990. [Google Scholar] [CrossRef]
- Aboudalle, A.; Djelal, H.; Fourcade, F.; Domergue, L.; Assadi, A.A.; Lendormi, T.; Taha, S.; Amrane, A. Metronidazole Removal by Means of a Combined System Coupling an Electro-Fenton Process and a Conventional Biological Treatment: By-Products Monitoring and Performance Enhancement. J. Hazard. Mater. 2018, 359, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Baiju, A.; Gandhimathi, R.; Ramesh, S.T.; Nidheesh, P.V. Combined Heterogeneous Electro-Fenton and Biological Process for the Treatment of Stabilized Landfill Leachate. J. Environ. Manag. 2018, 210, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, K.; Nélieu, S.; Van Hullebusch, E.D.; Labanowski, J.; Schmitz-Afonso, I.; Bermond, A.; Cassir, M. Electro-Fenton Removal of TNT: Evidences of the Electro-Chemical Reduction Contribution. Appl. Catal. B 2011, 104, 169–176. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).