Photo(solar)-Activated Hypochlorite Treatment: Radicals Analysis Using a Validated Model and Assessment of Efficiency in Organic Pollutants Degradation
Abstract
:1. Introduction
2. Experimental and Modeling Package
2.1. Reagents and Setup Specifications
2.2. Kinetic Model, Simulator, and Computational Approach
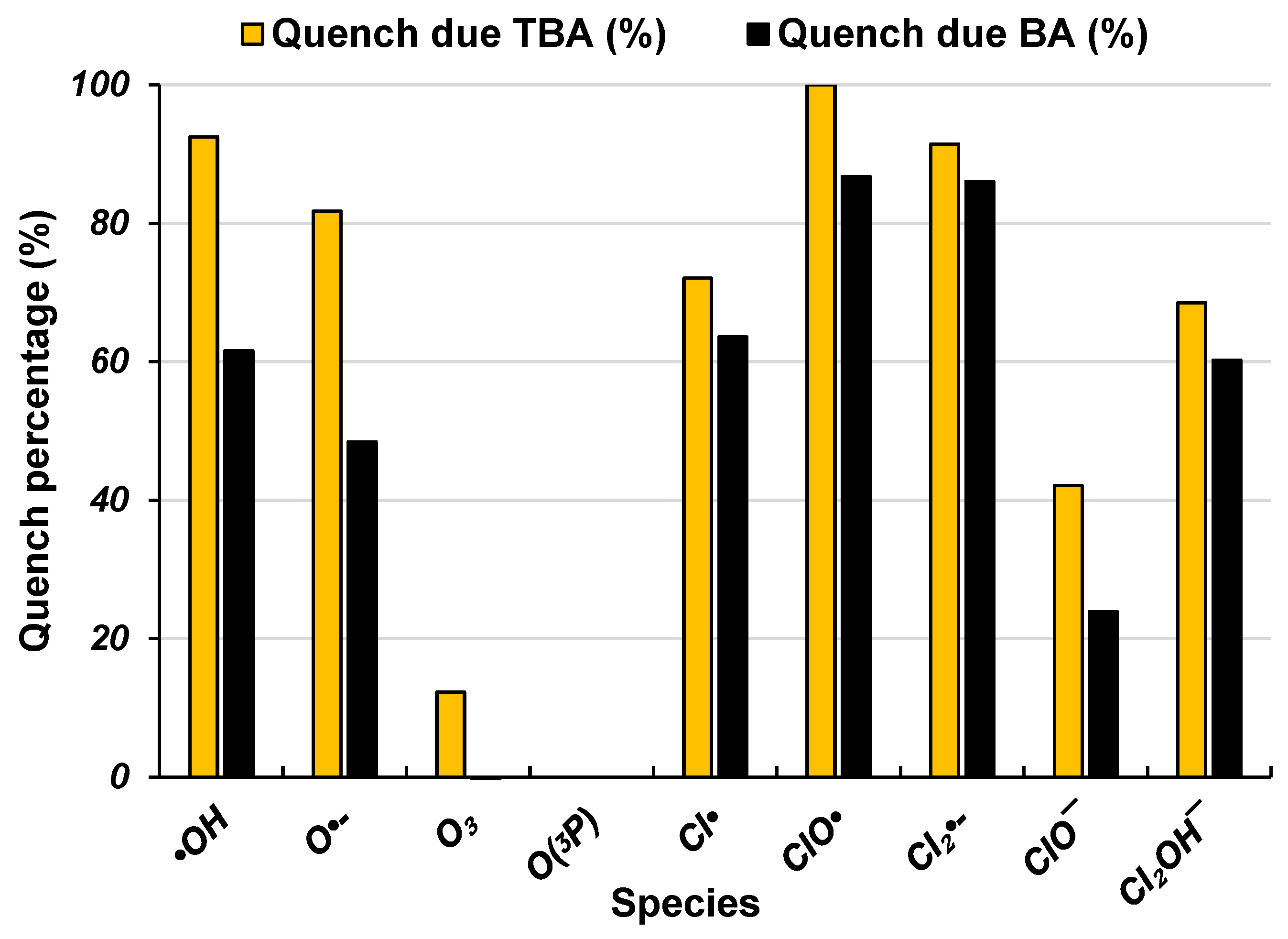
2.3. Reactive Species Contribution
3. Results and Discussion
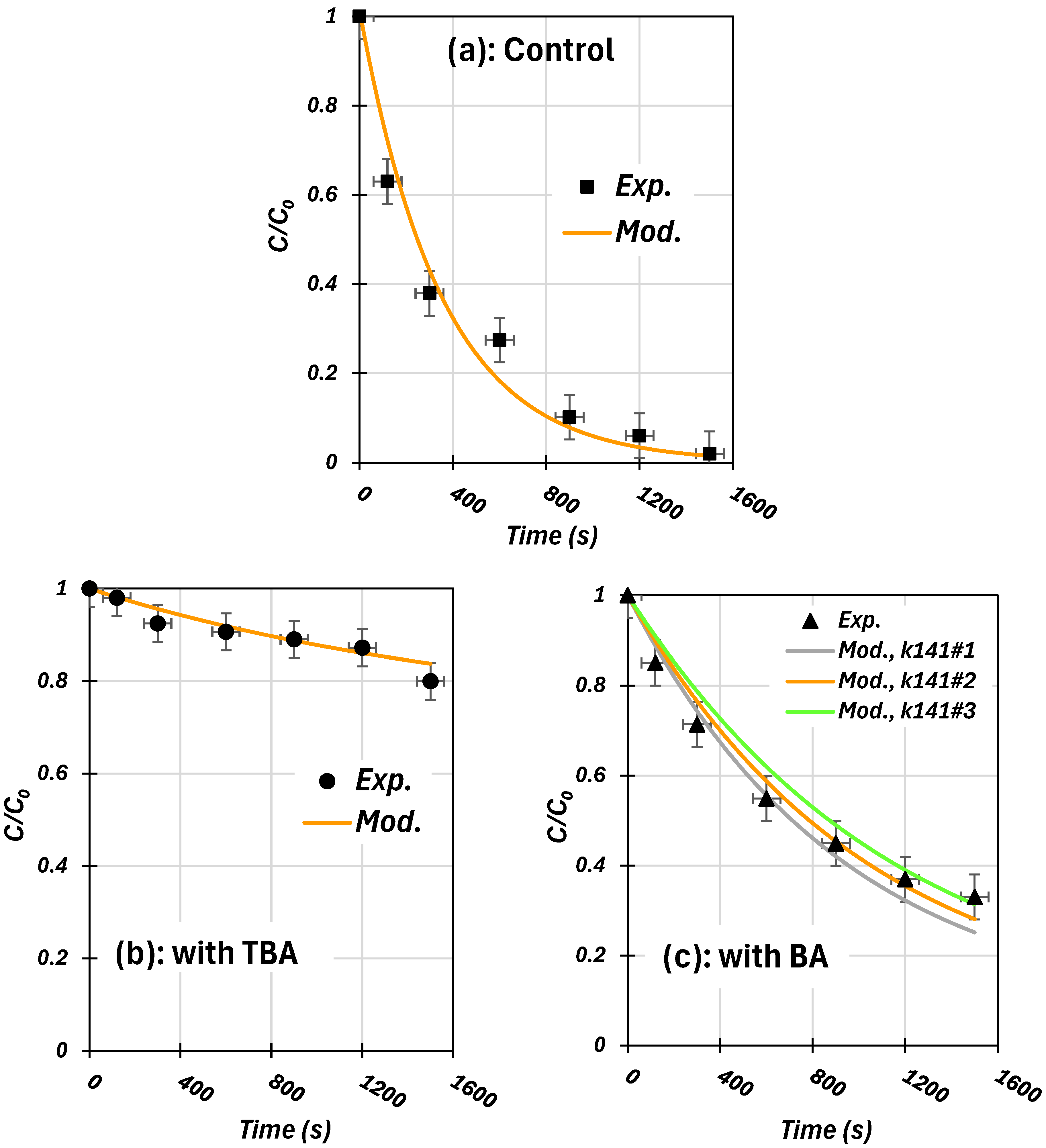
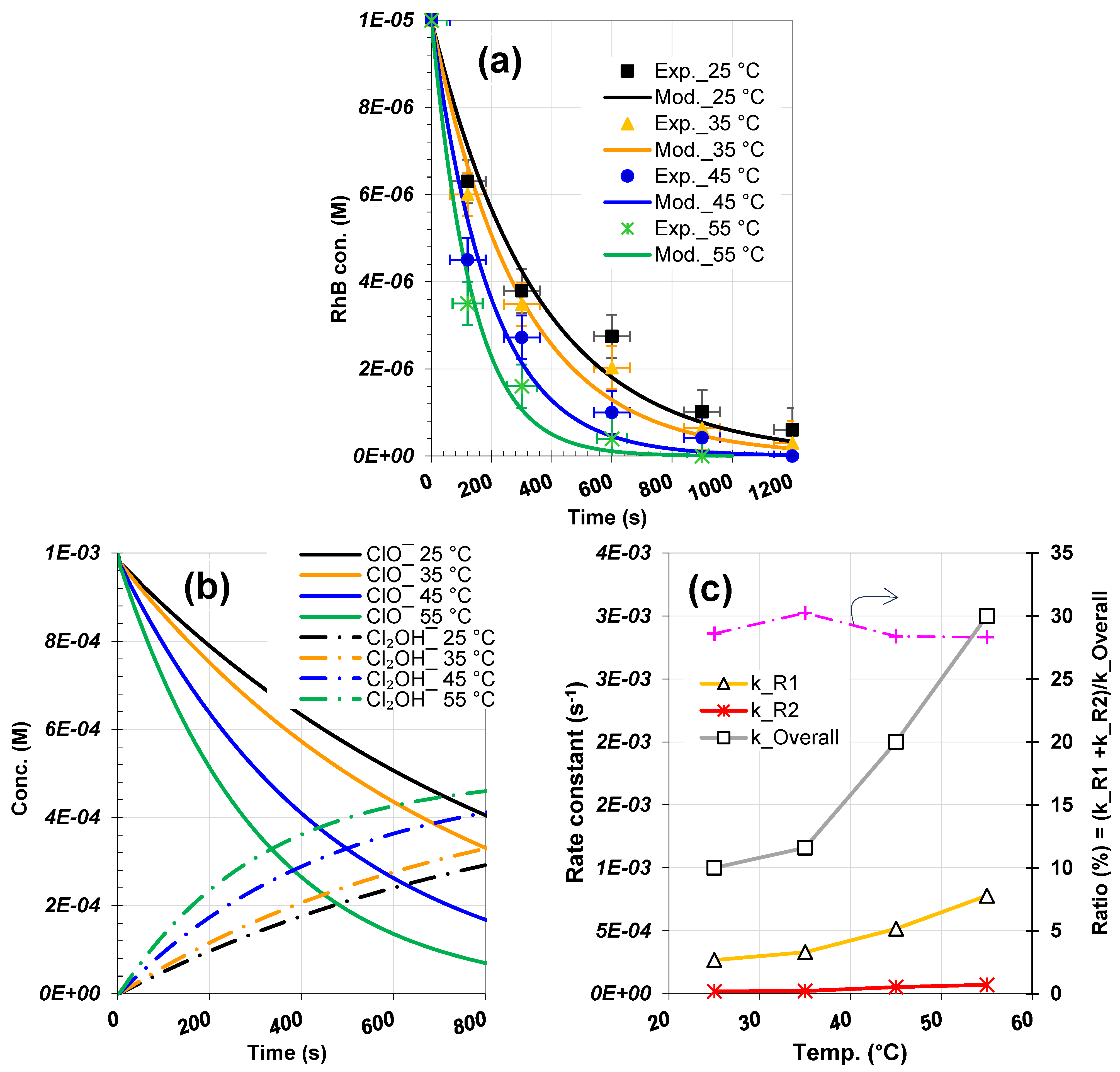
3.1. Validated RhB Degradation Kinetics Using Scavenger Tests
3.2. Determination of Unknown Rate Constants
3.3. Concentration Profiles of ClO−, Reactive Species and Degradation Products
3.3.1. Control Conditions (without Scavengers)
3.3.2. UVsolar/ClO−/TBA and UVsolar/ClO−/BA Systems
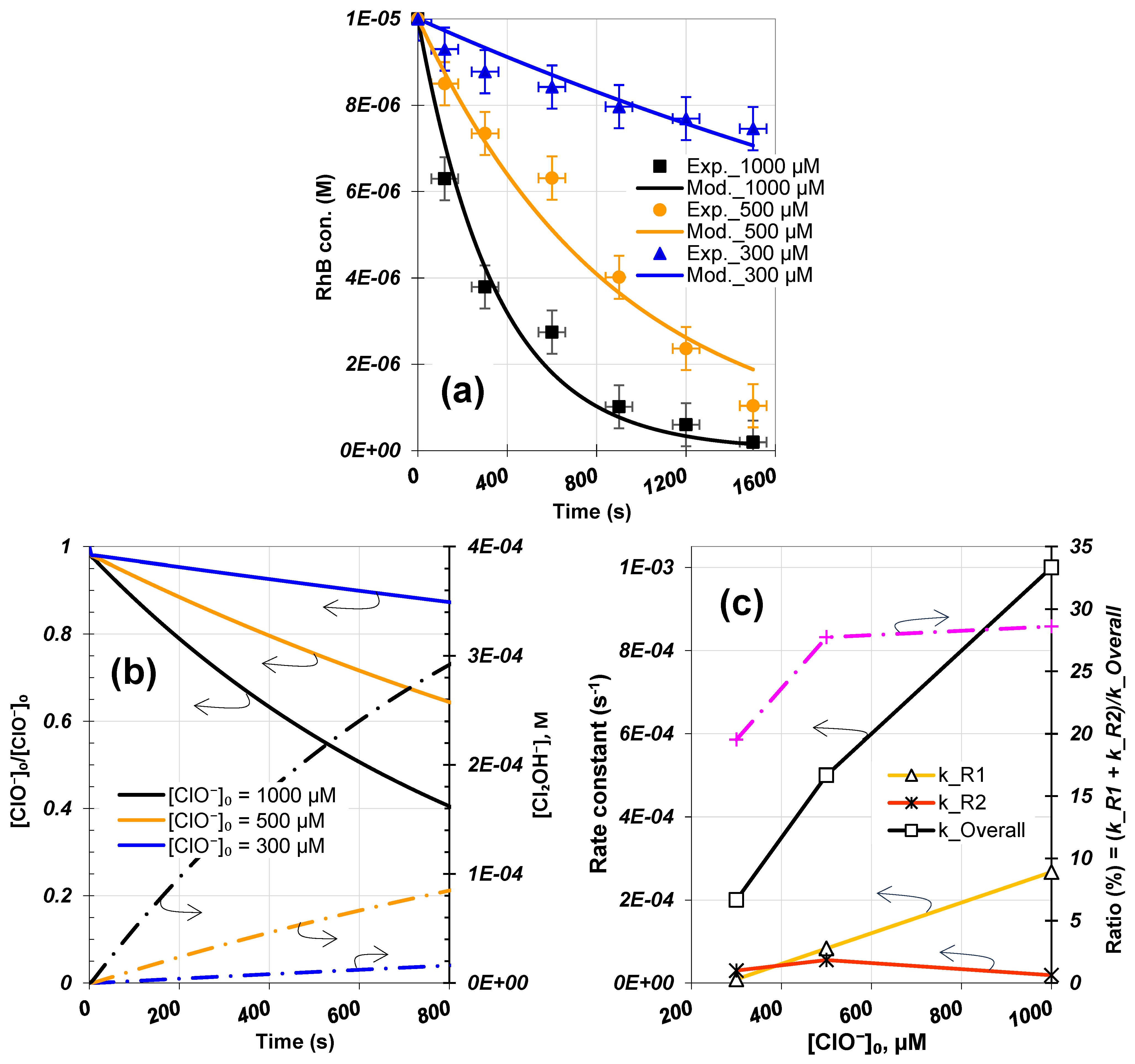
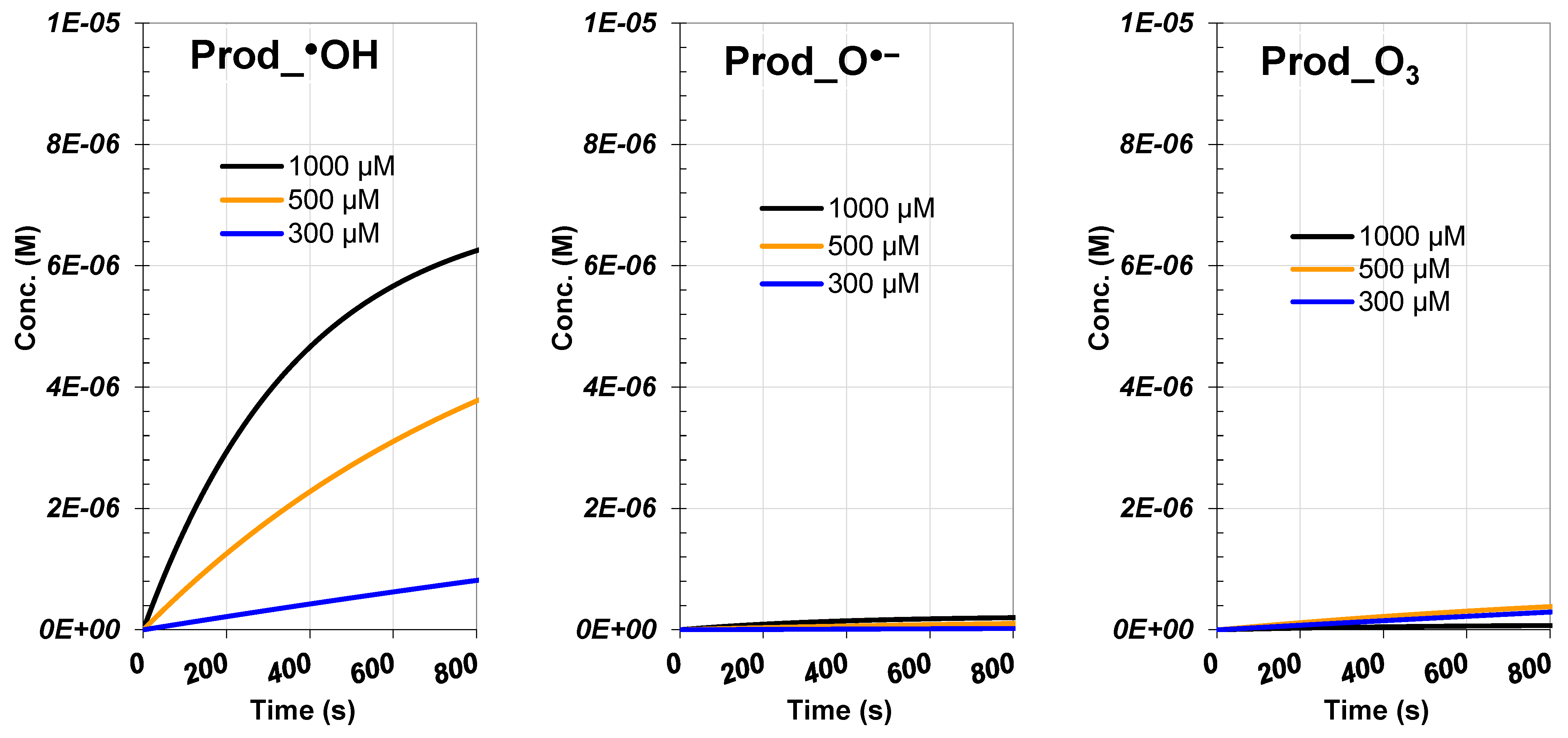
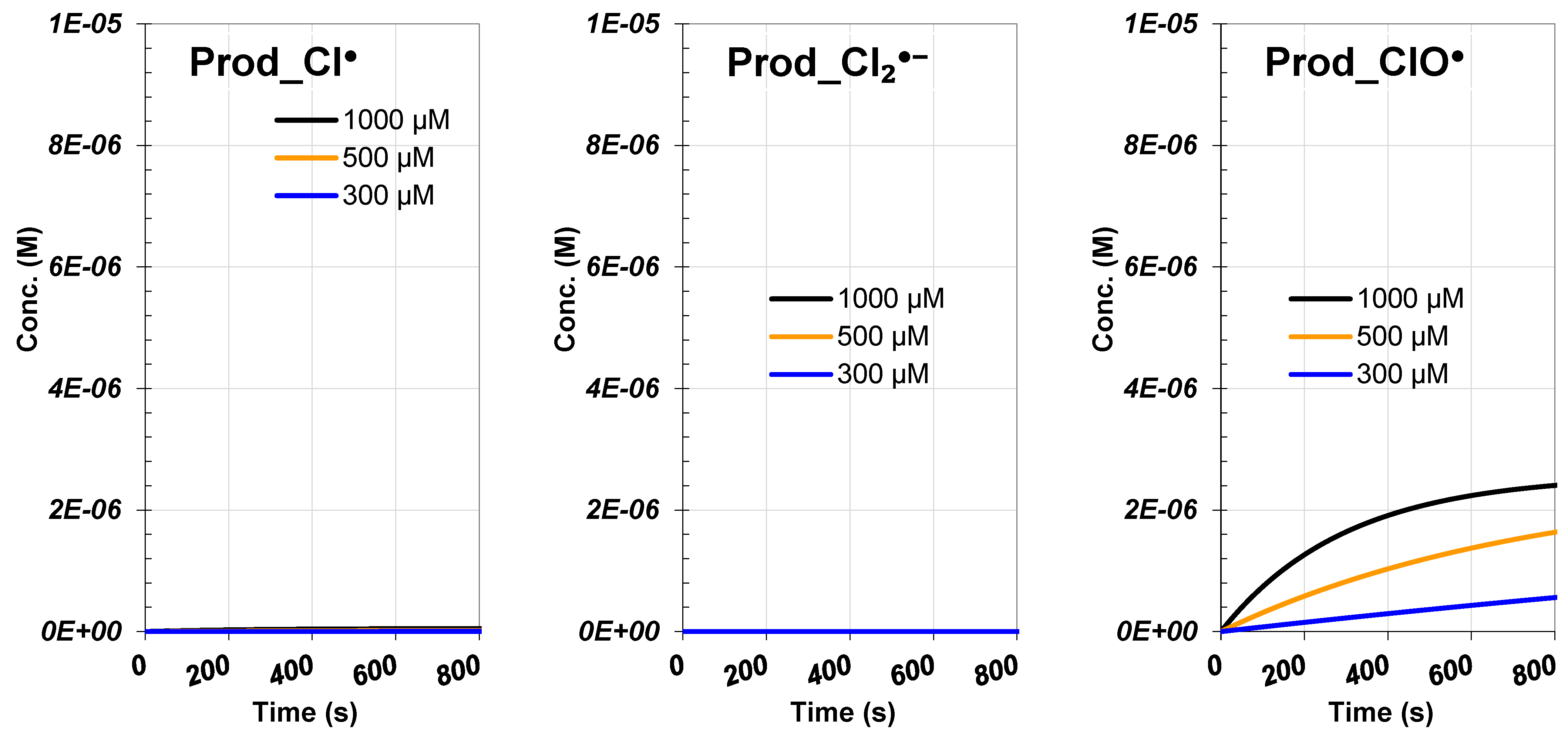
3.4. Analysis of Initial Hypochlorite Dosage
3.4.1. RhB and Hypochlorite Decay
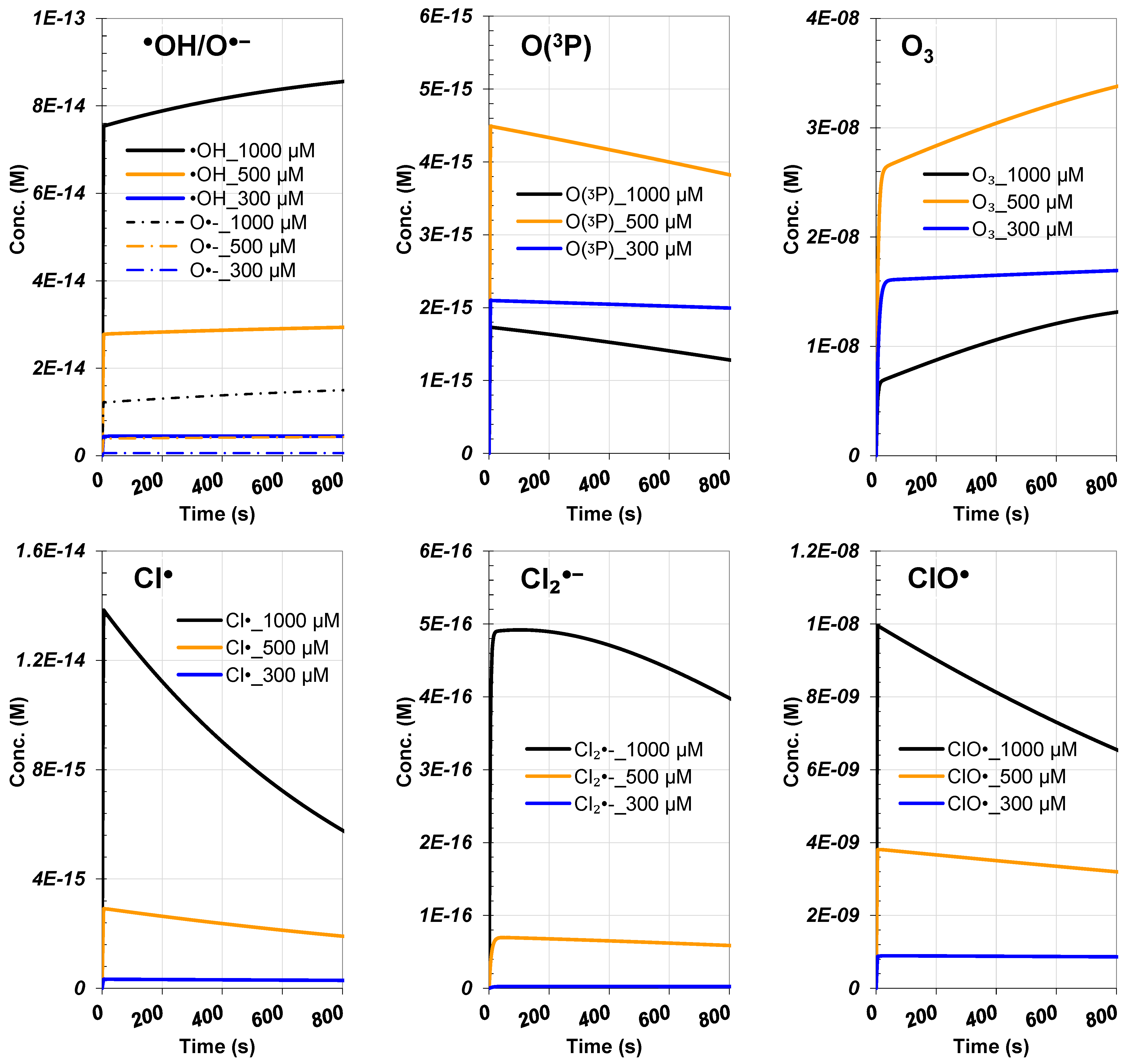
3.4.2. ROS and RCS Evolutions
3.4.3. Products and Reactive Species Contribution
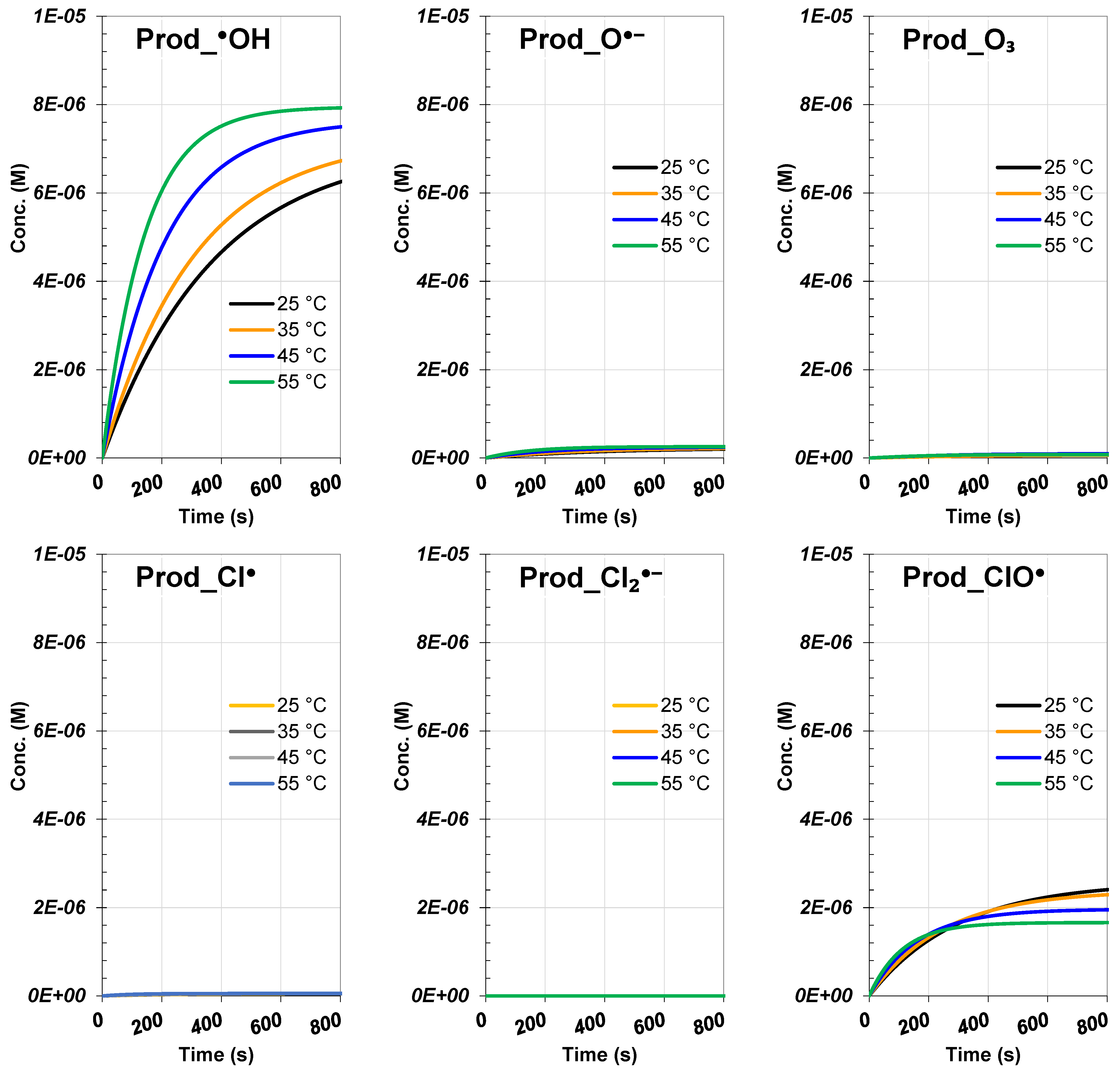
3.5. Analysis of Solution Temperature Impact
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masoner, J.R.; Kolpin, D.W.; Furlong, E.T.; Cozzarelli, I.M.; Gray, J.L. Landfill leachate as a mirror of today’s disposable society: Pharmaceuticals and other contaminants of emerging concern in final leachate from landfills in the conterminous United States. Environ. Toxicol. Chem. 2016, 35, 906–918. [Google Scholar] [CrossRef]
- Richardson, S.D.; Ternes, T.A. Water Analysis: Emerging Contaminants and Current Issues. Anal. Chem. 2018, 90, 398–428. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, D.J.; Arnold, W.A.; Barber, B.L.; Kaufenberg, E.F.; Koskinen, W.C.; Novak, P.J.; Rice, P.J.; Swackhamer, D.L. Contaminants of Emerging Concern: Mass Balance and Comparison of Wastewater Effluent and Upstream Sources in a Mixed-Use Watershed. Environ. Sci. Technol. 2016, 50, 36–45. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O. Sonochemical Treatment of Textile Wastewater. In Water Pollution and Remediation: Photocatalysis; Inamuddin, M.P., Asiri, A., Eds.; Springer-Nature: Cham, Switzerland, 2021; pp. 147–187. ISBN 978-3-030-54723-3. [Google Scholar]
- Bulman, D.M.; Mezyk, S.P.; Remucal, C.K. The Impact of pH and Irradiation Wavelength on the Production of Reactive Oxidants during Chlorine Photolysis. Environ. Sci. Technol. 2019, 53, 4450–4459. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Huie, R.E.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment—Fundamentals and Applications; IWA Publishing: London, UK, 2017; Volume 16, ISBN 9781780407180. [Google Scholar]
- Dehane, A.; Merouani, S. Dyes Sonolysis: An Industrial View of Process Intensification Using Carbon Tetrachloride. In Advanced Oxidation Processes in Dye-Containing Wastewater. Sustainable Textiles: Production, Processing, Manufacturing & Chemistry; Muthu, S.S., Khadir, A., Eds.; Springer Nature: Singapore, 2022; pp. 115–145. ISBN 978-981-19-0882-8. [Google Scholar]
- Merouani, S.; Dehane, A.; Hamdaoui, O.; Yasui, K.; Ashokkumar, M. Review on the impacts of external pressure on sonochemistry. Ultrason. Sonochem. 2024, 106, 106893. [Google Scholar] [CrossRef] [PubMed]
- Dehane, A.; Merouani, S. Chapter 13—Ultrasonic destruction of CCl4: A microscopic-scale analysis. In Development in Wastewater Treatment Research and Processes; Shah, M.P., Rodriguez-Couto, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 219–236. ISBN 978-0-323-95656-7. [Google Scholar]
- Bendjama, H.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient degradation method of emerging organic pollutants in marine environment using UV/periodate process: Case of chlorazol black. Mar. Pollut. Bull. 2018, 126, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Sadik, W.A. Effect of inorganic oxidants in photodecolourization of an azo dye. J. Photochem. Photobiol. A Chem. 2007, 191, 132–137. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Evgenidou, E.; Lambropoulou, D.; Konstantinou, I. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res. 2014, 53, 215–234. [Google Scholar] [CrossRef]
- Sun, P.; Lee, W.N.; Zhang, R.; Huang, C.H. Degradation of DEET and caffeine under UV/Chlorine and simulated sunlight/Chlorine conditions. Environ. Sci. Technol. 2016, 50, 13265–13273. [Google Scholar] [CrossRef]
- Laat, J.D.; Stefan, M. UV/chlorine process. In Advanced Oxidation Processes for Water Treatment; Stefan, M.I., Ed.; IWA Publishing: London, UK, 2017; pp. 383–428. [Google Scholar]
- Belghit, A.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Al-Zahrani, S. The multiple role of inorganic and organic additives in the degradation of reactive green 12 by UV/chlorine advanced oxidation process. Environ. Technol. 2022, 43, 835–847. [Google Scholar] [CrossRef]
- Yin, R.; Zhong, Z.; Ling, L.; Shang, C. The fate of dichloroacetonitrile in UV/Cl2 and UV/H2O2 processes: Implications on potable water reuse. Environ. Sci. Water Res. Technol. 2018, 4, 1295–1302. [Google Scholar] [CrossRef]
- Khajouei, G.; Finklea, H.O.; Lin, L. UV/chlorine advanced oxidation processes for degradation of contaminants in water and wastewater: A comprehensive review. J. Environ. Chem. Eng. 2022, 10, 107508. [Google Scholar] [CrossRef]
- Yeom, Y.; Han, J.; Zhang, X.; Shang, C.; Zhang, T.; Li, X.; Duan, X.; Dionysiou, D.D. A review on the degradation efficiency, DBP formation, and toxicity variation in the UV/chlorine treatment of micropollutants. Chem. Eng. J. 2021, 424, 130053. [Google Scholar] [CrossRef]
- Kishimoto, N. State of the art of UV/chlorine advanced oxidation processes: Their mechanism, byproducts formation, process variation, and applications. J. Water Environ. Technol. 2019, 17, 302–335. [Google Scholar] [CrossRef]
- Yin, R.; Ling, L.; Shang, C. Wavelength-dependent chlorine photolysis and subsequent radical production using UV-LEDs as light sources. Water Res. 2018, 142, 452–458. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Shang, C. Near-Ultraviolet Light-Driven Photocatalytic Chlorine Activation Process with Novel Chlorine Activation Mechanisms. ACS ES&T Water 2021, 1, 2067–2075. [Google Scholar] [CrossRef]
- Cheng, Z.; Ling, L.; Wu, Z.; Fang, J.; Westerho, P.; Shang, C. Novel Visible Light-Driven Photocatalytic Chlorine Activation Process for Carbamazepine Degradation in Drinking Water. Environ. Sci. Technol. 2020, 54, 11584–11593. [Google Scholar] [CrossRef] [PubMed]
- Remucal, C.K.; Manley, D. Emerging investigators series: The efficacy of chlorine photolysis as an advanced oxidation process for drinking water treatment. Environ. Sci. Water Res. Technol. 2016, 2, 565–579. [Google Scholar] [CrossRef]
- Chuang, Y.H.; Chen, S.; Chinn, C.J.; Mitch, W.A. Comparing the UV/Monochloramine and UV/Free Chlorine Advanced Oxidation Processes (AOPs) to the UV/Hydrogen Peroxide AOP under Scenarios Relevant to Potable Reuse. Environ. Sci. Technol. 2017, 51, 13859–13868. [Google Scholar] [CrossRef] [PubMed]
- Watts, M.J.; Linden, K.G. Chlorine photolysis and subsequent OH radical production during UV treatment of chlorinated water. Water Res. 2007, 41, 2871–2878. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Qiang, Z.; Hu, J.; Qu, J. Degradation of chloramphenicol by UV/chlorine treatment: Kinetics, mechanism and enhanced formation of halonitromethanes. Water Res. 2017, 121, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Wu, Z.; Shang, C.; Yao, B.; Hou, S.; Yang, X.; Song, W.; Fang, J. Radical Chemistry and Structural Relationships of PPCP Degradation by UV/Chlorine Treatment in Simulated Drinking Water. Environ. Sci. Technol. 2017, 51, 10431–10439. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Guo, K.; Fang, J.; Yang, X.X.; Xiao, H.; Hou, S.; Kong, X.; Shang, C.; Yang, X.X.; Meng, F.; et al. Factors affecting the roles of reactive species in the degradation of micropollutants by the UV/chlorine process. Water Res. 2017, 126, 351–360. [Google Scholar] [CrossRef]
- Belghit, A.A.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Alghyamah, A.; Bouhelassa, M. Influence of processing conditions on the synergism between UV irradiation and chlorine toward the degradation of refractory organic pollutants in UV/chlorine advanced oxidation system. Sci. Total Environ. 2020, 736, 139623_1–139623_10. [Google Scholar] [CrossRef]
- Kong, X.; Wu, Z.; Ren, Z.; Guo, K.; Hou, S.; Hua, Z.; Li, X.; Fang, J. Degradation of lipid regulators by the UV/chlorine process: Radical mechanisms, chlorine oxide radical (ClO•)-mediated transformation pathways and toxicity changes. Water Res. 2018, 137, 242–250. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Wu, Q.; Du, Y.; Hu, H. Degradation of natural organic matter by UV/chlorine oxidation: Molecular decomposition, formation of oxidation byproducts and cytotoxicity. Water Res. 2017, 124, 251–258. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated Electrons, hydrogen atoms and hydroxyl radicals (•OH/O-) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 515–886. [Google Scholar] [CrossRef]
- Matthew, B.M.; Anastasio, C. A chemical probe technique for the determination of reactive halogen species in aqueous solution: Part 2—Chloride solutions and mixed bromide/chloride solutions. Atmos. Chem. Phys. 2006, 6, 2439–2451. [Google Scholar] [CrossRef]
- Klaning, U.K.; Wolff, T. Laser flash photolysis of HCIO, CIO, HBrO, and BrO− in aqueous solution. Reactions of Cl− and Br− atoms. Berichte der Bunsengesellschaft/Physical Chem. Chem. Phys. 1985, 89, 243–245. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, W.; Sun, J.; Zhu, S.; Li, K.; Meng, X.; Luo, J.; Shi, Z.; Zhou, D.; Crittenden, J.C. Oxidation Mechanisms of the UV/Free Chlorine Process: Kinetic Modeling and Quantitative Structure Activity Relationships. Environ. Sci. Technol. 2019, 53, 4335–4345. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1973, 69, 1597–1607. [Google Scholar] [CrossRef]
- Celardin, F.; Marcantonatos, M. Kinetics of the Rhodamine B-Ozone Chemiluminescent Reaction in Acetic Acid. Zeitschrift Phys. Chemie Neue Folge 1975, 96, 109–124. [Google Scholar] [CrossRef]
- Meghlaoui, F.Z.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M.; Zohra, F.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Meghlaoui, F.Z.; et al. Rapid catalytic degradation of refractory textile dyes in Fe (II)/chlorine system at near neutral pH: Radical mechanism involving chlorine radical anion (Cl 2 %−)-mediated transformation pathways and impact of environmental matrices. Sep. Purif. Technol. 2019, 227, 115685. [Google Scholar] [CrossRef]
- Liang, C.; Su, H.W. Identification of sulfate and hydroxyl radicals in thermally activated persulfate. Ind. Eng. Chem. Res. 2009, 48, 5558–5562. [Google Scholar] [CrossRef]
- Wu, J.J.; Muruganandham, M.; Chen, S.H. Degradation of DMSO by ozone-based advanced oxidation processes. J. Hazard. Mater. 2007, 149, 218–225. [Google Scholar] [CrossRef]
- Alfassi, Z.B.; Huie, R.E.; Mosseri, S.; Neta, P. Kinetics of one-electron oxidation by the ClO radical. Int. J. Radiat. Appl. Instrument. Part C Radiat. Phys. Chem. 1988, 32, 85–88. [Google Scholar] [CrossRef]
- Fang, J.; Fu, Y.; Shang, C. The roles of reactive species in micropollutant degradation in the UV/free chlorine system. Environ. Sci. Technol. 2014, 48, 1859–1868. [Google Scholar] [CrossRef]
- Deng, J.; Wu, G.; Yuan, S.; Zhan, X.; Wang, W.; Hu, Z.H. Ciprofloxacin degradation in UV/chlorine advanced oxidation process: Influencing factors, mechanisms and degradation pathways. J. Photochem. Photobiol. A Chem. 2019, 371, 151–158. [Google Scholar] [CrossRef]
- Larbi Djaballah, M.; Belghit, A.; Dehane, A.; Merouani, S.; Hamdaoui, O.; Ashokkumar, M. Radicals (●OH, Cl●, ClO● and Cl2●–) concentration profiles in the intensified degradation of reactive green 12 by UV/chlorine process: Chemical kinetic analysis using a validated model. J. Photochem. Photobiol. A Chem. 2023, 439, 114557. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M. Sonochemical degradation of Rhodamine B in aqueous phase: Effects of additives. Chem. Eng. J. 2010, 158, 550–557. [Google Scholar] [CrossRef]
- Merouani, S.; Hamdaoui, O.; Saoudi, F.; Chiha, M.; Pétrier, C. Influence of bicarbonate and carbonate ions on sonochemical degradation of Rhodamine B in aqueous phase. J. Hazard. Mater. 2010, 175, 593–599. [Google Scholar] [CrossRef]
- Chiha, M.; Merouani, S.; Hamdaoui, O.; Baup, S.; Gondrexon, N.; Pétrier, C. Ultrasonics Sonochemistry Modeling of ultrasonic degradation of non-volatile organic compounds by Langmuir-type kinetics. Ultrason. Sonochem. 2010, 17, 773–782. [Google Scholar] [CrossRef]
- Bekkouche, S.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M. Efficient photocatalytic degradation of Safranin O by integrating solar-UV/TiO2/Persulfate treatment: Implication of sulfate radical in the oxidation process and effect of various water matrix components. J. Photochem. Photobiol. A Chem. 2017, 345, 80–91. [Google Scholar] [CrossRef]
- Djaballah, M.L.; Merouani, S.; Bendjama, H.; Hamdaoui, O. Development of a free radical-based kinetics model for the oxidative degradation of chlorazol black in aqueous solution using periodate photoactivated process. J. Photochem. Photobiol. A Chem. 2020, 408, 113102. [Google Scholar] [CrossRef]
- Wojnárovits, L.; Takács, E. Rate constants for the reactions of chloride monoxide radical (ClO •) and organic molecules of environmental interest. Water Sci. Technol. 2023, 87, 1925–1944. [Google Scholar] [CrossRef]
- Li, M.; Mei, Q.; Wei, B.; An, Z.; Sun, J.; Xie, J.; He, M. Mechanism and kinetics of ClO-mediated degradation of aromatic compounds in aqueous solution: DFT and QSAR studies. Chem. Eng. J. 2021, 412, 128728. [Google Scholar] [CrossRef]
- An, Z.; Li, M.; Huo, Y.; Jiang, J.; Zhou, Y.; Jin, Z.; Xie, J.; Zhan, J.; He, M. The pH-dependent contributions of radical species during the removal of aromatic acids and bases in light/chlorine systems. Chem. Eng. J. 2022, 433, 133493. [Google Scholar] [CrossRef]
- Meghlaoui, F.Z.; Merouani, S.; Hamdaoui, O.; Alghyamah, A.; Bouhelassa, M.; Ashokkumar, M. Fe(III)-catalyzed degradation of persistent textile dyes by chlorine at slightly acidic conditions: The crucial role of Cl2●− radical in the degradation process and impacts of mineral and organic competitors. Asia-Pacific J. Chem. Eng. 2020, 16, e2553. [Google Scholar] [CrossRef]
- Buxton, G.V.; Subhani, M.S. Radiation chemistry and photochemistry of oxychlorine ions. Part 2.—Photodecomposition of aqueous solutions of hypochlorite ions. J. Chem. Soc. Faraday Trans. 1972, 68, 958–969. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Concentration profiles of chlorine radicals and their significances in •OH-induced dye degradation: Kinetic modeling and reaction pathways. Chem. Eng. J. 2012, 209, 38–45. [Google Scholar] [CrossRef]
- Yuan, R.; Wang, Z.; Hu, Y.; Wang, B.; Gao, S. Probing the radical chemistry in UV/persulfate-based saline wastewater treatment: Kinetics modeling and byproducts identification. Chemosphere 2014, 109, 106–112. [Google Scholar] [CrossRef]
- Chia, L.H.; Tang, X.; Weavers, L.K. Kinetics and mechanism of photoactivated periodate reaction with 4-chlorophenol in acidic solution. Environ. Sci. Technol. 2004, 38, 6875–6880. [Google Scholar] [CrossRef] [PubMed]
- Chadi, N.E.; Merouani, S.; Hamdaoui, O.; Bouhelassa, M.; Ashokkumar, M. H2O2/periodate (IO4−): A novel advanced oxidation technology for the degradation of refractory organic pollutants. Environ. Sci. Water Res. Technol. 2019, 5, 1113–1123. [Google Scholar] [CrossRef]
- Watts, M.J.; Rosenfeldt, E.J.; Linden, K.G. Comparative OH radical oxidation using UV-Cl2 and UV-H2O2 processes. J. Water Supply Res. Technol.-AQUA 2007, 56, 469–477. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, Y.; Xu, B.; Huang, H.; Zhang, T.; Tian, F.; Gao, N. Degradation of chlortoluron during UV irradiation and UV/chlorine processes and formation of disinfection by-products in sequential chlorination. Chem. Eng. J. 2015, 283, 412–419. [Google Scholar] [CrossRef]
- Wang, W.L.; Wu, Q.Y.; Huang, N.; Wang, T.; Hu, H.Y. Synergistic effect between UV and chlorine (UV/chlorine) on the degradation of carbamazepine: Influence factors and radical species. Water Res. 2016, 98, 190–198. [Google Scholar] [CrossRef]





| Name | Reaction | Rate Constant | Ref. | |
|---|---|---|---|---|
| R1 | ClO− photolysis Rxns | ClO− O●− + Cl● | (0.028–2.67) × 10−4 s−1 | This study |
| R2 | ClO− O(3P) + Cl− | (1.88–2.46) × 10−5 s−1 | This study | |
| R3 | ClO− O(1D) + Cl− | 0 s−1 | This study | |
| R4 | O(1D)/O(3P) Rxns | O(1D) + H2O → 2●OH | 1.2 × 1011 M−1s−1 | [5] |
| R5 | O(3P) + O2 → O3 | 4.0 × 109 M−1s−1 | [5] | |
| R6 | O(3P) + ClO− → ClO2− | 9.4 × 109 M−1s−1 | [5] | |
| R7 | O(3P) + H2O2 → ●OH + HO2● | 1.6 × 109 M−1s−1 | [5] | |
| R8 | O(3P) + HO2− → ●OH + O2●− | 5.3 × 109 M−1s−1 | [5] | |
| R9 | O(3P) + OH− → HO2− | 4.2 × 108 M−1s−1 | [5] | |
| R10 | O3 → O2 + O(3P) | 4.5 × 10−6 s−1 | [5] | |
| R11 | Speciations Rxns | HOCl → H+ + ClO− | 1.41 × 103 s−1 | [5] |
| R12 | H+ + ClO− → HOCl | 5.0 × 1010 M−1s−1 | [33] | |
| R13 | HOCl + Cl− → Cl2 + H2O | 0.182 M−1s−1 | [5] | |
| R14 | Cl2 + H2O → HOCl + Cl− + H+ | 0.27 M−1s | [14] | |
| R15 | HOCl + Cl− → Cl2OH− | 1.5 × 104 M−1s−1 | [5] | |
| R16 | Cl2 + OH− → HOCl + Cl− | 1.0 × 109 M−1s−1 | [5] | |
| R17 | HCl → H+ + Cl− | 8.6 × 1016 s−1 | [14] | |
| R18 | H+ + Cl− → HCl | 5.0 × 1010 M−1s−1 | [14] | |
| R19 | H2O2 → H+ + HO2− | 0.13 s−1 | [33] | |
| R20 | H+ + HO2− → H2O2 | 5.0 × 1010 M−1s−1 | [33] | |
| R21 | H2O → H+ + OH− | 0.001 s−1 | [14] | |
| R22 | H+ + OH− → H2O | 1.0 × 1011 M−1s−1 | [14] | |
| R23 | ●OH Rxns | ●OH + HOCl → ClO● + H2O | 2.0 × 109 M−1s−1 | [34] |
| R24 | 2●OH → H2O2 | 5.5 × 109 M−1s−1 | [34] | |
| R25 | ●OH + ClO● → ClO2− + H+ | 1.0 × 109 M−1s−1 | [5] | |
| R26 | ●OH + H2O2 → HO2● + H2O | 2.7 × 107 M−1s−1 | [33] | |
| R27 | ●OH + O2●− → O2 + OH− | 7.0 × 109 M−1s−1 | [14] | |
| R28 | ●OH + Cl− → Cl● + OH− | 1.1 × 109 M−1s−1 | [5] | |
| R29 | ●OH + Cl2●− → HOCl + Cl− | 1.0 × 109 M−1s−1 | [5,14] | |
| R30 | ●OH + ClO− → ClO● + OH− | 8.8 × 109 M−1s−1 | [14] | |
| R31 | ●OH + HO2● → O2 + H2O | 6.6 × 109 M−1s−1 | [5,14] | |
| R32 | ●OH + Cl− → HOCl●− | 4.3 × 109 M−1s−1 | [14] | |
| R33 | ●OH + ClO2− → OH− + ClO2● | 4.5 × 109 M−1s−1 | [5] | |
| R34 | ●OH + ClO3− → ClO3● + OH− | 1.0 × 106 M−1s−1 | [5] | |
| R35 | ●OH + HO2− → HO2● + OH− | 7.5 × 109 M−1s−1 | [33] | |
| R36 | ●OH + HO2− → H2O + O2●− | 7.05 × 109 M−1s−1 | [33] | |
| R37 | ●OH + OH− → O●− + H2O | 1.25 × 1010 M−1s−1 | [5] | |
| R38 | ●OH + O●− → HO2− | 2.0 × 1010 M−1s−1 | [5] | |
| R39 | ●OH + ClO2● → ClO3− + H+ | 4.0 × 109 M−1s−1 | [5] | |
| R40 | ●OH + O3●− → HO2● + O2●− | 8.5 × 109 M−1s−1 | [5] | |
| R41 | ●OH + O3 → O2 + HO2● | 1.05 × 108 M−1s−1 | [5] | |
| R42 | Cl● Rxns | 2Cl● → Cl2. | 8.8.8 × 107 M−1s−1 | [5,14] |
| R43 | Cl● + HOCl → Cl− + ClO● + H+ | 3.0 × 109 M−1s−1 | [35] | |
| R44 | Cl● + ClO− → Cl− + ClO● | 8.3 × 109 M−1s−1 | [35] | |
| R45 | Cl● + Cl2 → Cl3● | 5.3 × 108 M−1s−1 | [5] | |
| R46 | Cl● + H2O → HOCl●− + H+ | 2.05 × 105 M−1s−1 | [5] | |
| R47 | Cl● + Cl− → Cl2●− | 8.5 × 109 M−1s−1 | [14] | |
| R48 | Cl● + OH− → HOCl●− | 1.8 × 1010 M−1s−1 | [14] | |
| R49 | Cl● + H2O2 → HO2● + Cl− + H+ | 2.0 × 109 M−1s−1 | [14] | |
| R50 | Cl● + ClO3− → Prod_1 | 1.0 × 106 M−1s−1 | [5] | |
| R51 | Cl● + ClO2● → Prod_2 | 1.0 × 109 M−1s−1 | [5] | |
| R52 | Cl● + Cl2●− → Cl2 + Cl− | 2.1 × 109 M−1s−1 | [5] | |
| R53 | Cl● + ClO2− → ClO2● + Cl− | 7.0 × 109 M−1s−1 | [5] | |
| R54 | Cl2●− Rxns | 2Cl2●− → Cl2 + 2Cl− | 8.0 × 108 M−1s−1 | [5] |
| R55 | Cl2●− + H2O → HOCl●− + Cl− + H+ | 1.3 × 103 M−1s−1 | [5] | |
| R56 | Cl2●− + OH− → HOCl●− + Cl− | 4.5 × 107 M−1s−1 | [5] | |
| R57 | Cl2●− + ClO− → ClO● + 2Cl− | 2.9 × 108 M−1s−1 | [5] | |
| R58 | Cl2●− + O2●− → O2 + 2Cl− | 2.0 × 109 M−1s−1 | [5,14] | |
| R59 | Cl2●− + HO2● → O2 + 2Cl− + H+ | 3.0 × 109 M−1s−1 | [14] | |
| R60 | Cl2●− + H2O2 → HO2● + 2Cl− + H+ | 1.4 × 105 M−1s−1 | [5] | |
| R61 | Cl2●− → Cl● + Cl− | 6.0 × 104 s−1 | [5] | |
| R62 | Cl2●− + ClO2● → Prod_3 | 1.0 × 109 M−1s−1 | [5] | |
| R63 | ClO● Rxns | 2ClO● → Cl2O2 | 2.5 × 109 M−1s−1 | [36] |
| R64 | Cl2O2 + H2O → HOCl + ClO2− + H+ | 4.5 × 107 M−1s−1 | [36] | |
| R65 | Cl2O2 + OH− → ClO− + ClO2− + H+ | 2.5 × 109 M−1s−1 | [36] | |
| R66 | ClO● + ClO2− → ClO2● + ClO− | 9.4 × 108 M−1s−1 | [5] | |
| R67 | Ozone Rxns | O3 + OH− → O2 + HO2− | 48 M−1s−1 | [6] |
| R68 | O3 + HO2− → O3●− + HO2● | 5.5 × 106 M−1s−1 | [5] | |
| R69 | O3 + HO2● → H+ + O2 + O3●− | 1.6 × 109 M−1s−1 | [5] | |
| R70 | O3 + ClO− → 2O2 + Cl− | 110 M−1s−1 | [5] | |
| R71 | O3 + ClO− → O2 + ClO2− | 30 M−1s−1 | [5] | |
| R72 | O3 + Cl− → O2 + ClO− | 0.0016 M−1s−1 | [5] | |
| R73 | O3 + H2O2 → O2 + ●OH + HO2● | 0.0272 M−1s−1 | [5] | |
| R74 | O3 + HO2− → O2 + ●OH + O2●− | 5.5 × 106 M−1s−1 | [5] | |
| R75 | O3 + Cl2●− → Prod_4 | 9.0 × 107 M−1s−1 | [5] | |
| R76 | O3 + O2●− → O2 + O3●− | 1.55 × 109 M−1s−1 | [5] | |
| R77 | O3 + ClO2● → O2 + ClO3● | 1230 M−1s−1 | [5] | |
| R78 | O3 + ClO3− → Prod_5 | 0.0001 M−1s−1 | [5] | |
| R79 | O3 + H+ → Prod_6 | 0.0004 M−1s−1 | [5] | |
| R80 | O3 + ClO2− → O3●− + ClO2● | 2.01 × 106 M−1s−1 | [5] | |
| R81 | ROS Rxns | H2O2 + Cl2 → 2HCl + O2 | 1.3 × 104 M−1s−1 | [5] |
| R82 | H2O2 + HOCl → HCl + H2O + O2 | 1.1 × 104 M−1s−1 | [5] | |
| R83 | H2O2 + ClO− → Cl− + H2O + O2 | 1.7 × 105 M−1s−1 | [5] | |
| R84 | H2O2 + HO2● → O2 + ●OH + H2O | 3 M−1s−1 | [5] | |
| R85 | H2O2 + O2●− → O2 + ●OH + OH− | 0.13 M−1s−1 | [5] | |
| R86 | H2O2 + O●− → O2●− + H2O | 4.0 × 108 M−1s−1 | [5] | |
| R87 | HO2− + O●− → HO2● + 2OH- | 5.0 × 108 M−1s−1 | [5] | |
| R88 | HO2− + O●− → ●OH + O2●− | 4.0 × 108 M−1s−1 | [5] | |
| R89 | HO2− + ClO2● → HO2● + ClO2- | 9.57 × 104 M−1s−1 | [5] | |
| R90 | HO2● + HOCl → Cl● + OH− + O2 + H+ | 7.5 × 106 M−1s−1 | [5] | |
| R91 | HO2● + Cl2 → Cl2●− + H+ + O2 | 1.0 × 109 M−1s−1 | [5] | |
| R92 | HO2● → H+ + O2●− | 1.6 × 105 M−1s−1 | [5] | |
| R93 | HO2● + O2●− → H2O2 + O2 + OH- | 7.9 × 107 M−1s−1 | [5] | |
| R94 | HO2● + O2●− → HO2− + O2 | 9.7 × 107 M−1s−1 | [5] | |
| R95 | 2 HO2● → H2O2 + O2 | 8.3 × 105 M−1s−1 | [5] | |
| R96 | HO2● + ClO2● → Prod_7 | 1.0 × 106 M−1s−1 | [5] | |
| R97 | O2●− + HOCl → Cl● + OH− + O2 | 7.5 × 106 M−1s−1 | [5] | |
| R98 | O2●− + HOCl → Cl− + ●OH + O2 | 7.5 × 106 M−1s−1 | [5] | |
| R99 | O2●− + ClO− → Cl● + 2 OH− + O2 | 2.0 × 108 M−1s−1 | [5] | |
| R100 | O2●− + Cl2 → Cl2●− + O2 | 1.0 × 109 M−1s−1 | [5] | |
| R101 | O2●− + O●− → 2OH− + O2 | 6.0 × 108 M−1s−1 | [5] | |
| R102 | O2●− + Cl− → Prod_8 | 140 M−1s−1 | [5] | |
| R103 | O2●− + ClO2● → O2 + ClO2- | 3.15 × 109 M−1s−1 | [5] | |
| R104 | O2●− + ClO2− → Prod_9 | 40 M−1s−1 | [5] | |
| R105 | O2●− + H+ → HO2● | 5.0 × 1010 M−1s−1 | [33] | |
| R106 | O2●− + ClO3− → Prod_10 | 3.2 × 103 M−1s−1 | [5] | |
| R107 | O3●− → O2 + O●− | 3.2 × 103 s−1 | [5,6] | |
| R108 | O3●− + ClO● → ClO− + O3 | 1.0 × 109 M−1s−1 | [5] | |
| R109 | O3●− + H+ → O2 + ●OH | 9.0 × 1010 M−1s−1 | [5] | |
| R110 | O3●− + O●− → 2O2●− | 7.0 × 108 M−1s−1 | [5] | |
| R111 | O3●− + ClO2● → O2 + ClO3− | 1.8 × 105 M−1s−1 | [5] | |
| R112 | 2O3●− → Prod_11 | 9.0 × 108 M−1s−1 | [5] | |
| R113 | O3●− + ClO2● → ClO2− + O3 | 3.15 × 109 M−1s−1 | [5] | |
| R114 | O●− + ClO− + H2O → ClO● + 2OH− | 2.3 × 108 M−1s−1 | [5] | |
| R115 | O●− + H2O → ●OH + OH− | 1.8 × 106 M−1s−1 | [14] | |
| R116 | O●− + O2 → O3●− | 3.5 × 109 M−1s−1 | [5] | |
| R117 | O●− + ClO2− → OH− + ClO2● | 1.95 × 108 M−1s−1 | [5] | |
| R118 | 2O●− → O22 − | 4.65 × 109 M−1s−1 | [5] | |
| R119 | O●− + ClO2● → ClO3− | 2.7 × 109 M−1s−1 | [5] | |
| R120 | Other RCS Rxns | HOCl●− → Cl− + ●OH | 6.1 × 109 M−1s−1 | [37] |
| R121 | HOCl●− + H+ → Cl● + H2O | 2.1 × 1010 M−1s−1 | [5] | |
| R122 | HOCl●− + Cl− → Cl2●− + OH− | 1.0 × 104 M−1s−1 | [5] | |
| R123 | ClO2● → Cl● + O2 | 6.7 × 109 M−1s−1 | [5] | |
| R124 | Pollutants Rxns | P + ●OH → Prod_●OH | 2.5 × 1010 M−1s−1 | [33] |
| R125 | P + O●− → Prod_O●− | 4.8 × 109 M−1s−1 | This study | |
| R126 | P + O3 → Prod_O3 | 2450 M−1s−1 | [38] | |
| R127 | P + Cl● → Prod_Cl● | 1.45 × 109 M−1s−1 | This study | |
| R128 | P + ClO● → Prod_ClO● | 8.70 × 104 M−1s−1 | This study | |
| R129 | P + Cl2●− → Prod_Cl2●− | 2.50 × 107 M−1s−1 | This study | |
| R130 | P + HOCl●− → Prod_HOCl●− | 4.10 × 104 M−1s−1 | This study | |
| R131 | P + HO2● → Prod_HO2● | 7.30 × 105 M−1s−1 | This study | |
| R132 | P + O2●− → Prod_O2●− | 3.60 × 104 M−1s−1 | This study | |
| R133 | P + O(3P) → Prod_O(3P) | 0.40 M−1s−1 | This study | |
| R134 | TBA Rxns | TBA + Cl● → Prod_Cl●-TBA | 3 × 108 M−1s−1 | [39] |
| R135 | TBA + ClO● → Prod_ClO●-TBA | 1.30 × 107 M−1s−1 | [39] | |
| R136 | TBA + Cl2●− → Prod_Cl2●−-TBA | 700 M−1s−1 | [39] | |
| R137 | TBA + ●OH → Prod_●OH-TBA | 3.80 × 108 M−1s−1 | [40] | |
| R138 | TBA + O3 → Prod_O3-TBA | 0.003 M−1s−1 | [41] | |
| R139 | TBA + O●− → Prod_O●−-TBA | 5 × 108 M−1s−1 | ||
| R140 | BA Rxns | BA + Cl● → Prod_Cl●-BA | 1.8 × 1010 M−1s−1 | [39] |
| R141 | BA + ClO● → Prod_ClO●-BA | <3 × 106 M−1s−1 | [42] | |
| R142 | BA + Cl2●− → Prod_Cl2●−-BA | 2 × 106 M−1s−1 | [5] | |
| R143 | BA + ●OH → Prod_●OH-BA | 5.27 × 109 M−1s−1 | [5] | |
| R144 | BA + O●− → Prod_O●−-BA | 4 × 107 M−1s−1 | [5] |
| Species | Rate (M/s) | Contribution (%) |
|---|---|---|
| [ClO−]₀ = 1000 µM | ||
| RhB | 2.89 × 10−6 | |
| Prod_Cl• | 1.93 × 10−8 | 0.67 |
| Prod_•OH | 1.94 × 10−6 | 67.01 |
| Prod_O•− | 6.05 × 10−8 | 2.09 |
| Prod_ClO• | 8.58 × 10−7 | 29.63 |
| Prod_O₃ | 1.75 × 10−8 | 0.60 |
| [ClO−]₀ = 500 µM | ||
| RhB | 1.25 × 10−6 | |
| Prod_Cl• | 4.61 × 10−9 | 0.37 |
| Prod_•OH | 7.84 × 10−7 | 62.78 |
| Prod_O•− | 2.12 × 10−8 | 1.70 |
| Prod_ClO• | 3.69 × 10−7 | 29.54 |
| Prod_O₃ | 7.00 × 10−8 | 5.61 |
| [ClO−]₀ = 300 µM | ||
| RhB | 2.70 × 10−7 | |
| Prod_Cl• | 5.60 × 10−10 | 0.21 |
| Prod_•OH | 1.31 × 10−7 | 48.43 |
| Prod_O•− | 3.32 × 10−9 | 1.23 |
| Prod_ClO• | 9.16 × 10−8 | 33.93 |
| Prod_O₃ | 4.38 × 10−8 | 16.21 |
| Species | Rate (M/s) | Contribution (%) |
|---|---|---|
| Temp. 25 °C | ||
| RhB | 2.89 × 10−6 | |
| Prod_Cl• | 1.93 × 10−8 | 0.67 |
| Prod_•OH | 1.94 × 10−6 | 67.01 |
| Prod_O•− | 6.05 × 10−8 | 2.09 |
| Prod_ClO• | 8.58 × 10−7 | 29.63 |
| Prod_O₃ | 1.75 × 10−8 | 0.60 |
| Temp. 35 °C | ||
| RhB | 3.35 × 10−6 | |
| Prod_Cl• | 2.27 × 10−8 | 0.68 |
| Prod_•OH | 2.32 × 10−6 | 69.25 |
| Prod_O•− | 7.25 × 10−8 | 2.16 |
| Prod_ClO• | 9.18 × 10−7 | 27.39 |
| Prod_O₃ | 1.72 × 10−8 | 0.51 |
| Temp. 45 °C | ||
| RhB | 4.58 × 10−6 | |
| Prod_Cl• | 3.18 × 10−8 | 0.69 |
| Prod_•OH | 3.38 × 10−6 | 73.77 |
| Prod_O•− | 1.06 × 10−7 | 2.31 |
| Prod_ClO• | 1.03 × 10−6 | 22.50 |
| Prod_O₃ | 3.33 × 10−8 | 0.73 |
| Temp. 55 °C | ||
| RhB | 5.87 × 10−6 | |
| Prod_Cl• | 4.07 × 10−8 | 0.69 |
| Prod_•OH | 4.55 × 10−6 | 77.54 |
| Prod_O•− | 1.43 × 10−7 | 2.44 |
| Prod_ClO• | 1.03 × 10−6 | 17.53 |
| Prod_O₃ | 3.50 × 10−8 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amichi, H.; Merouani, S.; Dehane, A.; Bouchoucha, H.; Hamdaoui, O. Photo(solar)-Activated Hypochlorite Treatment: Radicals Analysis Using a Validated Model and Assessment of Efficiency in Organic Pollutants Degradation. Processes 2024, 12, 1853. https://doi.org/10.3390/pr12091853
Amichi H, Merouani S, Dehane A, Bouchoucha H, Hamdaoui O. Photo(solar)-Activated Hypochlorite Treatment: Radicals Analysis Using a Validated Model and Assessment of Efficiency in Organic Pollutants Degradation. Processes. 2024; 12(9):1853. https://doi.org/10.3390/pr12091853
Chicago/Turabian StyleAmichi, Hayet, Slimane Merouani, Aissa Dehane, Hana Bouchoucha, and Oualid Hamdaoui. 2024. "Photo(solar)-Activated Hypochlorite Treatment: Radicals Analysis Using a Validated Model and Assessment of Efficiency in Organic Pollutants Degradation" Processes 12, no. 9: 1853. https://doi.org/10.3390/pr12091853






