Life Cycle Assessment of CO2-Based and Conventional Methanol Production Pathways in Thailand
Abstract
1. Introduction
2. Materials and Methods
2.1. Goal and Scope Definition
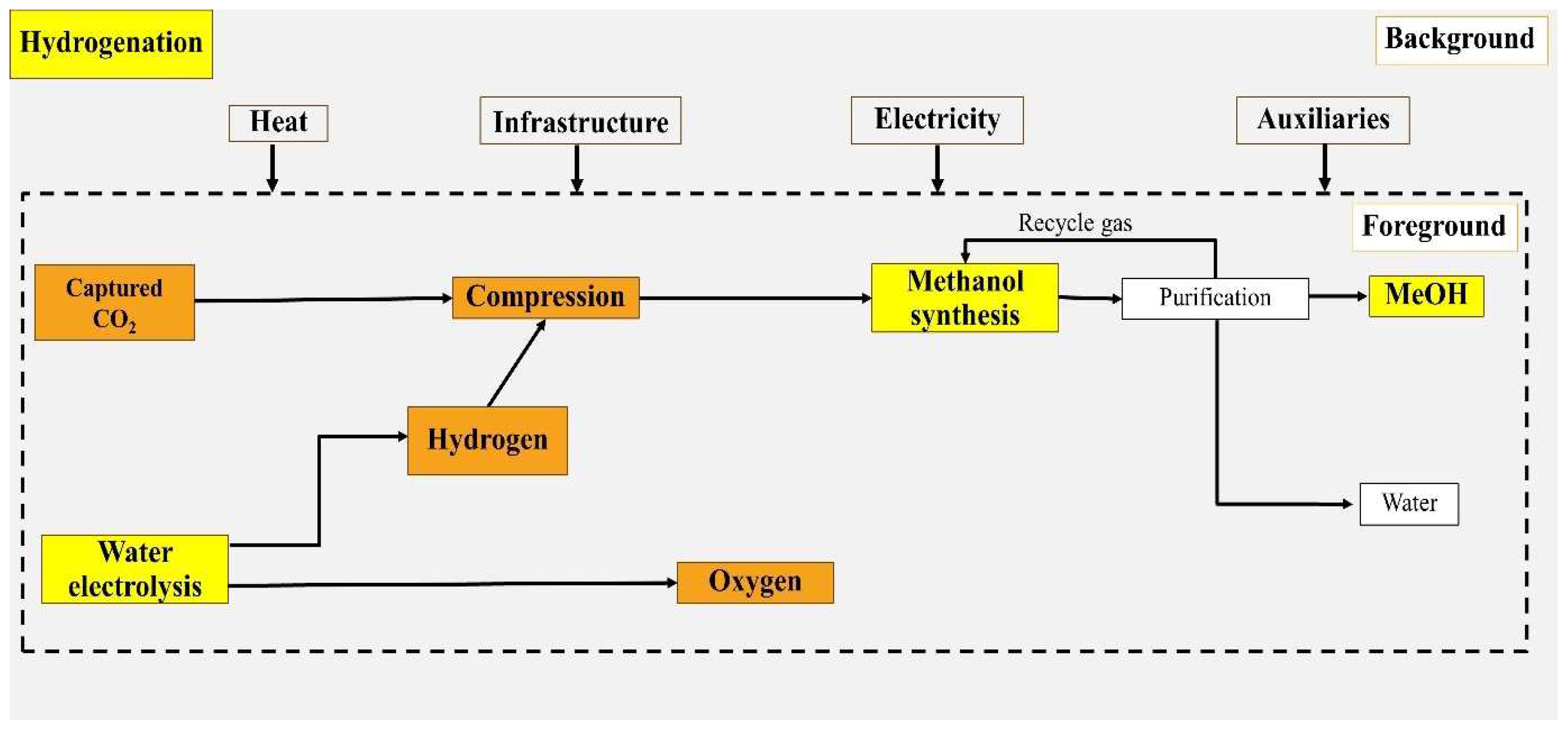
2.1.1. Route 1: Methanol Production from CO2 Hydrogenation
2.1.2. Route 2: Tri-Reforming of Methane with CO2 Utilization
2.1.3. Route 3: Co-Electrolysis of CO2 to Methanol
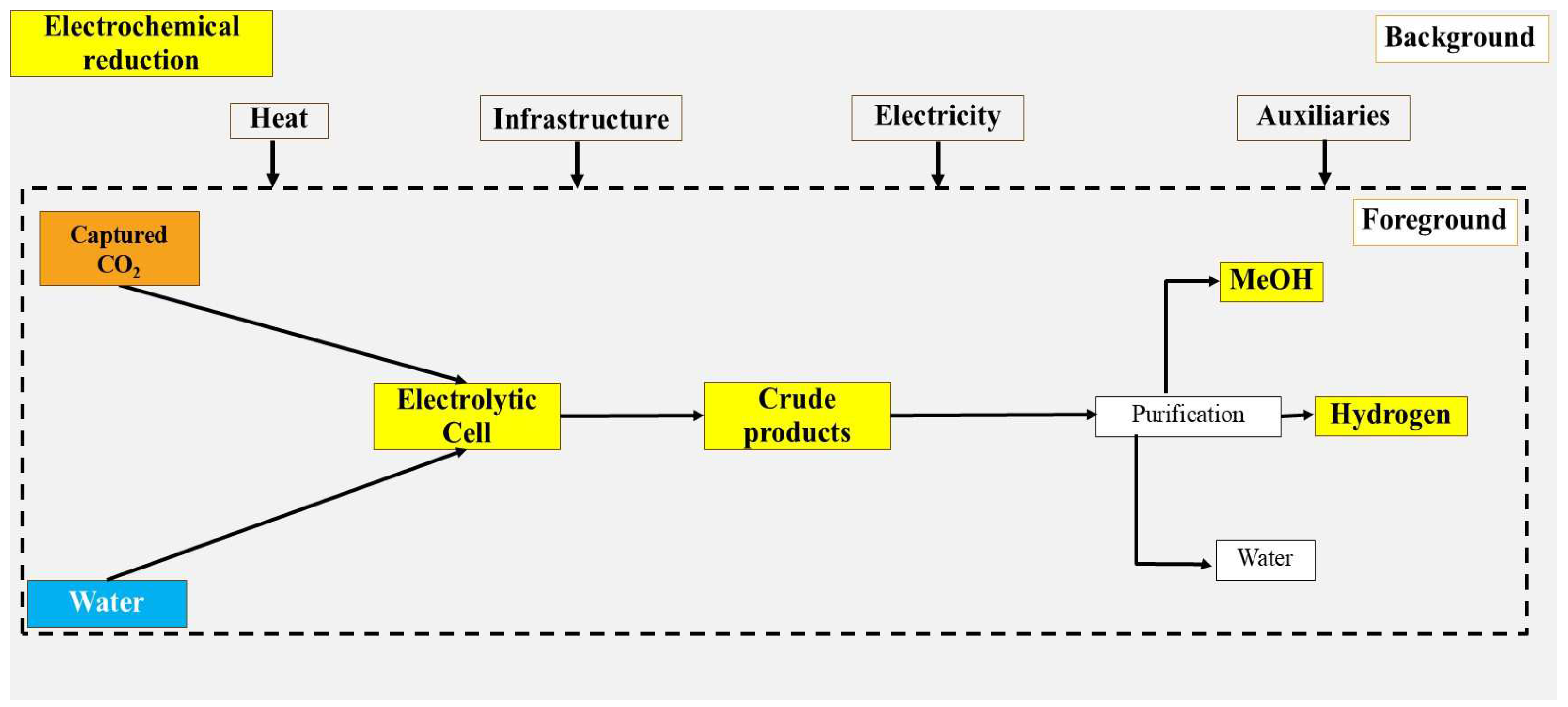
2.1.4. Route 4: Direct Electrochemical Reduction of CO2 to Methanol
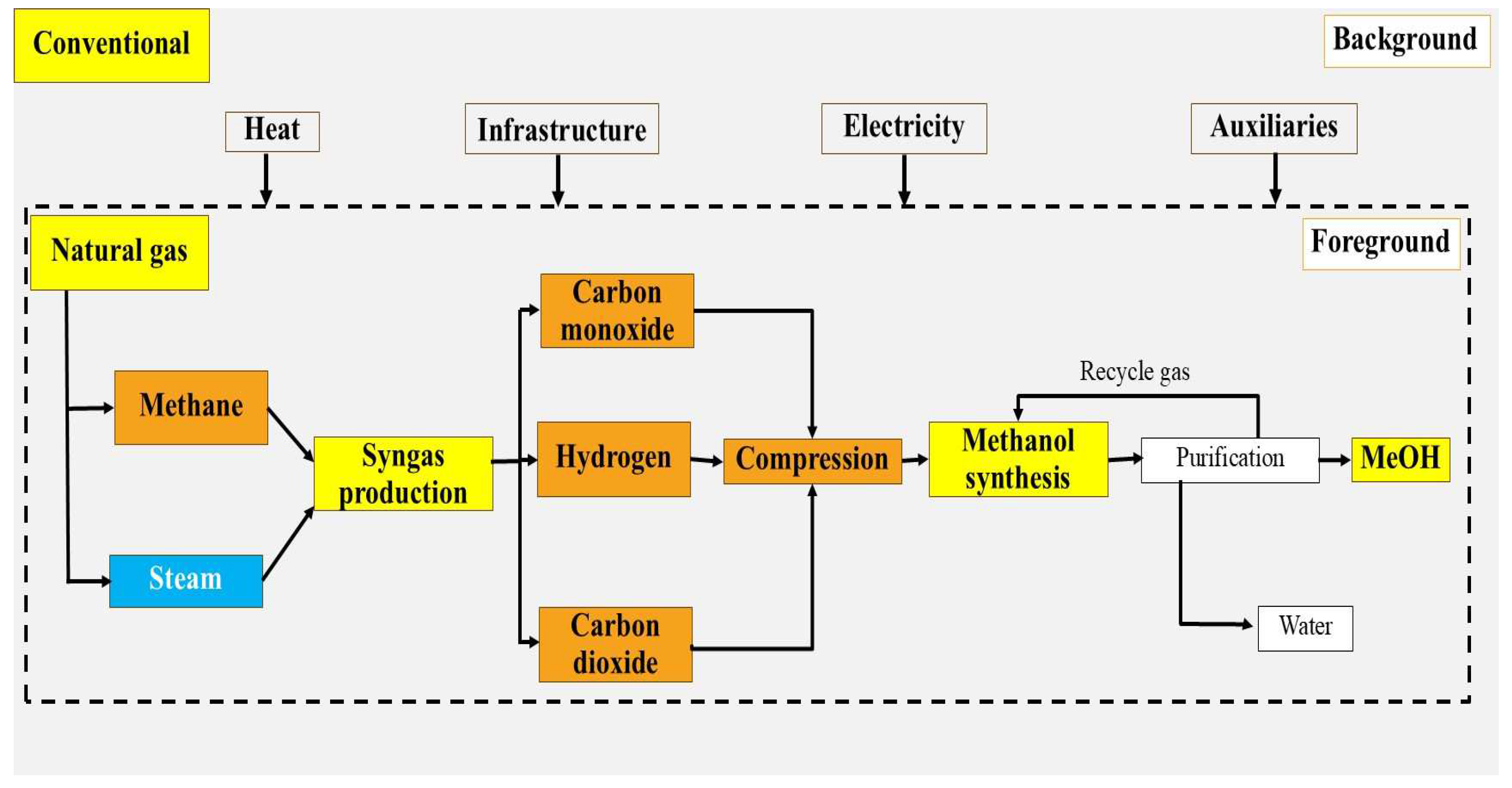
2.1.5. Route 5: Conventional Method
2.2. Source of Inputs and Allocation Method
2.3. Life Cycle Inventory (LCI) and Impact Categories
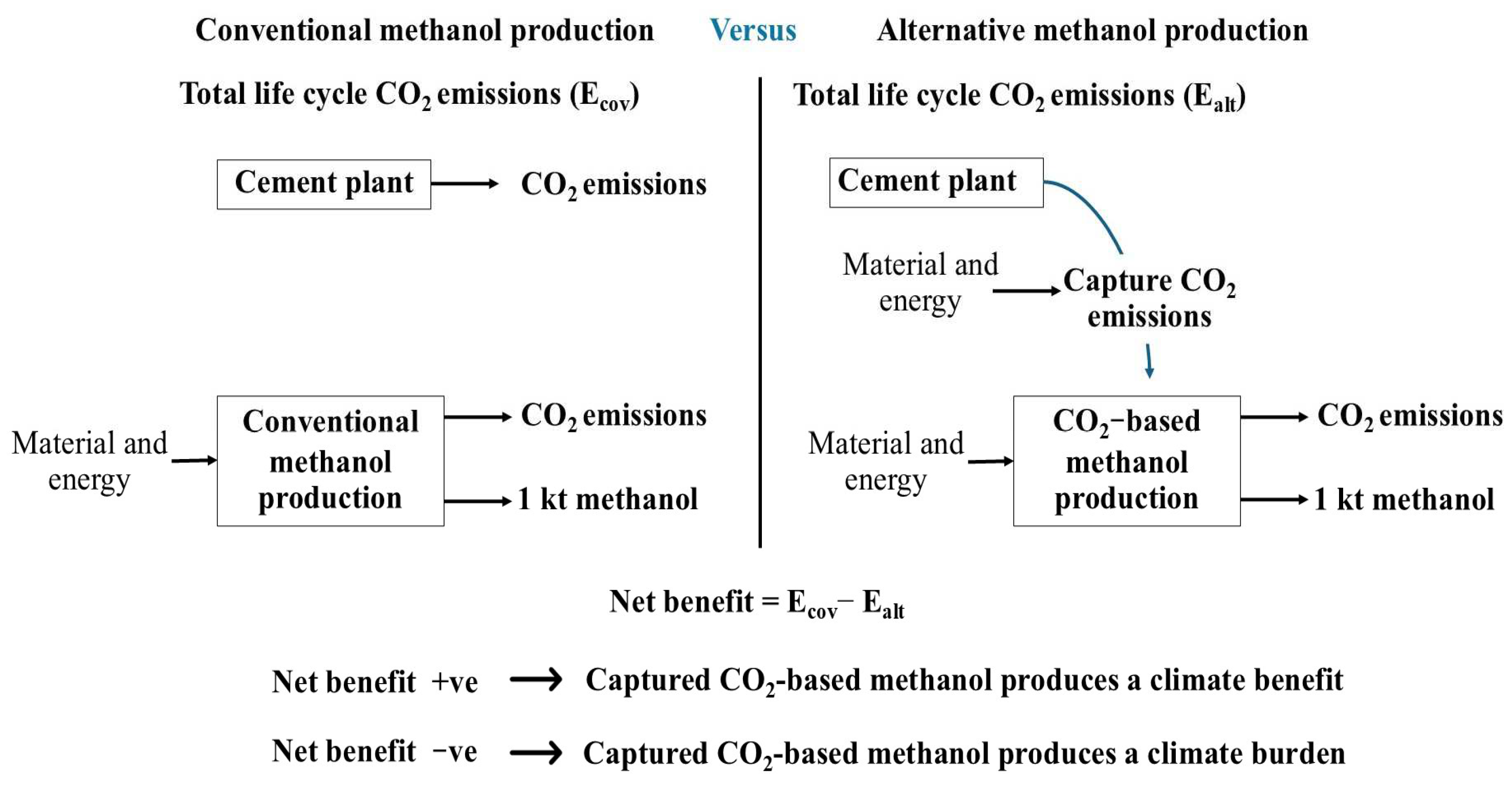
2.4. Net Reduction in Life Cycle CO2 Emissions
3. Results
3.1. Midpoint Impact Categories
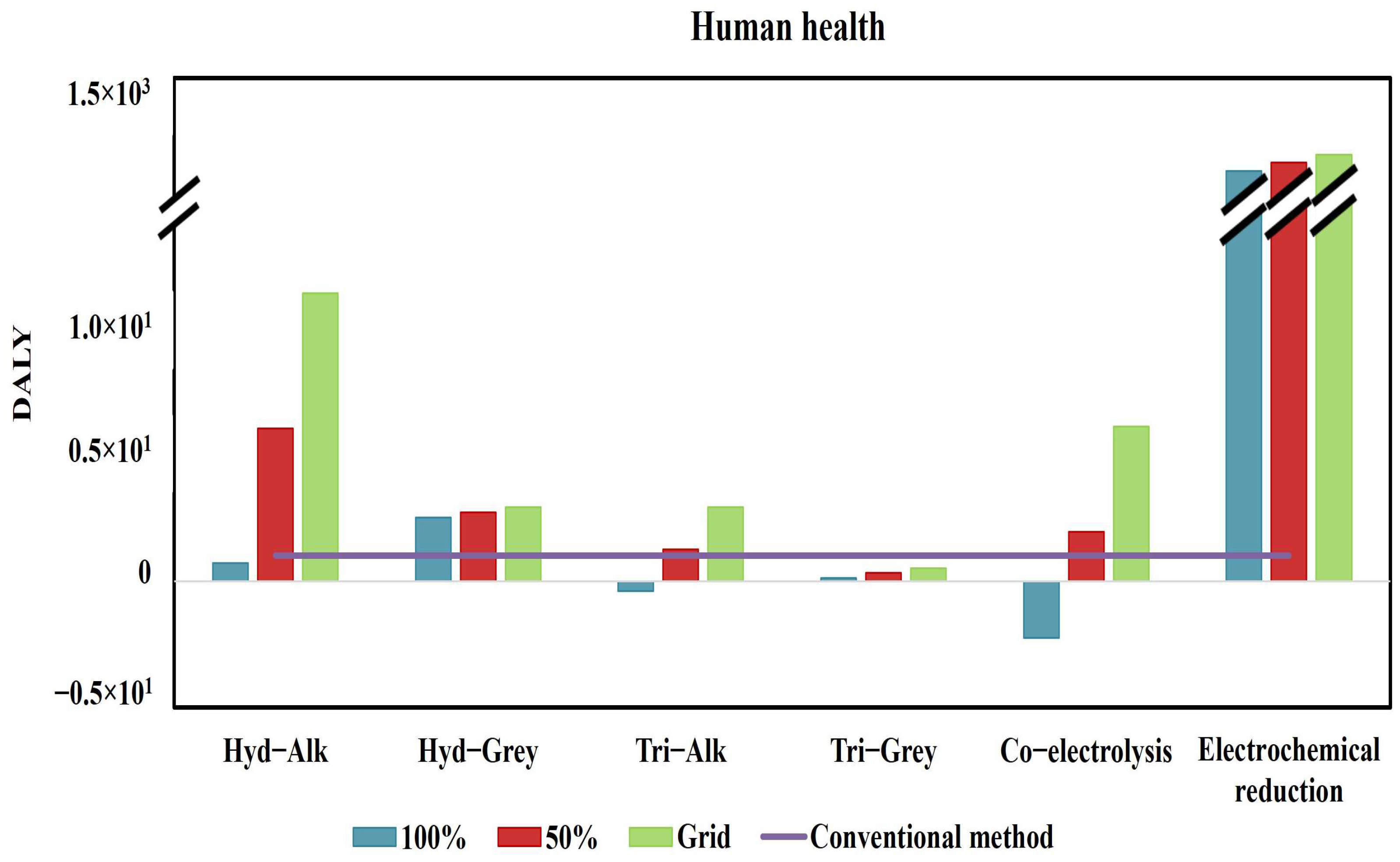
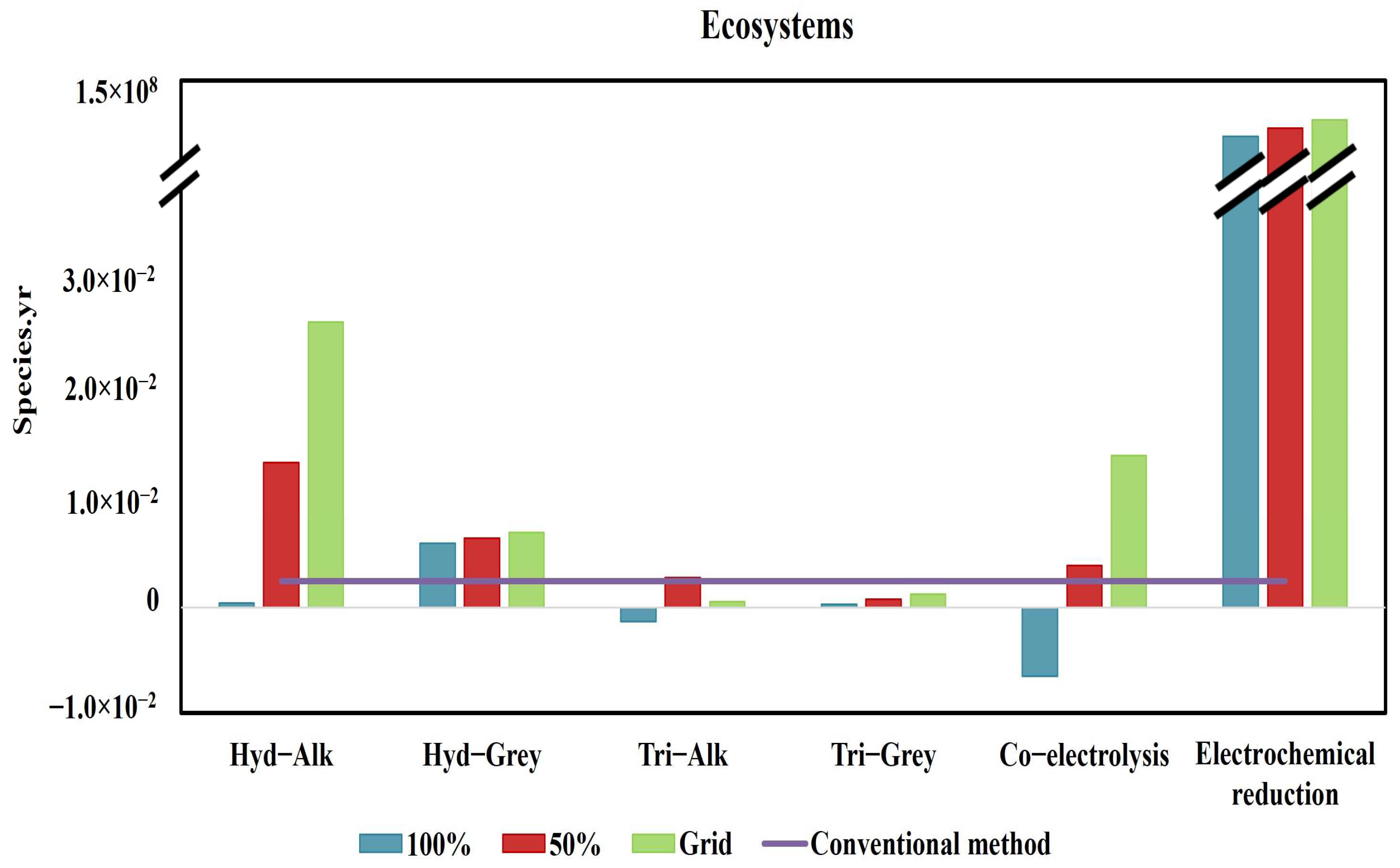
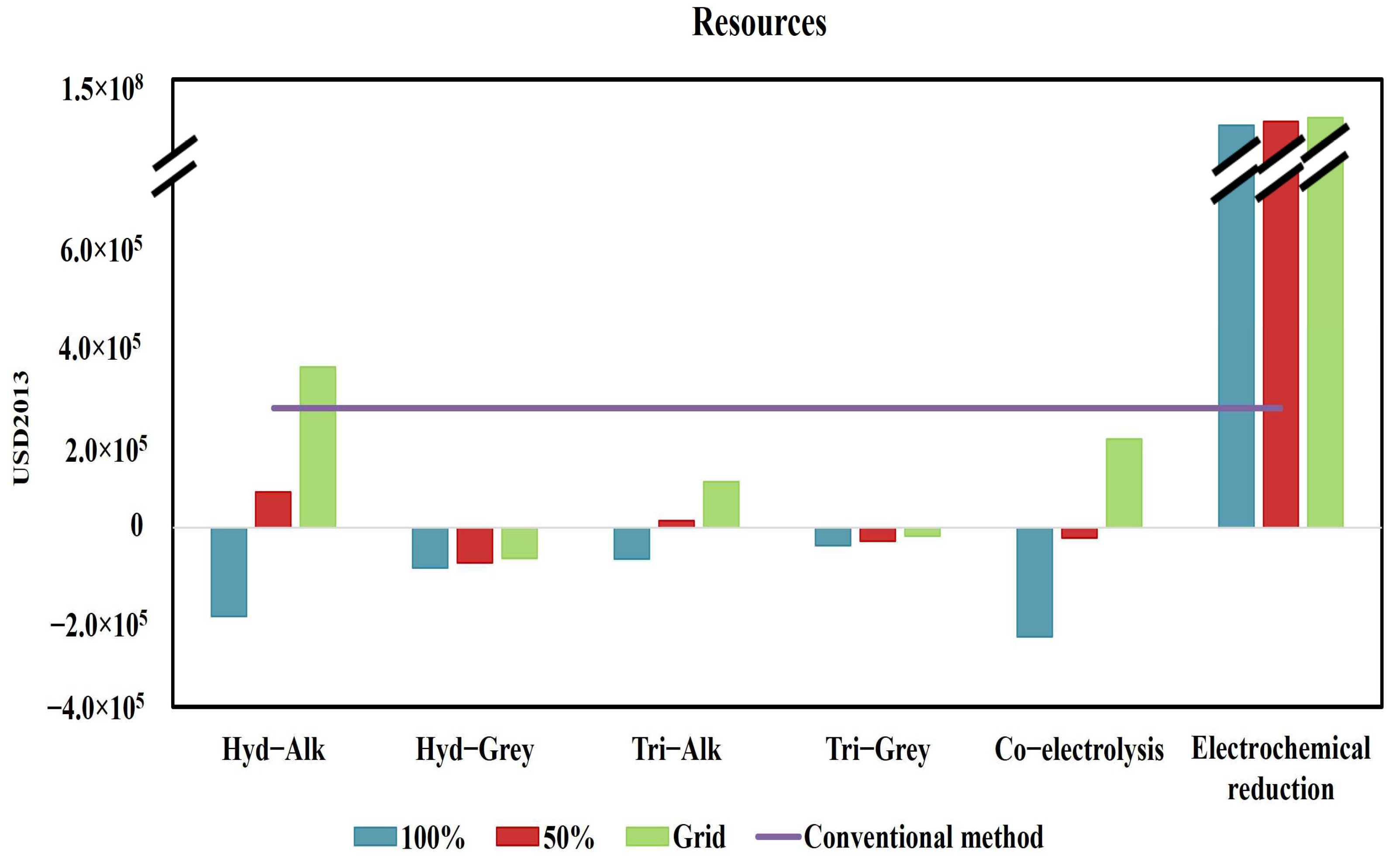
3.2. Endpoint Impact Categories
3.3. Application of Best-Case Scenario
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kreps, B.H. The rising costs of fossil-fuel extraction: An energy crisis that will not go away. Am. J. Econ. Sociol. 2020, 79, 695–717. [Google Scholar] [CrossRef]
- Day, C.; Day, G. Climate change, fossil fuel prices and depletion: The rationale for a falling export tax. Econ. Modell. 2017, 63, 153–160. [Google Scholar] [CrossRef]
- Wang, J.; Azam, W. Natural resource scarcity, fossil fuel energy consumption, and total greenhouse gas emissions in top emitting countries. Geosci. Front. 2024, 15, 101757. [Google Scholar] [CrossRef]
- Debating Science. The Effects of Arctic Offshore Drilling on Marine Ecosystems and Wildlife. 2016. Available online: https://websites.umass.edu/natsci397a-eross/the-effects-of-arctic-offshore-drilling-on-marine-ecosystems-and-wildlife/ (accessed on 2 August 2024).
- Organization of the Petroleum Exporting Countries. World Oil Outlook 2045. 2023. Available online: https://www.opec.org/opec_web/en/press_room/7042.htm (accessed on 24 August 2024).
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable energy and energy storage systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Chia, S.R.; Nomanbhay, S.; Ong, M.Y.; Chew, K.W.; Show, P.L. Renewable diesel as fossil fuel substitution in Malaysia: A review. Fuel 2022, 314, 123137. [Google Scholar] [CrossRef]
- Bertau, M.; Offermanns, H.; Plass, L.; Schmidt, F.; Wernicke, H.-J. Methanol: The Basic Chemical and Energy Feedstock of the Future; Springer: Berlin/Heidelberg, Germany, 2014; Volume 1. [Google Scholar]
- Dalena, F.; Senatore, A.; Marino, A.; Gordano, A.; Basile, M.; Basile, A. Chapter 1-Methanol Production and Applications: An Overview; Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 3–28. [Google Scholar]
- Simon Araya, S.; Liso, V.; Cui, X.; Li, N.; Zhu, J.; Sahlin, S.L.; Jensen, S.H.; Nielsen, M.P.; Kær, S.K. A review of the methanol economy: The fuel cell route. Energies 2020, 13, 596. [Google Scholar] [CrossRef]
- Olah, G.A.; Goeppert, A.; Prakash, G.S. Beyond Oil and Gas: The Methanol Economy; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Khojasteh-Salkuyeh, Y.; Ashrafi, O.; Mostafavi, E.; Navarri, P. CO2 utilization for methanol production; Part I: Process design and life cycle GHG assessment of different pathways. J. CO2 Util. 2021, 50, 101608. [Google Scholar] [CrossRef]
- Win, S.Y.; Opaprakasit, P.; Papong, S. Environmental and economic assessment of carbon capture and utilization at coal-fired power plant in Thailand. J. Clean. Prod. 2023, 414, 137595. [Google Scholar] [CrossRef]
- Tabibian, S.S.; Sharifzadeh, M. Statistical and analytical investigation of methanol applications, production technologies, value-chain and economy with a special focus on renewable methanol. Renew. Sust. Energy Rev. 2023, 179, 113281. [Google Scholar]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar]
- Solomon, M.B.; Rabha, S.S.; Fimbres-Weihs, G.; Goyal, H.; Taghikhah, F.R.; Varghese, J.J.; Wenger, S.R.; Liang, W.; Kearns, E.R.; Huang, J. Decarbonization in Australia and India: Bilateral opportunities and challenges for the net zero transformation. ACS Eng. Au 2024, 4, 295–311. [Google Scholar] [CrossRef]
- Langie, K.M.G.; Tak, K.; Kim, C.; Lee, H.W.; Park, K.; Kim, D.; Jung, W.; Lee, C.W.; Oh, H.-S.; Lee, D.K. Toward economical application of carbon capture and utilization technology with near-zero carbon emission. Nat. Commun. 2022, 13, 7482. [Google Scholar] [CrossRef]
- Song, B.; Liang, Y.; Zhou, Y.; Zhang, L.; Li, H.; Zhu, N.-X.; Tang, B.Z.; Zhao, D.; Liu, B. CO2-Based Stable Porous Metal–Organic Frameworks for CO2 Utilization. J. Am. Chem. Soc. 2024, 146, 14835–14843. [Google Scholar] [CrossRef]
- Pérez-Fortes, M.; Schöneberger, J.C.; Boulamanti, A.; Tzimas, E. Methanol synthesis using captured CO2 as raw material: Techno-economic and environmental assessment. Appl. Energy 2016, 161, 718–732. [Google Scholar] [CrossRef]
- Rajabloo, T.; Valee, J.; Marenne, Y.; Coppens, L.; De Ceuninck, W. Carbon capture and utilization for industrial applications. Energy Rep. 2023, 9, 111–116. [Google Scholar] [CrossRef]
- Moore, T.; Oyarzun, D.I.; Li, W.; Lin, T.Y.; Goldman, M.; Wong, A.A.; Jaffer, S.A.; Sarkar, A.; Baker, S.E.; Duoss, E.B. Electrolyzer energy dominates separation costs in state-of-the-art CO2 electrolyzers: Implications for single-pass CO2 utilization. Joule 2023, 7, 782–796. [Google Scholar] [CrossRef]
- Idem, R.; Supap, T.; Shi, H.; Gelowitz, D.; Ball, M.; Campbell, C.; Tontiwachwuthikul, P. Practical experience in post-combustion CO2 capture using reactive solvents in large pilot and demonstration plants. Int. J. Greenh. Gas Control 2015, 40, 6–25. [Google Scholar] [CrossRef]
- Rezaei, S.; Liu, A.; Hovington, P. Emerging technologies in post-combustion carbon dioxide capture & removal. Catal. Today 2023, 423, 114286. [Google Scholar]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar]
- Theofanidis, S.A.; Antzaras, A.N.; Lemonidou, A.A. CO2 as a building block: From capture to utilization. Curr. Opin. Chem. Eng. 2023, 39, 100902. [Google Scholar] [CrossRef]
- Dang, S.; Yang, H.; Gao, P.; Wang, H.; Li, X.; Wei, W.; Sun, Y. A review of research progress on heterogeneous catalysts for methanol synthesis from carbon dioxide hydrogenation. Catal. Today. 2019, 330, 61–75. [Google Scholar] [CrossRef]
- Rumayor, M.; Dominguez-Ramos, A.; Irabien, A. Innovative alternatives to methanol manufacture: Carbon footprint assessment. J. Clean. Prod. 2019, 225, 426–434. [Google Scholar] [CrossRef]
- Dueñas, D.M.A.; Riedel, M.; Riegraf, M.; Costa, R.; Friedrich, K.A. High temperature co-electrolysis for power-to-X. Chem. Ing. Tech. 2020, 92, 45–52. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y. Technological development of hydrogen production by solid oxide electrolyzer cell (SOEC). Int. J. Hydrogen Energy 2008, 33, 2337–2354. [Google Scholar] [CrossRef]
- Leonzio, G. Design and feasibility analysis of a Power-to-Gas plant in Germany. J. Clean. Prod. 2017, 162, 609–623. [Google Scholar]
- Sterner, M.; Stadler, I. Energiespeicher-Bedarf, Technologien, Integration; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Zhang, X.; Song, Y.; Wang, G.; Bao, X. Co-electrolysis of CO2 and H2O in high-temperature solid oxide electrolysis cells: Recent advance in cathodes. J. Energy Chem. 2017, 26, 839–853. [Google Scholar] [CrossRef]
- Hoppe, W.; Thonemann, N.; Bringezu, S. Life cycle assessment of carbon dioxide–based production of methane and methanol and derived polymers. J. Ind. Ecol. 2018, 22, 327–340. [Google Scholar] [CrossRef]
- Nimmanterdwong, P.; Piumsomboon, P.; Chalermsinsuwan, B. Emergy investigation of carbon dioxide utilization processes for methanol synthesis. J. Environ. Chem. Eng. 2022, 10, 108063. [Google Scholar] [CrossRef]
- Rosental, M.; Fröhlich, T.; Liebich, A. Life cycle assessment of carbon capture and utilization for the production of large volume organic chemicals. Front. Clim. 2020, 2, 586199. [Google Scholar] [CrossRef]
- Michailos, S.; Sanderson, P.; Villa Zaragoza, A.; McCord, S.; Armstrong, K.; Styring, P.; Mason, F.; Stokes, G.; Williams, E.; Zimmermann, A. Methanol Worked Examples for the TEA and LCA Guidelines for CO2 Utilization; University of Michigan Library: Ann Arbor, MI, USA, 2018. [Google Scholar] [CrossRef]
- ISO 14040; Environmental Management—Life Cycle Assessment: Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044; Environmental Management—Life Cycle Assessment: Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Kreuter, W.; Hofmann, H. Electrolysis: The important energy transformer in a world of sustainable energy. Int. J. Hydrogen Energy 1998, 23, 661–666. [Google Scholar] [CrossRef]
- Mergel, J.; Carmo, M.; Fritz, D. Status on technologies for hydrogen production by water electrolysis. In Transition to Renewable Energy Systems; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2013; pp. 423–450. [Google Scholar]
- Schiebahn, S.; Grube, T.; Robinius, M.; Tietze, V.; Kumar, B.; Stolten, D. Power to gas: Technological overview, systems analysis and economic assessment for a case study in Germany. Int. J. Hydrogen Energy 2015, 40, 4285–4294. [Google Scholar] [CrossRef]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydrogen Energy. 2013, 38, 4901–4934. [Google Scholar]
- Sarp, S.; Hernandez, S.G.; Chen, C.; Sheehan, S.W. Alcohol production from carbon dioxide: Methanol as a fuel and chemical feedstock. Joule 2021, 5, 59–76. [Google Scholar]
- Song, C.; Pan, W. Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today 2004, 98, 463–484. [Google Scholar] [CrossRef]
- Cho, W.; Song, T.; Mitsos, A.; McKinnon, J.T.; Ko, G.H.; Tolsma, J.E.; Denholm, D.; Park, T. Optimal design and operation of a natural gas tri-reforming reactor for DME synthesis. Catal. Today 2009, 139, 261–267. [Google Scholar] [CrossRef]
- Goeppert, A.; Czaun, M.; Jones, J.-P.; Prakash, G.S.; Olah, G.A. Recycling of carbon dioxide to methanol and derived products–closing the loop. Chem. Soc. Rev. 2014, 43, 7995–8048. [Google Scholar]
- Arora, S.; Prasad, R. An overview on dry reforming of methane: Strategies to reduce carbonaceous deactivation of catalysts. RSC Adv. 2016, 6, 108668–108688. [Google Scholar] [CrossRef]
- Speight, J.G. Gasification of Unconventional Feedstocks; Gulf Professional Publishing: Waltham, MA, USA, 2014. [Google Scholar]
- Kumar, R.; Kumar, K.; Pant, K.; Choudary, N. Tuning the metal-support interaction of methane tri-reforming catalysts for industrial flue gas utilization. Int. J. Hydrogen Energy 2020, 45, 1911–1929. [Google Scholar] [CrossRef]
- Challiwala, M.S.; Ghouri, M.M.; Sengupta, D.; El-Halwagi, M.M.; Elbashir, N.O. A process integration approach to the optimization of CO2 utilization via tri-reforming of methane. Comput. Aided Chem. Eng. 2017, 40, 1993–1998. [Google Scholar]
- Rezaei, E.; Catalan, L.J. Evaluation of CO2 utilization for methanol production via tri-reforming of methane. J. CO2 Util. 2020, 42, 101272. [Google Scholar] [CrossRef]
- Usman, M.; Daud, W.W. Recent advances in the methanol synthesis via methane reforming processes. RSC Adv. 2015, 5, 21945–21972. [Google Scholar] [CrossRef]
- Shi, C.; Labbaf, B.; Mostafavi, E.; Mahinpey, N. Methanol production from water electrolysis and tri-reforming: Process design and technical-economic analysis. J. CO2 Util. 2020, 38, 241–251. [Google Scholar] [CrossRef]
- Wang, L.; Rao, M.; Diethelm, S.; Lin, T.-E.; Zhang, H.; Hagen, A.; Maréchal, F. Power-to-methane via co-electrolysis of H2O and CO2: The effects of pressurized operation and internal methanation. Appl. Energy 2019, 250, 1432–1445. [Google Scholar] [CrossRef]
- Albarelli, J.Q.; Onorati, S.; Caliandro, P.; Peduzzi, E.; Meireles, M.A.A.; Marechal, F.; Ensinas, A.V. Multi-objective optimization of a sugarcane biorefinery for integrated ethanol and methanol production. Energy 2017, 138, 1281–1290. [Google Scholar] [CrossRef]
- Phillips, V.; Kinoshita, C.; Neill, D.; Takahashi, P. Thermochemical production of methanol from biomass in Hawaii. Appl. Energy 1990, 35, 167–175. [Google Scholar] [CrossRef]
- Becker, W.; Braun, R.; Penev, M.; Melaina, M. Production of Fischer–Tropsch liquid fuels from high temperature solid oxide co-electrolysis units. Energy 2012, 47, 99–115. [Google Scholar] [CrossRef]
- Wang, L.; Pérez-Fortes, M.; Madi, H.; Diethelm, S.; Maréchal, F. Optimal design of solid-oxide electrolyzer based power-to-methane systems: A comprehensive comparison between steam electrolysis and co-electrolysis. Appl. Energy 2018, 211, 1060–1079. [Google Scholar]
- Ertl, G.; Knözinger, H.; Schüth, F.; Weitkamp, J. Handbook of Heterogeneous Catalysis; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Tijm, P.; Waller, F.; Brown, D. Methanol technology developments for the new millennium. Appl. Catal. 2001, 221, 275–282. [Google Scholar] [CrossRef]
- Wilkinson, S.; Van De Water, L.; Miller, B.; Simmons, M.; Stitt, E.H.; Watson, M. Understanding the generation of methanol synthesis and water gas shift activity over copper-based catalysts–A spatially resolved experimental kinetic study using steady and non-steady state operation under CO/CO2/H2 feeds. J. Catal. 2016, 337, 208–220. [Google Scholar] [CrossRef]
- Albo, J.; Irabien, A. Cu2O-loaded gas diffusion electrodes for the continuous electrochemical reduction of CO2 to methanol. J. Catal. 2016, 343, 232–239. [Google Scholar] [CrossRef]
- Deka, T.J.; Osman, A.I.; Baruah, D.C.; Rooney, D.W. Methanol fuel production, utilization, and techno-economy: A review. Environ. Chem. Lett. 2022, 20, 3525–3554. [Google Scholar] [CrossRef]
- Pirola, C.; Bozzano, G.; Manenti, F. Chapter 3-Fossil or Renewable Sources for Methanol Production? Methanol; Elsevier: Amsterdam, The Netherlands, 2018; pp. 53–93. [Google Scholar]
- González-Garay, A.; Frei, M.S.; Al-Qahtani, A.; Mondelli, C.; Guillén-Gosálbez, G.; Pérez-Ramírez, J. Plant-to-planet analysis of CO2-based methanol processes. Energy Environ. Sci. 2019, 12, 3425–3436. [Google Scholar] [CrossRef]
- Maina, J.W.; Pringle, J.M.; Razal, J.M.; Nunes, S.; Vega, L.; Gallucci, F.; Dumée, L.F. Strategies for integrated capture and conversion of CO2 from dilute flue gases and the atmosphere. ChemSusChem 2021, 14, 1805–1820. [Google Scholar] [CrossRef]
- Zhao, H.; Luo, X.; Zhang, H.; Sun, N.; Wei, W.; Sun, Y. Carbon-based adsorbents for post-combustion capture: A review. Greenhouse Gas. Sci. Technol. 2018, 8, 11–36. [Google Scholar] [CrossRef]
- Wu, C.; Huang, Q.; Xu, Z.; Sipra, A.T.; Gao, N.; de Souza Vandenberghe, L.P.; Vieira, S.; Soccol, C.R.; Zhao, R.; Deng, S. A comprehensive review of carbon capture science and technologies. Carbon Capture Sci. Technol. 2024, 11, 100178. [Google Scholar]
- BUR4. The UNFCCC Secretariat (UN Climate Change). Thailand. 2022. Available online: https://unfccc.int/sites/default/files/resource/Thailand_BUR4_final_28122022.pdf (accessed on 15 June 2024).
- Rochelle, G.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar] [CrossRef]
- Abu-Zahra, M.R.; Schneiders, L.H.; Niederer, J.P.; Feron, P.H.; Versteeg, G.F. CO2 capture from power plants: Part I. A parametric study of the technical performance based on monoethanolamine. Int. J. Greenh. Gas Control 2007, 1, 37–46. [Google Scholar] [CrossRef]
- Liang, Z.H.; Rongwong, W.; Liu, H.; Fu, K.; Gao, H.; Cao, F.; Zhang, R.; Sema, T.; Henni, A.; Sumon, K. Recent progress and new developments in post-combustion carbon-capture technology with amine based solvents. Int. J. Greenh. Gas Control 2015, 40, 26–54. [Google Scholar]
- Wu, X.; Yu, Y.; Qin, Z.; Zhang, Z. The advances of post-combustion CO2 capture with chemical solvents: Review and guidelines. Energy Proc. 2014, 63, 1339–1346. [Google Scholar] [CrossRef]
- Dave, N.; Do, T.; Palfreyman, D.; Feron, P. Impact of liquid absorption process development on the costs of post-combustion capture in Australian coal-fired power stations. Chem. Eng. Res. Des. 2011, 89, 1625–1638. [Google Scholar] [CrossRef]
- Dumée, L.; Scholes, C.; Stevens, G.; Kentish, S. Purification of aqueous amine solvents used in post combustion CO2 capture: A review. Int. J. Greenh. Gas Control 2012, 10, 443–455. [Google Scholar] [CrossRef]
- Beysel, G. Enhanced cryogenic air separation—a proven process applied to oxyfuel. In Proceedings of the 1st Oxyfuel Combustion Conference, Cottbus, Germany, 8–11 September 2009. [Google Scholar]
- Patel, G.H.; Havukainen, J.; Horttanainen, M.; Soukka, R.; Tuomaala, M. Climate change performance of hydrogen production based on life cycle assessment. Green Chem. 2024, 26, 992–1006. [Google Scholar] [CrossRef]
- Rodríguez-Vallejo, D.F.; Valente, A.; Guillén-Gosálbez, G.; Chachuat, B. Economic and life-cycle assessment of OME 3–5 as transport fuel: A comparison of production pathways. Sustain. Energy Fuels 2021, 5, 2504–2516. [Google Scholar] [CrossRef]
- Gerloff, N. Comparative Life-Cycle-Assessment analysis of three major water electrolysis technologies while applying various energy scenarios for a greener hydrogen production. J. Energy Storage 2021, 43, 102759. [Google Scholar] [CrossRef]
- Koornneef, J.; van Keulen, T.; Faaij, A.; Turkenburg, W. Life cycle assessment of a pulverized coal power plant with post-combustion capture, transport and storage of CO2. Int. J. Greenh. Gas Control 2008, 2, 448–467. [Google Scholar] [CrossRef]
- Zhang, H.; Desideri, U. Techno-economic optimization of power-to-methanol with co-electrolysis of CO2 and H2O in solid-oxide electrolyzers. Energy 2020, 199, 117498. [Google Scholar] [CrossRef]
- Huijbregts, M.A.; Steinmann, Z.J.; Elshout, P.M.; Stam, G.; Verones, F.; Vieira, M.; Zijp, M.; Hollander, A.; Van Zelm, R. ReCiPe 2016: A Harmonized Life Cycle Impact Assessment Method at Midpoint and Endpoint Level, Report I: Characterization. Available online: https://www.rivm.nl/bibliotheek/rapporten/2016-0104.pdf (accessed on 21 July 2024).
- Ravikumar, D.; Keoleian, G.A.; Miller, S.A.; Sick, V. Assessing the relative climate impact of carbon utilization for concrete, chemical, and mineral production. Environ. Sci. Technol. 2021, 55, 12019–12031. [Google Scholar] [CrossRef]
- Reccessary. Thailand’s New Power Plan Aims for 51% Renewable Energy by 2037. 2024. Available online: https://www.reccessary.com/en/news/th-regulation/thailand-new-power-development-plan-raises-renewable-energy-targets (accessed on 8 August 2024).
- Chemanalyst. Decode the Future of Methanol. 2024. Available online: https://www.chemanalyst.com/industry-report/thailand-methanol-market-200 (accessed on 7 August 2024).
- Schorn, F.; Breuer, J.L.; Samsun, R.C.; Schnorbus, T.; Heuser, B.; Peters, R.; Stolten, D. Methanol as a renewable energy carrier: An assessment of production and transportation costs for selected global locations. Adv. Appl. Energy 2021, 3, 100050. [Google Scholar] [CrossRef]


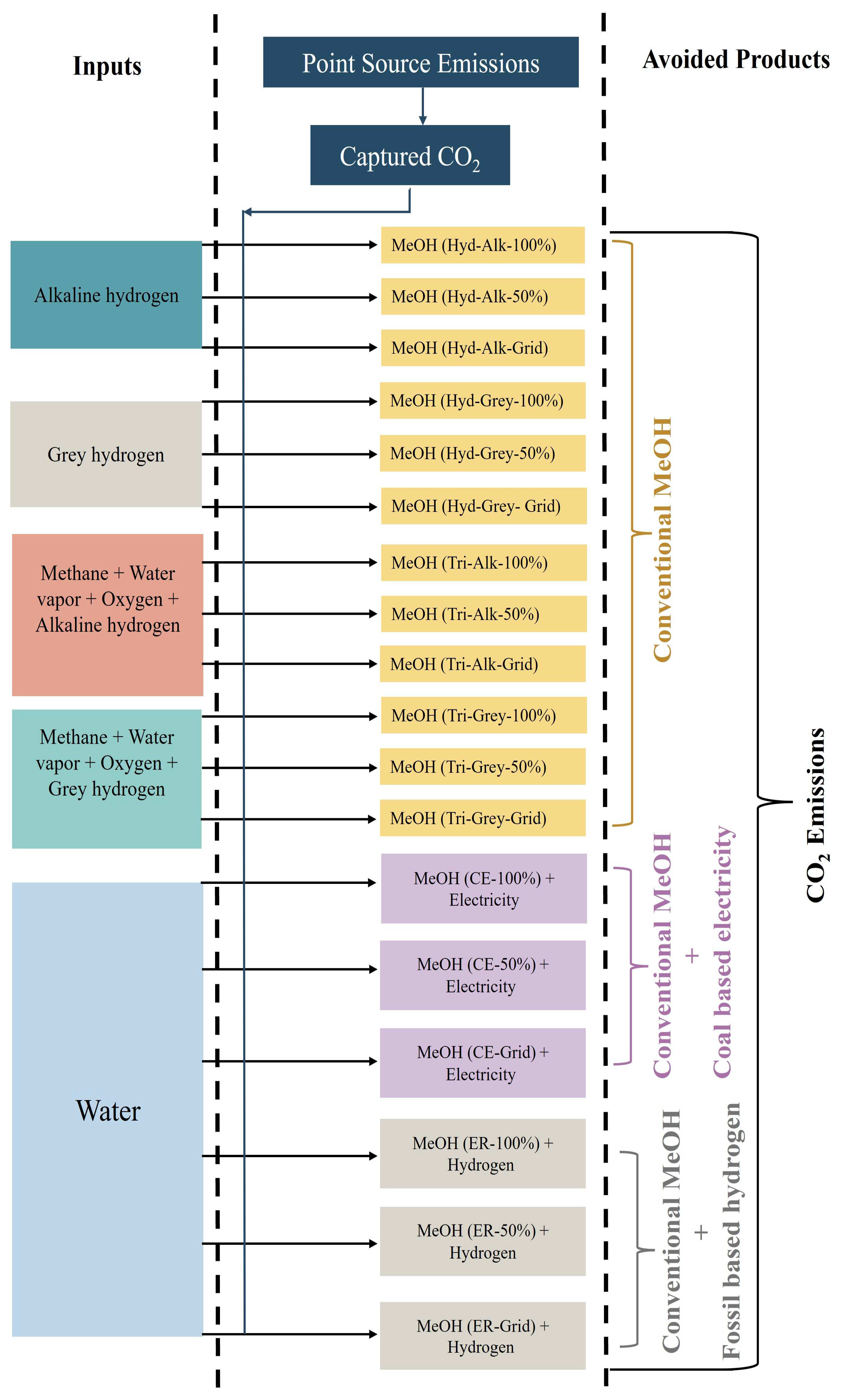


| Scenarios | 100% Renewable | 50% Renewable + 50% Grid | 100% Grid | Conventional Method |
|---|---|---|---|---|
| Hydrogenation with alkaline hydrogen | Scenario 1 (Hyd-Alk-100%) | Scenario 2 (Hyd-Alk-50%) | Scenario 3 (Hyd-Alk-Grid) | Scenario 19 (Conv-SMR) |
| Hydrogenation with grey hydrogen | Scenario 4 (Hyd-Grey-100%) | Scenario 5 (Hyd-Grey-50%) | Scenario 6 (Hyd-Grey-Grid) | |
| Tri-reforming with alkaline hydrogen | Scenario 7 (Tri-Alk-100%) | Scenario 8 (Tri-Alk-50%) | Scenario 9 (Tri-Alk-Grid) | |
| Tri-reforming with grey hydrogen | Scenario 10 (Tri-Grey-100%) | Scenario 11 (Tri-Grey-50%) | Scenario 12 (Tri-Grey-Grid) | |
| Co-electrolysis | Scenario 13 (CE-100%) | Scenario 14 (CE-50%) | Scenario 15 (CE-Grid) | |
| Electrochemical reduction | Scenario 16 (ER-100%) | Scenario 17 (ER-50%) | Scenario 18 (ER-Grid) |
| Technologies | Advantages | Disadvantages |
|---|---|---|
| Hydrogenation |
|
|
| Tri-reforming |
|
|
| Co-electrolysis |
|
|
| Electrochemical reduction |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafiq, A.; Farooq, A.; Gheewala, S.H. Life Cycle Assessment of CO2-Based and Conventional Methanol Production Pathways in Thailand. Processes 2024, 12, 1868. https://doi.org/10.3390/pr12091868
Rafiq A, Farooq A, Gheewala SH. Life Cycle Assessment of CO2-Based and Conventional Methanol Production Pathways in Thailand. Processes. 2024; 12(9):1868. https://doi.org/10.3390/pr12091868
Chicago/Turabian StyleRafiq, Adeel, Ahsan Farooq, and Shabbir. H. Gheewala. 2024. "Life Cycle Assessment of CO2-Based and Conventional Methanol Production Pathways in Thailand" Processes 12, no. 9: 1868. https://doi.org/10.3390/pr12091868
APA StyleRafiq, A., Farooq, A., & Gheewala, S. H. (2024). Life Cycle Assessment of CO2-Based and Conventional Methanol Production Pathways in Thailand. Processes, 12(9), 1868. https://doi.org/10.3390/pr12091868







