Scaling up the Natural Mode of Action of Macrophyte Allelochemicals and Their Effect on Toxic Cyanobacteria Using a Nitrogen-Limited Chemostat
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Microalgae Strain and Medium
2.3. Experimental Design of Continuous Culture in Chemostat Mode
2.4. Determination of M. aeruginosa Growth, Oxidative Stress Parameters, and Toxicity
2.5. Identification and Quantification of Phenolic Compounds by HPLC
2.6. Statistical Analysis
3. Results and Discussion
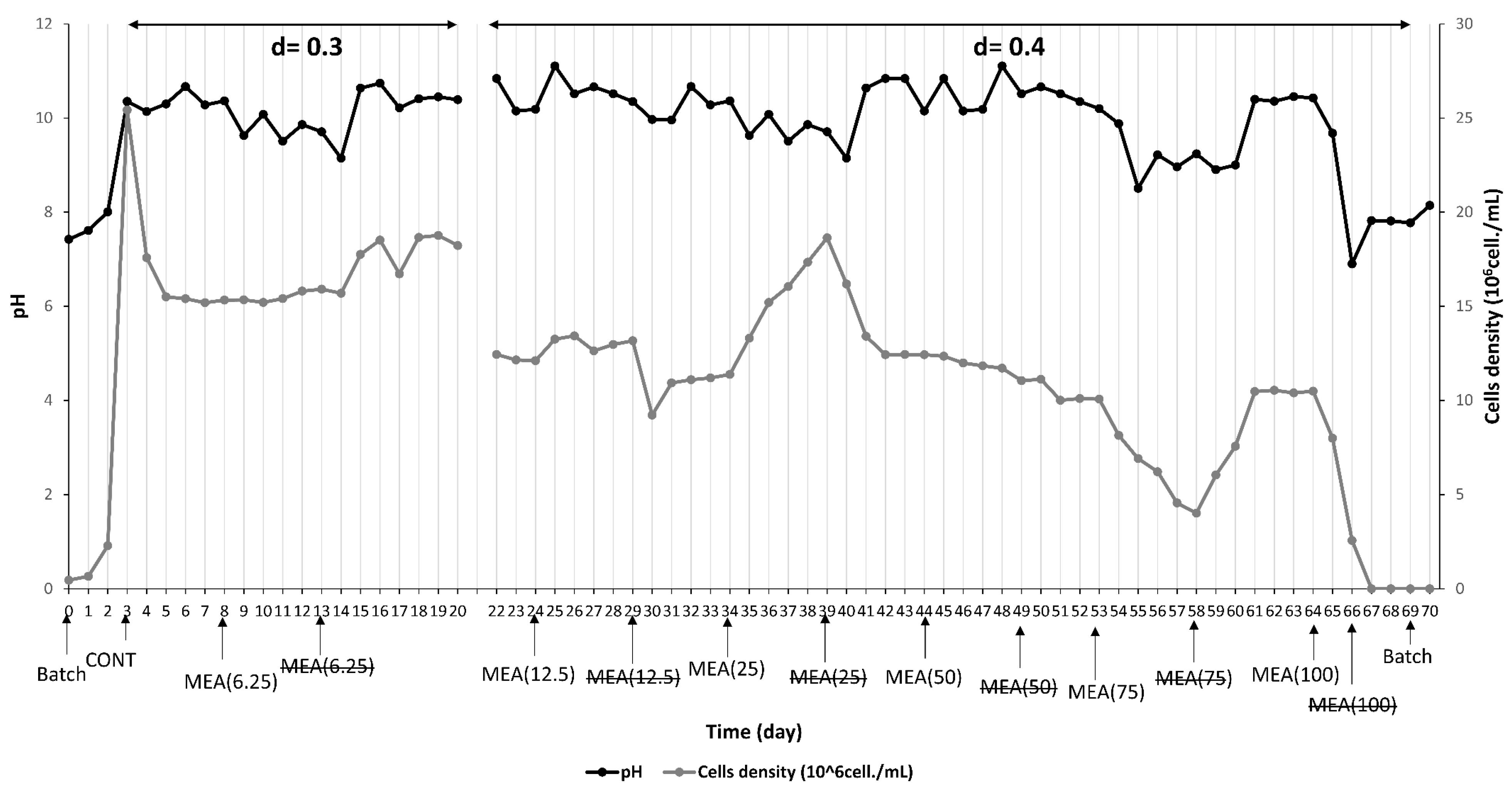
3.1. Effect of M. spicatum Extract on M. aeruginosa Growth
| Plant Material | Type of Extracts | Target Organism | Mode of Culture | Tested Concentration | Inhibitory Percentage | References |
|---|---|---|---|---|---|---|
| Acorus calamus | hexane extract of rhizome | M. aeruginosa | Batch | 20 mg/L | %IR = 100 | [33] |
| R. aquatilis and N. officinale | aqueous extract | M. aeruginosa | Batch | 75% of AE | %IR = 100 %IR = 95 | [4] |
| Spartina alterniflora | aqueous extract | M. aeruginosa | Batch | 150 mg/L | %IR = 99.4 | [28] |
| Cyperus alternifolius and Canna generalis | extracts of culture solutions | M. aeruginosa | Semi-continuous co-culture system | 2.34 mg/L | %IR = 99.6 | [34] |
| P. cristatus, P. maackianus, P. lucens, V. spinulosa, C. demersum, and H. verticillata | volatile compounds | M. aeruginosa | Batch | 50 mg/L | %IR = 30.2–41.7 | [35] |
| M. spicatum | ethyl acetate extract | M. aeruginosa | Continuous system | 75 mg/L 100 mg/L | %IR = 50 %IR = 100 | In this study |
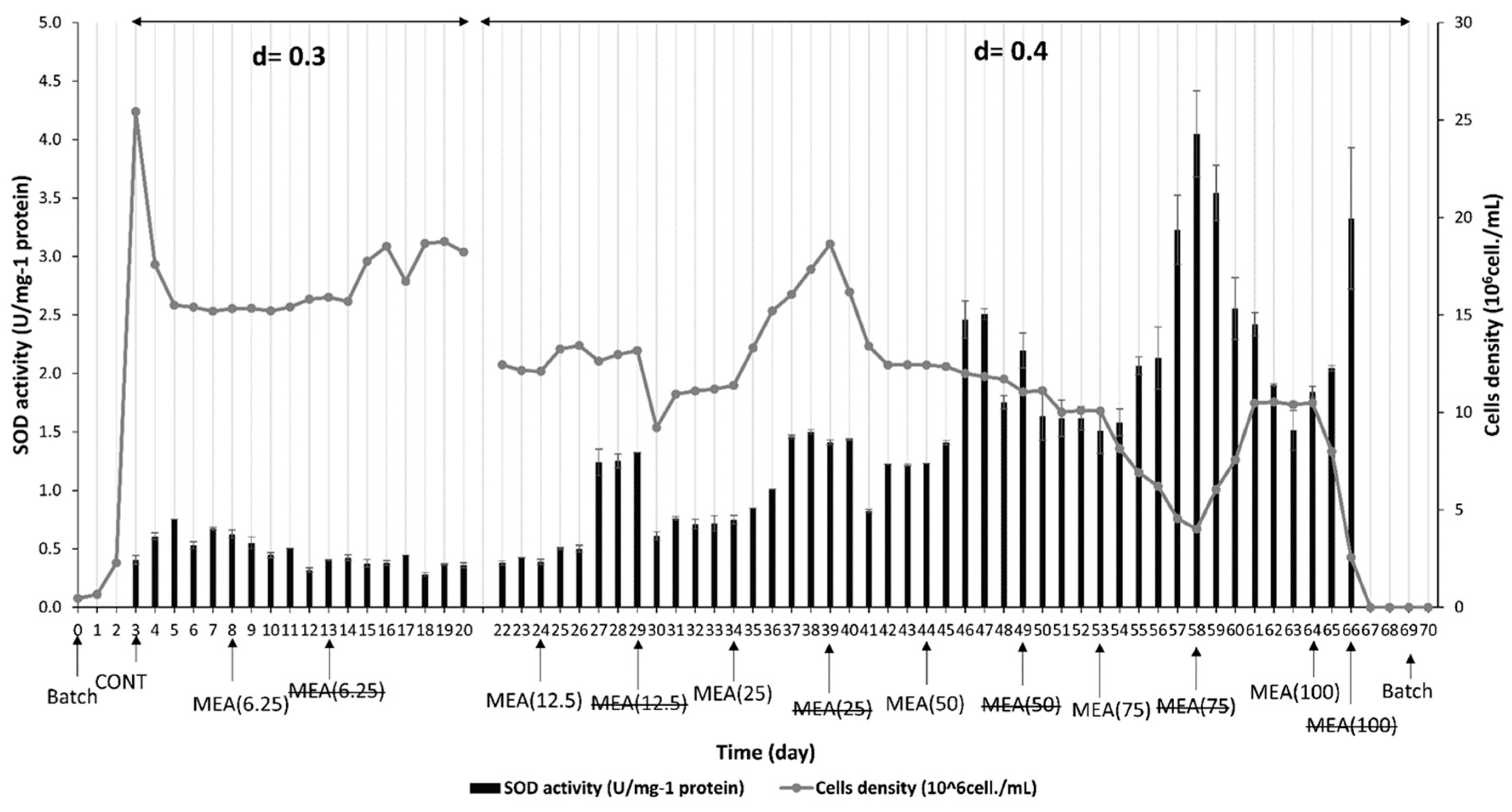
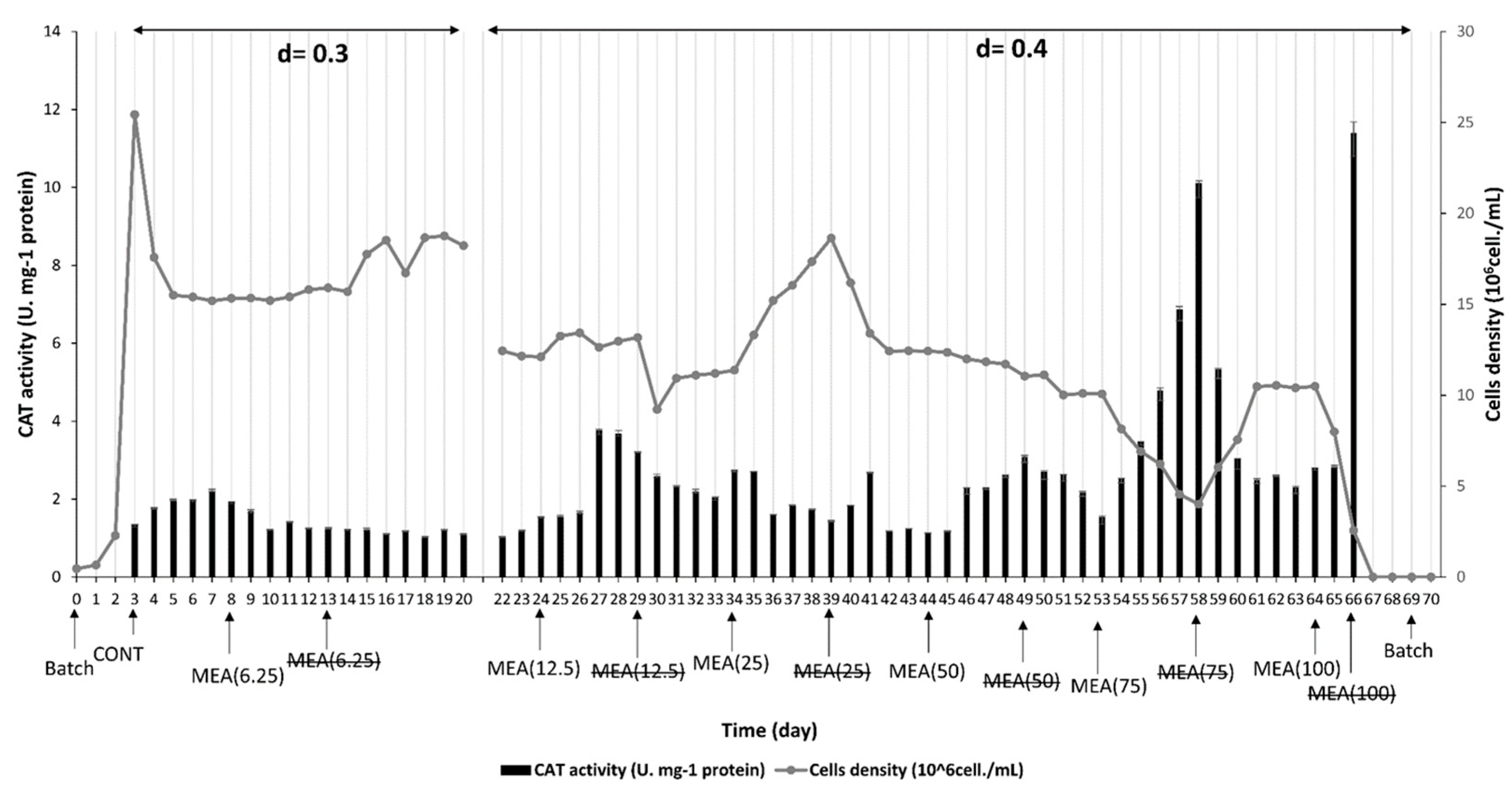
3.2. Effect of M. spicatum Extract on M. aeruginosa Physiological Responses
3.3. Microcystin Analysis
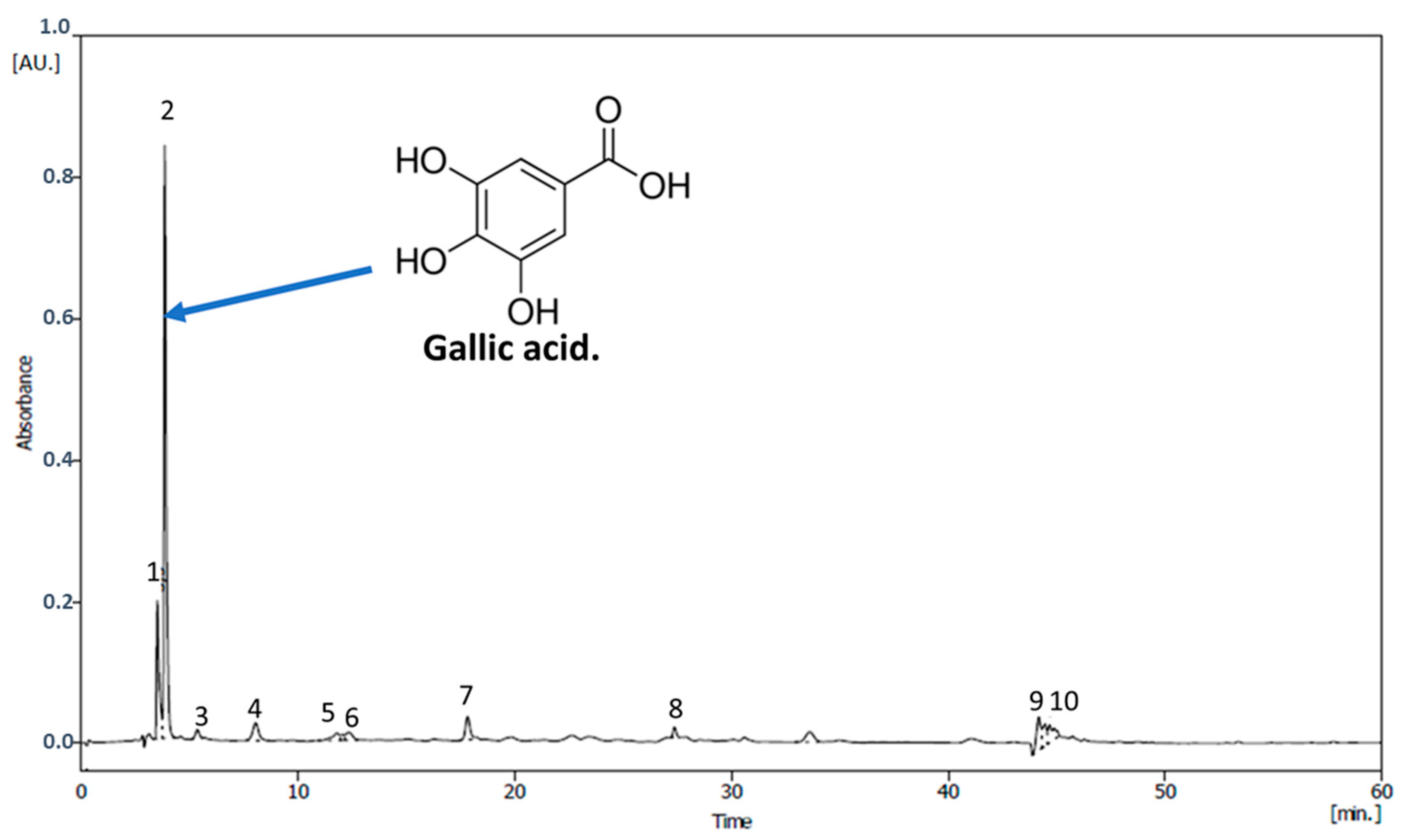
3.4. Phenolic Compounds of M. spicatum Extract
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carmichael, W.W.; Boyer, G.L. Health Impacts from Cyanobacteria Harmful Algae Blooms: Implications for the North American Great Lakes. Harmful Algae 2016, 54, 194–212. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Gobler, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.W. A Review of the Global Ecology, Genomics, and Biogeography of the Toxic Cyanobacterium, Microcystis Spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Ndlela, L.L.; Oberholster, P.J.; Van Wyk, J.H.; Cheng, P.-H.H. An Overview of Cyanobacterial Bloom Occurrences and Research in Africa over the Last Decade. Harmful Algae 2016, 60, 11–26. [Google Scholar] [CrossRef]
- Tazart, Z.; Douma, M.; Tebaa, L.; Loudiki, M. Use of Macrophytes Allelopathy in the Biocontrol of Harmful Microcystis Aeruginosa Blooms. Water Sci. Technol. Water Supply 2019, 19, 245–253. [Google Scholar] [CrossRef]
- Gallardo-Rodríguez, J.J.; Astuya-Villalón, A.; Llanos-Rivera, A.; Avello-Fontalba, V.; Ulloa-Jofré, V. A Critical Review on Control Methods for Harmful Algal Blooms. Rev. Aquac. 2019, 11, 661–684. [Google Scholar] [CrossRef]
- Nascimento, P.; Vasconcelos, V.; Campos, A.; Barreiro, A. Screening of Plant Extracts for a Microcosm in Vivo Test of Inhibition of the Toxic Bloom-Forming Cyanobacteria Chrysosporum Ovalisporum. Algal Res. 2024, 80, 103552. [Google Scholar] [CrossRef]
- Ma, X.; Wang, X.; Zhou, S.; Ma, J.; Wang, J.; Chen, J.; Zeng, Y.; Chen, Q.; Qin, B.; Li, M. Plant Allelochemicals Inhibit the Growth and Colony Formation of Microcystis. J. Oceanol. Limnol. 2024, 1–14. [Google Scholar] [CrossRef]
- Tazart, Z.; Douma, M.; Caldeira, A.T.; Tebaa, L.; Mouhri, K.; Loudiki, M. Highlighting of the Antialgal Activity of Organic Extracts of Moroccan Macrophytes: Potential Use in Cyanobacteria Blooms Control. Environ. Sci. Pollut. Res. 2020, 27, 19630–19637. [Google Scholar] [CrossRef]
- Tazart, Z.; Manganelli, M.; Scardala, S.; Buratti, F.M.; Di Gregorio, F.N.; Douma, M.; Mouhri, K.; Testai, E.; Loudiki, M. Remediation Strategies to Control Toxic Cyanobacterial Blooms: Effects of Macrophyte Aqueous Extracts on Microcystis Aeruginosa (Growth, Toxin Production and Oxidative Stress Response) and on Bacterial Ectoenzymatic Activities. Microorganisms 2021, 9, 1782. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Macrophytes-Cyanobacteria Allelopathic Interactions and Their Implications for Water Resources Management—A Review. Limnologica 2017, 63, 122–132. [Google Scholar] [CrossRef]
- Li, X.; Zhao, W.; Chen, J.; Wang, F. Dosage Impact of Submerged Plants Extracts on Microcystis Aeruginosa Growth: From Hormesis to Inhibition. Ecotoxicol. Environ. Saf. 2023, 268, 115703. [Google Scholar] [CrossRef]
- Gao, L.; Pan, X.; Zhang, D.; Mu, S.; Lee, D.J.; Halik, U. Extracellular Polymeric Substances Buffer against the Biocidal Effect of H2O2on the Bloom-Forming Cyanobacterium Microcystis Aeruginosa. Water Res. 2015, 69, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hong, Y. Algal-Bloom Control by Allelopathy of Aquatic Macrophytes—A Review Algal-Bloom Control by Allelopathy of Aquatic Macrophytes—A Review. Front. Environ. Sci. Eng. China 2008, 2, 421–438. [Google Scholar] [CrossRef]
- Bastien, C.; Cǒté, R. Variations Temporelles de l’ultrastructure de Scenedesmus Quadricauda Exposée Au Cuivre Lors d’une Expérience à Long Terme. Int. Rev. Der Gesamten Hydrobiol. Und Hydrogr. 1989, 74, 207–219. [Google Scholar] [CrossRef]
- Walsh, G.E. Principles of Toxicity Testing with Marine Unicellular Algae. Environ. Toxicol. Chem. Int. J. 1988, 7, 979–987. [Google Scholar] [CrossRef]
- Marsot, P.; Mouhri, K. Morphogenèse et Croissance de La Diatomée Marine Phaeodactylum Tricornutum En Cultures Continues Dialysante et Non Dialysante. Vie Et Milieu/Life Environ. 1995, 199–205. [Google Scholar] [CrossRef]
- Aiken, S.G.; Newroth, P.R.; Wile, I. The Biology of Canadian Weeds.: 34. Myriophyllum spicatum L. Can. J. Plant Sci. 1979, 59, 201–215. [Google Scholar] [CrossRef]
- Xian, Q.; Chen, H.; Zou, H.; Yin, D. Allelopathic Activity of Volatile Substance from Submerged Macrophytes on Microcystin Aeruginosa. Acta Ecol. Sin. 2006, 26, 3549–3554. [Google Scholar] [CrossRef]
- Rhee, G.Y. Continous Culture in Phytoplankton Ecology. Adv. Aquat. Microbiol. 1980, 151–203. [Google Scholar]
- Cho, D.-H.; Ramanan, R.; Heo, J.; Shin, D.-S.; Oh, H.-M.; Kim, H.-S. Influence of Limiting Factors on Biomass and Lipid Productivities of Axenic Chlorella Vulgaris in Photobioreactor under Chemostat Cultivation. Bioresour. Technol. 2016, 211, 367–373. [Google Scholar] [CrossRef]
- Cho, K.; Cho, D.-H.; Heo, J.; Kim, U.; Lee, Y.J.; Choi, D.-Y.; Kim, H.-S. Nitrogen Modulation under Chemostat Cultivation Mode Induces Biomass and Lipid Production by Chlorella Vulgaris and Reduces Antenna Pigment Accumulation. Bioresour. Technol. 2019, 281, 118–125. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lu, L.; Wu, Y.; Ding, H.; Zhang, W. The Combined and Second Exposure Effect of Copper (II) and Chlortetracycline on Fresh Water Algae, Chlorella Pyrenoidosa and Microcystis Aeruginosa. Environ. Toxicol. Pharmacol. 2015, 40, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide Dismutase: Improved Assays and an Assay Applicable to Acrylamide Gels. Anal Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and Ozone-Induced Biochemical Changes in Antioxidant Enzymes of Arabidopsis Thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef]
- Tazart, Z.; Caldeira, A.T.; Douma, M.; Salvador, C.; Loudiki, M. Inhibitory Effect and Mechanism of Three Macrophytes Extract on Microcystis Aeruginosa Growth and Physiology. Water Environ. J. 2021, 35, 580–592. [Google Scholar] [CrossRef]
- Wang, F.; Zhao, W.; Chen, J.; Zhou, Y. Allelopathic Inhibitory Effect on the Growth of Microcystis Aeruginosa by Improved Ultrasonic-Cellulase Extract of Vallisneria. Chemosphere 2022, 298, 134245. [Google Scholar] [CrossRef] [PubMed]
- Yuan, R.; Li, Y.; Li, J.; Ji, S.; Wang, S.; Kong, F. The Allelopathic Effects of Aqueous Extracts from Spartina Alterniflora on Controlling the Microcystis Aeruginosa Blooms. Sci. Total Environ. 2020, 712, 136332. [Google Scholar] [CrossRef] [PubMed]
- Hachelaf, A.; Touil, A.; Zellagui, A.; Rhouati, S. Antioxidant and Antibacterial Activities of Essential Oil Extracted from Ranunculus arvensis L. Der Pharma Chem. 2015, 7, 170–173. [Google Scholar]
- Zhang, S.H.; Zhang, S.Y.; Li, G. Acorus Calamus Root Extracts to Control Harmful Cyanobacteria Blooms. Ecol. Eng. 2016, 94, 95–101. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Zhang, P.; Zeng, G.; Cai, X.; Liu, S.; Yin, Y.; Hu, X.; Hu, X.; Tan, X. Growth Inhibition and Oxidative Damage of Microcystis Aeruginosa Induced by Crude Extract of Sagittaria Trifolia Tubers. J. Environ. Sci. 2016, 43, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Liu, Y.G.; Yan, Z.L.; Zeng, G.M.; Liu, S.B.; Wang, W.J.; Tan, X.F.; Deng, J.Q.; Tang, X.; Wang, Q.P. Allelopathic Effect of the Rice Straw Aqueous Extract on the Growth of Microcystis Aeruginosa. Ecotoxicol. Environ. Saf. 2018, 148, 953–959. [Google Scholar] [CrossRef]
- Zhang, S.; Benoit, G. Comparative Physiological Tolerance of Unicellular and Colonial Microcystis Aeruginosa to Extract from Acorus Calamus Rhizome. Aquat. Toxicol. 2019, 215, 105271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liu, Y.; Zhang, P.; Zeng, G.; Hu, X.; Li, H.; Guo, Y.; Guo, X. Co-Culture with Cyperus Alternifolius Induces Physiological and Biochemical Inhibitory Effects in Microcystis Aeruginosa. Biochem. Syst. Ecol. 2014, 56, 118–124. [Google Scholar] [CrossRef]
- Wang, H.Q.; Liang, F.; Qiao, N.; Dong, J.X.; Zhang, L.Y.; Guo, Y.F. Chemical Composition of Volatile Oil from Two Emergent Plants and Their Algae Inhibition Activity. Pol. J. Environ. Stud. 2014, 23, 2371–2374. [Google Scholar]
- Zhu, X.; Dao, G.; Tao, Y.; Zhan, X.; Hu, H. A Review on Control of Harmful Algal Blooms by Plant-Derived Allelochemicals. J. Hazard. Mater. 2021, 401, 123403. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, P.; Wang, Z.; Shang, R.; Wang, S. UV/Persulfate Preoxidation to Improve Coagulation Efficiency of Microcystis Aeruginosa. J. Hazard. Mater. 2017, 322, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Zhou, Y.; Wang, N.; Xu, L.; Zheng, Z.; Zhang, J. Allelopathic Effects of Harmful Algal Extracts and Exudates on Biofilms on Leaves of Vallisneria Natans. Sci. Total Environ. 2019, 655, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-L.; Yu, X.-B.; Zhang, C.; Wang, G.-X. Growth Inhibition and Microcystin Degradation Effects of Acinetobacter Guillouiae A2 on Microcystis Aeruginosa. Res. Microbiol. 2015, 166, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.N.; Ge, F.J.; Zhang, L.P.; He, Y.; Lu, Z.Y.; Zhang, Y.Y.; Liu, B.Y.; Zhou, Q.H.; Wu, Z.B. Enhanced Toxicity to the Cyanobacterium Microcystis Aeruginosa by Low-Dosage Repeated Exposure to the Allelochemical N-Phenyl-1-Naphthylamine. Chemosphere 2017, 174, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, Y.; Gao, Y.; Liu, B.; Sun, X.; He, F.; Zhou, Q.; Wu, Z. Effects of Pyrogallic Acid on Microcystis Aeruginosa: Oxidative Stress Related Toxicity. Ecotoxicol. Environ. Saf. 2016, 132, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zhao, L.; Liu, K.; Liu, W. Enantiomeric Environmental Behavior, Oxidative Stress and Toxin Release of Harmful Cyanobacteria Microcystis Aeruginosa in Response to Napropamide and Acetochlor. Environ. Pollut. 2019, 246, 728–733. [Google Scholar] [CrossRef]
- Singer, S.J.; Nicolson, G.L. The Fluid Mosaic Model of the Structure of Cell Membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Xu, J.; Lu, T.; Zhang, Q.; Qu, Q.; Yang, Z.; Pan, X. Responses of Unicellular Alga Chlorella Pyrenoidosa to Allelochemical Linoleic Acid. Sci. Total Environ. 2018, 625, 1415–1422. [Google Scholar] [CrossRef]
- Kong, Y.; Zou, P.; Yang, Q.; Xu, X.; Miao, L.; Zhu, L. Physiological Responses of Microcystis Aeruginosa under the Stress of Antialgal Actinomycetes. J. Hazard. Mater. 2013, 262, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, P.; Wang, C.; Hu, B.; Ren, L.; Yang, Y. Microcystin Biosynthesis in Microcystis Aeruginosa: Indirect Regulation by Iron Variation. Ecotoxicol. Environ. Saf. 2018, 148, 942–952. [Google Scholar] [CrossRef]
- Zuo, S.; Zhou, S.; Ye, L.; Ma, S. Synergistic and Antagonistic Interactions among Five Allelochemicals with Antialgal Effects on Bloom-Forming Microcystis Aeruginosa. Ecol. Eng. 2016, 97, 486–492. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, J.; Orr, P.T.; Chuang, A.; Franklin, H.M.; Michele, A. Enhanced Resistance of Co-Existing Toxigenic and Non-Toxigenic Microcystis Aeruginosa to Pyrogallol Compared with Monostrains. Toxicon 2020, 176, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Kang, P.; Hong, J.; Kim, E.; Kim, B. Effects of Extracts of Reed and Cattail on the Growth of a Cyanobacterium, Microcystis Aeruginosa. J. Freshw. Ecol. 2020, 35, 123–134. [Google Scholar] [CrossRef]
- Sun, Y.Y.; Zhou, W.J.; Wang, H.; Guo, G.L.; Su, Z.X.; Pu, Y.F. Antialgal Compounds with Antialgal Activity against the Common Red Tide Microalgae from a Green Algae Ulva Pertusa. Ecotoxicol. Environ. Saf. 2018, 157, 61–66. [Google Scholar] [CrossRef]
- Wang, Z.; Song, G.; Li, Y.; Yu, G.; Hou, X.; Gan, Z.; Li, R. Science of the Total Environment The Diversity, Origin, and Evolutionary Analysis of Geosmin Synthase Gene in Cyanobacteria. Sci. Total Environ. 2019, 689, 789–796. [Google Scholar] [CrossRef]
- Zhang, F.; Ye, Q.; Chen, Q.; Yang, K.; Zhang, D.; Chen, Z.; Lu, S.; Shao, X.; Fan, Y.; Yao, L.; et al. Crossm Algicidal Activity of Novel Marine Bacterium Paracoccus sp. Appl. Environ. Microbiol. 2018, 84, e01015-18. [Google Scholar]
- Wang, J.; Liu, Q.; Feng, J.; Lv, J.P.; Xie, S.L. Effect of High-Doses Pyrogallol on Oxidative Damage, Transcriptional Responses and Microcystins Synthesis in Microcystis Aeruginosa TY001 (Cyanobacteria). Ecotoxicol. Environ. Saf. 2016, 134, 273–279. [Google Scholar] [CrossRef]
- Mecina, G.F.; Chia, M.A.; Cordeiro-Araújo, M.K.; Bittencourt-Oliveira, M.d.C.; Rosa, M.V.; Torres, A.; Gonzales Molinillo, J.M.; Macías, F.A.; da Silva, R.M.G. Effect of Flavonoids Isolated from Tridax Procumbens on the Growth and Toxin Production of Microcystis Aeruginos. Aquat. Toxicol. 2019, 211, 81–91. [Google Scholar] [CrossRef]
- Sim, D.Z.H.; Mowe, M.A.D.; Mitrovic, S.M.; Tulsian, N.K.; Anand, G.S.; Yeo, D.C.J. Nutrient Conditions Influence Allelopathic Capabilities of Ludwigia Adscendens and Other Tropical Macrophytes against Microcystis Aeruginosa. Freshw. Biol. 2024, 69, 538–555. [Google Scholar] [CrossRef]
- Tao, C.; Niu, X.; Zhang, D.; Liu, Y.; Zhou, L.; Han, Y.; Mo, Y.; Wu, K.; Lin, Y.; Lin, Z. Inhibitory Effect of Malonic Acid on Microcystis Aeruginosa: Cell Morphology, Oxidative Stress and Gene Expression. Algal Res. 2024, 80, 103513. [Google Scholar] [CrossRef]
- Nakai, S.; Inoue, Y.; Hosomi, M.; Murakami, A. Myriophyllum Spicatum-Released Allelopathic Polyphenols Inhibiting Growth of Blue-Green Algae Microcystis Aeruginosa. Water Res. 2000, 34, 3026–3032. [Google Scholar] [CrossRef]
- Nakai, S.; Zou, G.; Okuda, T.; Nishijima, W.; Hosomi, M.; Okada, M. Polyphenols and Fatty Acids Responsible for Anti-Cyanobacterial Allelopathic Effects of Submerged Macrophyte Myriophyllum Spicatum. Water Sci. Technol. 2012, 66, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Hilt, S. Allelopathic Inhibition of Epiphytes by Submerged Macrophytes. Aquat. Bot. 2006, 85, 252–256. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, B.; Wang, J.; Gao, Y.; Wu, Z. Study on the Mechanism of Allelopathic Influence on Cyanobacteria and Chlorophytes by Submerged Macrophyte (Myriophyllum Spicatum) and Its Secretion. Aquat. Toxicol. 2010, 98, 196–203. [Google Scholar] [CrossRef]
- Zhiqun, T.; Jian, Z.; Junli, Y.; Chunzi, W.; Danju, Z. Allelopathic Effects of Volatile Organic Compounds from Eucalyptus Grandis Rhizosphere Soil on Eisenia Fetida Assessed Using Avoidance Bioassays, Enzyme Activity, and Comet Assays. Chemosphere 2017, 173, 307–317. [Google Scholar] [CrossRef] [PubMed]




| Treatment | TIME (day) | Intracellular (µg/L) | Extracellular (µg/L) | Reduction Rate for Intra-MCs in % | Reduction Rate for Extra-MCs in % |
|---|---|---|---|---|---|
| false positive | -- | 0.72 ± 0.14 | -- | -- | |
| continuous mode without plants | 5 | 30.47 ± 0.60 | 28.84 ± 0.57 | -- | -- |
| with 6.5 mg/L | 8 | 30.21 ± 0.47 | 15.4 ± 0.74 | No reduction | 28.51 |
| 13 | 30.84 ± 0.61 | 11.01 ± 0.22 * | |||
| with 12.5 mg/L | 24 | 8.85 ± 0.17 | 5.31 ± 0.10 | No reduction | 36.31 |
| 29 | 30.71 ± 0.30 * | 8.93 ± 0.08 * | |||
| with 25 mg/L | 34 | 21.71 ± 0.21 | 8.45 ± 0.08 | No reduction | 46.75 |
| 39 | 42.208 ± 0.42 * | 4.5 ± 0.04 * | |||
| with 50 mg/L | 45 | 10.199 ± 0.10 | 6.38 ± 0.06 | 18.37 | 54.70 |
| 50 | 8.26 ± 0.08 * | 2.89 ± 0.02 * | |||
| with 75 mg/L | 53 | 9.5 ± 0.32 | 3.52 ± 0.05 | 52.63 | 94.03 |
| 58 | 4.5 ± 0.2 * | 0.21 ± 0.12 * | |||
| with 100 mg/L | 64 | 6.199 ± 0.06 | 0.38 ± 0.003 | ~100 | ~100 |
| 66 | <0.1 * | <0.1 * | |||
| batch mode | 70 | <0.1 | <0.1 | -- | -- |
| Compounds | Retention Time [min] | Area [mAU.s] | Concentration [µg/g DW] |
|---|---|---|---|
| Ascorbic acid | 3.50 | 1622.20 | 185.19 |
| Gallic acid | 3.90 | 6056.40 | 362.56 |
| Fumaric acid | 5.40 | 161.90 | 126.78 |
| Tyrosol | 8.00 | 488.01 | 139.82 |
| Catechin | 11.80 | 238.77 | 129.85 |
| Chlorogenic acid | 12.10 | 89.45 | 123.88 |
| Tannic acid | 12.30 | 247.80 | 130.21 |
| Caffeic acid | 17.80 | 522.24 | 141.19 |
| Unknown | 27.40 | 137.30 | 125.79 |
| Unknown | 33.60 | 369.43 | 135.08 |
| Unknown | 44.20 | 658.72 | 146.65 |
| p-coumaric acid | 44.50 | 411.31 | 136.75 |
| Quercetin | 44.70 | 490.69 | 139.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tazart, Z.; Lazrak, K.; El Bouaidi, W.; Redouane, E.M.; Tebaa, L.; Douma, M.; Mouhri, K.; Loudiki, M. Scaling up the Natural Mode of Action of Macrophyte Allelochemicals and Their Effect on Toxic Cyanobacteria Using a Nitrogen-Limited Chemostat. Processes 2024, 12, 1883. https://doi.org/10.3390/pr12091883
Tazart Z, Lazrak K, El Bouaidi W, Redouane EM, Tebaa L, Douma M, Mouhri K, Loudiki M. Scaling up the Natural Mode of Action of Macrophyte Allelochemicals and Their Effect on Toxic Cyanobacteria Using a Nitrogen-Limited Chemostat. Processes. 2024; 12(9):1883. https://doi.org/10.3390/pr12091883
Chicago/Turabian StyleTazart, Zakaria, Khawla Lazrak, Widad El Bouaidi, El Mahdi Redouane, Lamiaa Tebaa, Mountasser Douma, Khadija Mouhri, and Mohammed Loudiki. 2024. "Scaling up the Natural Mode of Action of Macrophyte Allelochemicals and Their Effect on Toxic Cyanobacteria Using a Nitrogen-Limited Chemostat" Processes 12, no. 9: 1883. https://doi.org/10.3390/pr12091883
APA StyleTazart, Z., Lazrak, K., El Bouaidi, W., Redouane, E. M., Tebaa, L., Douma, M., Mouhri, K., & Loudiki, M. (2024). Scaling up the Natural Mode of Action of Macrophyte Allelochemicals and Their Effect on Toxic Cyanobacteria Using a Nitrogen-Limited Chemostat. Processes, 12(9), 1883. https://doi.org/10.3390/pr12091883







