Abstract
Nitrobenzene (NB) is one of the nitro-aromatic compounds that is extensively used in various chemical industries. Despite its potential applications, NB is considered to be a toxic compound that has significant hazardous effects on human health and the environment. Thus, it can be said that the NB level should be monitored to avoid its negative impacts on human health. In this vein, the electrochemical method has emerged as one of the most efficient sensing techniques for the determination of NB. The sensing performance of the electrochemical techniques depends on the electro-catalytic properties and conductivity of the electrode materials. In the past few years, various electrode materials, such as conductive metal ions, semiconducting metal oxides, metal–organic frameworks, and two-dimensional (2D) materials, have been used as the electrode material for the construction of the NB sensor. Thus, it is worth summarizing previous studies on the design and synthesis of electrode materials for the construction of the NB sensor. In this mini-review article, we summarize the previous reports on the synthesis of various advanced electrode materials, such as platinum (Pt) nanoparticles (NPs), silver (Ag) NPs, carbon dots (CDs), graphene, graphitic carbon nitride (g-C3N4), zinc stannate (ZnSnO3), cerium oxide (CeO2), zinc oxide (ZnO), and so on. Furthermore, the impacts of different electrode materials are systematically discussed for the sensing of NB. The advantages of, limitations of, and future perspectives on the construction of NB sensors are discussed. The aim of the present mini-review article is to enhance the knowledge and overall literature, working towards the construction of NB sensors.
1. Introduction
In the present scenario, environmental pollution is considered to be one of the major challenges globally [1,2]. Various organic and inorganic compounds, such as the nitro (-NO2) group containing aromatic compounds (nitrobenzene (NB; C6H5NO2), nitrophenol (NP), and nitrotoluene (NT)), hydrazine, catechol, hydrogen peroxide, etc., are widely used in the chemical and dye industries [3,4,5,6,7,8]. In particular, NB is widely used in various applications, such as rubber, chemicals, dyes, explosives, pesticides, urethane polymers, the pharmaceutical industry, and analgesic drugs [9,10,11,12]. Despite its various advantages and uses, NB has a significant negative impact on human health and the environment due to its hazardous nature. The United States Environmental Protection Agency classifies it as a group 2B carcinogen [13]. NB presents severe health risks as it can be easily inhaled, ingested, and absorbed through the skin [14,15]. In addition, NB may enter the environment through various routes, such as industrial discharge, land leaching, accidental spills, and chemical waste. Thus, it can be understood that NB has serious negative effects on human health and the environment. Therefore, it is of great significance to monitor the level of NB to avoid its hazardous effects. In this regard, conventional methods, such as gas chromatography (GC), surface plasmonic resonance (SPR), Raman spectroscopy (RS), and high-performance liquid chromatography (HPLC), are widely used for the determination of NB [13,14,15,16,17,18,19,20]. Unfortunately, these conventional methods are complex, expensive, and challenging to use for the determination of NB [20]. Thus, it is necessary to find an alternative sensing approach for the sensing of NB with high sensitivity and selectivity.
Recently, the electrochemical method has received a tremendous amount of attention because of its excellent sensitivity, simplicity, fast response, selectivity, storage stability, and repeatability [21]. These types of electrochemical sensors have a low environmental impact due to their excellent reusability and stability and the high stability of the catalyst. Thus, researchers are highly motivated towards the design and fabrication of highly efficient electrochemical sensors [22]. Electrochemical methods involve the use of electro-catalysts, which may significantly influence the performance of the fabricated sensors towards the detection of NB. In the past few years, various electro-catalytic materials, such as transition metal oxides, polymers, metal–organic frameworks (MOFs), carbon dots, metal carbides/nitrides, and composites, have been explored for electrochemical sensing applications [23,24,25,26].
In this mini-review article, we compiled previously published reports on the fabrication of the NB sensor using various nanostructured metal oxides, polymers, carbon-based materials, MOFs, and hybrid composites as electrode materials. To the best of our knowledge, in our literature survey, no review report is available which summarizes the different electrode materials towards the determination of NB. This is the first mini-review article to compile the advancements in the electrochemical fabrication of NB sensors. This review provides insights into the electrode materials for the construction of the NB sensor. It is believed that the present mini-review article will be useful to researchers working to improve the selectivity and sensitivity of NB sensors.
2. Progress in the Fabrication of NB Sensors
Previously, various advanced electrode materials based on transition metal oxides, polymers, carbon-based materials, MOFs, and hybrid composite materials were extensively used in the construction of NB sensors. In this section, we summarize previously reported articles on the fabrication of the NB sensor using metal oxides, carbon-based materials (carbon nanotubes, graphene, etc.), metal dichalcogenides, and other materials.
2.1. Metal-Oxide-Based Electrode Materials
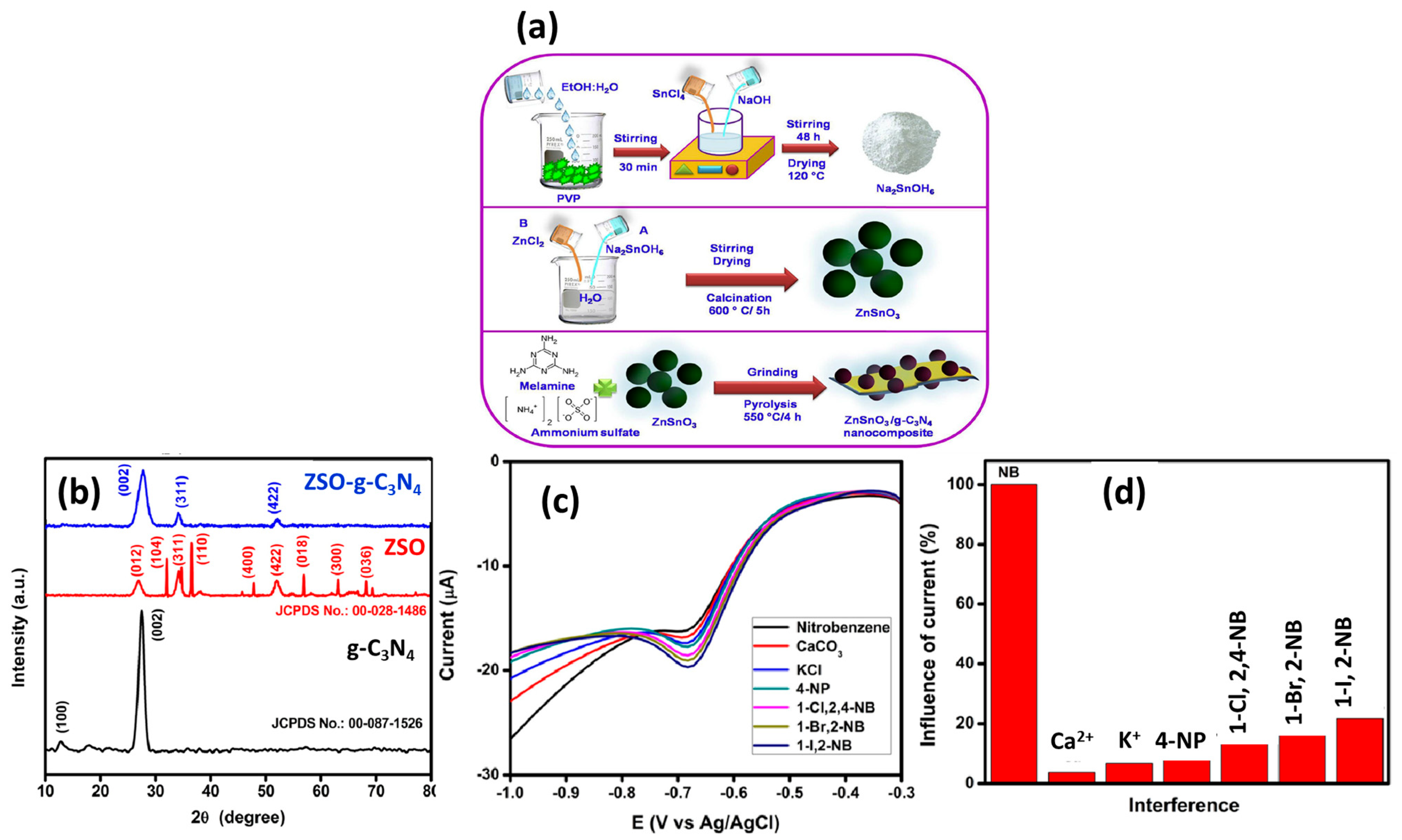
In previous years, metal oxides and their composites were extensively used for various electrochemical sensing applications due to their decent electro-catalytic properties, conductivity, eco-friendliness, and cost-effective properties. Cerium dioxide (CeO2) has good electro-catalytic properties and can be used in the fabrication of electrochemical sensors. It is worth exploring such materials for the fabrication of the NB sensor. In this context, Sangili et al. [27] synthesized CeO2 nanoparticles (NPs) by employing the hydrothermal method, and the morphological properties of the synthesized CeO2 NPs were characterized by transmission electron microscopy (TEM); the presence of Ce and O elements was confirmed via energy-dispersive X-ray spectroscopy (EDX). The authors reported that CeO2 NPs have a uniform spherical structure, and the size of the prepared particles lies in a range of 2–5 nm. The prepared CeO2 NPs were further proposed as an NB-sensing material, and the sensing capability of the CeO2-NP-modified electrode was studied by using the cyclic voltammetry (CV) and differential pulse voltammetry (DPV) techniques. The authors found that the fabricated electrode demonstrated an excellent LOD of 0.092 µM with a decent sensitivity of 1.1166 μA μM−1 cm−1. The proposed sensor also demonstrated a good linear dynamic range of 0.1 to 50 µM. This work also reported the real sample investigations of the fabricated electrode in tap water and river water with acceptable recoveries. Arul et al. [28] synthesized iron oxide (α-Fe2O3) by using the co-precipitation method at room temperature (RT). SEM studies demonstrated that α-Fe2O3 consists of micro/nanorods that may be beneficial for better electron transport. α-Fe2O3 micro/nanorods have a high specific surface area of 67 m2/g, and this may enhance electron transportation during redox reactions. The α-Fe2O3 micro/nanorods were deposited onto the surface of a GC electrode, and its electrochemical performance was checked by using the CV method. The α-Fe2O3/GC electrode exhibited an LOD of 30.4 ppb with a sensitivity of 446 nA/µM for the detection of NB. The reported articles showed that manganese ferrite (MnFe2O4; spinel ferrite) has excellent properties and can be used in various applications such as sensing and energy storage devices. In this vein, Sang et al. [29] reported the hydrothermal preparation of MnFe2O4 for the fabrication of an NB sensor. The synthesized MnFe2O4 colloid nanocrystals exhibited good physicochemical properties, and the authors used the synthesized material as an electro-catalyst towards the fabrication of an NB sensor. The MnFe2O4 colloid nanocrystal-modified electrode shows improved performance towards the sensing of NB, and an LOD of 4 mM was achieved. Zinc stannate (ZnSnO3) is a perovskite material, which has robust stability and decent electrical properties, suggesting its potential for electrochemical sensing applications. In this regard, Vinoth et al. [30] studied the role of ZnSnO3-incorporated graphitic carbon nitride (g-C3N4) as a sensing material for the detection of NB. The authors prepared a ZnSnO3/g-C3N4 composite by employing novel strategies, as shown in Figure 1a. The authors authenticated the formation of ZnSnO3/g-C3N4 via the XRD technique (Figure 1b). The high and sharp peak intensity suggest the good crystalline nature of the prepared ZnSnO3/g-C3N4. The GC electrode was modified with ZnSnO3/g-C3N4 for the sensing of NB. The ZnSnO3/g-C3N4-modified GC electrode exhibited a good LOD of 2.2 µM with a sensitivity of 0.05857 µA µM−1 cm−2. The ZnSnO3/g-C3N4-modified GC electrode also demonstrated a linear range of 30–100 µM towards the determination of NB using the LSV technique. The ZnSnO3/g-C3N4-modified GC electrode also exhibited excellent selectivity in the presence of various interfering substances.
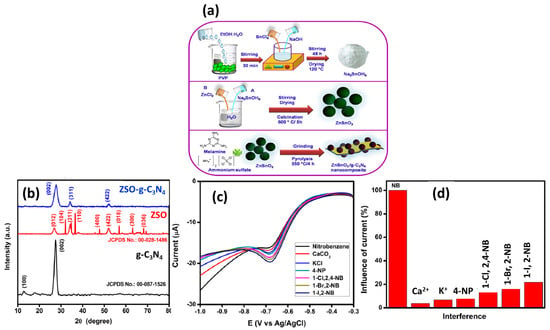
Figure 1.
(a) Schematic picture for the synthesis of ZnSnO3/g-C3N4 composite. (b) XRD pattern of the ZnSnO3, g-C3N4, and ZnSnO3/g-C3N4 composite. (c) Selectivity test (DPV curves) of the NB sensor in presence of various interfering substances. (d) Selectivity results for NB sensing. Reprinted with permission [30].
The DPV graphs are shown in Figure 1c, which indicate that the presence of interfering substances does not influence the performance of the ZnSnO3/g-C3N4-modified GC electrode. The ZnSnO3/g-C3N4-modified GC electrode demonstrated excellent selectivity for the detection of NB in the presence of interfering materials (K+, Ca2+, 4-nitrophenol, 1-bromo,2-nitrobenzene, 1-chloro,2,4-dinitrobenzene, 1-iodo, and 2-nitrobenzene), as shown in Figure 1d. The ZnSnO3/g-C3N4-modified GC electrode also exhibited good recovery in real samples, which suggested its application for practical purposes.
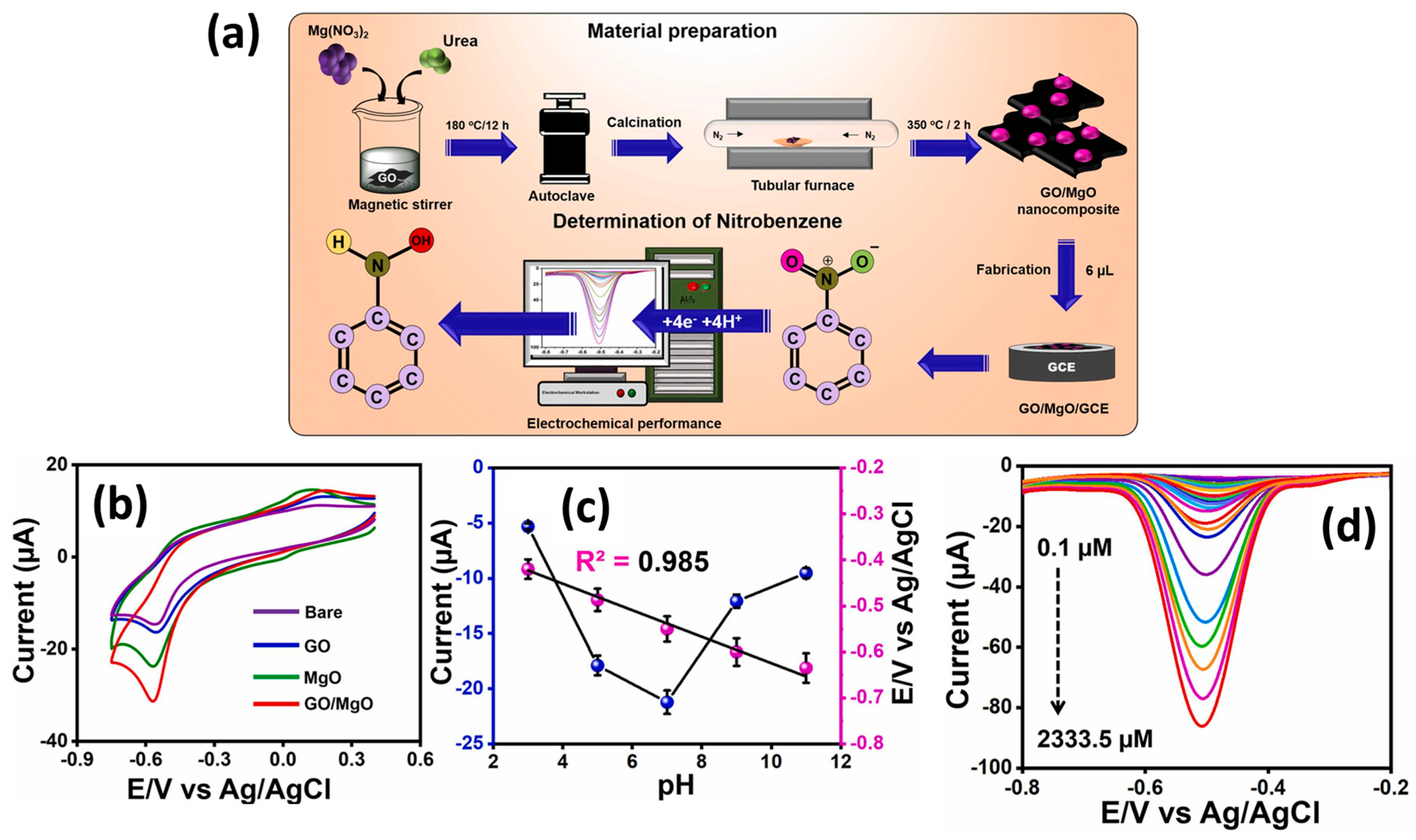
Magnesium oxide (MgO) is a fascinating alkaline metal oxide, which possesses excellent catalytic, adoption, chemical, and mechanical properties, which makes MgO an efficient electrode material for the construction of cost-effective electrochemical sensors. The catalytic properties of MgO can be further improved by combing it with carbon-based materials. Kokulnathan et al. [31] proposed the synthesis of a GO/MgO composite using a benign approach, as shown in Figure 2a. The authors modified the GC electrodes with GO, MgO, and GO/MgO as catalysts and evaluated their performance towards a reduction in NB using the CV and DPV methods (Figure 2b).
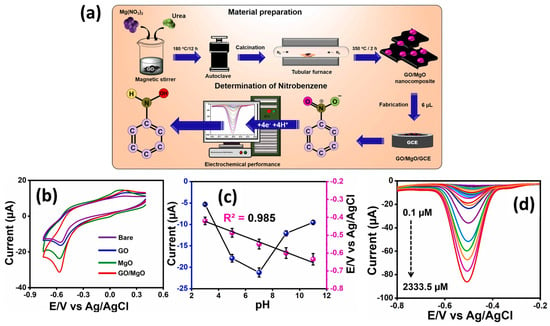
Figure 2.
(a) Schematic representation of the synthesis of GO/MgO composite, fabrication of GO/MgO/GC electrode and its working for NB sensing. (b) CV graphs of the different electrodes for NB sensing. (c) Current value versus pH graph for the sensing of NB using GO/MgO/GC electrode. (d) DPV graphs of the GO/MgO/GC electrode in presence of various concentrations of NB. Reprinted with permission [31].
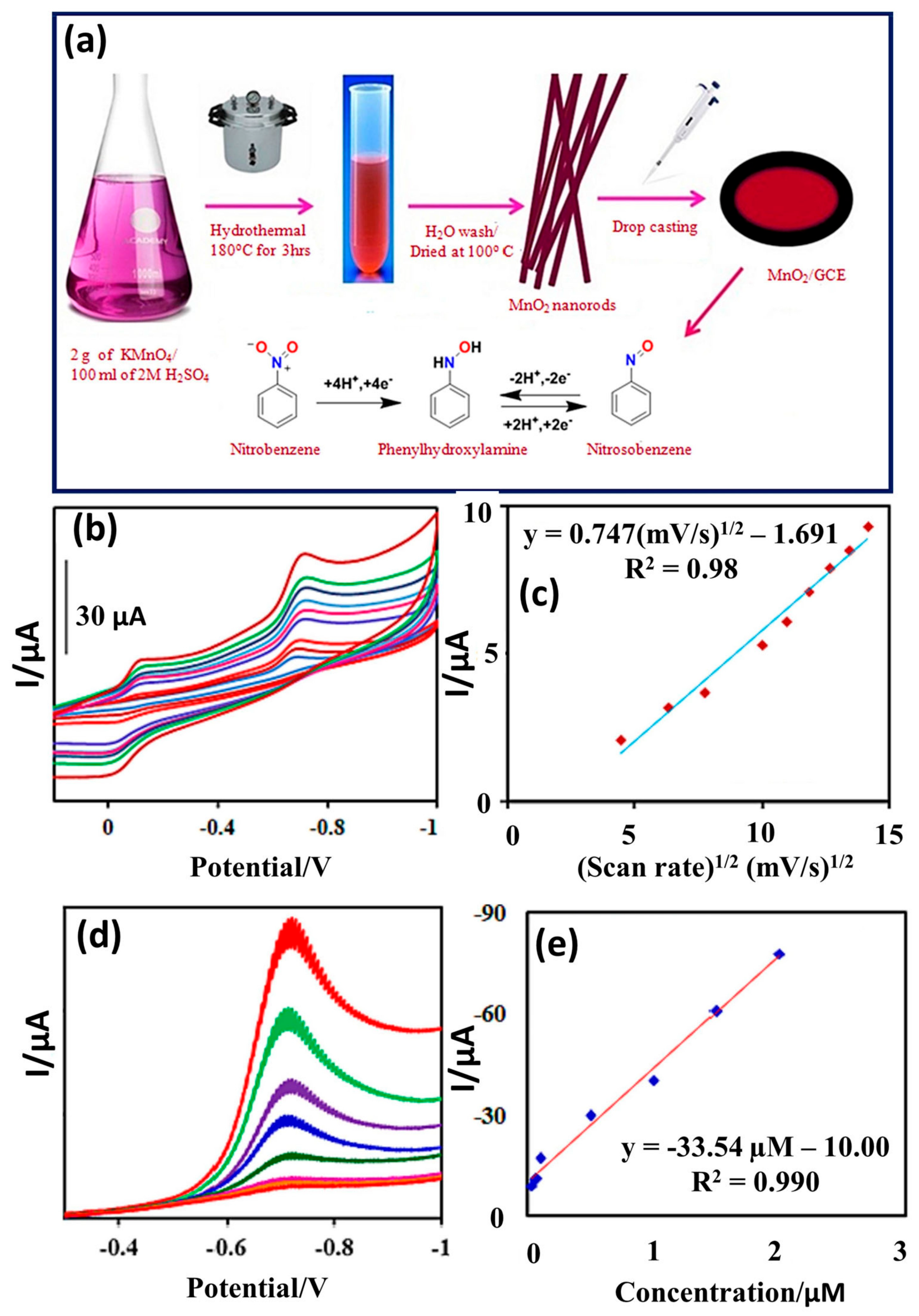
It was clearly seen that GO/MgO-modified GC electrode has higher catalytic activity for the improved NB reduction, as shown in Figure 2b. The pH of the solution was also optimized, and higher activity was observed at a pH of 7.0 (Figure 2c). The authors also adopted the DPV technique for further electrochemical investigations and found that DPV is more sensitive compared to the CV method. The effect of various concentrations was studied using a GO/MgO-modified GC electrode, as shown in Figure 2d. It is clear that synergistic interactions between GO and MgO enhanced the charge transfer properties of the modified electrode and improved the reduction in NB using the DPV method. The GO/MgO-modified GC electrode demonstrated a low LOD of 0.01 µM with two linear dynamic ranges of 0.1 to 38.9 µM and 58.5 to 2333.5 µM. The reported NB sensor exhibited excellent repeatability, stability, and selectivity. Acceptable recoveries of 99.35% to 99.80% were also observed for real sample investigations for the GO/MgO-modified GC electrode towards the detection of NB in real water samples. Manganese dioxide (MnO2) has good electro-catalytic properties and may be a good electrode for the fabrication of NB sensors. In this regard, Chellappa et al. [32] reported a simple hydrothermal method for the synthesis of MnO2 nanorods (NRs), as depicted in Figure 3a. The MnO2 NRs were coated onto the bare surface of the GC electrode, and its sensing activity for the sensing of NB was evaluated by using the CV and DPV methods.
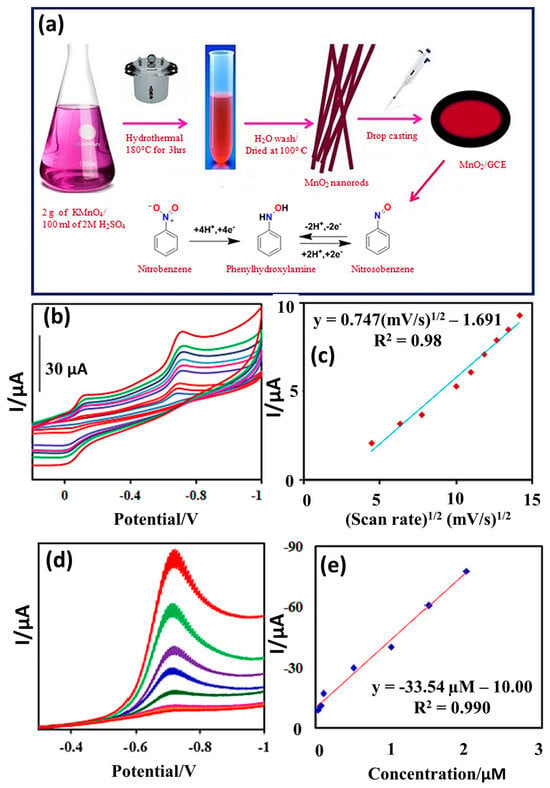
Figure 3.
(a) Schematic diagram presents the synthetic procedure for MnO2 NRs. (b) CV of the MnO2 NRs/GCE In presence of NB at different scan rates. (c) Calibration plot between current response and square root of scan rate. (d) DPV curves of the MnO2 NRs/GCE in presence of different concentrations of NB. (e) Calibration plot between current response and concentration of NB. Reprinted with permission [32].
CV graphs of the MnO2 NRs/GCE in the presence of NB at different applied scan rates are shown in Figure 3b. It is seen that the current response increases with respect to the applied scan rate. The calibration plot shows a regression coefficient (R2) value of 0.98 (Figure 3c). Thus, this suggests that the current response for redox reactions linearly increases. It is considered that DPV may be a more sensitive technique for the determination of NB. Thus, the authors studied the electro-catalytic activities of MnO2 NRs/GCE in the presence of different concentrations of NB. The DPV results for MnO2 NRs/GCE indicated that current response for the redox reactions also increases with increasing concentrations of NB (Figure 3d), and this response was found to be linear (Figure 3e), as suggested by the R2 value of 0.99. The authors proposed that DPV is relatively more sensitive for the detection for NB in comparison to CV. The MnO2 NRs/GCE demonstrated excellent recovery in real sample studies, with a reasonably good LOD of 0.025 µM and a linear range of 0.03 to 2 µM for the detection of NB, with high stability. Kokulnathan et al. [33] demonstrated that zinc oxide NRss/copper tin sulfide nanoflowers (ZnO-NRs@CTS-NFs) can be synthesized by using the hydrothermal route and used it as an NB sensing material. The proposed ZnO-NRs@CTS-NF-based NB sensor exhibited an LOD of 0.002 µM and sensitivity of 1.30 µA/µM cm2, with two linear ranges of 0.01–17.2 and 17.2–203 µM using the DPV method. The ZnO-NRs@CTS-NF-based NB sensor also exhibited acceptable recovery in real samples, such as rivers, ponds, and industrial water.
2.2. Carbon-Family-Based Electrode Materials
Carbon-based materials, such as graphene, carbon nanotubes, and graphitic carbon nitride, have decent catalytic properties and surface areas. Sang et al. [34] modified the surface area of the bare GCE using multi-walled carbon nanotubes (MWCNTs) as an electrode material for the determination of NB. The performance of the MWCNTs/GCE was evaluated in acid electrolytes using the CV and DPV methods. The authors found that hydroxyl-containing MWCNT-modified GCE (MWCNTs-OH/GCE) has excellent performance for the determination of NB compared to the pristine MWCNTs/GCE. This showed that the functionalization of MWCNTs with an OH group significantly improved the sensing properties of the pristine MWCNTs. Thus, it would be worth using the OH-functionalized MWCNT-modified electrodes as working electrodes for the detection of NB. The functionalized (f)-MWCNTs have an excellent conductive nature and improved catalytic properties due to the presence of functional groups on the surface of MWCNTs. Therefore, it is of great significance to utilize the f-MWCNTs as sensing materials for the construction of an NB sensor. Govindasamy et al. [35] proposed the use of f-MWCNTs as electrode materials for NB sensing applications.
The authors also used nafion as a binder to improve the adhesiveness/stability of the f-MWCNTs on the surface of a screen-printed carbon electrode (SPCE). The electrochemical performance of the f-MWCNT-modified SPCE (f-MWCNTs/SPCE) was determined in the presence of NB, and the obtained results suggested that f-MWCNTs/SPCE has good electro-catalytic properties. The f-MWCNTs/SPCE exhibited a wide dynamic linear range of 50 nm to 1170 µM. A sensitivity of 0.6685 µA/µM cm2 was obtained using f-MWCNTs/SPCE for the determination of NB. A decent LOD value of 45 nM was also reported for the sensing of NB using f-MWCNTs/SPCE. Furthermore, f-MWCNTs/SPCE also demonstrates satisfactory stability, repeatability, and reproducibility. The authors successfully recovered the NB from a human urine sample.
Thirumalraj et al. [36] demonstrated the role of electrochemically activated graphite (EAG) as catalysts for the sensing of NB. The authors prepared an EAG-modified screen-printed electrode (EAG-SPE) and explored this as the working electrode for the determination of NB. The sensing performance of EAG-SPE was determined by employing the CV method under different pH conditions of phosphate-buffered saline (PBS) solutions. The EAG-SPE exhibited higher catalytic properties under a pH of 7.0, and the authors reported a pH of 7.0 as the optimal condition. Furthermore, the authors checked the effects of different applied scan rates and found that current responses for the determination of NB increase with increasing applied scan rates. Chronoamperometry (CA) is one of the most sensitive methods for the sensing of toxic compounds and biomolecules. Thus, the authors also adopted the CA method for further sensing experiments. The effect of various concentrations of NB on the current response of EAG-SPE has been investigated using the CA method. The authors found that current responses/signals rapidly increase with the addition/spike of NB at different concentrations. EAG-SPE demonstrated an excellent LOD of 0.06 µM and sensitivity of 1.445 μA μM−1 cm−2 [36]. The EAG-SPE also demonstrated its excellent selective nature for the determination of NB in the presence of various interfering compounds. Ma et al. [37] also reported the fabrication of an NB sensor by employing macro-/meso-porous carbon material (MMPCM) catalysts. The MMPCMs were prepared via a sonication-assisted pyrolysis method using a silica template. The synthesized MMPCMs were characterized by the SEM method, which suggested the presence of a honeycomb-like surface morphology with lots of macro-pores (diameter range = ∼330 nm). These morphological features improve the electron transport and improve the sensing activity of modified GC electrodes. Thus, the authors modified the surface of the GC electrode using the MMPCM catalyst and employed linear sweep voltammetry (LSV) for the determination of NB. The authors studied the effects of various concentrations of NB (0.2, 0.4, 1, 2, 4, 6, 10, 15, 20, 30, 40 µM) on the performance of the MMPCM catalyst-modified GC electrode. They found that the current response increases with respect to the concentration of the NB. An LOD of 8 nM was obtained with a sensitivity of 2.36 µA µM−1. They also reported that the MMPCM catalyst-modified GC electrode has good selectivity for the detection of NB in the presence of various interfering molecules, such as NO3−, Mn2+, CO32−, SO42−, PO43−, CH3COO−, and Mg2+. The MMPCM catalyst-modified GC electrode also demonstrated good stability and reproducibility.
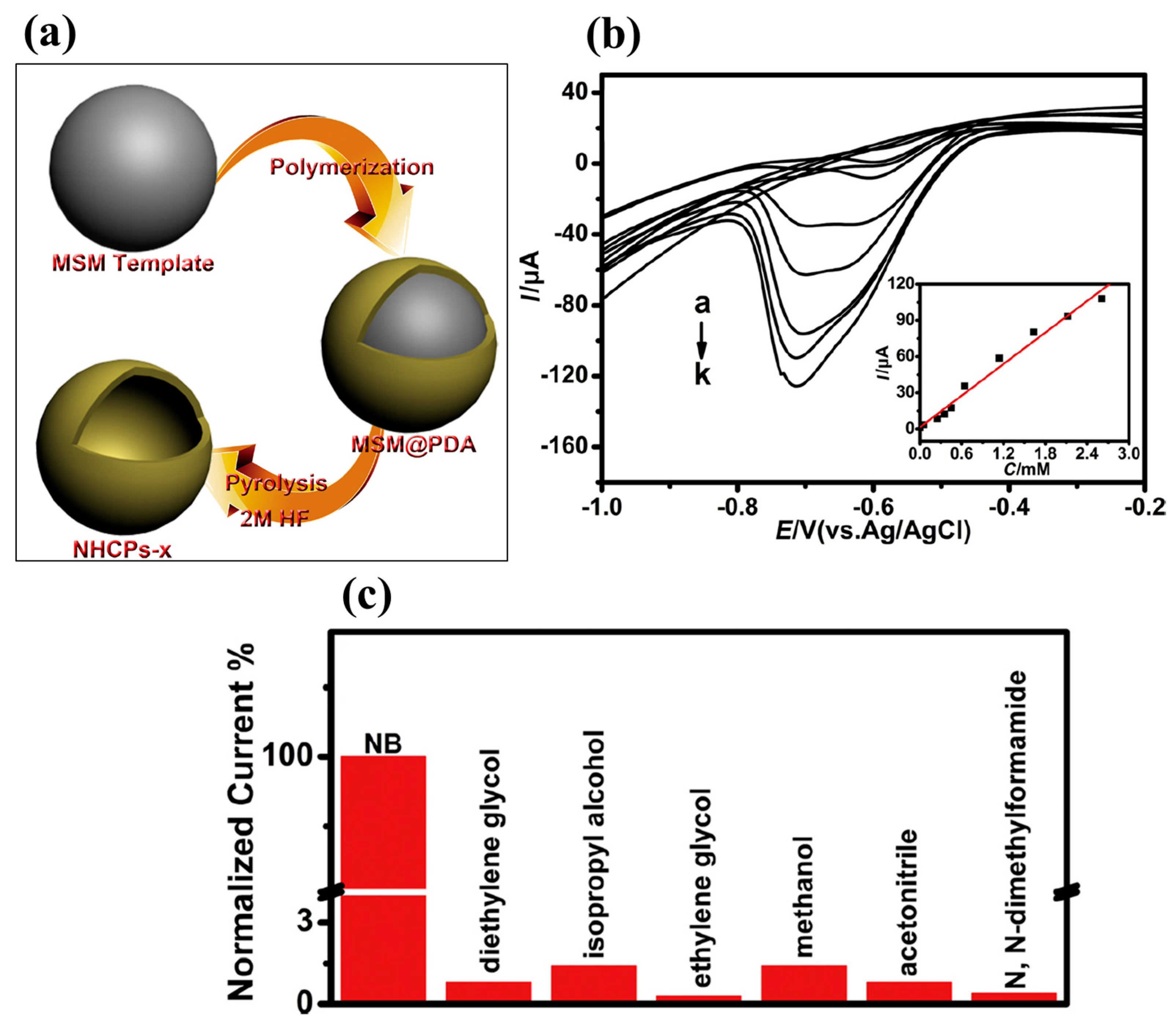
It is well known that nitrogen (N)-doped materials may have significantly improved catalytic properties due to the generation of active sites on N-doped materials. It is of great significance to prepare a N-doped catalyst for electrochemical sensing applications. In this context, Liu et al. [38] synthesized N-doped hollow carbon nanospheres (NHCPs) by employing novel strategies. The mono-dispersed SiO2 microspheres were used as a template with dopamine as the N source. The synthetic process for the preparation of NHCPs is described in Figure 4a.
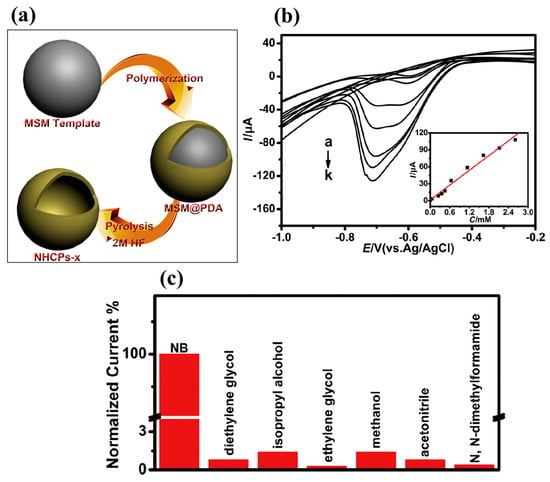
Figure 4.
(a) Schematic picture for the preparation of NHSPs-x. (b) DPV graphs of the NHCPs-750–GC electrode in different concentration of NB. (c) Selectivity nature of NHCPs-750–GC electrode for NB sensing. Reprinted with permission [38].
The authors authenticated the formation of NHCPs through XRD and Fourier-transform infrared (FT-IR) spectroscopy. The NHCPs were treated at a temperature of 750 ºC and denoted as NHCPs-750. The NHCPs-750 was coated on the GC surface, and the performance of the NHCPs-750–GC electrode was determined by the CV, electrochemical impedance spectroscopy (EIS), and DPV methods. The NHCPs-750–GC has a higher current response for the sensing of NB compared to the GC electrode. The DPV curves of the NHCPs-750–GC for different concentrations of NB are shown in Figure 4b. It is clear that the current linearly increases with increasing concentrations of NB (inset of Figure 4b). The NHCPs-750–GC demonstrated an LOD of 2.29 µM with high sensitivity of 436 μA mM−1. The NHCPs-750–GC also has an excellent linear range of 5–2610 µM for the sensing of NB. The presence of pyrindinic N in the NHCPs-750–GC electrode improved the catalytic behavior for the sensing of NB. The authors also evaluated the selectivity of the NHCPs-750–GC electrode for the sensing of NB in the presence of various interferences. Figure 4c shows the excellent selectivity of NHCPs-750–GC for the determination of NB in the presence of various interferences. In other work, Sakthivel et al. [39] prepared a novel catalyst material for the sensing of NB. The authors synthesized a chitin hydrogel-stabilized graphite (GR-CHI) composite using simple strategies and fabricated the surface of the GC electrode with the prepared GR-CHI as a catalyst material. The physiochemical investigations showed that synthesized GR-CHI is formed by strong interaction between GR and CHI. GR-CHI/GCE was further employed as a working electrode, and CV was adopted as the voltammetric sensing approach for the detection of NB. The CV results showed that the current response for the redox peaks increases with increasing scan rates, and sensing of NB is an adsorption-controlled process. The performance of the GR-CHI/GCE was further studied by employing the CA method. The current response increases with increasing concentrations of NB, and this increment was found to be linear. The GR-CHI/GCE exhibited an LOD of 37 nM and linear range of 0.1 to 594.6 µM using the amperometric (i–t) method. The sensor (GR-CHI/GCE) demonstrates good selectivity, excellent practicality, and consistent repeatability in detecting NB in laboratory water samples. β-cyclodextrin (β-CD) possesses notable physicochemical properties, which make it valuable in various applications, such as sensing, drug delivery, and environmental remediation. β-CD is a chemically stable molecular structure with biocompatibility and less toxicity, which make it a suitable candidate for the fabrication of electrochemical sensors to monitor toxic substances. In this vein, Velmurugan et al. [40] designed and prepared β-CD on the graphene oxide (GO) surface using a benign synthetic procedure (ultra-sonication method). The authors optimized the loading level of β-CD on the GC surface to improve the performance of the NB sensor. The β-CD/GO/SPCE was used as the NB sensor, and its performance was checked using the CV and LSV techniques. The authors reported that the presence of synergistic interactions between β-CD and GO improved the performance of β-CD/GO/SPCE towards the determination of NB. The β-CD1.2 mg/GO/SPCE was found to be an optimized electrode for the sensing of NB. The β-CD1.2 mg/GO/SPCE exhibited a linear range of 0.5 to 1000 µM, with an LOD of 0.184 µM. β-CD1.2 mg/GO/SPCE also has acceptable reproducibility, good sensitivity, and decent stability for the sensing of NB. The authors also proposed the potential of β-CD1.2 mg/GO/SPCE for practical purposes due to good recoveries in real samples.
Kubendhiran et al. [41] explored the potential of a reduced graphene oxide/nickel tetraphenyl porphyrin (GRGO/Ni-TPP) nanocomposite as a sensing material for the fabrication of an NB sensor. The authors synthesized GRGO via an eco-friendly method, utilizing caffeic acid as the reducing agent. Furthermore, the authors reported that the GRGO/Ni-TPP composite material was formed by π-π stacking interactions between RGO and Ni-TPP. The interaction and vibrational band in the prepared GRGO/Ni-TPP were confirmed by FTIR analysis. The synthesized GRGO/Ni-TPP was drop casted on the active surface of the bare GC electrode. The GRGO/Ni-TPP-modified GC electrode was further used for the sensing of NB. EIS studies also revealed that the GRGO/Ni-TPP/GC electrode has higher conductivity with a low charge transfer resistance value compared to the GC electrode. The CV graph of the GRGO/Ni-TPP/GC electrode was recorded in the absence and presence of NB.
Figure 5a reveals that no redox peaks appeared for GRGO/Ni-TPP/GCE in the absence of NB. However, sharp redox peaks were observed in the presence of NB. The GRGO/Ni-TPP/GCE demonstrated a higher current response compared to the bare GCE (b), GRGO/GCE (c), NiTPP/GCE (d), and GO/NiTPP/GCE (e), as shown in Figure 5a. The CV of the GRGO/Ni-TPP/GC was also obtained under different pH conditions, and the authors found that GRGO/Ni-TPP/GCE is highly active under pH conditions of 7.0 (Figure 5b). The GRGO/Ni-TPP/GCE exhibited an LOD of 0.14 µM and sensitivity of 1.277 µA µM−1 cm−2. A wide linear dynamic range of 0.5 to 878 µM was also observed for GRGO/Ni-TPP/GCE towards the sensing of NB. Figure 5c shows the DPV results for GRGO/Ni-TPP/GCE in the presence of different concentrations of NB. It is seen that the current response linearly increases with increasing concentrations of NB (Figure 5d). The DPV results demonstrated better performance compared to the CV results. The GRGO/Ni-TPP/GCE also demonstrated excellent selectivity towards NB detection. The real sample investigations in water samples suggested that the GRGO/Ni-TPP/GCE can be used for practical applications. The improved performance of GRGO/Ni-TPP/GCE may be ascribed to the presence of the synergism between the GRGO and Ni-TPP.
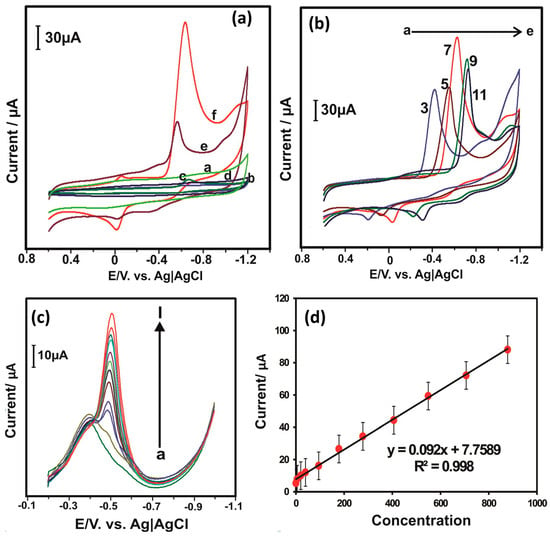
Figure 5.
(a) CV curve of the (a) GRGO/Ni-TPP/GCE in the absence of NB. CV of (b) bare GCE, (c) GRGO/GCE, (d) NiTPP/GCE, (e) GO/NiTPP/GCE and (f) GRGO/Ni-TPP/GCE in the presence of NB. (b) CV responses of the GRGO/Ni-TPP/GCE electrode in the presence of NB under different pH conditions. (c) DPV curves of the GRGO/Ni-TPP/GCE in the presence of different concentration of NB. (d) Corresponding calibration curve between current response and concentration of NB. Reprinted with permission [41].
In recent years, a new form of carbon material such as carbon dots (CDs) has received extensive attention from the scientific community because of its extraordinary optoelectronic properties, biocompatibility, ease of surface modification, and low toxicity. The CDs can be synthesized by hydrothermal methods using various C-sources. The utilization of waste materials to form CDs is of great significance. Thus, Bressi et al. [42] synthesized CDs using orange peel waste as a C source via a eco-friendly hydrothermal carbonization/electrochemical bottom-up synthetic process. The transformation of orange peel waste to CDs was confirmed by employing various sophisticated techniques. The authors reported that synthesized CDs have excellent electrochemical properties and deposited it onto the surface of an SPC electrode. The CD-modified SPC electrode was used as an NB sensor using DPV technology. The CD-modified SPC electrode exhibited an LOD, sensitivity, and linear range of 13 nM, 9.36 µA/µM cm2, and 0.1–200 µM, respectively. The excellent long-term stability, selectivity, and repeatability of the CD-modified SPC electrode suggested its potential for commercialization. Pandiyarajan et al. [43] also proposed the fabrication of a novel NB sensor using innovative strategies. In this vein, the authors proposed the synthesis of a Ag NP-decorated N-[3-(trimethoxysilyl) propyl]ethylenediamine (EDAS)-modified g-C3N4 composite for the construction of an NB sensor. The authors optimized the concentration of Ag NPs to improve the catalytic properties of the EDAS/(g-C3N4-Ag). The EDAS/(g-C3N4-Ag)-modified GC electrode demonstrated an LOD of 2 µM, sensitivity of 0.594 A M−1 cm−2, and linear range of 5 to 50 µM for the sensing of NB. Zhang et al. [44] reported the synthesis of 2D mesoporous carbon nitride (OMCN) using SBA-15 mesoporous silica as the template and melamine as the precursor. The synthesized OMCN was used as an electrode material for the modification of the GC electrode. The OMCN-modified GC electrode demonstrated an LOD of 1.52 µM for the sensing of NB.
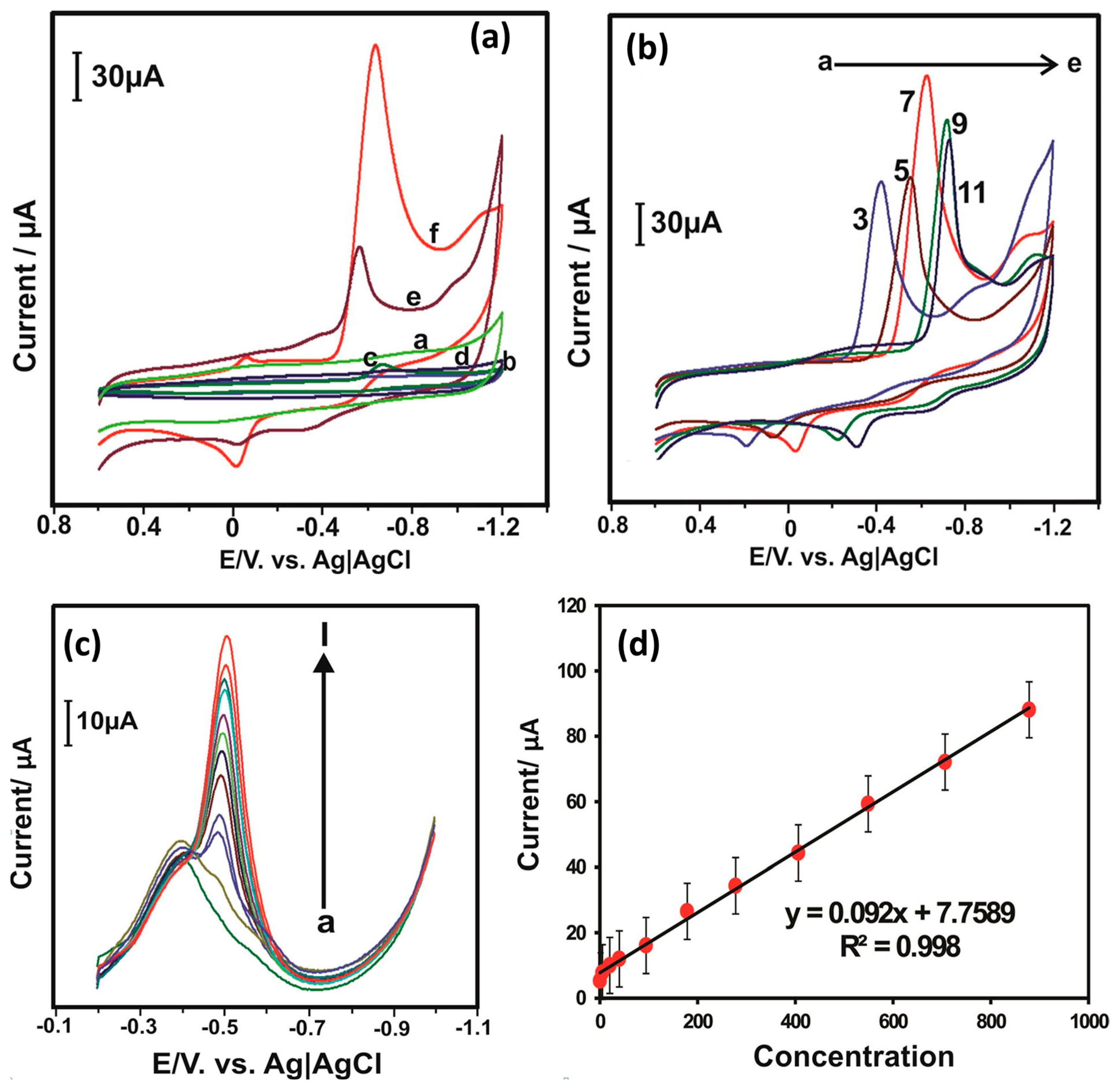
2.3. Bimetallic Materials and Metal Nanoparticle-Based Electrode Materials
Bimetallic materials with nanostructures have received great interest because of their excellent and unique chemical and physical properties and have been explored in various applications including sensors. Nickel–copper (Ni-Cu) is an environmental-friendly bimetallic material and has various advantages, such as low cost, high catalytic activity, and less-toxic nature. Previously, Yan et al. [45] adopted the Ni-Cu alloy electrode for the determination of NB using simple strategies. The authors used the electro-deposition method for the preparation of a Ni-Cu alloy electrode. The prepared Ni-Cu alloy electrode was characterized by X-ray diffraction (XRD) and scanning electron microscopy (SEM)-based analysis. The XRD results demonstrated the presence of (111) and (220) diffraction planes for the presence of the Ni-Cu alloy. The decent intensity of the diffraction peaks suggested a moderate crystalline nature of the prepared Ni-Cu alloy electrode. The SEM results also indicated the presence of small particles, which agglomerated with a rough surface. The authors stated that these surface properties may be useful for obtaining better electro-catalytic behavior of the Ni-Cu alloy electrode for the sensing of NB. A polarization method was used for the determination of NB, and the authors reported a limit of detection (LOD) of 4 × 10−5 M with a sensitivity of 298 µA/mM. The linear dynamic range of 0.1–20 mM has been reported for the sensing of NB with a correlation coefficient (R2) of 0.995 using the Ni-Cu alloy electrode. Platinum (Pt) NPs are a significant and highly active catalyst due to their excellent conductivity and catalytic properties. Zhang et al. [46] assembled Pt NPs on macroporous carbon (MPC) by using a simple and benign one-step microwave-assisted heating method. The synthesized Pt NPs/MPC was characterized by TEM, which revealed that the synthesized material has a uniform surface morphology. Further, the authors modified the GC electrode with Pt NPs/MPC (nafion was used as a binder) and investigated its performance for the determination of NB using the LSV method. The Pt NPs/MPC-modified GC electrode exhibited an LOD of 50 nM with a wide linear dynamic range of 1–200 µM. The Pt NPs/MPC-modified GC electrode also exhibited decent recovery in real samples. Rameshkumar et al. [47] prepared silver nanoparticles (Ag NPs) on an amine-functionalized SiO2 sphere-modified electrode. The Ag NPs/SiO2-modified electrode was then used as an NB sensor, and the electrochemical properties of this Ag NPs/SiO2-modified electrode was determined by CV and square wave voltammetry (SWV). The CV results indicated that the Ag NPs/SiO2-modified electrode has higher catalytic activity compared to the GC electrode. The effect of various concentrations of NB was studied by employing SWV, and the authors found that the increase in the current response of the Ag NPs/SiO2-modified electrode is directly proportional to the concentration of NB. The improved performance of the Ag NPs/SiO2-modified electrode may be ascribed to the presence of a large number of Ag NPs on the SiO2 surface. The Ag NPs/SiO2-modified electrode exhibited an LOD of 500 nm for the sensing of NB derivatives. Thirumalraj et al. [48] explored the potential of an alumina-polished GC electrode for the sensing of NB. The CV curves of the alumina/GC electrode are shown in Figure 6a.
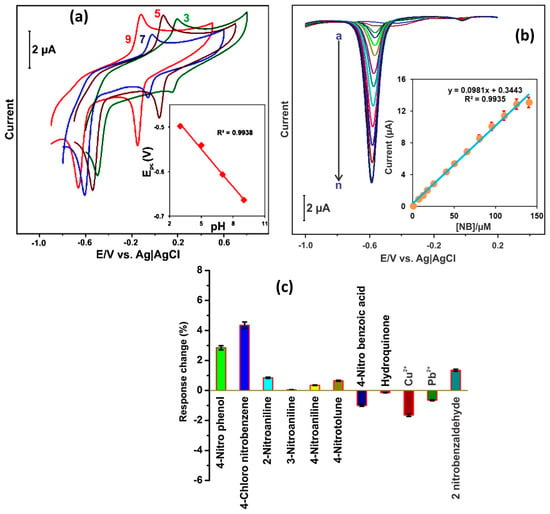
Figure 6.
(a) CV patterns of the alumina/GCE for the presence of 50 µM NB at different pHs. (b) DPV curves of the alumina/GCE in the presence of different concentrations of NB. (c) Selectivity of alumina/GCE for NB sensing. Reprinted with permission [48].
The authors optimized the pH of the solution to obtain higher electro-catalytic activity in the alumina/GC electrode. According to Figure 6a, it is clear that the alumina/GC electrode has higher catalytic properties under a pH of 5. The authors also used the DPV method for further investigations and found that DPV is a more efficient method for the sensing of NB. The current responses increase with increasing an concentration of NB (Figure 6b). Furthermore, the authors also investigated the selectivity of the alumina/GC electrode for the sensing of NB in the presence of various interfering materials. The selectivity studies are summarized in Figure 6c. It is suggested that alumina/GC electrodes have good selective nature for the sensing of NB in the presence of different interfering molecules. The alumina/GC electrode demonstrated an LOD of 0.15 µM. The alumina/GC electrode also showed good real sample studies in lake, tap, waste water, and drinking samples.
It is also reported that gold nanoparticles (Au NPs) may further improve the electrochemical performance of NB sensors. In this vein, Gupta et al. [49] reported the fabrication of an electrochemical sensing scaffold (ESS) for the determination of NB. The authors grew Au NPs on mesoporous SiO2 microspheres and used them as a catalyst for the determination of NB. The Au NPs/SiO2/GCE was employed as an NB sensor, and its electrochemical activities were checked by using the CV, DPV, and CA methods. The EIS also suggested the presence of a relatively high conductive nature and catalytic properties of the Au NPs/SiO2-modified GC electrode. The DPV technique suggested that the current response increases with increasing concentrations of NB. A wide linear dynamic range of 0.1 µM to 2.5 mM has been observed. An excellent LOD of 15 nM was achieved for the sensing of NB using Au NPs/SiO2/GCE. The authors also reported excellent selectivity of the Au NPs/SiO2/GCE towards the determination of NB in various interfering compounds. Rameshkumar et al. [50] proposed the fabrication of an NB sensor by employing simple protocols. The authors prepared silicate sol–gel-stabilized (SSG) Ag NPs using a benign one-pot synthetic process, and a mixture of hydrazine, nitric acid, and ammonium chloride was used as the reduction solution system. Initially, the authors used colorimetric methods for the determination of Hg (II) ions and reported an LOD of 5 µM. Furthermore, the authors explored the synthesized Ag NPs for the sensing of NB. The authors modified the active area of the bare GC electrode using Ag NPs as the catalyst and SWV as the sensing technique. The lowest LOD of 1 µM was reported by the authors for the sensing of NB using the square wave voltammetry (SWV) method. The enhanced performance of the NB sensor may be attributed to the presence of highly catalytic Ag NPs on the surface of the GC electrode.
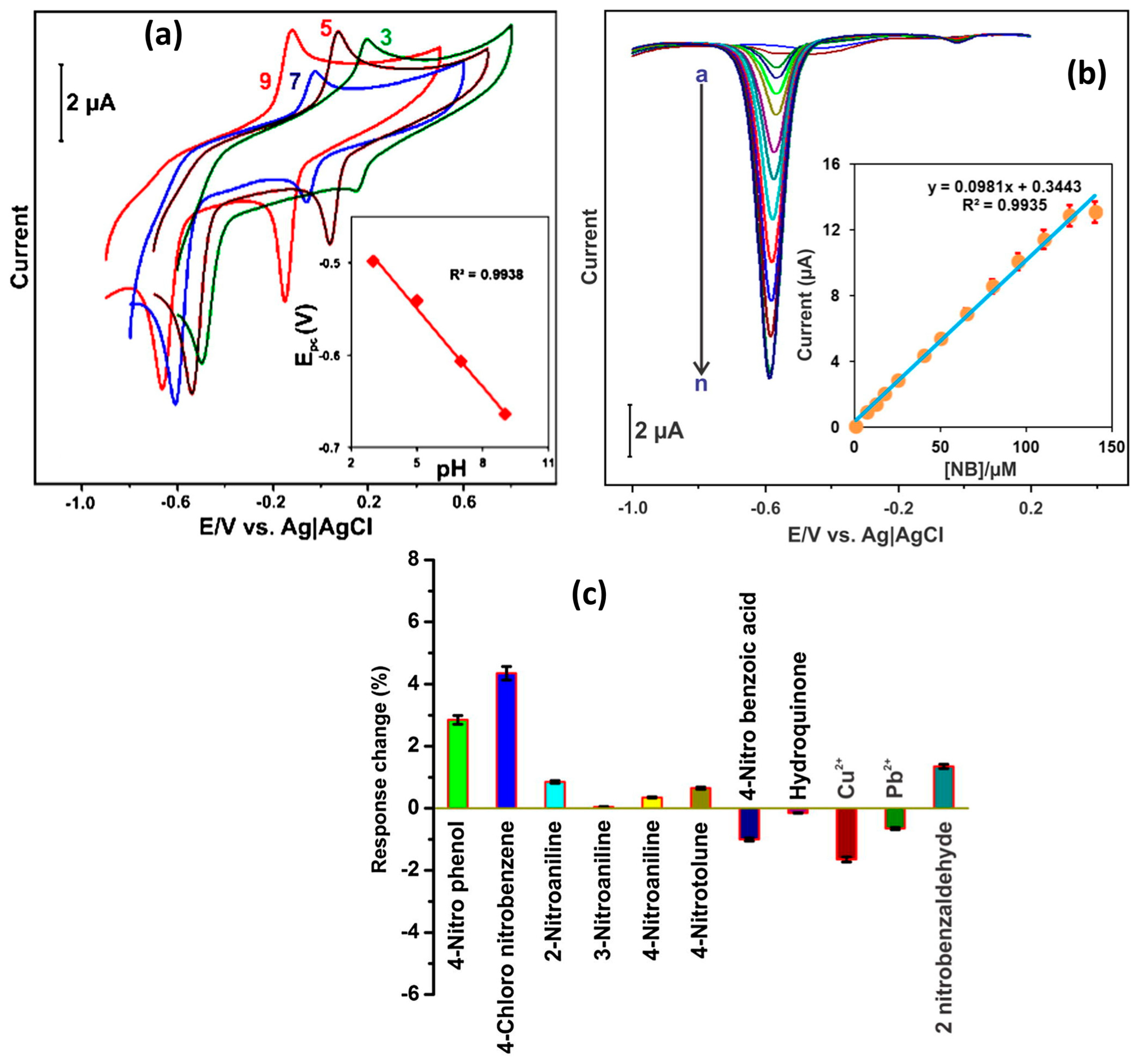
2.4. Metal Dichalcogenides, Polymers, MOF, Metal Sulfides, and Other Electrode Materials
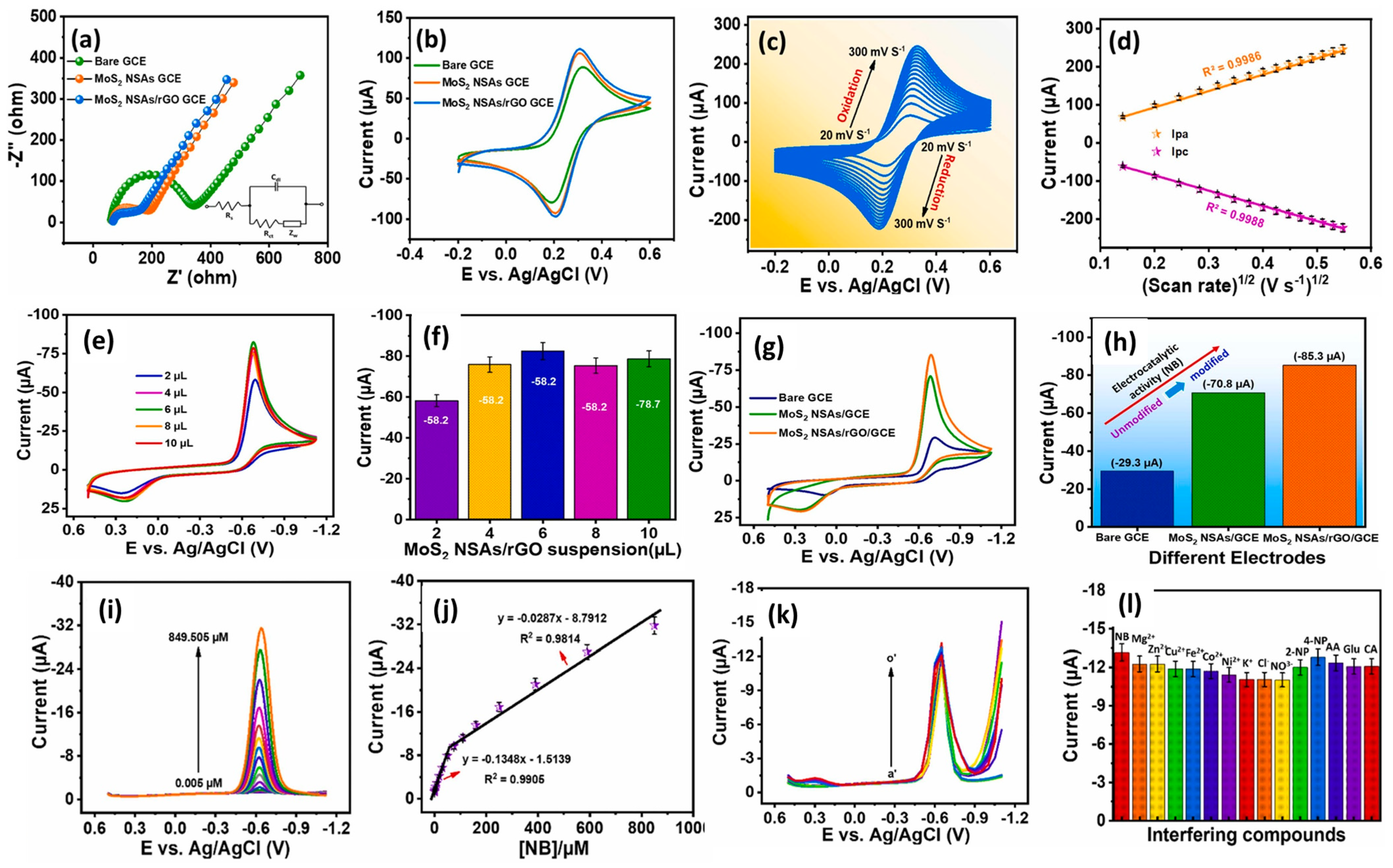
It is considered that nanoscale electrode materials play a crucial role in the design and fabrication of N2BHJU7 highly sensitive electrochemical sensors. Molybdenum disulfide (MoS2) is a widely used dichalcogenide in the construction of electrochemical sensors and energy storage applications. The catalytic properties of MoS2 can be further improved by incorporating MoS2 with rGO for electrochemical reactions. Nehru et al. [51] designed and prepared flower-like MoS2 nanosheet arrays (MoS2 NSA)/rGO hybrid composite material using the hydrothermal method. Furthermore, a GC electrode was modified with the prepared MoS2 NSAs/rGO as the sensing catalyst material, and CV/DPV techniques were adopted for the determination of NB. Figure 7a presents the EIS results for the differently modified electrodes under similar conditions. It is seen that MoS2 NSAs/rGO/GCE has low charge transfer resistance (Rct) and high conductivity. The obtained CV results for the different modified GCEs are shown in Figure 7b. The MoS2 NSAs/rGO/GCE exhibited a higher current response, which may be ascribed to the improved conductivity and better electro-catalytic properties. Figure 7c shows the CVs of the MoS2 NSAs/rGO/GCE at different scan rates, indicating that the current response linearly increases with increasing scan rates, and it is a diffusion-controlled process (Figure 7d). In terms of the effect of mass loading, as also studied, the authors reported 6 µL catalysts as the optimum amount for the sensing of NB (Figure 7e,f). The authors also recorded the CVs of the bare GCE, MoS2 NSAs/GCE, and MoS2 NSAs/rGO/GCE in the presence of NB. MoS2 NSAs/rGO/GCE exhibited a higher current response for the detection of NB compared to bare GCE and MoS2 NSAs/GCE (Figure 7g,h). Figure 7i shows DPV curves of the MoS2 NSAs/rGO/GCE at various concentrations of NB, and calibration curves between the current response and concentration of NB are depicted in Figure 7j. The authors found that the current value of the MoS2 NSAs/rGO/GCE increases linearly with increasing concentrations of NB. This proposed sensor demonstrated a linear range of 0.005 to 849.505 µM with a sensitivity of 1.8985 µA/µM cm2 for the determination of NB. The MoS2 NSAs/rGO/GCE also demonstrated an LOD of 0.0072 µM, with excellent cyclic stability, reproducibility, and repeatability. The authors investigated the selectivity test for the constructed MoS2 NSAs/rGO/GCE towards the determination of NB and found that MoS2 NSAs/rGO/GCE has excellent selectivity (Figure 7k,l). The MoS2 NSAs/rGO/GCE exhibits acceptable recoveries in real sample investigations, which suggests that MoS2 NSAs/rGO/GCE is a promising candidate for the sensing of NB in real life applications.
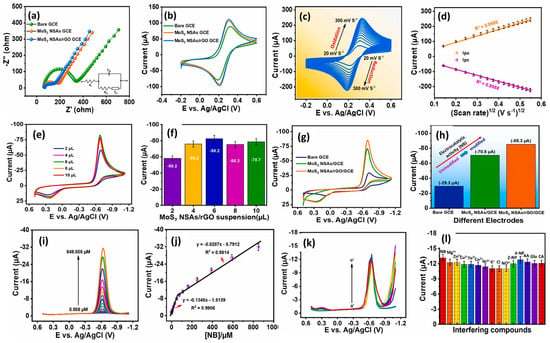
Figure 7.
(a) Nyquist curves and (b) CV graphs of bare GCE, MoS2 NSAs/GCE, and MoS2 NSAs/rGO/GCE in [Fe(CN)6]3−/4− redox probe (0.1 M KCl). (c) CV curves of MoS2 NSAs/rGO/GCE at different scan rates in the redox system and (d) corresponding calibration plot between peak current response and square root of scan rate. (e) CV curves and (f) cathodic current response of different volume of MoS2 NSAs/rGO/GCE in the presence of 200 µM NB in 0.05 M PBS. (g) CV curves and (h) cathodic current response of bare GCE, MoS2 NSAs/GCE, and MoS2 NSAs/rGO/GCE in the presence of 200 µM NB. (i) DPV curves of the MoS2/NSA/rGO/GCE in different concentrations of NB. (j) Calibration curve between current responses and concentration of NB. (k) DPV curves and (l) corresponding relative peak of the MoS2/NSA/rGO/GCE for selectivity test towards the sensing of NB in the presence of interfering substances. Reprinted with permission [51].
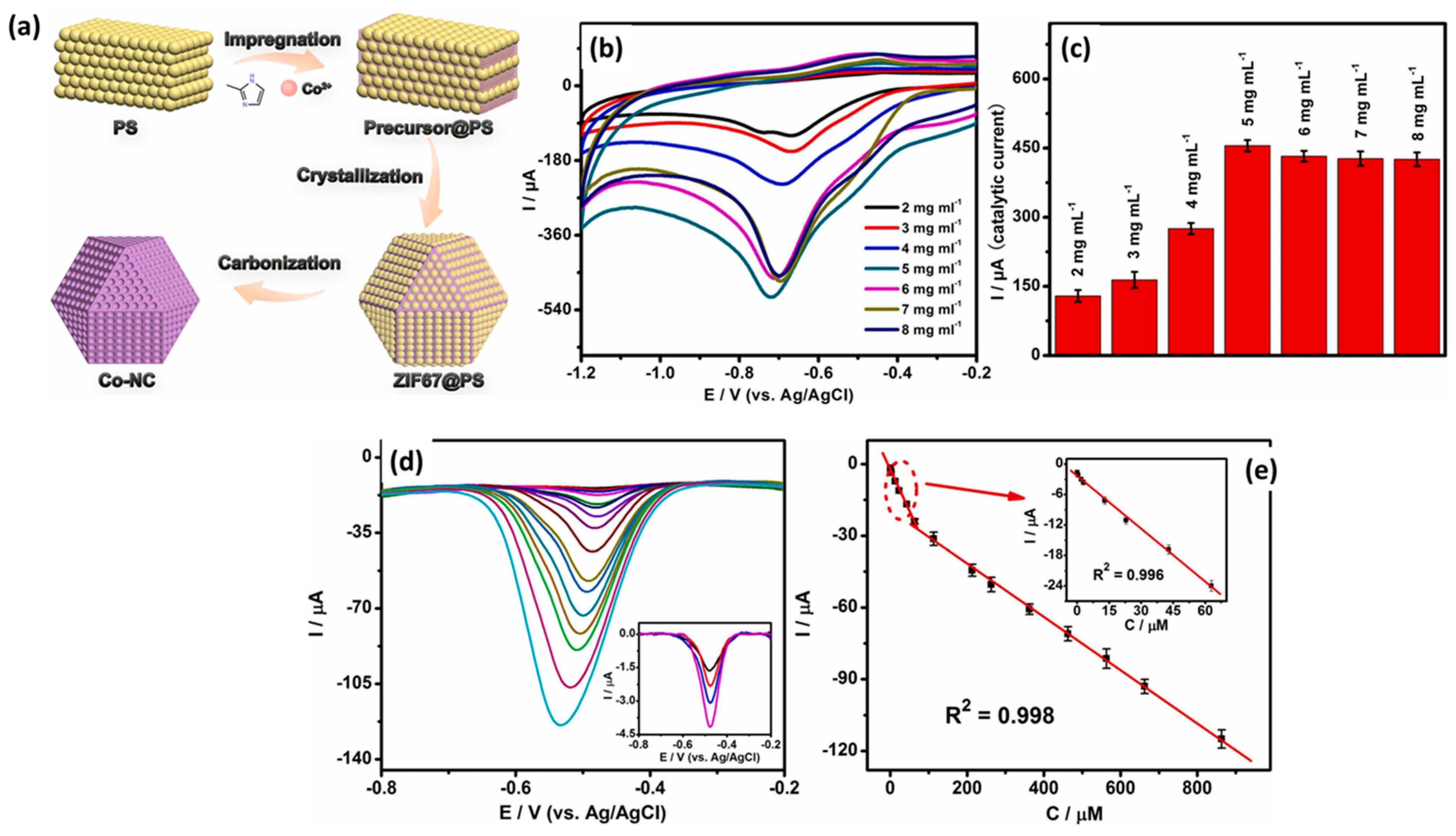
Recently, Papavasileiou et al. [52] reported the synthesis of 2D vanadium diselenide nanoflakes (VSe2 NFs) for the construction of an NB sensor. The VSe2 was coated on a GC electrode, and CV/DPV was used for the determination of NB. An LOD of 0.03 µM was reported with a linear range of 0.1 to 4 µM. The proposed sensor also showed acceptable recovery of 96% in real sample investigations. Karthik et al. [53] also reported strontium molybdate (SrMoO4) microflower/three-dimensional (3D) nitrogen-doped reduced graphene oxide aerogels (N-rGO) for the sensitive detection of NB in water samples. The SrMoO4/N-rGO-modified GC electrode demonstrated a remarkable LOD of 2.1 nM and linear range of 7.1 nM to 1.0 mM for the detection of NB. The performance of the SrMoO4/N-rGO-modified GC electrode also suggested that it can be used for real sample investigations with acceptable recoveries in a range of 96.1–99.6%. Polymers are well known for their excellent conductive nature, and their composite may be a promising electrode material for various electrochemical applications. In this context, Ramirez et al. [54] prepared a polymer nanocomposite (PNC) on flowers like the hierarchical rutile phase of the titanium dioxide (TiO2) nanorod microsphere. The authors optimized various conditions and reported the synthesis of unique surface morphologies (flower-like/cauliflower). The surface morphology of the synthesized PNC on TiO2/GO was characterized by employing SEM and TEM analysis. The authors modified the graphite electrode with the synthesized TiO2/GO and adopted this modified electrode as an NB sensor. The authors found that the sensing performance of the TiO2/GO-modified graphite electrode increases with increasing concentrations of NB. An improved LOD of 2.64 ppb was reported for the detection of NB using a TiO2/GO-modified graphite electrode. The TiO2/GO-modified graphite electrode also demonstrated acceptable repeatability, stability, and reproducibility towards the sensing of NB. The TiO2/GO-modified graphite electrode also exhibited excellent recovery in real sample applications. Yadav et al. [55] reported the synthesis of a Au NP-incorporated zinc-based metal–organic framework (MOF-5) and characterized by various sophisticated physicochemical techniques. The synthesized Au-MOF-5 was deposited onto the surface of a GC electrode and used as an electrochemical sensor towards the sensing of NB. The Au-MOF-5-modified GC electrode demonstrated improved electro-catalytic properties compared to the bare GC electrode, and this improved performance may be ascribed to the presence of the electrode material on the surface of the GC electrode. An interesting LOD of 1 µM with a sensitivity of 0.23 µA/µM cm2 was observed for the sensing of NB by using a Au-MOF-5-modified GC electrode. The excellent selectivity of the Au-MOF-5-modified GC electrode may be attributed to synergistic interactions. Zeolitic imidazole framework (ZIF) materials are well-known highly porous materials with a high surface area. The ZIF materials may be a promising material for various electrochemical applications. It would be of great significance to propose and fabricate ZIF-based hybrid or metal-doped materials for the construction of electrochemical sensors. An et al. [56] synthesized ZIF-67 material using simple protocols, and a synthetic process is shown in Figure 8a. The Co-NC was prepared by using ZIF-67 and PS nanospheres as the template and carbonizing process.
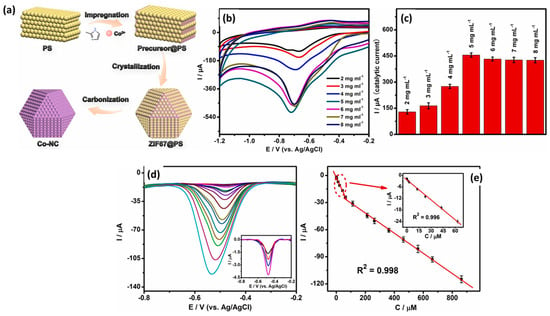
Figure 8.
(a) Schematic representation for the preparation of ZIF67@PS-derived Co-NC. (b) CV curves and (c) corresponding current values of the Co-NC-800-GCE (different mass loadings) in the presence of NB. (d) DPV curves of Co-NC-800-GCE in different concentrations of NB and (e) corresponding calibration plot between peak current and concentration of NB. Reprinted with permission [56].
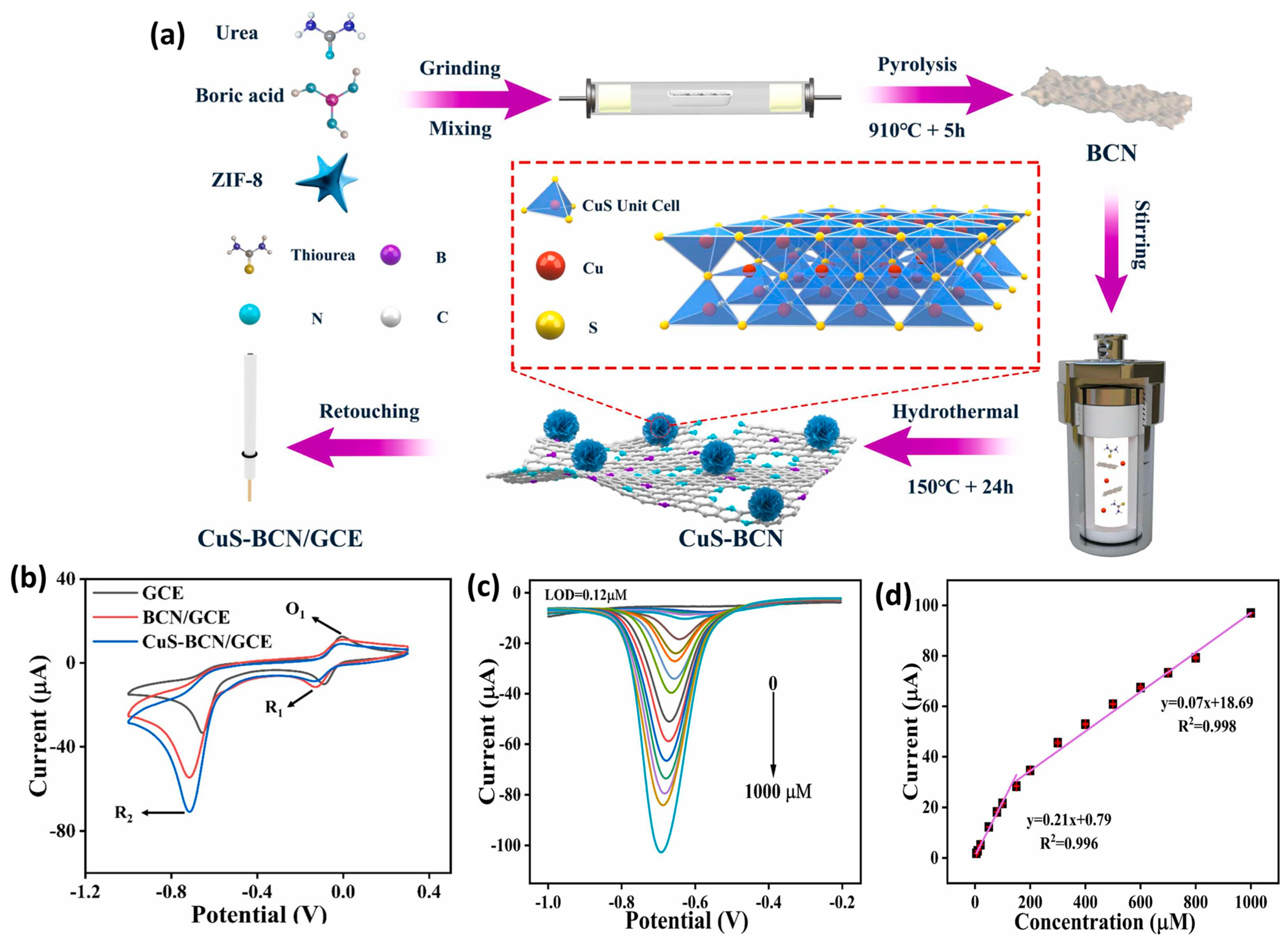
The authors also optimized the temperature of Co-NC synthesis and found that 800 °C is the suitable temperature for the preparation of a porous structure. The presence of N-atoms in the carbon framework improves the catalytic properties of the prepared materials. The Co-NC was coated on a GC electrode and used as a working electrode towards the sensing of NB. The loading mass of the catalyst was also optimized as 5 mg/mL using CV (Figure 8b,c). The effects of different concentrations were also studied using Co-NC-800-GCE. The DPV graphs demonstrated that the current linearly increases with increasing concentrations of NB (Figure 8d,e). The Co-NC-800-GCE demonstrated good selectivity for the determination of NB in various interfering substances. The Co-NC-800-GCE showed an LOD of 0.086 µM and linear range of 0.1 µM to 0.863 mM. The stability and better selectivity of the Co-NC-800-GCE was attributed to the presence of the robust electrode material on the GC surface. This work proposed the construction of a simple and highly selective NB sensor using robust electrode material. Li et al. [57] reported an MOF-conductive polymer composite film-modified electrode as an NB sensor, which demonstrated an LOD of 0.047 µM and linear range of 0.05 to 1 µM and 1 to 100 µM. Copper sulfide (CuS) is a semiconducting metal sulfide and has an optical band gap in the range of 1.23 to 2.0 eV. CuS has been widely used in various catalytic applications and energy-related applications such as lithium sulfur batteries and energy storage devices. It is also well reported that combining CuS may improve the properties of the hybrid composite materials. Carbon-based materials are desirable materials to form the composite materials. It is also known that doping with heteroatoms may further create defects and improve the catalytic properties of carbon-based materials. In this vein, Yuan et al. [58] designed and reported the facile synthesis of a CuS-loaded boron, nitrogen co-doped carbon composite (CuS-BCN) material using a simple synthetic method (Figure 9a). The XRD results suggested the formation of CuS-BCN, whereas SEM analysis revealed the presence of the surface structural morphology of the prepared CuS-BCN composite. The bare GC electrode was modified using CuS-BCN as a catalyst material, and its electrochemical performance was checked using the CV, EIS, and SWV methods. The EIS results showed that the CuS-BCN-coated GC electrode has a low resistance value compared to the bare GC electrode and revealed that the CuS-BCN-coated GC electrode has high conductivity. The CV results also demonstrated the presence of the relatively higher electro-catalytic behavior of the CuS-BCN-coated GC electrode (Figure 9b). The SWV demonstrated an excellent LOD of 0.12 µM using a CuS-BCN-coated GC electrode as an NB sensor. Two linear ranges of 0.5 to 150 µM and 150 to 1000 µM were reported for the sensing of NB using a CuS-BCN-coated GC electrode (Figure 9c,d). It was observed that SWV is more sensitive compared to the CV. Improved selectivity was observed for the detection of NB in the presence of various interfering substances by using a CuS-BCN coated GC electrode. The authors also reported excellent NB recovery in real water samples using a CuS-BCN-coated GC electrode. Thus, the authors proposed that the CuS-BCN-coated GC electrode is a promising sensing candidate for the determination of NB.
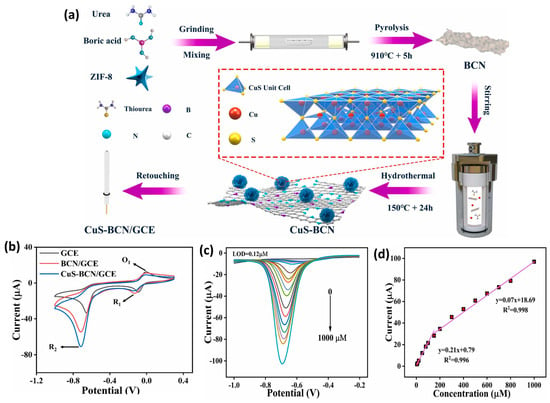
Figure 9.
(a) Schematic representation for the synthesis of CuS-BCN and surface modification of GC electrode for the sensing of NB. (b) CV curves of the GCE, BCN/GCE, and CuS/BCN/GCE in the presence of NB. (c) SWV curves of the CuS/BCN/GCE at different concentrations of NB and (d) corresponding calibration plot between peak current and concentration of NB. Reprinted with permission [58].
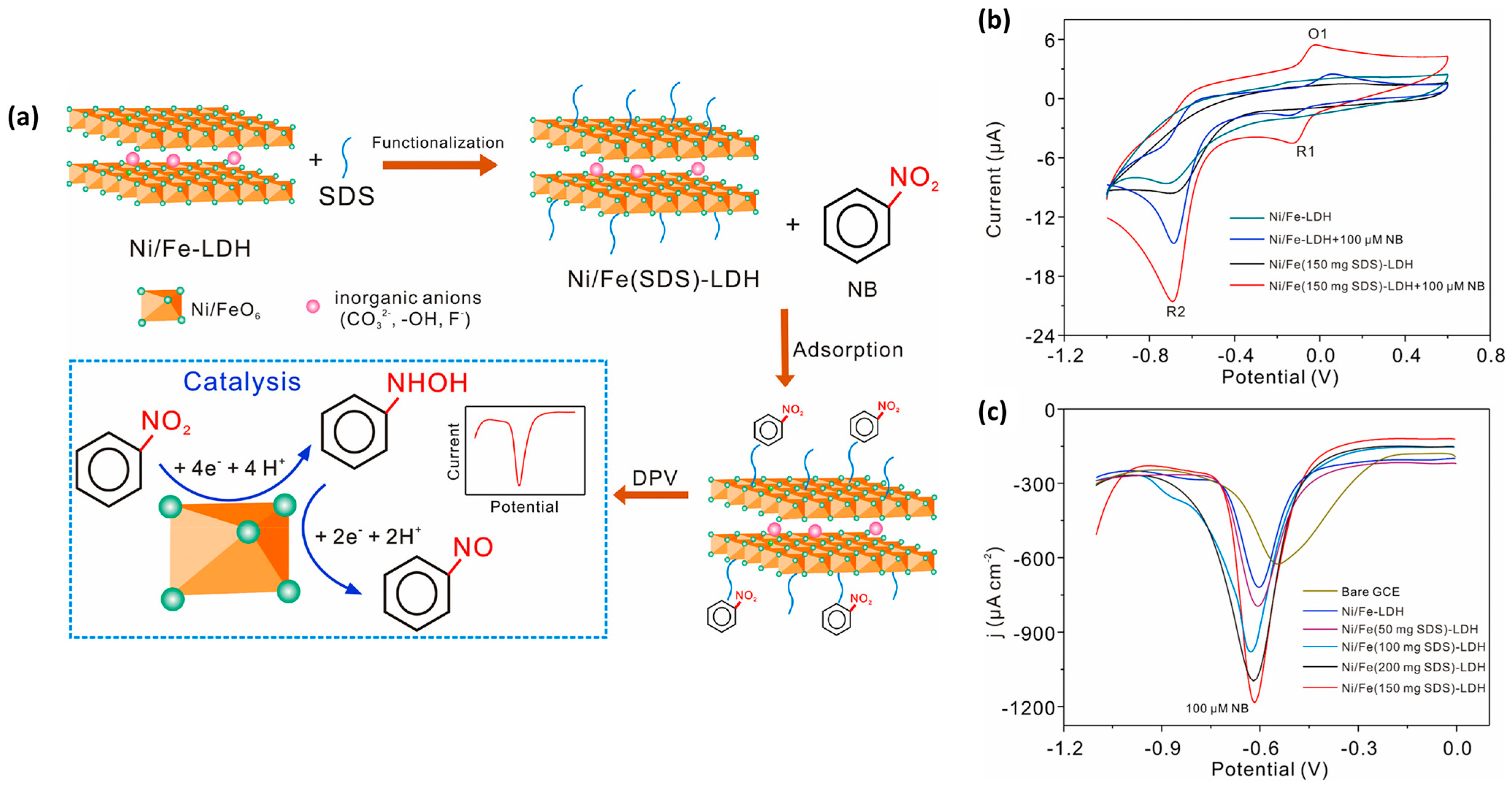
Recent years have witnessed a rapid surge in the synthesis and use of layered double hydroxides (LDHs) due to their excellent intrinsic properties for catalytic activity and interesting adsorption properties. LDH materials can be prepared via a hydrothermal method, and their properties can be tuned by incorporating with other materials. Li et al. [59] prepared a novel electrode material (Ni/Fe LDH) functionalized with sodium dodecyl sulfate (Ni/Fe(SDS)-LDH) for electrochemical sensing applications. This proposed 2D Ni/Fe(SDS)-LDH material was deposited on a GC electrode for electrochemical sensing studies. The electro-catalytic activities of the Ni/Fe(SDS)-LDH-modified GC electrode was evaluated by employing CV and DPV methods. The authors clearly found that the Ni/Fe(SDS)-LDH-modified GC electrode has higher catalytic properties compared to the bare GC electrode, suggesting that the presence of Ni/Fe(SDS)-LDH on the active surface of the GC electrode enhanced its catalytic behavior for the detection of NB. A low LOD of 0.093 µM was achieved using a Ni/Fe(SDS)-LDH-modified GC electrode for the determination of NB. The DPV results revealed that the Ni/Fe(SDS)-LDH-modified GC electrode has excellent selectivity for the sensing of NB, and this may be ascribed to the interactions/bonding between the NB and Ni/Fe(SDS)-LDH-modified GC electrode (Figure 10a). The CV curves of the different electrodes in the absence and presence of NB are shown in Figure 10b. It is seen that Ni/Fe(150 mg SDS)-LDH/GCE has high catalytic properties for the sensing of NB. The DPV graphs for Ni/Fe(0–200 mg SDS)-LDH/GCE are shown in Figure 10c. It is observed that Ni/Fe(150 mg SDS)-LDH/GCE has high electro-catalytic activities towards NB. The Ni/Fe(0–200 mg SDS)-LDH/GCE also demonstrated good recoveries for real sample investigations in tap water and underground water samples.
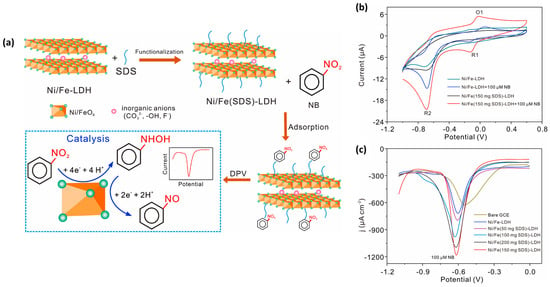
Figure 10.
(a) Schematic representation for the working mechanism of Ni/Fe(SDS)-LDH-modified GC electrode towards the determination of NB. (b) CV curves of the different electrodes in absence and presence of NB. (c) DPV curves of the different electrodes in the presence of NB. Reprinted with permission [59].
Perovskite materials have received extensive attention because of their excellent optoelectronic properties. Zhang et al. [60] synthesized an inorganic perovskite-based composite with high hydrophilicity. The synthesized CsPbBr3/TDPA exhibited high water stability due to the presence of oleylamine molecules. The ECL technique was used for the sensing of NB using CsPbBr3/TDPA as a sensing material. The authors reported an LOD of 0.05 µM with a linear range 1 mm to 0.1 µM towards the determination of NB. Rastogi et al. [61] reported a novel sensing system consisting of palladium nanoparticle-decorated polymer-silica (Pd-GG-g-PAM-silica). The authors modified the GC electrode using Pd-GG-g-PAM-silica as a sensing catalyst, and its performance was checked by employing the LSV, DPV, and CA techniques. The Pd-GG-g-PAM-silica-modified GC electrode demonstrated good reproducibility, acceptable repeatability, and high stability for the reduction/sensing of NB. An LOD of 0.06 mM with two linear ranges of 1 to 1900 mM and 1900 to 3900 mM were reported for the sensing of NB using a Pd-GG-g-PAM-silica-modified electrode. The authors reported that real sample recoveries are acceptable using the spike method. In the above sections, we described numerous electrode materials and their applications in the fabrication of NB sensors. The performance of the above-discussed NB sensors is summarized in Table 1 [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].

Table 1.
Electrochemical parameters of the published NB sensors [27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
3. Conclusions and Future Perspectives
We conclude that various electrode materials based on metal oxides (e.g., magnesium oxide, zinc oxide), graphene, carbon nitride, metal nanoparticles (e.g., silver or gold), metal–organic frameworks, or their composites have been reviewed for the determination of NB. The published articles demonstrated that the sensitivity and detection limit of NB sensors are the two major key parameters, which should be improved. In addition, electrochemical sensors should have excellent anti-interfering properties. Each material has its own advantages and disadvantages. From the published reports, it has been observed that metal oxide such as the cerium oxide-modified electrode demonstrated an excellent detection limit using the DPV method. On the other hand, the Ni-Cu-modified electrode exhibits a poor detection limit using the polarization method. Thus, the polarization method is not a highly efficient approach for the electrochemical sensing of NB. The CV-method-based sensing results demonstrated lower performance compared to the amperometry. This showed that CV is also a less sensitive method for the detection of NB compared to DPV or amperometry. It is clear that the DPV method is a more efficient and sensitive technique for the determination of NB. In addition, metal nanoparticles (gold, silver, platinum, etc.) have a highly conductive nature, but they are not suitable materials for NB sensing applications due to their high cost and poor performance. It is seen that metal oxides with hybrid composite materials are more efficient materials for the construction of NB sensors. However, some challenges still exist, such as poor adhesiveness of metal oxide particles on the surface of electrodes, the use of binders which reduce the catalytic activity and conductivity of the modified electrodes, and the low conductive nature of the metal oxides. It is required to combine metal oxides with highly conducting MXenes to form efficient electrode materials for the construction of NB sensors. Thus, it is believed that the performance of NB sensors can be further enhanced by incorporating metal oxides with MXenes.
Author Contributions
Conceptualization, K.A. and T.H.O.; writing—original draft preparation, K.A.; writing—review and editing, T.H.O.; supervision, T.H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wu, Y.; Qi, H.; Li, B.; Zhanhua, H.; Li, W.; Liu, S. Novel hydrophobic cotton fibers adsorbent for the removal of nitrobenzene in aqueous solution. Carbohydr. Polym. 2017, 155, 294–302. [Google Scholar] [CrossRef]
- Raza, W.; Ahmad, K.; Kim, H. Fabrication of defective graphene oxide for efficient hydrogen production and enhanced 4-nitro-phenol reduction. Nanotechnology 2021, 32, 495404. [Google Scholar] [CrossRef]
- Nemakal, M.; Aralekallu, S.; Mohammed, I.; Pari, M.; Reddy, K.R.V.; Sannegowda, L.K. Nanomolar detection of 4-aminophenol using amperometric sensor based on a novel phthalocyanine. Electrochim. Acta 2019, 318, 342–353. [Google Scholar] [CrossRef]
- Sajjan, V.A.; Aralekallu, S.; Nemakal, M.; Palanna, M.; Prabhu, C.P.K.; Sannegowda, L.K. Nanomolar detection of 4-nitrophenol using Schiff-base phthalocyanine. Microchem. J. 2021, 164, 105980. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Ansari, S.N.; Mobin, S.M. Construction of graphene oxide sheets based modified glassy carbon electrode (GO/GCE) for the highly sensitive detection of nitrobenzene. Mater. Res. Express 2018, 5, 075601. [Google Scholar] [CrossRef]
- Mohammad, A.; Ahmad, K.; Rajak, R.; Mobin, S.M. Binder Free Modification of Glassy Carbon Electrode by Employing Reduced Graphene Oxide/ZnO Composite for Voltammetric Determination of Certain Nitroaromatics. Electroanalysis 2018, 30, 274–282. [Google Scholar] [CrossRef]
- Ahmad, K.; Mohammad, A.; Mathur, P.; Mobin, S.M. Preparation of SrTiO3 perovskite decorated rGO and electrochemical detection of nitroaromatics. Electrochim. Acta 2016, 215, 435–446. [Google Scholar] [CrossRef]
- Kumar, P.; Khan, M.Q.; Khan, R.A.; Ahmad, K.; Kim, H. Hydrothermal Synthesis of MnO2/Reduced Graphene Oxide Composite for 4-Nitrophenol Sensing Applications. Inorganics 2022, 10, 219. [Google Scholar] [CrossRef]
- Beauchamp, R.O.; Irons, R.D.; Rickert, D.E.; Couch, D.B.; Hamm, T.E.; Lyon, J.P. A critical review of the literature on nitrobenzene toxicity. Crit. Rev. Toxicol. 1982, 11, 33–84. [Google Scholar] [CrossRef]
- Hanif, S.; Junaid, H.M.; Munir, F.; Waseem, M.T.; Majeed, S.; Shahzad, S.A. AIEE active new fluorescent and colorimetric probes for solution and vapor phase detection of Nitrobenzene: A reversible mechanochromism and application of logic gate. Microchem. J. 2022, 175, 107227. [Google Scholar] [CrossRef]
- Huang, X.L.; Liu, L.; Gao, M.L.; Han, Z.B. A luminescent metal-organic framework for highly selective sensing of nitrobenzene and aniline. RSC Adv. 2016, 6, 87945–87949. [Google Scholar] [CrossRef]
- Riskin, M.; Tel-Vered, R.; Lioubashevski, O.; Willner, I. Ultrasensitive surface plasmon resonance detection of trinitrotoluene by a bis-aniline-cross-linked Au nanoparticles composite. J. Am. Chem. Soc. 2009, 131, 7368–7378. [Google Scholar] [CrossRef]
- Rajeevan, G.; Ramesh, A.; Madanan, A.S.; Varghese, S.; Abraham, M.K.; Shkhair, A.I.; Indongo, G.; Arathy, B.K.; George, S. Efficient nanostructured Cs2CuBr2Cl2 perovskite as a fluorescent sensor for the selective “Switch Off” detection of nitrobenzene. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2024, 318, 124481. [Google Scholar] [CrossRef]
- Babaee, S.; Beiraghi, A. Micellar extraction and high performance liquid chromatography-ultra violet determination of some explosives in water samples. Anal. Chim. Acta 2010, 662, 9–13. [Google Scholar] [CrossRef]
- Berg, M.; Bolotin, J.; Hofstetter, T.B. Compound-specific nitrogen and carbon isotope analysis of nitroaromatic compounds in aqueous samples using solid-phase microextraction coupled to GC/IRMS. Anal. Chem. 2007, 79, 2386–2393. [Google Scholar] [CrossRef]
- Kumar, V.; Maiti, B.; Chini, M.K.; De, P.; Satapathi, S. Multimodal Fluorescent Polymer Sensor for Highly Sensitive Detection of Nitroaromatics. Sci. Rep. 2019, 9, 7269. [Google Scholar] [CrossRef]
- Furstenberg, R.; Kendziora, C.A.; Stepnowski, J.; Stepnowski, S.V.; Rake, M.; Papantonakis, M.R.; Nguyen, V.; Hubler, G.K.; McGill, R.A. Stand-off detection of trace explosives via resonant infrared photothermal imaging. Appl. Phys. Lett. 2008, 93, 224103. [Google Scholar] [CrossRef]
- Ko, H.; Chang, S.; Tsukruk, V.V. Porous substrates for label-free molecular level detection of nonresonant organic molecules. ACS Nano 2009, 3, 181–188. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Sikka, R.; Kumar, P. Optical Sensing Capability Evaluation for Methylammonium Based Perovskites for Explosive. J. Fluoresc. 2023, 33, 1677–1682. [Google Scholar] [CrossRef]
- Ahmad, K.; Khan, R.A. Design and fabrication of nitrogen-doped tungsten trioxide-based 4-nitrobenzene sensor. J. Mater Sci. Mater. Electron. 2024, 35, 1061. [Google Scholar] [CrossRef]
- Moro, G.; Bottari, F.; Loon, J.V.; Bois, E.D.; Wael, K.D.; Moretto, L.M. Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019, 146, 111758. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Li, Y.; Chen, X.; Qin, H.; Yang, J.; Fan, S.; Wu, H. Construction of porphyrinic manganese-organic frameworks based on structural regulation for electrochemical determination of nitrobenzene in water and vegetable samples. Front Chem. 2024, 12, 1380551. [Google Scholar] [CrossRef]
- Niyitanga, T.; Chaudhary, A.; Ahmad, K.; Kim, H. Titanium Carbide (Ti3C2Tx) MXene as Efficient Electron/Hole Transport Material for Perovskite Solar Cells and Electrode Material for Electrochemical Biosensors/Non-Biosensors Applications. Micromachines 2023, 14, 1907. [Google Scholar] [CrossRef]
- Niyitanga, T.; Ahmad, K.; Chaudhary, A.; Kim, H. Carbon dots as efficient electrode material for hydrogen peroxide sensing applications: A mini review. Inorg. Chem. Commun. 2023, 156, 111249. [Google Scholar] [CrossRef]
- Rhouati, A.; Berkani, M.; Vasseghian, Y.; Golzadeh, N. MXene-based electrochemical sensors for detection of environmental pollutants: A comprehensive review. Chemosphere 2022, 291, 132921. [Google Scholar] [CrossRef]
- Sangili, A.; Annalakshmi, M.; Chen, S.M.; Chen, T.W.; Kumaravel, S.; Govindasamy, M. A Facile synthesis of ultra-small cerium oxide nanoparticles for enhanced Electrochemical Detection of Nitrobenzene in water samples. Int. J. Electrochem. Sci. 2018, 13, 6135–6143. [Google Scholar] [CrossRef]
- Arul, N.S.; Mangalaraj, D.; Kumar, P.N.; Kim, E.; Devi, P.; Han, J.I. Synthesis and characterization of α-Fe2O3 Micro-/Nanorods-modified glassy carbon electrode for electrochemical sensing of nitrobenzene. Ceram. Int. 2015, 41, 5568–5573. [Google Scholar] [CrossRef]
- Sang, Y.; Cui, Y.; Li, Z.; Ye, W.; Li, H.; Zhao, X.S.; Guo, P. Electrochemical reaction of nitrobenzene and its derivatives on glassy carbon electrode modified with MnFe2O4 colloid nanocrystal assemblies. Sens. Actuators B Chem. 2016, 234, 46–52. [Google Scholar] [CrossRef]
- Vinoth, S.; Rajaitha, P.M.; Pandikumar, A. In-situ pyrolytic processed zinc stannate incorporated graphitic carbon nitride nanocomposite for selective and sensitive electrochemical determination of nitrobenzene. Compos. Sci. Technol. 2020, 195, 108192. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Jothi, A.I.; Chen, S.M.; Almutairi, G.; Ahmed, F.; Arshi, N.; AlOtaibi, B. Integrating graphene oxide with magnesium oxide nanoparticles for electrochemical detection of nitrobenzene. J. Environ. Chem. Eng. 2021, 9, 106310. [Google Scholar] [CrossRef]
- Chellappa, V.; Meenakshisundaram, N.; Annaraj, J.; Sagadevan, S. Hydrothermal synthesis of MnO2 nanorods for efficient electrochemical detection of environmental anthropogenic pollutants and nitrobenzene. Inorg. Chem. Commun. 2024, 160, 112015. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Vishnuraj, R.; Wang, T.J.; Pullithadathil, B.; Rangarajan, M.; Ahmed, F.; Alshahrani, T. Strongly coupled design of zinc oxide-nanorods/copper tin sulfide-nanoflowers nanostructures: An electrochemical study in 4-nitrochlorobenzene detection. Chem. Eng. J. 2024, 479, 147747. [Google Scholar] [CrossRef]
- Sang, Y.; Wang, B.; Wang, Q.; Zhao, G.; Guo, P. Insights into the electrocatalysis of nitrobenzene using chemically-modified carbon nanotube electrodes. Sci. Rep. 2014, 4, 6321. [Google Scholar] [CrossRef]
- Govindasamy, M.; Mani, V.; Chen, S.M.; Subramani, B.; Devasenathipathy, R.; Tamilarasan, S. Highly Sensitive Amperometric Sensor for Nitrobenzene Detection Using Functionalized Multiwalled-Carbon Nanotubes Modified Screen Printed Carbon Electrode. Int. J. Electrochem. Sci. 2016, 11, 10837–10846. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.M. An Amperometric Nitrobenzene Electrochemical Sensor Based on Electrochemically Activated Graphite Modified Screen Printed Carbon Electrode. Int. J. Electrochem. Sci. 2015, 10, 4173–4182. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, Y.; Zhang, X.; Zhu, G.; Liu, B.; Chen, J. Sensitive electrochemical detection of nitrobenzene based on macro-/meso-porous carbon materials modified glassy carbon electrode. Talanta 2012, 88, 696–700. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, T.; Ren, H.; Wang, L.; Meng, T.; Zhao, J.; Wang, H.; Zhang, Y. Nitrogen-doped hollow carbon nanospheres for highly sensitive electrochemical sensing of nitrobenzene. Mater. Res. Bull. 2018, 104, 15–19. [Google Scholar] [CrossRef]
- Sakthivel, R.; Palanisamy, S.; Chen, S.M.; Ramaraj, S.; Velusamy, V.; Yi-Fan, P.; Hall, J.M.; Ramaraj, S.K. A robust nitrobenzene electrochemical sensor based on chitin hydrogel entrapped graphite composite. J. Taiwan Inst. Chem. Eng. 2017, 80, 663–668. [Google Scholar] [CrossRef]
- Velmurugan, M.; Karikalan, N.; Chen, S.M.; Dai, Z.C. Studies on the influence of β-cyclodextrin on graphene oxide and its synergistic activity to the electrochemical detection of nitrobenzene. J. Colloid Interface Sci. 2017, 490, 365–371. [Google Scholar] [CrossRef]
- Kubendhiran, S.; Sakthinathan, S.; Chen, S.M.; Tamizhdurai, P.; Shanthi, K.; Karuppiah, C. Green reduction of reduced graphene oxide with nickel tetraphenyl porphyrin nanocomposite modified electrode for enhanced electrochemical determination of environmentally pollutant nitrobenzene. J. Colloid Interface Sci. 2017, 497, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bressi, V.; Chiarotto, I.; Ferlazzo, A.; Celesti, C.; Michenzi, C.; Len, T.; Iannazzo, D.; Neri, G.; Espro, C. Voltammetric Sensor Based on Waste-Derived Carbon Nanodots for Enhanced Detection of Nitrobenzene. ChemElectroChem 2023, 10, e202300004. [Google Scholar] [CrossRef]
- Pandiyarajan, C.; Rameshkumar, P.; Murugesan, S.; Selvaraj, M. Silver nanoparticles-supported graphitic-like carbon nitride for the electrochemical sensing of nitrobenzene and its derivatives. J. Mater. Sci. Mater. Electron. 2021, 32, 19912–19924. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, X.; Nsabimana, A.; Luhana, C.; Wang, G.; Wang, H.; Li, M.; Guo, L. Fabrication of 2D ordered mesoporous carbon nitride and its use as electrochemical sensing platform for H2O2, nitrobenzene, and NADH detection. Biosens. Bioelectron. 2014, 53, 250–256. [Google Scholar] [CrossRef]
- Yan, Z.; Xu, Z.; Zhang, W.; Zhao, S.; Xu, Y. A Novel Electrochemical Nitrobenzene Sensor Based on NiCu Alloy Electrode. Int. J. Electrochem. Sci. 2012, 7, 2938–2946. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, L.; Bo, X.; Wang, H.; Guo, L. Electrochemical study of nitrobenzene reduction using novel Pt nanoparticles/macroporous carbon hybrid nanocomposites. Anal. Chim. Acta 2012, 752, 45–52. [Google Scholar] [CrossRef]
- Rameshkumar, P.; Ramaraj, R. Electroanalysis of nitrobenzene derivatives and nitrite ions using silver nanoparticles deposited silica spheres modified electrode. J. Electroanal. Chem. 2014, 731, 72–77. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Palanisamy, S.; Chen, S.M.; Thangavelu, K.; Periakaruppan, P.; Liu, X.H. A simple electrochemical platform for detection of nitrobenzene in water samples using an alumina polished glassy carbon electrode. J. Colloid Interface Sci. 2016, 475, 154–160. [Google Scholar] [CrossRef]
- Gupta, R.; Rastogi, P.K.; Ganesan, V.; Yadav, D.K.; Sonkar, P.K. Gold nanoparticles decorated mesoporous silica microspheres: A proficient electrochemical sensing scaffold for hydrazine and nitrobenzene. Sens. Actuators B Chem. 2017, 239, 970–978. [Google Scholar] [CrossRef]
- Rameshkumar, P.; Viswanathan, P.; Ramaraj, R. Silicate sol–gel stabilized silver nanoparticles for sensor applications toward mercuric ions, hydrogen peroxide and nitrobenzene. Sens. Actuators B Chem. 2014, 202, 1070–1077. [Google Scholar] [CrossRef]
- Nehru, R.; Kumar, B.S.; Chen, C.W.; Dong, C.W. Sphere-like MoS2 nanosheet arrays/reduced graphene oxide hybrid electrocatalyst for accurate electrochemical monitoring of toxic pollutant. J. Environ. Chem. Eng. 2022, 10, 108687. [Google Scholar] [CrossRef]
- Papavasileiou, A.V.; Antonatos, N.; Luxa, J.; Děkanovský, L.; Ashtiani, S.; Fomekong, R.L.; Sofer, Z. Two-dimensional VSe2 nanoflakes as a promising sensing electrocatalyst for nitrobenzene determination in water samples. Electrochim. Acta 2024, 475, 143653. [Google Scholar] [CrossRef]
- Karthik, R.; Chavan, P.R.; Sukanya, R.; Dhakal, G.; Shim, J.-J.; Breslin, C.B. Flower-like strontium molybdate anchored on 3D N-rich reduced graphene oxide aerogel composite: An efficient catalyst for the detection of lethal pollutant nitrobenzene in water samples. Compos. Part B Eng. 2023, 256, 110649. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, M.M.; Silva-Carrillo, C.; Hinostroza-Mojarro, J.J.; Rivera-Lugo, Y.Y.; Valle-Trujillo, P.; Trujillo-Navarrete, B. Electrochemical sensor for determination of nitrobenzene in aqueous solution based on nanostructures of TiO2/GO. Fuel 2021, 283, 119326. [Google Scholar] [CrossRef]
- Yadav, D.K.; Ganesan, V.; Sonkar, P.K.; Gupta, R.; Rastogi, P.K. Electrochemical investigation of gold nanoparticles incorporated zinc based metal-organic framework for selective recognition of nitrite and nitrobenzene. Electrochim. Acta 2016, 200, 276–282. [Google Scholar] [CrossRef]
- An, S.; Shang, N.; Zhang, J.; Nsabimana, A.; Su, M.; Zhang, S.; Zhang, Y. Fabrication of electrocatalytically active, cobalt-embedded nitrogen-doped ordered macroporous carbon for sensitive detection of nitrobenzene. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130078. [Google Scholar] [CrossRef]
- Li, Y.P.; Zhuge, R.X.; Zhang, T. MOF-Conductive polymer composite electrode as electrochemical sensor of nitrobenzene. Inorg. Chem. Commun. 2023, 154, 110904. [Google Scholar] [CrossRef]
- Yuan, C.; Li, N.; Zhang, X.; Wang, Y.; Zhou, Z.; Zhang, L.; Zhou, M.; Hu, G. Flower-like copper sulfide-decorated boron-nitrogen co-doped carbon-modified glassy carbon electrode for selective and sensitive electrochemical detection of nitrobenzene in natural water. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 132011. [Google Scholar] [CrossRef]
- Li, S.S.; Fang, J.H.; Li, L.; Zhu, M.; Zhang, F.; Zhang, B.Y.; Jiang, T.J.; Zhang, Y.X. An ultra-sensitive electrochemical sensor of Ni/Fe-LDH toward nitrobenzene with the assistance of surface functionalization engineering. Talanta 2021, 225, 122087. [Google Scholar] [CrossRef]
- Zhang, W.X.; Chen, J.S.; Liu, X.P.; Mao, C.J.; Jin, B.-K. An electrochemiluminescent sensor based on hydrophilic CsPbBr3/TDPA nanocrystals for sensitive detection of nitrobenzene. Sens. Diagn. 2023, 2, 445–456. [Google Scholar] [CrossRef]
- Rastogi, P.K.; Ganesan, V.; Krishnamoorthi, S. Palladium nanoparticles incorporated polymer-silica nanocomposite based electrochemical sensing platform for nitrobenzene detection. Electrochim. Acta 2014, 147, 442–450. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).