The Application of Organic and Inorganic Nanoparticles Incorporated in Edible Coatings and Their Effect on the Physicochemical and Microbiological Properties of Seafood
Abstract
:1. Introduction
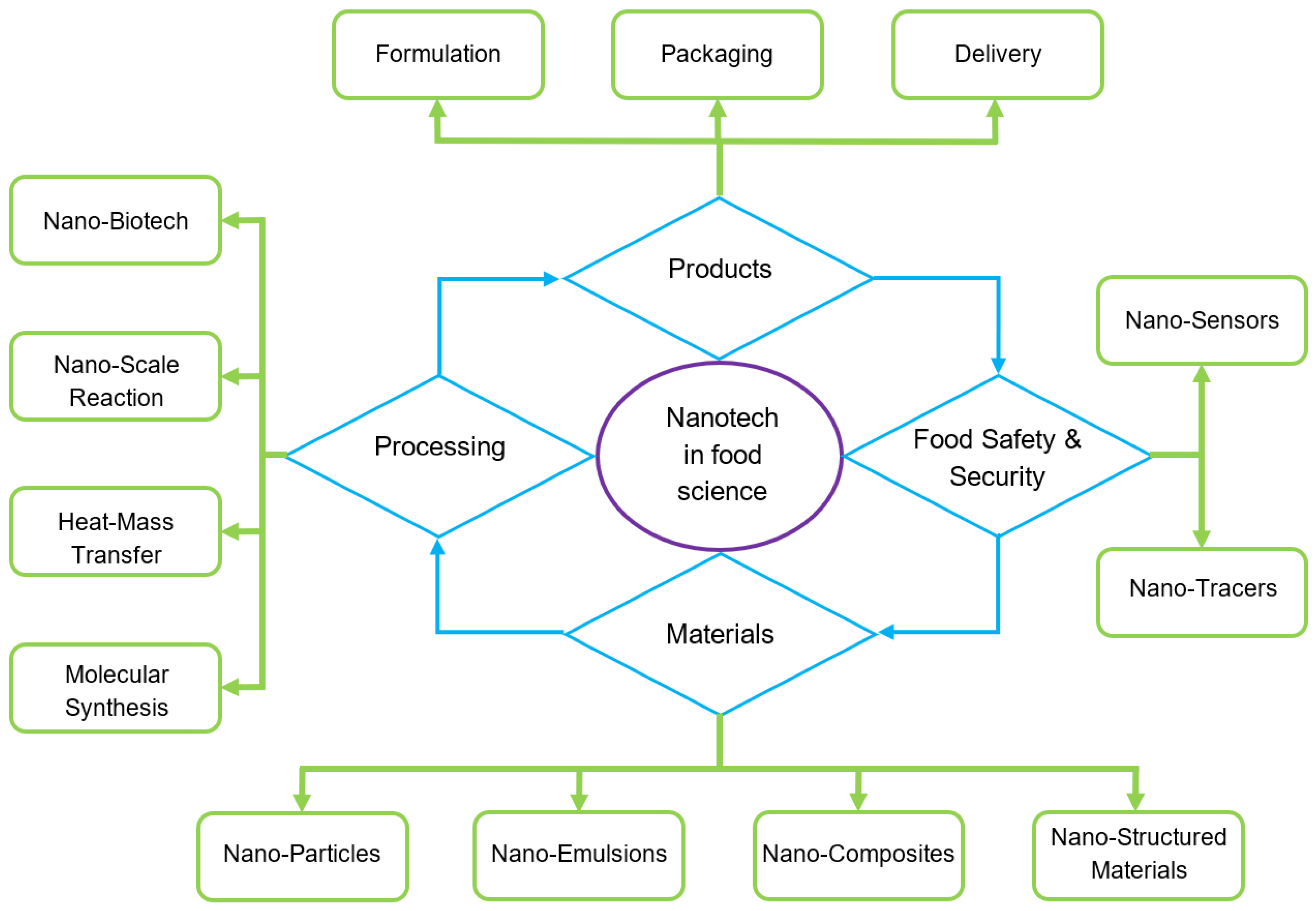
2. Nanotechnology
2.1. Potential Application of Nanoparticles in Food
2.2. Materials Used for the Development of Nanoparticles
2.2.1. Organic Materials
2.2.2. Inorganic Materials
2.3. Potential Toxicity of Nanoparticles
3. Methodologies Used for the Development of Nanoparticles
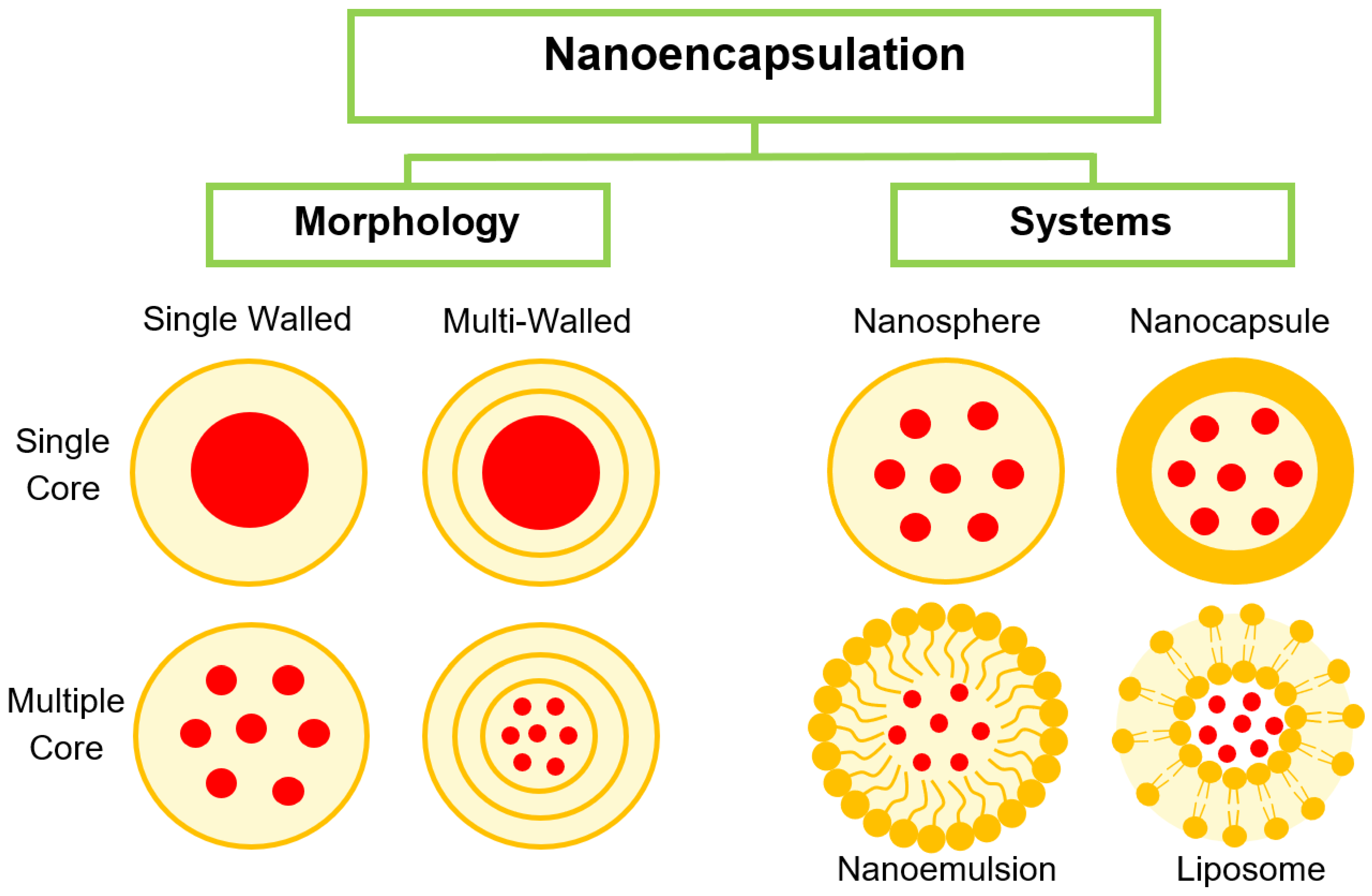
3.1. Nanoencapsulation
3.2. Nanoprecipitation
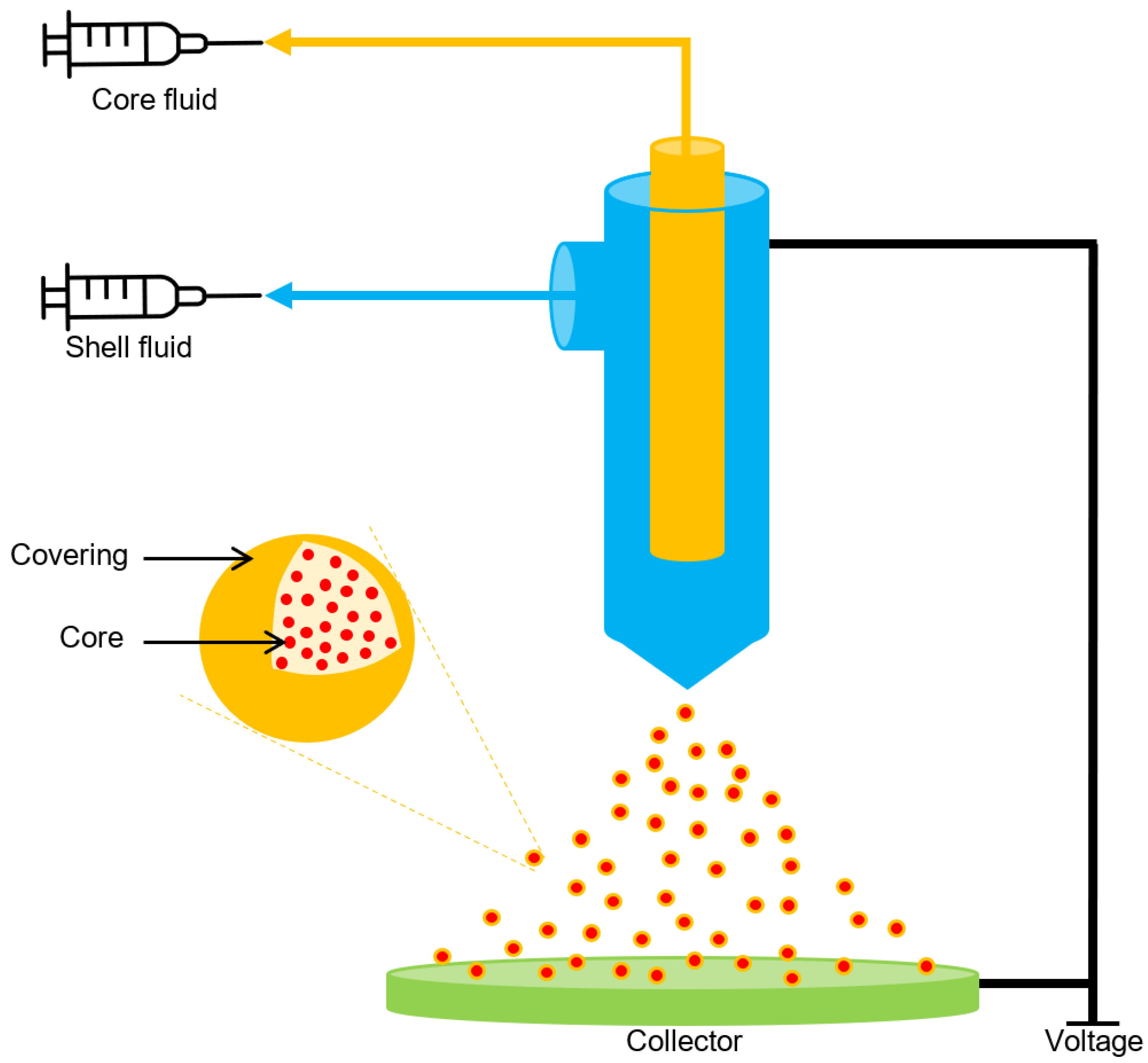
3.3. Electrospraying
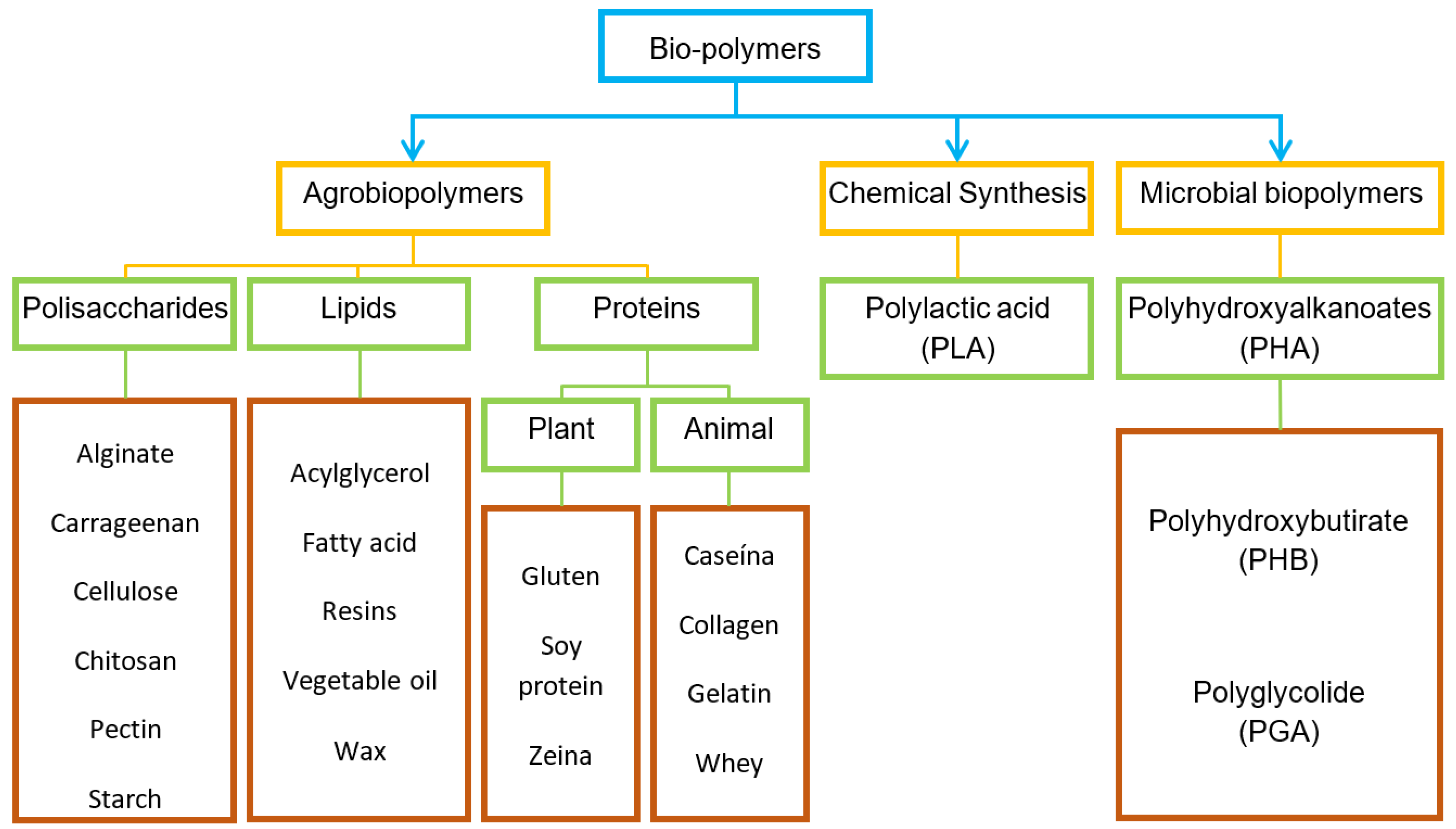
4. Biopolymers as a Base for Coatings and Films
4.1. Biopolymers Produced by Biomass
4.1.1. Polysaccharides
4.1.2. Proteins
4.1.3. Lipids
4.2. Biopolymers Produced by Chemical Synthesis
Polylactic acid (PLA)
4.3. Biopolymers Produced by Microorganisms
Polyhydroxyalkanoates (PHA)
5. Composite Coatings with Nanoparticles
5.1. Intelligent and Active Packaging with Nanoparticles
5.1.1. Intelligent Packaging
5.1.2. Active Packaging
5.1.3. Recent Research Highlights the Capabilities of Active Packaging Systems Utilizing Nanoparticles and Their Effects on Food Preservation
| Polymer | Nanoparticle | Properties | Coating or Film | Reference |
|---|---|---|---|---|
| Bovine serum albumin (BSA) | Selenium (Se) | Exhibited antimicrobial activity against several food-borne bacteria, with inhibition observed at 0.5 µg/mL for Listeria monocytogenes and Staphylococcus epidermidis. The average diameter of SeNPs was 22.8 ± 4.7 nm. | Coating | [121] |
| Carboxymethyl cellulose | Silver (Ag), zinc oxide (ZnO) and copper oxide (CuO) | The incorporation of metallic nanoparticles enhances mechanical properties. Films containing Ag and ZnO inhibited the growth of both S. aureus and E. coli, while those with CuO only inhibited E. coli | Film | [122] |
| Cellulose paper | Silver (Ag) | Enhanced antimicrobial efficacy against Bacillus cereus and Staphylococcus aureus, with maximum inhibition of 24 mm and 22 mm, respectively. Coated paper maintained the appearance and firmness of stored tomato fruit | Coating | [123] |
| Chitosan | Titanium dioxide (TiO2) | Greater antioxidant power compared to the use of TiO2 macroparticles and chitosan alone | Film | [51] |
| Resveratrol nanoencapsulated | The NPs significantly enhanced the aqueous solubility of trans-resveratrol by 150-fold and increased its bioavailability by 3.5-fold. The coating diminished dehydration, inhibited microbe growth, and extended shelf life of strawberries | Edible coating | [124] | |
| Silver (Ag) with tea polyphenols | Excellent antioxidant and antibacterial activity, better than chitosan film | Film | [125] | |
| Silver (Ag) | Showed high antimicrobial activity against Fusarium oxysporum and other fungi with minimum inhibitory concentrations of 41.7 μg/mL. They were non-phytotoxic, enhancing germination and chlorophyll levels in early plant development. | Coating | [126] | |
| Silver (Ag) | High antifungal activity | Film | [127] | |
| Rosemary essential oil nanoemulsions | High antimicrobial activity, helped inhibit the growth of pathogenic microorganisms | Coating | [11] | |
| Turmeric essential oil nanoemulsions | High antimicrobial activity, helped inhibit the growth of pathogenic microorganisms, in smaller quantities compared to rosemary | Coating | ||
| Gelatin and silver (Ag) | The shelf life of carrot pieces was enhanced by composite films containing nanoparticles, which exhibited ideal characteristics for food packaging | Film | [128] | |
| Essential oil of citrus extract and cinnamon and silver | The composite films exhibited strong activity against L. monocytogenes, S. Typhimurium, and A. niger, effectively delaying the decomposition of irradiated strawberries | Film | [64] | |
| Ethyl vinyl acetate (EVA) | Zinc oxide (ZnO), rosemary and montmorillonite extract | High antioxidant and antimicrobial activity compared to EVA | Film | [129] |
| Eugenol vinyl-based resins | Silica | Coatings exhibited adequate antioxidant capacity and reduced eugenol release to prolong beneficial effects, scavenging free radicals effectively during testing with food simulants | Coating | [130] |
| Glucose oxidase modified (GO) | Silver (Ag) and zinc oxide (ZnO) | The incorporation of GO with Ag and ZnO enhanced the activity of the enzymes, maintaining fruit quality parameters such as total suspended solids and firmness. GO/ZnO showed the best results in extending shelf life | Spray coating | [131] |
| Methylcellulose | Copper oxide (CuO)/gelatin as stabilized | Presented antibacterial activity and a small migration of the nanoparticles when evaluating the storage of cheese with the films | Film | [132] |
| Molecularly imprinted polymer (MIP) | Gold (AuNPs) and black phosphorus nanocomposites (BPNS) | The MIP/BPNS-AuNPs provided a broad detection range (0.005–10 μM) for pefloxacin with a low detection limit (0.80 nM) and high sensitivity (3.199 μA μM−1). The sensor maintained stable signals over 35 days | Sensor platform | [133] |
| Nanocellulose | Dextran-coated silver | Dextran enhanced the dispersion of silver nanoparticles and helped preserve food by inhibiting bacterial growth | Film | [134] |
| Pectin | Cellulose nanocrystals (CNC-NPs) | The incorporation of CNC-NPs significantly improved barrier properties, reducing water vapor and oxygen permeability by 12.6% and 22.3%, respectively, while also providing antioxidant properties | Coating | [135] |
| Chlorophyll of black mulberry leaf encapsulated with carboxymethylcellulose/silica | Antioxidant and antibacterial activity concerning DPPH radical and E. coli and S. aureus bacteria, respectively | Film | [136] | |
| Polydimethylsiloxane (PDMS) | Silicon dioxide (SiO2) | The spiky SiO2 nanoparticles created superhydrophobic coatings with contact angles of 165.4° and high transparency (96.93% transmittance). The coatings were resilient to UV irradiation, water, and elevated temperatures | Coating | [137] |
| Polylactic acid (PLA) | Zinc oxide (ZnO) with zataria essential oil | Higher antioxidant and antimicrobial power than PLA alone | Film | [45] |
| Zinc oxide (ZnO) with peppermint essential oil | Higher antioxidant and antimicrobial power than PLA alone but lower than PLA with zataria oil | Film | ||
| Starch | PVA and Ag NPs | Enhanced antibacterial and antiviral activities, with complete virus inactivation within 1 min. The coated paper also exhibited improved water resistance and tensile strength | Coating | [138] |
| Starch and carboxymethylcellulose | Epigallocatechin-3-gallate, cysteine, and cinnamaldehyde (ECCNPs) | The incorporation of ECCNPs improved UV resistance, physical properties, antioxidative, and antibacterial activity of the coating. It effectively prolonged the shelf life of strawberries and oranges | Coating | [139] |
| Zein | Turmeric nanocapsules with chitosan | High oxidation resistance | Film | [140] |
| Chitosan/cinnamon essential oil | The addition of nanoparticles increased the antibacterial activity against S. aureus and E. coli bacterial | Film | [141] | |
| Catechin/β-cyclodextrin inclusion complex NPs | The addition of nanoparticles enhanced the film structure and exhibited strong antioxidant activity | Film | [142] |
5.2. Properties of Composite Coatings with Nanoparticles
5.2.1. Mechanical Properties
5.2.2. Barrier Properties
5.3. Coating Application Methods
5.3.1. Immersion
5.3.2. Spraying
6. Effect of Nanoparticles Incorporated into Coatings Applied to Seafood
6.1. Physicochemical Properties
6.1.1. pH
6.1.2. Total Nitrogenous Volatile Bases
6.1.3. Thiobarbituric Acid Reactive Substances
6.2. Microbiological Properties
6.2.1. Deteriorating Bacteria
6.2.2. Pathogenic Microorganisms
7. Future Directions
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CONAPESCA. La pesca mexicana, una actividad inmensa como el mar. Gobierno de México. 21 November 2019. Available online: https://www.gob.mx/conapesca/articulos/la-pesca-mexicana-una-actividad-inmensa-como-el-mar-227722?idiom=es (accessed on 4 October 2023).
- FAO. Fishing Grows in Mexico, Driven by Freshwater Fishing. 2018. Available online: http://www.fao.org/mexico/noticias/detail-events/es/c/1144778/ (accessed on 4 October 2023).
- SAGARHPA. Programa de Mediano Plazo Desarrollo Pesquero y Acuícola 2016–2021. Available online: http://desaladora.sonora.gob.mx/images/transparencia/programas/programa-sectorial-mediano-plazo-sagarhpa.pdf (accessed on 4 October 2023).
- Mathew, S.; Raman, M.; Kalarikkathara Parameswaran, M.; Rajan, D.P. Fish and Fishery Products: Quality Indices. In Fish and Fishery Products Analysis; Springer: Singapore, 2019; pp. 63–144. [Google Scholar] [CrossRef]
- Soares, M.G.; Bevilaqua, G.C.; de Lima, M. Potential Applications of Environmentally Friendly Nanoparticles in Food Matrices: A Review. Food Bioprocess Technol. 2023, 16, 2742–2760. [Google Scholar] [CrossRef]
- Gupta, R.K.; El Gawad, F.A.; Ali, E.A.E.; Karunanithi, S.; Yugiani, P.; Srivastav, P.P. Nanotechnology: Current applications and future scope in food packaging systems. Meas. Food 2024, 13, 100131. [Google Scholar] [CrossRef]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Baskar, V.; Selvaraj, D.; Nile, A.; Xiao, J.; Kai, G. Nanotechnologies in Food Science: Applications, Recent Trends, and Future Perspectives. Nano-Micro Lett. 2020, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeon, Y.; Han, T.; Kim, S.; Gwon, Y.; Kim, J. Nanoscale manufacturing as an enabling strategy for the design of smart food packaging systems. Food Packag. Shelf Life 2020, 26, 100570. [Google Scholar] [CrossRef]
- Ahmad, K.; Li, Y.; Tu, C.; Guo, Y.; Yang, X.; Xia, C.; Hou, H. Nanotechnology in food packaging with implications for sustainable outlook and safety concerns. Food Biosci. 2024, 58, 103625. [Google Scholar] [CrossRef]
- Ceylan, Z.; Meral, R.; Kose, S.; Sengor, G.; Akinay, Y.; Durmus, M.; Ucar, Y. Characterized nano-size curcumin and rosemary oil for the limitation microbial spoilage of rainbow trout fillets. LWT 2020, 134, 109965. [Google Scholar] [CrossRef]
- Echeverría, I.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Active nanocomposite films based on soy proteins-montmorillonite- clove essential oil for the preservation of refrigerated bluefin tuna (Thunnus thynnus) fillets. Int. J. Food Microbiol. 2018, 266, 142–149. [Google Scholar] [CrossRef]
- De Sousa, M.S.; Schlogl, A.E.; Estanislau, F.R.; Souza, V.G.L.; dos Reis Coimbra, J.S.; Santos, I.J.B. Nanotechnology in Packaging for Food Industry: Past, Present, and Future. Coatings 2023, 13, 1411. [Google Scholar] [CrossRef]
- Montes-de-Oca-Ávalos, J.M.; Altamura, D.; Herrera, M.L.; Huck-Iriart, C.; Scattarella, F.; Siliqi, D.; Giannini, C.; Candal, R.J. Physical and structural properties of whey protein concentrate-Corn oil-TiO2 nanocomposite films for edible food-packaging. Food Packag. Shelf Life 2020, 26, 100590. [Google Scholar] [CrossRef]
- Grujic, R.; Vukic, M.; Gojkovic, V. Application of Biopolymers in the Food Industry. In Advances in Applications of Industrial Biomaterials; Pellicer, E., Nikolic, D., Sort, J., Baró, M., Zivic, F., Grujovic, N., Grujic, R., Pelemis, S., Eds.; Springer: Cham, Switzerland, 2017; pp. 103–120. [Google Scholar]
- Yochabedh, C.A.; Nandhini, L.; Preetha, R.; Rejish Kumar, V.J. Nanomaterials in aquatic products and aquatic systems, and its safety aspects. Appl. Nanosci. 2023, 13, 5435–5448. [Google Scholar] [CrossRef]
- Han, H.S.; Song, K.B. Antioxidant properties of watermelon (Citrullus lanatus) rind pectin films containing kiwifruit (Actinidia chinensis) peel extract and their application as chicken thigh packaging. Food Packag. Shelf Life 2021, 28, 100636. [Google Scholar] [CrossRef]
- Kuswandi, B.; Moradi, M. Improvement of food packaging based on functional nanomaterial. In Nanotechnology: Applications in Energy, Drug and Food; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Herrera-Rivera, M.d.R.; Torres-Arellanes, S.P.; Cortés-Martínez, C.I.; Navarro-Ibarra, D.C.; Hernández-Sánchez, L.; Solis-Pomar, F.; Pérez-Tijerina, E.; Román-Doval, R. Nanotechnology in food packaging materials: Role and application of nanoparticles. RSC Adv. 2024, 14, 21832–21858. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wei, J.; An, M.; Zhang, L.; Lin, S.; Shu, G.; Yuan, Z.; Lin, J.; Peng, G.; Liang, X.; et al. Casein nanoparticles as oral delivery carriers of mequindox for the improved bioavailability. Colloids Surf. B Biointerfaces 2020, 195, 111221. [Google Scholar] [CrossRef] [PubMed]
- Rakhimol, K.R.; Thomas, S.; Kalarikkal, N.; Jayachandran, K. Casein mediated synthesis of stabilized metal/metal-oxide nanoparticles with varied surface morphology through pH alteration. Mater. Chem. Phys. 2020, 246, 122803. [Google Scholar] [CrossRef]
- Nieto-Maldonado, A.; Bustos-Guadarrama, S.; Espinoza-Gomez, H.; Flores-López, L.Z.; Ramirez-Acosta, K.; Alonso-Nuñez, G.; Cadena-Nava, R.D. Green synthesis of copper nanoparticles using different plant extracts and their antibacterial activity. J. Environ. Chem. Eng. 2022, 10, 107130. [Google Scholar] [CrossRef]
- Alao, I.I.; Oyekunle, I.P.; Iwuozor, K.O.; Emenike, E.C. Green synthesis of Copper Nanoparticles and Investigation of its Antimicrobial Properties. Adv. J. Chem.-Sect. B Nat. Prod. Med. Chem. 2022, 4, 39–52. [Google Scholar] [CrossRef]
- Moreno-Vásquez, M.J.; Plascencia-Jatomea, M.; Sánchez-Valdes, S.; Tanori-Córdova, J.C.; Castillo-Yañez, F.J.; Quintero-Reyes, I.E.; Graciano-Verdugo, A.Z. Characterization of epigallocatechin-gallate-grafted chitosan nanoparticles and evaluation of their antibacterial and antioxidant potential. Polymers 2021, 13, 1375. [Google Scholar] [CrossRef]
- Ahmadi, S.; Fazilati, M.; Nazem, H.; Mousavi, S.M. Green Synthesis of Magnetic Nanoparticles Using Satureja hortensis Essential Oil toward Superior Antibacterial/Fungal and Anticancer Performance. BioMed Res. Int. 2021, 2021, 8822645. [Google Scholar] [CrossRef]
- Zakeri, Z.; Allafchian, A.; Vahabi, M.R.; Jalali, S.A.H. Synthesis and characterization of antibacterial silver nanoparticles using essential oils of crown imperial leaves, bulbs and petals. Micro Nano Lett. 2021, 16, 533–539. [Google Scholar] [CrossRef]
- Jan, M.T.; Ali, A.; Uddin, K.; Said, G.; Islam, M.G.; Uddin, S.H.; Muhammad, A. Antimicrobial and antioxidant activities of silver nanoparticles synthesized by novel biogenic method using mixed reductants. Pak. J. Pharm. Sci. 2021, 34, 995–1001. [Google Scholar]
- Rodríguez-Félix, F.; López-Cota, A.G.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z.; Quintero-Reyes, I.E.; Del-Toro-Sánchez, C.L.; Tapia-Hernández, J.A. Sustainable-green synthesis of silver nanoparticles using safflower (Carthamus tinctorius L.) waste extract and its antibacterial activity. Heliyon 2021, 7, e06923. [Google Scholar] [CrossRef]
- Liu, Q.; Sun, Y.; Cheng, J.; Guo, M. Development of whey protein nanoparticles as carriers to deliver soy isoflavones. LWT 2022, 155, 112953. [Google Scholar] [CrossRef]
- Shen, P.; Zhou, F.; Zhang, Y.; Yuan, D.; Zhao, Q.; Zhao, M. Formation and characterization of soy protein nanoparticles by controlled partial enzymatic hydrolysis. Food Hydrocoll. 2020, 105, 105844. [Google Scholar] [CrossRef]
- Ahmad, M.; Gani, A.; Hassan, I.; Huang, Q.; Shabbir, H. Production and characterization of starch nanoparticles by mild alkali hydrolysis and ultra-sonication process. Sci. Rep. 2020, 10, 3533. [Google Scholar] [CrossRef] [PubMed]
- Charles, A.P.R.; Mu, R.; Jin, T.Z.; Li, D.; Pan, Z.; Rakshit, S.; Cui, S.W.; Wu, Y. Application of yellow mustard mucilage and starch in nanoencapsulation of thymol and carvacrol by emulsion electrospray. Carbohydr. Polym. 2022, 298, 120148. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Feng, B.; Bi, Y.; Kong, F.; Wang, Z.; Tan, S. Application of nanoencapsulation technology to improve the stability and bioactivity of tea polyphenols. Food Biosci. 2023, 55, 103076. [Google Scholar] [CrossRef]
- Hamed, M.T.; Bakr, B.A.; Shahin, Y.H.; Elwakil, B.H.; Abu-Serie, M.M.; Aljohani, F.S.; Bekhit, A.A. Novel Synthesis of Titanium Oxide Nanoparticles: Biological Activity and Acute Toxicity Study. Bioinorg. Chem. Appl. 2021, 2021, 8171786. [Google Scholar] [CrossRef]
- Pragathiswaran, C.; Smitha, C.; Barabadi, H.; Al-Ansari, M.M.; Al-Humaid, L.A.; Saravanan, M. TiO2@ZnO nanocomposites decorated with gold nanoparticles: Synthesis, characterization and their antifungal, antibacterial, anti-inflammatory and anticancer activities. Inorg. Chem. Commun. 2020, 121, 108210. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, Y.; Jiang, B.; Chen, J.; Zhang, T. Development of self-assembled zein-fucoidan complex nanoparticles as a delivery system for resveratrol. Colloids Surf. B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef]
- Ye, W.; Zhang, G.; Liu, X.; Ren, Q.; Huang, F.; Yan, Y. Fabrication of polysaccharide-stabilized zein nanoparticles by flash nanoprecipitation for doxorubicin sustained release. J. Drug Deliv. Sci. Technol. 2022, 70, 103183. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Alegbeleye, O.O.; Strateva, M.; Stratev, D. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.P.; Consulting, C.; Hartel, R.W.; Ibarz, A.; Kokini, J.; Mccarthy, M.; Niranjan, K.; Kingdom, U.; Peleg, M.; Rahman, S.; et al. Food Nanoscience and Nanotechnology; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Bharti, S.K.; Pathak, V.; Alam, T.; Arya, A.; Basak, G.; Awasthi, M.G. Materiality of Edible Film Packaging in Muscle Foods: A Worthwhile Conception. J. Packag. Technol. Res. 2020, 4, 117–132. [Google Scholar] [CrossRef]
- Siddiquee, S.; Melvin, G.J.H.; Rahman, M.M. Nanotechnology: Applications in Energy, Drug and Food; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Wei, A.; Li, H.; Zhang, H.; Zheng, L.; Xia, N.; Wang, J. Protein-based active films: Raw materials, functions, and food applications. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13302. [Google Scholar] [CrossRef]
- Jeevahan, J.; Chandrasekaran, M. Nanoedible films for food packaging: A review. J. Mater. Sci. 2019, 54, 12290–12318. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, S.V.; Regenstein, J.M. Edible films and coatings in seafood preservation: A review. Food Chem. 2018, 240, 505–513. [Google Scholar] [CrossRef]
- Heydari-Majd, M.; Ghanbarzadeh, B.; Shahidi-Noghabi, M.; Najafi, M.A.; Hosseini, M. A new active nanocomposite film based on PLA/ZnO nanoparticle/essential oils for the preservation of refrigerated Otolithes ruber fillets. Food Packag. Shelf Life 2019, 19, 94–103. [Google Scholar] [CrossRef]
- Yuan, Y.; Ma, M.; Xu, Y.; Wang, D. Surface coating of zein nanoparticles to improve the application of bioactive compounds: A review. Trends Food Sci. Technol. 2022, 120, 1–15. [Google Scholar] [CrossRef]
- Benjakul, S.; Singh, A.; Chotphruethipong, L.; Mittal, A. Protein-polyphenol conjugates: Preparation, functional properties, bioactivities and applications in foods and nutraceuticals. In Advances in Food and Nutrition Research, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; Braga, A.R.C.; de Oliveira, B.R.; Gomes, F.P.; Moreira, V.L.; Pereira, V.A.C.; Egea, M.B. The potential of anthocyanins in smart, active, and bioactive eco-friendly polymer-based films: A review. Food Res. Int. 2021, 142, 110202. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Tzegkas, S.G.; Danezis, G.P. Nanomaterials in food packaging: State of the art and analysis. J. Food Sci. Technol. 2018, 55, 2862–2870. [Google Scholar] [CrossRef]
- Abbasi, R.; Shineh, G.; Mobaraki, M.; Doughty, S.; Tayebi, L. Structural parameters of nanoparticles affecting their toxicity for biomedical applications: A review. J. Nanoparticle Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Enescu, D.; Dehelean, A.; Gonçalves, C.; Cerqueira, M.A.; Magdas, D.A.; Fucinos, P.; Pastrana, L.M. Evaluation of the specific migration according to EU standards of titanium from Chitosan/Metal complexes films containing TiO2 particles into different food simulants. A comparative study of the nano-sized vs micro-sized particles. Food Packag. Shelf Life 2020, 26, 100579. [Google Scholar] [CrossRef]
- Xuan, L.; Ju, Z.; Skonieczna, M.; Zhou, P.K.; Huang, R. Nanoparticles-induced potential toxicity on human health: Applications, toxicity mechanisms, and evaluation models. MedComm 2023, 4, e327. [Google Scholar] [CrossRef]
- Jaswal, T.; Gupta, J. A review on the toxicity of silver nanoparticles on human health. Mater. Today: Proc. 2021, 81, 859–863. [Google Scholar] [CrossRef]
- Gao, M.; Chang, J.; Wang, Z.; Zhang, H.; Wang, T. Advances in transport and toxicity of nanoparticles in plants. J. Nanobiotechnology 2023, 21, 75. [Google Scholar] [CrossRef]
- Awashra, M.; Młynarz, P. The toxicity of nanoparticles and their interaction with cells: An in vitro metabolomic perspective. Nanoscale Adv. 2023, 5, 2674–2723. [Google Scholar] [CrossRef] [PubMed]
- Anandharamakrishnan, C. Liquid-Based Nanoencapsulation Techniques. In Techniques for Nanoencapsulation of Food Ingredients; Springer: New York, NY, USA, 2014. [Google Scholar] [CrossRef]
- Rahim, R.A.; Jayusman, P.A.; Muhammad, N.; Ahmad, F.; Mokhtar, N.; Mohamed, I.N.; Mohamed, N.; Shuid, A.N. Recent advances in nanoencapsulation systems using plga of bioactive phenolics for protection against chronic diseases. Int. J. Environ. Res. Public Health 2019, 16, 4962. [Google Scholar] [CrossRef]
- Ghayempour, S.; Montazer, M. Micro/nanoencapsulation of essential oils and fragrances: Focus on perfumed, antimicrobial, mosquito-repellent and medical textiles. J. Microencapsul. 2016, 33, 497–510. [Google Scholar] [CrossRef]
- Brandelli, A.; Brum, L.F.W.; dos Santos, J.H.Z. Nanostructured bioactive compounds for ecological food packaging. Environ. Chem. Lett. 2017, 15, 193–204. [Google Scholar] [CrossRef]
- Lammari, N.; Louaer, O.; Meniai, A.H.; Elaissari, A. Encapsulation of essential oils via nanoprecipitation process: Overview, progress, challenges and prospects. Pharmaceutics 2020, 12, 431. [Google Scholar] [CrossRef]
- Barreras, C.G.; Ramírez, B.; López, G.A.; Burruel, S.E.; Martínez, O.; Tapia, J.A.; Rodríguez, F. Nano- and Micro-Particles by Nanoprecipitation: Possible Application in the Food and Agricultural Industries. Int. J. Food Prop. 2016, 19, 1912–1923. [Google Scholar] [CrossRef]
- Hernández-Téllez, C.N.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O. Chitosan-Based Bionanocomposites: Development and Perspectives in Food and Agricultural Applications. In Chitosan in the Preservation of Agricultural Commodities; Academic Press: Cambridge, MA, USA, 2016; pp. 315–338. [Google Scholar] [CrossRef]
- Yus, C.; Arruebo, M.; Irusta, S.; Sebastián, V. Microflow nanoprecipitation of positively charged gastroresistant polymer nanoparticles of Eudragit® RS100: A study of fluid dynamics and chemical parameters. Materials 2020, 13, 2925. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Khodaei, D.; Lacroix, M. Effect of chitosan/essential oils/silver nanoparticles composite films packaging and gamma irradiation on shelf life of strawberries. Food Hydrocoll. 2021, 117, 106750. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Del-Toro-Sánchez, C.L.; Cinco-Moroyoqui, F.J.; Ruiz-Cruz, S.; Juárez, J.; Castro-Enríquez, D.D.; Barreras-Urbina, C.G.; López-Ahumada, G.A.; Rodríguez-Félix, F. Gallic Acid-Loaded Zein Nanoparticles by Electrospraying Process. J. Food Sci. 2019, 84, 818–831. [Google Scholar] [CrossRef] [PubMed]
- Barrimi, M.; Aalouane, R.; Aarab, C.; Hafidi, H.; Baybay, H.; Soughi, M.; Tachfouti, N.; Nejjari, C.; Mernissi, F.Z.; Rammouz, I.; et al. Electrospray and MALDI Mass Spectrometry. Encephale 2013, 53, 59–65. [Google Scholar] [CrossRef]
- Zhu, Y.; Chiarot, P.R. Structure of nanoparticle aggregate films built using pulsed-mode electrospray atomization. J. Mater. Sci. 2019, 54, 6122–6139. [Google Scholar] [CrossRef]
- Ghaffarzadegan, R.; Khoee, S.; Rezazadeh, S. Fabrication, characterization and optimization of berberine-loaded PLA nanoparticles using coaxial electrospray for sustained drug release. DARU J. Pharm. Sci. 2020, 28, 237–252. [Google Scholar] [CrossRef]
- Estrella-Osuna, D.E.; Tapia-Hernández, J.A.; Ruíz-Cruz, S.; Márquez-Ríos, E.; Ornelas-Paz, J.d.J.; Del-Toro-Sánchez, C.L.; Ocaño-Higuera, V.M.; Rodríguez-Félix, F.; Estrada-Alvarado, M.I.; Cira-Chávez, L.A. Nanoencapsulation of Eggplant (Solanum melongena L.) Peel Extract in Electrospun Gelatin Nanofiber: Preparation, Characterization, and In Vitro Release. Nanomaterials 2022, 12, 2303. [Google Scholar] [CrossRef]
- Gan, I.; Chow, W.S. Antimicrobial poly(lactic acid)/cellulose bionanocomposite for food packaging application: A review. Food Packag. Shelf Life 2018, 17, 150–161. [Google Scholar] [CrossRef]
- Gaspar, M.C.; Leocádio, J.; Mendes, C.V.T.; Cardeira, M.; Fernández, N.; Matias, A.; Carvalho, M.G.V.S.; Braga, M.E.M. Biodegradable film production from agroforestry and fishery residues with active compounds. Food Packag. Shelf Life 2021, 28, 100661. [Google Scholar] [CrossRef]
- Kwon, C.W.; Chang, P.S. Influence of alkyl chain length on the action of acetylated monoglycerides as plasticizers for poly (vinyl chloride) food packaging film. Food Packag. Shelf Life 2021, 27, 100619. [Google Scholar] [CrossRef]
- Nascimento, K.M.; Cavalheiro, J.B.; Monge Netto, A.Á.; da Silva Scapim, M.R.; Bergamasco, R.d.C. Properties of alginate films incorporated with free and microencapsulated Stryphnodendron adstringens extract (barbatimão). Food Packag. Shelf Life 2021, 28, 100637. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Han Lyn, F.; Chin Ping, T.; Zawawi, R.M.; Nur Hanani, Z.A. Effect of sonication time and heat treatment on the structural and physical properties of chitosan/graphene oxide nanocomposite films. Food Packag. Shelf Life 2021, 28, 100663. [Google Scholar] [CrossRef]
- Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Development of anacardic acid incorporated biopolymeric film for active packaging applications. Food Packag. Shelf Life 2021, 28, 100656. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Vatankhah, M.; Hassanisaadi, M.; Shafiei-Hematabad, Z.; Kennedy, J.F. Advancements in coating technologies: Unveiling the potential of chitosan for the preservation of fruits and vegetables. Int. J. Biol. Macromol. 2024, 254, 127677. [Google Scholar] [CrossRef]
- Ribeiro, A.M.; Estevinho, B.N.; Rocha, F. Preparation and Incorporation of Functional Ingredients in Edible Films and Coatings. Food Bioprocess Technol. 2021, 14, 209–231. [Google Scholar] [CrossRef]
- da Rocha, M.; de Souza, M.M.; Prentice, C. Biodegradable Films: An Alternative Food Packaging. In Food Packaging and Preservation; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Siddiqui, S.A.; Ariyanto, H.D.; Sasmitaloka, K.S.; Rathod, N.B.; Wahono, S.K.; Jaiswal, S.; Goksen, G.; Indrianingsih, A.W. Cellulose-Based Coating for Tropical Fruits: Method, Characteristic and Functionality. Food Rev. Int. 2024, 40, 1069–1092. [Google Scholar] [CrossRef]
- Floros, J.D. Food packaging and preservation: Edited by M. Mathlouthi, Blackie, 1994. £69.00 (xvi + 275 pages) ISBN 0 7514 0182 X. Trends Food Sci. Technol. 1996, 7, 69. [Google Scholar] [CrossRef]
- Gothandam, K.M.; Ranjan, S.; Dasgupta, N.; Ramalingam, C.; Lichtfouse, E. (Eds.) Nanotechnology, Food Security and Water Treatment; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Majeed, T.; Dar, A.H.; Pandey, V.K.; Dash, K.K.; Srivastava, S.; Shams, R.; Jeevarathinam, G.; Singh, P.; Echegaray, N.; Pandiselvam, R. Role of additives in starch-based edible films and coating: A review with current knowledge. Prog. Org. Coat. 2023, 181, 107597. [Google Scholar] [CrossRef]
- Shih, Y.T.; Zhao, Y. Development, characterization and validation of starch based biocomposite films reinforced by cellulose nanofiber as edible muffin liner. Food Packag. Shelf Life 2021, 28, 100655. [Google Scholar] [CrossRef]
- Hammam, A.R.A. Technological, applications, and characteristics of edible films and coatings: A review. SN Appl. Sci. 2019, 1, 632. [Google Scholar] [CrossRef]
- Hashemi, M.; Adibi, S.; Hojjati, M.; Razavi, R.; Noori, S.M.A. Impact of alginate coating combined with free and nanoencapsulated Carum copticum essential oil on rainbow trout burgers. Food Sci. Nutr. 2023, 11, 1521–1530. [Google Scholar] [CrossRef]
- Moustafa, H.; Youssef, A.M.; Darwish, N.A.; Abou-Kandil, A.I. Eco-friendly polymer composites for green packaging: Future vision and challenges. Compos. Part B: Eng. 2019, 172, 16–25. [Google Scholar] [CrossRef]
- Kumar, S.; Reddy, A.R.L.; Basumatary, I.B.; Nayak, A.; Dutta, D.; Konwar, J.; Purkayastha, M.D.; Mukherjee, A. Recent progress in pectin extraction and their applications in developing films and coatings for sustainable food packaging: A review. Int. J. Biol. Macromol. 2023, 239, 124281. [Google Scholar] [CrossRef]
- Calva, S.J.; Jiménez, M.; Lugo, E. Protein-Based Films: Advances in the Development of Biomaterials Applicable to Food Packaging. Food Eng. Rev. 2019, 11, 78–92. [Google Scholar] [CrossRef]
- Severo, C.; Anjos, I.; Souza, V.G.L.; Canejo, J.P.; Bronze, M.R.; Fernando, A.L.; Coelhoso, I.; Bettencourt, A.F.; Ribeiro, I.A.C. Development of cranberry extract films for the enhancement of food packaging antimicrobial properties. Food Packag. Shelf Life 2021, 28, 100646. [Google Scholar] [CrossRef]
- Martins, F.C.O.L.; Sentanin, M.A.; De Souza, D. Analytical methods in food additives determination: Compounds with functional applications. Food Chem. 2019, 272, 732–750. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Jamróz, E.; Kasprzak, M.; Zając, M.; Pająk, P.; Grzebieniarz, W.; Nowak, N.; Juszczak, L. Edible Coatings Based on a Furcellaran and Gelatin Extract with Herb Addition as an Active Packaging for Carp Fillets. Food Bioprocess Technol. 2023, 16, 1009–1021. [Google Scholar] [CrossRef]
- Lan, X.; Zhang, X.; Wang, L.; Wang, H.; Hu, Z.; Ju, X.; Yuan, Y. A review of food preservation based on zein: The perspective from application types of coating and film. Food Chem. 2023, 424, 136403. [Google Scholar] [CrossRef]
- Rodríguez-Félix, F.; Corte-Tarazón, J.A.; Rochín-Wong, S.; Fernández-Quiroz, J.D.; Garzón-García, A.M.; Santos-Sauceda, I.; Plascencia-Martínez, D.F.; Chan-Chan, L.H.; Vásquez-López, C.; Barreras-Urbina, C.G.; et al. Physicochemical, structural, mechanical and antioxidant properties of zein films incorporated with no-ultrafiltered and ultrafiltered betalains extract from the beetroot (Beta vulgaris) bagasse with potential application as active food packaging. J. Food Eng. 2022, 334, 111153. [Google Scholar] [CrossRef]
- Akrami, S.; Saki, M.; Marashi Hossaeini, S.M.; Sabahi, S.; Noori, S.M.A. Application of soy protein-based films and coatings on the shelf life of food products: A mini-review of recent publications with emphasis on nanotechnology. Food Meas. 2023, 17, 1393–1401. [Google Scholar] [CrossRef]
- Ma, Q.; Liang, S.; Xu, S.; Li, J.; Wang, L. Characterization of antioxidant properties of soybean protein-based films with Cortex Phellodendri extract in extending the shelf life of lipid. Food Packag. Shelf Life 2019, 22, 100413. [Google Scholar] [CrossRef]
- Nilsen-Nygaard, J.; Fernandez, E.N.; Radusin, T.; Rotabakk, B.T.; Sarfraz, J.; Sharmin, N.; Sivertsvik, M.; Sone, I.; Pettersen, M.K. Current status of biobased and biodegradable food packaging materials: Impact on food quality and effect of innovative processing technologies. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1333–1380. [Google Scholar] [CrossRef]
- Göksen, G.; Fabra, M.J.; Pérez-Cataluña, A.; Ekiz, H.I.; Sanchez, G.; López-Rubio, A. Biodegradable active food packaging structures based on hybrid cross-linked electrospun polyvinyl alcohol fibers containing essential oils and their application in the preservation of chicken breast fillets. Food Packag. Shelf Life 2021, 27, 100613. [Google Scholar] [CrossRef]
- Nurul Syahida, S.; Ismail-Fitry, M.R.; Ainun, Z.M.A.; Nur Hanani, Z.A. Effects of gelatin/palm wax/lemongrass essential oil (GPL)-coated Kraft paper on the quality shelf life of ground beef stored at 4 °C. Food Packag. Shelf Life 2021, 28, 100640. [Google Scholar] [CrossRef]
- Reyes, L.M.; Landgraf, M.; Sobral, P.J.A. Gelatin-based films activated with red propolis ethanolic extract and essential oils. Food Packag. Shelf Life 2021, 27, 100607. [Google Scholar] [CrossRef]
- Shahbazi, Y.; Shavisi, N. Effects of sodium alginate coating containing Mentha spicata essential oil and cellulose nanoparticles on extending the shelf life of raw silver carp (Hypophthalmichthys molitrix) fillets. Food Sci. Biotechnol. 2019, 28, 433–440. [Google Scholar] [CrossRef]
- Villada, H.S.; Acosta, H.; Velasco, R. Natural biopolymers used in biodegradable packaging. Agrar. Top. 2007, 12, 5–13. [Google Scholar] [CrossRef]
- Khosravi, A.; Fereidoon, A.; Khorasani, M.M.; Naderi, G.; Ganjali, M.R.; Zarrintaj, P.; Saeb, M.R.; Gutiérrez, T.J. Soft and hard sections from cellulose-reinforced poly(lactic acid)-based food packaging films: A critical review. Food Packag. Shelf Life 2020, 23, 100429. [Google Scholar] [CrossRef]
- Luís, Â.; Domingues, F.; Silva, F. Bio-Based Materials for Active Food Packaging. In Food Packaging; Rangappa, S.M., Parameswaranpillai, J., Thiagamani, S.M.K., Krishnasamy, S., Siengchin, S., Eds.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and intelligent biodegradable packaging films using food and food waste-derived bioactive compounds: A review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Gomes, V.; Souza, L.; Fernando, A.L. Nanoparticles in food packaging : Biodegradability and potential migration to food—A review. Food Packag. Shelf Life 2016, 8, 63–70. [Google Scholar] [CrossRef]
- Kuswandi, B. Environmental friendly food nano-packaging. Environ. Chem. Lett. 2017, 15, 205–221. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2021, 19, 583–611. [Google Scholar] [CrossRef]
- Liu, S.; Yang, J.; Yu, Y.; Liang, D.; Li, Y.; Si, X.; Song, S.; Meng, M.; Zhang, J.; Zhang, Y. Multifunctional flexible carbon fiber felt@nickel composite films with core–shell heterostructure: Exceptional Joule heating capability, thermal management, and electromagnetic interference shielding. Chem. Eng. J. 2024, 494, 153221. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Bildik, F.; Altay, F.; Ceylan, Z. Gelatin nanofibers with black elderberry, Au nanoparticles and SnO2 as intelligent packaging layer used for monitoring freshness of Hake fish. Food Chem. 2024, 437, 137843. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, A.; Radoor, S.; Shin, G.H.; Siengchin, S.; Kim, J.T. Active and intelligent packaging films based on PVA/Chitosan/Zinc oxide nanoparticles/Sweet purple potato extract as pH sensing and antibacterial wraps. Food Biosci. 2023, 56, 103432. [Google Scholar] [CrossRef]
- Yang, G.; Phua, S.Z.F.; Bindra, A.K.; Zhao, Y. Degradability and Clearance of Inorganic Nanoparticles for Biomedical Applications. Adv. Mater. 2019, 31, 1805730. [Google Scholar] [CrossRef]
- Yang, C.; Tang, H.; Wang, Y.; Liu, Y.; Wang, J.; Shi, W.; Li, L. Development of PLA-PBSA based biodegradable active film and its application to salmon slices. Food Packag. Shelf Life 2019, 22, 100393. [Google Scholar] [CrossRef]
- Alkan, B.; Sehit, E.; Tas, C.E.; Unal, S.; Cebeci, F.C. Carvacrol loaded halloysite coatings for antimicrobial food packaging applications. Food Packag. Shelf Life 2019, 20, 100300. [Google Scholar] [CrossRef]
- Ali, S.; Chen, X.; Ajmal, M.; Ali, M.; Zareef, M.; Arslan, M.; Ahmad, S.; Jiao, T.; Li, H.; Chen, Q. The avenue of fruit wastes to worth for synthesis of silver and gold nanoparticles and their antimicrobial application against foodborne pathogens : A review. Food Chem. 2021, 359, 129912. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of Nanomaterials in Food Packaging and its Advanced Future Prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef]
- Sultan, M.; Abdelhakim, A.A.; Nassar, M.; Hassan, Y.R. Active packaging of chitosan film modified with basil oil encapsulated in silica nanoparticles as an alternate for plastic packaging materials. Food Biosci. 2023, 51, 102298. [Google Scholar] [CrossRef]
- Bumbudsanpharoke, N.; Harnkarnsujarit, N.; Chongcharoenyanon, B.; Kwon, S.; Ko, S. Enhanced properties of PBAT/TPS biopolymer blend with CuO nanoparticles for promising active packaging. Food Packag. Shelf Life 2023, 37, 101072. [Google Scholar] [CrossRef]
- Lin, L.; Mei, C.; Shi, C.; Li, C.; Abdel-Samie, M.A.; Cui, H. Preparation and characterization of gelatin active packaging film loaded with eugenol nanoparticles and its application in chicken preservation. Food Biosci. 2023, 53, 102778. [Google Scholar] [CrossRef]
- Han, Y.; Zhou, M.; McClements, D.J.; Liu, F.; Cheng, C.; Xiong, J.; Zhu, M.; Chen, S. Investigation of a novel smart and active packaging materials: Nanoparticle-filled carrageenan-based composite films. Carbohydr. Polym. 2023, 301, 120331. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Xiao, R.; Afolabi, M.; Bomma, M.; Xiao, Z. Evaluation of Antibacterial Activity of Selenium Nanoparticles against Food-Borne Pathogens. Microorganisms 2023, 11, 1519. [Google Scholar] [CrossRef]
- Ebrahimi, Y.; Peighambardoust, S.J.; Peighambardoust, S.H.; Karkaj, S.Z. Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J. Food Sci. 2019, 84, 2537–2548. [Google Scholar] [CrossRef]
- Sharma, S.; Sharma, N.; Kaushal, N. Utilization of novel bacteriocin synthesized silver nanoparticles (AgNPs) for their application in antimicrobial packaging for preservation of tomato fruit. Front. Sustain. Food Syst. 2023, 7, 1072738. [Google Scholar] [CrossRef]
- Hu, Q.; Zhou, F.; Ly, N.K.; Ordyna, J.; Peterson, T.; Fan, Z.; Wang, S. Development of Multifunctional Nanoencapsulated trans-Resveratrol/Chitosan Nutraceutical Edible Coating for Strawberry Preservation. ACS Nano 2023, 17, 8586–8597. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, W. Antioxidant and antibacterial chitosan film with tea polyphenols-mediated green synthesis silver nanoparticle via a novel one-pot method. Int. J. Biol. Macromol. 2020, 155, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- Mondéjar-López, M.; López-Jimenez, A.J.; Ahrazem, O.; Gómez-Gómez, L.; Niza, E. Chitosan coated-biogenic silver nanoparticles from wheat residues as green antifungal and nanoprimig in wheat seeds. Int. J. Biol. Macromol. 2023, 225, 964–973. [Google Scholar] [CrossRef]
- Ortiz-Duarte, G.; Martínez-Hernández, G.B.; Casillas-Peñuelas, R.; Pérez-Cabrera, L.E. Evaluation of Biopolymer Films Containing Silver–Chitosan Nanocomposites. Food Bioprocess Technol. 2021, 14, 492–504. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Mahmoudian, M.; Rahmani, K.; Abdali, A.; Nozad, E.; Enayati, M. Active intelligent packaging film based on ethylene vinyl acetate nanocomposite containing extracted anthocyanin, rosemary extract and ZnO/Fe-MMT nanoparticles. Food Packag. Shelf Life 2019, 22, 100389. [Google Scholar] [CrossRef]
- Orlo, E.; Nerín, C.; Lavorgna, M.; Wrona, M.; Russo, C.; Stanzione, M.; Nugnes, R.; Isidori, M. Antioxidant activity of coatings containing eugenol for flexible aluminium foils to preserve food shelf-life. Food Packag. Shelf Life 2023, 39, 101145. [Google Scholar] [CrossRef]
- Shouket, S.; Khurshid, S.; Khan, J.; Batool, R.; Sarwar, A.; Aziz, T.; Alhomrani, M.; Alamri, A.S.; Sameeh, M.Y.; Zubair Filimban, F. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports. A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar] [CrossRef]
- Gvozdenko, A.A.; Siddiqui, S.A.; Blinov, A.V.; Golik, A.B.; Nagdalian, A.A.; Maglakelidze, D.G.; Statsenko, E.N.; Pirogov, M.A.; Blinova, A.A.; Sizonenko, M.N.; et al. Synthesis of CuO nanoparticles stabilized with gelatin for potential use in food packaging applications. Sci. Rep. 2022, 12, 12843. [Google Scholar] [CrossRef]
- Li, G.; Qi, X.; Wu, J.; Wan, X.; Wang, T.; Liu, Y.; Chen, Y.; Xia, Y. Highly stable electrochemical sensing platform for the selective determination of pefloxacin in food samples based on a molecularly imprinted-polymer-coated gold nanoparticle/black phosphorus nanocomposite. Food Chem. 2024, 436, 137753. [Google Scholar] [CrossRef]
- Lazić, V.; Vivod, V.; Peršin, Z.; Stoiljković, M.; Ratnayake, I.S.; Ahrenkiel, P.S.; Nedeljković, J.M.; Kokol, V. Dextran-coated silver nanoparticles for improved barrier and controlled antimicrobial properties of nanocellulose films used in food packaging. Food Packag. Shelf Life 2020, 26, 100575. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, W.; Farag, M.A.; Shao, P. Functionalized cellulose nanocrystal embedded into citrus pectin coating improves its barrier, antioxidant properties and potential application in food. Food Chem. 2023, 401, 134079. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, K.A.; Pirsa, S. Biodegradable film of black mulberry pulp pectin/chlorophyll of black mulberry leaf encapsulated with carboxymethylcellulose/silica nanoparticles: Investigation of physicochemical and antimicrobial properties. Mater. Chem. Phys. 2021, 267, 124580. [Google Scholar] [CrossRef]
- Nguyen, N.B.; Ly, N.H.; Tran, H.N.; Son, S.J.; Joo, S.W.; Vasseghian, Y.; Osman, S.M.; Luque, R. Transparent Oil–Water Separating Spiky SiO2 Nanoparticle Supramolecular Polymer Superhydrophobic Coatings. Small Methods 2023, 7, 2201257. [Google Scholar] [CrossRef] [PubMed]
- Srikhao, N.; Ounkaew, A.; Srichiangsa, N.; Phanthanawiboon, S.; Boonmars, T.; Artchayasawat, A.; Theerakulpisut, S.; Okhawilai, M.; Kasemsiri, P. Green-synthesized silver nanoparticle coating on paper for antibacterial and antiviral applications. Polym. Bull. 2023, 80, 9651–9668. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, J.; Chen, X.; Zhou, X.; Zhou, J.; Sun, H.; Wang, S.; Liu, Y. Organic nanoparticles incorporated starch/carboxymethylcellulose multifunctional coating film for efficient preservation of perishable products. Int. J. Biol. Macromol. 2024, 275, 133357. [Google Scholar] [CrossRef] [PubMed]
- Xin, S.; Xiao, L.; Dong, X.; Li, X.; Wang, Y.; Hu, X.; Sameen, D.E.; Qin, W.; Zhu, B. International Journal of Biological Macromolecules Preparation of chitosan/curcumin nanoparticles based zein and potato starch composite films for Schizothorax prenati fillet preservation. Int. J. Biol. Macromol. 2020, 164, 211–221. [Google Scholar] [CrossRef]
- Vahedikia, N.; Garavand, F.; Tajeddin, B.; Cacciotti, I.; Jafari, S.M.; Omidi, T.; Zahedi, Z. Biodegradable zein film composites reinforced with chitosan nanoparticles and cinnamon essential oil: Physical, mechanical, structural and antimicrobial attributes. Colloids Surf. B Biointerfaces 2019, 177, 25–32. [Google Scholar] [CrossRef]
- Jiang, L.; Jia, F.; Han, Y.; Meng, X.; Xiao, Y.; Bai, S. Development and characterization of zein edible films incorporated with catechin/β-cyclodextrin inclusion complex nanoparticles. Carbohydr. Polym. 2021, 261, 117877. [Google Scholar] [CrossRef]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-based edible films and coatings for food packaging applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef]
- Katiyar, V.; Ghosh, T. Nanotechnology in Edible Food Packaging; Springer: Singapore, 2021; Available online: http://link.springer.com/10.1007/978-981-33-6169-0 (accessed on 4 October 2023).
- Casp, A.; Abril, J. Food Preservation Processes; Ediciones Mundi Prensa: Madrid, Spain, 2003. [Google Scholar]
- Cetinkaya, T.; Wijaya, W. Advanced nanomaterials for enhancing the shelf life and quality of seafood products. Food Biosci. 2024, 59, 104018. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Benjakul, S. Natural Preservatives for Extending the Shelf-Life of Seafood: A Revisit. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1595–1612. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Cui, Z.; Shang, M.; Zhong, Y. A colorimetric film based on polyvinyl alcohol/sodium carboxymethyl cellulose incorporated with red cabbage anthocyanin for monitoring pork freshness. Food Packag. Shelf Life 2021, 28, 100641. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent advances in the preparation, physical and functional properties, and applications of anthocyanins-based active and intelligent packaging films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Lee, B.H.; Wu, S.C.; Shen, T.L.; Hsu, Y.Y.; Chen, C.H.; Hsu, W.H. The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna fish. Food Chem. 2021, 340, 128104. [Google Scholar] [CrossRef] [PubMed]
- Medina, S.; Pereira, J.A.; Silva, P.; Perestrelo, R.; Câmara, J.S. Food fingerprints—A valuable tool to monitor food authenticity and safety. Food Chem. 2019, 278, 144–162. [Google Scholar] [CrossRef]
- Vicente, R.; Rodríguez, E.A.; Marrero, D.; González, V.; Sierra, R.; Morales, C. Preliminary determination of degradation products formed by auto-oxidation of the lipid extract of Roystonea regia. Rev. CENIC Cienc. Químicas 2016, 47, 49–55. [Google Scholar]
- Semeniuc, C.A.; Mandrioli, M.; Rodriguez-Estrada, M.T.; Muste, S.; Lercker, G. Thiobarbituric acid reactive substances in flavored phytosterol-enriched drinking yogurts during storage: Formation and matrix interferences. Eur. Food Res. Technol. 2016, 242, 431–439. [Google Scholar] [CrossRef]
- Davies, A.M.C.; Boley, N.P. Food analysis. Anal. Proc. 2017, 21, 64–68. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- de la Caba, K.; Guerrero, P.; Trung, T.S.; Cruz-Romero, M.; Kerry, J.P.; Fluhr, J.; Maurer, M.; Kruijssen, F.; Albalat, A.; Bunting, S.; et al. From seafood waste to active seafood packaging: An emerging opportunity of the circular economy. J. Clean. Prod. 2019, 208, 86–98. [Google Scholar] [CrossRef]
- Mahajan, P.V.; Caleb, O.J.; Gil, M.I.; Izumi, H.; Colelli, G.; Watkins, C.B.; Zude, M. Quality and safety of fresh horticultural commodities: Recent advances and future perspectives. Food Packag. Shelf Life 2017, 14, 2–11. [Google Scholar] [CrossRef]
- Odeyemi, O.A.; Burke, C.M.; Bolch, C.C.J.; Stanley, R. Seafood spoilage microbiota and associated volatile organic compounds at different storage temperatures and packaging conditions. Int. J. Food Microbiol. 2018, 280, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Pathakoti, K.; Manubolu, M.; Hwang, H.M. Nanostructures: Current uses and future applications in food science. J. Food Drug Anal. 2017, 25, 245–253. [Google Scholar] [CrossRef]
- Sullivan, D.J.; Cruz-romero, M.C.; Hernandez, A.B.; Cummins, E.; Kerry, J.P.; Morris, M.A. A novel method to deliver natural antimicrobial coating materials to extend the shelf-life of European hake (Merluccius merluccius) fillets. Food Packag. Shelf Life 2020, 25, 100522. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Sarkar, P. Composite edible films and coatings from food-grade biopolymers. J. Food Sci. Technol. 2018, 55, 4369–4383. [Google Scholar] [CrossRef]
- Shanaghi, E.; Aghajani, M.; Esmaeli, F.; Faramarzi, M.A.; Jahandar, H.; Amani, A. Application of electrospray in preparing solid lipid nanoparticles and optimization of nanoparticles using artificial neural networks. Avicenna J. Med. Biotechnol. 2020, 12, 251–254. [Google Scholar] [PubMed]
- Shehabeldine, A.M.; Amin, B.H.; Hagras, F.A.; Ramadan, A.A.; Kamel, M.R.; Ahmed, M.A.; Atia, K.H.; Salem, S.S. Potential Antimicrobial and Antibiofilm Properties of Copper Oxide Nanoparticles: Time-Kill Kinetic Essay and Ultrastructure of Pathogenic Bacterial Cells. Appl. Biochem. Biotechnol. 2023, 195, 467–485. [Google Scholar] [CrossRef] [PubMed]
- Barabadi, H.; Jounaki, K.; Pishgahzadeh, E.; Morad, H.; Sadeghian-Abadi, S.; Vahidi, H.; Hussain, C.M. Antiviral potential of green-synthesized silver nanoparticles. In Handbook of Microbial Nanotechnology; Academic Press: Cambridge, MA, USA, 2022; pp. 285–310. [Google Scholar] [CrossRef]
- Plotniece, A.; Sobolev, A.; Supuran, C.T.; Carta, F.; Björkling, F.; Franzyk, H.; Yli-Kauhaluoma, J.; Augustyns, K.; Cos, P.; De Vooght, L.; et al. Selected strategies to fight pathogenic bacteria. J. Enzym. Inhib. Med. Chem. 2023, 38, 2155816. [Google Scholar] [CrossRef]
- Wang, X.; Xia, Z.; Wang, H.; Wang, D.; Sun, T.; Hossain, E.; Pang, X.; Liu, Y. Cell-membrane-coated nanoparticles for the fight against pathogenic bacteria, toxins, and inflammatory cytokines associated with sepsis. Theranostics 2023, 13, 3224–3244. [Google Scholar] [CrossRef]


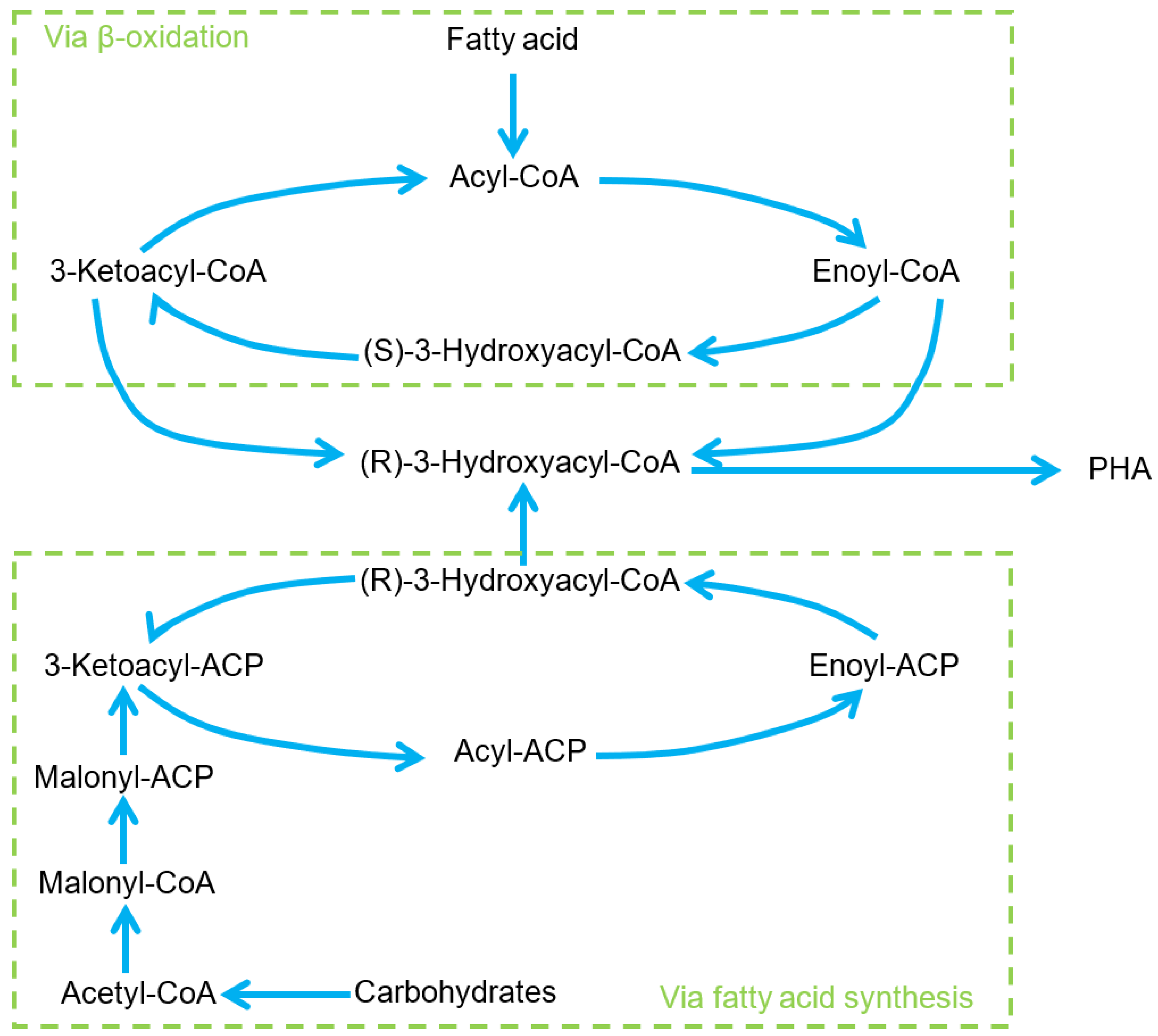



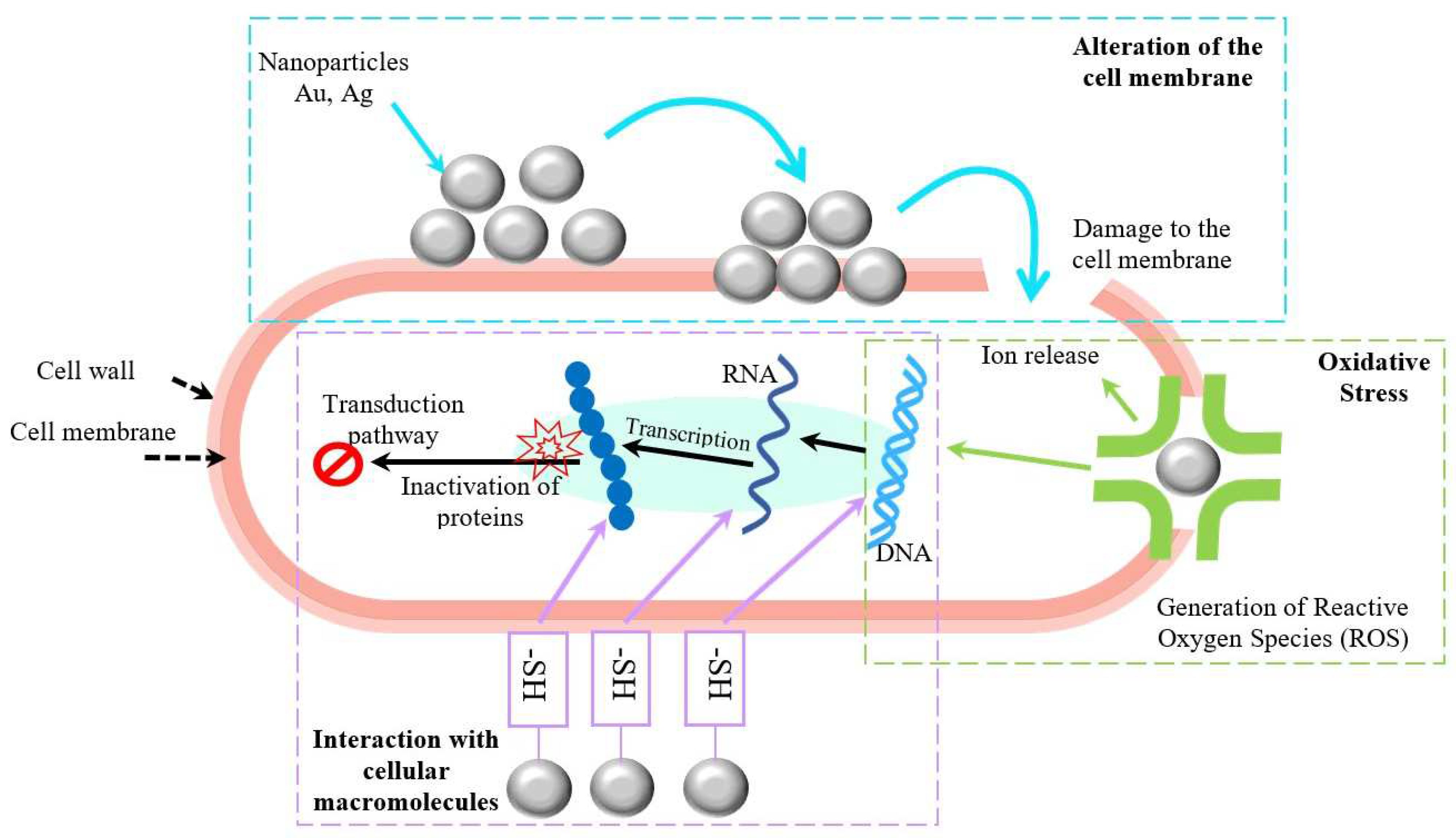
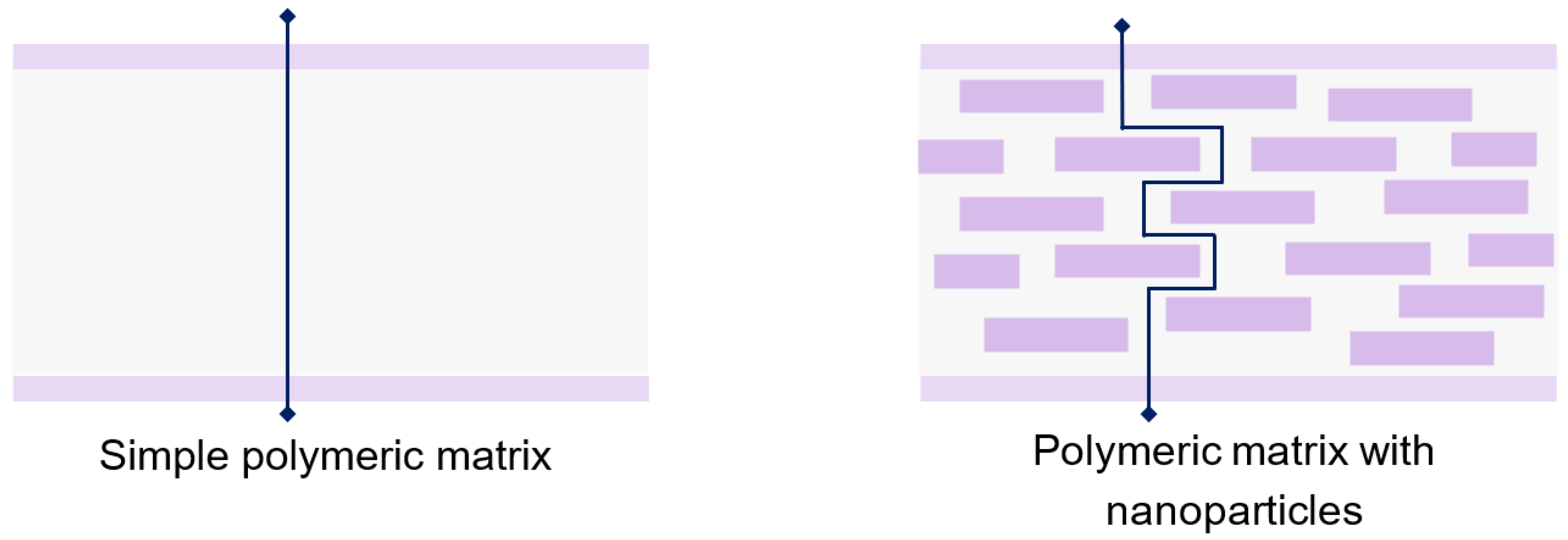
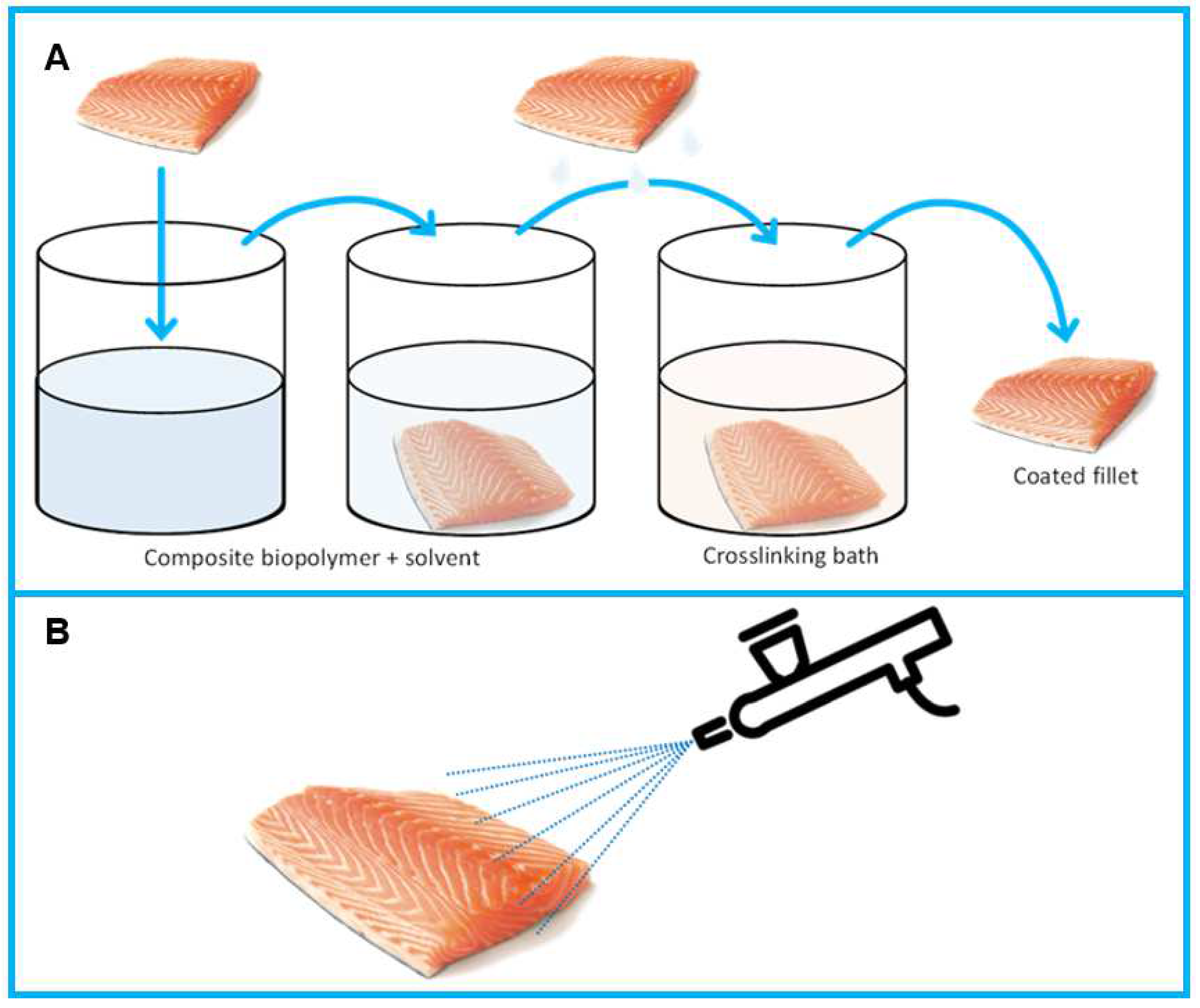

| Materials | Type/Technique | Characteristics | Application | Ref. |
|---|---|---|---|---|
| Casein/mequindox | Nanoencapsulation by sonication technique | The development of nanoparticles enhances the encapsulation efficiency and bioavailability of mequindox | Use of these nanoparticles in oral pharmaceuticals | [20] |
| Casein/silver (Ag), gold (Au) and copper oxide (CuO) | Three types of nanoparticles (Ag, Au, and Cu) were synthesized using casein as a reducing agent in a green process | Casein serves as a stabilizer in nanoparticle preparation, but each nanoparticle exhibits different morphologies, indicating dependence on the precursor used | - | [21] |
| Copper/aqueous extract of Rosa Andeli or Gardenia jasminoides leaves | Nanoparticles by green synthesis method | Rosa Andeli nanoparticles demonstrate superior antibacterial activity compared to those from Gardenia jasminoides | Potential application in drug-resistant bacteria | [22] |
| Copper (Cu)/Kigelia Africana fruit extract | Nanoparticles by green synthesis method | The extract enhanced nanoparticle synthesis and demonstrated strong antimicrobial activity | Potential application as therapeutic drug for microbial infections | [23] |
| Curcumin/rosemary oil | Nanoemulsions by a single sonication technique | The nanoemulsions inhibit efficiently the pathogenic bacteria P. aeruginosa, E. coli, and S. typhimurium | The nanoemulsions are used to increase the shelf life of rainbow trout fillets | [11] |
| Epigallocatechin gallate-grafted-chitosan | Nanoparticle by nanoprecipitation | The nanoparticle is made from chitosan modification to improve antioxidant and antibacterial activity compared to chitosan nanoparticles | Potential uses in biomedical, food packaging, nutraceutical, and pharmaceutical fields | [24] |
| Iron (Fe3O4) | Nanoparticles by biosynthesis using Satureja hortensis essential oil | The nanoparticles present cubic morphological structure and exhibit antimicrobial activity and anticancer effect against selected cell lines | Potential medicine drug | [25] |
| Silver (Ag)/essential oil of crown imperial leaves, bulbs and petals | Nanoparticle synthesis with essential oil as reduction agent | Essential oils may be an effective component in nanoparticle production and their application possess significant antibacterial activity | Potential application in medicine, pharmaceutical and food industry | [26] |
| Silver (Ag)/extract of (Mentha viridis) plant and Prunus domestica gum | Nanoparticle by green synthesis method | This NP presents an excellent antifungal and antibacterial activity and a moderate antioxidant activity | Future applications in medicine, medical devices, and antioxidant system | [27] |
| Silver (Ag)/safflower extract | Nanoparticles by green synthesis using safflower (Carthamus tinctorius L.) waste extract | Green synthesis enhances nanoparticle development and shows strong activity against S. aureus and P. fluorescens | Potential use in the food and medicine sectors | [28] |
| Soy isoflavone/whey protein | Soy isoflavone nanoencapsulated by emulsification evaporation method | The nanoencapsulation of soy isoflavone is improved with whey protein and presents an excellent stability, bioactivity and bioaccessibility | Potential application in functional food and pharmaceutical | [29] |
| Soy protein | Nanoparticles by enzymatic hydrolysis | The inclusion of three enzymes Flavorzyme, Alcalase and Protamex improve the development of the nanoparticles and exhibit better antioxidant activity than peptides of soy protein | Potential application in food, cosmetics and pharmaceuticals | [30] |
| Starch | Nanoparticles by ultra-sonication and mild alkali hydrolysis | This methodology proved to be useful and simple for the preparation of nanoparticles, also demonstrated enhanced stability and antioxidant activity | Potential application in food and pharmaceuticals | [31] |
| Starch/water soluble yellow mustard mucilage | Nanocapsules loaded with thymol and carvacrol by electrospray | Spherical structure, with high efficiency of encapsulation and uniform diameter | Elaboration of antimicrobial food packaging | [32] |
| Tea polyphenol, soybean oil | Nanoencapsulation of tea polyphenol to form a nanoemulsion by ultrasound-assisted method | The nanoemulsions present excellent antioxidant activity, inhibition of α-glucosidase and α-amylase and inhibit bacterial growth better than poly phenols | Food and nutraceutical industry | [33] |
| Titanium oxide (TiO2) | Nanoparticles synthesis with propolis extract | The nanoparticles showed antimicrobial activity, anticancer activity against human cancer cells and proved safe in low doses in albino male rats | Biomedical applications | [34] |
| Titanium oxide (TiO2) and zinc oxide (ZnO) with silver (Au) decorated | Nanoparticle by hydrothermal method | The nanocomposites exhibited antimicrobial activity against E. coli, S. aureus and C. albicans, anti-inflammatory activity using in vitro assays and significant anticancer activity through in vitro cytotoxicity assay | Biomedical applications | [35] |
| Zein/fucoidan complex and resveratrol | Nanoencapsulation of resveratrol | The zein/fucoidan nanocarrier system demonstrated controlled resveratrol release during in vitro digestion and showed low cytotoxicity | Potential use in the nutraceutical and pharmaceutical industries | [36] |
| Zein stabilized with pectin, xanthan gum and sodium alginate | Nanoencapsulation doxorubicin by flash nanoprecipitation | These nanoparticles demonstrated good stability for two weeks, and using alginate as a stabilizer showed excellent encapsulation efficiency | Potential use for delivering hydrophobic drugs | [37] |
| Zinc oxide (ZnO) | Nanoparticles by biosynthesis from essential oil of Eucalyptus globulus | The nanoparticles exhibited a potential antibacterial activity and biofilm inhibition. The use of essential oil showed an improvement in green synthesis | Potential application in medicine to combat resistant bacteria and inhibit bacterial biofilms | [38] |
| Bacterium | Characteristics |
|---|---|
| Brochuthrix | Hemophilic, non-sporulated and immobile |
| Carnobacterium | Psychotropic, immobile and oxidase positive |
| Photobacterium | Psychotropic |
| Pseudoalteromonas | Aerobic, heterotopic, oxidase and catalase positive |
| Pseudomonas | Positive and non-fermentative oxidase |
| Shewanella | Oxidase positive, catalase positive and does not ferment glucose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozuna-Valencia, K.H.; Moreno-Vásquez, M.J.; Graciano-Verdugo, A.Z.; Rodríguez-Félix, F.; Robles-García, M.Á.; Barreras-Urbina, C.G.; Quintero-Reyes, I.E.; Cornejo-Ramírez, Y.I.; Tapia-Hernández, J.A. The Application of Organic and Inorganic Nanoparticles Incorporated in Edible Coatings and Their Effect on the Physicochemical and Microbiological Properties of Seafood. Processes 2024, 12, 1889. https://doi.org/10.3390/pr12091889
Ozuna-Valencia KH, Moreno-Vásquez MJ, Graciano-Verdugo AZ, Rodríguez-Félix F, Robles-García MÁ, Barreras-Urbina CG, Quintero-Reyes IE, Cornejo-Ramírez YI, Tapia-Hernández JA. The Application of Organic and Inorganic Nanoparticles Incorporated in Edible Coatings and Their Effect on the Physicochemical and Microbiological Properties of Seafood. Processes. 2024; 12(9):1889. https://doi.org/10.3390/pr12091889
Chicago/Turabian StyleOzuna-Valencia, Karla Hazel, María Jesús Moreno-Vásquez, Abril Zoraida Graciano-Verdugo, Francisco Rodríguez-Félix, Miguel Ángel Robles-García, Carlos Gregorio Barreras-Urbina, Idania Emedith Quintero-Reyes, Yaeel Isbeth Cornejo-Ramírez, and José Agustín Tapia-Hernández. 2024. "The Application of Organic and Inorganic Nanoparticles Incorporated in Edible Coatings and Their Effect on the Physicochemical and Microbiological Properties of Seafood" Processes 12, no. 9: 1889. https://doi.org/10.3390/pr12091889
APA StyleOzuna-Valencia, K. H., Moreno-Vásquez, M. J., Graciano-Verdugo, A. Z., Rodríguez-Félix, F., Robles-García, M. Á., Barreras-Urbina, C. G., Quintero-Reyes, I. E., Cornejo-Ramírez, Y. I., & Tapia-Hernández, J. A. (2024). The Application of Organic and Inorganic Nanoparticles Incorporated in Edible Coatings and Their Effect on the Physicochemical and Microbiological Properties of Seafood. Processes, 12(9), 1889. https://doi.org/10.3390/pr12091889











