Experimental Device for the “Green” Synthesis of Unbranched Aliphatic Esters C4–C8 Using an Audio Frequency Electric Field
Abstract
1. Introduction
2. Results and Discussions
2.1. The Experimental Device Based on Green Technology
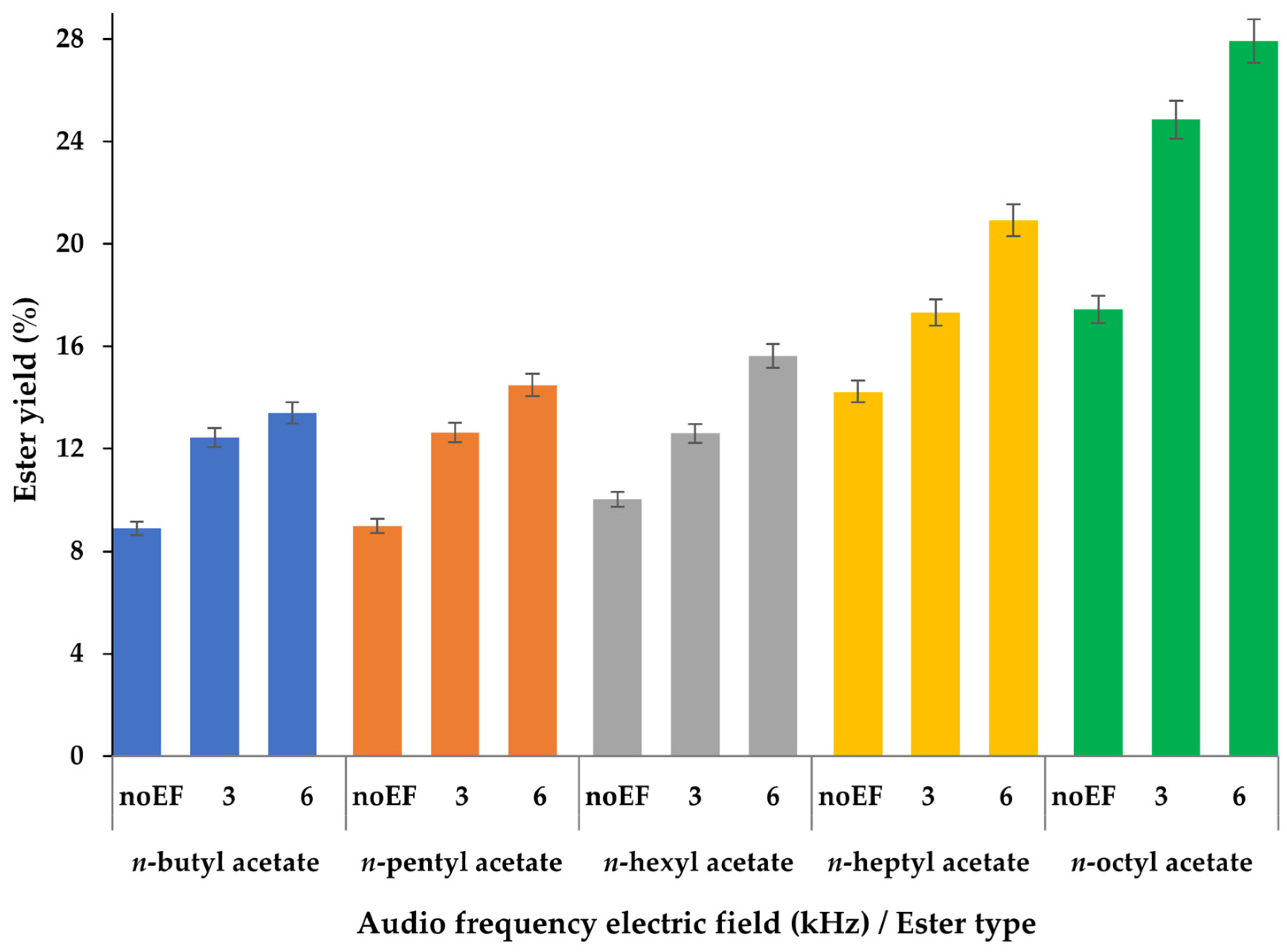
2.2. Synthesis of C4–C8 Acetate Esters Using an Audio Frequency Electric Field
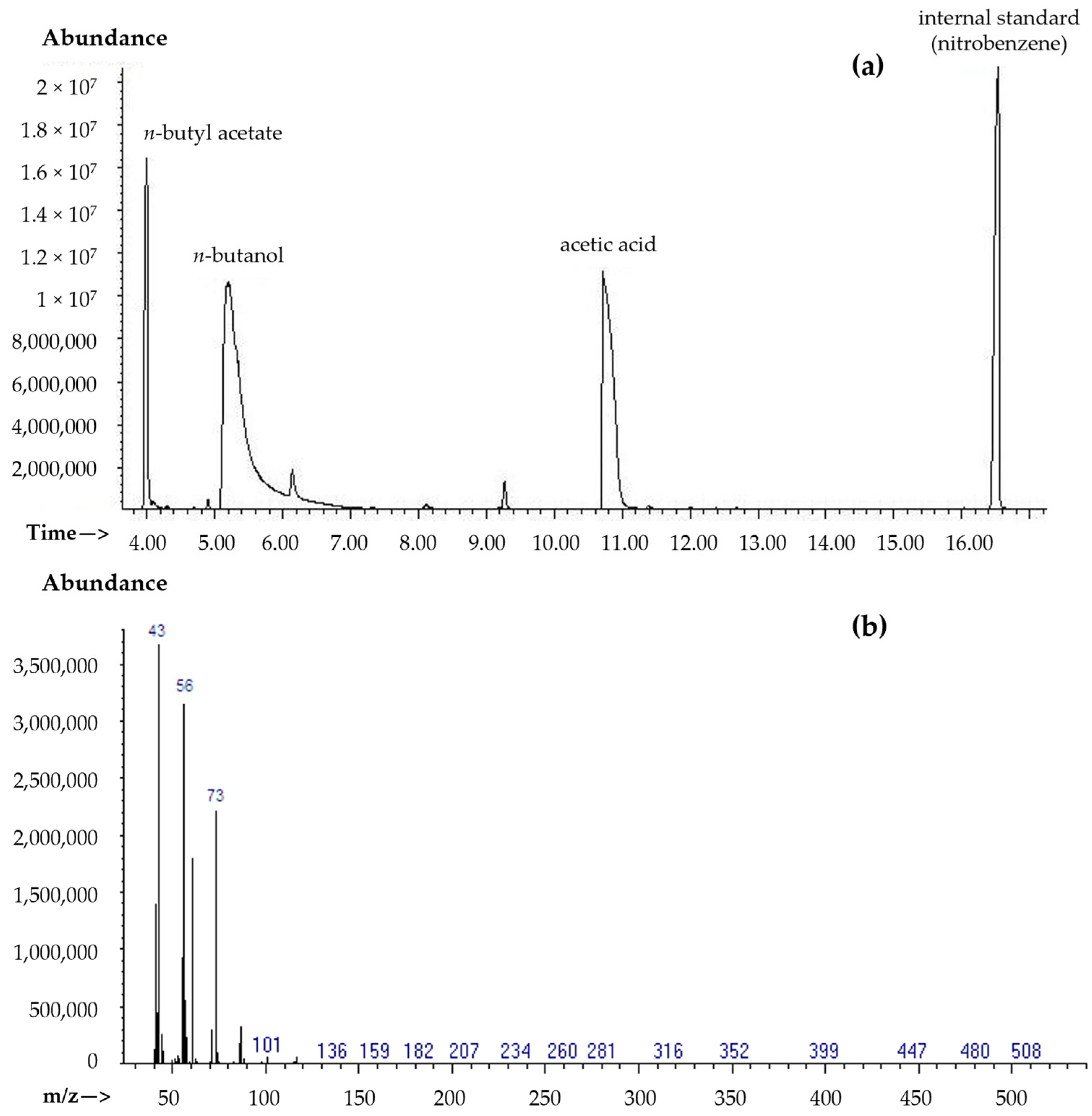
2.3. C4–C8 Ester Analysis Using Gas Chromatography Techniques
2.4. Mechanism of Esterification Using an Audio Frequency Electric Field
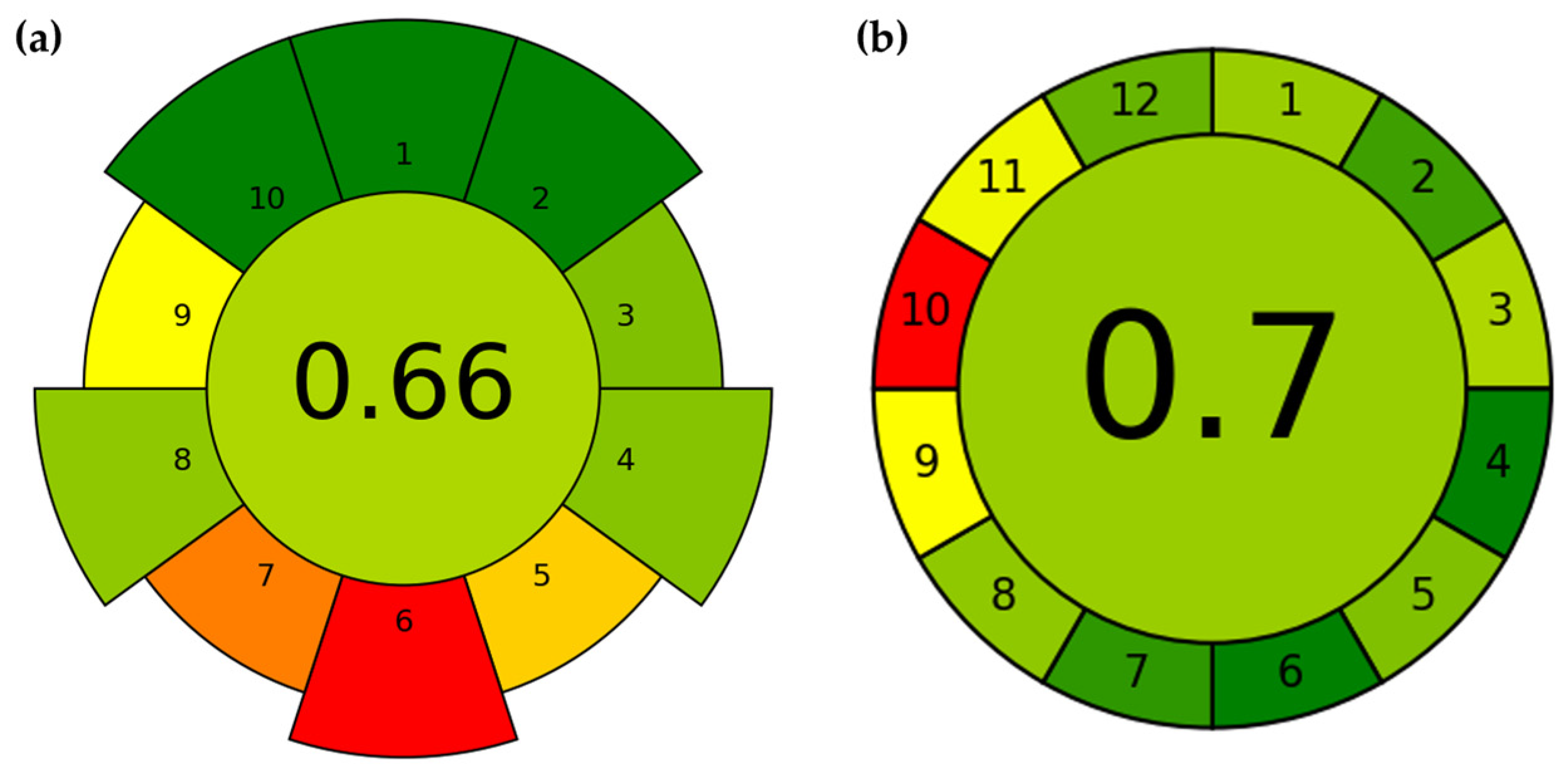
2.5. AGREE Analysis
2.6. Strengths, Limitations, and Future Perspectives
3. Materials and Methods
3.1. The Experimental Device
3.2. Synthesis of Aliphatic Esters Using an Audio Frequency Electric Field
3.3. GC-FID Analysis
3.4. GC-MS Analysis
3.5. Evaluation of Preparation and Analytical Methods Using AGREE Tools
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, R.; Barbosa, A.; Advinha, B.; Sales, H.; Pontes, R.; Nunes, J. Green Extraction Techniques of Bioactive Compounds: A State-of-the-Art Review. Processes 2023, 11, 2255. [Google Scholar] [CrossRef]
- Udrea, I.-A.; Ordodi, V.; Paul, C.; Stănese, C.; Vaszilcsin, N. Effects of Pulsed Electric Field on the Esterification Reactions. Stud. UBB Chem. 2023, 68, 49–58. [Google Scholar] [CrossRef]
- El Hadi, M.; Zhang, F.-J.; Wu, F.-F.; Zhou, C.-H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed]
- Gómez, E.; Ledbetter, C.A. Development of Volatile Compounds during Fruit Maturation: Characterization of Apricot and Plum×Apricot Hybrids. J. Sci. Food Agric. 1997, 74, 541–546. [Google Scholar] [CrossRef]
- Vasilescu, C.; Todea, A.; Nan, A.; Circu, M.; Turcu, R.; Benea, I.-C.; Peter, F. Enzymatic Synthesis of Short-Chain Flavor Esters from Natural Sources Using Tailored Magnetic Biocatalysts. Food Chem. 2019, 296, 1–8. [Google Scholar] [CrossRef]
- Babali, B.; Tuter, M.; Ustun, G. Enzymatic Esterification of (−)-menthol with Lauric Acid in Isooctane by Sorbitan Monostearate-coated Lipase from Candida Rugosa. J. Americ Oil Chem. Soc. 2001, 78, 173–175. [Google Scholar] [CrossRef]
- Lopresto, C.G.; Calabrò, V.; Woodley, J.M.; Tufvesson, P. Kinetic Study on the Enzymatic Esterification of Octanoic Acid and Hexanol by Immobilized Candida Antarctica Lipase B. J. Mol. Catal. B Enzym. 2014, 110, 64–71. [Google Scholar] [CrossRef]
- Khan, Z.; Javed, F.; Shamair, Z.; Hafeez, A.; Fazal, T.; Aslam, A.; Zimmerman, W.B.; Rehman, F. Current Developments in Esterification Reaction: A Review on Process and Parameters. J. Ind. Eng. Chem. 2021, 103, 80–101. [Google Scholar] [CrossRef]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F.F. Comparative Study of Four Greenness Assessment Tools for Selection of Greenest Analytical Method for Assay of Hyoscine N-Butyl Bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef]
- Van Aken, K.; Strekowski, L.; Patiny, L. EcoScale, a Semi-Quantitative Tool to Select an Organic Preparation Based on Economical and Ecological Parameters. Beilstein J. Org. Chem. 2006, 2, 3. [Google Scholar] [CrossRef]
- Nowak, P.M. What Does It Mean That “Something Is Green”? The Fundamentals of a Unified Greenness Theory. Green Chem. 2023, 25, 4625–4640. [Google Scholar] [CrossRef]
- Pipus, G.; Plazl, I.; Koloini, T. Esterification of Benzoic Acid in Microwave Tubular Flow Reactor. Chem. Eng. J. 2000, 76, 239–245. [Google Scholar] [CrossRef]
- Pathak, G.; Das, D.; Rokhum, S.L. A Microwave-Assisted Highly Practical Chemoselective Esterification and Amidation of Carboxylic Acids. RSC Adv. 2016, 6, 93729–93740. [Google Scholar] [CrossRef]
- Mahapatra, P.; Kumari, A.; Kumar Garlapati, V.; Banerjee, R.; Nag, A. Enzymatic Synthesis of Fruit Flavor Esters by Immobilized Lipase from Rhizopus Oligosporus Optimized with Response Surface Methodology. J. Mol. Catal. B Enzym. 2009, 60, 57–63. [Google Scholar] [CrossRef]
- Berger, R.G. Biotechnology of Flavours—The next Generation. Biotechnol. Lett. 2009, 31, 1651–1659. [Google Scholar] [CrossRef]
- Martins, A.B.; Graebin, N.G.; Lorenzoni, A.S.G.; Fernandez-Lafuente, R.; Ayub, M.A.Z.; Rodrigues, R.C. Rapid and High Yields of Synthesis of Butyl Acetate Catalyzed by Novozym 435: Reaction Optimization by Response Surface Methodology. Process Biochem. 2011, 46, 2311–2316. [Google Scholar] [CrossRef]
- Calinescu, I.; Vartolomei, A.; Gavrila, I.-A.; Vinatoru, M.; Mason, T.J. A Reactor Designed for the Ultrasonic Stimulation of Enzymatic Esterification. Ultrason. Sonochem. 2019, 54, 32–38. [Google Scholar] [CrossRef]
- Duong, N.T.C.; Uthairatanakij, A.; Laohakunjit, N.; Jitareerat, P.; Kaisangsri, N. Cross-Linked Alginate Edible Coatings Incorporated with Hexyl Acetate: Film Characteristics and Its Application on Fresh-Cut Rose Apple. Food Biosci. 2023, 52, 102410. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Rathod, V.K. Intensification of Enzyme Catalysed Synthesis of Hexyl Acetate Using Sonication. Green Process. Synth. 2017, 6, 55–62. [Google Scholar] [CrossRef]
- Lanciotti, R.; Belletti, N.; Patrignani, F.; Gianotti, A.; Gardini, F.; Guerzoni, M.E. Application of Hexanal, (E)-2-Hexenal, and Hexyl Acetate To Improve the Safety of Fresh-Sliced Apples. J. Agric. Food Chem. 2003, 51, 2958–2963. [Google Scholar] [CrossRef]
- Sá, A.G.A.; Meneses, A.C.D.; Araújo, P.H.H.D.; Oliveira, D.D. A Review on Enzymatic Synthesis of Aromatic Esters Used as Flavor Ingredients for Food, Cosmetics and Pharmaceuticals Industries. Trends Food Sci. Technol. 2017, 69, 95–105. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 8159, Heptyl Acetate. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Heptyl-acetate (accessed on 6 August 2024).
- Fahlbusch, K.; Hammerschmidt, F.; Panten, J.; Pickenhagen, W.; Schatkowski, D.; Bauer, K.; Garbe, D.; Surburg, H. Flavors and Fragrances. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH, Ed.; Wiley: Hoboken, NJ, USA, 2003; ISBN 978-3-527-30385-4. [Google Scholar]
- Balci, S.; Sefa, I.; Altin, N. An Investigation of Ferrite and Nanocrystalline Core Materials for Medium-Frequency Power Transformers. J. Electron. Mater. 2016, 45, 3811–3821. [Google Scholar] [CrossRef]
- Lara-Reyes, J.; Ponce-Silva, M.; Cortés-García, C.; Lozoya-Ponce, R.E.; Parrilla-Rubio, S.M.; García-García, A.R. High-Power-Factor LC Series Resonant Converter Operating Off-Resonance with Inductors Elaborated with a Composed Material of Resin/Iron Powder. Electronics 2022, 11, 3761. [Google Scholar] [CrossRef]
- Liu, Z.; Winands, G.J.J.; Yan, K.; Pemen, A.J.M.; Van Heesch, E.J.M. A High-Voltage Pulse Transformer with a Modular Ferrite Core. Rev. Sci. Instrum. 2008, 79, 015104. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wei, Y.; Xu, L. Electrochemical Synthesis of Sulfinic Esters from Alcohols and Thiophenols. Tetrahedron Lett. 2020, 61, 151631. [Google Scholar] [CrossRef]
- De Barros, D.P.C.; Fonseca, L.P.; Fernandes, P.; Cabral, J.M.S.; Mojovic, L. Biosynthesis of Ethyl Caproate and Other Short Ethyl Esters Catalyzed by Cutinase in Organic Solvent. J. Mol. Catal. B Enzym. 2009, 60, 178–185. [Google Scholar] [CrossRef]
- Su, L.; Hong, R.; Guo, X.; Wu, J.; Xia, Y. Short-Chain Aliphatic Ester Synthesis Using Thermobifida Fusca Cutinase. Food Chem. 2016, 206, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, W. Superabsorbent Materials. In Kirk-Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 1–34. [Google Scholar] [CrossRef]
- Serra, S.; Fuganti, C.; Brenna, E. Biocatalytic Preparation of Natural Flavours and Fragrances. Trends Biotechnol. 2005, 23, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Schrader, J.; Etschmann, M.M.W.; Sell, D.; Hilmer, J.-M.; Rabenhorst, J. Applied Biocatalysis for the Synthesis of Natural Flavour Compounds—Current Industrial Processes and Future Prospects. Biotechnol. Lett. 2004, 26, 463–472. [Google Scholar] [CrossRef]
- Alenezi, R.; Leeke, G.A.; Winterbottom, J.M.; Santos, R.C.D.; Khan, A.R. Esterification Kinetics of Free Fatty Acids with Supercritical Methanol for Biodiesel Production. Energy Convers. Manag. 2010, 51, 1055–1059. [Google Scholar] [CrossRef]
- Gang, L.; Xinzong, L.; Eli, W. Solvent-Free Esterification Catalyzed by Surfactant-Combined Catalysts at Room Temperature. New J. Chem. 2007, 31, 348. [Google Scholar] [CrossRef]
- Kawabata, T.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Highly Efficient Esterification of Carboxylic Acids with Alcohols by Montmorillonite-Enwrapped Titanium as a Heterogeneous Acid Catalyst. Tetrahedron Lett. 2003, 44, 9205–9208. [Google Scholar] [CrossRef]
- Pandian, S.; Sakthi Saravanan, A.; Sivanandi, P.; Santra, M.; Booramurthy, V.K. 4-Application of Heterogeneous Acid Catalyst Derived from Biomass for Biodiesel Process Intensification: A Comprehensive Review. In Refining Biomass Residues for Sustainable Energy and Bioproducts; Kumar, R.P., Gnansounou, E., Raman, J.K., Baskar, G., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 87–109. [Google Scholar]
- Jiang, Y.; Lu, J.; Sun, K.; Ma, L.; Ding, J. Esterification of Oleic Acid with Ethanol Catalyzed by Sulfonated Cation Exchange Resin: Experimental and Kinetic Studies. Energy Convers. Manag. 2013, 76, 980–985. [Google Scholar] [CrossRef]
- Zang, Y.; Zou, Q.; Fu, T.; Ng, F.; Fowler, B.; Yang, J.; Li, H.; Steigerwald, M.L.; Nuckolls, C.; Venkataraman, L. Directing isomerization reactions of cumulenes with electric fields. Nat. Commun. 2019, 10, 4482. [Google Scholar] [CrossRef]
- Al-Arafi, N.; Salimon, J. Production of Oleic Acid Based Wax Ester Using Acidic Homogeneous Catalysts. E-J. Chem. 2012, 9, 99–106. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Experimental and Kinetic Study of Esterification of Acrylic Acid with Ethanol Using Homogeneous Catalyst. Int. J. Chem. React. Eng. 2016, 14, 571–578. [Google Scholar] [CrossRef]
- Gui, J.; Cong, X.; Liu, D.; Zhang, X.; Hu, Z.; Sun, Z. Novel Brønsted Acidic Ionic Liquid as Efficient and Reusable Catalyst System for Esterification. Catal. Commun. 2004, 5, 473–477. [Google Scholar] [CrossRef]
- Zang, Y.; Shi, J.; Zhang, F.; Zhong, Y.; Zhu, W. Sulfonic Acid-Functionalized MIL-101 as a Highly Recyclable Catalyst for Esterification. Catal. Sci. Technol. 2013, 3, 2044–2049. [Google Scholar] [CrossRef]
- Schmickler, W.; Santos, E. Electrocatalysis. In Interfacial Electrochemistry, 2nd ed.; Springer: Heidelberg, Germany, 2010; ISBN 978-3-642-04929-8. [Google Scholar]
- Lin, Z.-R.; Zeng, X.-A.; Yu, S.-J.; Sun, D.-W. Enhancement of Ethanol–Acetic Acid Esterification Under Room Temperature and Non-catalytic Condition via Pulsed Electric Field Application. Food Bioprocess Technol. 2011, 5, 2637–2645. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness Metric Approach and Software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Wojnowski, W.; Tobiszewski, M.; Pena-Pereira, F.; Psillakis, E. AGREEprep–Analytical Greenness Metric for Sample Preparation. TrAC Trends Anal. Chem. 2022, 149, 116553. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Tobiszewski, M.; Wojnowski, W.; Psillakis, E. A Tutorial on AGREEprep an Analytical Greenness Metric for Sample Preparation. Adv. Sample Prep. 2022, 3, 100025. [Google Scholar] [CrossRef]
- Kim, J.; Kim, D.-H.; Kim, J.; Park, Y.-J. Wireless Power Transfer between Two Self-Resonant Coils over Medium Distance Supporting Optimal Impedance Matching Using Ferrite Core Transformers. Energies 2021, 14, 8540. [Google Scholar] [CrossRef]




| Ester | Chemical Structure | Characteristics/Usage | References |
|---|---|---|---|
| n-Butyl acetate | 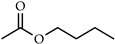 | Sweet scent of banana or apple employed in various industries, including food, beverage, fragrance, cosmetics, and pharmaceutical industries | [14,15,16] |
| n-Pentyl acetate |  | Scent like bananas and apples used as a flavoring in foods and as a scent in perfumes | [17] |
| n-Hexyl acetate | 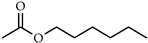 | Fruity smelling scent used as a flavoring agent or as a scent in perfumes; exhibits antimicrobial activity and can be used to improve the safety of minimally processed fruits | [18,19,20] |
| n-Heptyl acetate |  | Pear scent/essence used as flavoring in foods and as a scent in perfumes | [21,22] |
| n-Octyl acetate |  | Scent like oranges, grapefruits, and other citrus products used as a flavoring in foods and as a scent in perfumes | [23] |
| Reactants | Catalyst | Temperature (°C) | Reaction Time (min) | Yield (%) | Product | Experiment Setup | References |
|---|---|---|---|---|---|---|---|
| Oleic acid/ Oleyl alcohol | H2SO4 Perchloric acid Phosphoric acid | 90 | 300 | 93.88 54.9 52.7 | Oleyl oleate | Oil bath stirring | [39] |
| Acrylic acid/ Ethanol | H2SO4 | 70 | 360 | 83.99 | Ethyl acrylate | Bath reactor | [40] |
| Oleic acid/ 1-Octanol | DBSA (dodecylbenzene sulfonic acid) | 23 | 1440 | 98.7 | Octyl oleate | Dean–Stark apparatus | [34] |
| Acetic acid/ Ethanol | Ionic liquid (1-(4-sulfonic acid) butylpyridinium hydrogen sulfate) | 100 | 240 | 99 | Ethyl acetate | Oil bath stirring | [41] |
| Acetic acid/ n-Hexanol | Sulfonic acid-functionalized MIL-101 | 110 | 300 | 99 | Hexyl acetate | Dean–Stark apparatus | [42] |
| Octanoic acid/ Hexanol | Candida antarctica lipase (Novozym 435) | 35 | 60 | 90 | Hexyl octanoate | Glass vials and thermoshaker | [7] |
| Benzoic acid/1-Propanol | Triphenylphosphine and iodine | 85 | 30 | 93 | Propyl benzoate | Microwave reactor | [13] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udrea, I.-A.; Lukinich-Gruia, A.T.; Paul, C.; Pricop, M.-A.; Dan, M.; Păunescu, V.; Băloi, A.; Tatu, C.A.; Vaszilcsin, N.; Ordodi, V.L. Experimental Device for the “Green” Synthesis of Unbranched Aliphatic Esters C4–C8 Using an Audio Frequency Electric Field. Processes 2024, 12, 1891. https://doi.org/10.3390/pr12091891
Udrea I-A, Lukinich-Gruia AT, Paul C, Pricop M-A, Dan M, Păunescu V, Băloi A, Tatu CA, Vaszilcsin N, Ordodi VL. Experimental Device for the “Green” Synthesis of Unbranched Aliphatic Esters C4–C8 Using an Audio Frequency Electric Field. Processes. 2024; 12(9):1891. https://doi.org/10.3390/pr12091891
Chicago/Turabian StyleUdrea, Ioan-Alexandru, Alexandra Teodora Lukinich-Gruia, Cristina Paul, Maria-Alexandra Pricop, Mircea Dan, Virgil Păunescu, Alexandru Băloi, Călin A. Tatu, Nicolae Vaszilcsin, and Valentin L. Ordodi. 2024. "Experimental Device for the “Green” Synthesis of Unbranched Aliphatic Esters C4–C8 Using an Audio Frequency Electric Field" Processes 12, no. 9: 1891. https://doi.org/10.3390/pr12091891
APA StyleUdrea, I.-A., Lukinich-Gruia, A. T., Paul, C., Pricop, M.-A., Dan, M., Păunescu, V., Băloi, A., Tatu, C. A., Vaszilcsin, N., & Ordodi, V. L. (2024). Experimental Device for the “Green” Synthesis of Unbranched Aliphatic Esters C4–C8 Using an Audio Frequency Electric Field. Processes, 12(9), 1891. https://doi.org/10.3390/pr12091891







