Recent Developments in Application of Nanofibers
Abstract
:1. Introduction
Electrospinning
2. Non-Electrospinning Methods
- (i)
- Drawing
- (ii)
- Template
- (iii)
- Self-assembly
- (iv)
- Phase separation
2.1. Health Applications
2.2. Wastewater Treatment
2.3. Protection
2.4. Electronics
2.5. Packaging
2.6. Apparel and Technical Textiles
2.7. Future Research
3. Conclusions
Funding
Conflicts of Interest
References
- Yang, G.; Yang, H.; Wang, M.; Sun, Y.; Wang, M. Application of nanofiber material based on electrospinning technology in sports rehabilitation of basketball player’s wrist joint. J. Nanomater. 2022, 2022, 1–9. [Google Scholar] [CrossRef]
- Yu, X.-C.; Li, B.-B.; Wang, P.; Tong, L.; Jiang, X.-F.; Li, Y.; Gong, Q.; Xiao, Y.-F. Single nanoparticle detection and sizing using a nanofiber pair in an aqueous environment. Adv. Mater. 2014, 26, 7462–7467. [Google Scholar] [CrossRef]
- Beachley, V.; Wen, X. Effect of electrospinning parameters on the nanofiber diameter and length. Mater. Sci. Eng. C 2009, 29, 663–668. [Google Scholar] [CrossRef]
- Rafiq, M.; Rather, A.H.; Khan, R.S.; Rehman, R.; Qureashi, A.; Khan, H.A.; Alhomida, A.S.; Triphathi, R.M.; Rather, S.; Majeed, S.; et al. Enhancing nanofiber characteristics through solvothermal approaches for environmental cleaning and energy-saving applications. Mater. Today Commun. 2024, 40, 110120. [Google Scholar] [CrossRef]
- Huang, Y.; Song, J.; Yang, C.; Long, Y.; Wu, H. Scalable manufacturing and applications of nanofibers. Mater. Today 2019, 28, 98–113. [Google Scholar] [CrossRef]
- Coleman, B.F.; Street, R.M.; Fitzsimons, T.; Schauer, C.L. Touchspinning: Mechanically drawing polyacrylonitrile nanofibers. J. Appl. Polym. Sci. 2022, 139, e52339. [Google Scholar] [CrossRef]
- Dahlin, R.L.; Kasper, F.K.; Mikos, A.G. Polymeric nanofibers in tissue engineering. Tissue Eng. Part Rev. 2011, 17, 349–364. [Google Scholar] [CrossRef]
- Agrawal, S.; Ranjan, R.; Lal, B.; Rahman, A.; Singh, S.P.; Selvaratnam, T.; Nawaz, T. Synthesis and water treatment applications of nanofibers by electrospinning. Processes 2021, 9, 1779. [Google Scholar] [CrossRef]
- Tayebi-Khorrami, V.; Rahmanian-Devin, P.; Fadaei, M.R.; Movaffagh, J.; Askari, V.R. Advanced applications of smart electrospun nanofibers in cancer therapy: With insight into material capabilities and electrospinning parameters. Int. J. Pharm. X 2024, 100265, 8. [Google Scholar] [CrossRef]
- Anusiya, G.; Jaiganesh, R. A review on fabrication methods of nanofibers and a special focus on application of cellulose nanofibers. Carbohydr. Polym. Technol. Appl. 2022, 4, 100262. [Google Scholar] [CrossRef]
- Almetwally, A.A.; El-Sakhawy, M.; Elshakankery, M.H.; Kasem, M.H. Technology of nanofibers: Production techniques and properties-Critical review. J. Text. Assoc. 2017, 78, 5–14. [Google Scholar]
- Chen, S.; Tian, H.; Mao, J.; Ma, F.; Zhang, M.; Chen, F. Preparation and application of chitosan-based medical electrospun nanofibers. Int. J. Biol. Macromol. 2023, 226, 410–422. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the fabrication and application of electrospun nanofiber composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef] [PubMed]
- Khandanga, V.; Mishra, P.C. A review on toxicity mechanism and risk factors of nanoparticles in respiratory tract. Toxicology 2024, 504, 153781. [Google Scholar] [CrossRef] [PubMed]
- Zamwar, K.P.; Tonge, S.; Raut, J.S.; Parshive, P.S.; Bharsakale, R.R.; Jain, N. A review on synthesis, advantages and disadvantages of nanofibers. Mukt Shabd J. 2020, 915, 2347–3150. [Google Scholar]
- Zhang, M.; Xu, L.; Liu, F. Batch preparation and characterization of zein-based beaded nanofiber membranes for active food packaging. Int. J. Biol. Macromol. 2024, 276, 133966. [Google Scholar] [CrossRef]
- Hassabo, A.G.; Elmorsy, H.M.; Gamal, N.; Sedik, A.; Saad, F.; Hegazy, B.M.; Othman, H.A. Applications of nanotechnology in the creation of smart sportswear for enhanced sports performance: Efficiency and comfort. J. Text. Color. Polym. Sci. 2022, 20, 11–28. [Google Scholar] [CrossRef]
- Liu, M.; Lake-Thompson, G.; Wescott, A.; Beeby, S.; Tudor, J.; Yang, K. Design and development of a stretchable electronic textile and its application in a knee sleeve targeting wearable pain management. Sens. Actuators A Phys. 2024, 369, 115102. [Google Scholar] [CrossRef]
- Mei, X.; Zhu, L.; Peng, X.; Yang, J.; Li, Y. Wearable Janus nanofiber membrane-based colorimetric sensors for directional transfer and analysis of sweat. Biomed. Anal. 2024, 1, 64–72. [Google Scholar] [CrossRef]
- Wu, H.; Yu, Y.; Yu, Y.; Shi, J.; Morikawa, H.; Zhu, C. Hyperthermal management core-sheath polyurethane nanofiber yarns hyperthermal management core-sheath polyurethane nanofiber yarns based on vanadium dioxide toward commercialization. J. Energy Storage 2024, 86, 111311. [Google Scholar]
- Gavande, V.; Nagappan, S.; Seo, B.; Lee, W. A systematic review on green and natural polymeric nanofibers for biomedical applications. Int. J. Biol. Macromol. 2024, 262, 130135. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, X. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-N. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Fadil, F.; Affandi, N.D.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-Applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Kailasa, S.; Reddy, M.S.; Maurya, M.R.; Rani, B.C.; Rao, K.V.; Sadasivun, K.K. Electrospun nanofibers: Materials, synthesis parameters, and their role in sensing applications. Macromol. Mater. Eng. 2021, 306, 2100410. [Google Scholar] [CrossRef]
- Song, J.; Kim, M.; Lee, H. Recent advances on nanofiber fabrications: Unconventional state-of-the-art spinning techniques. Polymers 2020, 12, 1386. [Google Scholar] [CrossRef] [PubMed]
- Lou, L.; Chen, H.; Zhang, L. Biodegradable gelatin/pectin films containing cellulose nanofibers and biguanide polymers: Characterization and application in sweet cherry packaging. Int. J. Biol. Macromol. 2024, 274, 133530. [Google Scholar] [CrossRef]
- Yusuf, J.; Sapuan, S.M.; Ansari, M.A.; Siddiqui, V.U.; Jamal, T.; Ilyas, R.A.; Hassan, M.R. Exploring nanocellulose frontiers: A comprehensive review of its extraction, properties, and pioneering applications in the automotive and biomedical industries. Int. J. Biol. Macromol. 2024, 255, 128121. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Bildik, F.; Altay, F.; Ceylan, Z. Gelatin nanofibers with black elderberry, Au nanoparticles and SnO2 as intelligent packaging layer used for monitoring freshness of Hake fish. Food Chem. 2024, 437, 137843. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Said, N.S.; Yosri, N.; Hawash, H.B.; El-Sherif, D.M.; Abouzid, M.; Abdel-Daim, M.M.; Yaseen, M.; Omar, H.; Shou, Q.; et al. Gelatin nanofibers: Recent insights in synthesis, bio-medical applications and limitations. Heliyon 2023, 9, e26228. [Google Scholar] [CrossRef]
- Wu, X.; Cao, S.; Ghim, D.; Jiang, Q.; Singamaneni, S.; Jun, Y.-S. A thermally engineered polydopamine and bacterial nanocellulose bilayer membrane for photothermal membrane distillation with bactericidal capability. Nano Energy 2021, 79, 105353. [Google Scholar] [CrossRef]
- Seddiqi, H.; Oliaei, E.; Honarkar, H.; Jin, J.; Geonzon, L.C.; Bacabac, R.G.; Klein-Nulend, J. Cellulose and its derivatives: Towards biomedical applications. Cellulose 2021, 18, 1893–1931. [Google Scholar] [CrossRef]
- Pennels, J.; Godwin, I.D.; Amiralian, N.; Martin, D.J. Trends in the production of cellulose nanofibers from nonwood sources. Cellulose 2020, 27, 575–593. [Google Scholar] [CrossRef]
- Apelgren, P.; Samfors, S.; Saljo, K.; Molne, J.; Gatenholm, P.; Troedsson, C.; Thompson, E.M.; Kolby, L. Biomaterial and biocompatibility evaluation of tunicate nanocellulose for tissue engineering. Biomater. Adv. 2022, 137, 212828. [Google Scholar] [CrossRef]
- Patil, T.V.; Patel, D.K.; Dutta, S.D.; Ganguly, K.; Santra, T.S.; Lim, K.-T. Nanocellulose, a versatile platform: From the delivery of active molecules to tissue engineering applications. Bioact. Mater. 2022, 9, 556–589. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.V.; Otoni, C.G.; De France, K.J.; Barud, H.S.; Lona, L.M.; Cranston, E.D.; Rojas, O.J. Porous nanocellulose gels and foams: Breakthrough status in the development of scaffolds for tissue engineering. Mater. Today 2020, 37, 126–141. [Google Scholar] [CrossRef]
- Talukder, E.; Talukder, R.; Pervez, N.; Song, H.; Naddeo, V. Bead-containing superhydrophobic nanofiber membrane for membrane distillation. Membranes 2024, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Mehrasa, M.; Doostmohamadi, M.; Forootanfar, H.; Amini, S.; Davari, N.; Salehi, H.; Amirpour, N. Silica nano particles embedded in random and aligned PLGA/gelatin electrospun nano fibers improve growth and differentiation of human adipose-derived stem cells into anterior neuroectodermal cells. Mater. Today Commun. 2022, 31, 103461. [Google Scholar] [CrossRef]
- Yan, T.; Wang, X.; Qiao, Y. Strategy to Antibacterial, High-Mechanical, and Degradable polylactic acid/chitosan composite film through reactive compatibilization via epoxy chain extender. ACS Omega 2024, 9, 27312–27320. [Google Scholar] [CrossRef]
- Chang, S.-H.; Chen, Y.-J.; Tseng, H.-J.; Hsiao, H.-I.; Chai, H.-J.; Shang, K.-C.; Pan, C.-L.; Tsai, G.-J. Antibacterial activity of chitosan–polylactate fabricated plastic film and its application on the preservation of fish fillet. Polymers 2021, 13, 696. [Google Scholar] [CrossRef]
- Osali, S.; Ghiyasi, Y.; Esfahani, H.; Jose, R.; Ramakrishna, S. Electrospun nanomembranes at the liquid–liquid and solid–liquid interface-a review. Mater. Today 2023, 76, 151–177. [Google Scholar] [CrossRef]
- Zhou, J.; Cui, Z.; Liu, X.; Wang, Q.; Chen, B.; Zeng, S.; Si, J. Multifunctional nanofiber membrane with antifouling properties for efficient seawater desalination and wastewater purification. Desalination 2024, 586, 117812. [Google Scholar] [CrossRef]
- Rasal, R.K.; Badsha, I.; Thiyagarajan, D.; Rajendran, N.; Sundaresan, M.B.; Nallathambi, G. Statistically engineered self-cleaning neoteric multifunctional PAN-GOCM and PAN-BDMCAQD electrospun nanofibers for ultrafiltration, heavy metal removal and corrosion mitigation. J. Ind. Eng. Chem. 2024, 138, 208–236. [Google Scholar]
- Radoor, S.; Karayil, J.; Jayakumar, A.; Siengchin, S. Efficient removal of dyes, heavy metals and oil-water from wastewater using electrospun nanofiber membranes: A review. J. Water Process Eng. 2024, 59, 104983. [Google Scholar] [CrossRef]
- Yao, J.; Bastiaansen, C.W.; Peijs, T. High strength and high modulus electrospun nanofibers. Fibers 2014, 2, 158–187. [Google Scholar] [CrossRef]
- Lu, X.; Meng, J.; He, J.; Qi, N.; Lu, Y. Numerical investigation of the effect of static and dynamic air gap on heat. Int. Commun. Heat Mass Transf. 2024, 156, 107665. [Google Scholar] [CrossRef]
- Colburn, D.; Russo, L.; Burkard, R.; Hostler, D. Firefighter protective clothing and self-contained breathing apparatus does not alter balance testing using a standard sensory organization test or motor control test in healthy, rested individuals. Appl. Ergon. 2019, 80, 187–192. [Google Scholar] [CrossRef]
- Abtew, M.A.; Boussu, F.; Bruniaux, P.; Loghin, C.; Cristian, I. Ballistic impact mechanisms–A review on textiles and fiber-reinforced composites impact responses. Compos. Struct. 2019, 223, 110966. [Google Scholar] [CrossRef]
- Su, Y.; Yao, J.; Li, J.; Tian, M. Numerical analysis of heat and moisture transfer in waterproof and breathable composite fabric used for steam protective clothing. Int. Commun. Heat Mass Transf. 2024, 152, 107336. [Google Scholar] [CrossRef]
- Kumar, V.; Bhanja, D.; Acharya, J. Performance assessment of firefighter protective clothing under different thermal environments: Impact of the variation of thickness in inter-layer airgap and microclimate. Int. J. Therm. Sci. 2024, 205, 109293. [Google Scholar] [CrossRef]
- Roossien, C.C.; Heus, R.; Reneman, M.F.; Verkerke, G.J. Monitoring core temperature of firefighters to validate a wearable non-invasive core thermometer in different types of protective clothing: Concurrent in-vivo validation. Appl. Ergon. 2020, 83, 103001. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Li, R.; Yang, I.; Song, G.; Li, J. Developing a test device to analyze heat transfer through firefighter protective clothing. Int. J. Therm. Sci. 2019, 138, 1–11. [Google Scholar] [CrossRef]
- Kolka, M.A.; Levine, L.; Stephenson, L.A. Use of an ingestible telemetry sensor to measure core temperature under chemical protective clothing. J. Therm. Biol. 1997, 22, 343–349. [Google Scholar] [CrossRef]
- Abdalla, I.; Cai, J.; Lu, W.; Yu, J.; Li, Z.; Ding, B. Recent progress on electromagnetic wave absorption materials enabled by electrospun carbon nanofibers. Carbon 2023, 213, 118300. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, X.; Jiang, Q.; Duan, J.; Hou, B.; Jin, Z.; Ma, F.; Deng, J. Research progress in the preparation of electromagnetic wave absorbing and corrosion resistant nanofiber materials by electrospinning. J. Ind. Eng. Chem. 2024, 138, 34–48. [Google Scholar] [CrossRef]
- Rahman, S.; Al Haque, M.; Solaiman, M.; Ratul, R.H.; Ahmed, I.; Tabassum, S.; Ciesielska-Wrobel, I. Wireless power transfer using electronic textiles: A comparative review. J. Eng. Res. 2024. [Google Scholar] [CrossRef]
- Lang, K.; Liu, T.; Padilla, D.J.; Nelson, M.; Landorf, C.W.; Patel, J.; Ballentine, M.L.; Kennedy, A.J.; Shih, W.-S.; Scotch, A.; et al. Nanofibers enabled advanced gas sensors: A review. Adv. Sens. Energy Mater. 2024, 3, 1000. [Google Scholar] [CrossRef]
- Wang, Y.; Yokota, T.; Someya, T. Electrospun nanofiber-based soft electronics. NPG Asia Mater. 2021, 13, 22. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, T.; Tian, G.; Lan, B.; Deng, W.; Tang, L.; Ao, Y.; Sun, Y.; Zeng, W.; Ren, X.; et al. Spatially Confined MXene/PVDF nanofiber piezoelectric electronics. Adv. Fiber Mater. 2024, 6, 133–144. [Google Scholar] [CrossRef]
- Guberman-Pfeffer, M.J.; Dorval Courchesne, N.M.; Lovley, D.R. Microbial nanowires for sustainable electronics. Nat. Rev. Bioeng. 2024, 1–18. [Google Scholar] [CrossRef]
- Oyehan, T.A.; Salami, B.A.; Abdulrasheed, A.A.; Hambali, H.U.; Gbadamosi, A.; Valsami-Jones, E.; Saleh, T.A. MXenes: Synthesis, properties, and applications for sustainable energy and environment. Appl. Mater. Today 2023, 35, 101993. [Google Scholar] [CrossRef]
- Shapiro, D.M.; Mandava, G.; Yalcin, S.E.; Arranz-Gibert, P.; Dahl, P.J.; Shipps, C.; Gu, Y.; Srikanth, V.; Salazar-Morales, A.I.; O’Brien, J.P.; et al. Protein nanowires with tunable functionality and programmable self-assembly using sequence controlled. Nat. Commun. 2022, 13, 1–10. [Google Scholar]
- Lekbach, Y.; Ueki, T.; Liu, X.; Woodard, T.; Yao, J.; Lovley, D.R. Microbial nanowires with genetically modified peptide ligands to sustainably fabricate electronic sensing devices. Biosens. Bioelectron. 2023, 226, 115147. [Google Scholar] [CrossRef]
- Jin, J.; Luo, B.; Xuan, S.; Shen, P.; Jin, P.; Wu, Z.; Zheng, Y. Degradable chitosan-based bioplastic packaging: Design, preparation and applications. Int. J. Biol. Macromol. 2024, 266, 131253. [Google Scholar] [CrossRef]
- Shahreen, L.; Chase, G.G. Effects of electrospinning solution properties on formation of beads in TIO2 fibers with PDO particles. J. Eng. Fibers Fabr. 2015, 10, 3. [Google Scholar] [CrossRef]
- Nanda, A.; Pandey, P.; Rajinikanth, P.S.; Singh, N. Revolution of nanotechnology in food packaging: Harnessing electrospun zein nanofibers for improved preservation-A review. Int. J. Biol. Macromol. 2024, 260, 129416. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, S.; Almasi, H.; Amjadi, S.; Moradi, M.; Alamdari, N.G.; Salmasi, S.; Divsala, E. Development and characterization of active packaging system based on zein nanofibers mat incorporated with geraniol-loaded nanoliposomes. Food Sci. Nutr. 2024, 12, 5373–5387. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, H.; Toivakka, M.; Xu, C. Current progress in functionalization of cellulose nanofibers (CNFs) for active food packaging. Int. J. Biol. Macromol. 2024, 267, 131490. [Google Scholar] [CrossRef]
- Lu, P.; Yang, Y.; Liu, R.; Liu, X.; Ma, J.; Wu, M.; Wang, S. Preparation of sugarcane bagasse nanocellulose hydrogel as a colourimetric freshness indicator for intelligent food packaging. Carbohydr. Polym. 2020, 249, 116831. [Google Scholar] [CrossRef]
- Joshi, M.; Aayush, K.; Sharma, K.; Bose, I.; Khan, A.A.; Atanassova, M.; Yang, T.; Murariu, O.C.; Sharma, S.; Caruso, G. Fiber and nanofiber based edible packaging for enhancing the shelf life of food: A review. Food Biosci. 2024, 59, 103970. [Google Scholar] [CrossRef]
- Zhao, X.; Zou, D.; Liu, Y.; Xia, Y.; Tao, J.; Zeng, Q.; Hou, Y.; Liu, M. Electrospun polylactic acid nanofibers membrane with copper ion-loaded clay nanotubes for fresh-keeping packaging. Int. J. Biol. Macromol. 2024, 267, 131651. [Google Scholar] [CrossRef] [PubMed]
- Stramarkou, M.; Tzegiannakis, I.; Christoforidi, E.; Krokida, M. Use of electrospinning for sustainable production of nanofibers: A comparative assessment of smart textiles-related applications. Polymers 2024, 16, 514. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhu, H.; Chao, Y. Innovative material applications in clothing design research. Mater. Express 2024, 14, 820–827. [Google Scholar] [CrossRef]
- Hossain, M.T.; Shahid, M.A. Hybrid structured silk-rPET nanotechnical cloth for advanced air purification. Discov. Appl. Sci. 2024, 6, 296. [Google Scholar] [CrossRef]
- Moreno, M.; Herrera, E. Evaluation on phantoms of the feasibility of a smart bra to detect breast cancer in young adults. Sensors 2019, 19, 5491. [Google Scholar] [CrossRef]
- Tang, J.; Wu, T.; Ma, S.; Yan, T.; Pan, Z. Flexible strain sensor based on CNT/TPU composite nanofiber yarn for smart sports bandage. Compos. Part B 2022, 232, 109605. [Google Scholar] [CrossRef]
- Kenry; Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, M.; Yuan, J.; Cai, J.; Xie, H.; Zhu, M.; Cai, L.; Wei, P.; Chang, C. High-strength and recyclable hydroplastic films from hydrophobic cellulose nanofibers produced via deep eutectic solvents. Chem. Eng. J. 2023, 476, 146771. [Google Scholar] [CrossRef]
- Nicolau, G.D.; Weber, R.P.; Monteiro, S.N.; Monsores, K.C.; da Silva, A.O. Influence of solution concentration on recycled pol-ycarbonate nanofibers produced by solution blow-spinning process: A short communication. J. Mater. Res. Technol. 2022, 21, 1454–1460. [Google Scholar] [CrossRef]
- Mogharbel, R.T.; Almahri, A.; Alaysuy, O.; Alzahrani, S.O.; Alorabi, A.Q.; Al-Qahtani, S.D.; El-Metwaly, N.M. Preparation of photochromic solution blow spun polycarbonate nanofibers from recycled plastic for optical anti-counterfeiting. Opt. Mater. 2023, 141, 113936. [Google Scholar] [CrossRef]

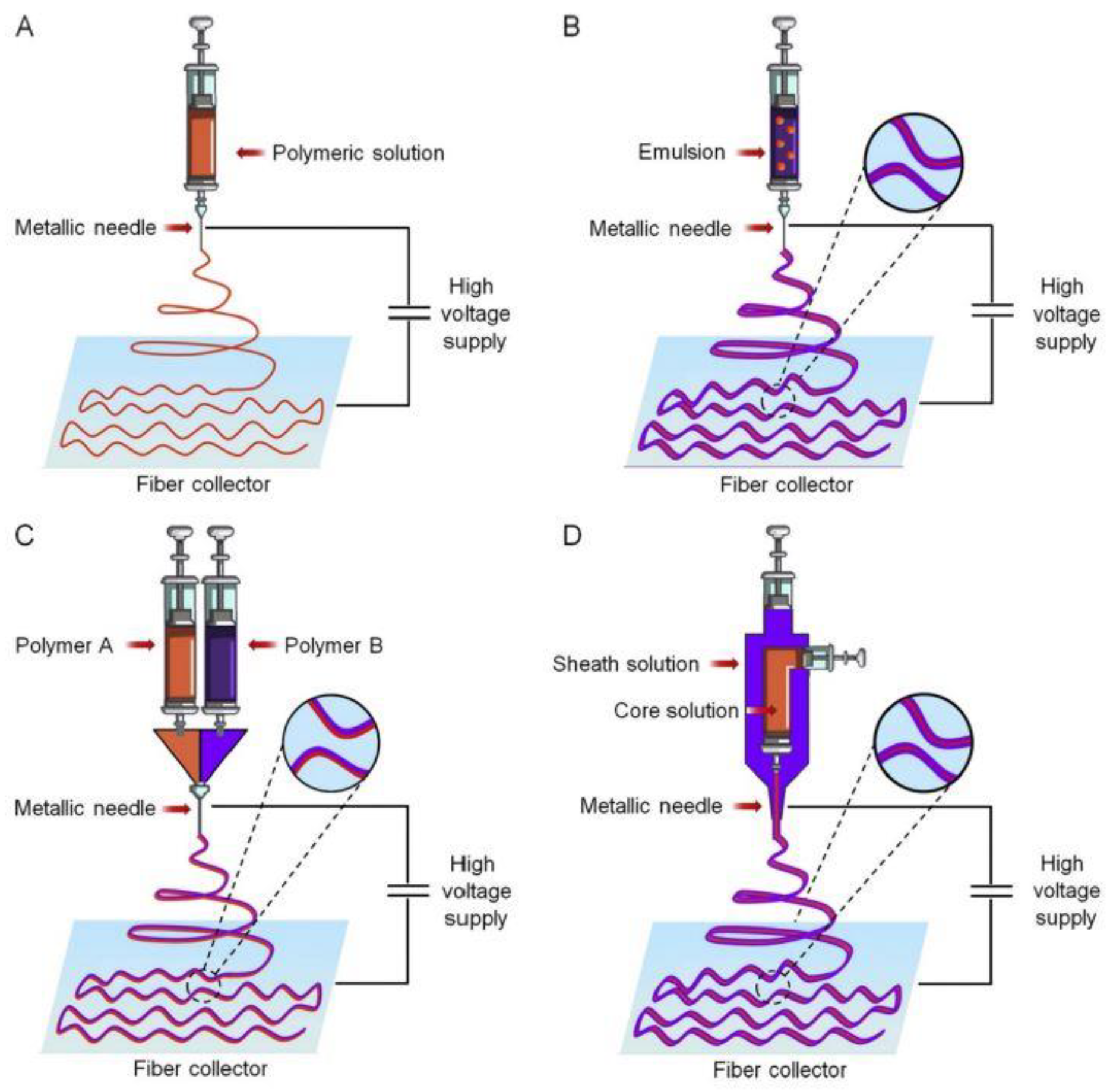
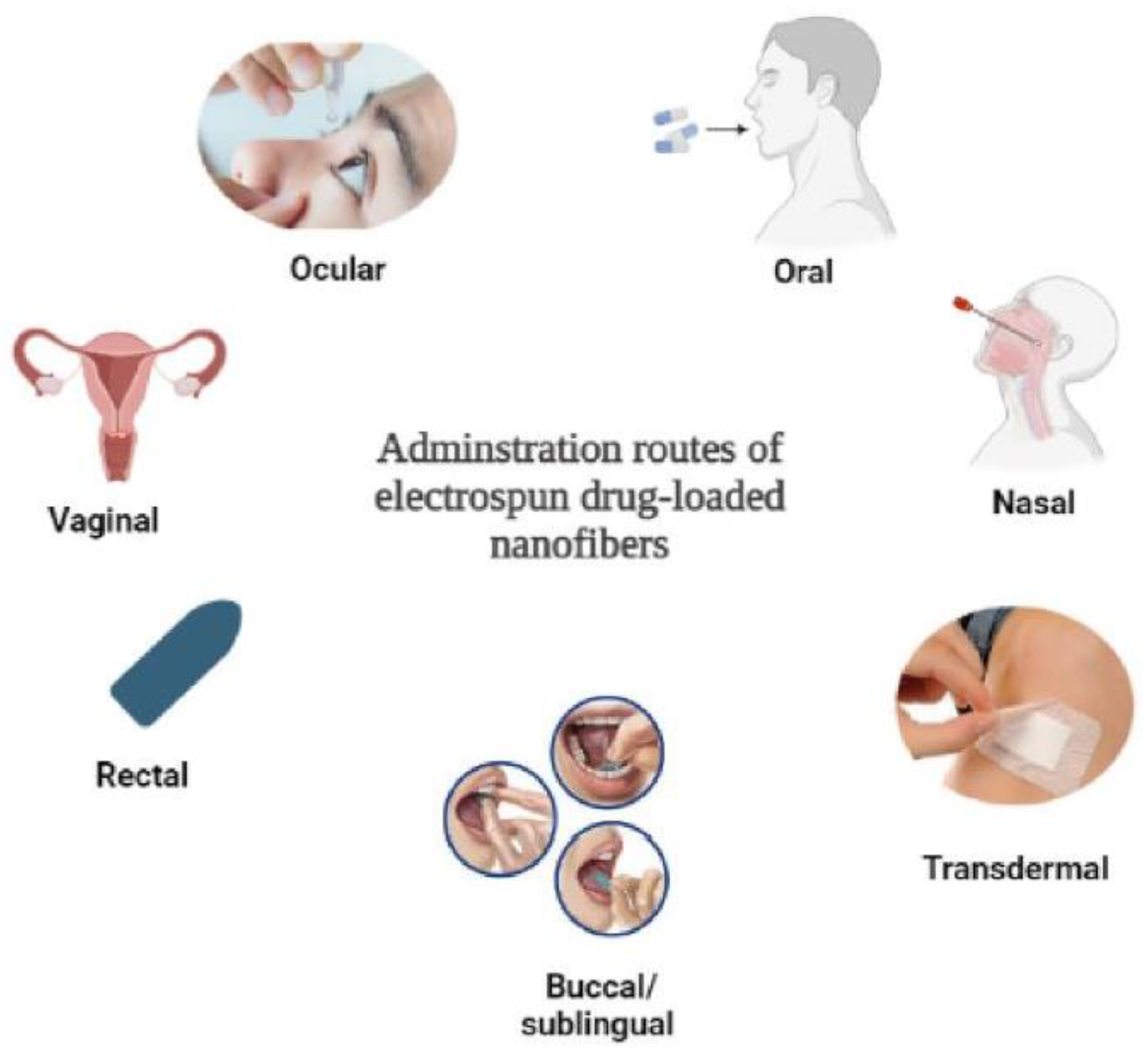


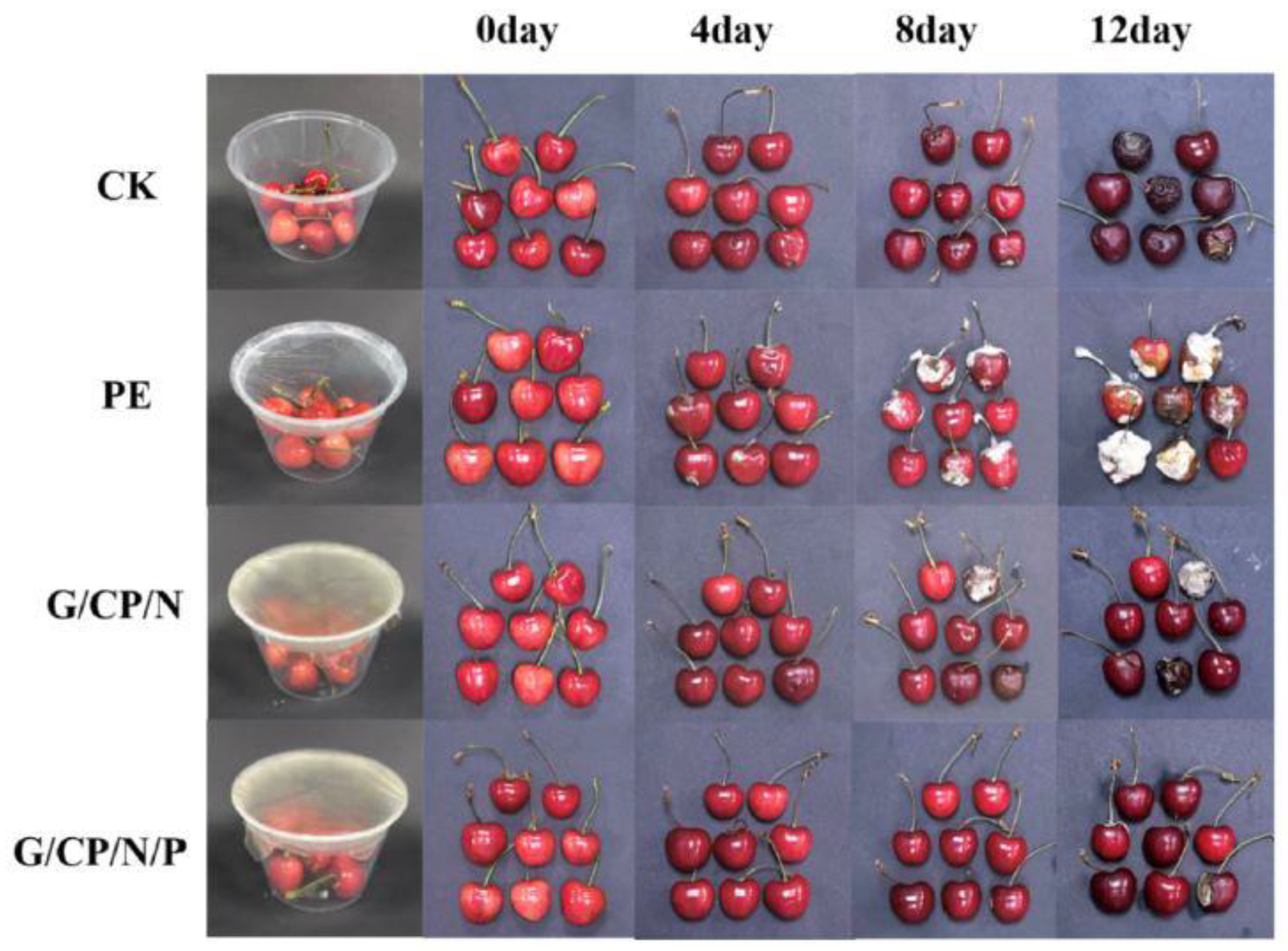
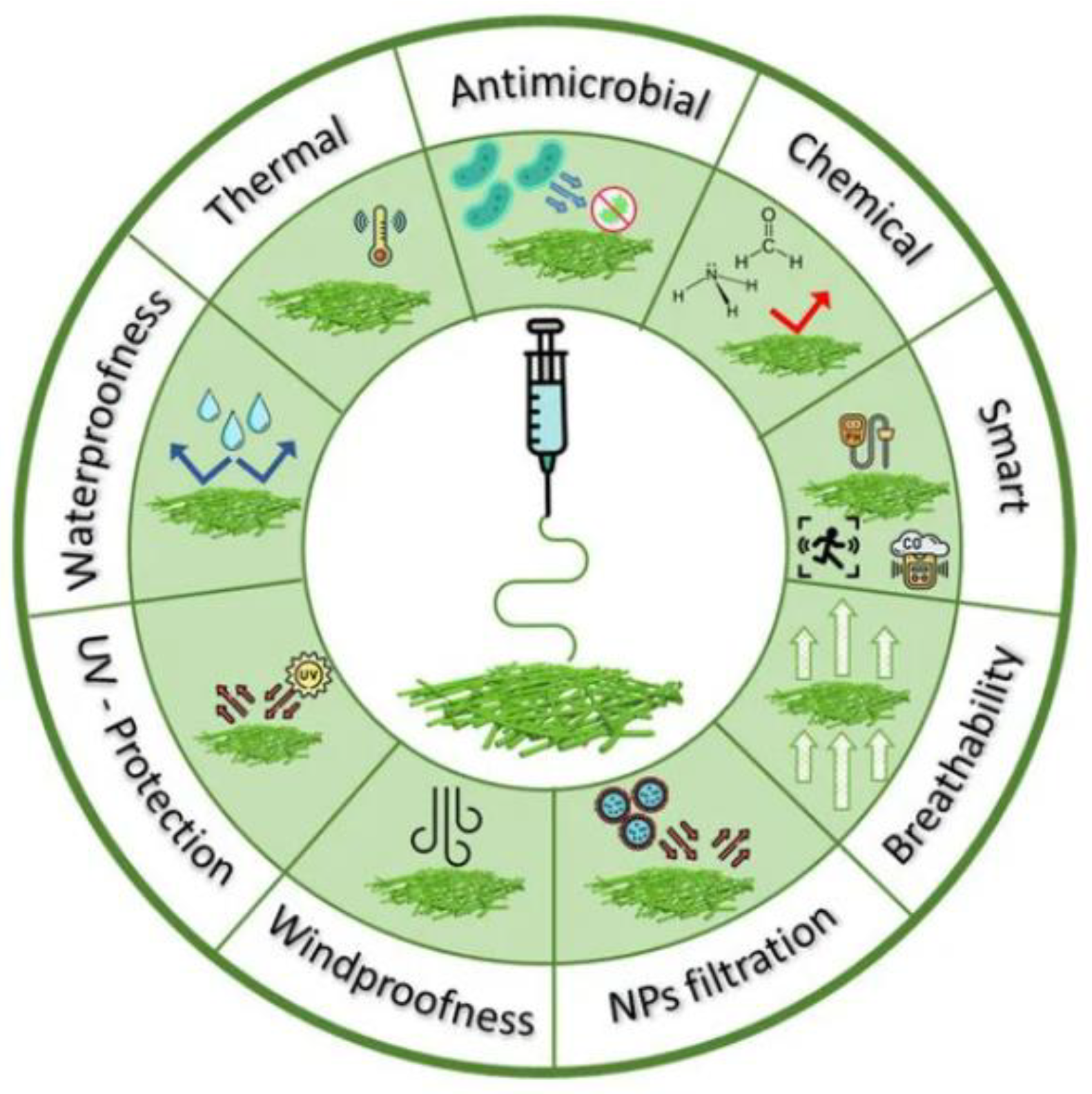
| Factors | Electrospinning Fiber Formation |
|---|---|
| Polymer type and concentration | Low concentrations lead to the formation of beads, whereas excessively high concentrations lead to the formation of coarse fibers or discontinuous fibers. The optimum feed rate is maintained to a stable Taylor cone shape, and the solvents have enough time to evaporate before the polymer is collected on the collector. |
| Polymer molecular weight | The entanglement of low-molecular-weight polymeric chains is not effective, which leads to the formation of beads. Both a dilute solution of a high-molecular-weight polymer and a concentrated solution of a low-molecular-weight polymer can produce fibers of identical fineness. |
| Solution viscosity | Different polymers have different viscosities. Viscosity is affected by the solvent used and the polymer concentration. Low solution viscosity promotes the formation of beads. When the viscosity is excessively high, coarse fibers are formed. |
| Solution conductivity | High conductivity promotes the formation of finer nanofibers, whereas a lower conductivity will promote beads formation. |
| Solvent | The solvent must have a good boiling point to evaporate from the polymers. Highly volatile solvents have a low boiling point and quickly evaporate, which can cause the jet to dry. Less volatile will not evaporate quickly to allow the jet to solidify. Precaution should be taken as most of the volatile solvents are toxic to the environment. |
| Voltage | The voltage applied to the solution differs from polymer to polymer. Excessive voltage can cause the formation of beads. |
| Collector type | The collector needs to have strong conductivity to effectively remove charges from the plate. If charges accumulate, they will repel incoming fibers and hinder membrane formation |
| Distance between nozzle and collector | The distance influences the fiber diameter. Shorter distances produce coarse fibers as the distance is too small to stretch the polymer to reduce its diameter |
| Surrounding conditions | High temperatures decrease viscosity, causing the formation of finer fibers. High humidity leads to the formation of coarse fibers. |
| Phase Separation | Drawing | Template | Self-Assembly | Electrospinning |
|---|---|---|---|---|
| No voltage is required. | No voltage is required. | No voltage is required. | No voltage is required. | High voltage is required. |
| Limited to certain polymers. | Limited to elastic polymers. | Production of continuous, long nanofibers is a challenge. | Limited to certain polymers. | Limited to conductive polymers, and solvent evaporation is required. |
| Low scalability and time-consuming | Limited to laboratory production | Limited to laboratory production. | Low scalability and complex processes | Can be scalable, but it also comes with its own challenges. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patnaik, A. Recent Developments in Application of Nanofibers. Processes 2024, 12, 1894. https://doi.org/10.3390/pr12091894
Patnaik A. Recent Developments in Application of Nanofibers. Processes. 2024; 12(9):1894. https://doi.org/10.3390/pr12091894
Chicago/Turabian StylePatnaik, Asis. 2024. "Recent Developments in Application of Nanofibers" Processes 12, no. 9: 1894. https://doi.org/10.3390/pr12091894





