The Synergistic Effect of CeO2 and Micron-Cu Enhances the Hydrogenation of CO2 to CO
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of Catalysts
2.2. Characterization
2.3. Evaluation of Catalytic Performance
3. Results and Discussion
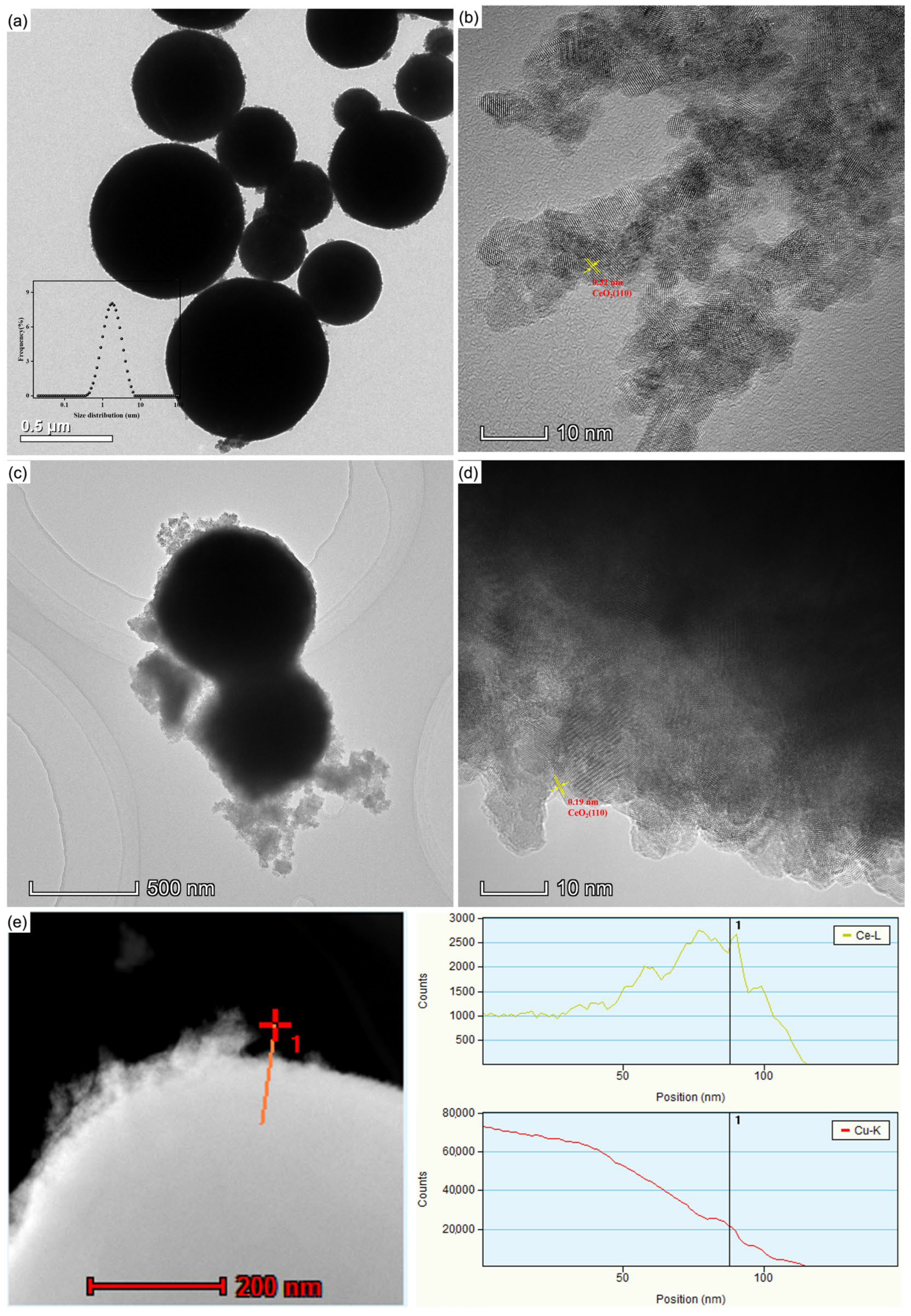
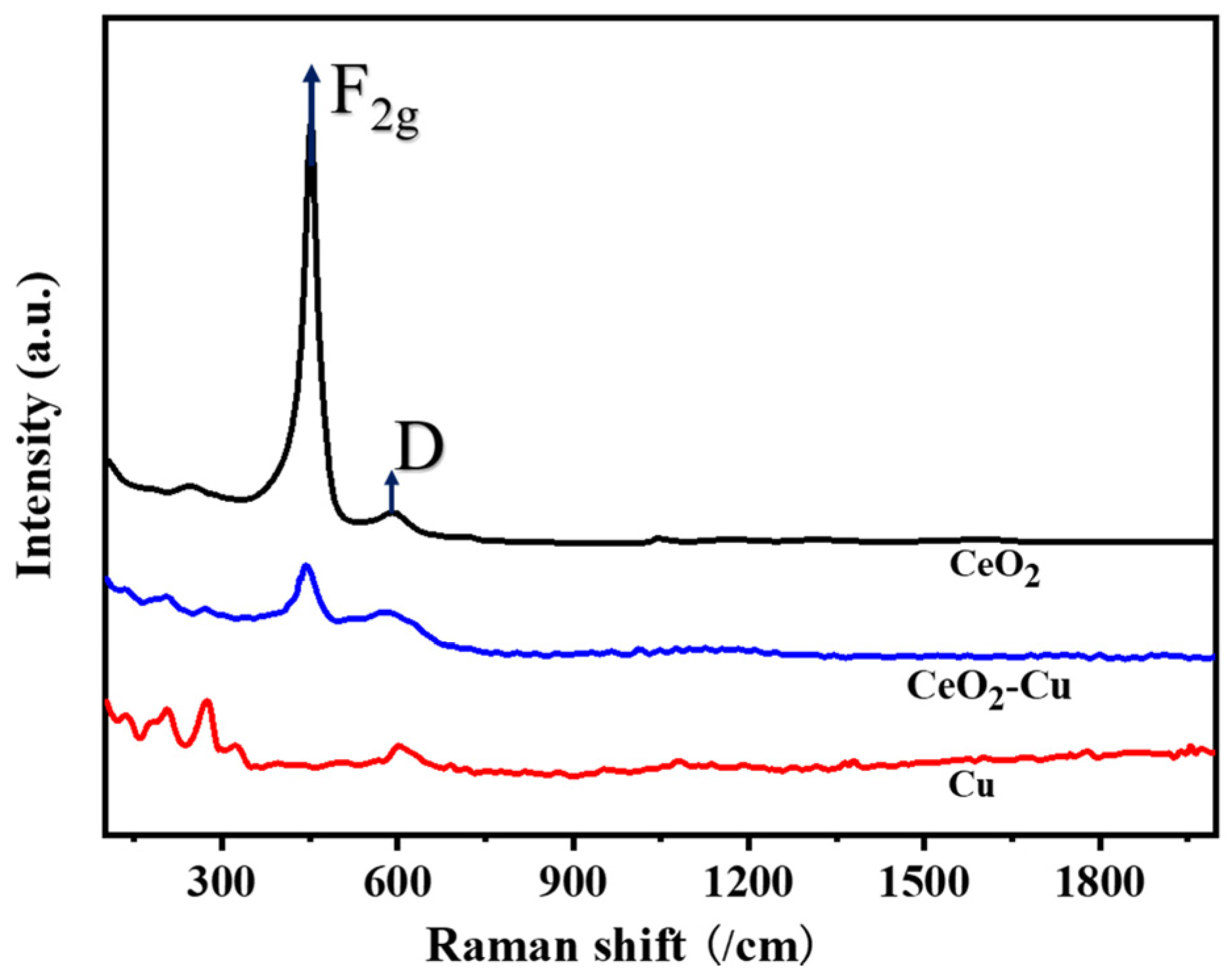
3.1. Characterization
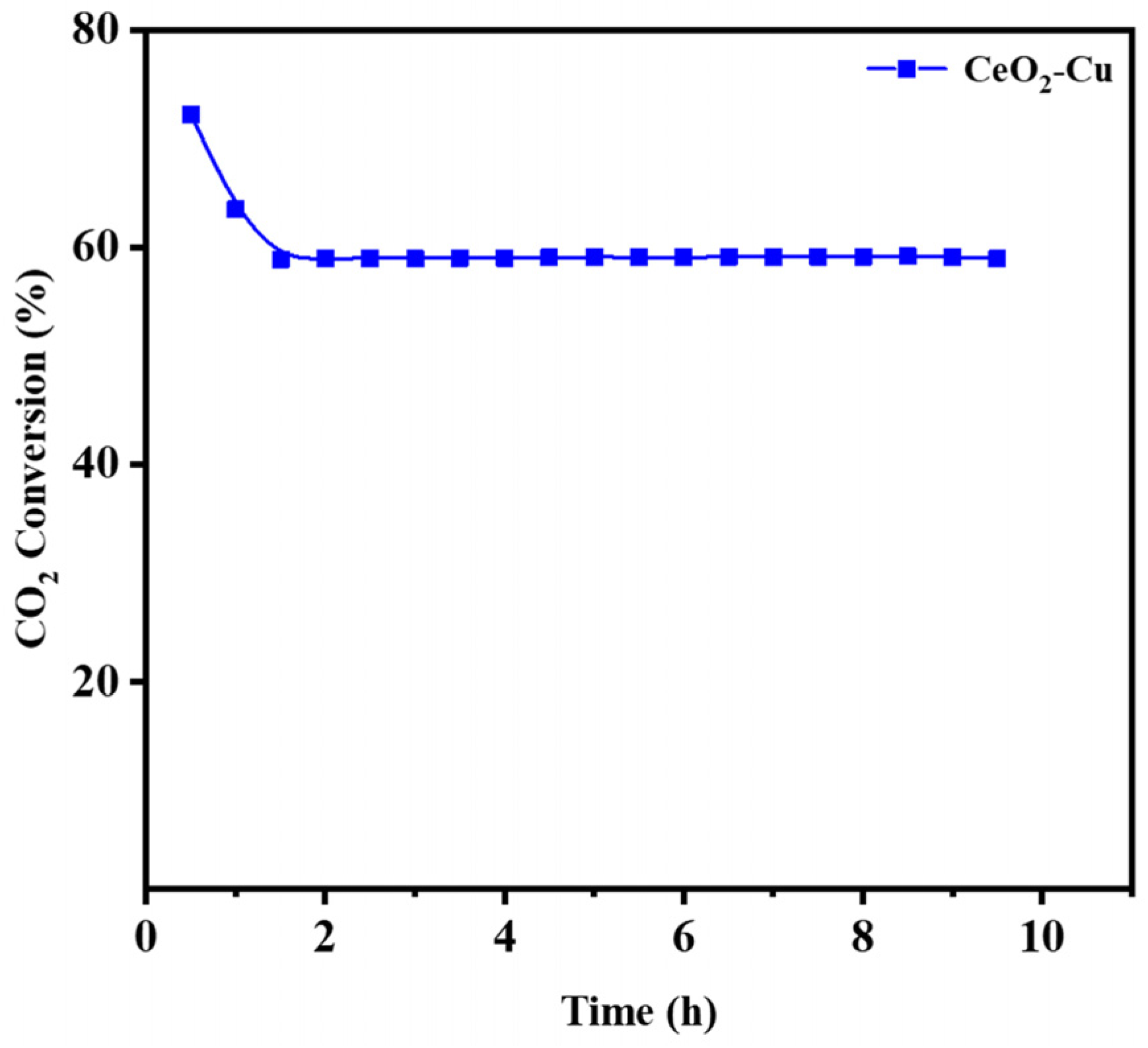
3.2. CO2 Hydrogenation Performance
3.3. CeO2 Effect on Micro-Cu
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, J.; Xu, Y.; Liao, P.; Wang, H.; Zhou, H. Recent progress in integrated CO2 capture and conversion process using dual function materials: A state-of-the-art review. Carbon Capt. Sci. Technol. 2022, 4, 100052. [Google Scholar] [CrossRef]
- Ma, J.; Kong, H.; Wang, J.; Zhong, H.; Li, B.; Song, J.; Kammen, D.M. Carbon-neutral pathway to mitigating transport-power grid cross-sector effects. Innovation 2024, 5, 100611. [Google Scholar] [CrossRef] [PubMed]
- Mathias Dautzenberg, F.; Lu, Y.; Xu, B. Controlling the global mean temperature by decarbonization. Acta Phys.-Chim. Sin. 2020, 37, 2008066. [Google Scholar] [CrossRef]
- Dai, A.; Luo, D.; Song, M.; Liu, J. Arctic amplification is caused by sea-ice loss under increasing CO2. Nat. Commun. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Scanes, E.; Scanes, P.R.; Ross, P.M. Climate change rapidly warms and acidifies Australian estuaries. Nat. Commun. 2020, 11, 1803. [Google Scholar] [CrossRef]
- Geng, T.; Jia, F.; Cai, W.; Wu, L.; Gan, B.; Jing, Z.; Li, S.; McPhaden, M.J. Increased occurrences of consecutive la niña events under global warming. Nature 2023, 619, 774–781. [Google Scholar] [CrossRef]
- Zantye, M.S.; Arora, A.; Hasan, M.M.F. Renewable-integrated flexible carbon capture: A synergistic path forward to clean energy future. Energy Environ. Sci. 2021, 14, 3986–4008. [Google Scholar] [CrossRef]
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef]
- Shi, Y.; Shou, H.; Li, H.; Zhan, G.; Liu, X.; Yang, Z.; Mao, C.; Cheng, J.; Zhang, X.; Jiang, Y.; et al. Visible light-driven conversion of carbon-sequestrated seawater into stoichiometric co and HClO with nitrogen-doped BiOCl atomic layers. Angew. Chem. Int. Ed. 2023, 62, e202302286. [Google Scholar] [CrossRef]
- Jing, R.; Wang, R.; Xing, L.; Li, Q.; Wang, L. Industrial perspective on the current status of carbon capture application in china’s nonpower industries. Sep. Purif. Technol. 2024, 334, 125993. [Google Scholar] [CrossRef]
- Porosoff, M.D.; Yan, B.; Chen, J.G. Catalytic reduction of CO2 by H2 for synthesis of CO, methanol and hydrocarbons: Challenges and opportunities. Energy Environ. Sci. 2016, 9, 62–73. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning selectivity of CO2 hydrogenation reactions at the metal/oxide interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Winter, L.R.; Chen, J.G.; Yan, B. CO2 hydrogenation over heterogeneous catalysts at atmospheric pressure: From electronic properties to product selectivity. Green Chem. 2021, 23, 249–267. [Google Scholar] [CrossRef]
- Wang, I.; Huang, S.; Wang, S.; Bie, X.; Zhou, H.; Li, Z. Mechanistic study of integrated CO2 capture and utilization over cu and al-modified calcined limestone with high stability using mfb-tga-ms. Sep. Purif. Technol. 2024, 333, 125975. [Google Scholar] [CrossRef]
- Fatimah, M.; Qyyum, M.A.; Lee, M.; Alshareef, R.S.; Aslam, M.; Saeed, B.; Dai, L.; Gilani, M.A.; Bazmi, A.A.; Chang, I.S.; et al. Industrial waste gases as a resource for sustainable hydrogen production: Resource availability, production potential, challenges, and prospects. Carbon Capt. Sci. Technol. 2024, 12, 100228. [Google Scholar] [CrossRef]
- Davies, W.G.; Babamohammadi, S.; Yan, Y.; Clough, P.T.; Masoudi Soltani, S. Exergy analysis in intensification of sorption-enhanced steam methane reforming for clean hydrogen production: Comparative study and efficiency optimisation. Carbon Capt. Sci. Technol. 2024, 12, 100202. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.; Ma, X.; Gong, J. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Centi, G.; Quadrelli, E.A.; Perathoner, S. Catalysis for CO2 conversion: A key technology for rapid introduction of renewable energy in the value chain of chemical industries. Energy Environ. Sci. 2013, 6, 1711. [Google Scholar] [CrossRef]
- Zhang, C.; Li, Y.; Chu, Z.; Fang, Y.; Han, K.; He, Z. Analysis of integrated CO2 capture and utilization via calcium-looping in-situ dry reforming of methane and fischer-tropsch for synthetic fuels production. Sep. Purif. Technol. 2024, 329, 125109. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Tang, X.; Yin, H.; Kang, J.; Zhang, Q.; Wang, Y. Zn and na promoted fe catalysts for sustainable production of high-valued olefins by CO2 hydrogenation. Fuel 2022, 309, 122105. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Kazumi, S.; Fang, Y.; Shi, L.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Solvent-free anchoring nano-sized zeolite on layered double hydroxide for highly selective transformation of syngas to gasoline-range hydrocarbons. Fuel 2019, 253, 249–256. [Google Scholar] [CrossRef]
- Yang, L.; Pastor-Pérez, L.; Gu, S.; Sepúlveda-Escribano, A.; Reina, T.R. Highly efficient Ni/CeO2-Al2O3 catalysts for CO2 upgrading via reverse water-gas shift: Effect of selected transition metal promoters. Appl. Catal. B Environ. 2018, 232, 464–471. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Braga, A.H.; Arpini, B.H.; Szanyi, J.; Goncalves, R.V.; Zornio, B.F.; Miranda, C.R.; Rossi, L.M. Optimizing active sites for high co selectivity during CO2 hydrogenation over supported nickel catalysts. J. Am. Chem. Soc. 2021, 143, 4268–4280. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Zhang, Z.; Li, X.; Luo, C.; Xu, Y.; Zhang, L. High-efficiency CuCe(rod) catalysts for CO2 hydrogenation with high Cu content. Fuel 2020, 276, 118135. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, W.; Lin, S. Mechanism of CO formation in reverse water–gas shift reaction over Cu/Al2O3 catalyst. Catal. Lett. 2000, 68, 45–48. [Google Scholar] [CrossRef]
- Chen, C.-S.; Cheng, W.-H.; Lin, S.-S. Study of iron-promoted Cu/SiO2 catalyst on high temperature reverse water gas shift reaction. Appl. Catal. A Gen. 2004, 257, 97–106. [Google Scholar] [CrossRef]
- Chen, C.S.; Cheng, W.H.; Lin, S.S. Study of reverse water gas shift reaction by TPD, TPR and CO2 hydrogenation over potassium-promoted Cu/Sio2 catalyst. Appl. Catal. A Gen. 2003, 238, 55–67. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.; Guo, X.; Song, C.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to methanol via heterogeneous catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Chen, A.; Yu, X.; Zhou, Y.; Miao, S.; Li, Y.; Kuld, S.; Sehested, J.; Liu, J.; Aoki, T.; Hong, S.; et al. Structure of the catalytically active copper–ceria interfacial perimeter. Nat. Catal. 2019, 2, 334–341. [Google Scholar] [CrossRef]
- Konsolakis, M. The role of copper–ceria interactions in catalysis science: Recent theoretical and experimental advances. Appl. Catal. B Environ. 2016, 198, 49–66. [Google Scholar] [CrossRef]
- Lu, B.; Xu, Y.; Zhang, Z.; Wu, F.; Li, X.; Luo, C.; Zhang, L. CO2 hydrogenation on CeO2@Cu catalyst synthesized via a solution auto-combustion method. J. CO2 Util. 2021, 54, 101757. [Google Scholar] [CrossRef]
- Muravev, V.; Spezzati, G.; Su, Y.-Q.; Parastaev, A.; Chiang, F.-K.; Longo, A.; Escudero, C.; Kosinov, N.; Hensen, E.J.M. Interface dynamics of Pd–CeO2 single-atom catalysts during co oxidation. Nat. Catal. 2021, 4, 469–478. [Google Scholar] [CrossRef]
- Dong, L.; Yao, X.; Chen, Y. Interactions among supported copper-based catalyst components and their effects on performance: A review. Chin. J. Catal. 2013, 34, 851–864. [Google Scholar] [CrossRef]
- Zhou, G.; Dai, B.; Xie, H.; Zhang, G.; Xiong, K.; Zheng, X. Cecu composite catalyst for co synthesis by reverse water–gas shift reaction: Effect of Ce/Cu mole ratio. J. CO2 Util. 2017, 21, 292–301. [Google Scholar] [CrossRef]
- Lin, L.; Yao, S.; Liu, Z.; Zhang, F.; Li, N.; Vovchok, D.; Martínez-Arias, A.; Castañeda, R.; Lin, J.; Senanayake, S.D.; et al. In situ characterization of Cu/CeO2 nanocatalysts for CO2 hydrogenation: Morphological effects of nanostructured ceria on the catalytic activity. J. Phys. Chem. C 2018, 122, 12934–12943. [Google Scholar] [CrossRef]
- Lu, B.; Zhang, T.; Zhang, L.; Xu, Y.; Zhang, Z.; Wu, F.; Li, X.; Luo, C. Promotion effects of oxygen vacancies on activity of na-doped CeO2 catalysts for reverse water gas shift reaction. Appl. Surf. Sci. 2022, 587, 152881. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Xu, H.; Han, Y. Reverse water–gas shift reaction over ceria nanocube synthesized by hydrothermal method. Catal. Commun. 2016, 76, 1–6. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, Y.; Wang, Z.; Yan, J.; Ge, Q.; Liu, C. Reverse water gas shift over In2O3–CeO2 catalysts. Catal. Today 2016, 259, 402–408. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, A.; Ning, J.; Shen, W. Electronic and geometric structure of the copper-ceria interface on Cu/CeO2 catalysts. Chin. J. Catal. 2020, 41, 928–937. [Google Scholar] [CrossRef]
- Lu, B.; Wu, F.; Li, X.; Luo, C.; Zhang, L. Reconstruction of interface oxygen vacancy for boosting CO2 hydrogenation by Cu/CeO2 catalysts with thermal treatment. Carbon Capt. Sci. Technol. 2024, 10, 100173. [Google Scholar] [CrossRef]
- Li, W.; Feng, X.; Zhang, Z.; Jin, X.; Liu, D.; Zhang, Y. A controllable surface etching strategy for well-defined spiny yolk@shell CuO@CeO2 cubes and their catalytic performance boost. Adv. Funct. Mater. 2018, 28, 1802559. [Google Scholar] [CrossRef]
- Cao, F.; Xiao, Y.; Zhang, Z.; Li, J.; Xia, Z.; Hu, X.; Ma, Y.; Qu, Y. Influence of oxygen vacancies of CeO2 on reverse water gas shift reaction. J. Catal. 2022, 414, 25–32. [Google Scholar] [CrossRef]





| Catalyst | gCeO2/gCu (Wt%) | SBET (m2/g) | Pore Volume (mL/g) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| Cu | 2.68 | 0.22 | 12.36 | |
| CeO2 | 149.87 | 0.407 | 5.02 | |
| CeO2–Cu | 20 | 13.54 | 0.0277 | 6.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, B.; Sang, H.; Liu, L.; Yu, Z.; Guo, Y.; Xu, Y. The Synergistic Effect of CeO2 and Micron-Cu Enhances the Hydrogenation of CO2 to CO. Processes 2024, 12, 1912. https://doi.org/10.3390/pr12091912
Lu B, Sang H, Liu L, Yu Z, Guo Y, Xu Y. The Synergistic Effect of CeO2 and Micron-Cu Enhances the Hydrogenation of CO2 to CO. Processes. 2024; 12(9):1912. https://doi.org/10.3390/pr12091912
Chicago/Turabian StyleLu, Bowen, Huiying Sang, Liang Liu, Zhijian Yu, Yaqin Guo, and Yongqing Xu. 2024. "The Synergistic Effect of CeO2 and Micron-Cu Enhances the Hydrogenation of CO2 to CO" Processes 12, no. 9: 1912. https://doi.org/10.3390/pr12091912







