The Effects of Sodium Silicate and Sodium Citrated on the Properties and Structure of Alkali-Activated Foamed Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mix Ratio of Experiment
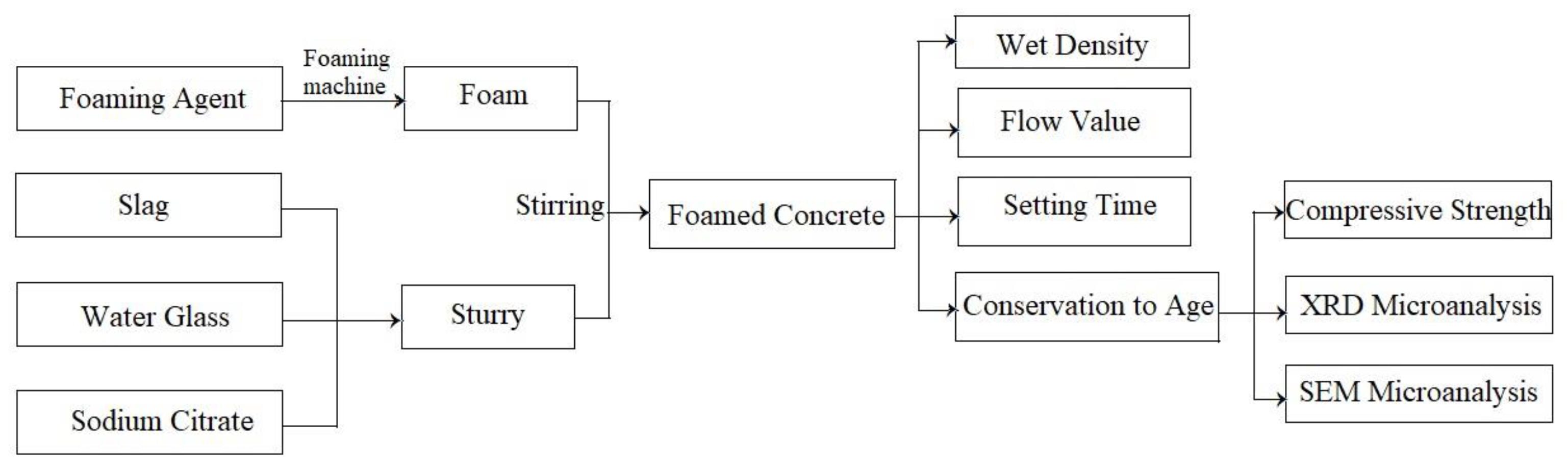
2.3. Preparation Method
2.4. Technical Indicators and Test Methods
2.4.1. Requirements for Technical Specifications for FC
2.4.2. Test Methods
3. Results and Discussion
3.1. Effects of Activator on Flowability, Setting Time, and Compressive Strength of AASC Foamed Concrete
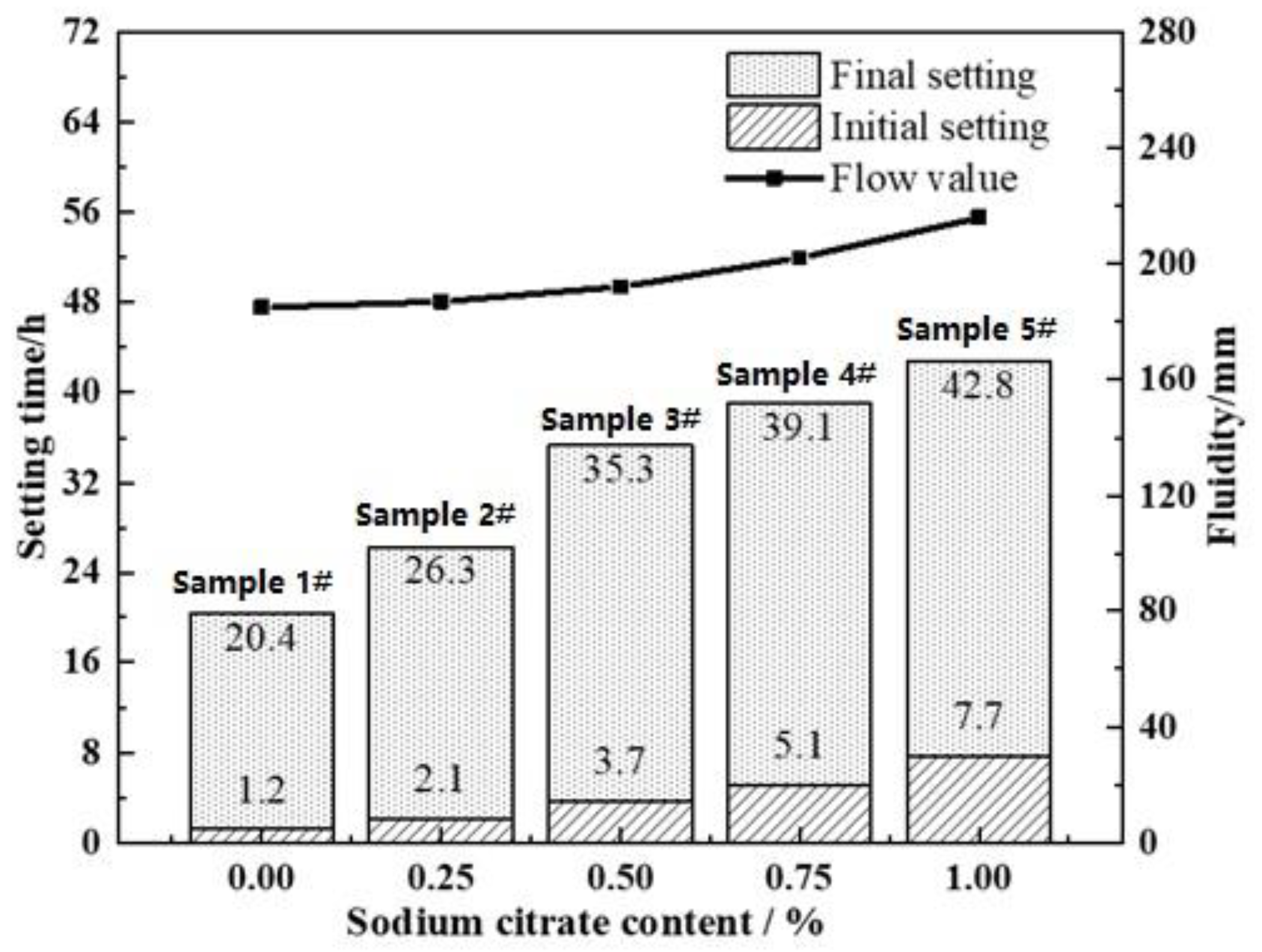
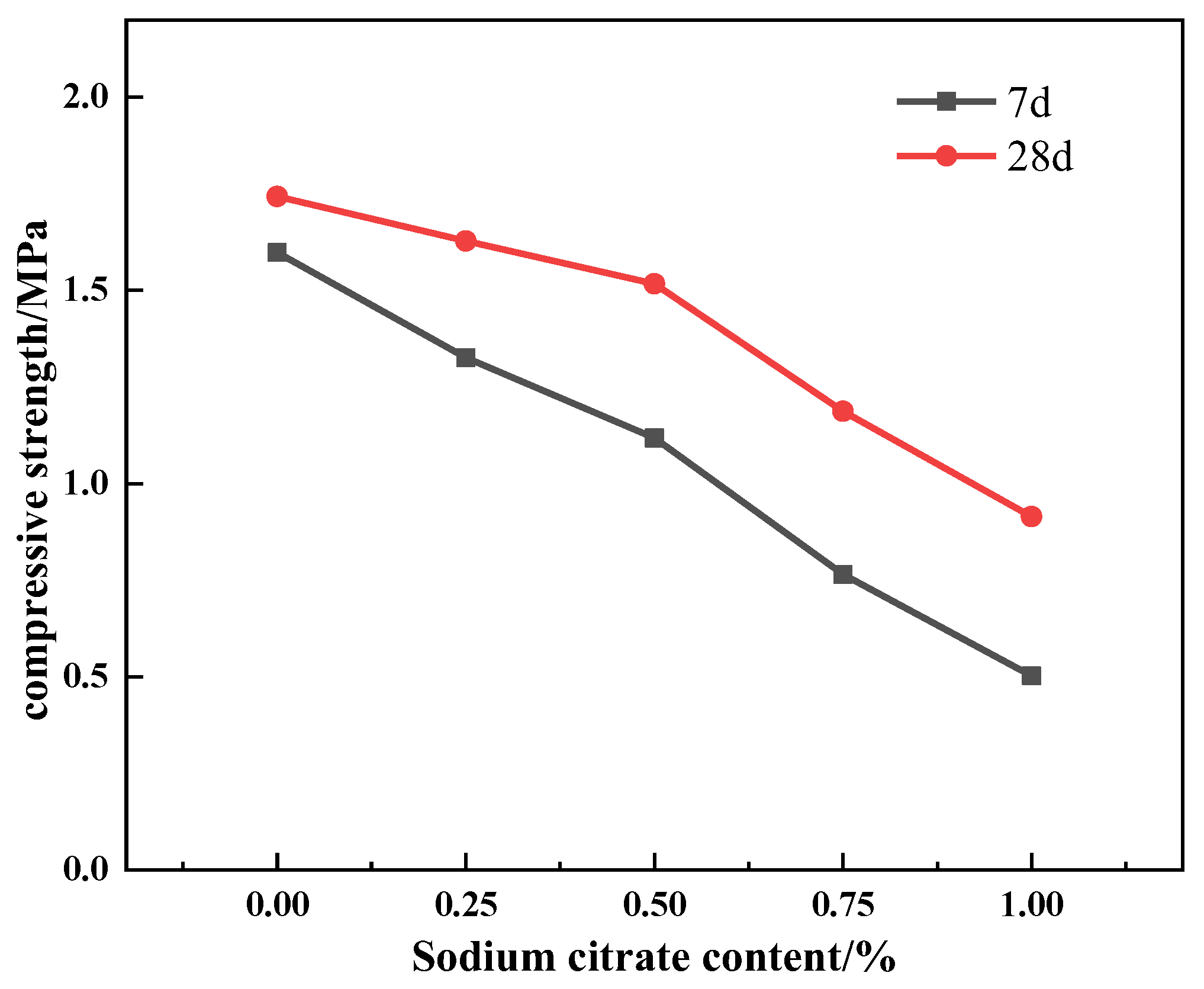
3.2. Effects of Na3Cit on Flowability, Setting Time, and Compressive Strength of AASC Foamed Concrete
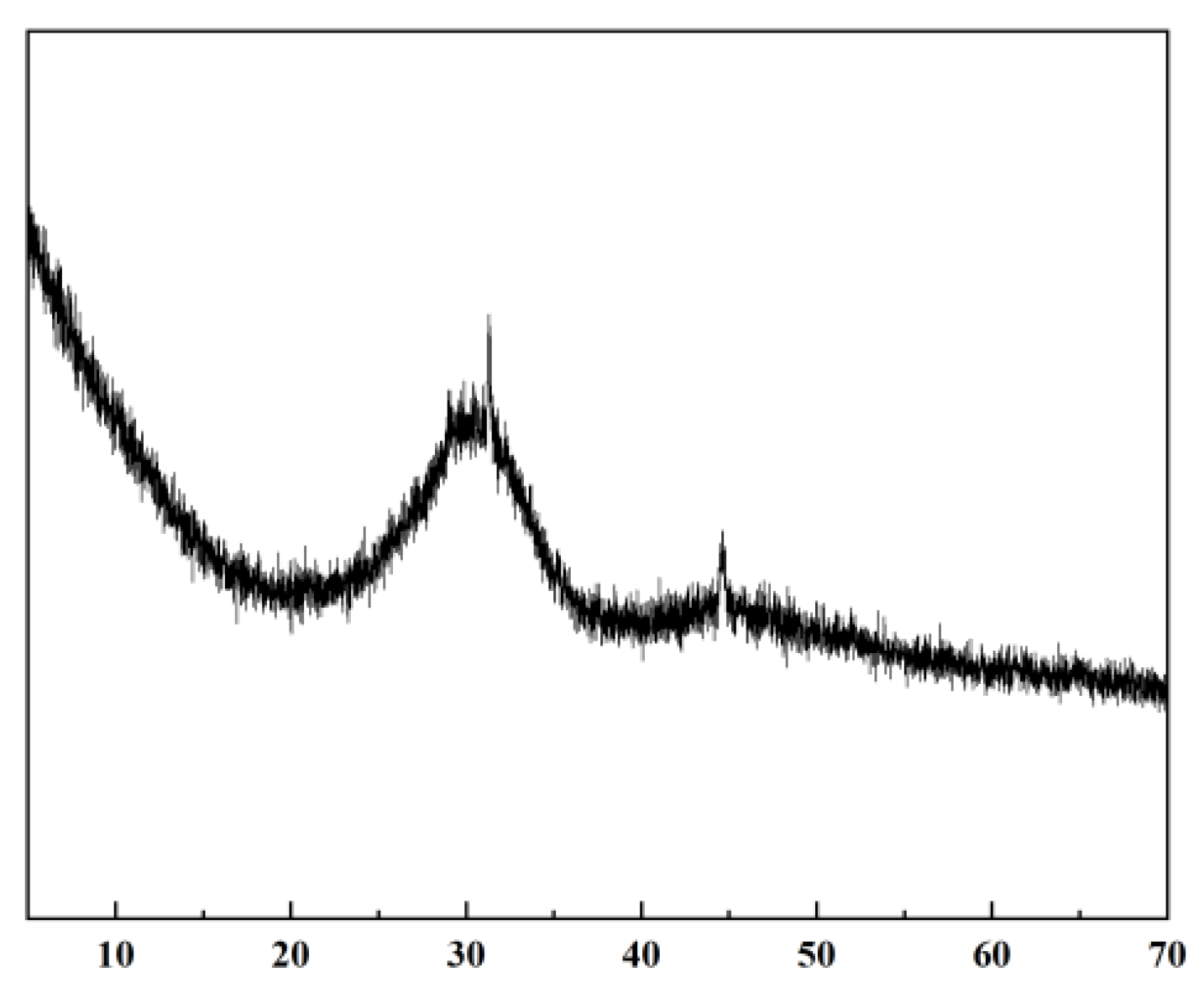
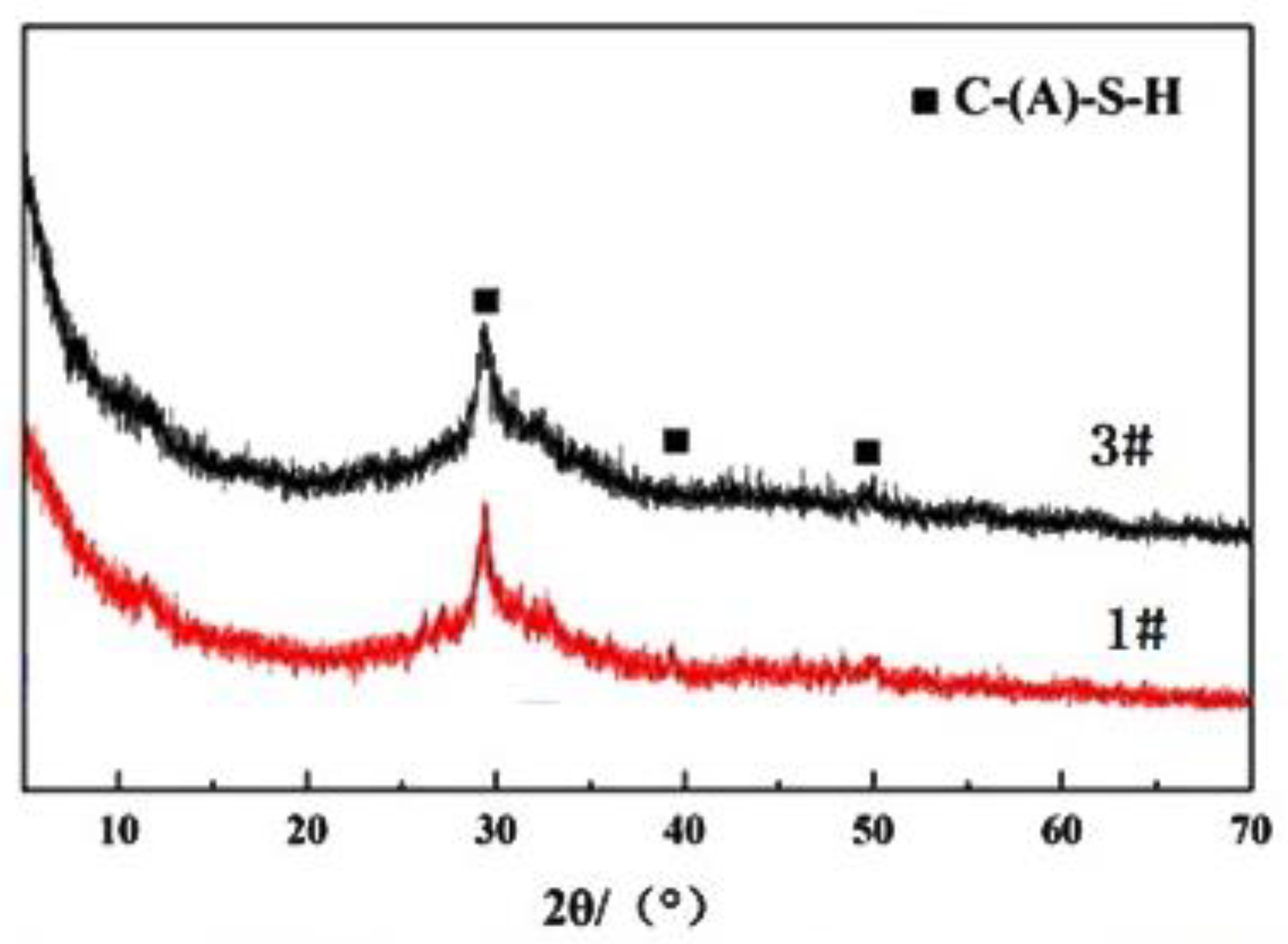
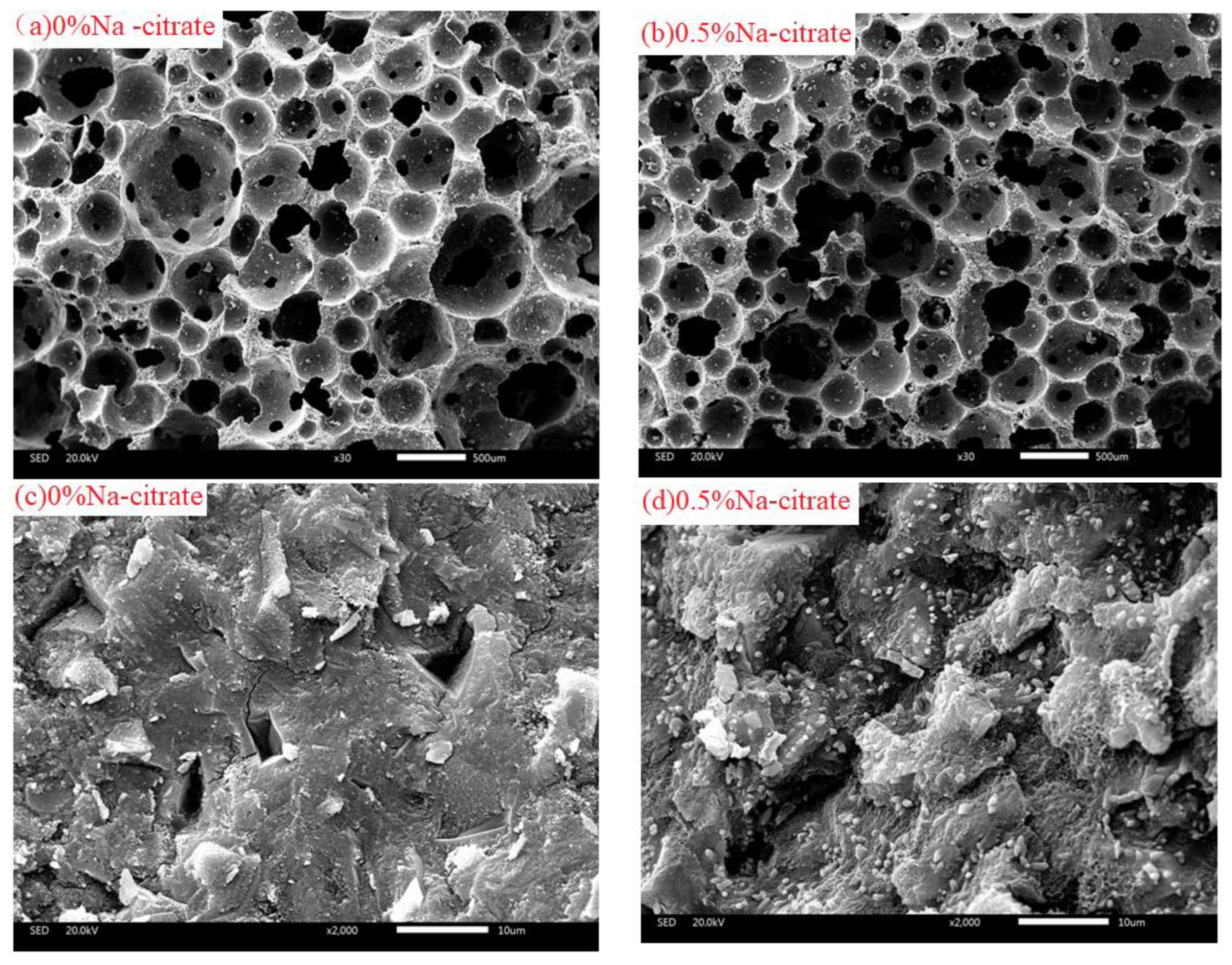
3.3. Microstructure and Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amran, Y.M.; Farzadnia, N. Properties and applications of foamed concrete; A review. Constr. Build. Mater. 2015, 101, 990–1005. [Google Scholar] [CrossRef]
- Jalal, M.D.; Tanveer, A. Foam concrete. Int. J. Civ. Eng. Res. 2017, 8, 1–14. [Google Scholar]
- Hou, M.Y.; Zhu, X.C. Review on the Research and Application of Foam Concrete. Bull. Chin. Ceram. Soc. 2019, 38, 410–416. [Google Scholar]
- Zhang, J. Foam Concrete; Harbin Institute of Technology: Harbin, China, 2016. [Google Scholar]
- Ma, Z.; Wang, X. Light Aggregate Concrete; Chemical Industry Press: Beijing, China, 2014. [Google Scholar]
- Yan, Z.; He, Y. Production and Application Technology of Foam Agent for Foam Concrete; China Building Materials Industry Press: Beijing, China, 2021. [Google Scholar]
- Hashim, M.; Tantray, M. Comparative study on the performance of protein and synthetic-based foaming agents used in foamed concrete. Case Stud. Constr. Mater. 2021, 14, e00524. [Google Scholar] [CrossRef]
- Hou, L.; Li, J.; Lu, Z.; Niu, Y. Influence of foaming agent on cement and foam concrete. Constr. Build. Mater. 2021, 280, 122399. [Google Scholar] [CrossRef]
- Liu, X.M.; Wang, L.Y.; Li, S.L.; Wang, J.P. Study on high-efficiency complexed foaming agent for lightweight foamed concrete. Adv. Mater. Res. 2021, 250–253, 569–573. [Google Scholar] [CrossRef]
- Guo, J. Influence analysis of foamed light soil on differential settlement of new and old roadbed in expressway reconstruction and expansion. Build. Technol. 2024, 55, 676–679. [Google Scholar]
- Hu, J. Application of foam light soil in backfilling of bridge platform. Jiangsu Build. Mater. 2023, 5, 35–37. [Google Scholar]
- Yu, J.Y. Research on Foam Lightweight Soil Subgrade for Reconstruction and Expansion of Binlai Expressway. Master’s Thesis, Shandong University, Jinan, China, 2018. [Google Scholar]
- Nong, F.B. Study on Mechanical Properties of Lightweight Foam Soil and Its Application in Liunan Expressway Reconstruction and Expansion Project. Master’s Thesis, Guangxi University, Nanning, China, 2018. [Google Scholar]
- Kim, T.H.; Kim, T.H.; Kang, G.C. Performance evaluation of road embankment constructed using lightweight soils on an unimproved soft soil layer. Eng. Geol. 2013, 160, 34–43. [Google Scholar] [CrossRef]
- Wang, Y.; Wan, H.; Liu, H.; Zhang, G.; Xu, X.; Shen, C. Preparation and Properties of Low-Carbon Foamed Lightweight Soil with High Resistance to Sulphate Erosion Environments. Materials 2023, 16, 4604. [Google Scholar] [CrossRef]
- Wang, L.L.; Si, C.Y. Effect of Potassium Hydroxide-Sodium Water Glass Activator on Propert of Alkali-Activated Slag Cementitious Materials. Bull. Chin. Ceram. Soc. 2022, 41, 2654–2662. [Google Scholar]
- Zheng, W.Z.; Zou, M.N. Literature review of alkali-activated cementitious materials. J. Build. Struct. 2019, 40, 28–39. [Google Scholar]
- Wang, L.; Si, C.; Li, C.; Sun, X.; Zhou, H.; Guo, S. Effect of potassium-sodium hydroxide and water glass activator on properties of alkali-excited slag cementing materials. Chin. J. Ceram. 2002, 41, 2654–2662. [Google Scholar]
- Ji, Y.; Wu, Y.W. Reinforcing for Slag-based Lightweight Foamed Concrete. Bull. Chin. Ceram. Soc. 2018, 37, 1861–1867. [Google Scholar]
- Liguori, B.; Capasso, I. Hybrid geopolymeric foams with diatomite addition: Effect on chemico-physical properties. J. Cell. Plast. 2017, 53, 525–536. [Google Scholar] [CrossRef]
- Wang, H.F.; Lu, J.L. Study of geopolymer-based foamed concrete prepared from construction waste. New Build. Mater. 2016, 43, 73–75+94. [Google Scholar]
- Wang, Q.; Ding, J.N. Research and preparation on polymer base foam concrete of inorganic mineral. Concrete 2018, 11, 118–121+126. [Google Scholar]
- Hao, Y.; Yang, G. Development of fly ash and slag based high-strength alkali-activated foam concrete. Cem. Concr. Compos. 2022, 128, 104447. [Google Scholar] [CrossRef]
- Yang, K.H.; Lee, K.H. Properties and sustainability of alkali-activated slag foamed concrete. J. Clean. Prod. 2014, 68, 226–233. [Google Scholar] [CrossRef]
- Wang, P.M.; Jin, Z.P. Research on composite exciter for alkali slag cementitious materials. New Build. Mater. 2005, 8, 32–34. [Google Scholar]
- Liu, T.; Gong, C.; Duan, L.C.; Qu, B. Effects of sodium citrate on compressive strength and microstructure of NaOH-activated fly ash/slag cement exposed to high temperature. Constr. Build. Mater. 2023, 363, 129852. [Google Scholar] [CrossRef]
- Chen, M.M.; Yu, Y.W.; Feng, C.H.; Li, D.X. Study on Flue Gas Desulphurization Gypsum Plaster. Appl. Mech. Mater. 2011, 71, P842–P846. [Google Scholar] [CrossRef]
- Wu, H.; Zha, Y.; Wang, J.; Wang, E.; Li, Y.; Wang, X. Study on the retarding effect and mechanism analysis of different retarders. Concr. Cem. Prod. 2023, 9, 23–26. [Google Scholar]
- Gao, G.B.; Yan, S.T.; Wang, Y.W.; Liu, C.B. Study on Compatibility of Polycarboxylates Superplasticizer with Different kinds of Retarders. Adv. Mater. Res. 2012, 450, P543–P547. [Google Scholar] [CrossRef]
- Xuan, J.Q.; Liu, H. Preparation of foamed concrete by alkali activated mineral powder and performance influence mechanism. New Build. Mater. 2023, 83–89. [Google Scholar]
- Provis, J.L.; Deventer, J. Alkali Activated Materials; State-of-the-Art Report, RILEM TC 224-AAM; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Collins, F.; Sanjayan, J.G. Microcracking and strength development of alkali activated slag concrete. Cem. Concr. Compos. 2001, 23, 345–352. [Google Scholar] [CrossRef]
- Yu, X.G.; Wei, J.R. Influence of test methods on the setting time of foam concrete. In Proceedings of the 2010 Annual Meeting of CCPA China Foam Concrete Branch and the 2nd National Foam Concrete Technology Exchange Conference, Yantai, China, 12 December 2010; pp. 181–186. [Google Scholar]
- Fan, L.L.; Yang, Y. Experimental study on the setting time of foam concrete. New Build. Mater. 2012, 7, 46–48. [Google Scholar]
- Zhu, H.; Dong, R.; Ma, B.; Liu, S. Effect of Amount of NaOH and Performance of Water Glass on Performance of Alkali-Activated Cementing Materials (AASC); Research Progress in Chemically activated Cementing Materials; Southeast University Press: Nanjing, China, 2005; pp. 146–150. [Google Scholar]
- Jiang, L. Study on Mix Ratio Optimization and Retarding and Toughening Modification of Alkali-Excited Slag Gelling Materials. Master’s Thesis, Qingdao University of Technology, Qingdao, China, 2021. [Google Scholar]
- Hu, Z. Study on Preparation and Properties of Mixed Alkali Excited Mineral Powder Foam Concrete. Master’s Thesis, Yantai University, Yantai, China, 2024. [Google Scholar]
- Shi, C. Early hydration and microstructure development of alkali-activated slag cement pastes. X Intern. Cong. Chem. Cem (Goteborg) 1997, 3, 267–272. [Google Scholar]
- Burciaga-Díaz, O.; Escalante-García, J.I. Structure, mechanisms of reaction, and strength of an alkali-activated blast-furnace slag. J. Am. Ceram. Soc. 2013, 96, 3939–3948. [Google Scholar] [CrossRef]
- Huang, L.P.; Ma, Q.M. Experimental Study on Hydration Products of Alkali-Activated Slag. Bull. Chin. Ceram. Soc. 2020, 39, 1194–1200. [Google Scholar]
- Sun, C.; Zhu, Y.; Guo, J.; Zhang, Y.; Sun, G. Effects of foaming agent type on the workability, drying shrinkage, frost resistance and pore distribution of foamed concrete. Constr. Build. Mater. 2018, 186, P833–P839. [Google Scholar] [CrossRef]
- Zeng, Y.W.; Fang, Y.H. Proceedings of the 1st China National Workshop on Chemically-Activated Cementing Materials; Southeast University Press: Nanjing, China, 2005; pp. 184–193. [Google Scholar]
- Qu, B.; Liu, T. The effect of sodium citrate on NaOH-activated BFS cement: Hydration, mechanical property, and micro/nanostructure. Cem. Concr. Compos. 2022, 133, 104703. [Google Scholar] [CrossRef]

| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | K2O | Na2O | TiO2 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| 39.92 | 31.23 | 14.12 | 0.78 | 7.34 | 2.23 | 0.61 | 0.72 | 0.76 | −0.29 |
| No. | WG/% | Modulus | Sodium Citrate/% | GGBS/g | Water Binder Ratio | Foam */g |
|---|---|---|---|---|---|---|
| M1 | 12 | 1.0 | 0 | 1200 | 0.4 | 115 |
| M2 | 12 | 1.2 | 0 | |||
| M3 | 12 | 1.5 | 0 | |||
| N1 | 15 | 1.0 | 0 | |||
| N2 | 15 | 1.2 | 0 | |||
| N3 | 15 | 1.5 | 0 | |||
| P1 | 18 | 1.0 | 0 | |||
| P2 | 18 | 1.2 | 0 | |||
| P3 | 18 | 1.5 | 0 | |||
| 1# | 15 | 1.2 | 0 | 1200 | 0.4 | 115 |
| 2# | 15 | 1.2 | 0.25 | |||
| 3# | 15 | 1.2 | 0.5 | |||
| 4# | 15 | 1.2 | 0.75 | |||
| 5# | 15 | 1.2 | 1.0 |
| No. | WG/% | Modulus | Flow Value/mm | Setting Time/h | Compressive Strength/MPa | ||
|---|---|---|---|---|---|---|---|
| Initial Setting | Final Setting | 7 d | 28 d | ||||
| M1 | 12 | 1.0 | 230 | 1.3 | 18.4 | 0.72 | 1.50 |
| M2 | 12 | 1.2 | 195 | 2.3 | 24.5 | 0.95 | 1.08 |
| M3 | 12 | 1.5 | 210 | 3.1 | 27.3 | 0.61 | 1.29 |
| N1 | 15 | 1.0 | 222 | 0.7 | 15.2 | 1.22 | 1.67 |
| N2 | 15 | 1.2 | 185 | 1.2 | 20.4 | 1.62 | 1.77 |
| N3 | 15 | 1.5 | 215 | 2.7 | 26.6 | 0.91 | 1.21 |
| P1 | 18 | 1.0 | 205 | 0.4 | 12.9 | 1.35 | 1.72 |
| P2 | 18 | 1.2 | 175 | 0.8 | 14.3 | 1.71 | 2.03 |
| P3 | 18 | 1.5 | 210 | 2.6 | 24.1 | 0.92 | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, G.; Li, J.; Xuan, J.; Wang, Y.; Wan, H.; Huang, Y. The Effects of Sodium Silicate and Sodium Citrated on the Properties and Structure of Alkali-Activated Foamed Concrete. Processes 2024, 12, 1927. https://doi.org/10.3390/pr12091927
Liu H, Zhang G, Li J, Xuan J, Wang Y, Wan H, Huang Y. The Effects of Sodium Silicate and Sodium Citrated on the Properties and Structure of Alkali-Activated Foamed Concrete. Processes. 2024; 12(9):1927. https://doi.org/10.3390/pr12091927
Chicago/Turabian StyleLiu, Hao, Gaoke Zhang, Jixin Li, Jiaqi Xuan, Yongsheng Wang, Huiwen Wan, and Yun Huang. 2024. "The Effects of Sodium Silicate and Sodium Citrated on the Properties and Structure of Alkali-Activated Foamed Concrete" Processes 12, no. 9: 1927. https://doi.org/10.3390/pr12091927
APA StyleLiu, H., Zhang, G., Li, J., Xuan, J., Wang, Y., Wan, H., & Huang, Y. (2024). The Effects of Sodium Silicate and Sodium Citrated on the Properties and Structure of Alkali-Activated Foamed Concrete. Processes, 12(9), 1927. https://doi.org/10.3390/pr12091927





