Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection
Abstract
1. Introduction
2. Experimental Work
2.1. Materials
2.2. PCLNPG Synthesis
2.3. Membrane Preparation
2.4. Membrane Characterization
2.5. Performance Tests
3. Results and Discussion
3.1. Membrane Morphology
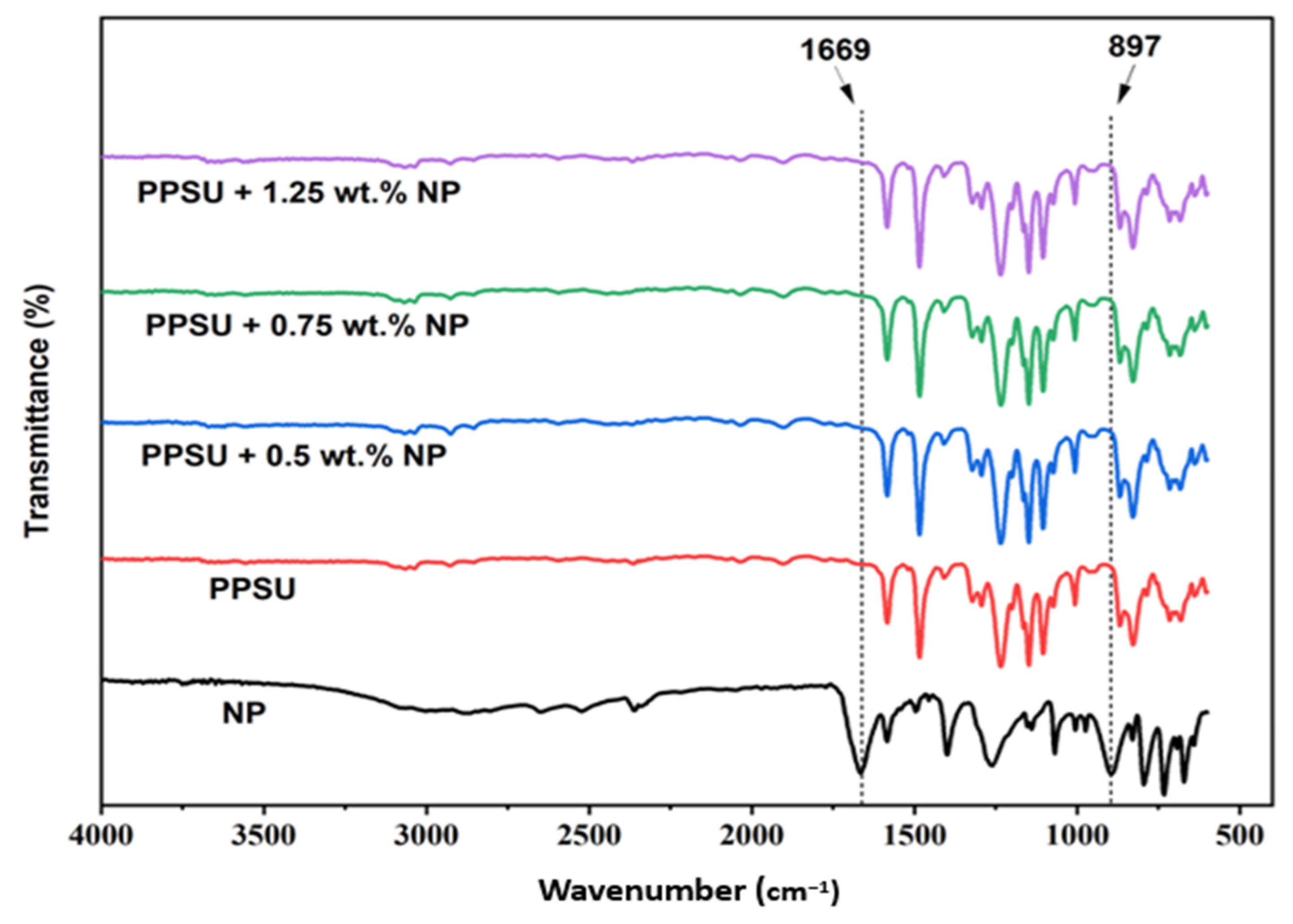
3.2. FTIR Analysis
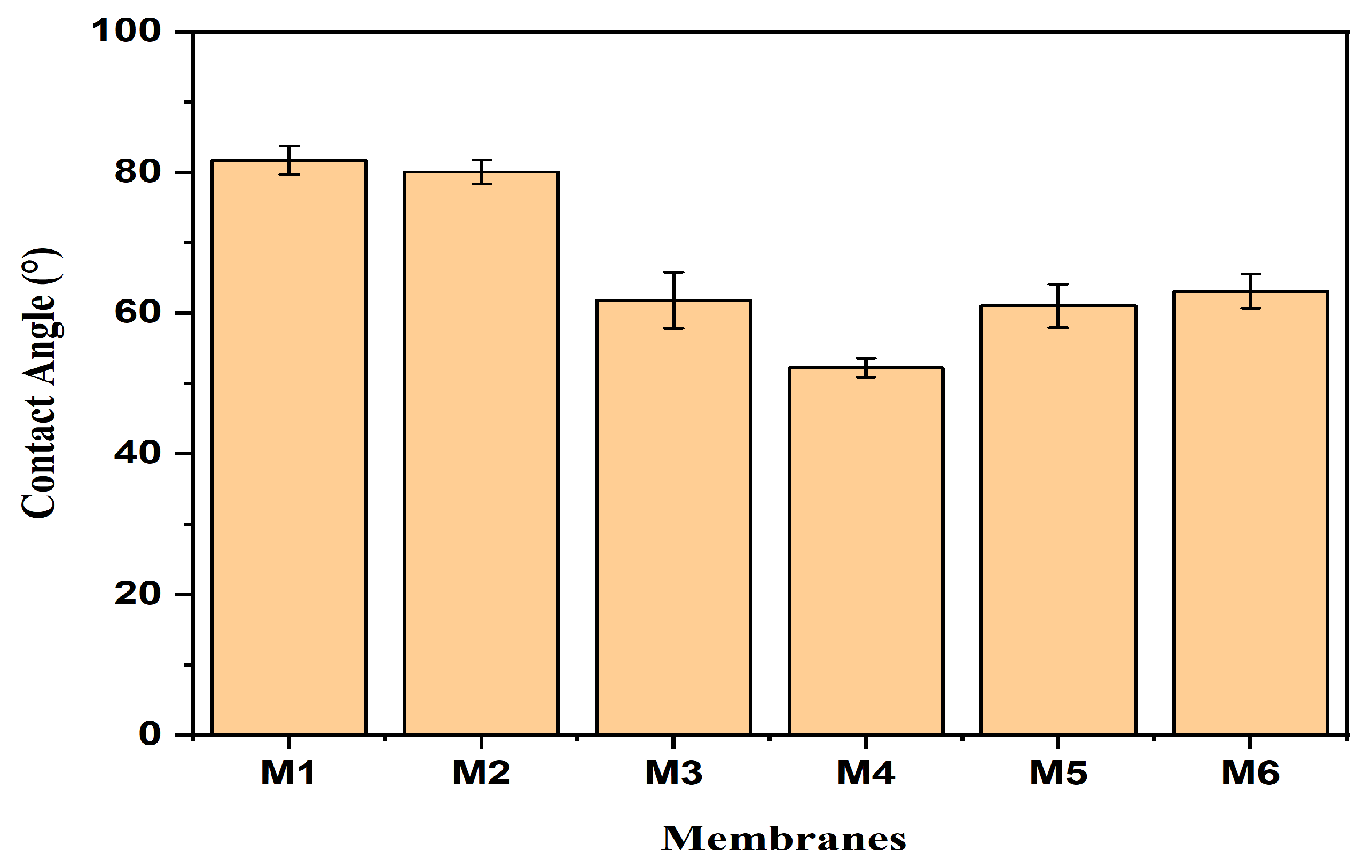
3.3. Hydrophilicity of Membranes
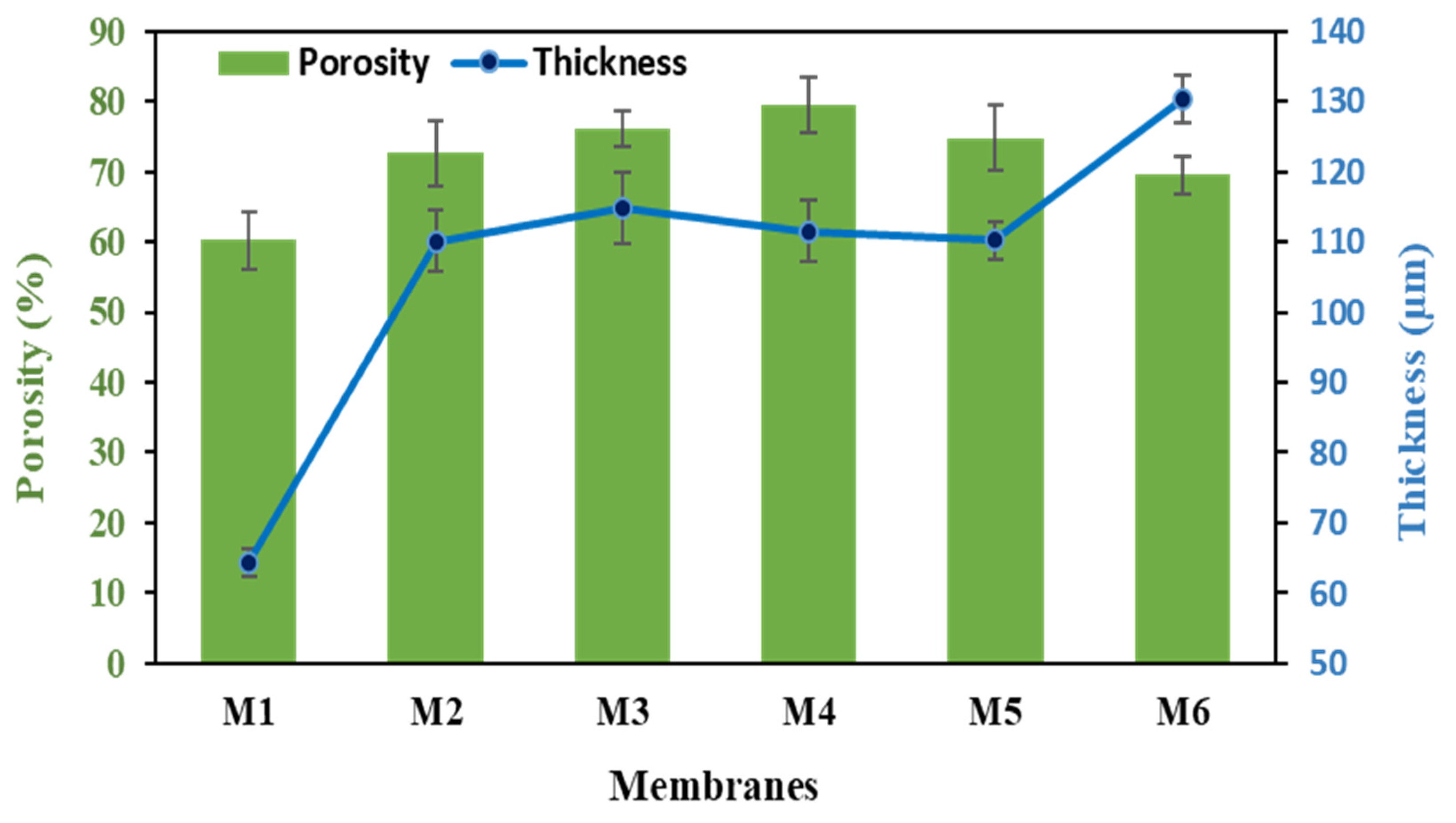
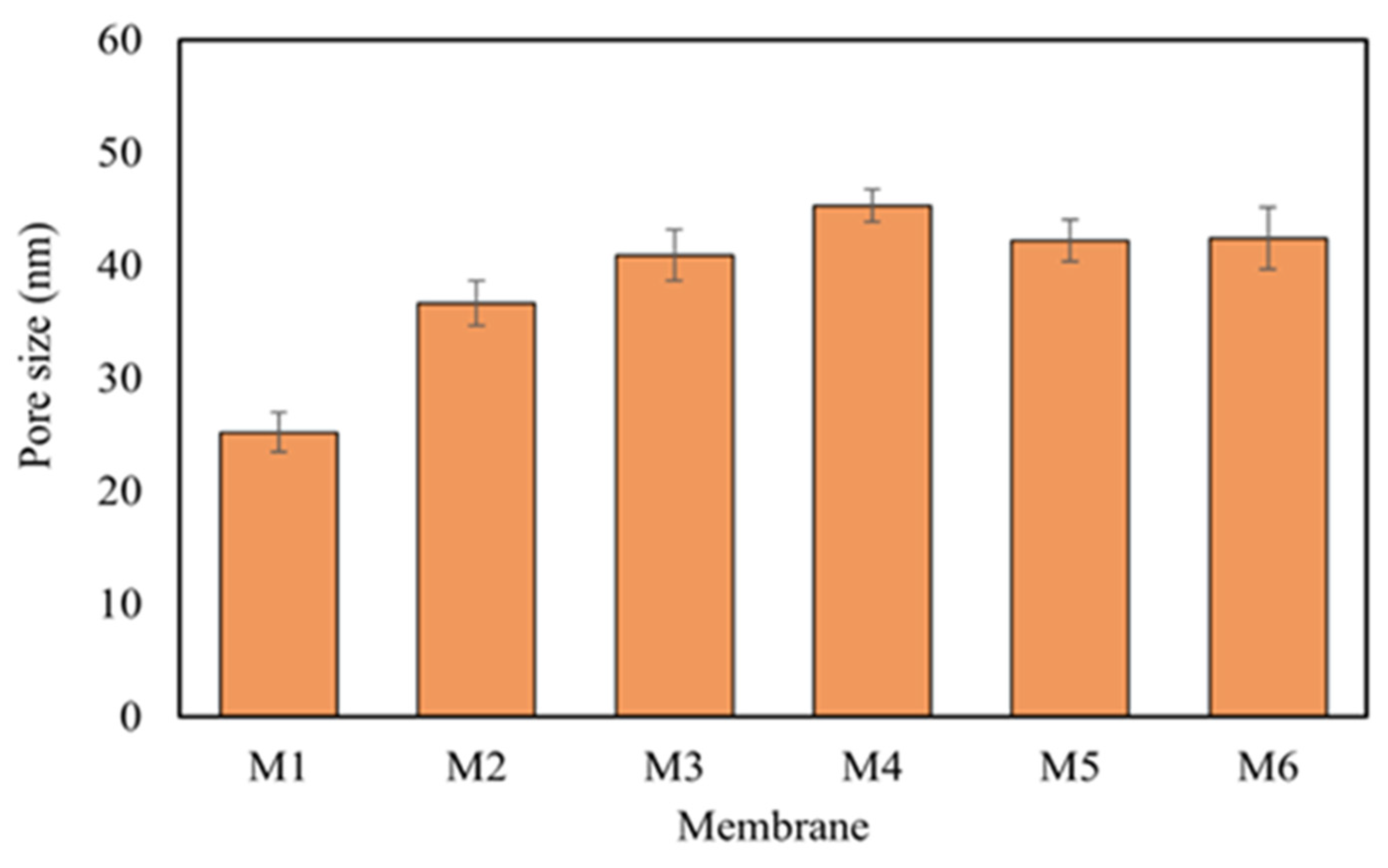
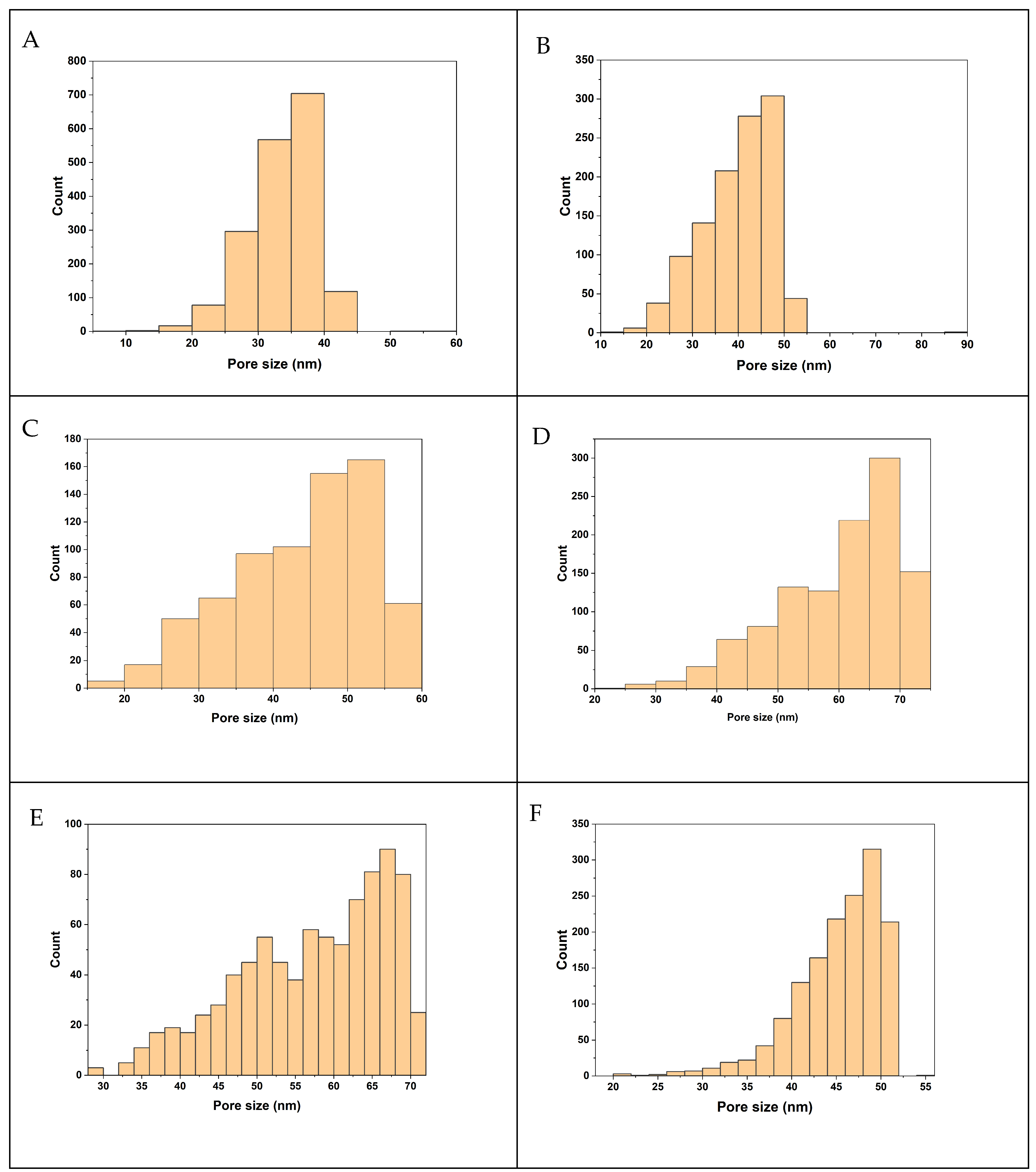
3.4. Thickness, Porosity, and Pore Size of Membranes
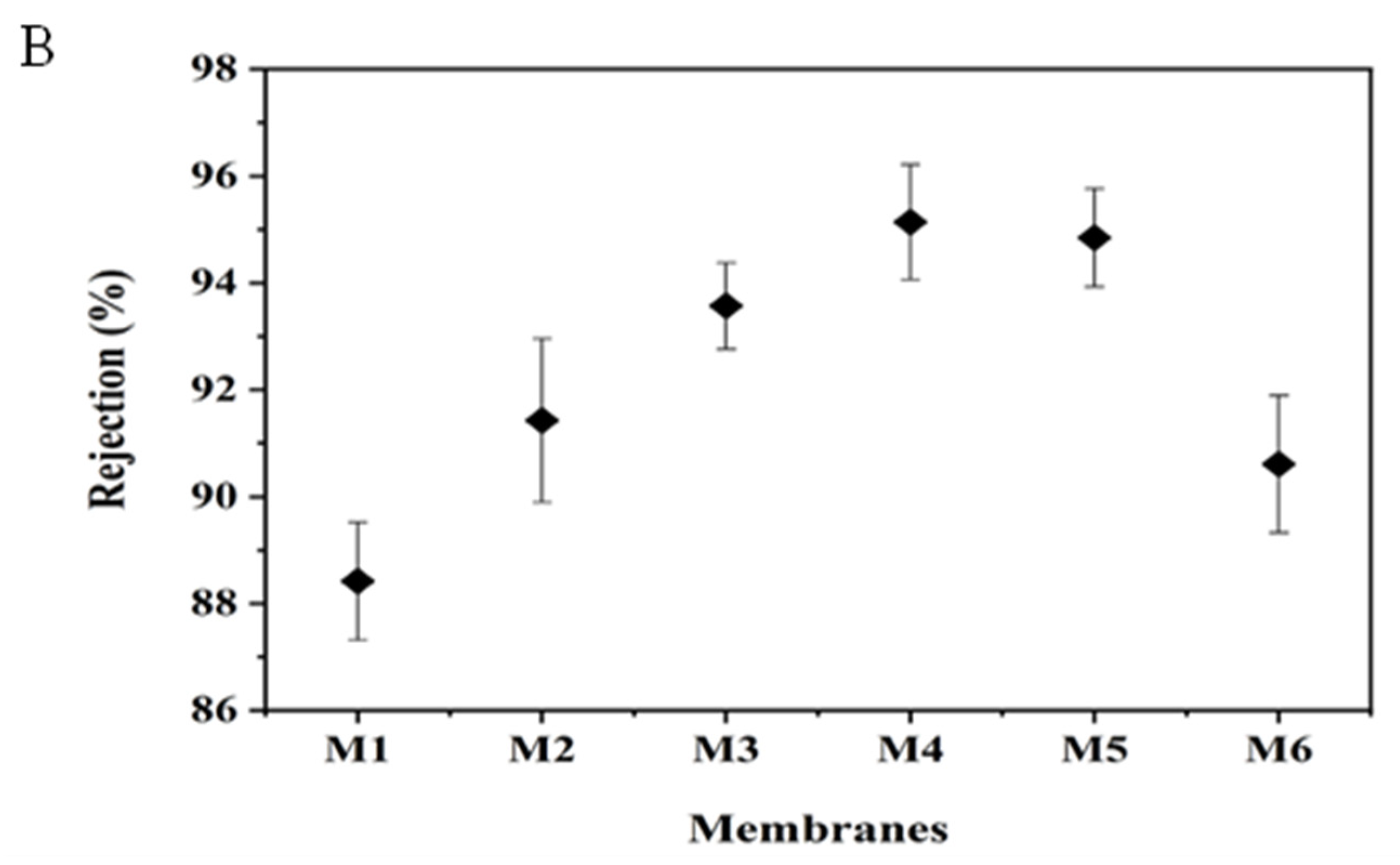
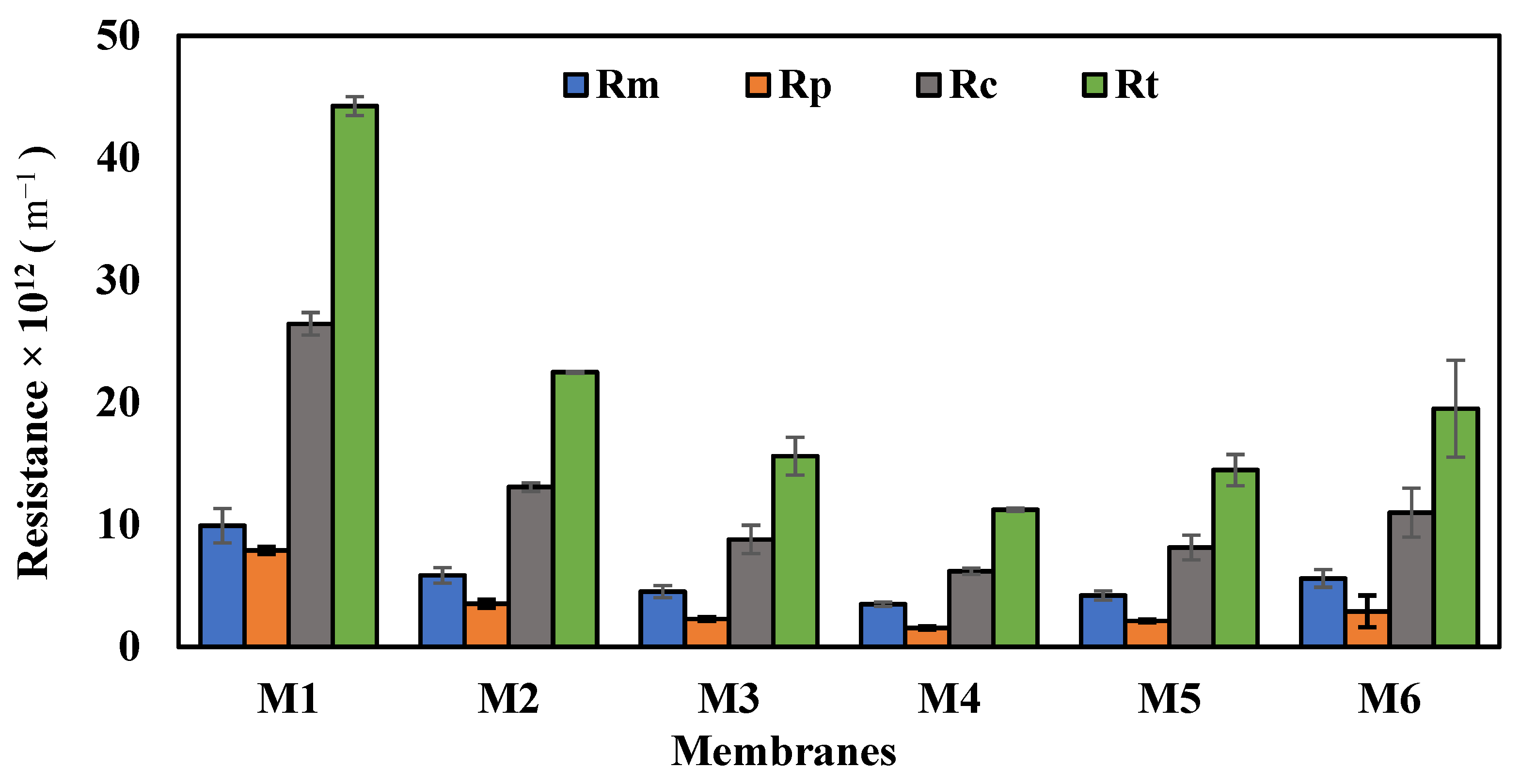
3.5. Membrane Performance
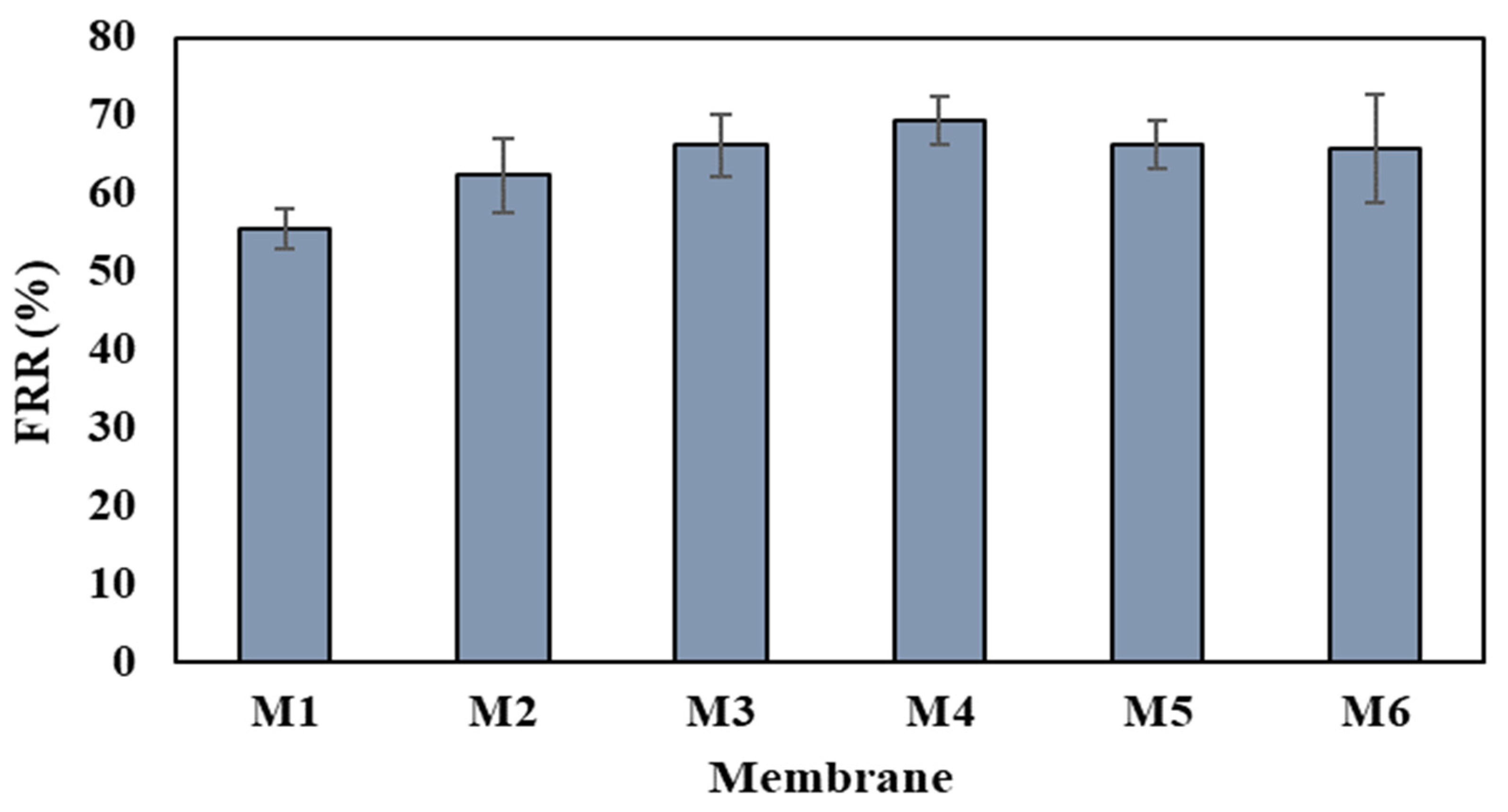
3.6. Antifouling Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Qadir, D.; Mukhtar, H.; Keong, L.K. Mixed Matrix Membranes for Water Purification Applications. Sep. Purif. Rev. 2017, 46, 62–80. [Google Scholar] [CrossRef]
- Wee, T.; Kim, C.; Hanif, M. Review on Wastewater Treatment Technologies. Int. J. Appl. Environ. Sci. 2016, 11, 111–126. [Google Scholar]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration membranes for wastewater and water process engineering: A comprehensive statistical review over the past decade. J. Water Process. Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Li, Q.; Xu, Z.; Pinnau, I. Fouling of reverse osmosis membranes by biopolymers in wastewater secondary effluent: Role of membrane surface properties and initial permeate flux. J. Membr. Sci. 2007, 290, 173–181. [Google Scholar] [CrossRef]
- Hilal, N.; Ismail, A.F.; Wright, C. Membrane Fabrication; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Al Aani, S.; Gomez, V.; Wright, C.J.; Hilal, N. Fabrication of antibacterial mixed matrix nanocomposite membranes using hybrid nanostructure of silver coated multi-walled carbon nanotubes. Chem. Eng. J. 2017, 326, 721–736. [Google Scholar] [CrossRef]
- Sonune, A.; Ghate, R. Developments in wastewater treatment methods. Desalination 2004, 167, 55–63. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, Z.; Wei, X.; Tian, X.; Wang, J.; Yang, S.; Wang, S. Comparison study of the effect of PVP and PANI nanofibers additives on membrane formation mechanism, structure and performance. J. Membr. Sci. 2011, 385–386, 110–122. [Google Scholar] [CrossRef]
- Abdullah, R.R.; Shabeeb, K.M.; Alzubaydi, A.B.; Figoli, A.; Criscuoli, A.; Drioli, E.; Alsalhy, Q. Characterization of the Efficiency of Photo-Catalytic Ultrafiltation PES Membrane Modified with Tungsten Oxide in the Removal of Tinzaparin Sodium. Eng. Technol. J. 2022, 40, 1–10. [Google Scholar] [CrossRef]
- Al-Araji, D.D.; Al-Ani, F.H.; Alsalhy, Q.F. The permeation and Separation Characteristics of Polymeric Membranes Incorporated with Nanoparticles for Dye Removal and Interaction Mechanisms between Polymer and Nanoparticles: A Mini Review. Eng. Technol. J. 2022, 40, 1399–1411. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Li, C.; Hou, L.-A. Fabrication of GO modified PVDF membrane for dissolved organic matter removal: Removal mechanism and antifouling property. Sep. Purif. Technol. 2019, 209, 482–490. [Google Scholar] [CrossRef]
- Dipheko, T.D.; Matabola, K.P.; Kotlhao, K.; Moutloali, R.M.; Klink, M. Fabrication and Assessment of ZnO Modified Polyethersulfone Membranes for Fouling Reduction of Bovine Serum Albumin. Int. J. Polym. Sci. 2017, 2017, 3587019. [Google Scholar] [CrossRef]
- Pakizeh, M.; Azinfar, F.; Safarnia, M.; Raji, F. The effects of TiO2 nanoparticles and polydopamine on the structure, separation, and antifouling properties of PPSU membrane. Sep. Sci. Technol. 2022, 57, 1788–1799. [Google Scholar] [CrossRef]
- Rashid, H.-O.; Ralph, S.F. Carbon nanotube membranes: Synthesis, properties, and future filtration applications. Nanomaterials 2017, 7, 99. [Google Scholar] [CrossRef] [PubMed]
- Plisko, T.; Karslyan, Y.; Bildyukevich, A. Effect of polyphenylsulfone and polysulfone incompatibility on the structure and performance of blend membranes for ultrafiltration. Materials 2021, 14, 5740. [Google Scholar] [CrossRef] [PubMed]
- Ghadhban, M.Y.; Majdi, H.S.; Rashid, K.T.; Alsalhy, Q.F.; Lakshmi, D.S.; Salih, I.K.; Figoli, A. Removal of dye from a leather tanning factory by flat-sheet blend ultrafiltration (UF) membrane. Membranes 2020, 10, 47. [Google Scholar] [CrossRef]
- Hussein, S.S.; Ibrahim, S.S.; Toma, M.A.; Alsalhy, Q.F.; Drioli, E. Novel chemical modification of polyvinyl chloride membrane by free radical graft copolymerization for direct contact membrane distillation (DCMD) application. J. Membr. Sci. 2020, 611, 118266. [Google Scholar] [CrossRef]
- Mansouri, J.; Harrisson, S.; Chen, V. Strategies for controlling biofouling in membrane filtration systems: Challenges and opportunities. J. Mater. Chem. 2010, 20, 4567–4586. [Google Scholar] [CrossRef]
- Fang, X.; Li, J.; Li, X.; Sun, X.; Shen, J.; Han, W.; Wang, L. Polyethyleneimine, an effective additive for polyethersulfone ultrafiltration membrane with enhanced permeability and selectivity. J. Membr. Sci. 2014, 476, 216–223. [Google Scholar] [CrossRef]
- Shabeeb, K.M.; Noori, W.A.; Abdulridha, A.A.; Majdi, H.S.; Al-Baiati, M.N.; Yahya, A.A.; Rashid, K.T.; Németh, Z.; Hernadi, K.; Alsalhy, Q.F. Novel partially cross-linked nanoparticles graft co-polymer as pore former for polyethersulfone membranes for dyes removal. Heliyon 2023, 9, e21958. [Google Scholar] [CrossRef]
- Garcia-Ivars, J.; Iborra-Clar, M.-I.; Alcaina-Miranda, M.-I.; Van der Bruggen, B. Comparison between hydrophilic and hydrophobic metal nanoparticles on the phase separation phenomena during formation of asymmetric polyethersulphone membranes. J. Membr. Sci. 2015, 493, 709–722. [Google Scholar] [CrossRef]
- Kang, Y.; Obaid, M.; Jang, J.; Ham, M.-H.; Kim, I.S. Novel sulfonated graphene oxide incorporated polysulfone nanocomposite membranes for enhanced-performance in ultrafiltration process. Chemosphere 2018, 207, 581–589. [Google Scholar] [CrossRef]
- Xiao, S.; Yu, S.; Yan, L.; Liu, Y.; Tan, X. Preparation and properties of PPSU/GO mixed matrix membrane. Chin. J. Chem. Eng. 2017, 25, 408–414. [Google Scholar] [CrossRef]
- Gebru, K.A.; Das, C. Removal of bovine serum albumin from wastewater using fouling resistant ultrafiltration membranes based on the blends of cellulose acetate, and PVP-TiO 2 nanoparticles. J. Environ. Manag. 2017, 200, 283–294. [Google Scholar] [CrossRef]
- Liu, J.; Zhong, Z.; Ma, R.; Zhang, W.; Li, J. Development of high-antifouling PPSU ultrafiltration membrane by using compound additives: Preparation, morphologies, and filtration resistant properties. Membranes 2016, 6, 35. [Google Scholar] [CrossRef]
- Arumugham, T.; Kaleekkal, N.J.; Doraiswamy, M. Development of new hybrid ultrafiltration membranes by entanglement of macromolecular PPSU-SO3H chains: Preparation, morphologies, mechanical strength, and fouling resistant properties. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Alsalhy, Q.F.; Al-Ani, F.H.; Al-Najar, A.E.; Jabuk, S.I. A study of the effect of embedding ZnO-NPs on PVC membrane performance use in actual hospital wastewater treatment by membrane bioreactor. Chem. Eng. Process. Process. Intensif. 2018, 130, 262–274. [Google Scholar] [CrossRef]
- Zwane, S.; Kuvarega, A.T.; Mhlanga, S.D.; Dlamini, D.S. Hydrophilic polysulfone/Lantana camara mixed matrix membranes for the removal of dyes from water. Surf. Interfaces 2018, 13, 216–223. [Google Scholar] [CrossRef]
- Saljoughi, E.; Amirilargani, M.; Mohammadi, T. Effect of PEG additive and coagulation bath temperature on the morphology, permeability and thermal/chemical stability of asymmetric CA membranes. Desalination 2010, 262, 72–78. [Google Scholar] [CrossRef]
- Farjami, M.; Vatanpour, V.; Moghadassi, A. Effect of nanoboehmite/poly(ethylene glycol) on the performance and physiochemical attributes EPVC nano-composite membranes in protein separation. Chem. Eng. Res. Des. 2020, 156, 371–383. [Google Scholar] [CrossRef]
- Al-Ani, D.M.; Al-Ani, F.H.; Alsalhy, Q.F.; Ibrahim, S. Preparation and characterization of ultrafiltration membranes from PPSU-PES polymer blend for dye removal. Chem. Eng. Commun. 2021, 208, 41–59. [Google Scholar] [CrossRef]
- Kadhim, R.J.; Al-Ani, F.H.; Al-Shaeli, M.; Alsalhy, Q.F.; Figoli, A. Removal of dyes using graphene oxide (Go) mixed matrix membranes. Membranes 2020, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.S.; Torbati, S.F.; Shahmirzadi, M.A.A.; Tavangar, T. Fabrication, characterization, and performance evaluation of polyethersulfone/TiO2 nanocomposite ultrafiltration membranes for produced water treatment. Polym. Adv. Technol. 2018, 29, 2619–2631. [Google Scholar] [CrossRef]
- Mushtaq, A.; Bin Mukhtar, H.; Shariff, A.M. FTIR study of enhanced polymeric blend membrane with amines. Res. J. Appl. Sci. Eng. Technol. 2014, 7, 1811–1820. [Google Scholar] [CrossRef]
- Ali, A.M.; Rashid, K.T.; Yahya, A.A.; Majdi, H.S.; Salih, I.K.; Yusoh, K.; Alsalhy, Q.F.; AbdulRazak, A.A.; Figoli, A. Fabrication of gum arabic-graphene (Gga) modified polyphenylsulfone (ppsu) Mixed Matrix Membranes: A Systematic Evaluation Study for Ultrafiltration (UF) Applications. Membranes 2021, 11, 542. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Díez-Vicente, A.L. Effect of TiO2nanoparticles on the performance of polyphenylsulfone biomaterial for orthopaedic implants. J. Mater. Chem. B 2014, 2, 7502–7514. [Google Scholar] [CrossRef]
- Haghighat, N.; Vatanpour, V.; Sheydaei, M.; Nikjavan, Z. Preparation of a novel polyvinyl chloride (PVC) ultrafiltration membrane modified with Ag/TiO2 nanoparticle with enhanced hydrophilicity and antibacterial activities. Sep. Purif. Technol. 2020, 237, 116374. [Google Scholar] [CrossRef]
- Al-Ani, F.H.; Alsalhy, Q.F.; Raheem, R.S.; Rashid, K.T.; Figoli, A. Experimental investigation of the effect of implanting tio2-nps on pvc for long-term uf membrane performance to treat refinery wastewater. Membranes 2020, 10, 77. [Google Scholar] [CrossRef]
- Safarpour, M.; Vatanpour, V.; Khataee, A. Preparation and characterization of graphene oxide/TiO2 blended PES nanofiltration membrane with improved antifouling and separation performance. Desalination 2016, 393, 65–78. [Google Scholar] [CrossRef]
- Hong, J.; He, Y. Polyvinylidene fluoride ultrafiltration membrane blended with nano-ZnO particle for photo-catalysis self-cleaning. Desalination 2014, 332, 67–75. [Google Scholar] [CrossRef]
- Maximous, N.; Nakhla, G.; Wan, W.; Wong, K. Performance of a novel ZrO2/PES membrane for wastewater filtration. J. Membr. Sci. 2010, 352, 222–230. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Moradian, R.; Zinadini, S.; Astinchap, B. Fabrication and characterization of novel antifouling nanofiltration membrane prepared from oxidized multiwalled carbon nanotube/polyethersulfone nanocomposite. J. Membr. Sci. 2011, 375, 284–294. [Google Scholar] [CrossRef]
- Aljanabi, A.A.A.; Mousa, N.E.; Aljumaily, M.M.; Majdi, H.S.; Yahya, A.A.; Al-Baiati, M.N.; Hashim, N.; Rashid, K.T.; Al-Saadi, S.; Alsalhy, Q.F. Modification of Polyethersulfone Ultrafiltration Membrane Using Poly(terephthalic acid-co-glycerol-g-maleic anhydride) as Novel Pore Former. Polymers 2022, 14, 3408. [Google Scholar] [CrossRef]
- Balta, S.; Sotto, A.; Luis, P.; Benea, L.; Van der Bruggen, B.; Kim, J. A new outlook on membrane enhancement with nanoparticles: The alternative of ZnO. J. Membr. Sci. 2012, 389, 155–161. [Google Scholar] [CrossRef]
- Vatanpour, V.; Madaeni, S.S.; Khataee, A.R.; Salehi, E.; Zinadini, S.; Monfared, H.A. TiO 2 embedded mixed matrix PES nanocomposite membranes: Influence of different sizes and types of nanoparticles on antifouling and performance. Desalination 2012, 292, 19–29. [Google Scholar] [CrossRef]
- Arthanareeswaran, G.; Mohan, D.; Raajenthiren, M. Preparation and performance of polysulfone-sulfonated poly(ether ether ketone) blend ultrafiltration membranes. Part I. Appl. Surf. Sci. 2007, 253, 8705–8712. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Alberto, M.; Vijayaraghavan, A.; Fan, X.; Holmes, S.M.; Souaya, E.R.; Badawy, M.I.; Gorgojo, P. High flux and fouling resistant flat sheet polyethersulfone membranes incorporated with graphene oxide for ultrafiltration applications. Chem. Eng. J. 2018, 334, 789–799. [Google Scholar] [CrossRef]
- Zhou, D.; Rong, G.; Huang, S.; Pang, J. Preparation of a novel sulfonated polyphenlene sulfone with flexible side chain for ultrafiltration membrane application. Sep. Purif. Technol. 2019, 210, 817–823. [Google Scholar] [CrossRef]
- Falsanisi, D.; Liberti, L.; Notarnicola, M. Ultrafiltration (UF) pilot plant for municipal wastewater reuse in agriculture: Impact of the operation mode on process performance. Water 2010, 2, 872–885. [Google Scholar] [CrossRef]
- Cosenza, A.; Gulhan, H.; Maida, C.M.; Mannina, G. Nutrient recovery from wastewater treatment by ultrafiltration membrane for water reuse in view of a circular economy perspective. Bioresour. Technol. 2022, 363, 127929. [Google Scholar] [CrossRef]




| Membrane Code | PPSU wt.% | NMP wt.% | PCLNPG wt.% |
|---|---|---|---|
| M1 | 16 | 84 | 0 |
| M2 | 16 | 83.75 | 0.25 |
| M3 | 16 | 83.5 | 0.5 |
| M4 | 16 | 83.25 | 0.75 |
| M5 | 16 | 83 | 1.0 |
| M6 | 16 | 82.75 | 1.25 |
| Membrane | Pore Size (nm) | Porosity (%) | Contact Angle (°) | Thickness (µm) |
|---|---|---|---|---|
| M1 | 25.22 | 60.16 | 81.7 | 64.33 |
| M2 | 36.68 | 72.61 | 80.0 | 110.1 |
| M3 | 40.92 | 76.02 | 61.8 | 114.8 |
| M4 | 45.32 | 79.49 | 52.2 | 111.53 |
| M5 | 42.22 | 74.81 | 61.0 | 110.2 |
| M6 | 42.42 | 69.48 | 63.1 | 130.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taha, Y.R.; Zrelli, A.; Hajji, N.; Al-Juboori, R.A.; Alsalhy, Q. Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection. Processes 2024, 12, 1930. https://doi.org/10.3390/pr12091930
Taha YR, Zrelli A, Hajji N, Al-Juboori RA, Alsalhy Q. Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection. Processes. 2024; 12(9):1930. https://doi.org/10.3390/pr12091930
Chicago/Turabian StyleTaha, Younus Rashid, Adel Zrelli, Nejib Hajji, Raed A. Al-Juboori, and Qusay Alsalhy. 2024. "Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection" Processes 12, no. 9: 1930. https://doi.org/10.3390/pr12091930
APA StyleTaha, Y. R., Zrelli, A., Hajji, N., Al-Juboori, R. A., & Alsalhy, Q. (2024). Impact of PCLNPG Nanopolymeric Additive on the Surface and Structural Properties of PPSU Ultrafiltration Membranes for Enhanced Protein Rejection. Processes, 12(9), 1930. https://doi.org/10.3390/pr12091930








