Heat Pump Performance Mapping for Energy Recovery from an Industrial Building
Abstract
:1. Introduction
2. Methodology
2.1. Description of the System
2.2. Case of Study
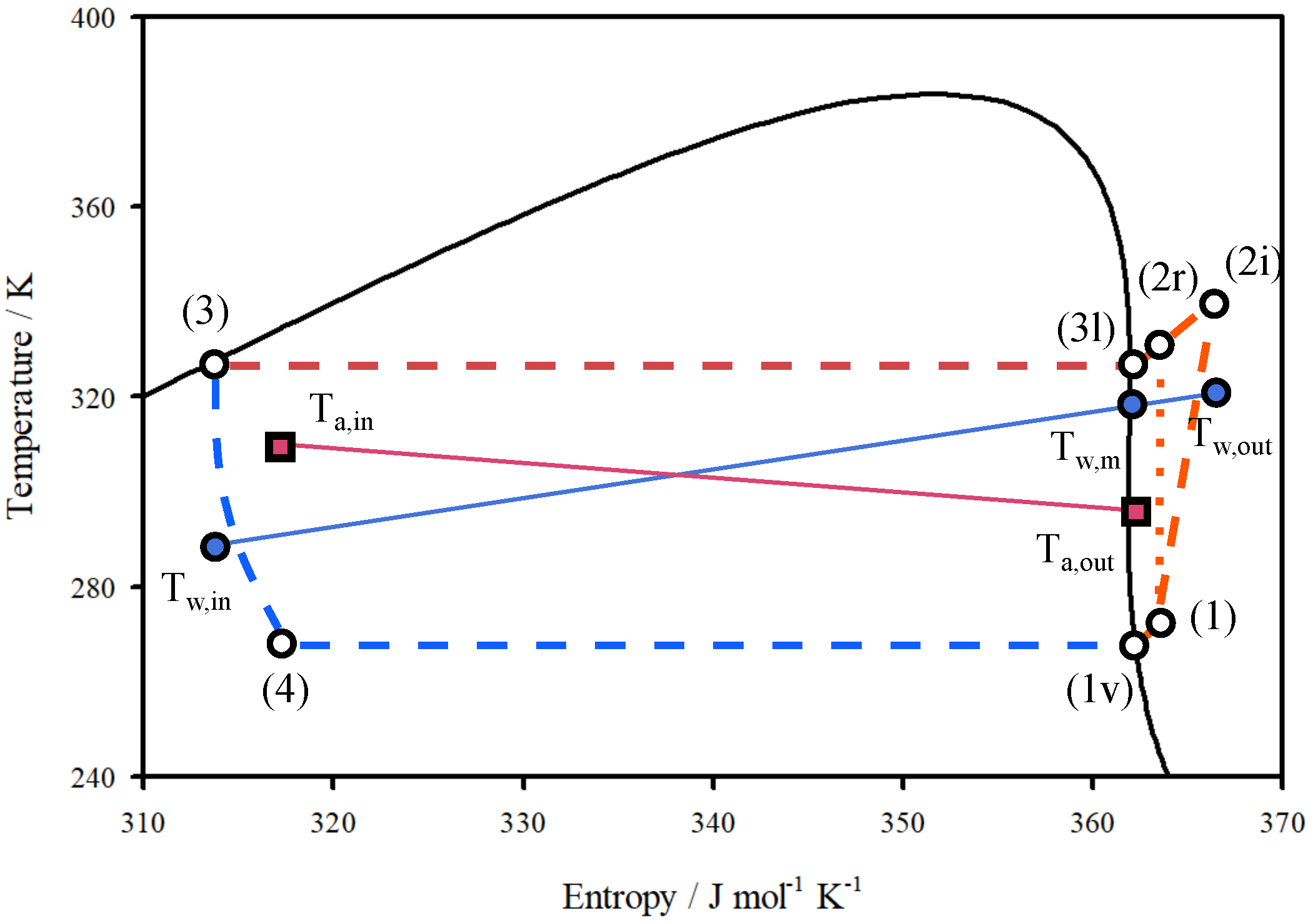
2.3. Modelling
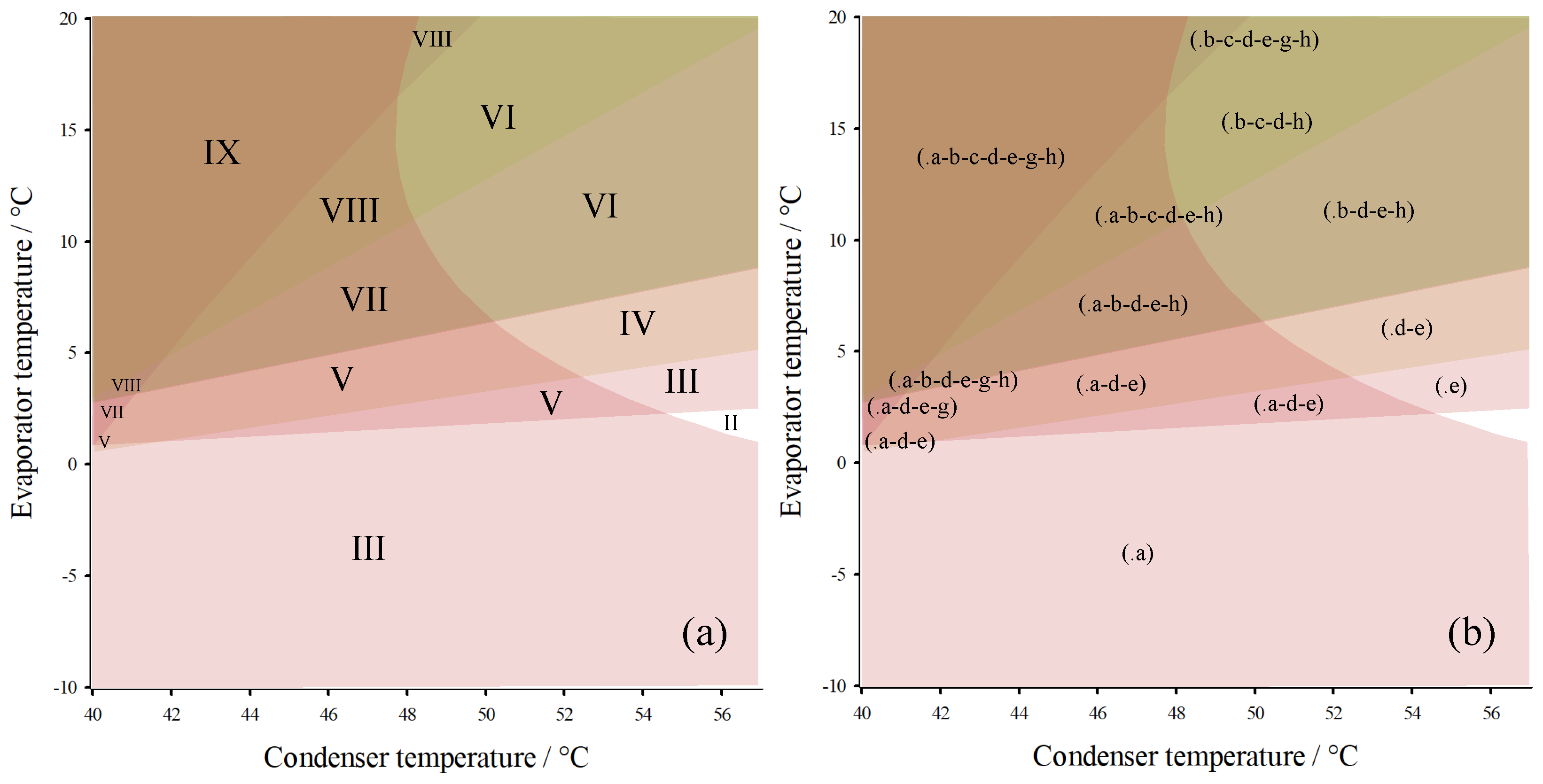
3. Results
3.1. Validation
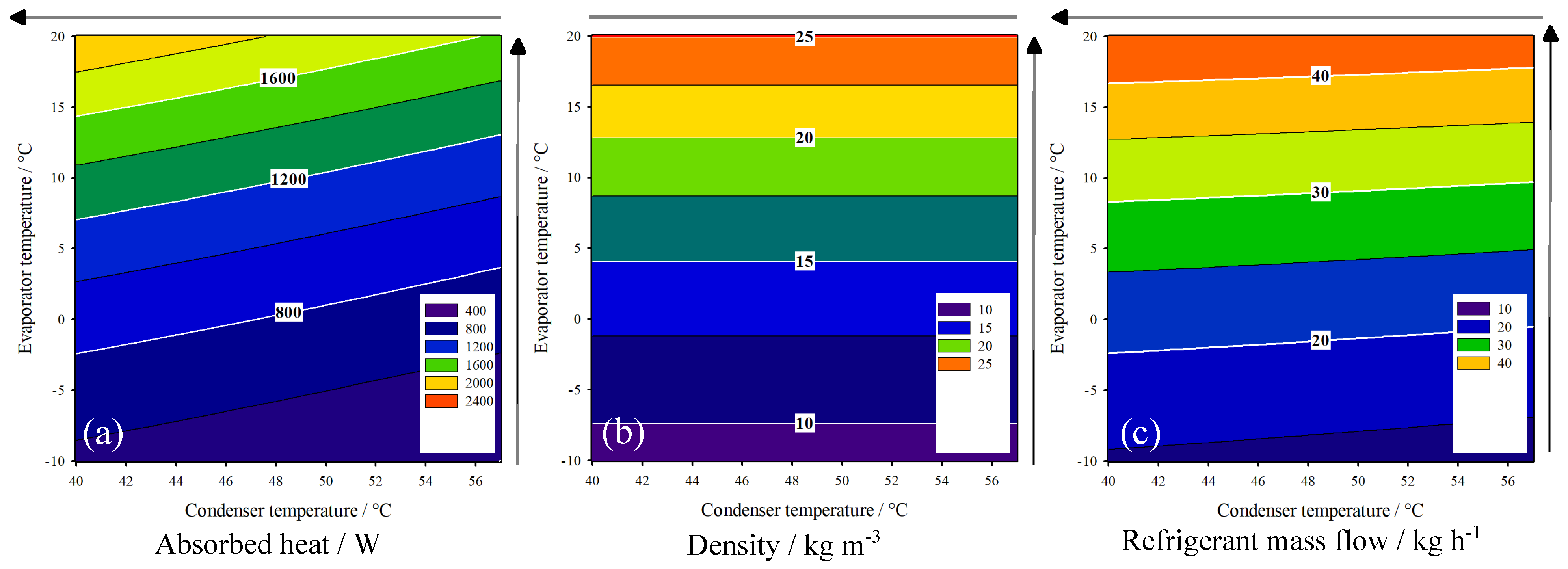
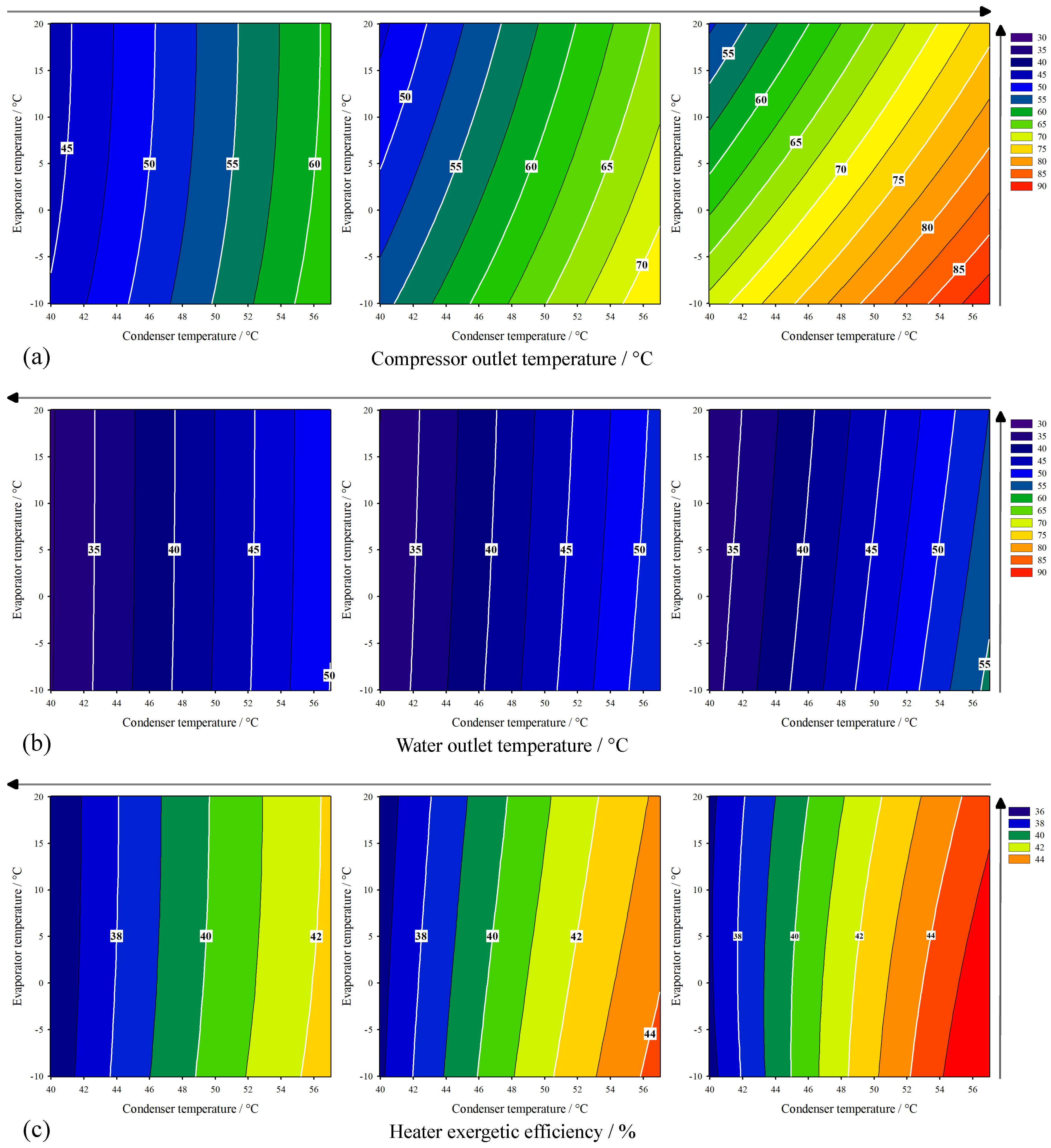
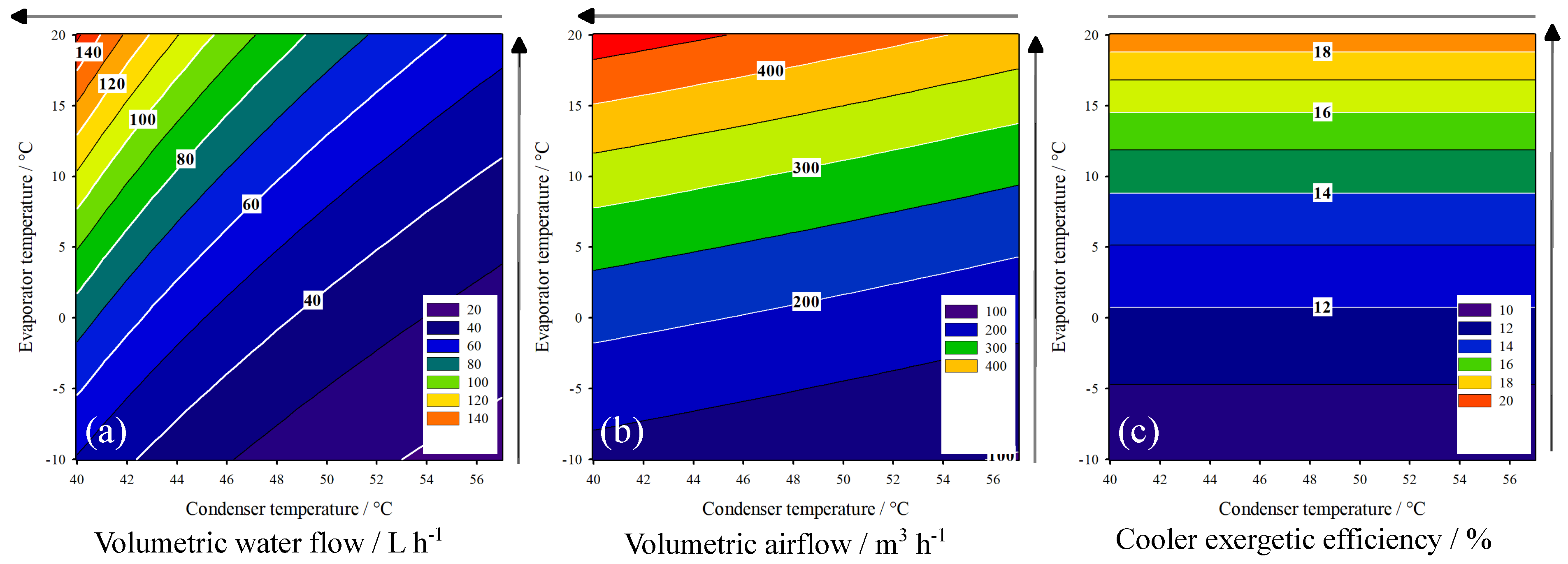
3.2. HP-System Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| COP | Coefficient of performance |
| EOS | Equation of state |
| HP | Heat pump |
| LTTE | Low-temperature thermal energy |
| PC-SAFT | Perturbed-Chain Statistical Associating Fluid Theory |
References
- Al-Mansour, F. Energy efficiency trends and policy in Slovenia. Energy 2011, 36, 1868–1877. [Google Scholar] [CrossRef]
- de la Rue du Can, S.; Letschert, V.; Agarwal, S.; Park, W.Y.; Kaggwa, U. Energy efficiency improves energy access affordability. Energy Sustain. Dev. 2022, 70, 560–568. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, Y.; Jiang, C.; Li, H. How does energy efficiency affect employment? Evidence from Chinese cities. Energy 2023, 280, 128071. [Google Scholar] [CrossRef]
- González, J.; Llovell, F.; Garrido, J.M.; Quinteros-Lama, H. A rigorous approach for characterising the limiting optimal efficiency of working fluids in organic Rankine cycles. Energy 2022, 124191, 254. [Google Scholar] [CrossRef]
- Yekoladio, P.J.; Bello-Ochende, T.; Meyer, J.P. Thermodynamic analysis and performance optimization of organic rankine cycles for the conversion of low-to-moderate grade geothermal heat. Int. J. Energy Res. 2015, 39, 1256–1271. [Google Scholar] [CrossRef]
- Peyrovedin, H.; Haghbakhsh, R.; Duarte, A.R.C.; Shariati, A. Deep Eutectic Solvents as Phase Change Materials in Solar Thermal Power Plants: Energy and Exergy Analyses. Molecules 2022, 27, 1427. [Google Scholar] [CrossRef] [PubMed]
- Daniarta, S.; Nemś, M.; Kolasiński, P.; Pomorski, M. Sizing the Thermal Energy Storage Device Utilizing Phase Change Material (PCM) for Low-Temperature Organic Rankine Cycle Systems Employing Selected Hydrocarbons. Energies 2022, 15, 956. [Google Scholar] [CrossRef]
- Bellocchi, S.; Guizzi, G.L.; Manno, M.; Pentimalli, M.; Salvatori, M.; Zaccagnini, A. Adsorbent materials for low-grade waste heat recovery: Application to industrial pasta drying processes. Energy 2017, 140, 729–745. [Google Scholar] [CrossRef]
- Urbano, D.G.; Aquino, A.; Scrucca, F. Energy Performance, Environmental Impacts and Costs of a Drying System: Life Cycle Analysis of Conventional and Heat Recovery Scenarios. Energies 2023, 16, 1523. [Google Scholar] [CrossRef]
- Hervás-Blasco, E.; Navarro-Peris, E.; Barceló-Ruescas, F.; Corberán, J.M. Improved water to water heat pump design for low-temperature waste heat recovery based on subcooling control. Int. J. Refrig. 2019, 106, 374–383. [Google Scholar] [CrossRef]
- Ammar, Y.; Joyce, S.; Norman, R.; Wang, Y.; Roskilly, A.P. Low grade thermal energy sources and uses from the process industry in the UK. Appl. Energy 2012, 89, 3–20. [Google Scholar] [CrossRef]
- Zendehboudi, A. Optimal discharge pressure and performance characteristics of a transcritical CO2 heat pump system with a tri-partite gas cooler for combined space and water heating. Renew. Energy 2024, 226, 120359. [Google Scholar] [CrossRef]
- Zendehboudi, A. Experimental analysis of a tri-partite brazed plate gas cooler for CO2 heat pump water heaters. Appl. Therm. Eng. 2024, 241, 122376. [Google Scholar] [CrossRef]
- Zendehboudi, A. Energy, exergy, and exergoeconomic analyses of an air source transcritical CO2 heat pump for simultaneous domestic hot water and space heating. Energy 2024, 290, 130295. [Google Scholar] [CrossRef]
- Sun, D.; Wang, C.; Liu, Z.; Qin, J.; Liu, Z. Experimental and simulation study on R134a/RE170/R152a mixture as R134a replacement in a moderately-high temperature heat pump. Appl. Therm. Eng. 2024, 236, 121643. [Google Scholar] [CrossRef]
- Song, Y.; Li, D.; Yang, D.; Jin, L.; Cao, F.; Wang, X. Comparaison de performances entre une pompe à chaleur combinée au R134a/CO2 et une pompe à chaleur en cascade au R134a/CO2 pour chauffage de locaux. Int. J. Refrig. 2017, 74, 590–603. [Google Scholar] [CrossRef]
- Bianchi, M.; De Pascale, A. Bottoming cycles for electric energy generation: Parametric investigation of available and innovative solutions for the exploitation of low and medium temperature heat sources. Appl. Energy 2011, 88, 1500–1509. [Google Scholar] [CrossRef]
- Brogioli, D.; La Mantia, F. Heat recovery in energy production from low temperature heat sources. AIChE J. 2019, 65, 980–991. [Google Scholar] [CrossRef]
- Linton, J.W.; Snelson, W.K.; Hearty, P.F.; Triebe, A.R.; Linton, J.W.; Snelson, W.K.; Hearty, P.F. The Potential of HFC-134a and HFC-152a to Replace CFC-12 in Medium Temperature Heat Pump Applications. In Proceedings of the International Refrigeration and Air Conditioning Conference, West Lafayette, IN, USA, 14–17 July 1992; pp. 203–209. [Google Scholar]
- Carrington, C.G.; Bannister, P.; Liu, Q. Performance of a scroll compressor with R134a at medium temperature heat pump conditions. Int. J. Energy Res. 1996, 20, 733–743. [Google Scholar] [CrossRef]
- Lee, J.Y.; Chen, P.Y. Optimization of Heat Recovery Networks for Energy Savings in Industrial Processes. Processes 2023, 11, 321. [Google Scholar] [CrossRef]
- Pandey, G.K.; Sikha, S.S.; Thakur, A.; Yarlagadda, S.S.; Thatikonda, S.S.; Baiju suja, B.; Mystkowski, A.; Dragašius, E.; Gundabattini, E. Thermal Mapping and Heat Transfer Analysis of an Induction Motor of an Electric Vehicle Using Nanofluids as a Cooling Medium. Sustainability 2023, 15, 8124. [Google Scholar] [CrossRef]
- Borgeson, S.; Brager, G. Comfort standards and variations in exceedance for mixed-mode buildings. Build. Res. Inf. 2011, 39, 118–133. [Google Scholar] [CrossRef]
- Singh, S.; Hanna, E.G.; Kjellstrom, T. Working in Australia’s heat: Health promotion concerns for health and productivity. Health Promot. Int. 2015, 30, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Mi, H.; Zhou, Y.; Zhang, Y. Experimental research of a water-source heat pump water heater system. Energies 2018, 11, 1205. [Google Scholar] [CrossRef]
- Janghorban Esfahani, I.; Ifaei, P. Optimal Design of a Renewable-Energy-Driven Integrated Cooling–Freshwater Cogeneration System. Processes 2024, 12, 1164. [Google Scholar] [CrossRef]
- Masip, X.; Navarro-Peris, E.; Corberán, J.M. Influence of the thermal energy storage strategy on the performance of a booster heat pump for domestic hot water production system based on the use of low temperature heat source. Energies 2020, 13, 6576. [Google Scholar] [CrossRef]
- Dávila, P.; Bourouis, M.; Francisco Nicolalde, J.; Martínez-Gómez, J. Modelling and analysis of a compression/resorption heat pump system with a zeotropic mixture of acetone/CO2. Appl. Therm. Eng. 2023, 227, 120388. [Google Scholar] [CrossRef]
- Min, M.; Kim, Y.; Lee, S.; Ko, C. Performance evaluation of a water-to-water heat pump with a floor panel heating system. KSME Int. J. 1999, 13, 642–649. [Google Scholar] [CrossRef]
- Ritchie, M.J.; Engelbrecht, J.A.; Booysen, M.J. Which strategy saves the most energy for stratified water heaters? Energies 2021, 14, 4859. [Google Scholar] [CrossRef]
- Oh, S.; Cho, Y.; Yun, R. Raw-water source heat pump for a vertical water treatment building. Energy Build. 2014, 68, 321–328. [Google Scholar] [CrossRef]
- Pitarch, M.; Hervas-Blasco, E.; Navarro-Peris, E.; Corberán, J.M. Exergy analysis on a heat pump working between a heat sink and a heat source of finite heat capacity rate. Int. J. Refrig. 2019, 99, 337–350. [Google Scholar] [CrossRef]
- Stene, J. Residential CO2 heat pump system for combined space heating and hot water heating. Int. J. Refrig. 2005, 28, 1259–1265. [Google Scholar] [CrossRef]
- SECOP. Technical Data Model SC10GHH. 2023. Available online: https://www.secop.com/fileadmin/user_upload/SEPS/datasheets/en/sc1212g_104g8280_r134a_220v_50hz_03-2024_ds.pdf (accessed on 1 June 2024).
- Portal, E.E. Electrical Characteristic of the Motor. 2024. Available online: https://electrical-engineering-portal.com/electrical-characteristics-motor (accessed on 3 June 2024).
- Moshinsky, M. Chapter 3. In Manual de Refrigeración; Reverté: Barcelona, Spain, 2012. [Google Scholar]
- SECOP. SECOP LBP-MBP-HBP COMPRESSORS: Evaporation Pressures. 2023. Available online: https://www.secop.com/fileadmin/user_upload/products/product-selector/secop-lbp-mbp-hbp.pdf (accessed on 5 June 2024).
- Wagner, W.; Pruß, A. The IAPWS Formulation 1995 for the Thermodynamic Properties of Ordinary Water Substance for General and Scientific Use. J. Phys. Chem. Ref. Data 2002, 31, 387–535. [Google Scholar] [CrossRef]
- Keenan, J.; Chao, J.; Kaye, J. Gas Tables: Thermodynamic Properties of Air Products of Combustion and Component Gases, Compressible Flow Functions; Wiley: Hoboken, NJ, USA, 1980. [Google Scholar]
- Siddiqui, M.U.; Owes, A.; Al-Amri, F.G.; Saeed, F. Recent developments in the search for alternative low-global-warming-potential refrigerants: A review. Int. J.-Air-Cond. Refrig. 2020, 28, 2030004. [Google Scholar] [CrossRef]
- Shaik, S.V.; Babu, T.P. Theoretical Performance Investigation of Vapour Compression Refrigeration System Using HFC and HC Refrigerant Mixtures as Alternatives to Replace R22. Energy Procedia 2017, 109, 235–242. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Perturbed-Chain SAFT: An equation of state based on a perturbation theory for chain molecules. Ind. Eng. Chem. Res. 2001, 40, 1244–1260. [Google Scholar] [CrossRef]
- Gross, J.; Sadowski, G. Application of the Perturbed-Chain SAFT equation of state to associating systems. Ind. Eng. Chem. Res. 2002, 41, 5510–5515. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. SAFT: Equation-of-state solution model for associating fluids. Fluid Ph. Equilibria 1989, 52, 31–38. [Google Scholar] [CrossRef]
- Chapman, W.G.; Gubbins, K.E.; Jackson, G.; Radosz, M. New reference equation of state for associating liquids. Ind. Eng. Chem. Res. 1990, 29, 1709–1721. [Google Scholar] [CrossRef]
- González, J.; Llovell, F.; Garrido, J.M.; Quinteros-Lama, H. Accurate and Model-Free Control Function for a Single Stage Transcritical Refrigerator Cycle. ACS Omega 2020, 5, 19217–19226. [Google Scholar] [CrossRef]
- Parvaneh, K.; Rasoolzadeh, A.; Shariati, A. Modeling the phase behavior of refrigerants with ionic liquids using the QC-PC-SAFT equation of state. J. Mol. Liq. 2019, 274, 497–504. [Google Scholar] [CrossRef]
- Cea-Klapp, E.; Polishuk, I.; Canales, R.I.; Quinteros-Lama, H.; Garrido, J.M. Estimation of Thermodynamic Properties and Phase Equilibria in Systems of Deep Eutectic Solvents by PC-SAFT EoS. Ind. Eng. Chem. Res. 2020, 59, 22292–22300. [Google Scholar] [CrossRef]
- González, J.; Llovell, F.; Garrido, J.M.; Quinteros-Lama, H. Selection of a suitable working fluid for a combined organic Rankine cycle coupled with compression refrigeration using molecular approaches. Fluid Ph. Equilibria 2023, 572, 113847. [Google Scholar] [CrossRef]
- Standards and Technology National Institute. Search for Species Data by Chemical Name. Available online: https://webbook.nist.gov/chemistry/name-ser/ (accessed on 2 June 2024).
- Yang, L.; Zhao, L.X.; Zhang, C.L.; Gu, B. Loss-efficiency model of single and variable-speed compressors using neural networks. Int. J. Refrig. 2009, 32, 1423–1432. [Google Scholar] [CrossRef]
- Willem, H.; Lin, Y.; Lekov, A. Review of energy efficiency and system performance of residential heat pump water heaters. Energy Build. 2017, 143, 191–201. [Google Scholar] [CrossRef]



| Value | Range | |
|---|---|---|
| HP system compressor technical conditions | ||
| Displaced volume | 10.29 | – |
| Revolutions per minute | 2900.00 rpm | 2900.00 to 3500.00 rpm |
| Pressure operation maximum | 1600.00 kPa | – |
| Temperature operation maximum | 330.15 K | – |
| Isentropic performance | – | 60 to 100% |
| General boundary and limit conditions | ||
| Condenser temperature | – | 313.15 to 330.15 K |
| Evaporator temperature | – | 263.15 to 293.15 K |
| Initial water temperature | 293.15 K | – |
| Initial industrial building temperature | 313.15 K | – |
| Comfort industrial building temperature | 297.15 K | – |
| i | |||
|---|---|---|---|
| 1 | J | J | J |
| 5.8341 | 9.6110 | 6.6642 | |
| 2 | J | J | J |
| 0.4713 | 4.9931 | −2.9897 |
| R134a | m | |||
|---|---|---|---|---|
| Residual | K | |||
| 3.2483 | 3.0157 | 170.60 | ||
| Ideal gas | ||||
| 7.4912 | 0.01707 | −6.0249 | −115,348.62 |
| AARE of the Equilibrium Properties/% | ||||
|---|---|---|---|---|
| Pressure | Liquid Density | Vapor Density | Enthalpy Difference | Entropy Difference |
| 0.0800 | 0.3299 | 3.3205 | 3.5697 | 3.5706 |
| AARE of the Equilibrium Properties/% | ||||
|---|---|---|---|---|
| Pressure | Liquid Density | Vapor Density | Enthalpy Difference | Entropy Difference |
| 0.1057 | 0.5328 | 4.0941 | 4.6929 | 4.6935 |
| AARE of the Key Parameters/% | ||
|---|---|---|
| Heat Delivery | Coefficient of Performance | Compression Relation |
| 3.8219 | 3.7433 | 1.3864 |
| of the Key Parameters/% | ||||||
|---|---|---|---|---|---|---|
| Temperature Increase | Compressor Power/ | Heat Delivery/ | Coefficient of Performance/COP | |||
| = 80% | = 60% | = 80% | = 60% | = 80% | = 60% | |
| Condenser | 25.000 | 66.670 (o) | 5.960 | 15.892 (+) | 15.232 | 30.465 (−) |
| Evaporator | 25.000 | 66.670 (o) | 3.332 | 8.884 (−) | 17.335 | 34.670 (+) |
| of the Key Parameters/% | ||||||
|---|---|---|---|---|---|---|
| Temperature Increase | Compressor Outlet Temperature/ | Water Outlet Temperature/ | Heater Exergetic Efficiency/ | |||
| = 80% | = 60% | = 80% | = 60% | = 80% | = 60% | |
| Condenser | 17.780 | 47.340 (−) | 3.053 | 8.142 (+) | 3.106 | 5.264 (+) |
| Evaporator | 13.078 | 34.775 (−) | 1.269 | 3.383 (−) | 1.339 | 1.879 (−) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, L.; Romero, J.; Saavedra, N.; Garrido, J.M.; Quinteros-Lama, H.; González, J. Heat Pump Performance Mapping for Energy Recovery from an Industrial Building. Processes 2024, 12, 1955. https://doi.org/10.3390/pr12091955
González L, Romero J, Saavedra N, Garrido JM, Quinteros-Lama H, González J. Heat Pump Performance Mapping for Energy Recovery from an Industrial Building. Processes. 2024; 12(9):1955. https://doi.org/10.3390/pr12091955
Chicago/Turabian StyleGonzález, Leonardo, Jerson Romero, Nicolás Saavedra, José Matías Garrido, Héctor Quinteros-Lama, and Johan González. 2024. "Heat Pump Performance Mapping for Energy Recovery from an Industrial Building" Processes 12, no. 9: 1955. https://doi.org/10.3390/pr12091955






