Key Components Degradation in Proton Exchange Membrane Fuel Cells: Unraveling Mechanisms through Accelerated Durability Testing
Abstract
:1. Introduction
2. Experimental Section
2.1. MEA Fabrication
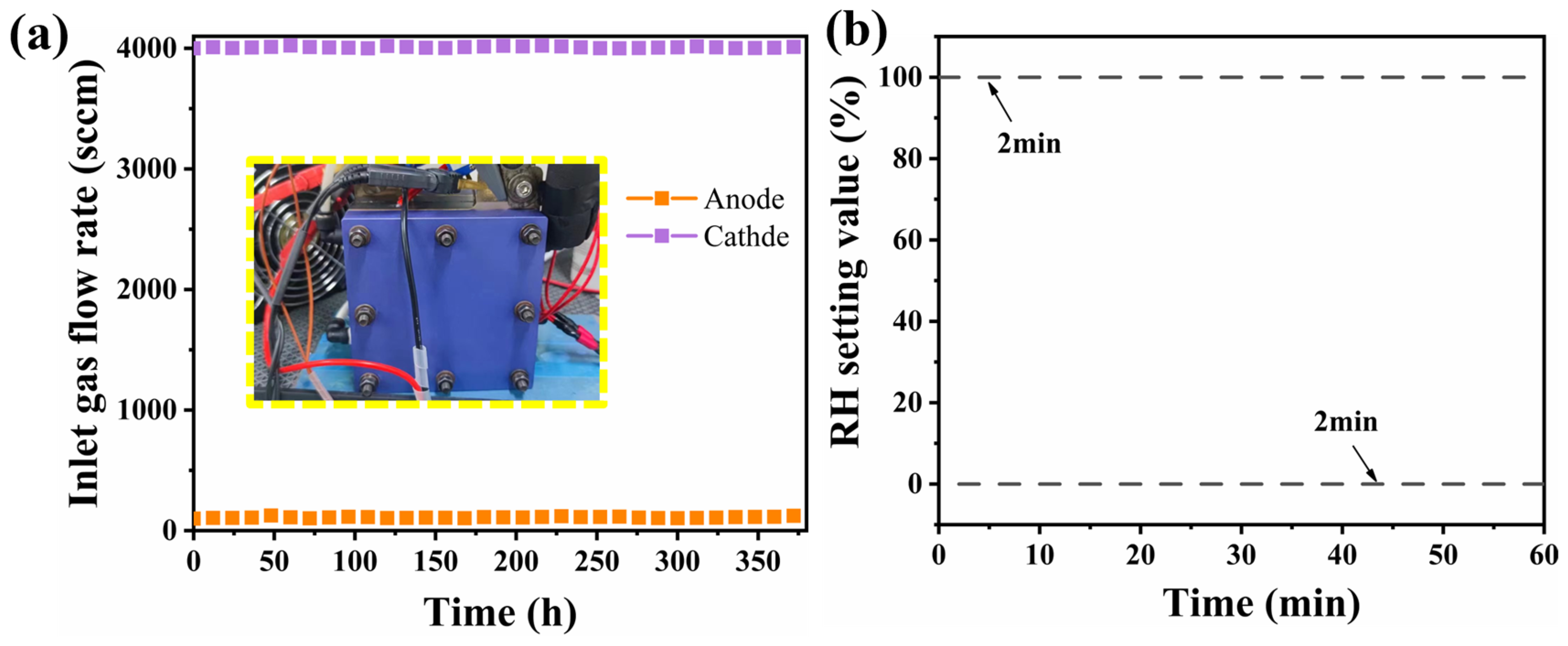
2.2. Electrochemical Measurements
2.3. Structural Characterization
3. Results and Discussion
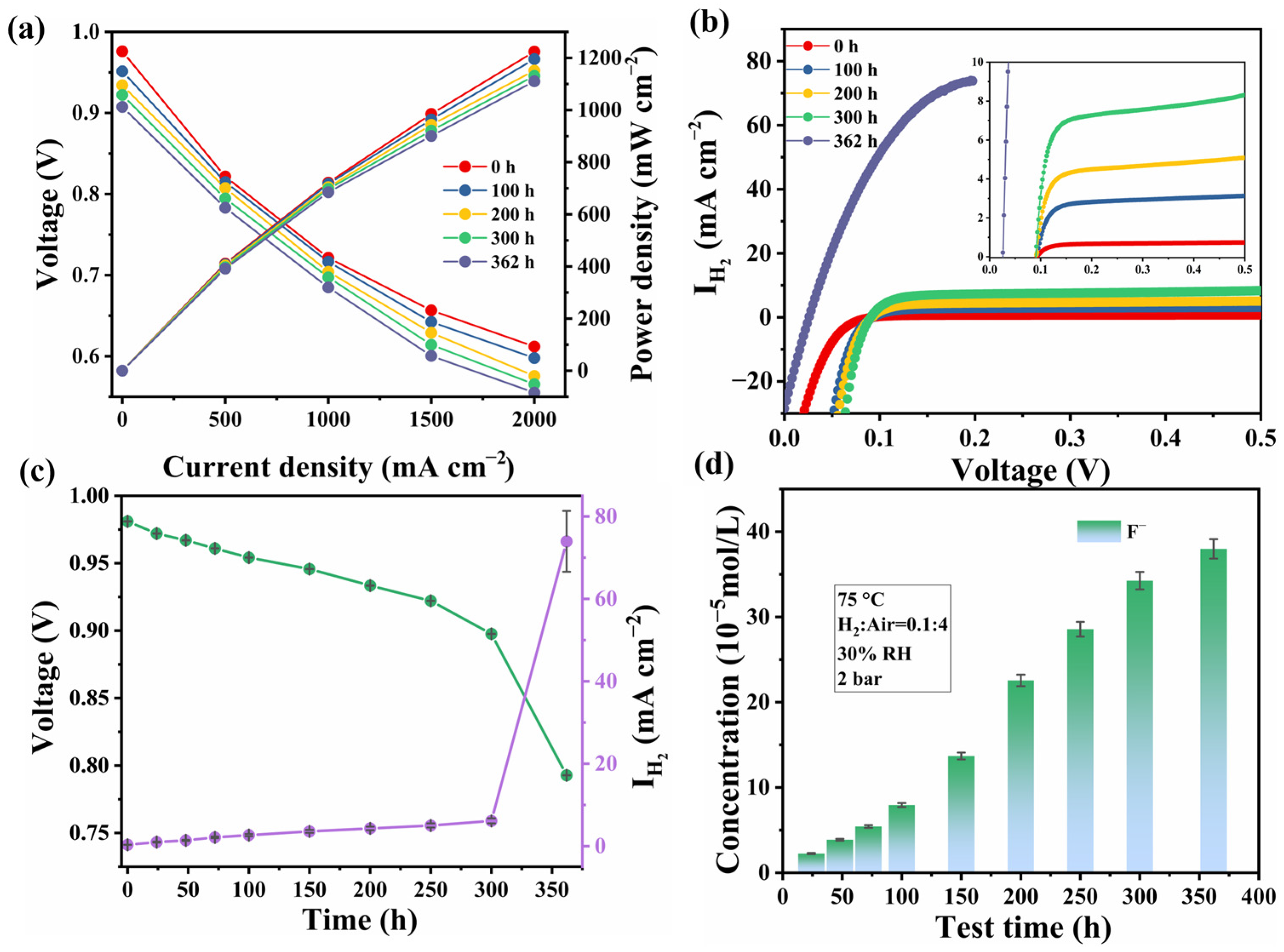
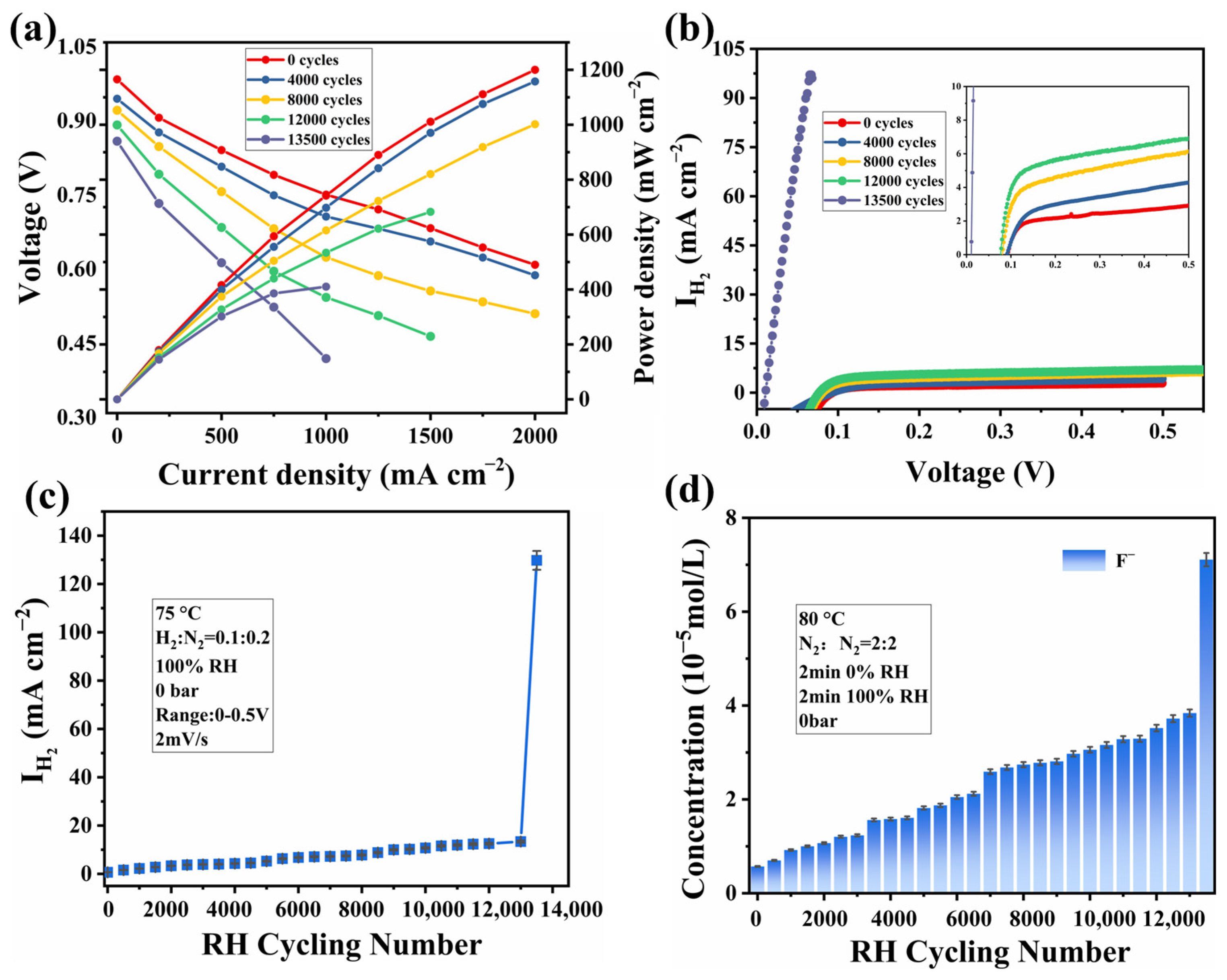
3.1. Electrochemical Performance Analysis
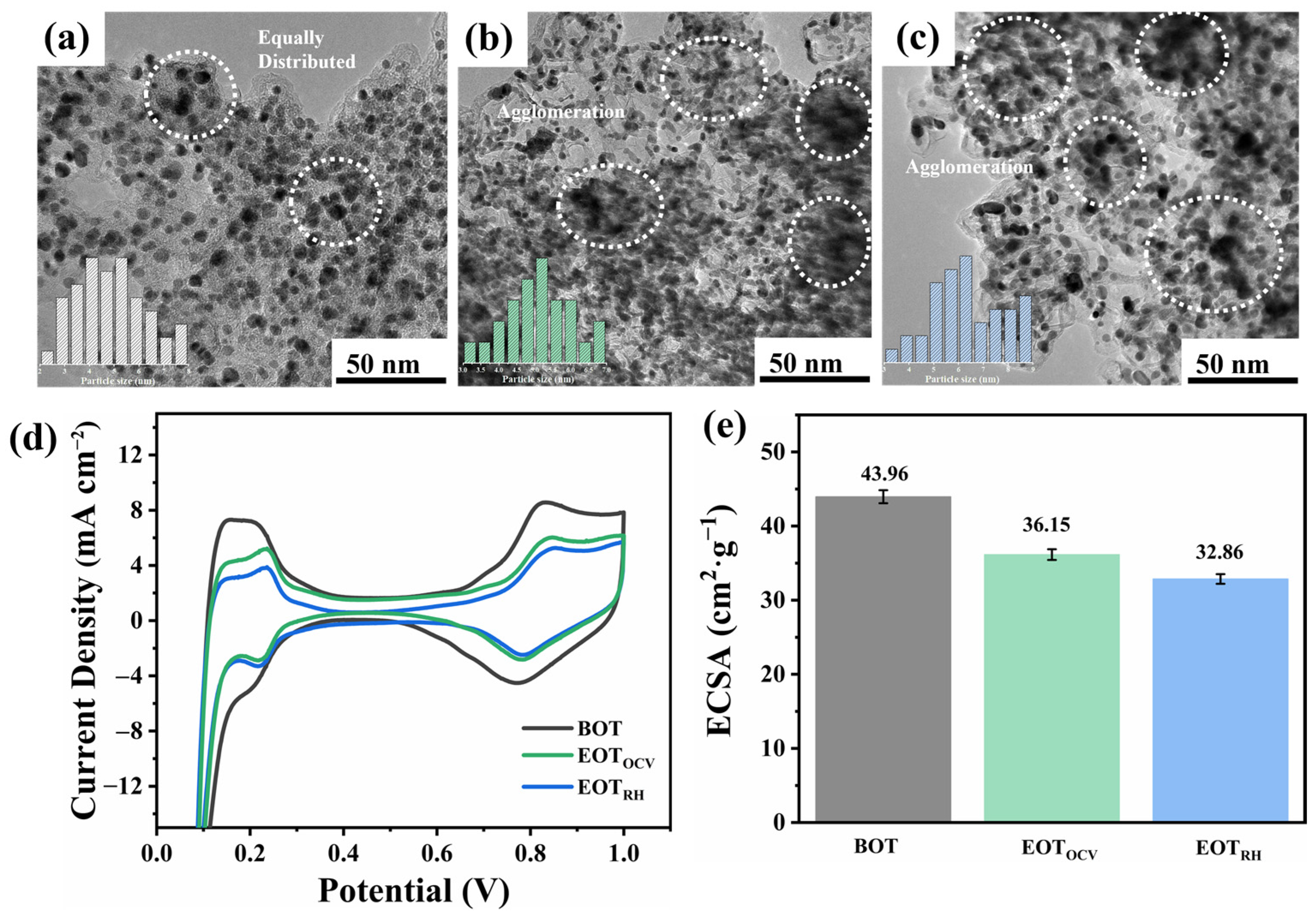
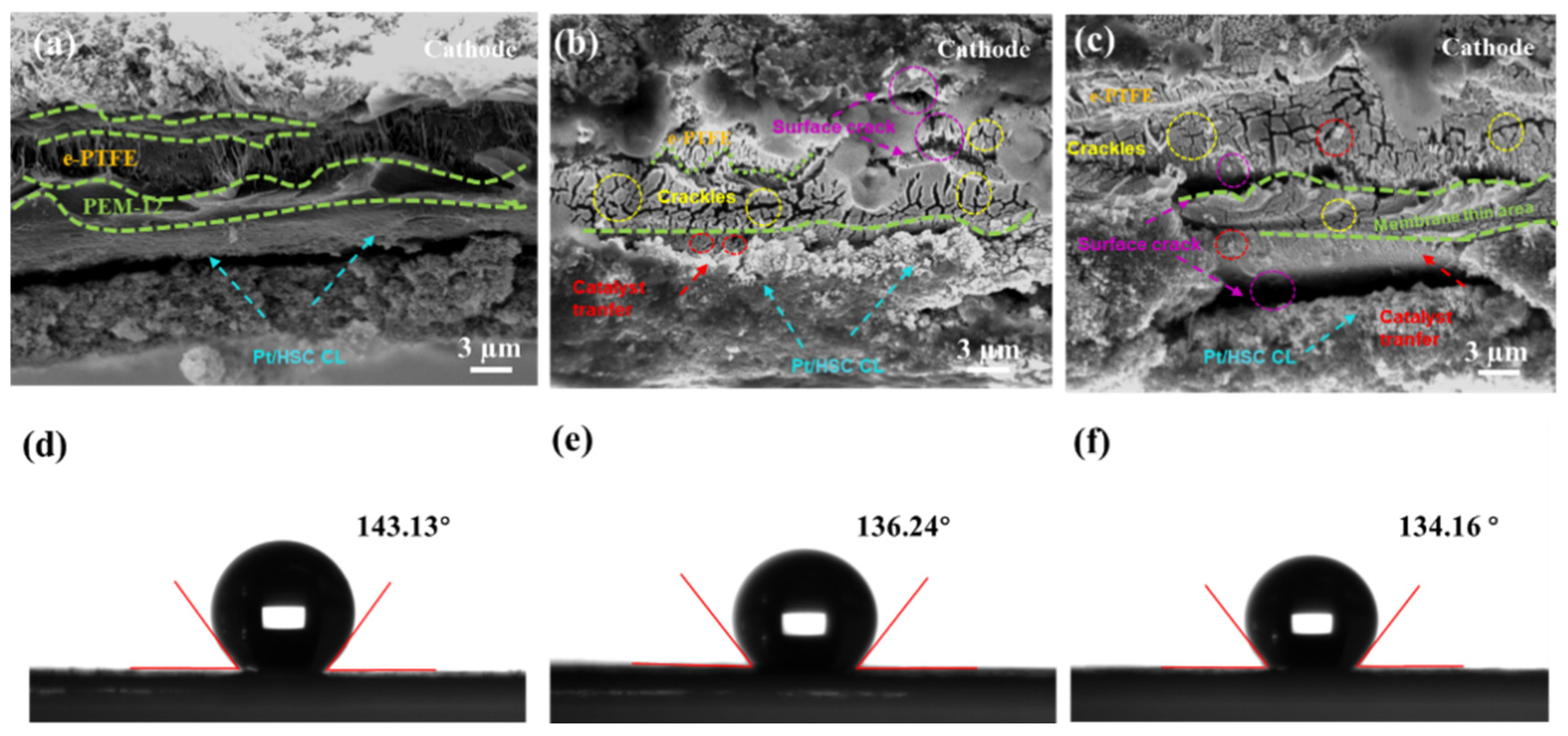
3.2. Degradation Behavior Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stariha, S.; Macauley, N.; Sneed, B.T.; Langlois, D.; More, K.L.; Mukundan, R.; Borup, R.L. Recent Advances in Catalyst Accelerated Stress Tests for Polymer Electrolyte Membrane Fuel Cells. J. Electrochem. Soc. 2018, 165, 492–501. [Google Scholar] [CrossRef]
- Choi, S.R.; Lim, M.; Kim, D.Y.; An, W.Y.; Lee, S.W.; Choi, S.; Bae, S.J.; Yim, S.-D.; Park, J.-Y. Life prediction of membrane electrode assembly through load and potential cycling accelerated degradation testing in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2022, 47, 17379–17392. [Google Scholar] [CrossRef]
- Qu, E.; Hao, X.; Xiao, M.; Han, D.; Huang, S.; Huang, Z.; Wang, S.; Meng, Y. Proton exchange membranes for high temperature proton exchange membrane fuel cells: Challenges and perspectives. J. Power Sources 2022, 533, 231386. [Google Scholar] [CrossRef]
- Qiu, D.; Peng, L.; Liang, P.; Yi, P.; Lai, X. Mechanical degradation of proton exchange membrane along the MEA frame in proton exchange membrane fuel cells. Energy 2018, 165, 210–222. [Google Scholar] [CrossRef]
- Huang, X.; Russo, C.; Zou, Y.; Solasi, R.; Reifsnider, K.; Condit, D. Degradation and Failure Mechanisms of PEMFC Membrane Electrode Assembly under RH Cycling Conditions. ECS Meet. Abstr. 2007, 1, 402. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Yamazaki, S.; Yasuda, K. Yasuda Electrocatalysts for PEM Fuel Cells. Adv. Energy Mater. 2019, 9, 1801284. [Google Scholar] [CrossRef]
- Liu, S.; Rasinski, M.; Rahim, Y.; Zhang, S.; Wippermann, K.; Reimer, U.; Lehnert, W. Influence of operating conditions on the degradation mechanism in high-temperature polymer electrolyte fuel cells. J. Power Sources 2019, 439, 227090. [Google Scholar] [CrossRef]
- Pei, P.; Wu, Z.; Li, Y.; Jia, X.; Chen, D.; Huang, S. Improved methods to measure hydrogen crossover current in proton exchange membrane fuel cell. Appl. Energy 2018, 215, 338–347. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, L.; Hu, Z.; Zhao, Y.; Li, J.; Ouyang, M. Dynamic modeling of chemical membrane degradation in polymer electrolyte membrane fuel cells: Effect of precipitated Pt particles. Int. J. Hydrogen Energy 2024, 56, 1111–1119. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, L.; Hu, Z.; Zhao, Y.; Li, J.; Ouyang, M. Dynamic modeling of Pt degradation and mitigation strategies in polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2022, 12, 100171. [Google Scholar] [CrossRef]
- Wang, M.; Park, J.H.; Kabir, S.; Neyerlin, K.C.; Kariuki, N.N.; Lv, H.; Stamenkovic, V.R.; Myers, D.J.; Ulsh, M.; Mauger, S.A. Impact of Catalyst Ink Dispersing Methodology on Fuel Cell Performance Using in-Situ X-ray Scattering. ACS Appl. Energy Mater. 2019, 2, 6417–6427. [Google Scholar] [CrossRef]
- Yang, B.; Xiang, Z. Affiliation Nanostructure Engineering of Cathode Layers in Proton Exchange Membrane Fuel Cells: From Catalysts to Membrane Electrode Assembly. ACS Nano 2020, 5, 100075. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, S.; Zhong, Q.; Jin, A.; Pan, M. Mechanism of improving oxygen transport resistance of polytetrafluoroethylene in catalyst layer for polymer electrolyte fuel cells. Int. J. Hydrogen Energy 2018, 43, 7456–7464. [Google Scholar] [CrossRef]
- Fan, L.; Zhao, J.; Luo, X.; Tu, Z. Comparison of the performance and degradation mechanism of PEMFC with Pt/C and Pt black catalyst. Int. J. Hydrogen Energy 2022, 47, 5418–5428. [Google Scholar] [CrossRef]
- Chen, Z.; Zuo, W.; Zhou, K.; Li, Q.; Huang, Y.; Jiaqiang, E. Multi-factor impact mechanism on the performance of high temperature proton exchange membrane fuel cell. Energy 2023, 278, 127982. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Tang, F.; Li, B.; Ming, P.; Zhang, C. Failure behavior of gas diffusion layer in proton exchange membrane fuel cells. J. Power Sources 2021, 515, 230655. [Google Scholar] [CrossRef]
- Colombo, E.; Baricci, A.; Mora, D.; Guetaz, L.; Casalegno, A. An innovative accelerated stress test representative of automotive PEMFC degradation mechanisms validated on 1000 hours real-world operation. J. Power Sources 2023, 580, 233376. [Google Scholar] [CrossRef]
- Choi, D. Three-Dimensional Modeling and Numerical Analysis for PEM Fuel Cells. ACS Sustain. Chem. Eng. 2023, 11, 063. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, S.; Guo, S. High-Potential Control for Durability Improvement of the Vehicle Fuel Cell System Based on Oxygen Partial Pressure Regulation under Low-Load Conditions. Int. J. Hydrogen Energy 2022, 47, 32607–32627. [Google Scholar] [CrossRef]
- Eskin, M.G.; Yeşilyurt, S. Anode Bleeding Experiments to Improve the Performance and Durability of Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2019, 44, 11047–11056. [Google Scholar] [CrossRef]
- Li, Y.; Pei PMa, Z.; Ren, P.; Huang, H. Analysis of Air Compression, Progress of Compressor and Control for Optimal Energy Efficiency in Proton Exchange Membrane Fuel Cell. Renew. Sustain. Energy Rev. 2020, 133, 110304. [Google Scholar] [CrossRef]
- Lapicque, F.; Belhadj, M.; Bonnet, C.; Pauchet, J.; Thomas, Y. A Critical Review on Gas Diffusion Micro and Macroporous Layers Degradations for Improved Membrane Fuel Cell Durability. J. Power Sources 2016, 336, 40–53. [Google Scholar] [CrossRef]
- Sun, X.; Xu, H.; Zhu, Q.; Lu, L.; Zhao, H. Synthesis of Nafion®-Stabilized Pt Nanoparticles to Improve the Durability of Proton Exchange Membrane Fuel Cell. J. Energy Chem. 2015, 24, 359–365. [Google Scholar] [CrossRef]
- Wang, M.; Rome, G.; Medina, S.; Pfeilsticker, J.R.; Kang, Z.; Pylypenko, S.; Ulsh, M.; Bender, G. Impact of electrode thick spot irregularities on polymer electrolyte membrane fuel cell initial performance. J. Power Sources 2020, 466, 228344. [Google Scholar] [CrossRef]
- Xiao, Y.; Li, X.; Wang, Q.; Yang, Y.; Li, B.; Ming, P.; Zhang, C.; Dai, H. A Super Uniform Hydrophobic Gas Diffusion Layer for a Proton Exchange Membrane Fuel Cell. ACS Appl. Mater. 2023, 15, 38090–38099. [Google Scholar] [CrossRef]
- Xu, K.; Di, Q.; Sun, F.; Chen, M.; Wang, H. Degradation mechanism analysis of substrate and microporous layer of gas diffusion layer in proton exchange membrane fuel cell. Fuel 2024, 358, 130198. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Huang, P.; Meng, C.; Tian, J. Comparative study on carbon corrosion characteristics of gas diffusion layer in PEMFCs under two accelerated stress tests. Int. J. Electrochem. Sci. 2024, 19, 100581. [Google Scholar] [CrossRef]
- Sim, J.; Chun, H.; Chang, J.; Kim, J.; Kim, B.; Kim, Y.; Kang, S.; Min, K. Impacts of characteristics of carbon black comprising microporous layer on performance and durability of gas diffusion layer under newly developed accelerated stress tests. J. Power Sources 2024, 594, 233989. [Google Scholar] [CrossRef]
- Sim, J.; Kang, M.; Oh, H.; Lee, E.; Min, K. The effect of gas diffusion layer on electrochemical effective reaction area of catalyst layer and water discharge capability. Renew. Energy 2022, 197, 932–942. [Google Scholar] [CrossRef]
- Sim, J.; Kang, M.; Min, K.; Lee, E.; Young, J. Effects of microporous layer penetration ratio and substrate carbonization temperature on the performance of proton exchange membrane fuel cells. J. Mech. Sci. Technol. 2022, 36, 4825–4838. [Google Scholar] [CrossRef]
- Meyer, Q.; Pivac, I.; Barbir, F.; Zhao, C. Detection of oxygen starvation during carbon corrosion in proton exchange membrane fuel cells using low-frequency electrochemical impedance spectroscopy. J. Power Sources 2020, 470, 228285. [Google Scholar] [CrossRef]
- Yao, Z.; Zhou, F.; Tu, C.; Tan, J.; Pan, M. Decay behaviour of ultrathin reinforced membranes in PEMFCs subjected to the combination of mechanical/chemical accelerated stress testing. Int. J. Hydrogen Energy 2024, 50, 200–208. [Google Scholar] [CrossRef]
- Zheng, W.; Xu, L.; Hu, Z.; Ding, Y.; Li, J.; Ouyang, M. Dynamic modeling of chemical membrane degradation in polymer electrolyte fuel cells: Effect of pinhole formation. J. Power Sources 2021, 487, 229367. [Google Scholar] [CrossRef]
- Ham, K.; Chung, S.; Lee, J. A Comprehensive Review of PEMFC Durability Test Protocol of Pt Catalyst and MEA. Appl. Chem. Eng. 2019, 30, 659–666. [Google Scholar] [CrossRef]
- Prithi, J.; Vedarajan, R.; Rao, G.R.; Rajalakshmi, N. Functionalization of carbons for Pt electrocatalyst in PEMFC. Int. J. Hydrogen Energy 2021, 2, 186. [Google Scholar] [CrossRef]
- Liu, H.; Wang, X.; Lao, K.; Wen, L.; Huang, M.; Liu, J.; Hu, T.; Hu, B.; Xie, S.; Li, S.; et al. Optimizing Ionomer Distribution in Anode Catalyst Layer for Stable Proton Exchange Membrane Water Electrolysis. Adv. Mater. 2024, 36, 2402780. [Google Scholar] [CrossRef]
- Wang, M.; Medina, S.; Ochoa-Lozano, J.; Mauger, S.; Pylypenko, S.; Ulsh, M.; Bender, G. Visualization, understanding, and mitigation of process-induced-membrane irregularities in gas diffusion electrode-based polymer electrolyte membrane fuel cells. Int. J. Hydrogen Energy 2021, 46, 14699–14712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, K.; Wang, L.; Wang, X.; Xue, X.; Li, S.; Zhang, H.; Li, Z.; Li, Y.; Peng, G.; Wang, M.; et al. Key Components Degradation in Proton Exchange Membrane Fuel Cells: Unraveling Mechanisms through Accelerated Durability Testing. Processes 2024, 12, 1983. https://doi.org/10.3390/pr12091983
Yao K, Wang L, Wang X, Xue X, Li S, Zhang H, Li Z, Li Y, Peng G, Wang M, et al. Key Components Degradation in Proton Exchange Membrane Fuel Cells: Unraveling Mechanisms through Accelerated Durability Testing. Processes. 2024; 12(9):1983. https://doi.org/10.3390/pr12091983
Chicago/Turabian StyleYao, Keguang, Li Wang, Xin Wang, Xiaowu Xue, Shuai Li, Hanwen Zhang, Zhengnan Li, Yanpu Li, Gangping Peng, Min Wang, and et al. 2024. "Key Components Degradation in Proton Exchange Membrane Fuel Cells: Unraveling Mechanisms through Accelerated Durability Testing" Processes 12, no. 9: 1983. https://doi.org/10.3390/pr12091983
APA StyleYao, K., Wang, L., Wang, X., Xue, X., Li, S., Zhang, H., Li, Z., Li, Y., Peng, G., Wang, M., & Wang, H. (2024). Key Components Degradation in Proton Exchange Membrane Fuel Cells: Unraveling Mechanisms through Accelerated Durability Testing. Processes, 12(9), 1983. https://doi.org/10.3390/pr12091983




