Abstract
Water-based fracturing fluid has recently garnered increasing attention as an alternative oilfield working fluid for propagating reservoir fractures and transporting sand. However, the low temperature resistance and stability of water-based fracturing fluid is a significant limitation, restricting the fracture propagation and gravel transport. To effectively ameliorate the temperature resistance and sand-carrying capacity, a modified cross-linker with properties adaptable to varying reservoir conditions and functional groups was synthesized and chemically characterized. Meanwhile, a multifunctional collaborative progressive evaluation device was developed to investigate the rheology and sand-carrying capacity of fracturing fluid. Utilizing molecular dynamics simulations, the thickening mechanism of the modified cross-linker and the sand-carrying mechanism of the fracturing fluid were elucidated. Results indicate that the designed cross-linker provided a high viscosity stability of 130 mPa·s and an excellent sand-carrying capacity of 15 cm2 at 0.3 wt% cross-linker content. Additionally, increasing reservoir pressure exhibited enhanced thickening and sand-carrying capacities. However, a significant inverse relationship was observed between reservoir temperature and sand-carrying capacity, attributed to changes in the drag coefficient and thickener adsorption. These results verified the effectiveness of the cross-linker in enhancing fluid viscosity and sand-carrying capacity as a modified cross-linker for water-based fracturing fluid.
1. Introduction
Energy shortages resulting from economic development are regarded as a major impediment to social progress and human civilization [1,2,3]. The extraction of conventional fossil energy sources, including oil, natural gas, and coal, has become increasingly difficult and cumbersome due to uncontrolled mining in the early stages [4,5]. Energy engineers are gradually shifting energy substitution to two hot areas. (1) Clean energy such as hydrogen, wind, and solar energy to replace conventional fossil energy [6,7,8]. This can not only effectively make up for the shortage of fossil energy, but also effectively improve the greenhouse effect due to the use of clean energy. However, the limitations of energy storage equipment and the backwardness of energy collection technology prevent clean energy from being used on a large scale and replacing fossil fuels [9,10,11]. (2) Unconventional energy and natural gas are replacing conventional fossil fuels, not only addressing the limitations of conventional energy but also promoting economic development [12,13,14]. Nevertheless, conventional energy reservoir transformation measures, including fracturing technology, acidizing and plugging removal techniques, and heavy oil viscosity reduction methods [15,16], are completely unsuitable for the unique environment and geological characteristics of unconventional reservoirs. As the most commonly used reservoir transformation measure for conventional oil reservoirs, water-based fracturing technology effectively achieves fracture expansion and enhances crude oil recovery [17,18]. The gel-like substance formed by guar gum, cross-linker, and water in the water-based fracturing fluid will become thinner due to the influence of reservoir temperature, which hinders the excellent performance of water-based fracturing fluid in fracturing cracks [19]. Numerous research results show that the temperature resistance of water-based fracturing fluid has become an important reason for the reduction of fracturing performance, and the high-temperature environment of shale reservoirs poses a great challenge to the high viscosity stability of water-based fracturing fluid [20]. The high temperature of shale reservoirs may cause conventional water-based fracturing fluids to rapidly break the hydrogen bonds formed between guar gum and water molecules at the microscopic level, resulting in a significant reduction in fluid viscosity [21,22]. Additionally, the sand-carrying ability of fracturing fluids, hindered by extremely low apparent viscosity, has been considered as a critical factor limiting the application of water-based fracturing fluids in unconventional reservoirs [23,24].
An important solution for water-based fracturing fluid to meet the fracturing needs of shale reservoir is to choose an effective cross-linker, which can more strongly connect guar gum and water molecules with chemical bonds [25,26]. Organoboron, organozirconium, and organotitanium are currently important cross-linkers that effectively enhance the performance of water-based fracturing fluids, leading to increased apparent viscosity and stability [27,28,29]. However, the extremely low price, low residue, and excellent viscoelasticity of organic boron make it suitable for fracturing operations in medium and low-temperature reservoirs up to 120 °C [30]. In addition, the high temperatures of shale reservoirs cause the fracturing fluid to break down easily and exhibit low stability, hindering the use of organic boron in reservoir exploitation [31]. Although organic zirconium is capable of adapting to higher-temperature reservoir environments in comparison to organic boron, the viscosity of a water-based fracturing fluid containing organic zirconium will decrease significantly at 160 °C [32]. Organic titanium can achieve water-based fracturing in acidic environments and exhibits better apparent viscosity and fluid stability compared to organic zirconium and organic boron cross-linker [33]. Nevertheless, the large usage amount and weak sand-carrying capacity are important reasons why organic titanium cross-linking agents continue to be analyzed and studied.
Herein, a water-based fracturing fluid performance evaluation device that can realize multifunctional collaborative measurement is established and used to explore the phase behavior, apparent viscosity, rheological parameters, and sand-carrying capacity of fracturing fluid in shale reservoirs. Moreover, a modified organic titanium cross-linker containing numerous active groups was synthesized and characterized, which not only improves the rheological properties of water-based fracturing fluids but also enhances sand-carrying capacity. In addition, the effects of cross-linker characteristics and reservoir conditions (temperature, pressure, and shear rate) on the viscosity and sand-carrying performance of water-based fracturing fluids were also analyzed, and the sand-carrying mechanism of the improved organic titanium in fracturing fluid is also revealed in detail. The water-based fracturing fluid, which contains synthetic cross-linkers, exhibits heat and shear resistance, thereby enabling it to adapt more effectively to the fracturing exploitation of unconventional high-temperature shale reservoirs. Furthermore, it can effectively avoid the change of fracturing fluid viscosity caused by the high-temperature environment of shale. Concurrently, the enhanced sand-carrying capacity of the water-based fracturing fluid can effectively reinforce the fissures within shale reservoirs, facilitating the influx of a considerable volume of reservoir crude oil into the production well via the reinforced shale fissures, thereby enhancing the recovery rate of crude oil. This work can provide the most basic data support and theoretical basis for the application of water-based fracturing fluids in energy extraction from unconventional reservoirs.
2. Materials and Methods
2.1. Chemicals and Device
Chemicals: All chemicals were analytically pure before use and could be used to synthesize cross-linkers without any purification. Titanium tetrachloride, ethanol, hydrochloric acid, sodium silicate, and γ-aminopropyltrimethoxysilane were purchased from Xilong Chemical Co., Ltd. (Shantou, Guangdong, China). Xylene and Nano Silica were obtained from Aladdin China Chemical Reagent Co., Ltd. (Shanghai, China) Deionized water was homemade in the laboratory. In addition, 4 mm of quartz sand is a significant raw material for proppant particles, and its pertinent physical and chemical properties are presented in Table 1.

Table 1.
Physical and chemical properties of proppants for fracturing.
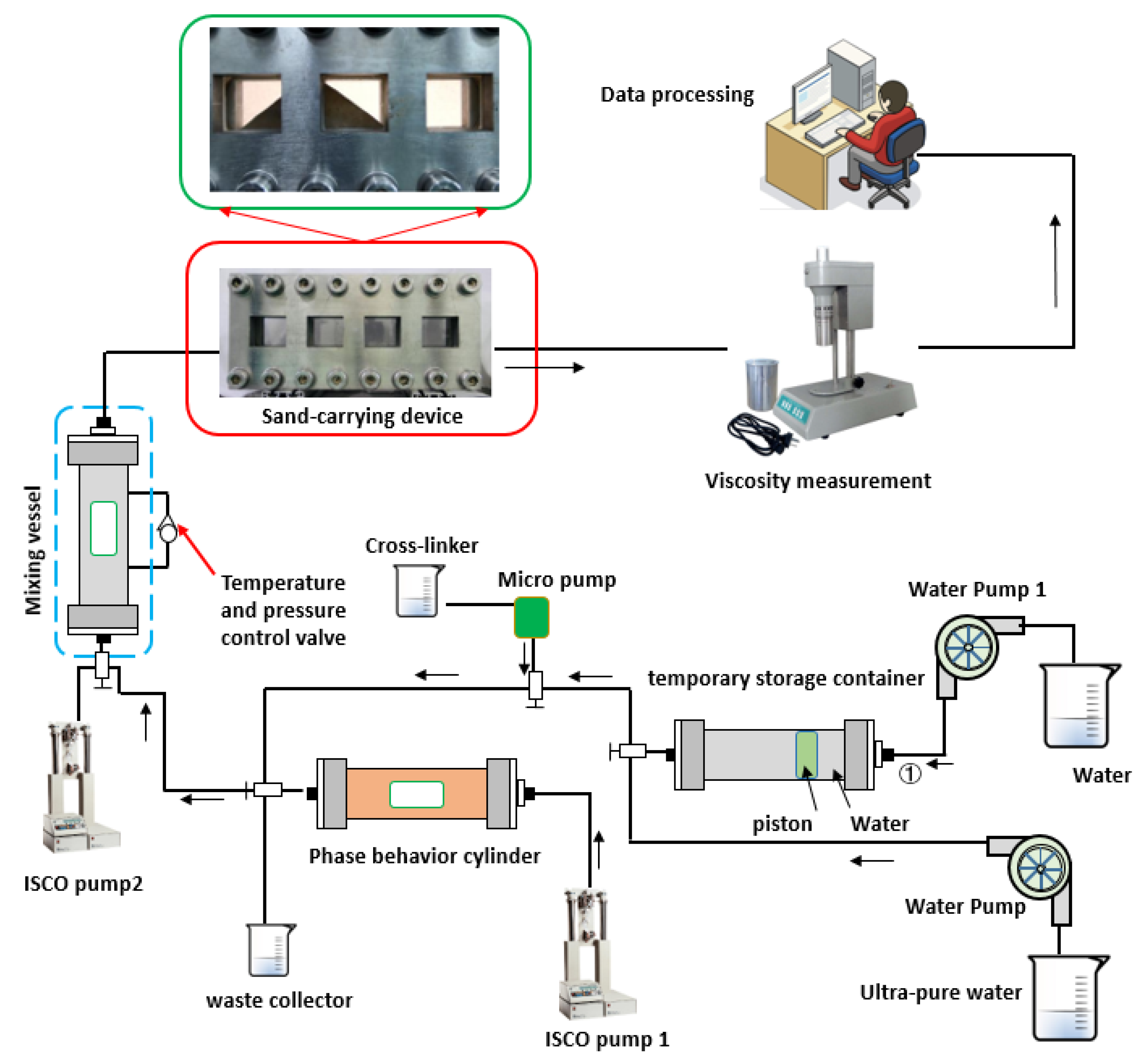
Apparatus: A collaborative evaluation device that can measure multiple properties of water-based fracturing fluid was designed and assembled by us (Figure 1). A collaborative evaluation device capable of measuring multiple properties of water-based fracturing fluid was designed and assembled in-house. This device can evaluate various performance metrics of water-based fracturing fluids, including stirring, phase behavior, solubility, apparent viscosity, sand-carrying capacity, and friction resistance. Additionally, the measurement data can be analyzed and screened using artificial intelligence.
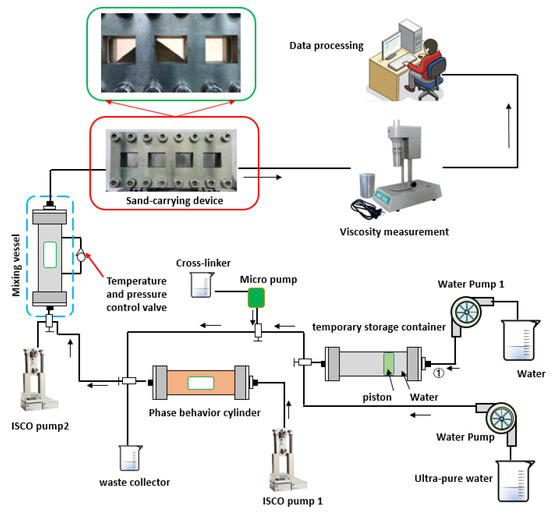
Figure 1.
Collaborative evaluation device of water-based fracturing fluid performance.
2.2. Design and Synthesis of Titanium Cross-Linker
First, 3.5 g of γ-aminopropyltrimethoxysilane and 45 g of sodium silicate solution (35%) were poured into a three-necked flask containing 40 mL of deionized water and 50 mL of ethanol. Simultaneously, hydrochloric acid was then added dropwise to the flask to adjust the pH to 9. The solid particles were washed after reacting at 60 °C for 3 h. Immediately, 20 mL of xylene, 15 g of surface-modified nano-silica and 5 g of titanium tetrachloride were added into a 250 mL three-necked flask and reacted for 5 h at 100 °C. The prepared nano titanium cross-linker is a white granular solid with a few protrusions on the surface, and the synthesis process (Figure 2a) and chemical characterization (Figure 2b) are shown in Figure 2.
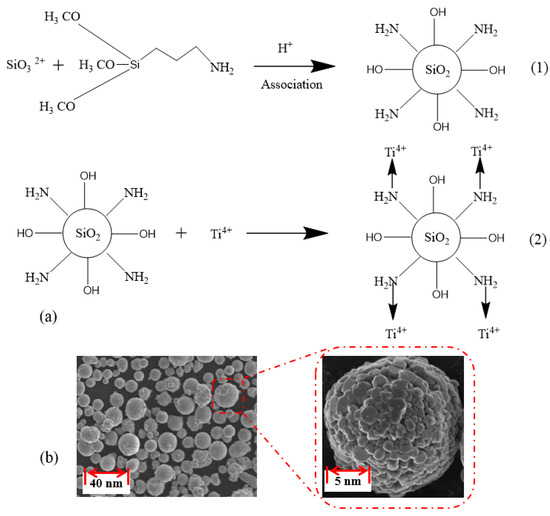
Figure 2.
Synthesis process (a) and chemical characterization (b) of nano-titanium cross-linker.
2.3. Assembly and Viscosity Calculation of Water-Based Fracturing Fluid
A small amount of nano-titanium cross-linker was poured into a beaker (250 mL) containing 30 mL of deionized water and stirred at 500 r/s for 3 min. The evenly stirred cross-linking agent solution is pressurized into a pressure-resistant steel cylinder using the device shown in Figure 1. Simultaneously, another pipeline of the pressure-resistant steel cylinder is injected with a fracturing base fluid containing 0.4% displacement aid, 0.01% coagulant, and a pH adjuster. The solubility and phase behavior of the well-stirred water-based fracturing fluid can be evaluated using the device with the pressure-resistant glass skylight. Additionally, the viscosity and rheological parameters of the water-based fracturing fluid can be analyzed using the collaborative evaluation device shown in Figure 1. Moreover, the viscosity calculation of water-based fracturing fluid is evaluated according to SY-T 5107-2005 and SY-T 6074-94 [34,35]. In addition, the temperature change is primarily regulated by controlling the resistance wire surrounding the viscometer and monitoring it in real time through the temperature sensor. The pressure is regulated by sealing and pressurizing the instrument at the viscometer measuring device. Additionally, the shear rate is regulated by changing the rotation speed of the rotary viscometer, and the temperature and pressure at this time are maintained at 20 MPa and 453 K. Each viscosity test time is maintained at 30 s and the experimental conditions are kept constant.
The fracturing fluid is injected into the modified closed sample cup of the rotary viscometer. The pressure of the fracturing fluid changes with the increase of the injection volume. At the same time, the resistance wire around the sample cup controls the temperature of the fracturing fluid at an increase rate of 3 °C/min. In addition, the shear rate is generally changed from the basic value of 170 s−1 to show a gradient decrease or increase to measure the fluid viscosity value at different temperatures and pressures. The shear time of the water-based fracturing fluid is maintained at 30 s for each measurement, and the corresponding data value can be recorded after the shear rate is stable.
The viscosity calculation formula for water-based fracturing fluid is primarily based on Equation (1).
where μ is the viscosity of water-based fracturing fluid, in mPa·s. α presents the pointer data of rotational viscometer. p is the revolutions per minute of rotational viscometer. In addition, 5.077 is considered as the shear stress value when α = 1, and 1.704 is the shear rate at 1 rpm.
2.4. Sand-Carrying Capacity of Water-Based Fracturing Fluid
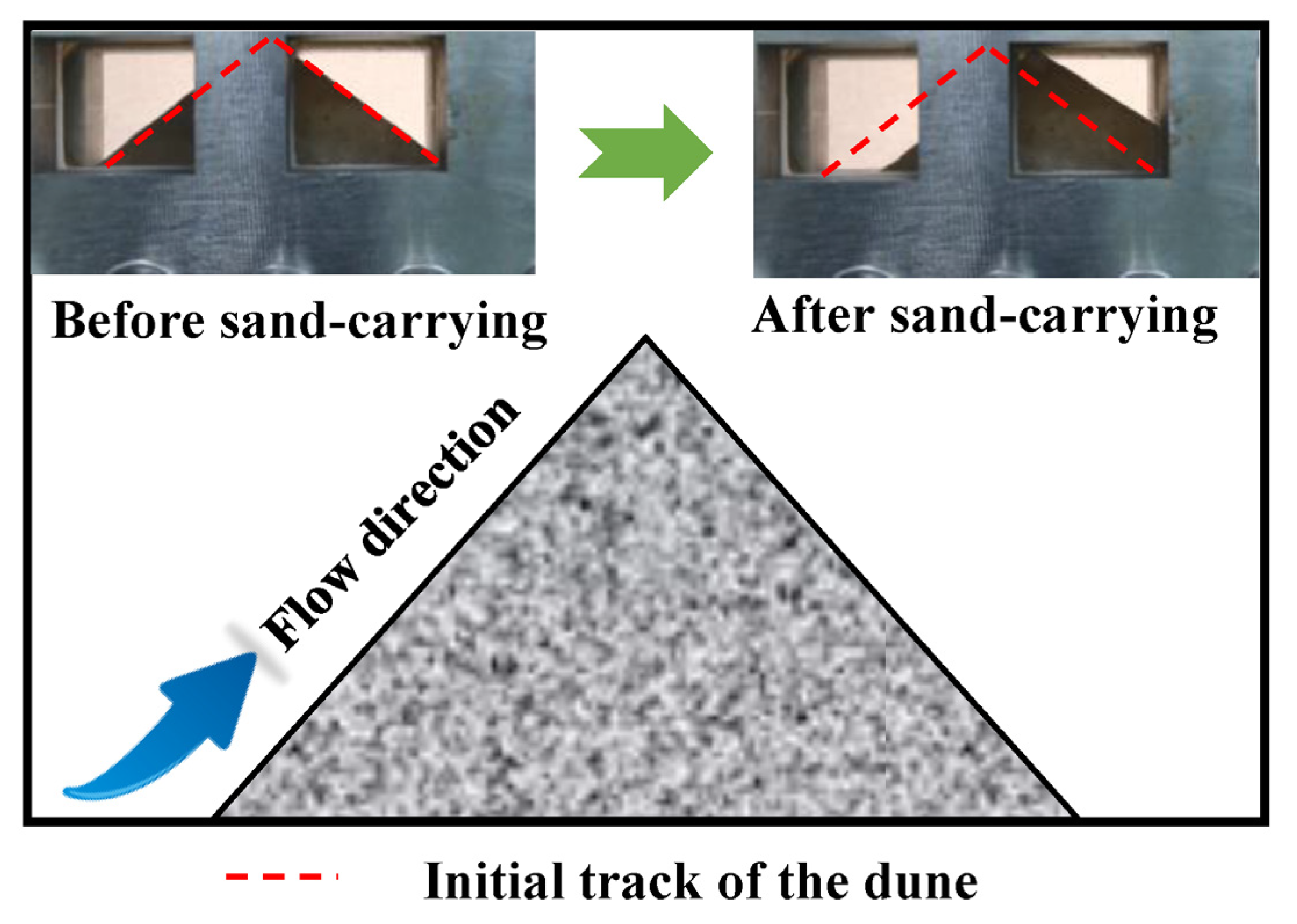
The sand-carrying experimental device for water-based fracturing fluid uses sand as a proppant to simulate the fracturing process. These proppants effectively support reservoir fractures after the fracturing operation. The sand is placed in a pressure-resistant container under normal pressure, and the dunes are then stabilized for 20 min. After the high-pressure water-based fracturing fluid is injected, the sand flows with the fluid, and the sand-carrying process can be observed through the pressure-resistant glass window. The increase in the back area of the dune before and after sand carrying was dedicated to estimate the sand-carrying capacity of water-based fracturing fluid, and the significant area change in the back part of the dune indicates that the water-based fracturing fluid has excellent sand-carrying performance. This excellent sand-carrying capacity displayed important implications for improving the sweep coefficient of fracturing fluid in the underground reservoir.
The pre-pressurized water-based fracturing fluid is injected at a constant flow rate from one side of the sand-carrying device. If the shear rate is to be evaluated, the injection flow rate needs to be changed to maintain a constant temperature and pressure. First, the positive area of the sand pile before carrying the sand is measured. After the fracturing fluid carries the sand for 5 min, the experiment is stopped, and the area change value of the back of the sand pile is measured (Figure 3). The calculation of the sand-carrying performance of water-based fracturing fluid is shown in Equation (2).
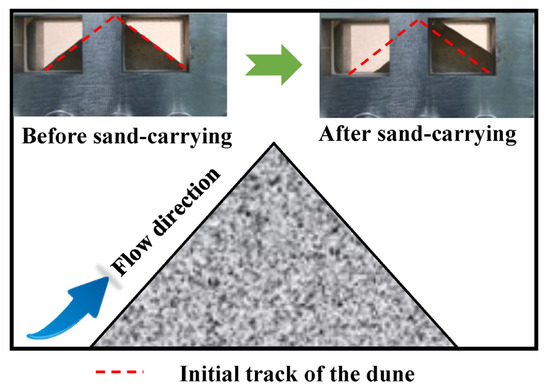
Figure 3.
Sand-carrying experimental device and schematic diagram of water-based fracturing fluid.
In which θ is the sand-carrying capacity of water-based fracturing fluid, in cm2. SBefore and SAfter present the back area of the dune before and after the injection of fracturing fluid respectively, in cm2.
3. Results and Discussion
3.1. Effect of Titanium Cross-Linker Content on the Fluid Viscosity and Sand-Carrying Capacity
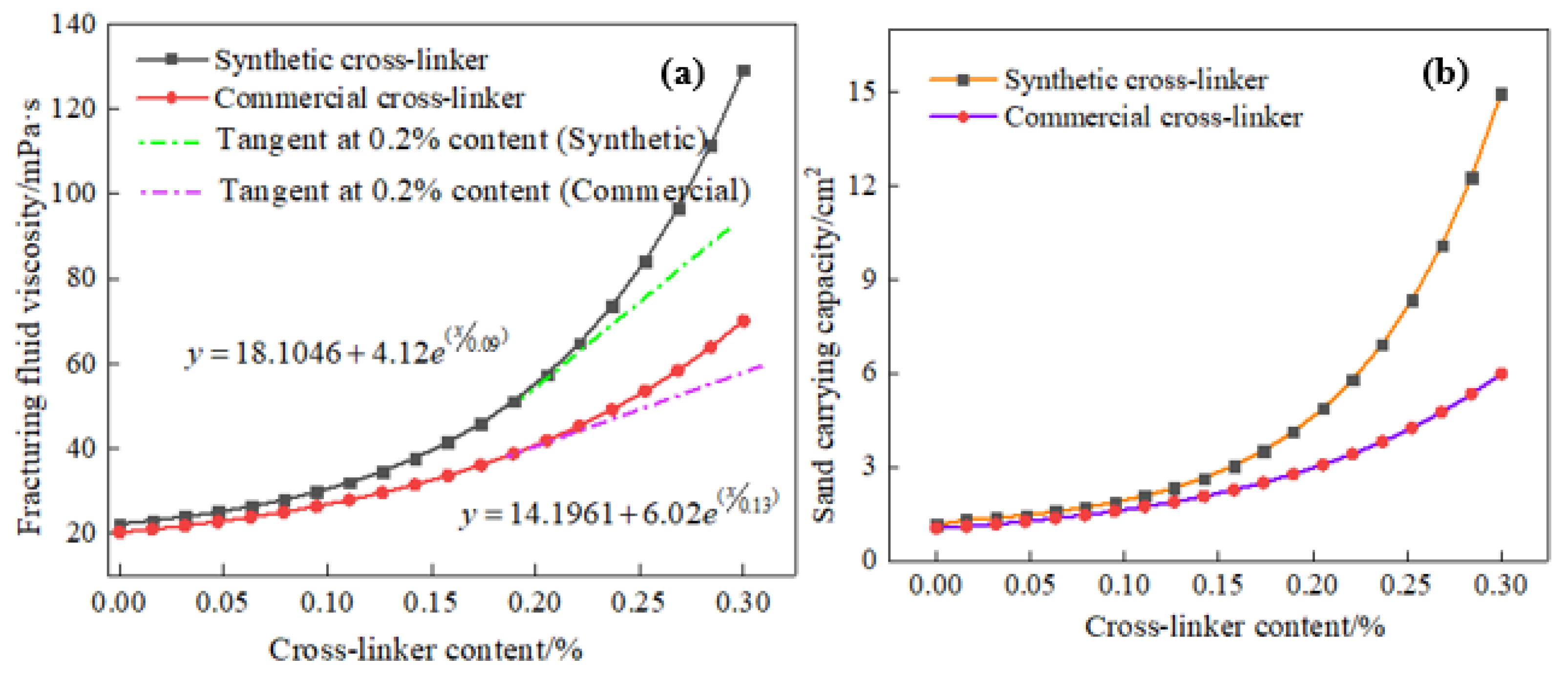
Previous research [31] has examined the influence of cross-linker composition on the viscosity of water-based fracturing fluids, resulting in notable alterations to the properties of the cross-linker. The sand-carrying capacity of water-based fracturing fluids is of paramount importance, as it has the potential to significantly impact a number of crucial aspects related to the proppant’s journey to the reservoir, the dynamics of fracture opening and closing, and ultimately, the oil recovery. Figure 4 displays the effect of cross-linker content on fracturing fluid viscosity and sand-carrying capacity. It can be seen from Figure 4 that fluid viscosity gradually increases with a higher cross-linker content. Conversely, a lower cross-linker content results in a more gradual viscosity increase. In addition, the fluid viscosity is observed to increase significantly when the cross-linker content exceeds 0.20%, and a 0.3% cross-linker (synthetic chemicals) content can increase the viscosity of water-based fracturing fluid to 130 mPa·s, demonstrating the excellent cross-linking ability of the synthetic chemical. However, a commercial cross-linker under the same evaluation conditions showed a poorer crosslinking ability than that of this synthetic cross-linker, and 0.3% of commercially available crosslinking agent can only increase the viscosity of water-based fracturing fluid to 70 mPa·s. The viscosity curves of the two cross-linkers in Figure 4 present that the slope of the synthetic cross-linker’s curve is significantly higher than that of the commercially available cross-linker. Moreover, the relationship between the viscosity of the fracturing fluid and the content of the two cross-linkers can be divided into two distinct phases. When the cross-linking agent content is below 0.20%, the fracturing fluid viscosity exhibits a weaker growth rate. However, when the cross-linking agent content exceeds 0.20%, the fluid viscosity shows a very significant exponential upward trend. A mere 0.3% of commercially available cross-linkers can elevate the viscosity of fracturing fluid to 105 mPa·s at 353 K, while the reservoir temperature of 453 K makes the viscosity of fracturing fluid drop rapidly to 70 mPa·s.
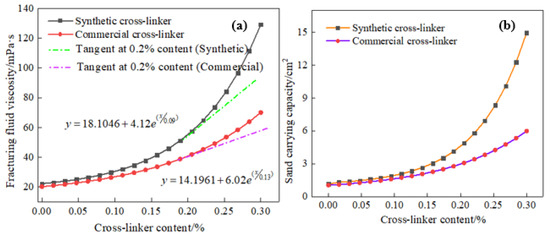
Figure 4.
Effect of cross-linker content on fracturing fluid viscosity and sand-carrying capacity (453 K, 170 s−1 and 20 MPa). (a): fracturing fluid viscosity. (b): sand-carrying capacity.
Figure 4 also shows the relationship between the cross-linker content and the sand-carrying capacity of the fracturing fluid, and an excellent positive correlation is exhibited. Furthermore, the sand-carrying capacity of the fracturing fluid also increases with the fluid viscosity, and numerous gravels can traverse and reach the rear of the sand dune. Both the commercial cross-linker and the synthetic cross-linker exhibited comparable sand-carrying performance for gravel, but the synthetic cross-linker demonstrated a significantly excellent sand-carrying capacity compared to the commercial cross-linker. A 0.3% content of synthetic cross-linker increases the particle area on the back of the dune by 15 cm2, whereas the commercial product increases it by only 6 cm2. The substantial discrepancy in sand-carrying capacity between the two thickeners can be attributed to the analysis of the forces between the fracturing fluid and the particles, as well as the fluid mechanics properties, shown in (Equations (3)–(5)) [36].
where Fd is the drag force of fracturing fluid on particles, in N. CD is a drag coefficient. up and uc represent the shear rate of the fracturing fluid and particle, respectively, in m/s. ρc is the density of the fracturing fluid, in kg/m3. dp is considered as a particle diameter, in m. Rep is a Reynolds number of water-based fracturing fluid. η is the fluid viscosity, in mPa·s.
The theory presented in Equations (1)–(3) clearly demonstrates that the drag force Fd exerted by water-based fracturing fluids on particles is directly proportional to the viscosity of the fluid. Simultaneously, the increase in viscosity of the fracturing fluid facilitates the drag of particles by the fluid, which enables the particles to ascend the sand dune and subsequently reach the opposite side. However, the drag force of water-based fracturing fluid on particles is closely related to the formation, quantity, and bond energy of chemical bonds at a microscopic level (Figure 5). Chemical kinetics suggests that the numerous hydroxyl groups around the nano-titanium cross-linker can interact with the hydroxyl groups in guar gum molecules to form hydrogen bonds [37]. These bonds facilitate the connection between guar gum molecules and the cross-linker, acting as a medium. The microscopic grid formed by hydrogen bonds between molecules of the fracturing fluid can carry particles forward. A smaller content of cross-linker results in an insufficient number of hydrogen bonds and micro-grid density, which, in turn, impedes the migration of particles over long distances with the fracturing fluid. However, a high content of cross-linker facilitates the formation of additional hydrogen bonds with an increased number of guar molecules, thereby contributing to an enhancement in micro-grid density. The grid constructed by chemical bonds between molecules is like a huge fishing net that can drag particles forward. Thus, the cross-linker content affects the viscosity of the fracturing fluid on a macroscopic level to facilitate particle carrying, while on a microscopic level, it primarily increases the drag force of the fracturing fluid on the particles by forming a microscopic grid. Nevertheless, the limited number of hydroxyl groups present in commercially available cross-linker molecules is insufficient to form additional hydrogen bonds with guanidine gum molecules, which markedly reduces the micro-grid density. This reduction in micro-grid density may result in a decrease in macroscopic viscosity and sand-carrying capacity.
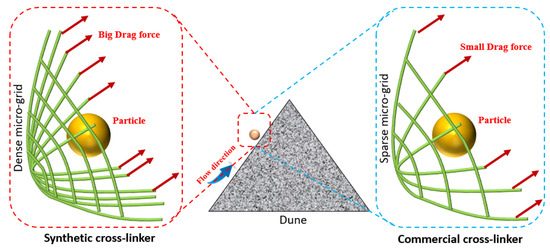
Figure 5.
Micro mechanism of sand carrying in fracturing fluid by the cross-linker type and cross-linker content.
3.2. Effects of Reservoir Temperature on the Fluid Viscosity and Sand-Carrying Capacity
The non-Newtonian fluid characteristics of water-based fracturing fluids [38,39] indicated that reservoir temperature impacts their rheological properties and sand-carrying capacity, and Figure 6 demonstrates the impact of reservoir temperature on fracturing fluid viscosity and sand-carrying capacity. We can conclude from Figure 6 that a very significant reduction in the viscosity of water-based fracturing fluids is demonstrated with an increase in the reservoir temperature, and the effect of reservoir temperature on fracturing fluid viscosity shows a completely different trend from that of cross-linker content. The aforementioned phenomenon indicates that the continuously increasing reservoir temperature is detrimental to the viscosity stability of water-based fracturing fluids, significantly impacting fracture expansion and the improvement of the oil recovery. In addition, a low reservoir temperature can maintain a high viscosity of the fracturing fluid, with a relatively slow decrease in viscosity. Conversely, a high temperature will cause a more rapid decrease in the fluid viscosity than a low temperature. Figure 6, additionally, illustrates that the synthesized cross-linker exhibits enhanced temperature resistance for water-based fracturing fluids in comparison to commercially available cross-linkers. Therefore, 0.3% of commercially available cross-linking agent can make the viscosity of fracturing fluid reach 105 mPa·s at 353 K, while the reservoir temperature of 453 K makes the viscosity of fracturing fluid drop rapidly to 70 mPa·s. Meanwhile, 0.3% of synthetic cross-linker only reduces the viscosity of water-based fracturing fluid from 130 mPa·s at 353 K to 110 mPa·s at 453 K. The smaller viscosity reduction value of the synthetic cross-linker demonstrates its superior temperature resistance to water-based fracturing fluids than commercial cross-linkers.
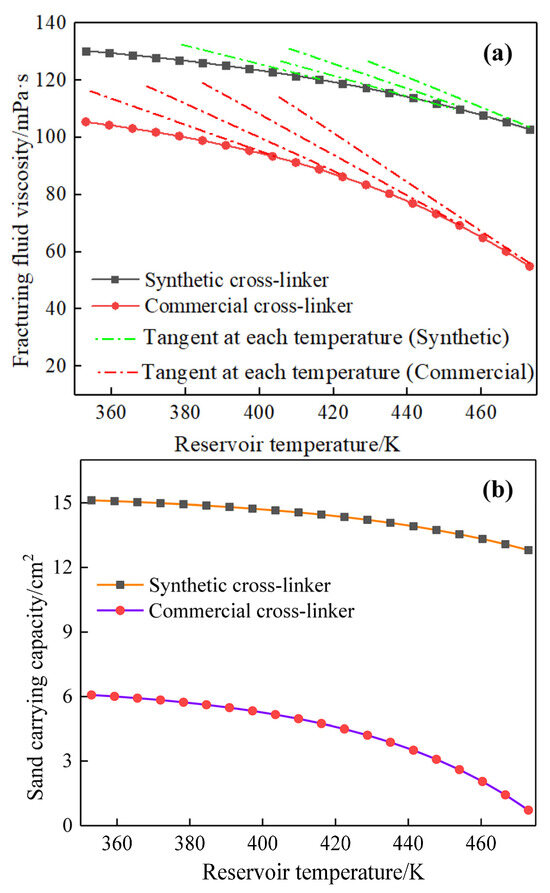
Figure 6.
Effect of reservoir temperature on fracturing fluid viscosity and sand-carrying capacity (0.3% cross-linker content, 170 s−1 and 20 MPa). (a): fracturing fluid viscosity. (b): sand-carrying capacity.
Figure 6b shows that the sand-carrying capacity gradually decreases with the increase in reservoir temperature, and the sand-carrying capacity of water-based fracturing fluid at high temperatures is significantly reduced. A 15.1 cm2 sand-carrying capacity was demonstrated with a water-based fracturing fluid containing 0.3% synthetic cross-linker, but a reservoir temperature of 453 K only showed a 13.6 cm2 sand-carrying capacity. Commercial cross-linkers exhibit weaker sand-carrying capacity for water-based fracturing fluids than synthetic cross-linkers, and an increase in reservoir temperature from 353 K to 453 K has been observed to result in a reduction in the sand-carrying capacity of water-based fracturing fluid containing 0.3% commercial cross-linking agent from 6 cm2 to 2.5 cm2. The data presented in Figure 6a,b demonstrate that the synthetic cross-linker is capable of not only enhancing the temperature resistance of the water-based fracturing fluid, but also of exhibiting an exceptional capacity to transport sand and a versatility that allows for a multitude of potential applications, largely due to the minimal viscosity alteration.
The effect of reservoir temperature on the viscosity and sand-carrying performance of water-based fracturing fluid is mainly evaluated based on the strength and number of microscopic hydrogen bonds between chemical molecules [18]. The density of the microscopic grid constructed by water, cross-linking agent, and guar gum connected by intermolecular hydrogen bonds is affected by many factors, such as molecular spacing, intermolecular repulsion, and reservoir conditions [40]. The reservoir temperature can enhance the activity of individual molecules, leading to conspicuous irregular motion of a significant number of molecules. The increasing molecular activity causes the chemical bonds and microscopic grids to behave as follows. (1) The random motion of molecules and the repulsion between them increase as the reservoir temperature rises, directly leading to an increase in the distance between molecules. The increasing distance between molecules will lengthen the hydrogen bonds, resulting in a decrease in the bond energy of hydrogen bonds [41]. (2) Some molecules that were originally more energetic become larger due to the increase in reservoir temperature, which will cause the hydrogen bonds with smaller bond energy to break due to the separation between molecules. The breaking or lengthening of hydrogen bonds between molecules will result in a gradual decrease in the density of the microscopic grid, and the macroscopic fluid viscosity and sand-carrying capacity will deteriorate significantly due to the reduction of microscopic grid density [42].
Furthermore, the synthesized cross-linker molecules possess a greater number of active groups that are capable of attracting water and guar gum, thereby forming stronger and shorter hydrogen bonds with these substances [43]. The robust hydrogen bonds prevent the micro-grids from undergoing any deformation or rupture as a consequence of an elevated reservoir temperature. However, commercially available cross-linkers that lack active groups cannot form a large number of strong hydrogen bonds with guar gum molecules. Even when these cross-linkers form hydrogen bonds with other molecules, the resulting bonds are long-range chemical bonds with extremely low bond energy. Fracturing fluids containing commercially available cross-linkers either cannot form dense micro-grids, or the weak strength of the formed grids is demonstrated. Thus, the reservoir temperature is primarily regulated by controlling molecular activity, which, in turn, affects the formation of intermolecular hydrogen bonds and micro grid density. This enables the temperature resistance of water-based fracturing fluids to be achieved (Figure 7).
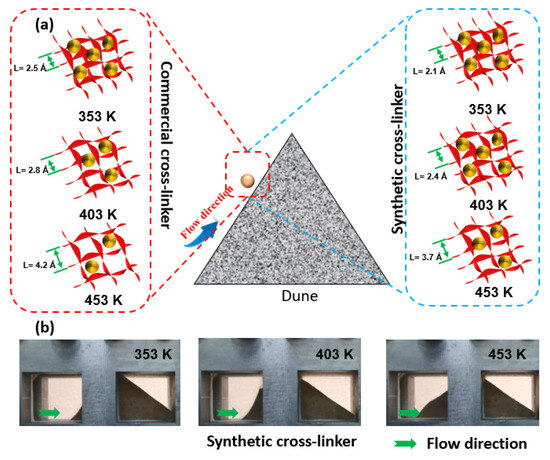
Figure 7.
Microscopic model differences of fracturing fluid at different reservoir temperatures. (a): Microscopic grid variation. (b): sand-carrying capacity of synthetic crosslinker.
3.3. Effects of Reservoir Pressure on the Fluid Viscosity and Sand-Carrying Capacity
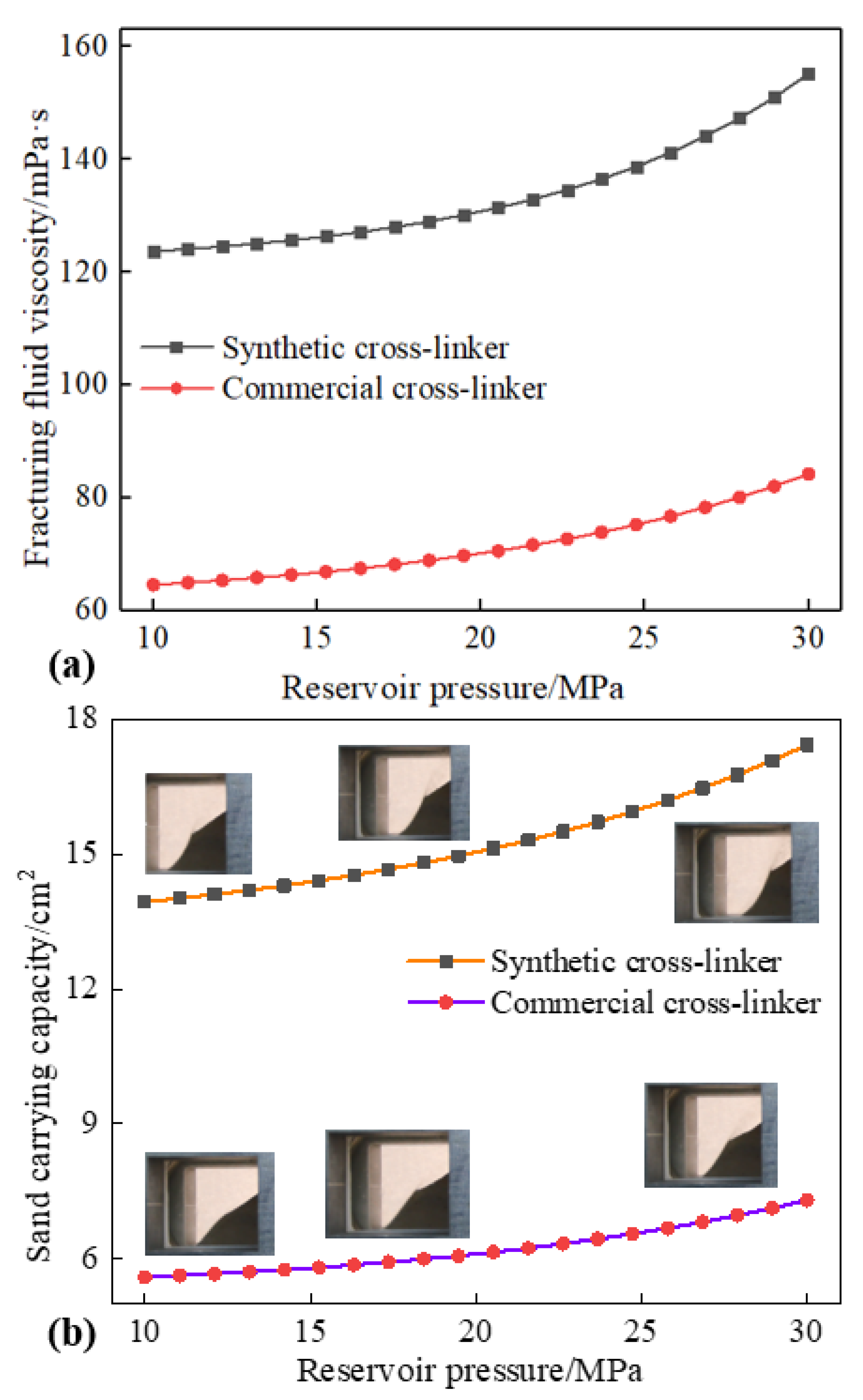
Prior research [44] has indicated that reservoir pressure exerts a considerable influence on the rheology and other characteristics of water-based fracturing fluids. However, there is a paucity of literature examining the sand-carrying capacity of water-based fracturing fluids. Figure 8 shows the effects of reservoir pressure on the fluid viscosity and sand-carrying capacity of water-based fracturing fluids, and the changes in the above properties of water-based fracturing fluid due to reservoir pressure show a completely opposite trend to that of reservoir temperature. The viscosity of water-based fracturing fluid increases with the rise in reservoir pressure. Additionally, a consistent positive proportional relationship between sand-carrying capacity and reservoir pressure was presented at 0.3% cross-linker content, 453 K and 170 s−1. A 10 MPa of reservoir pressure can make the viscosity and sand-carrying capacity of water-based fracturing fluid (containing 0.3% synthetic cross-linker) reach 124 mPa·s and 14 cm2, respectively. The positive upward trend makes the reservoir pressure of 15 MPa more advantageous for the rheology and sand-carrying capacity of the fracturing fluid, and a fluid viscosity of 126 mPa·s and the sand-carrying capacity of 14.3 cm2, respectively, was shown with a reservoir pressure of 15 MPa, but the slight change in the magnitude of the rise trend expressed a transition from a weak effect of reservoir pressure to an excellent improvement. The change in the sand-carrying capacity of fracturing fluids resulting from the increased reservoir pressure was attributed to the fluid viscosity, and the accuracy of the above description can be proven by Equations (1)–(3). An increase in reservoir pressure will result in an elevated viscosity of the water-based fracturing fluid, thereby facilitating a considerable enhancement in the quantity of sand transported by the fracturing fluid. Concurrently, the continued increase of the reservoir pressure from 15 MPa to 20 MPa results in a promotion in the observed fluid viscosity and sand-carrying capacity, and the performance parameters of water-based fracturing fluid are increased to 130 mPa·s and 15 cm2, respectively. Nevertheless, reservoir pressure greater than 25 MPa will significantly increase the many properties of water-based fracturing fluid. A reservoir pressure of 25 MPa will increase the viscosity and sand-carrying capacity of water-based fracturing fluid to 140 mPa·s and 16.1 cm2, respectively. The compression of microscopic molecules by reservoir pressure is considered to be an important reason for promoting the performance of water-based fracturing fluid. A reservoir pressure of 25 MPa will significantly increase the density of water-based fracturing fluid. High-content fracturing fluid inevitably leads to an increase in viscosity and an improvement in sand-carrying capacity. However, a weak reservoir pressure may result in a decrease in the external pressure experienced by water-based fracturing fluids, which could lead to a reduction in fluid density variation.
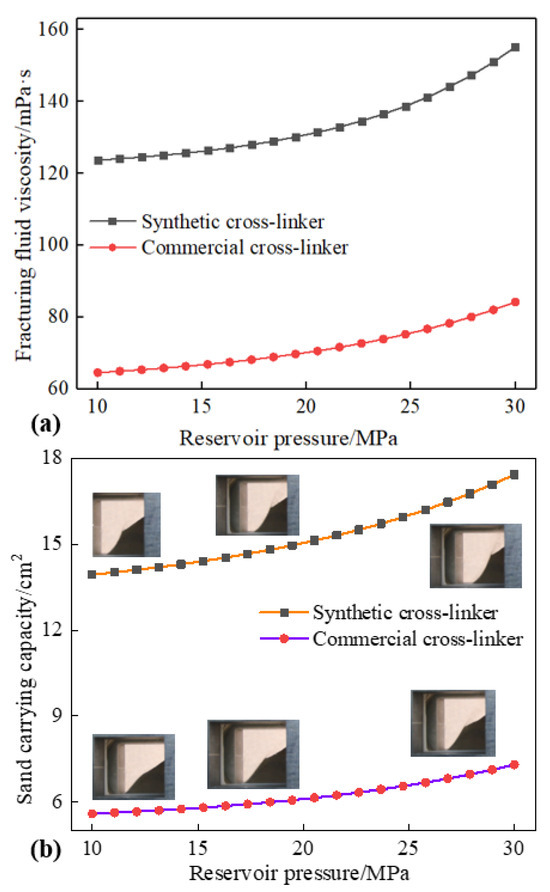
Figure 8.
Effect of reservoir pressure on fracturing fluid viscosity and sand-carrying capacity (0.3% cross-linker content, 170 s−1 and 453 K). (a): fracturing fluid viscosity. (b): sand-carrying capacity.
In addition, Figure 7a,b also show the performance change trend of water-based fracturing fluid containing 0.3% commercial cross-linker under the same reservoir pressure. Water-based fracturing fluids containing commercially available cross-linking agents not only gradually increase in viscosity as reservoir pressure rises but also exhibit a significant direct proportional relationship between sand-carrying capacity and reservoir pressure. Similarly, low reservoir pressure also causes a relatively weak fluid viscosity and sand-carrying capacity of water-based fracturing fluids containing commercial cross-linkers, and the above two parameters can be expressed as 65 mPa·s and 5.6 cm2, respectively, in a reservoir environment of 10 MPa. However, reservoir pressure of 25 MPa will increase fluid viscosity and sand-carrying capacity to 77 mPa·s and 6.6 cm2. Although the fracturing fluid containing a commercial cross-linker will continue to improve with the increase in reservoir pressure, the increase is still lower than that of the synthetic cross-linker. Low reservoir pressure will cause the distances between water, guar gum, and cross-linkers in water-based fracturing fluid to be relatively large, which is not conducive to the formation of a substantial number of hydrogen bonds and microscopic grids [45]. Smaller micro-grid density at low pressure leads to a weaker fluid viscosity and sand-carrying capacity of water-based fracturing fluid. However, the molecules that were previously unable to form chemical bonds due to the substantial molecular distance gradually interact with each other as a consequence of the augmented reservoir pressure, and more intermolecular hydrogen bonds and a greater micro-grid density were shown with the increasing reservoir pressure [46]. Furthermore, the synthetic cross-linker molecules contain more hydroxyl and functional groups, enabling the formation of more intermolecular hydrogen bonds under the same cross-linker content and reservoir pressure. Additionally, high reservoir pressure will cause the synthetic cross-linker to form more hydrogen bonds, resulting in significant changes in many properties of the fracturing fluid [47]. Increasing reservoir pressure will not cause commercial cross-linker molecules to quickly form a large number of hydrogen bonds with water and guar gum, thereby reducing the impact of reservoir pressure on the performance of fracturing fluids containing commercial cross-linkers.
3.4. Effects of Shear Rate on the Reservoir Fractures
The shear rate of water-based fracturing fluid is not only regarded as a significant factor changing the self-parameters of water-based fracturing fluid (fluid viscosity), but also affects the carrying capacity of fracturing fluid on proppant particles. It can be illustrated from Figure 9a,b that a gradually decreasing fluid viscosity is demonstrated with increasing the shear rate of water-based fracturing fluid, and the sand-carrying capacity of fracturing fluid is gradually increasing when an increasing fluid velocity is displayed. The obvious inverse relationship between the viscosity and shear rate of the fracturing fluid also indicates that water-based fracturing fluid has a very unique shear thinning property, and the fracturing fluids containing cross-linkers are also considered as a non-Newtonian fluid. The low shear rates will result in a relatively minor reduction in the viscosity of water-based fracturing fluids. The viscosity of the fracturing fluid containing 0.3% synthetic cross-linker was observed to decrease from 142 mPa·s at 130 s−1 to 137 mPa·s at 150 s−1. The viscosity reduction value of 5 mPa·s indicates that low shear rates have an extremely weak shear capacity on water-based fracturing fluids. Nevertheless, an increase in the viscosity of the fracturing fluid to 170 s−1 will result in a relatively rapid decrease in fluid viscosity.
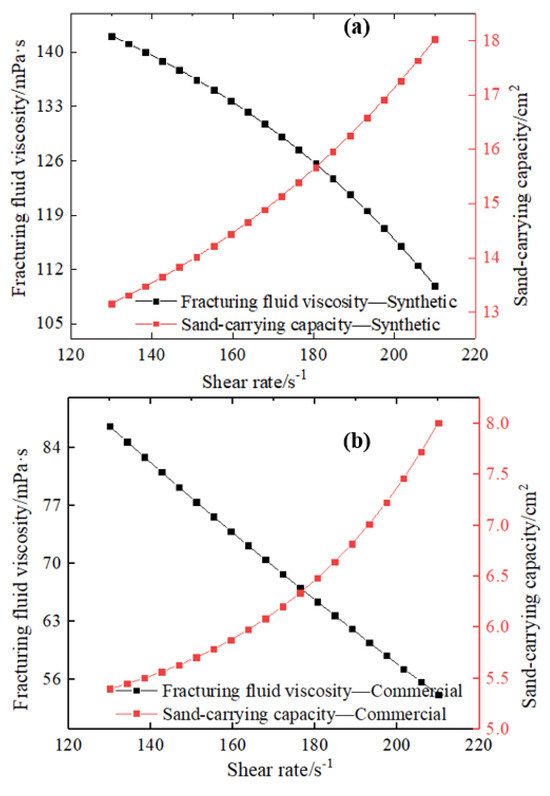
Figure 9.
Effect of reservoir pressure on fracturing fluid viscosity and sand -carrying capacity (0.3% cross-linker content, 20 MPa and 453 K). (a): Synthetic. (b): Commercial.
A decrease in viscosity of 7 mPa·s is evident in the fluid viscosity when the shear rate is changed from 150 s−1 to 170 m s−1. A higher shear rate indicates a lower fluid viscosity than a lower shear rate, with the reduction occurring at a significantly faster rate. Nevertheless, the capacity of water-based fracturing fluids to transport sand increases in proportion to the shear rate, exhibiting a clear linear relationship between these two variables. Although a lower fracturing fluid shear rate facilitates higher fluid viscosity, the sand-carrying capacity remains relatively limited. Conversely, water-based fracturing fluid can achieve considerable sand-carrying capacity. However, the fluid viscosity is relatively low at a high shear rate. A water-based fracturing fluid containing 0.3% synthetic cross-linker can show a sand-carrying area of 12.9 cm2 at a shear rate of 130 s−1, but a sand-carrying capacity of 13.5 cm2 will appear at a shear rate of 150 s−1. Subsequently, an enhanced augmentation in the sand-carrying capacity (15 cm2) was demonstrated at a fracturing fluid shear rate of 170 s−1.
Furthermore, water-based fracturing fluids containing 0.3% commercially available cross-linker also showed a similar trend, and the fluid viscosity and the sand-carrying capacity show a very perfect inverse relationship with an increasing in the shear rate of the fracturing fluid. The alteration in shear rate demonstrates a comparable shift in the sand-carrying capacity of the fracturing fluids derived from the two cross-linkers. However, the fracturing fluid comprising the commercial cross-linker exhibits a diminished sand-carrying capacity relative to the synthetic cross-linker at an equivalent shear rate. The formation and alteration of chemical bonds and microscopic grids remain the primary microscopic factors influencing the change in shear rate and performance of water-based fracturing fluids [48]. The flow of fracturing fluid can effectively shear microscopic intermolecular hydrogen bonds, leading to the potential disruption of certain chemical bonds under shear stress. Even if complete shearing of hydrogen bonds does not occur, the stretching effect may still result in the formation of chemical bonds with reduced bond energy. The shearing or elongation of hydrogen bonds leads to a gradual decrease in the density of the microscopic network, which directly results in a continuous reduction in the viscosity of the fracturing fluid. The low shear effect of a fracturing fluid’s low shear rate can disrupt or extend a limited number of low-energy hydrogen bonds, whereas a higher shear rate can swiftly and efficiently sever the majority of chemical bonds. Thus, the high-flow water-based fracturing fluid not only exhibits a lower microscopic grid density at the microscopic level, but also exhibits an extremely low fluid viscosity at the macroscopic level. However, the excellent sand-carrying capacity caused by a high shear rate is mainly attributed to the drag of micro-grid and the boost of fluid. Although the reduced micro-grid drag force formed at high shear rates is not conducive to the sand-carrying capacity of the fracturing fluid, the enhanced boost force can offset the weakened grid drag and enhance the momentum of particle forward movement. In addition, the synthesized cross-linker exhibits a distinctive three-dimensional spherical configuration at the molecular level, which facilitates the formation of intermolecular hydrogen bonds between its hydroxyl groups and those of the guar gum, as well as between the hydroxyl groups and water molecules. More hydrogen bonds in fracturing fluids comprising synthetic cross-linkers can effectively resist shearing at elevated shear rates, thereby enhancing the shear resistance and sand-carrying capacity of fracturing fluids. However, commercially available cross-linker molecules contain a limited number of functional groups, which restricts their ability to form a significant number of hydrogen bonds. This has a notable impact on the shear resistance and sand-carrying capacity of fracturing fluids containing commercially available cross-linkers.
The markedly low heat and shear resistance of water-based fracturing fluids comprising commercially available cross-linkers gives rise to a notably low fluid viscosity in high-temperature shale reservoirs. This is not conducive to rock cracking and crack expansion in shale fractures. Furthermore, the extremely low fluid viscosity is not conducive to the transportation of proppants into the fractures, resulting in the expansion and subsequent reclosure of a considerable number of cracks. However, the cross-linker synthesized in this study has the potential to address the aforementioned limitations, demonstrating heat and shear resistance that is sufficient for the high-temperature environment of shale reservoirs. An increase in fluid viscosity can facilitate both the expansion of shale fractures and the rapid transportation of proppants to reinforce the fractures. Additionally, shale oil can be transported to production wells via the propped-up fractures, thereby increasing crude oil recovery.
The viscosity of water-based fracturing fluids containing synthetic cross-linkers remains relatively stable when subjected to elevated reservoir temperatures. Furthermore, the viscosity decline of water-based fracturing fluids within the same reservoir temperature and shear rate range is less pronounced than that observed with commercially available cross-linkers. Furthermore, the sand-carrying capacity will remain largely unaffected by high temperatures or high shear rates. While synthetic cross-linkers still fall short of fully meeting the actual fracturing and sand-carrying requirements of shale reservoirs, their ongoing chemical structure improvements serve as a benchmark for the continuous advancement and evolution of water-based fracturing fluids, facilitating the efficient development of shale reservoirs.
4. Conclusions
This study synthesized a cross-linker comprising a substantial number of functional groups, which is capable of achieving the temperature and shear resistance observed in water-based fracturing fluids. Furthermore, this study also constructed a multifunctional collaborative device that is able to simulate the rheology and sand-carrying capacity of water-based fracturing fluids in shale reservoirs and analyzed the effects of different factors on the viscosity and sand-carrying capacity of water-based fracturing fluids. The sand-carrying capacity of nano cross-linkers for water-based fracturing fluids is wholly distinct from that of commercially available cross-linkers, and the temperature and shear resistance will also be markedly enhanced. Concurrently, water-based fracturing fluids comprising nano-cross-linking agents can also demonstrate markedly elevated fluid viscosity, which is of considerable consequence for enhancing the attributes of water-based fracturing fluids. The three-dimensional structure of the nano cross-linker facilitates the formation of more directional functional groups on the surface of the sphere, which is a significant factor contributing to the nano cross-linker’s enhanced sand-carrying capacity. Furthermore, the heat and shear resistance are primarily attributed to the nano-cross-linker’s capacity to form numerous intermolecular hydrogen bonds. However, the exceedingly simple structure of the commercially available cross-linker is unable to interact with a greater quantity of water and guar gum. In addition, a reduction in cross-linker content to enhance the efficacy of sand-carrying capacity and temperature and shear resistance remains a pivotal avenue for the utilization of water-based fracturing fluids in shale reservoir transformation.
Author Contributions
Conceptualization, Q.L. (Qiang Li), Q.L. (Qingchao Li) and F.W.; methodology, J.W. and F.W.; validation, Y.W.; formal analysis, Q.L. (Qiang Li) and Q.L. (Qingchao Li). All authors have read and agreed to the published version of the manuscript.
Funding
This research sponsored by Henan Provincial Science and Technology Research Project (242102320342), the Fundamental Research Funds for the Universities of Henan Province (NSFRF240616).
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, Q.; Li, Y.; Cheng, Y.; Li, Q.; Wang, F.; Wei, J.; Liu, Y.; Zhang, C.; Song, B.; Yan, C.; et al. Numerical simulation of fracture reorientation during hydraulic fracturing in perforated horizontal well in shale reservoirs. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 1807–1813. [Google Scholar] [CrossRef]
- Lv, Q.; Li, Z.; Li, B.; Zhang, C.; Shi, D.; Zheng, C.; Zhou, T. Experimental study on the dynamic filtration control performance of N2/liquid CO2 foam in porous media. Fuel 2017, 202, 435–445. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Wang, S.; Guo, Y.; Han, X.; Li, Q.; Cheng, Y.; Dong, Z.; Li, X.; Zhang, X. Numerical insights into factors affecting collapse behavior of horizontal wellbore in clayey silt hydrate-bearing sediments and the accompanying control stratege. Ocean. Eng. 2024, 297, 117029. [Google Scholar] [CrossRef]
- Sorrell, S.; Speirs, J.; Bentley, R.; Brandt, A.; Miller, R. Global oil depletion: A review of the evidence. Energy Policy 2010, 38, 5290–5295. [Google Scholar] [CrossRef]
- Sekar, L.K.; Kiran, R.; Okoroafor, E.R.; Wood, D.A. Review of reservoir challenges associated with subsurface hydrogen storage and recovery in depleted oil and gas reservoirs. J. Energy Storage 2023, 72, 108605. [Google Scholar] [CrossRef]
- Hu, J.; Tang, Q.; Wu, Z.; Zhang, B.; He, C.; Chen, Q. Optimization and assessment method for total energy system retrofit in the petrochemical industry considering clean energy substitution for fossil fuel. Energy Convers. Manag. 2023, 284, 116967. [Google Scholar] [CrossRef]
- Zastempowski, M. Analysis and modeling of innovation factors to replace fossil fuels with renewable energy sources-Evidence from European Union enterprises. Renew. Sustain. Energy Rev. 2023, 178, 113262. [Google Scholar] [CrossRef]
- Rehm, T.E. Advanced nuclear energy: The safest and most renewable clean energy. Curr. Opin. Chem. Eng. 2023, 39, 100878. [Google Scholar] [CrossRef]
- Iyke, B.N. Climate change, energy security risk, and clean energy investment. Energy Econ. 2024, 129, 107225. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Xiao, Z.; Jiang, B.; Ren, J.; Jin, Z.; Li, X. Conversion of rice husk biomass into electrocatalyst for oxygen reduction reaction in Zn-air battery: Effect of self-doped Si on performance. J. Colloid Interface Sci. 2022, 606, 1014–1023. [Google Scholar] [CrossRef]
- Aydin, M.; Degirmenci, T. The impact of clean energy consumption, green innovation, and technological diffusion on environmental sustainability: New evidence from load capacity curve hypothesis for 10 European Union countries. Sustain. Dev. 2024, 32, 2358–2370. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Wang, Y.; Forson, K.; Cao, L.; Zhang, C.; Chen, J. Experimental investigation on the high-pressure sand suspension and adsorption capacity of guar gum fracturing fluid in low-permeability shale reservoirs: Factor analysis and mechanism disclosure. Environ. Sci. Pollut. Res. 2022, 29, 53050–53062. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cheng, Y.; Li, Q.; Zhang, C.; Ansari, U.; Song, B. Establishment and evaluation of strength criterion for clayey silt hydrate-bearing sediments. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 742–750. [Google Scholar] [CrossRef]
- Li, Q.; Wang, F.; Wang, Y.; Zhang, J.; Yu, X.; Zhao, M.; Li, W. Influence of organoboron cross-linker and reservoir characteristics on filtration and reservoir residual of guar gum fracturing fluid in low-permeability shale gas reservoirs. Environ. Sci. Pollut. Res. 2022, 29, 82975–82985. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, Y.; Zhou, T.; Li, Y.; Li, Q. Enhanced toughness and mechanical property of epoxy resins with good shape memory behaviors. Fibers Polym. 2020, 21, 1187–1194. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Z.; Zeng, X.; Li, D.; An, H.; Zhao, L.; Li, Q. Influence of Supercritical CO2 Fracturing Fluid on the Permeability of Shale Reservoir and Mechanism Analysis. ACS Omega 2024, 9, 23294–23302. [Google Scholar] [CrossRef]
- Xue, S.; Huang, Q.; Wang, G.; Bing, W.; Li, J. Experimental study of the influence of water-based fracturing fluids on the pore structure of coal. J. Nat. Gas Sci. Eng. 2021, 88, 103863. [Google Scholar] [CrossRef]
- Papavasileiou, K.D.; Michalis, V.K.; Peristeras, L.D.; Vasileiadis, M.; Striolo, A.; Economou, I.G. Molecular dynamics simulation of water-based fracturing fluids in kaolinite slit pores. J. Phys. Chem. C 2018, 122, 17170–17183. [Google Scholar] [CrossRef]
- Wu, G.; Pan, J.; Anwaier, M.; Wu, J.; Xiao, P.; Zheng, L.; Wang, W.; Meng, X.; Wang, P.; Liu, J.; et al. Effect of nano-SiO2 on the flowback-flooding integrated performance of water-based fracturing fluids. J. Mol. Liq. 2023, 379, 121686. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Zuo, Z.; Liao, M.; Peng, P. Preparation and property evaluation of a temperature-resistant Zr-crosslinked fracturing fluid. J. Ind. Eng. Chem. 2021, 96, 121–129. [Google Scholar] [CrossRef]
- Al-Hajri, S.; Negash, B.M.; Rahman, M.M.; Haroun, M.; Al-Shami, T.M. Perspective Review of polymers as additives in water-based fracturing fluids. ACS Omega 2022, 7, 7431–7443. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Lai, X.; Tang, M.; Li, J.; Wang, L.; Gao, J. Preparation and properties of a clean, low-damage waterproof locking damage multifunctional integrated water-based fracturing fluid. J. Appl. Polym. Sci. 2022, 139, e53207. [Google Scholar] [CrossRef]
- Peng, H.; Li, W.; Liu, J.; Peng, J.; Han, H.; Liu, J.; Liu, D.; Yang, Z. Experimental Study for the Effects of Different Factors on the Sand-Carrying Capacity of Slickwater. Geofluids 2023, 2023, 7897165. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, F.; Bai, H.; Li, Y.; Yang, H. A Comprehensive method to evaluate the viscous slickwater as fracturing fluids for hydraulic fracturing applications. J. Pet. Sci. Eng. 2020, 193, 107359. [Google Scholar] [CrossRef]
- Yang, X.; Mao, J.; Zhang, W.; Zhang, H.; Zhang, Y.; Zhang, C.; Ouyang, D.; Chen, Q.; Lin, C.; Zhao, J. Tertiary cross-linked and weighted fracturing fluid enables fracture stimulations in ultra high pressure and temperature reservoir. Fuel 2020, 268, 117222. [Google Scholar] [CrossRef]
- Ge, Y.; Zhao, Z.; Cheng, X.; Chen, T.; Liu, T.; Guo, X. Research of a novel double cross-linking fracturing fluid. J. Pet. Explor. Prod. Technol. 2021, 11, 2191–2197. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Xu, N.; Lan, J.; Jiang, B.; Meng, L. Synthesis and properties of organoboron functionalized nanocellulose for crosslinking low polymer fracturing fluid system. RSC Adv. 2021, 11, 13466–13474. [Google Scholar] [CrossRef]
- Zhou, M.; Liu, Y.; Li, C.; Chen, Z.; Li, C.; Zhang, L. Fe3+-crosslinked alkyl phosphate ester as thickener for oil-based fracturing fluids. Mater. Chem. Phys. 2024, 313, 128748. [Google Scholar] [CrossRef]
- Fan, Y.; Duan, W.; Xu, K.; Yan, C.; Zheng, C. Zr, N-Co-Doped Carbon Quantum Dot Crosslinking Agents for Use in Fracturing Fluids. ACS Appl. Nano Mater. 2023, 6, 7920–7930. [Google Scholar] [CrossRef]
- Kreipl, M.P.; Kreipl, A.T. Hydraulic fracturing fluids and their environmental impact: Then, today, and tomorrow. Environ. Earth Sci. 2017, 76, 160. [Google Scholar] [CrossRef]
- Nianyin, L.; Yu, J.; Daocheng, W.; Chao, W.; Jia, K.; Pingli, L.; Chengzhi, H.; Ying, X. Development status of crosslinking agent in high-temperature and pressure fracturing fluid: A review. J. Nat. Gas Sci. Eng. 2022, 107, 104369. [Google Scholar] [CrossRef]
- Kalam, S.; Afagwu, C.; Al Jaberi, J.; Siddig, O.M.; Tariq, Z.; Mahmoud, M.; Abdulraheem, A. A review on non-aqueous fracturing techniques in unconventional reservoirs. J. Nat. Gas Sci. Eng. 2021, 95, 104223. [Google Scholar] [CrossRef]
- Elsner, M.; Hoelzer, K. Quantitative survey and structural classification of hydraulic fracturing chemicals reported in unconventional gas production. Environ. Sci. Technol. 2016, 50, 3290–3314. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Tang, J.; Yan, S.; Wang, Y.; Han, J.; Shi, S. Preparation and performance of novel temperature-resistant thickening agent. Polym. Adv. Technol. 2018, 29, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, K.; Cao, M. Experimental study on modified polyacrylamide coated self-suspending proppant. Fuel 2017, 199, 185–190. [Google Scholar] [CrossRef]
- Hou, L.; Sun, B.; Wang, Z.; Li, Q. Experimental study of particle settling in supercritical carbon dioxide. J. Supercrit. Fluids 2015, 100, 121–128. [Google Scholar] [CrossRef]
- Hurnaus, T.; Plank, J. Behavior of titania nanoparticles in cross-linking hydroxypropyl guar used in hydraulic fracturing fluids for oil recovery. Energy Fuels 2015, 29, 3601–3608. [Google Scholar] [CrossRef]
- Fu, C.; Liu, N. Waterless fluids in hydraulic fracturing–A review. J. Nat. Gas Sci. Eng. 2019, 67, 214–224. [Google Scholar] [CrossRef]
- Li, Q.; Han, Y.; Liu, X.; Ansari, U.; Cheng, Y.; Yan, C. Hydrate as a by-product in CO2 leakage during the long-term sub-seabed sequestration and its role in preventing further leakage. Environ. Sci. Pollut. Res. 2022, 29, 77737–77754. [Google Scholar] [CrossRef]
- Shan, H.; Sun, Y.; Li, B.; Shen, Y.; Qi, Y.; Zhang, G. Low-temperature gel breaking fracturing fluid for marine hydrate reservoir fracturing and its effect on phase equilibrium of methane hydrates. Geoenergy Sci. Eng. 2024, 233, 212583. [Google Scholar] [CrossRef]
- Mao, H.; Yang, Y.; Zhang, H.; Zhang, J.; Huang, Y. A critical review of the possible effects of physical and chemical properties of subcritical water on the performance of water-based drilling fluids designed for ultra-high temperature and ultra-high pressure drilling applications. J. Pet. Sci. Eng. 2020, 187, 106795. [Google Scholar] [CrossRef]
- Huang, Q.; Li, M.; Li, J.; Gui, Z.; Du, F. Comparative experimental study on the effects of water-and foam-based fracturing fluids on multiscale flow in coalbed methane. J. Nat. Gas Sci. Eng. 2022, 103, 104648. [Google Scholar] [CrossRef]
- Chu, Q.; Lin, L.; Su, J. Amidocyanogen silanol as a high-temperature-resistant shale inhibitor in water-based drilling fluid. Appl. Clay Sci. 2020, 184, 105396. [Google Scholar] [CrossRef]
- Zhang, C.P.; Cheng, P.; Ma, Z.Y.; Ranjith, P.G.; Zhou, J.P. Comparison of fracturing unconventional gas reservoirs using CO2 and water: An experimental study. J. Pet. Sci. Eng. 2021, 203, 108598. [Google Scholar] [CrossRef]
- Li, X.; Feng, Z.; Han, G.; Elsworth, D.; Marone, C.; Saffer, D.; Cheon, D.S. Breakdown pressure and fracture surface morphology of hydraulic fracturing in shale with H2O, CO2 and N2. Geomech. Geophys. Geo-Energy Geo-Resour. 2016, 2, 63–76. [Google Scholar] [CrossRef]
- Luo, X.; Wang, S.; Wang, Z.; Jing, Z.; Lv, M.; Zhai, Z.; Han, T. Experimental investigation on rheological properties and friction performance of thickened CO2 fracturing fluid. J. Pet. Sci. Eng. 2015, 133, 410–420. [Google Scholar] [CrossRef]
- Lyu, Q.; Long, X.; Ranjith, P.G.; Tan, J.; Kang, Y. Experimental investigation on the mechanical behaviours of a low-clay shale under water-based fluids. Eng. Geol. 2018, 233, 124–138. [Google Scholar] [CrossRef]
- Wang, G.; Huang, T.; Yan, S.; Liu, X. Experimental study of the fracturing-wetting effect of VES fracturing fluid for the coal seam water injection. J. Mol. Liq. 2019, 295, 111715. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).