Abstract
The increasing concern over greenhouse gas emissions, particularly CO2, has emphasized the urgency for practical solutions to mitigate the environmental impacts of climate change. This study assessed the technical, economic, and environmental feasibility of producing dimethyl carbonate (DMC) through an integrated route using methanol derived from biomass gasification in sugarcane-based industries. Unlike previous studies that analyzed isolated aspects of DMC production, this research was conducted through process modeling and simulation in Aspen Plus® V12.1, evaluating key performance indicators such as conversion rates, product purity, capital and operating expenses, and CO2 emissions. A DMC conversion rate of 78.06% and a purity level of 96.80% were achieved. However, the integration of methanol production increased both CAPEX and OPEX, leading to a net present value (NPV) of R$ 36.7 million over 10 years, lower than alternative routes using commercially available methanol. Additionally, the process resulted in a net CO2 emission of 3.41 kg CO2 per kg of DMC, exceeding conventional methods. These findings suggest that under the evaluated conditions, process integration did not offer economic advantages, despite many environmental advantages over commercially available methanol.
1. Introduction
The increase in greenhouse gas emissions, particularly carbon dioxide (CO2), has raised growing concerns about the global environmental impacts in recent years, underscoring the urgency of addressing climate change. These impacts are widely observed across various domains, including the economy and policy-making, making the search for effective solutions to reduce greenhouse gas emissions increasingly urgent and relevant [1]. Furthermore, the effects are more pronounced in the environmental domain, with rising CO2 concentrations—largely driven by human activities such as fossil fuel combustion and land-use changes—directly contributing to an increase in average global temperatures [2].
A promising approach to reducing CO2 emissions lies in its capture, storage, and utilization technologies [3]. This strategy mitigates emissions and offers economic opportunities, particularly for industries with significant CO2 capture potential, such as the sugarcane-based ethanol industry, where CO2 is a byproduct of fermentation [4]. In this context, dimethyl carbonate (DMC) production is a promising opportunity [5,6]. DMC is a versatile chemical solvent highly valued for its molecular properties, which enable a wide range of applications (e.g., polycarbonate production, intermediate in the synthesis of pharmaceuticals, fuel additive, and lithium-ion batteries component, among others) [7,8,9]. Producing DMC from CO2 offers numerous environmental advantages as it represents a clean and safe process without generating toxic or hazardous waste. Souza et al. (2013) [10] documented a 92% reduction in CO2 emissions through this route.
This route has been extensively studied and has shown promising results from the point of view of catalysis and their respective kinetics for CO2 conversion into DMC [11,12,13]. Kohli et al. (2022) [10] highlight the role of catalysts in process efficiency and selectivity under a well-done review, evaluating various catalytic systems used in direct and indirect routes for DMC production. Compounds based on metal salts (Zn, Sn, and Ce) showed good catalytic activity, especially in direct reactions of CO2 and methanol. For example, Zn-based catalysts achieved CO2 conversions higher than 30% under optimized conditions, highlighting their high selectivity for DMC. CeO2-based catalysts achieved up to 25% conversions with selectivity above 90% for DMC. Zhao et al. [14] examined the catalytic conversion of CO2 and methanol into DMC using nickel acetate under near-supercritical conditions. Under 305 K, 2 h, and 10.3 MPa conditions, the reaction achieved 100% selectivity for DMC and a yield of 350% per mol of catalyst. However, the extensively studied direct reactive pathways typically exhibit low conversions and require complex operating conditions. Consequently, indirect synthesis presents an attractive alternative involving two reaction steps in separate systems. Such a route demonstrates significant potential for industrial application [15], beginning with the carbonylation of ethylene oxide (EO) with CO2 to produce ethylene carbonate (EC). The EC then undergoes a transesterification step with excess methanol, yielding DMC and ethylene glycol (EG) [16].
Due to the need for methanol as an intermediate in this route, the alignment of methanol production through a route that also aims to reduce CO2 emissions is necessary. In this context, producing methanol from biomass has gained traction to enhance the environmental benefits of this process and reduce the dependence on non-renewable resources [17,18]. Biomass materials, such as residual bagasse from sugar and ethanol industries, can be converted into a gas and subsequently reformed to produce synthesis gas and methanol [19]. Initially, the biomass biodigestion process produces biogas, which can be purified to biomethane so that the known reforming reaction can be carried out to generate synthesis gas. An alternative to generating this gas is the biomass gasification or pyrolysis processes [20]. However, regardless of how this gas is obtained, it can be processed to produce a range of chemical derivatives using different routes, including conversion to methanol [21,22,23].
Therefore, these two intermediates (EO and methanol) are critical for DMC production and, when derived from waste, contribute to the process’s sustainability and circularity. Since both can be obtained sustainably in line with green chemistry, the DMC production process has the potential to align with global sustainability goals, particularly in countries with growing biogas production, such as Brazil, Germany, India, and China, among others [24]. However, a significant challenge lies in predicting the behavior of this scenario within the context of a biorefinery, where additional products may be available for processing and compete for the utilization of synthesis gas. Previous studies have predominantly examined specific aspects of DMC production, such as the techno-economic evaluation of standalone production routes. However, research integrating methanol production from biomass with DMC synthesis in a comprehensive process assessment remains limited.
Considering the potential of this alternative for CO2 mitigation and addressing this gap, this study examines the indirect route for CO2 reutilization by evaluating the technical, economic, and environmental feasibility of dimethyl carbonate (DMC) production through process modeling and simulation methodologies. The proposed approach integrates and analyzes methanol production from syngas obtained through the gasification of residual biomass from sugarcane-based industries, combined with CO2 captured and stored by the same facilities. This evaluation enables an analysis of how the process aligns with economic feasibility and environmental performance considering the CO2 net emission when implemented in industrial contexts.
2. Materials and Methods
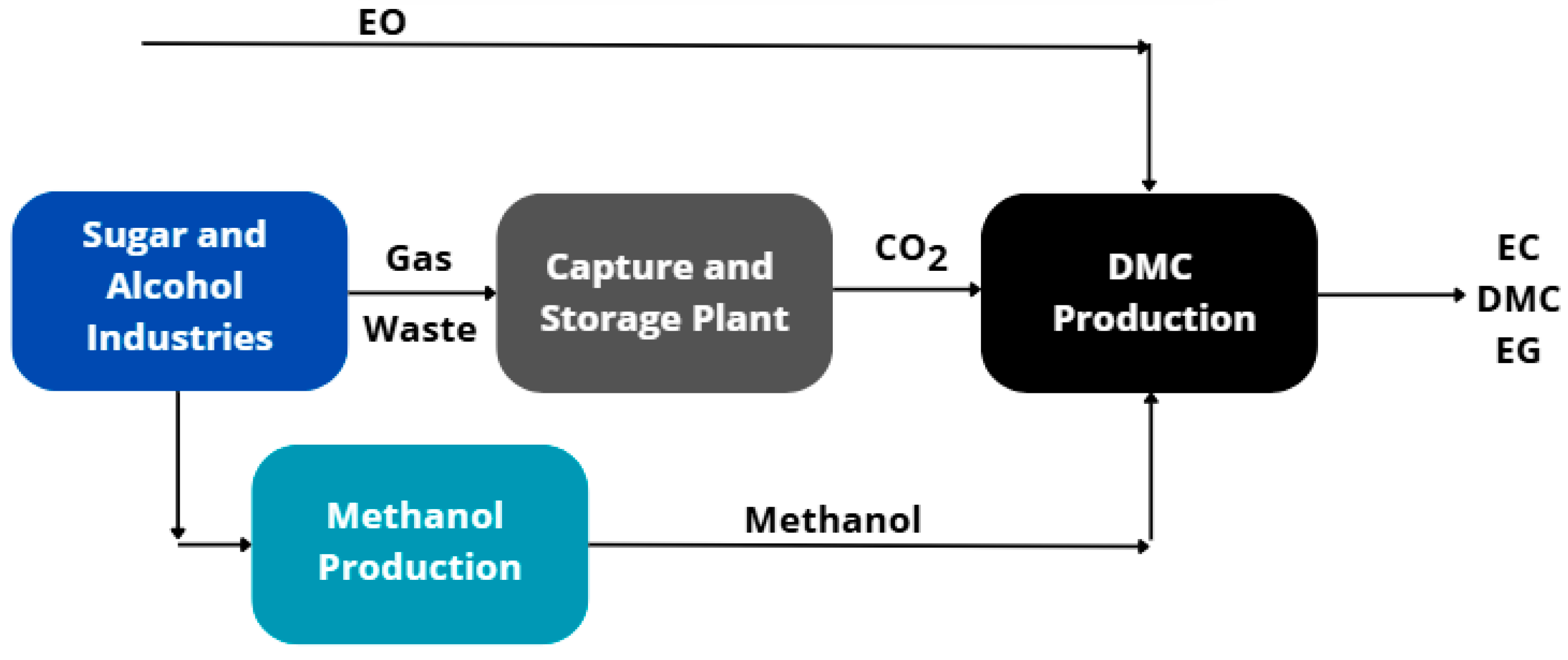
The simulation of DMC production routes and methanol synthesis from biomass was performed and validated using the commercial software Aspen Plus® V12.1. The sugarcane industry was considered the source of two key components for the studied process: CO2, captured from the fermentation process and stored at low pressure, and sugarcane bagasse, a byproduct of the milling process. As a starting point for the DMC production route, the study by Kontou et al. (2021) [15] was used as a reference to establish the feed conditions of the process components and the operational parameters. Similarly, Motta et al. (2019) [19] provided the initial framework for methanol production. These conditions were subsequently analyzed and adjusted to assess the route investigated, with a flowsheet presented in Figure 1.
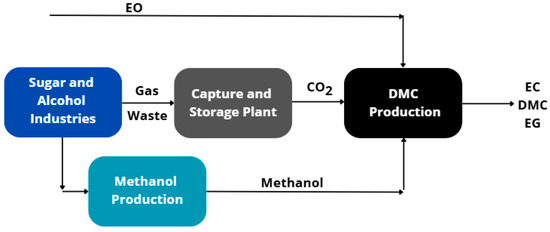
Figure 1.
Flowsheet of the DMC production process using CO2 and sugarcane bagasse from the sugar and alcohol industry.
Additionally, based on the references, the UNIQUAC thermodynamic model was selected for DMC production, while the Peng–Robinson model was applied to methanol synthesis. These models were used to predict the parameters required to describe the vapor–liquid equilibrium present in most separation stages of the process. The proposed design envisions a continuously operating production plant with 2200 kg∙h−1 of CO2 and EO feed, which also determined the required feedstock of approximately 36,500 kg∙h−1 of sugarcane bagasse for methanol synthesis via gasification.
2.1. Process Simulation
2.1.1. Dimethyl Carbonate Production
As previously mentioned, the most efficient route for dimethyl carbonate (DMC) synthesis is the indirect pathway, which involves the production of ethylene carbonate (EC) as an intermediate step toward obtaining DMC. Equations (1) and (2) represent the overall reactions in this route.
For kinetic modeling, the first reaction was based on the study by Wang et al. (2011) [25], which investigated a catalyst composed of potassium iodide supported on potassium carbonate (KI∙K2CO3−1). Their findings indicated that the reaction follows second-order kinetics, with the rate law described by Equations (3) and (4). This catalyst demonstrated high selectivity and stability over multiple cycles, making it a promising option for continuous processes. However, its performance can be influenced by the reaction temperature and CO2 adsorption, which may lead to catalyst deactivation over extended use.
For the second reaction, Fang and Xiao (2004) [26] proposed using a homogeneous catalyst, CH3Ona, which facilitates the transesterification of ethylene carbonate (EC) with methanol. This reaction is reversible, and Equations (5) and (6) present its corresponding rate law. The advantage of CH3ONa lies in its high catalytic activity, but it requires efficient separation strategies to avoid contamination of the final product. Furthermore, the presence of water or CO2 in the system can lead to catalyst deactivation, reducing overall efficiency [26].
In these equations, rEO and rEC represent ethylene oxide (EO) and ethylene carbonate (EC) reaction rates, respectively. CEO, CCO2, CEC, CMeOH, and CDMC denote the concentrations of EO, CO2, EC, methanol, ethylene glycol (EG), and DMC, respectively. R is the universal gas constant, T is the system temperature, and k0, k+1, and k−1 are the specific reaction rate parameters. The activation energies (E) and pre-exponential factors (A) for these parameters were obtained from the respective studies and are presented in Table 1.

Table 1.
Kinetic parameters of the reactions.
The selection of these catalysts and kinetic models was based on their efficacy in promoting high conversion rates, their reported stability, and their industrial feasibility. The study parameters, such as temperature, reactant concentrations, and catalyst loading, were optimized to balance reaction kinetics and process efficiency while minimizing by-product formation and catalyst degradation.
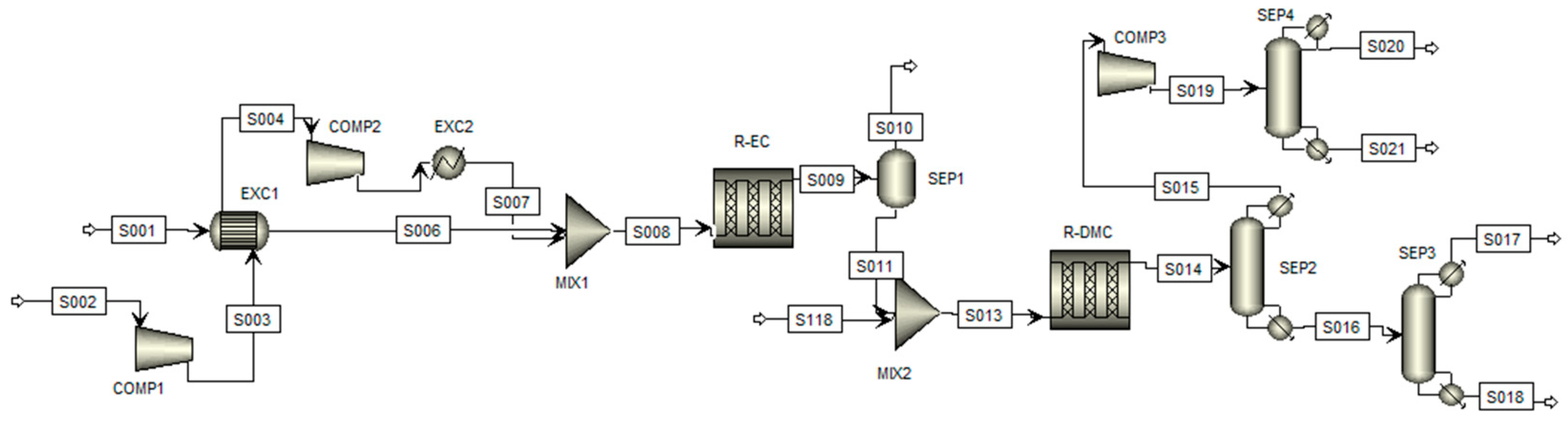
The synthesized process flowsheet for the simulation of this route is illustrated in Figure 2, where the arrows indicate the process steams and blocks represent the process equipment; the process steps will be described hereafter according to Kongpanna et al. (2015) [27].
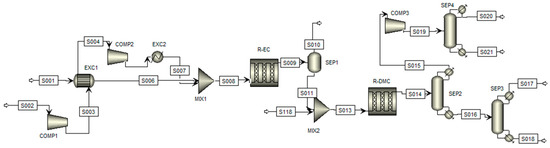
Figure 2.
Flowsheet of the simulation of the DMC production process by indirect synthesis.
Initial Reaction Stage: At the process’s beginning, CO2 and ethylene oxide (EO) are fed into the first plug flow reactor (R-EC) in a 1:1 molar ratio. Since the CO2 is stored at low pressure in the capture units, it must be compressed before entering the DMC production section. The compression occurs in two stages. In the first stage (COMP1), the pressure is raised to 10 bar, causing a simultaneous increase in temperature. At this point, the CO2 provides sufficient energy through a heat exchanger (EXC1) to bring the EO to the necessary temperature for the reactor. The CO2 is then compressed again (COMP2) to a pressure of 39.5 bar, and, via a second heat exchanger (EXC2), its temperature is adjusted to 100 °C.
The reactor (R-EC) is a single tube, 4.11 m long and 0.91 m in diameter, operating at a constant temperature of 150 °C. The product stream from this reaction (S009) is sent to a flash separator (SEP1) operating at 1.5 bar and 210 °C to recover unreacted CO2. The resulting liquid stream, predominantly ethylene carbonate (EC, S011), is then directed to the transesterification stage.
Transesterification Stage: In this stage, the EC stream is mixed with methanol and sent to the second plug flow reactor (R-DMC). Methanol is added in excess, as recommended by several studies, to enhance conversion rates [4,11]. The second reactor is multitubular, with tubes 10 m long and 5 cm in diameter, maintained at a constant temperature of 30 °C.
Due to the significant presence of impurities in the outlet stream (S014), much of this stage is dedicated to product separation and purification. Initially, the process passes through a distillation column (SEP2) with 9 stages and a distillate-to-feed ratio of 0.84. This column separates a mixture of DMC and methanol at the top (S015), while the bottom stream (S016) predominantly contains ethylene glycol (EG).
The bottom stream is directed to a second distillation column (SEP3), featuring 25 stages and a reflux ratio of 2.4, which enables the complete separation of EG (S017). Meanwhile, the top stream from the first column (S015) is sent to a third distillation column (SEP4), equipped with 18 stages and a reflux ratio of 15. This column separates the excess methanol from DMC, with the bottom stream (S021) composed mainly of purified DMC and the top stream (S020), rich in methanol, which can be recycled for use in the second reactor or other processes.
2.1.2. Methanol Production
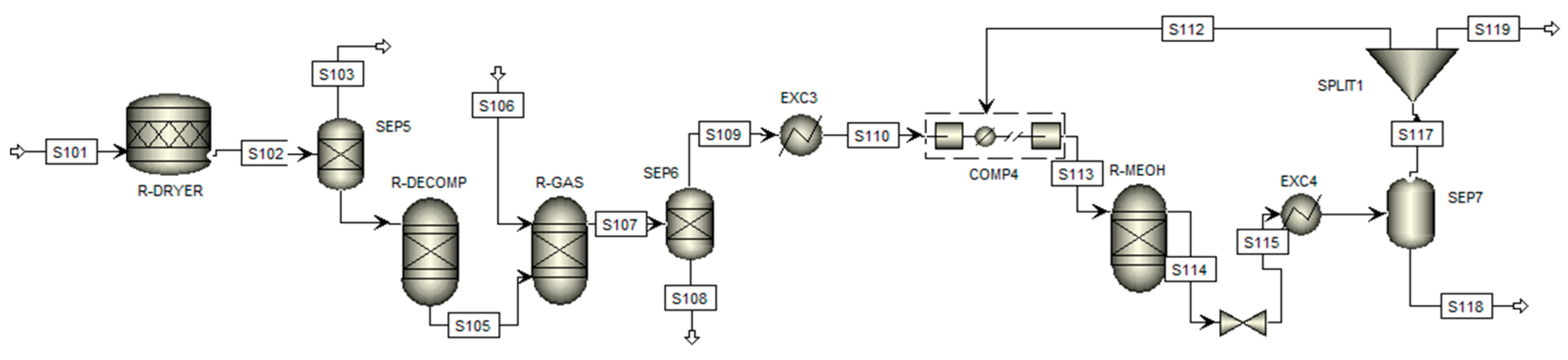
On the other hand, the complementary process of methanol production via biomass gasification is initially divided into three main stages: raw material pretreatment, gasification, and, finally, methanol synthesis. The flowchart of this process is shown in Figure 3, where the arrows indicate the process steams and blocks represent the process equipment, with the conditions described in detail below.
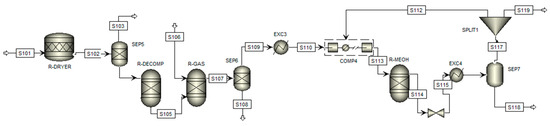
Figure 3.
Flowsheet of the simulation of the methanol production process by gasification.
The initial biomass parameters were based on the study by Rey et al. (2021) [28], which characterized biomass components from the combination of sugarcane bagasse and straw. In addition, adjustments and normalizations were made to improve the accuracy of the feedstock simulation, representing coal as carbon in its entirety. The details of the parameters used are described in Table 2 and Table 3.

Table 2.
Parameters of biomass analysis on a dry basis.

Table 3.
Biomass composition on a dry basis.
The process begins with introducing biomass into a conversion reactor (R-DRYER) at 373.15 K, representing the dehumidification stage, where the moisture content is reduced based on the stoichiometric biomass–water ratio of 1:0.055. Phase separation occurs in a representative manner using a flash separator (SEP5), directing the vapor to the upper stream (S103) and the dried biomass to the lower stream (S104).
In the subsequent stage, this stream is fed into a Gibbs reactor (R-DECOMP) at 1073.15 K, where calcination occurs to yield elemental fractions. The products are then directed to the gasification reactor (R-GAS), also a Gibbs reactor, where reactions occur in controlled steam under high pressure. The nitrogen (N), sulfur (S), and chlorine (Cl) compounds in the biomass are converted into ammonia (NH3), hydrogen sulfide (H2S), and hydrochloric acid (HCl), respectively. At the same time, carbon (C) is predominantly transformed into carbon monoxide (CO) and carbon dioxide (CO2). Phase separation is performed using another separator (SEP6), which removes the ash via the bottom stream (S108) and collects the purified syngas in the top stream (S109).
The third stage begins with the purified stream, which is cooled (EXC3) and compressed to 2.5 MPa (COMP4) to achieve optimal conditions for the methanol production reactor (R-MEOH), which, like the two previous ones, is a Gibbs reactor. Methanol synthesis occurs through the hydrogenation of carbon oxides using specific catalysts such as copper oxide and zinc oxide, which also promote the water–gas shift reaction. Consequently, methanol is produced from carbon monoxide and carbon dioxide, as described in Equations (7) and (8).
This final reactor operates under high pressure at 493.15 K, excluding reactions involving methane (CH4) and ammonia. The output stream (S114) undergoes a pressure and temperature reduction process, utilizing a valve and a heat exchanger before being introduced into a flash separator (SEP7), which separates methanol from the residual gases. The bottom stream (S118) contains the desired product, while the residual gases are subjected to a purge (SPLIT1) before being recirculated into the process (S112) to participate in the reaction again.
2.2. Economic Feasibility Analysis
The financial investment required to implement the facilities from the analyzed scenario was assessed using the Aspen Process Economic Analyzer (APEA), an integrated supplement to Aspen Plus that allows users to map, size, and estimate equipment costs directly from process simulators. This tool is based on estimates obtained from models to evaluate the capital and operational costs of the project, providing valuable information for comparing and selecting various process options.
Considering that the methanol production process simulation utilized some blocks merely as representatives, an additional methodology also had to be applied. The work conducted by Moura et al. (2023) [29] was considered for such equipment. Given the similarity of the process involved with this study, the equipment cost estimates were determined by applying the six-tenths rule [30], used to estimate acquisition costs based on the proportionality between equipment capacities, as shown in Equation (9).
In this equation, C represents the cost for a given equipment capacity P. Furthermore, it should be noted that the values used in this study are expressed in the Brazilian Real (R$), obtained after conversion using exchange rates of 5.66 for the US Dollar (US$) and 6.12 for the Euro (€), valid as of July 2024 [31].
Additionally, the economic evaluation methodology proposed by Peters and Timmerhaus (2003) [31] was applied to estimate indirect expenses, with the assumption that the acquisition of piping and auxiliary systems corresponds to 32% of the equipment cost [8]. In this analysis, the capital expenditure (CAPEX) covers the costs of acquiring and installing equipment and facilities, including indirect expenses such as legal fees, safety measures, and contingencies. On the other hand, operational expenditure (OPEX) includes the ongoing costs required for the plant’s daily operations, encompassing direct production costs such as labor, utilities, and raw materials.
It is worth noting that the equipment dimensions were estimated based on the operating conditions and utility requirements of the proposed plant, which was designed to operate for 300 working days per year (equivalent to 7200 h∙year−1), a standard period typically considered when evaluating new plants [32].
As mentioned earlier, conducting the economic analysis requires both CAPEX and OPEX values, which can be determined and related by converting CAPEX into an Equivalent Annual Capital Cost (EACC). This approach distributes the capital costs across the operational lifespan of the plant to represent an annual cost, as defined by Equation (10).
In this equation, n represents the estimated plant lifespan and i is the investment interest rate, which was assumed to be 10 years and 10.5% per annum (based on the current Brazilian interest rate of return (IRR)), respectively, in the base case.
Additionally, in process synthesis and investment project evaluation, the Net Present Value (NPV) is frequently used as a financial indicator [33]. The Gross Operating Margin (GOM) is also employed for its calculation. It assesses cash flow based on annual gross profits and operating expenses, such as OPEX and utilities, as shown in Equation (11). For the scenario under consideration, the NPV, as presented in Equation (12), was used to evaluate the process feasibility. Moreover, the payback period was determined, considering adjustments for the interest rate, which indicates the time required to achieve a positive cash flow.
In order to verify the sensitivity of the process’s economic viability, different economic scenarios were evaluated, varying the IRR to 8.5%, 10.5%, and 12.5%, and the reactant prices (EO and CO2) in ±10%. The NPV profile for all scenarios assessed was plotted, and the influence of market fluctuations can be assessed.
2.3. CO2 Emissions Assessment
Since one of the objectives of this study is to evaluate a CO2 utilization process that is also environmentally viable, the process’s net CO2 emission (CO2, Net) is one of the criteria that can be analyzed (Bonfim-Rocha et al., 2018) [33]. This net balance can be assessed using Equation (13).
In this equation, CO2,stream refers to the CO2 flows emitted in the output streams, which were not converted and were purged from the process and vent to the atmosphere (e.g., streams S010, S017, S020, S113, and S119); CO2,utilities refers to the CO2 generated when utility is consumed (i.e., low-pressure steam, high pressure steam, and electricity, among others), which is estimated by Aspen Plus in the Energy Analysis supplement; and CO2,consumption refers to the amount of CO2 consumed from streams fed into the process or contained in the syngas produced (i.e., S002, S117). At the same time, CO2,Net represents the net emissions value.
When the value obtained is negative, the amount of CO2 the process utilizes exceeds the amount generated, indicating a net gas reduction. On the other hand, if the net emissions are positive, they should be compared to existing conventional processes, as both would emit more CO2 than they consume. It is important to emphasize that, beyond direct emissions, it is crucial to consider the indirect contributions of all material and utility streams entering and leaving the process, such as those associated with heat and energy generation.
3. Results and Discussion
3.1. Technical Performance of the Process
The results and the sensitivity and optimization analyses were obtained after the process simulation converged. Table 4 details the main currents.

Table 4.
Characteristics of the currents after the convergence of the simulation.
The resulting physical properties—phase, temperature, and pressure—were consistent, demonstrating the technical feasibility of the process, as the conditions of the streams and the equipment involved are not overly complex. The conversion and purity of the DMC obtained were 78.06% and 96.80%, respectively, values close to those reported in previously cited studies. Furthermore, when comparing the molar quantities to those studies, there is potential for even greater production, especially in Brazil, considering the abundance of raw materials available in sugarcane ethanol plants and the growing interest in DMC, which proves to be a promising product.
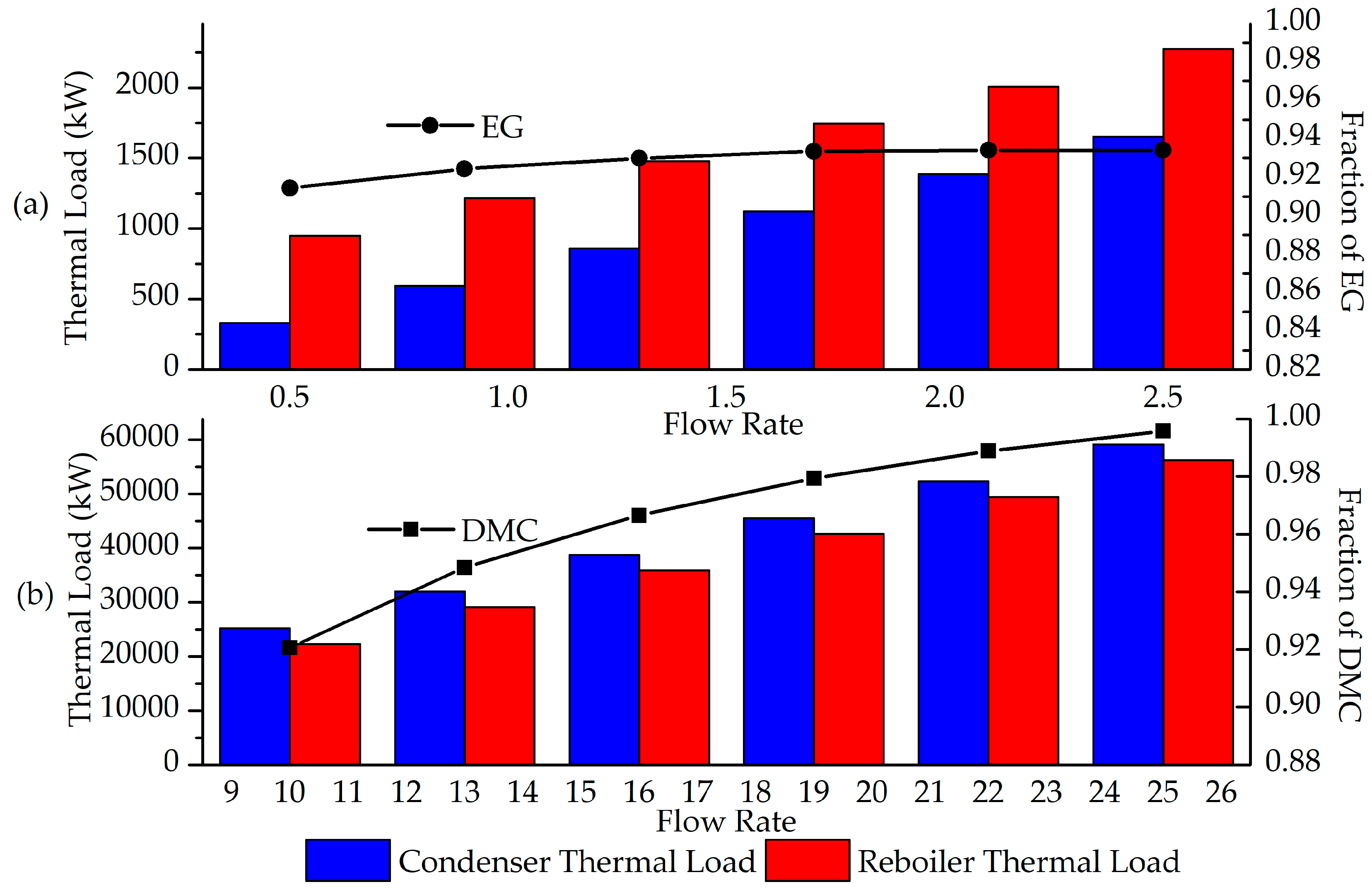
Additionally, the simulation enabled a sensitivity analysis of the separation system responsible for purifying EG and DMC, corresponding to separators SEP3 and SEP4, respectively, to evaluate the energy consumption of these units in relation to the purity of each product. As such, the molar reflux ratio (RR) required in these distillation columns was considered, along with the condenser and reboiler heat duties for each. Figure 4 presents the variations in the molar fractions of EG and DMC in their main output streams (S021 and S017) and the associated heat duties as a function of RR.
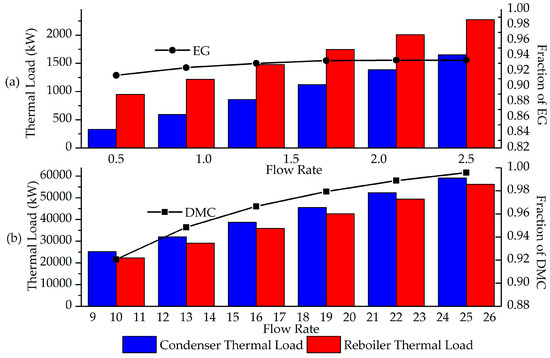
Figure 4.
Analysis of the purity of the streams with main products as a function of the reflux ratio of the distillation columns SEP3 (a) and SEP4 (b).
Initially, it is observed that, in both cases, the reflux ratio (RR) is directly proportional to the fraction of DMC and EG in the streams and to the energy demand in the condensers and reboilers. This occurs because the RR is related to the amount of saturated liquid returning to the top of the column after condensation, aiming to retain the heavier compounds present in the vapor phase, thereby aiding in the purification of both the distillate and bottom streams, especially when the less volatile component is the desired product in the liquid phase exiting the column.
Another relevant point is the impact of the RR value on the different columns, which leads to higher energy consumption. For the separation of the mixture containing DMC, which, after the initial separation (SEP2), consists predominantly of DMC and methanol, more complex operating conditions are required due to the close boiling points of these compounds at the pressure used in the column, compared to the other stream, which mainly consists of EC and EG.
Finally, when specifically analyzing the purity of DMC, it is noted that it could exceed the value obtained in the simulation, which utilized an RR of 15 for this column. However, this increase in purity must be weighed against the higher energy consumption required, considering the economic feasibility when comparing the value of a purer product to the cost of utilities. It is also necessary to assess whether a higher purity fraction is required by regulations or by subsequent process demands.
However, the transition from simulation-based evaluation to industrial-scale implementation requires the consideration of several key factors. One of the primary challenges is ensuring process stability and energy efficiency at large scales, particularly in maintaining optimal reaction conditions while minimizing operational costs. Separation and purification challenges also play a critical role in the industrial feasibility of DMC production. As highlighted by Kongpanna et al. (2015) [27], the presence of azeotropic mixtures between DMC and methanol complicates downstream separation, increasing energy consumption and requiring advanced distillation techniques or hybrid separation methods.
3.2. Economic Results
The simulation also allowed us to verify the economic viability of the DMC production process using methanol synthesized in an adjunct system, providing conditions for sizing equipment, demand for inputs, and utilities. Initially, it was possible to stipulate the investment required to acquire equipment, which is part of the CAPEX. Such equipment and their respective costs, in millions (MM), are presented in Table 5, which also includes the total investment.

Table 5.
Investment in the acquisition of equipment for the implementation of the process.
With the acquisition cost of the components, it is possible, following the aforementioned methodology (Peters and Timmerhaus, 2003) [30], to also calculate the total investment (CAPEX), which is presented in Table 6, together with its annualized value following Equation (10).

Table 6.
Total investment (CAPEX) and its annualized value.
At a later stage, based on the quantity of raw material consumed and other operating expenses, also calculated according to the above methods, it was possible to find the operating expenses per year, compiled in Table 7. The reference for costs, when necessary, is also presented.

Table 7.
Operating costs (OPEX) per year.
Finally, to assess the economic viability of the process, it was also necessary to list its revenue, which is obtained by selling the products and by-products generated in the process. For the scenario studied, the methanol recovered in the DMC production process was considered a marketable product, not reinserting it into the system. Therefore, Table 8 presents the revenue obtained by each product and the total, which corresponds to the Gross Profit shown in Equation (11). The references used to obtain the prices are also shown; however, the price charged will consider the purity of the product in question in the output streams.

Table 8.
Total revenue by year.
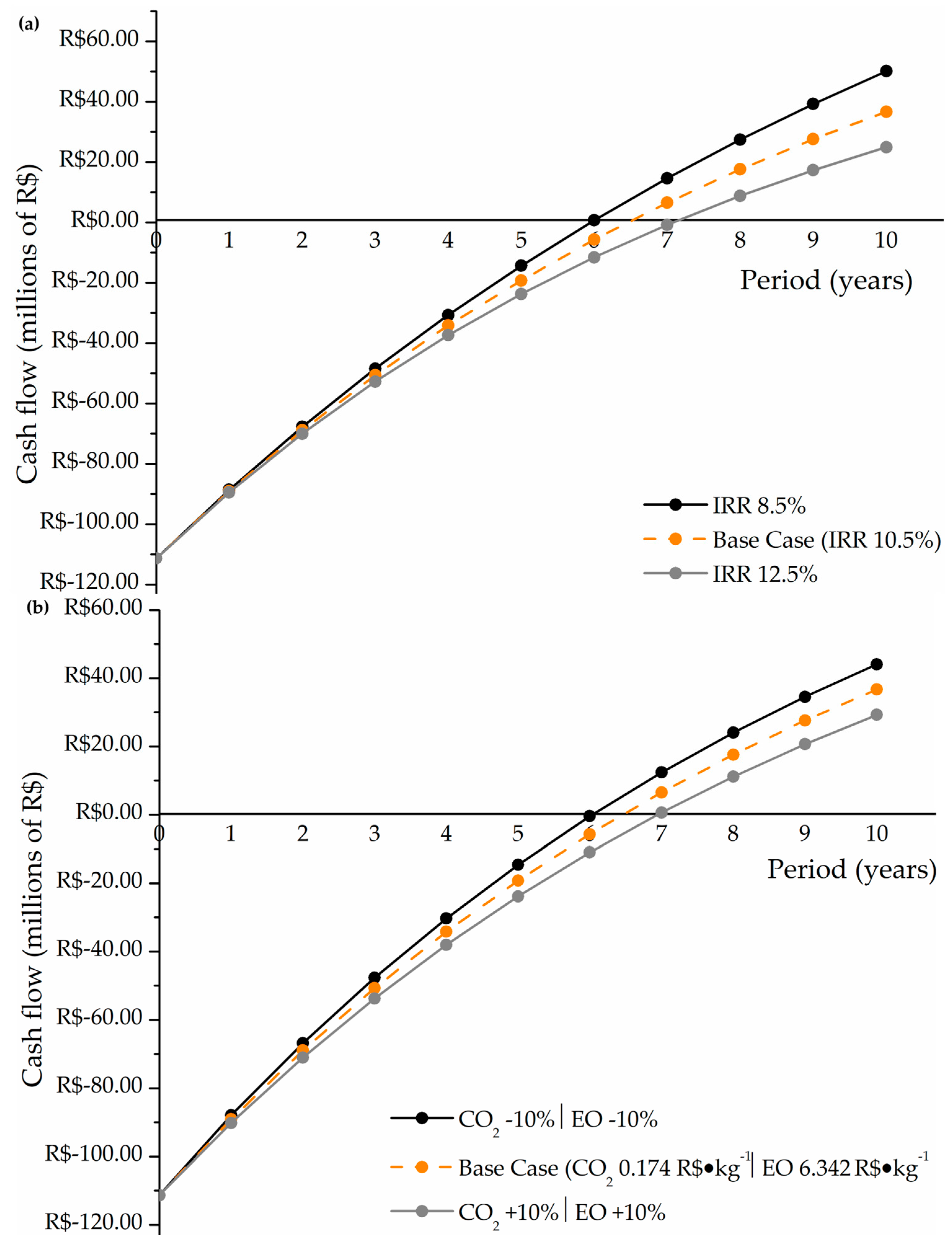
With the CAPEX, OPEX, and revenue values obtained, it was possible to find the financial indicators to assess the project’s viability, the NPV, and the discounted payback, which considers the interest rate adjustment in future years. These results, including the behavior of the sensitivity analysis purposed for the economic assessment, can be observed in Figure 5.
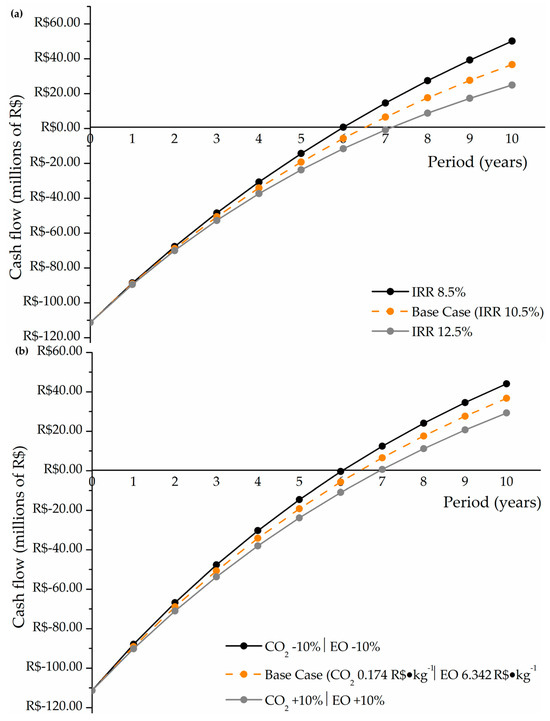
Figure 5.
(a) Return on investment sensitivity analysis for the interest rate adjustment; (b) return on investment sensitivity analysis for the raw materials prices.
In these figures, year 0 corresponds to the start of the plant operation, and the initial negative values describe the plant construction costs. For the base case, the final economic outcome obtained was positive and viable, considering that the project paid for itself within the estimated lifespan of the plant and resulted in an NPV of approximately R$36.7 million at the end of the projected 10 years, with a payback of 6.47 years. The recovery and commercialization of excess methanol contributed to a positive cash flow starting in the second half of the sixth year, accelerating the recovery of the initial investment and yielding a positive net present value. However, considering the sensitivity analysis, Figure 5a shows that the results of the economic analyses varying the IRR are more sensitive and significant than the results of changes in raw material prices (Figure 5b). In both analyses, the plant makes a valuable profit between MMR$20 and MMR$50 at the end of its lifetime, indicating that small market fluctuations in the stipulated range can double the return on investment. Also, in Figure 5, it is noted that for a plant lifetime of 10 years, all economic scenarios evaluated are economically feasible, with payback times ranging from 6 to 7 years.
For comparison purposes, a similar study conducted by Kontou et al. (2021) [15] can be evaluated, in which the authors assessed DMC production via the same synthetic route, but with methanol obtained commercially and reused after its passage through the reactor. The economic results reported included a shorter payback period of 2.69 years and a significantly higher NPV of R$977.85 million over a 20-year plant lifespan. These results indicate that, from an economic standpoint, the integration of methanol production within the plant was not advantageous, as the specific CAPEX and OPEX (in terms of hourly DMC production) in the current study were R$42.26 MM∙(t/h)−1 and R$113.95 MM∙(t/h)−1, respectively, compared to R$22.03 MM/(t/h) and R$65.87 MM∙(t/h)−1 reported in the literature.
The contributions of the integrated methanol synthesis process to the elevated CAPEX and OPEX values are noteworthy. The equipment for this process accounts for approximately 41% of the total equipment acquisition cost, with particular emphasis on the gasifier and the reactor for methanol production, which together amount to R$16.9 million. Additionally, operational costs associated with electricity for methanol production are more than 10 times higher than for the DMC production process, indirectly impacting other operational expenses.
Another important factor influencing the economic feasibility of the process is its integration into existing industrial facilities. Given the significant contribution of methanol synthesis to CAPEX and OPEX, strategies such as heat recovery, raw material recycling, and energy efficiency improvements become essential to offset costs and enhance profitability. In particular, the high electricity consumption of methanol production, which indirectly increases operational expenses, could be mitigated through process integration with cogeneration systems or renewable energy sources. Additionally, optimizing reactor configurations and separation processes with a focus on economic efficiency could reduce the impact of equipment-related costs, while maintaining high selectivity and minimizing catalyst deactivation. These factors must be carefully considered to improve the economic competitiveness of the integrated production route compared to conventional alternatives.
3.3. CO2 Emissions Resulting from Net Balance
To evaluate the processes from an environmental point of view, Table 9 presents the CO2 input and output flows for both, considering the criteria listed in Equation (13) to obtain the net value of gas emissions.

Table 9.
Net value of CO2 emissions from process integration.
The CO2 emissions balance revealed net values of −13.35 MMt∙year−1 for DMC and 78.06 MMt∙year−1 for methanol, resulting in a total of 64.71 MMt∙year−1, which corresponds to a specific generation of 3.41 kgCO2∙(kgDMC)−1. These results indicate that the methanol production process negatively impacted the environmental profile of the integrated process, as net emissions became positive primarily due to the high electricity consumption required for methanol production.
When comparing these results with the literature, a significant disparity is observed. Kontou et al. (2021) [15] reported a specific emission of 0.49 kgCO2∙(kgDMC)−1, while Kongpanna et al. (2014) [27] reported 0.45 kgCO2∙(kgDMC)−1. Both studies considered DMC production using commercially acquired methanol, contributing to significantly lower emissions than the process analyzed in this study. Thus, without considering the complete product lifecycle, it is evident that integration was not environmentally advantageous in the current configuration of the synthetic routes for DMC and methanol.
4. Conclusions
The present study assessed the technical, economic, and environmental feasibility of DMC and methanol’s integrated production from sugarcane industries’ biomass gasification, contributing to the broader discussion on sustainable chemical production. The proposed integration, which reuses CO2 captured from the fermentation process and uses sugarcane bagasse as feedstock, demonstrated promising technical results, with product conversions and purities close to the values reported in the literature. Economic feasibility was also confirmed, with an NPV of approximately R$36.7 million at the end of 10 years, indicating potential financial feasibility under certain conditions. Also, compared to other existing routes, the EC-based synthesis shows lower net CO2 emissions (0.452 kg CO2/kg DMC) than the Bayer process (0.522 kg CO2/kg DMC) and urea route (2.938 kg CO2/kg DMC). Additionally, its energy consumption (9.13 MJ/kg DMC) is significantly lower than alternatives such as the urea route (59.36 MJ/kg DMC) [27].
However, despite these environmental advantages, the integration of methanol production from biomass introduces economic challenges, increasing CAPEX and OPEX, especially due to the high energy consumption of the reformer and gasifier. Then, a comparative analysis with other studies revealed that, while technically feasible, the integration within the plant was not economically advantageous. The high CAPEX and OPEX, primarily due to the cost of equipment for methanol production, contributed to a lower financial return than alternative routes that utilize commercially acquired methanol. Furthermore, the integration proved environmentally unfavorable, with net CO2 emissions exceeding those reported in studies that exclude alcohol synthesis. The high electricity consumption for methanol production negatively impacted the environmental profile of the process, resulting in specific emissions of 3.41 kgCO2∙(kgDMC)−1.
These findings suggest that although the integrated route offers advantages regarding waste utilization and CO2 capture, its competitiveness is strongly influenced by capital costs, energy efficiency, and process integration strategies. Future research should focus on optimizing energy consumption, exploring alternative feedstocks, and improving methanol synthesis efficiency to enhance the overall sustainability of this production route. Expanding the analysis to different energy sources and regional economic conditions could further refine the assessment of its viability in various industrial contexts.
Author Contributions
Conceptualization, L.B.-R.; methodology, R.F.C.; writing—original draft preparation, O.V.J.; writing—original draft preparation, F.L.d.A.B.; writing—original draft preparation, T.L.d.S.; writing—review and editing A.L.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stern, N. The Economics of Climate Change: The Stern Review; Cambridge University Press: Cambridge, UK, 2007; pp. 1–692. ISBN 9780521877. [Google Scholar] [CrossRef]
- Leung, J.Y.S.; Russell, B.D.; Connell, S.D. Adaptive Responses of Marine Gastropods to Heatwaves. One Earth 2019, 1, 374–381. [Google Scholar] [CrossRef]
- U.S. Department of Energy, National Energy Technology Laboratory. Carbon Dioxide Capture Handbook; DOE/NETL Carbon Capture Program: Wilsonville, AL, USA, August 2015. Available online: https://netl.doe.gov/sites/default/files/netl-file/Carbon-Dioxide-Capture-Handbook-2015.pdf (accessed on 21 January 2025).
- Rochedo, P.R.R.; Costa, I.V.L.; Império, M.; Hoffmann, B.S.; Merschmann, P.R.D.C.; Oliveira, C.C.N.; Szklo, A.; Schaeffer, R. Carbon Capture Potential and Costs in Brazil. J. Clean. Prod. 2016, 131, 280–295. [Google Scholar] [CrossRef]
- Chaban, V.V.; Andreeva, N.A.; dos Santos, L.M.; Einloft, S. Revolutionary Valorization of Carbon Dioxide into Dimethyl Carbonate is Catalyzed by Sodium Chloride: Cheap, Clean, One-Pot, and Water-Free Synthesis. arXiv 2023, arXiv:2312.02582. [Google Scholar]
- Ohno, H.; Ikhlayel, M.; Tamura, M.; Nakao, K.; Suzuki, K.; Morita, K.; Kato, Y.; Tomishige, K.; Fukushima, Y. Direct Dimethyl Carbonate Synthesis from CO2 and Methanol Catalyzed by CeO2 and Assisted by 2-Cyanopyridine: A Cradle-to-Gate Greenhouse Gas Emission Study. Green Chem. 2021, 23, 457–469. [Google Scholar] [CrossRef]
- Gu, X.; Zhang, X.; Yang, Z.; Shen, W.; Deng, C.; Zeng, S.; Zhang, X. Technical-Environmental Assessment of CO2 Conversion Process to Dimethyl Carbonate/Ethylene Glycol. J. Clean. Prod. 2021, 288, 125598. [Google Scholar] [CrossRef]
- Pohjakallio, M.; Kordas, M. Dimethyl Carbonate: Green Solvent and Ambident Reagent. In Green Chemical Reactions; Morandi, A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 215–230. [Google Scholar] [CrossRef]
- Fiorani, G.; Perosa, A.; Selva, M. Dimethyl Carbonate: A Versatile Reagent for a Sustainable Valorization of Renewables. Green Chem. 2018, 20, 288–322. [Google Scholar] [CrossRef]
- Souza, L.F.S.; Ferreira, P.R.R.; De Medeiros, J.L.; Alves, R.M.B.; Araújo, O.Q.F. Production of DMC from CO2 via Indirect Route: Technical-Economical-Environmental Assessment and Analysis. ACS Sustain. Chem. Eng. 2014, 2, 62–69. [Google Scholar] [CrossRef]
- Kohlii, K.; Sharma, B.; Panchal, C.B. Dimethyl Carbonate: Review of Synthesis Routes and Catalysts Used. Energies 2022, 15, 5133. [Google Scholar] [CrossRef]
- Lopes, E.J.C.; Ribeiro, A.P.C.; Martins, M.D.R.S.L. New Trends in the Conversion of CO2 to Cyclic Carbonates. Catalysts 2020, 10, 479. [Google Scholar] [CrossRef]
- Gao, D.; Li, W.; Wang, H.; Wang, G.; Cai, R. Heterogeneous Catalysis for CO2 Conversion into Chemicals and Fuels. Trans. Tianjin Univ. 2022, 28, 245–264. [Google Scholar] [CrossRef]
- Zhao, T.; Han, Y.; Sun, Y. Novel reaction route for dimethyl carbonate synthesis from CO2 and methanol. Fuel Process. Technol. 2000, 62, 187–194. [Google Scholar] [CrossRef]
- Kontou, V.; Grimekis, D.; Braimakis, K.; Karellas, S. Techno-Economic Assessment of Dimethyl Carbonate Production Based on Carbon Capture and Utilization and Power-to-Fuel Technology. Renew. Sustain. Energy Rev. 2022, 157, 112006. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Wang, Q.-Q.; Wang, F.; Xu, J.; Xue, B. Efficient Synthesis of Dimethyl Carbonate via Transesterification of Ethylene Carbonate Catalyzed by CN-SiAlO Composites. Catal. Lett. 2024, 154, 47–55. [Google Scholar] [CrossRef]
- Rooney, S.; Smith, J.; Brown, T. Methanol Fuel Production, Utilization, and Techno-Economy: A Review. Environ. Chem. Lett. 2022, 20, 45–60. [Google Scholar] [CrossRef]
- Kumar, S.; Patel, A.; Ramesh, V. A Critical Review of the Sustainable Production and Application of Methanol as a Biochemical and Bioenergy Carrier. Biomass 2022, 5, 1. [Google Scholar] [CrossRef]
- Motta, I.L.; Miranda, N.T.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane Bagasse Gasification: Simulation and Analysis of Different Operating Parameters, Fluidizing Media, and Gasifier Types. Biomass Bioenergy 2019, 122, 433–445. [Google Scholar] [CrossRef]
- Bustan, M.D.; Haryati, S.; Hadiah, F.; Selpiana, S.; Huda, A. Syngas Production Improvement of Sugarcane Bagasse Conversion Using an Electromagnetic Field-Assisted Pyrolysis Reactor. Processes 2020, 8, 252. [Google Scholar] [CrossRef]
- Deng, R.; Wu, J.; Huang, Z.; Feng, Z.; Hu, W.; Tang, Y.; Tan, H.; Zhang, H.; Zairov, R.; Pan, Z. Biogas to Chemicals: A Review of the State-of-the-Art Conversion Technologies. Biomass Convers. Biorefin. 2024, 14, 243–259. [Google Scholar] [CrossRef]
- López, A.F.; Rodríguez, T.L.; Abdolmaleki, S.F.; Matínez, M.G.; Bugallo, P.M.B. From Biogas to Biomethane: An In-Depth Review of Upgrading Technologies That Enhance Sustainability and Reduce Greenhouse Gas Emissions. Appl. Sci. 2024, 14, 2342. [Google Scholar] [CrossRef]
- Meena, P.K.; Pal, A. A comprehensive review on methane enrichment in biogas through the purification process using biomass-based adsorbents. Biomass Convers. Biorefin. 2024, 14, 605–618. [Google Scholar] [CrossRef]
- IEA Bioenergy. Country Report Summary 2024: Biogas Developments Across the World. Available online: https://www.ieabioenergy.com/wp-content/uploads/2024/10/IEA_Bioenergy_T37_CountryReportSummary_2024.pdf (accessed on 21 January 2025).
- Wang, J.Q.; Sun, J.; Shi, C.Y.; Cheng, W.G.; Zhang, X.P.; Zhang, S.J. Synthesis of Dimethyl Carbonate from CO2 and Ethylene Oxide Catalyzed by K2CO3-Based Binary Salts in the Presence of H2O. Green Chem. 2011, 13, 3213–3217. [Google Scholar] [CrossRef]
- Fang, Y.J.; Xiao, W. De Experimental and Modeling Studies on a Homogeneous Reactive Distillation System for Dimethyl Carbonate Synthesis by Transesterification. Sep. Purif. Technol. 2004, 34, 255–263. [Google Scholar] [CrossRef]
- Kongpanna, P.; Pavarajarn, V.; Gani, R.; Assabumrungrat, S. Techno-Economic Evaluation of Different CO2-Based Processes for Dimethyl Carbonate Production. Chem. Eng. Res. Des. 2015, 93, 496–510. [Google Scholar] [CrossRef]
- Copa Rey, J.R.; Tamayo Pacheco, J.J.; António da Cruz Tarelho, L.; Silva, V.; Cardoso, J.S.; Silveira, J.L.; Tuna, C.E. Evaluation of Cogeneration Alternative Systems Integrating Biomass Gasification Applied to a Brazilian Sugar Industry. Renew. Energy 2021, 178, 318–333. [Google Scholar] [CrossRef]
- Moura, C.P.C.; de Araujo Filho, M.A.; Villardi, H.G.D.; Cavalcante, R.M.; Young, A.F. Process Simulation and Economic Evaluation of an Integrated Production Plant for Methanol, Acetic Acid and DME Synthesis via Sugarcane Bagasse Gasification. Energy Convers. Manag. 2023, 286, 117051. [Google Scholar] [CrossRef]
- Peters, M.S.; Timmerhaus, K.D. Plant Design and Economics for Chemical Engineers; McGraw-Hill: New York, NY, USA, 2003; Volume 5. [Google Scholar] [CrossRef]
- BCB Banco Central Do Brasil. Available online: https://www.bcb.gov.br (accessed on 4 July 2024).
- Turton, R.; Bailie, R.C.; Whiting, W.B.; Shaeiwitz, J.; Bhattacharrya, D. Analysis, Synthesis, and Design of Chemical Processes; Prentice Hall: Hoboken, NJ, USA, 2018; Volume 5. [Google Scholar] [CrossRef]
- Bonfim-Rocha, L.; Gimenes, M.L.; Bernardo de Faria, S.H.; Silva, R.O.; Esteller, L.J. Multi-Objective Design of a New Sustainable Scenario for Bio-Methanol Production in Brazil. J. Clean. Prod. 2018, 187, 1043–1056. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).