Optimized Biodiesel Production from Pumpkin (Cucurbita pepo L.) Seed Oil: A Response Surface Methodology for Microwave-Assisted Transesterification
Abstract
1. Introduction
2. Materials and Methods
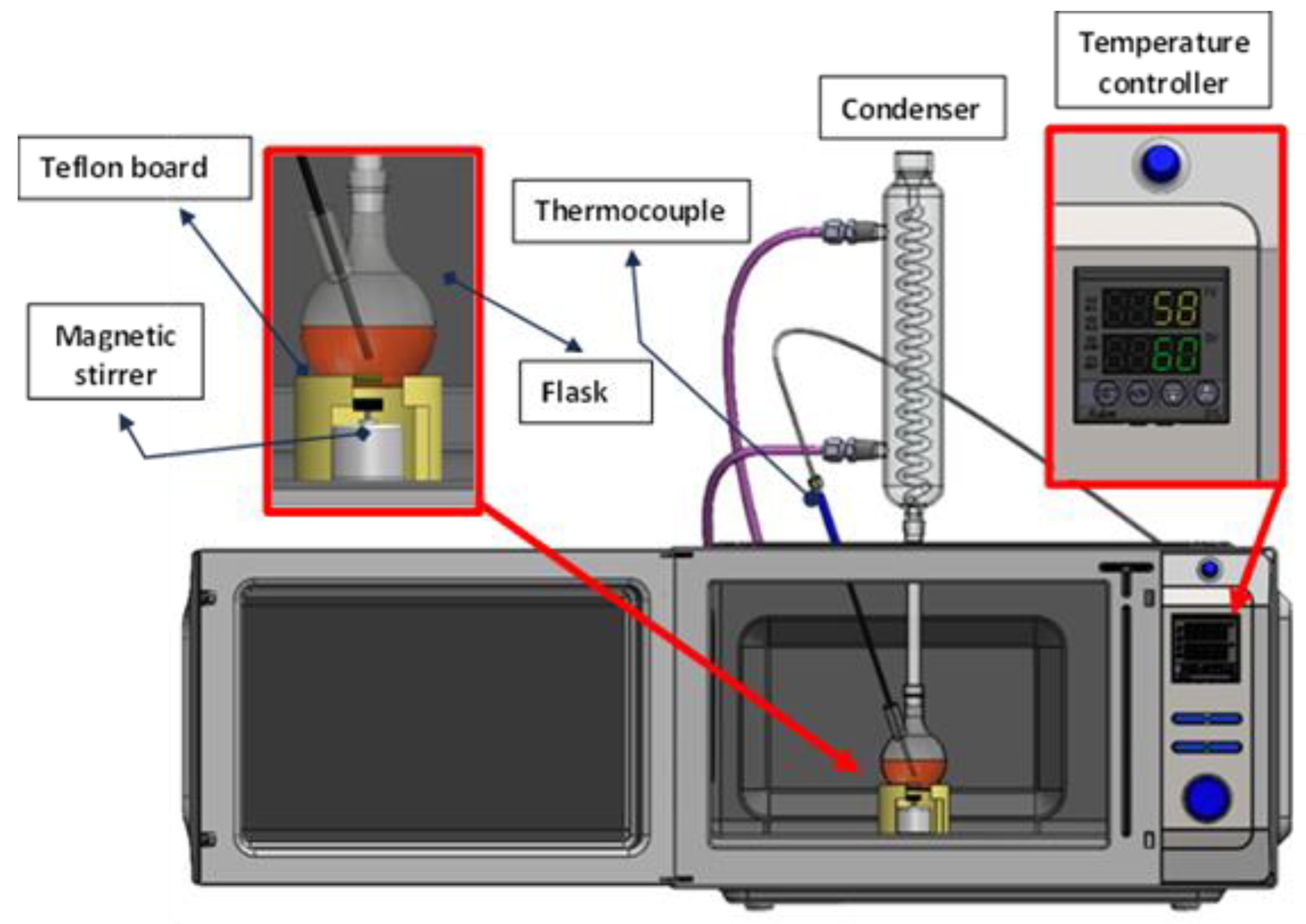
2.1. Experimental Set-Up and Procedure
2.2. Determination of Fuel Properties
2.3. Box–Behnken Experimental Design
3. Results and Discussion
A2 + 1.63875 × B2 − 4.36625 × C2
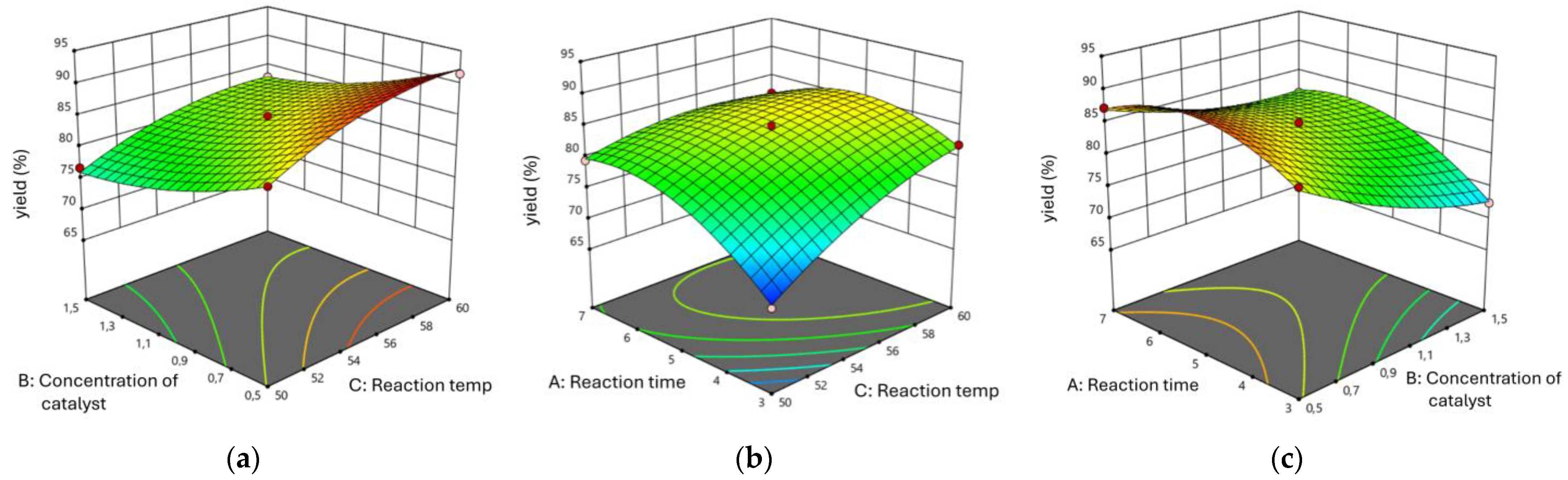
3.1. Effect of Reaction Parameters on Biodiesel Yield
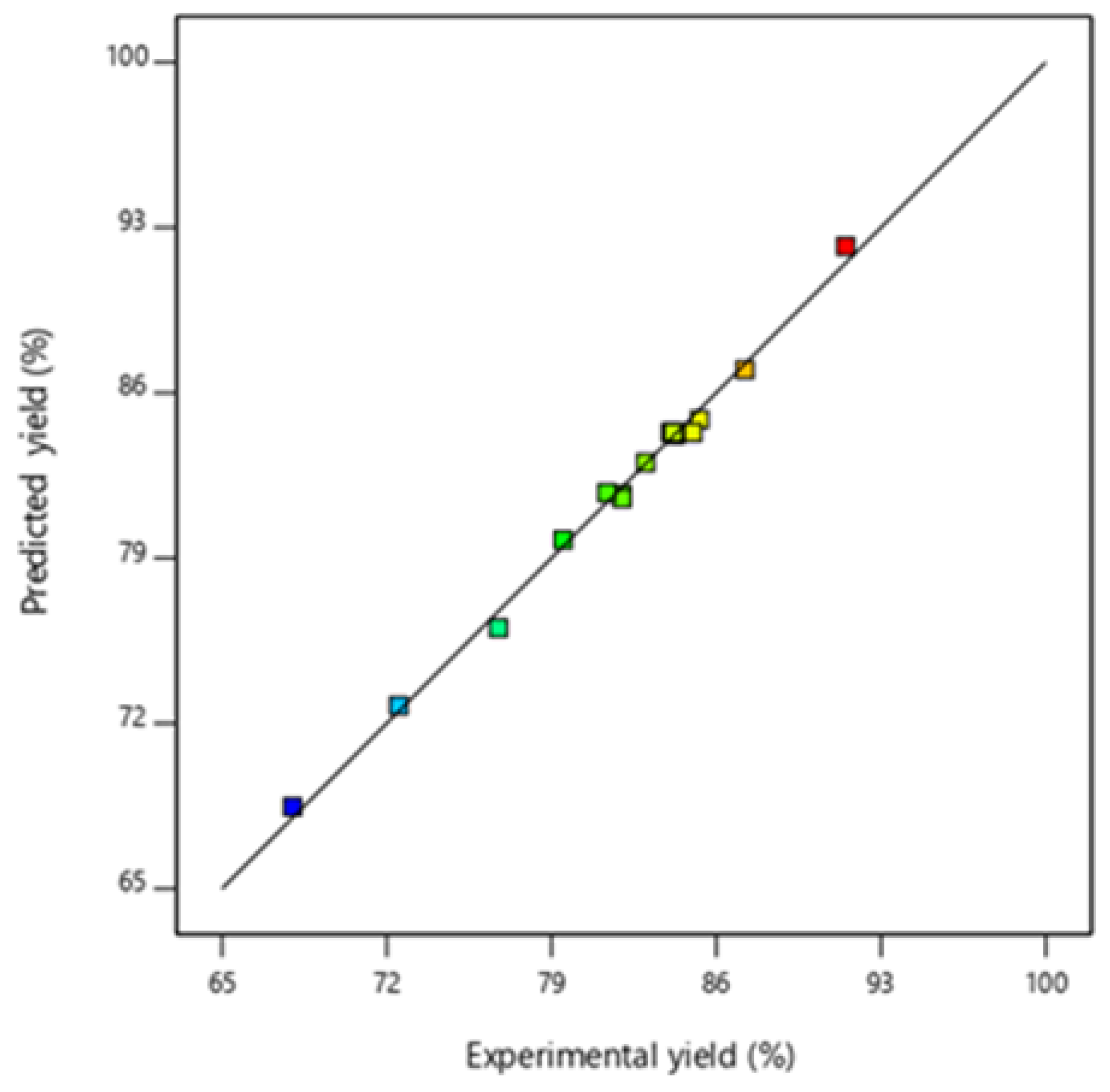
3.2. Optimization and Model Validation (Esterification)
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pasias, S.; Barakos, N.; Alexopoulos, C.; Papayannakos, N. Heterogeneously Catalyzed Esterification of FFAs in Vegetable Oils. Chem. Eng. Technol. 2006, 29, 1365–1371. [Google Scholar] [CrossRef]
- Rhithuparna, D.; Ghosh, N.; Lalthazuala Rokhum, S.; Halder, G. Current Progress and Future Outlooks of Microwave-Irradiated Biodiesel Production: A Holistic Review. Chem. Eng. J. 2024, 482, 149033. [Google Scholar] [CrossRef]
- Visković, J.; Dunđerski, D.; Adamović, B.; Jaćimović, G.; Latković, D.; Vojnović, Đ. Toward an Environmentally Friendly Future: An Overview of Biofuels from Corn and Potential Alternatives in Hemp and Cucurbits. Agronomy 2024, 14, 1195. [Google Scholar] [CrossRef]
- Schinas, P.; Karavalakis, G.; Davaris, C.; Anastopoulos, G.; Karonis, D.; Zannikos, F.; Stournas, S.; Lois, E. Pumpkin (Cucurbita pepo L.) Seed Oil as an Alternative Feedstock for the Production of Biodiesel in Greece. Biomass Bioenergy 2009, 33, 44–49. [Google Scholar] [CrossRef]
- Ramli, A.; Farooq, M.; Naeem, A.; Khan, S.N.; Hummayun, M.; Iqbal, A.; Ahmed, S.A.; Shah, L.A. Bifunctional Heterogeneous Catalysts for Biodiesel Production Using Low Cost Feedstocks: A Future Perspective. Front. Bioenergy Biofuels 2017, 285, 285–299. [Google Scholar] [CrossRef]
- Ogbu, I.M.; Ajiwe, V.I.E. Biodiesel Production via Esterification of Free Fatty Acids from Cucurbita Pepo L. Seed Oil: Kinetic Studies. Int. J. Sci. Technol. 2013, 2, 616–621. [Google Scholar]
- Nüchter, M.; Müller, U.; Ondruschka, B.; Tied, A.; Lautenschläger, W. Microwave-Assisted Chemical Reactions. Chem. Eng. Technol. 2003, 26, 1207–1216. [Google Scholar] [CrossRef]
- Bogdal, D. Microwave Assisted Organic Synthesis-One Hundred Reaction Procedures; Elsevier Publications: Amsterdam, The Netherlands, 2005; pp. 13–14. [Google Scholar]
- Loupy, A. Microwave in Organic Synthesis; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Azcan, D.N. Yemeklik Atık Yağın Mikrodalga Destekli Transesterifikasyonu İle Biyodizel Eldesi Ve Moleküler Distilasyonla Saflaştırılması Proje No: 110M011; TUBITAK: Eskişehir, Türkiye, 2011. [Google Scholar]
- Azcan, N.; Danisman, A. Alkali Catalyzed Transesterification of Cottonseed Oil by Microwave Irradiation. Fuel 2007, 86, 2639–2644. [Google Scholar] [CrossRef]
- Sinha, S.; Agarwal, A.K.; Garg, S. Biodiesel Development from Rice Bran Oil: Transesterification Process Optimization and Fuel Characterization. Energy Convers. Manag. 2008, 49, 1248–1257. [Google Scholar] [CrossRef]
- Azzena, U.; Montenero, A.; Carraro, M.; Crisafulli, R.; De Luca, L.; Gaspa, S.; Muzzu, A.; Nuvoli, L.; Polese, R.; Pisano, L.; et al. Recovery, Purification, Analysis and Chemical Modification of a Waste Cooking Oil. Waste Biomass Valorization 2023, 14, 145–157. [Google Scholar] [CrossRef]
- Rani, S.; Kaur, S.; Kaur, H. Production of Biodiesel from Non- Edible Oil (WCO). Int. Res. J. Sci. Technol. 2020, 1, 268–271. [Google Scholar] [CrossRef]
- Özgür, C. Soya ve M ı s ı r Ya Ğı Ndan Biyodizel Üretiminin Yan ı t Yüzey Metodu Kullan ı Larak Optimizasyonu Using Response Surface Methodology to Optimize Biodiesel Production from Soybean and Corn Oil. Çukurova Univ. J. Fac. Eng. 2021, 36, 197–206. [Google Scholar]
- Ansori, A.; Mahfud, M. Box-Behnken Design for Optimization on Biodiesel Production from Palm Oil and Methyl Acetate Using Ultrasound Assisted Interesterification Method. Period. Polytech. Chem. Eng. 2022, 66, 30–42. [Google Scholar] [CrossRef]
- Durgut, M.R.; Aktaş, T.; Kayİşoğlu, S. Research Article Determination of Optimum Reaction Conditions in Biodiesel Production from Microalgae Oil Using Microwave Irradiation. J. Sci. Eng. Res. 2017, 4, 111–115. [Google Scholar]
- Danisman, A. Bitkisel Yağlardan Değerli Kimyasallar ve Biyodizel Üretimi. Master’s Thesis, Anadolu University, Eskişehir, Türkiye, 2008. [Google Scholar]
- Noureddini, H. Kinetics of Transesterification of Soybean Oil. J. Am. Oil Soc. Chem. 1997, 74, 1457. [Google Scholar] [CrossRef]
- Lou, W.Y.; Zong, M.H.; Duan, Z.Q. Efficient Production of Biodiesel from High Free Fatty Acid-Containing Waste Oils Using Various Carbohydrate-Derived Solid Acid Catalysts. Bioresour. Technol. 2008, 99, 8752–8758. [Google Scholar] [CrossRef]
- Zheng, S.; Kates, M.; Dubé, M.A.; McLean, D.D. Acid-Catalyzed Production of Biodiesel from Waste Frying Oil. Biomass Bioenergy 2006, 30, 267–272. [Google Scholar] [CrossRef]
- Abbah, E.C.; Nwandikom, G.I.; Egwuonwu, C.C.; Nwakuba, N.R. Effect of Reaction Temperature on the Yield of Biodiesel from Neem Seed Oil. Am. J. Energy Sci. 2016, 3, 16–20. [Google Scholar]
- Narwal, S.K.; Saun, N.K.; Dogra, P.; Chauhan, G.; Gupta, R. Production and Characterization of Biodiesel Using Nonedible Castor Oil by Immobilized Lipase from Bacillus Aerius. Biomed. Res. Int. 2015, 2015, 281934. [Google Scholar] [CrossRef]
- Kumari, A.; Mahapatra, P.; Garlapati, V.K.; Banerjee, R. Enzymatic Transesterification of Jatropha Oil. Biotechnol. Biofuels 2009, 2, 1. [Google Scholar] [CrossRef]
- Hundie, K.B. Optimization of Biodiesel Production Parameters from Cucurbita Maxima Waste Oil Using Microwave Assisted via Box-Behnken Design Approach. J. Chem. 2022, 2022, 8516163. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Guo, Y. Transesterification of Neat and Used Frying Oil: Optimization for Biodiesel Production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel Production Process Optimization and Characterization to Assess the Suitability of the Product for Varied Environmental Conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Vicente, G.; Martínez, M.; Aracil, J. Integrated Biodiesel Production: A Comparison of Different Homogeneous Catalysts Systems. Bioresour. Technol. 2004, 92, 297–305. [Google Scholar] [CrossRef]
- Anantapinitwatna, A.; Ngaosuwan, K.; Kiatkittipong, W.; Wongsawaeng, D.; Anantpinijwatna, A.; Quitain, A.T.; Assabumrungrat, S. Water Influence on the Kinetics of Transesterification Using CaO Catalyst to Produce Biodiesel. Fuel 2021, 296, 120653. [Google Scholar] [CrossRef]
- Encinar, J.M.; González, J.F.; Rodríguez-Reinares, A. Biodiesel from Used Frying Oil. Var. Affect. Yields Charact. Biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Meher, L.C.; Dharmagadda, V.S.S.; Naik, S.N. Optimization of Alkali-Catalyzed Transesterification of Pongamia Pinnata Oil for Production of Biodiesel. Bioresour. Technol. 2006, 97, 1392–1397. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of Biodiesel through Base-Catalyzed Transesterification of Safflower Oil Using an Optimized Protocol. Energy Fuels 2008, 22, 1306–1312. [Google Scholar] [CrossRef]
- Buasri, A.; Sirikoom, P.; Pattane, S.; Buachum, O.; Loryuenyong, V. Process Optimization of Biodiesel from Used Cooking Oil in a Microwave Reactor: A Case of Machine Learning and Box–Behnken Design. ChemEngineering 2023, 7, 65. [Google Scholar] [CrossRef]
- Sterpu, A.E.; Simedrea, B.G.; Chis, T.V.; Săpunaru, O.V. Corrosion Effect of Biodiesel-Diesel Blend on Different Metals/Alloy as Automotive Components Materials. Fuels 2024, 5, 17–32. [Google Scholar] [CrossRef]



| Process Parameters | Units | Levels | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| (A) Reaction Temp | °C | 50 | 55 | 60 |
| (B) KOH catalyst concentration | (w/w)% | 0.5 | 1 | 1.5 |
| (C) Reaction time | min | 3 | 5 | 7 |
| No | Temp (°C) | Loading KOH Catalyst (% wt) | Time (min) | Reaction Yield % | Predicted Yield % |
|---|---|---|---|---|---|
| 1 | 55 | 1 | 5 | 84.26 | 84.32 |
| 2 | 60 | 1 | 7 | 82.00 | 81.53 |
| 3 | 55 | 0.5 | 7 | 87.22 | 86.98 |
| 4 | 50 | 1.5 | 5 | 76.75 | 76.04 |
| 5 | 50 | 0.5 | 5 | 84.26 | 84.21 |
| 6 | 60 | 1,5 | 5 | 83.00 | 83.05 |
| 7 | 55 | 1 | 5 | 84.06 | 84.32 |
| 8 | 55 | 1.5 | 7 | 81.35 | 81.77 |
| 9 | 60 | 1 | 3 | 82.00 | 81.71 |
| 10 | 55 | 1 | 5 | 85.00 | 84.32 |
| 11 | 55 | 0.5 | 3 | 85.29 | 84.87 |
| 12 | 55 | 1 | 5 | 84.17 | 84.32 |
| 13 | 60 | 0.5 | 5 | 91.50 | 92.21 |
| 14 | 55 | 1.5 | 3 | 72.51 | 72.75 |
| 15 | 50 | 1 | 3 | 68.00 | 68.47 |
| 16 | 50 | 1 | 7 | 79.49 | 79.78 |
| 17 | 55 | 1 | 5 | 84.11 | 84.32 |
| R2 (R-Squared) | R2-Adjusted | R2-Pred. | Adeq Prec. | Std. Dev. | Mean | C.V. % |
|---|---|---|---|---|---|---|
| 0.9945 | 0.9873 | 0.9292 | 50.0600 | 0.6183 | 82.06 | 0.7535 |
| Source * | SS | df | MS | F-Value | p Value | |
|---|---|---|---|---|---|---|
| Model | 479.56 | 9 | 53.28 | 139.37 | <0.0001 | Significant |
| A-A | 112.50 | 1 | 112.50 | 294.26 | <0.0001 | |
| B-B | 150.16 | 1 | 150.16 | 392.77 | <0.0001 | |
| C-C | 61.94 | 1 | 61.94 | 162.01 | <0.0001 | |
| AB | 0.2450 | 1 | 0.2450 | 0.6409 | 0.4497 | |
| AC | 33.01 | 1 | 33.01 | 86.33 | <0.0001 | |
| BC | 11.94 | 1 | 11.94 | 31.22 | 0.0008 | |
| A2 | 18.24 | 1 | 18.24 | 47.70 | 0.0002 | |
| B2 | 11.31 | 1 | 11.31 | 29.58 | 0.0010 | |
| C2 | 80.27 | 1 | 80.27 | 209.95 | <0.0001 | |
| Residual | 2.68 | 7 | 0.3823 | |||
| Lack of Fit | 2.08 | 3 | 0.6920 | 4.61 | 0.0869 | Not significant |
| Pure Error | 0.6002 | 4 | 0.1501 | |||
| Cor Total | 482.23 | 16 |
| Properties | Unit | European Committee for Standardization | Biodiesel Experiments | |
|---|---|---|---|---|
| Min | Max | |||
| Efficiency | % | 90 | - | 91.7 ± 0.56 |
| Density, 25 °C | kg/m3 | 860 | 900 | 884 ± 0.02 |
| Viscosity, 40 °C | mm2/s | 3.5 | 5.0 | 4.17 ± 0.17 |
| Flash Point | °C | 120 | - | 175 ± 0.37 |
| Cloud Point | °C | - | - | 3 ± 0.08 |
| Pour Point | °C | - | - | −1 ± 0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Durgut, M.R. Optimized Biodiesel Production from Pumpkin (Cucurbita pepo L.) Seed Oil: A Response Surface Methodology for Microwave-Assisted Transesterification. Processes 2025, 13, 572. https://doi.org/10.3390/pr13020572
Durgut MR. Optimized Biodiesel Production from Pumpkin (Cucurbita pepo L.) Seed Oil: A Response Surface Methodology for Microwave-Assisted Transesterification. Processes. 2025; 13(2):572. https://doi.org/10.3390/pr13020572
Chicago/Turabian StyleDurgut, Mehmet Recai. 2025. "Optimized Biodiesel Production from Pumpkin (Cucurbita pepo L.) Seed Oil: A Response Surface Methodology for Microwave-Assisted Transesterification" Processes 13, no. 2: 572. https://doi.org/10.3390/pr13020572
APA StyleDurgut, M. R. (2025). Optimized Biodiesel Production from Pumpkin (Cucurbita pepo L.) Seed Oil: A Response Surface Methodology for Microwave-Assisted Transesterification. Processes, 13(2), 572. https://doi.org/10.3390/pr13020572






