Recent Advances in Cellulose Nanocrystal Production from Green Methods
Abstract
:1. Introduction
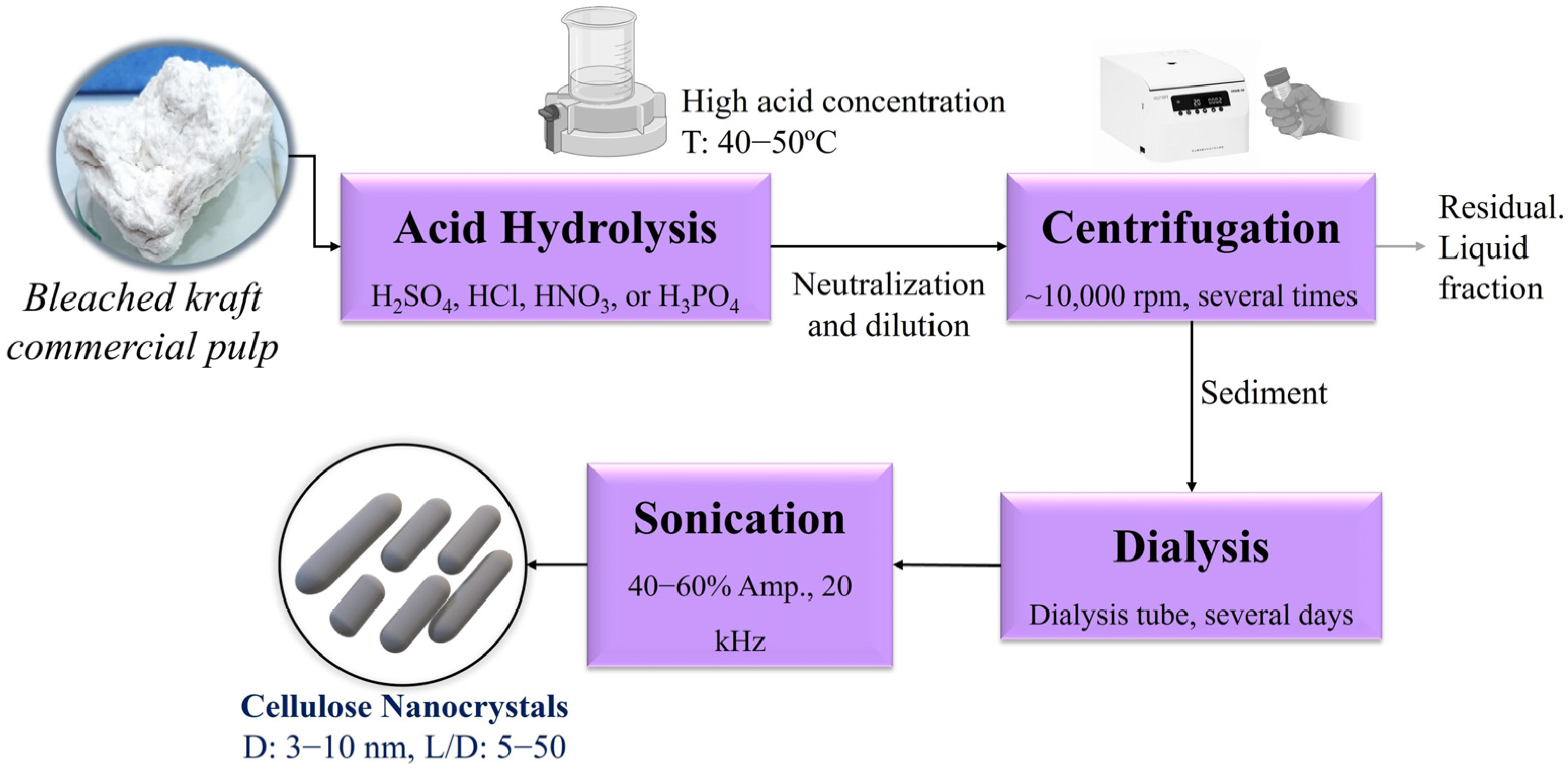
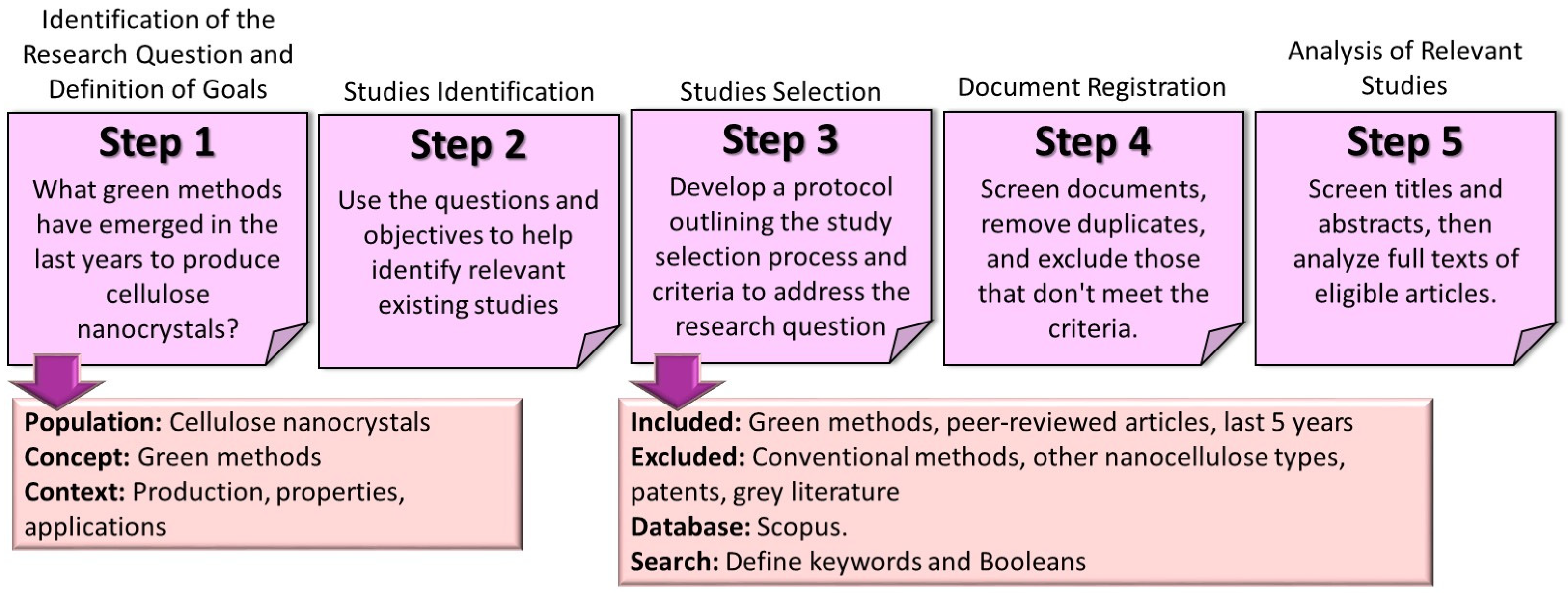
2. Materials and Methods
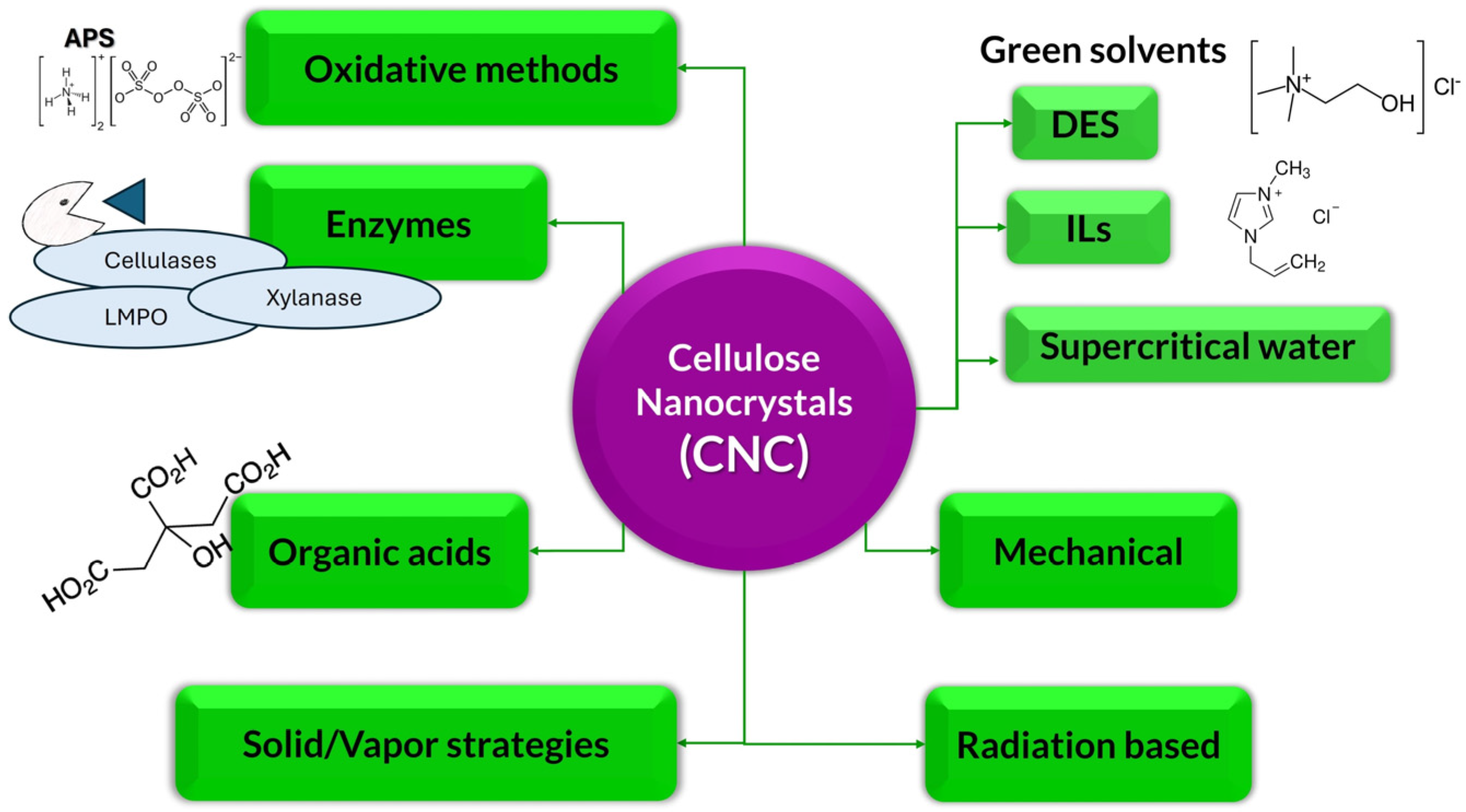
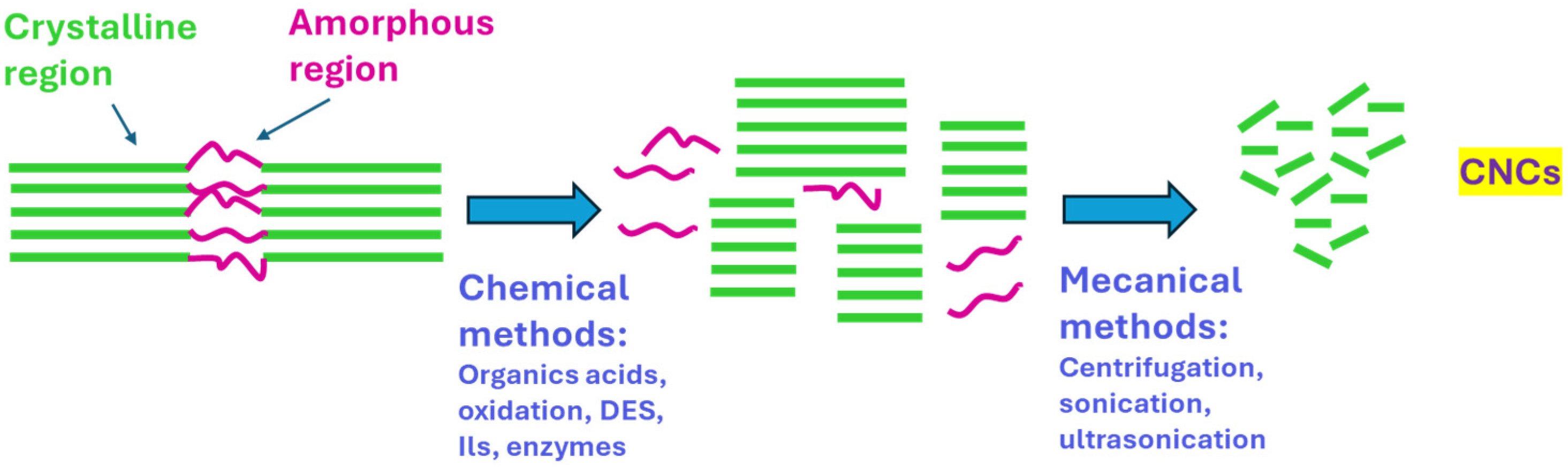
3. Results
3.1. Organic Acids
3.2. Oxidative Methods
3.3. Enzymes
3.4. Green Solvents
3.4.1. Deep Eutectic Solvents
- Recyclable IL dilution ([Hmim] [(HSO4) (H2SO4)]/H2O, 64 wt% IL);
- Recyclable dilution ([Hmim] [(HSO4) (H2SO4)]/H2O, 80 wt% IL);
- Non-recyclable ternary DES (60 wt%/ChCl: OA/30 wt%: PA/10 wt% water).
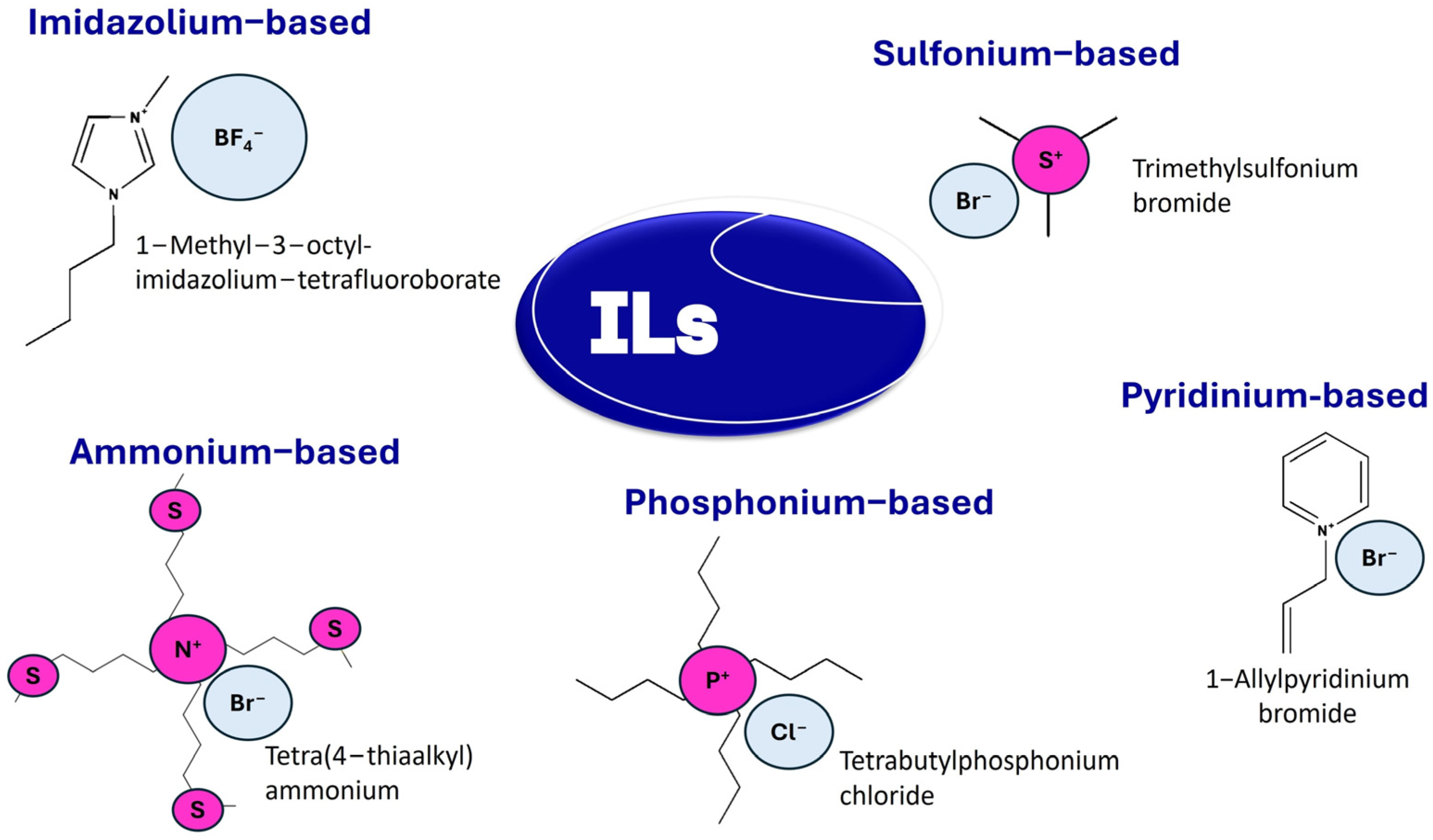
3.4.2. Ionic Liquids
3.4.3. Subcritical Water
3.5. Mechanical Treatments
3.6. Solid/Vapor Strategies
3.7. Radiation-Based Treatments
4. Discussion
4.1. Advantages of the Green Methods
4.2. Current Limitations
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Acetic Acid |
| CA | Citric Acid |
| CNC | Cellulose nanocrystals |
| CNF | Cellulose nanofibers |
| ChCl | Choline Chloride |
| DES | Deep eutectic solvent |
| EBI | Electron beam irradiation |
| FA | Formic Acid |
| GWP | Global warming potential |
| HBA | Hydrogen bond acceptor |
| HBD | Hydrogen bond donor |
| ILs | Ionic liquids |
| MA | Maleic Acid |
| OA | Oxalic Acid |
| SW | Subcritical water |
References
- Arockiasamy, F.S.; Manoharan, B.; Santhi, V.M.; Prakalathan, K.; Periasamy, D.; Dhandapani, A.; Natarajan, V.; Senthilkumar, K.; Muthu Kumar, T.S.; Ilyas, R.A. Navigating the Nano-World Future: Harnessing Cellulose Nanocrystals from Green Sources for Sustainable Innovation. Heliyon 2024, 11, e41188. [Google Scholar] [CrossRef]
- ISO/TS 20477; Standard Terms and Their Definition for Cellulose Nanomaterial. International Organization for Standardization (ISO): Geneva, Switzerland, 2017.
- Noremylia, M.B.; Hassan, M.Z.; Ismail, Z. Recent Advancement in Isolation, Processing, Characterization and Applications of Emerging Nanocellulose: A Review. Int. J. Biol. Macromol. 2022, 206, 954–976. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradji, M.; Hamidon, T.S.; Masruchin, N.; Brosse, N.; Hussin, M.H. Nanocellulose: From Fundamentals to Advanced Applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef]
- Aziz, T.; Fan, H.; Zhang, X.; Haq, F.; Ullah, A.; Ullah, R.; Khan, F.U.; Iqbal, M. Advance Study of Cellulose Nanocrystals Properties and Applications. J. Polym. Environ. 2020, 28, 1117–1128. [Google Scholar] [CrossRef]
- Bondancia, T.J.; de Aguiar, J.; Batista, G.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Production of Nanocellulose Using Citric Acid in a Biorefinery Concept: Effect of the Hydrolysis Reaction Time and Techno-Economic Analysis. Ind. Eng. Chem. Res. 2020, 59, 11505–11516. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Liu, H.; Xie, H.; Xu, T.; Zhao, X.; Liu, Y.; Zhang, X.; Si, C. Highly Efficient and Sustainable Preparation of Carboxylic and Thermostable Cellulose Nanocrystals via FeCl3 -Catalyzed Innocuous Citric Acid Hydrolysis. ACS Sustain. Chem. Eng. 2020, 8, 16691–16700. [Google Scholar] [CrossRef]
- Lv, D.; Du, H.; Che, X.; Wu, M.; Zhang, Y.; Liu, C.; Nie, S.; Zhang, X.; Li, B. Tailored and Integrated Production of Functional Cellulose Nanocrystals and Cellulose Nanofibrils via Sustainable Formic Acid Hydrolysis: Kinetic Study and Characterization. ACS Sustain. Chem. Eng. 2019, 7, 9449–9463. [Google Scholar] [CrossRef]
- Wang, H.; Du, H.; Liu, K.; Liu, H.; Xu, T.; Zhang, S.; Chen, X.; Zhang, R.; Li, H.; Xie, H.; et al. Sustainable Preparation of Bifunctional Cellulose Nanocrystals via Mixed H2SO4/Formic Acid Hydrolysis. Carbohydr. Polym. 2021, 266, 118107. [Google Scholar] [CrossRef]
- Henschen, J.; Li, D.; Ek, M. Preparation of Cellulose Nanomaterials via Cellulose Oxalates. Carbohydr. Polym. 2019, 213, 208–216. [Google Scholar] [CrossRef]
- Tang, F.; Li, Y.; Huang, J.; Tang, J.; Chen, X.; Yu, H.-Y.; Zhou, Y.; Tang, D. An Environmentally Friendly and Economical Strategy to Cyclically Produce Cellulose Nanocrystals with High Thermal Stability and High Yield. Green Chem. 2021, 23, 4866–4872. [Google Scholar] [CrossRef]
- Yu, H.; Abdalkarim, S.Y.H.; Zhang, H.; Wang, C.; Tam, K.C. Simple Process to Produce High-Yield Cellulose Nanocrystals Using Recyclable Citric/Hydrochloric Acids. ACS Sustain. Chem. Eng. 2019, 7, 4912–4923. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Z.; Lin, Y.; Qin, Y.; He, R.; Wang, M.; Sun, Q.; Peng, Y. Nanocellulose from Agro-Industrial Wastes: A Review on Sources, Production, Applications, and Current Challenges. Food Res. Int. 2024, 192, 114741. [Google Scholar] [CrossRef] [PubMed]
- Magagula, L.P.; Masemola, C.M.; Ballim, M.A.; Tetana, Z.N.; Moloto, N.; Linganiso, E.C. Lignocellulosic Biomass Waste-Derived Cellulose Nanocrystals and Carbon Nanomaterials: A Review. Int. J. Mol. Sci. 2022, 23, 4310. [Google Scholar] [CrossRef]
- Nickerson, R.F.; Habrle, J.A. Cellulose Intercrystalline Structure. Ind. Eng. Chem. 1947, 39, 1507–1512. [Google Scholar] [CrossRef]
- Almashhadani, A.Q.; Leh, C.P.; Chan, S.-Y.; Lee, C.Y.; Goh, C.F. Nanocrystalline Cellulose Isolation via Acid Hydrolysis from Non-Woody Biomass: Importance of Hydrolysis Parameters. Carbohydr. Polym. 2022, 286, 119285. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Shang, Y.; Li, B.; Du, H. Sustainable Preparation of Cellulose Nanocrystals: State of the Art and Perspectives. Green Chem. 2022, 24, 9346–9372. [Google Scholar] [CrossRef]
- Gu, J.; Catchmark, J.M.; Kaiser, E.Q.; Archibald, D.D. Quantification of Cellulose Nanowhiskers Sulfate Esterification Levels. Carbohydr. Polym. 2013, 92, 1809–1816. [Google Scholar] [CrossRef]
- Kassab, Z.; Syafri, E.; Tamraoui, Y.; Hannache, H.; Qaiss, A.E.K.; El Achaby, M. Characteristics of Sulfated and Carboxylated Cellulose Nanocrystals Extracted from Juncus Plant Stems. Int. J. Biol. Macromol. 2020, 154, 1419–1425. [Google Scholar] [CrossRef]
- Wang, H.; Xie, H.; Du, H.; Wang, X.; Liu, W.; Duan, Y.; Zhang, X.; Sun, L.; Zhang, X.; Si, C. Highly Efficient Preparation of Functional and Thermostable Cellulose Nanocrystals via H2SO4 Intensified Acetic Acid Hydrolysis. Carbohydr. Polym. 2020, 239, 116233. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, R.K.; Tadesse, M.G. Preparation and Characterization of Carboxylated Cellulose Nanocrystals from Oxytenanthera abyssinica (Ethiopian Lowland Bamboo) Cellulose via Citric Acid Anhydrous Hydrolysis Catalyzed by Sulfuric Acid. Biomass Biorefin. 2024, 14, 28807–28823. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Emerging Technologies for the Production of Nanocellulose from Lignocellulosic Biomass. Carbohydr. Polym. 2022, 285, 119258. [Google Scholar] [CrossRef] [PubMed]
- Arksey, H.; O’Malley, L. Scoping Studies: Towards a Methodological Framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Levac, D.; Colquhoun, H.; O’Brien, K.K. Scoping Studies: Advancing the Methodology. Implement. Sci. 2010, 5, 69. [Google Scholar] [CrossRef]
- Lin, C.; Wang, P.; Liu, Y.; Lv, Y.; Ye, X.; Liu, M.; Zhu, J.Y. Chiral Self-Assembly Behavior of Carboxylated Cellulose Nanocrystals Isolated by Recyclable Oxalic Acid from Degreasing Cotton. ACS Sustain. Chem. Eng. 2023, 11, 8035–8043. [Google Scholar] [CrossRef]
- Bello, F.; Chimphango, A. Non-Catalyzed Formic Acid-Based Process for Preparing Thermally Stable Spherical Cellulose Nanocrystals from Mango Seed Husk. Biomass Convers. Biorefin. 2024, 14, 1133–1148. [Google Scholar] [CrossRef]
- Jia, W.; Liu, Y. Two Characteristic Cellulose Nanocrystals (CNCs) Obtained from Oxalic Acid and Sulfuric Acid Processing. Cellulose 2019, 26, 8351–8365. [Google Scholar] [CrossRef]
- Xu, J.; Wu, Z.; Wu, Q.; Kuang, Y. Acetylated Cellulose Nanocrystals with High-Crystallinity Obtained by One-Step Reaction from the Traditional Acetylation of Cellulose. Carbohydr. Polym. 2020, 229, 115553. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, H.; Mu, T.; Li, Q.; Richel, A. Preparation of Cellulose Nanocrystals from Purple Sweet Potato Peels by Ultrasound-Assisted Maleic Acid Hydrolysis. Food Chem. 2023, 403, 134496. [Google Scholar] [CrossRef]
- Seta, F.T.; An, X.; Liu, L.; Zhang, H.; Yang, J.; Zhang, W.; Nie, S.; Yao, S.; Cao, H.; Xu, Q.; et al. Preparation and Characterization of High Yield Cellulose Nanocrystals (CNC) Derived from Ball Mill Pretreatment and Maleic Acid Hydrolysis. Carbohydr. Polym. 2020, 234, 115942. [Google Scholar] [CrossRef]
- Ji, H.; Xiang, Z.; Qi, H.; Han, T.; Pranovich, A.; Song, T. Strategy towards One-Step Preparation of Carboxylic Cellulose Nanocrystals and Nanofibrils with High Yield, Carboxylation and Highly Stable Dispersibility Using Innocuous Citric Acid. Green Chem. 2019, 21, 1956–1964. [Google Scholar] [CrossRef]
- Bondancia, T.J.; Batista, G.; de Aguiar, J.; Lorevice, M.V.; Cruz, A.J.G.; Marconcini, J.M.; Mattoso, L.H.C.; Farinas, C.S. Cellulose Nanocrystals from Sugar Cane Bagasse Using Organic and/or Inorganic Acids: Techno-Economic Analysis and Life Cycle Assessment. ACS Sustain. Chem. Eng. 2022, 10, 4660–4676. [Google Scholar] [CrossRef]
- Tamo, A.K. Nanocellulose-Based Hydrogels as Versatile Materials with Interesting Functional Properties for Tissue Engineering Applications. J. Mater. Chem. B 2024, 12, 7692–7759. [Google Scholar] [CrossRef]
- Martinsson, A.; Hasani, M.; Potthast, A.; Theliander, H. Modification of Softwood Kraft Pulp Fibres Using Hydrogen Peroxide at Acidic Conditions. Cellulose 2020, 27, 7191–7202. [Google Scholar] [CrossRef]
- Vallejos, M.E.; Olmos, G.V.; Taleb, M.C.; Felissia, F.E.; Ehman, N.V.; Peresin, M.S.; Area, M.C.; Maximino, M.G. Dissolving Pulp from Eucalyptus Sawdust for Regenerated Cellulose Products. Cellulose 2022, 29, 4645–4659. [Google Scholar] [CrossRef]
- Koshani, R.; van de Ven, T.G.M.; Madadlou, A. Characterization of Carboxylated Cellulose Nanocrytals Isolated through Catalyst-Assisted H2O2 Oxidation in a One-Step Procedure. J. Agric. Food Chem. 2018, 66, 7692–7700. [Google Scholar] [CrossRef]
- Kumari, P.; Kumar, A. Advanced Oxidation Process: A Remediation Technique for Organic and Non-Biodegradable Pollutant. Results Surf. Interfaces 2023, 11, 100122. [Google Scholar] [CrossRef]
- Koshani, R.; van de Ven, T.G.M. Carboxylated Cellulose Nanocrystals Developed by Cu-Assisted H2O2 Oxidation as Green Nanocarriers for Efficient Lysozyme Immobilization. J. Agric. Food Chem. 2020, 68, 5938–5950. [Google Scholar] [CrossRef]
- Valls, C.; Cusola, O.; Roncero, M.B. Evaluating the Potential of Ozone in Creating Functional Groups on Cellulose. Cellulose 2022, 29, 6595–6610. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Li, Y.; Xu, H.; Liu, R.; Zhang, Y.; Zhang, Z.; Yuan, Y.; Zong, L.; Zhou, L.; et al. Oxidation with Potassium Ferrate for the One-Pot Preparation of Carboxylated Cellulose II Nanocrystals. Carbohydr. Polym. 2024, 329, 121796. [Google Scholar] [CrossRef]
- Zielińska, D.; Szentner, K.; Waśkiewicz, A.; Borysiak, S. Production of Nanocellulose by Enzymatic Treatment for Application in Polymer Composites. Materials 2021, 14, 2124. [Google Scholar] [CrossRef]
- Kumari, P.; Seth, R.; Meena, A.; Sharma, D. Enzymatic Synthesis of Cellulose Nanocrystals from Lemongrass and Its Application in Improving Anti-Cancer Drug Release, Uptake and Efficacy. Ind. Crops. Prod. 2023, 192, 115933. [Google Scholar] [CrossRef]
- Yupanqui-Mendoza, S.L.; Prado, C.A.; dos Santos, J.C.; Arantes, V. Hydrodynamic Cavitation as a Promising Pretreatment Technology to Enhance the Efficiency of Cellulose Nanocrystal Production via Enzymatic Hydrolysis. Chem. Eng. J. 2023, 472, 144821. [Google Scholar] [CrossRef]
- Pota, G.; Gallucci, N.; Cavasso, D.; Krauss, I.R.; Vitiello, G.; López-Gallego, F.; Costantini, A.; Paduano, L.; Califano, V. Controlling the Adsorption of β-Glucosidase onto Wrinkled SiO2 Nanoparticles To Boost the Yield of Immobilization of an Efficient Biocatalyst. Langmuir 2023, 39, 1482–1494. [Google Scholar] [CrossRef]
- Pota, G.; Sapienza Salerno, A.; Costantini, A.; Silvestri, B.; Passaro, J.; Califano, V. Co-Immobilization of Cellulase and β-Glucosidase into Mesoporous Silica Nanoparticles for the Hydrolysis of Cellulose Extracted from Eriobotrya Japonica Leaves. Langmuir 2022, 38, 5481–5493. [Google Scholar] [CrossRef]
- Xu, C.; Tong, S.; Sun, L.; Gu, X. Cellulase Immobilization to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass: An All-Inclusive Review. Carbohydr. Polym. 2023, 321, 121319. [Google Scholar] [CrossRef]
- De Souza Lima, J.; Boemo, A.P.S.I.; de Araújo, P.H.H.; de Oliveira, D. Immobilization of Endoglucanase on Kaolin by Adsorption and Covalent Bonding. Bioprocess Biosyst. Eng. 2021, 44, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Meng, X.; Ragauskas, A.J.; Lai, C.; Ling, Z.; Huang, C.; Yong, Q. Unlocking the Secret of Lignin-Enzyme Interactions: Recent Advances in Developing State-of-the-Art Analytical Techniques. Biotechnol. Adv. 2022, 54, 107830. [Google Scholar] [CrossRef]
- Dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin–Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kermanshahi-pour, A.; Brar, S.K.; Xu, C.C.; He, Q.S.; Evans, S.; Rainey, J.K. Enzymatic Digestibility of Lignocellulosic Wood Biomass: Effect of Enzyme Treatment in Supercritical Carbon Dioxide and Biomass Pretreatment. Heliyon 2023, 9, e21811. [Google Scholar] [CrossRef]
- De Oliveira Júnior, S.D.; Asevedo, E.A.; de Araújo, J.S.; Brito, P.B.; dos Santos Cruz Costa, C.L.; de Macedo, G.R.; dos Santos, E.S. Enzymatic Extract of Aspergillus Fumigatus CCT 7873 for Hydrolysis of Sugarcane Bagasse and Generation of Cellulose Nanocrystals (CNC). Biomass Convers. Biorefin. 2022, 12, 5515–5526. [Google Scholar] [CrossRef]
- Waghmare, P.; Xu, N.; Waghmare, P.; Liu, G.; Qu, Y.; Li, X.; Zhao, J. Production and Characterization of Cellulose Nanocrystals from Eucalyptus Dissolving Pulp Using Endoglucanases from Myceliophthora thermophila. Int. J. Mol. Sci. 2023, 24, 10676. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Xu, N.; Guo, Y.; Liu, G.; Zhao, J. Preparation of Cellulose Nanocrystals from Commercial Dissolving Pulp Using an Engineered Cellulase System. Bioresour. Bioprocess. 2023, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Sikder, B.H.; Rashid, S.S.; Ab Rahim, M.H.; Ramli, A.N.M.; Roslan, R.; Sasi, A.A.; Mustafa, A.H. Enzymatic Cellulose Nanocrystal Production from Pretreated Palm Oil Empty Fruit Bunch Fibers. Mater. Today Proc. 2023, 107, 249–253. [Google Scholar] [CrossRef]
- Spagnuolo, L.; Beneventi, D.; Dufresne, A.; Operamolla, A. High Yield Synthesis of Cellulose Nanocrystals from Avicel by Mechano-Enzymatic Approach. ChemistrySelect 2024, 9, e202401511. [Google Scholar] [CrossRef]
- Dias, I.K.R.; Lacerda, B.K.; Arantes, V. High-Yield Production of Rod-like and Spherical Nanocellulose by Controlled Enzymatic Hydrolysis of Mechanically Pretreated Cellulose. Int. J. Biol. Macromol. 2023, 242, 125053. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Park, S.; Jung, D.; Oh, K.K.; Lee, S.H. Effect of Hydrogen Bond Donor on the Choline Chloride-Based Deep Eutectic Solvent-Mediated Extraction of Lignin from Pine Wood. Int. J. Biol. Macromol. 2020, 165, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Cai, K.; Liu, M.; Xu, M.; Zhao, T. Deep Eutectic Solvents with Multiple Hydroxyl Sites for Efficient and Reversible Absorption of SF6. J. Mol. Liq. 2022, 356, 119052. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Zeng, X.; Tang, X.; Sun, Y.; Lei, T.; Lin, L. Extraction of Cellulose Nanocrystals Using a Recyclable Deep Eutectic Solvent. Cellulose 2020, 27, 1301–1314. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Dong, C.; Li, R.; Zhang, X.; Wang, T.; Zhang, K. Transparent, Thermal Stable, Water Resistant and High Gas Barrier Films from Cellulose Nanocrystals Prepared by Reactive Deep Eutectic Solvents. Int. J. Biol. Macromol. 2024, 276, 134107. [Google Scholar] [CrossRef]
- Zhang, X.; Ni, H.; Xu, X.; Li, L.; Kang, H.; Li, D. Recent Advancements in the Synthesis, Functionalization, and Utilization of Cellulose Nanocrystals. Resour. Chem. Mater. 2024, 10073. [Google Scholar] [CrossRef]
- Mariño, M.A.; Rueda-Ordonez, D.; Paredes, M.G.; Tapia, R.A.; Pita, R.; Pavez, P. Recycled Ionic Liquid vs. Deep Eutectic Solvent in Cellulose Nanocrystals Production: Characterization, Techno-Economic Analysis, and Life Cycle Assessment. J. Clean. Prod. 2024, 472, 143461. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent Advances of Greener Pretreatment Technologies of Lignocellulose. Curr. Res. Green Sustain. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Cellulose Hydrolysis Using Ionic Liquids and Inorganic Acids under Dilute Conditions: Morphological Comparison of Nanocellulose. RSC Adv. 2020, 10, 39413–39424. [Google Scholar] [CrossRef] [PubMed]
- Haron, G.A.S.; Mahmood, H.; Bin Noh, H.; Moniruzzaman, M. Fabrication and Characterization of 3D Printable Nanocomposite Filament Based on Cellulose Nanocrystals and Polylactic Acid Using Ionic Liquids. J. Appl. Polym. Sci. 2024, 141, e54780. [Google Scholar] [CrossRef]
- Ma, L.; Xu, Y.; Chen, J.; Dong, C.; Pang, Z. Preparation of Cellulose Nanocrystals by Synergistic Action of Ionic Liquid and Recyclable Solid Acid under Mild Conditions. Molecules 2023, 28, 3070. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Bin Noh, H.; Goto, M.; Moniruzzaman, M. Cellulose Nanocrystals Preparation from Microcrystalline Cellulose Using Ionic Liquid-DMSO Binary Mixture as a Processing Medium. J. Mol. Liq. 2022, 346, 118208. [Google Scholar] [CrossRef]
- Da Silva, J.B.A.; Vieira, S.R.; Pessôa, L.C.; Santana, J.S.; Lemos, P.V.F.; de Souza, C.O.; Cardoso, L.G.; de Assis, D.J.; Mussagy, C.U.; Santos Ebinuma, V.C.; et al. Impact of Ionic Liquid’s Cation Alkyl Chain Length and Reaction Time on Cellulose Nanocrystals Preparation. Carbohydr. Polym. Technol. Appl. 2023, 6, 100390. [Google Scholar] [CrossRef]
- Kulkarni, S.P. Supercritical Water Hydrolysis of Cellulose: State-of-the-Art of Green Depolymerisation Technique. Biomass Bioenergy 2024, 184, 107182. [Google Scholar] [CrossRef]
- Osei-Bonsu, R.; Hoque, M.; McMichael, P.S.; Foster, E.J. Subcritical Water Digestion of Woody Biomass: Extraction of Cellulose Nanomaterials under Acid-Lean Condition. Nanoscale Adv. 2024, 6, 3923–3933. [Google Scholar] [CrossRef]
- Singh, S.; Bhardwaj, S.; Tiwari, P.; Dev, K.; Ghosh, K.; Maji, P.K. Recent Advances in Cellulose Nanocrystals-Based Sensors: A Review. Mater. Adv. 2024, 5, 2622–2654. [Google Scholar] [CrossRef]
- Lan, L.; Chen, H.; Lee, D.; Xu, S.; Skillen, N.; Tedstone, A.; Robertson, P.; Garforth, A.; Daly, H.; Hardacre, C.; et al. Effect of Ball-Milling Pretreatment of Cellulose on Its Photoreforming for H2 Production. ACS Sustain. Chem. Eng. 2022, 10, 4862–4871. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Varela, J.D.; Chanona-Pérez, J.J.; Calderón Benavides, H.A.; Cervantes Sodi, F.; Vicente-Flores, M. Effect of Ball Milling on Cellulose Nanoparticles Structure Obtained from Garlic and Agave Waste. Carbohydr. Polym. 2021, 255, 117347. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Wang, T.; Makarem, M.; Santiago Cintrón, M.; Cheng, H.N.; Kang, X.; Bacher, M.; Potthast, A.; Rosenau, T.; King, H.; et al. Effects of Ball Milling on the Structure of Cotton Cellulose. Cellulose 2019, 26, 305–328. [Google Scholar] [CrossRef]
- Kano, F.S.; de Souza, A.G.; Rosa, D.d.S. Variation of the Milling Conditions in the Obtaining of Nanocellulose from the Paper Sludge. Matéria 2019, 24, e12406. [Google Scholar] [CrossRef]
- Ferreira, R.R.; Souza, A.G.; Nunes, L.L.; Shahi, N.; Rangari, V.K.; Rosa, D.d.S. Use of Ball Mill to Prepare Nanocellulose from Eucalyptus Biomass: Challenges and Process Optimization by Combined Method. Mater. Today Commun. 2020, 22, 100755. [Google Scholar] [CrossRef]
- Wu, C.; McClements, D.J.; He, M.; Zheng, L.; Tian, T.; Teng, F.; Li, Y. Preparation and Characterization of Okara Nanocellulose Fabricated Using Sonication or High-Pressure Homogenization Treatments. Carbohydr. Polym. 2021, 255, 117364. [Google Scholar] [CrossRef]
- Tang, Y.; Yang, H.; Vignolini, S. Recent Progress in Production Methods for Cellulose Nanocrystals: Leading to More Sustainable Processes. Adv. Sustain. Syst. 2022, 6, 2100100. [Google Scholar] [CrossRef]
- Gao, A.; Chen, H.; Tang, J.; Xie, K.; Hou, A. Efficient Extraction of Cellulose Nanocrystals from Waste Calotropis Gigantea Fiber by SO42−/TiO2 Nano-Solid Superacid Catalyst Combined with Ball Milling Exfoliation. Ind. Crops. Prod. 2020, 152, 112524. [Google Scholar] [CrossRef]
- Gui, X.; Wan, Z.; Zhang, H.; Niu, M.; Guo, Y.; Li, H. Preparation of Cellulose Nanocrystals by Ultrasonication-Assisted Phosphotungstic Acid Method: An Effective Method of High Yield and Friendly Environment. Ind. Crops. Prod. 2024, 222, 119780. [Google Scholar] [CrossRef]
- Chen, B.-H.; Wang, Z.-Q.; Jin, Z.-C.; Gou, Z.-C.; Tang, S.-S.; Yu, X.-X.; Chen, H.; Chen, G.; Su, Y.-J. Optimized Phosphotungstic Acid Pretreatment for Enhancing Cellulase Adsorption and Biomass Saccharification in Corn Stover. Biomass Convers. Biorefin. 2023, 13, 9249–9264. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, Q.; An, X.; Ji, X.; Tian, Z.; Liu, S.; Yang, G. Recent Advances in Sustainable Preparation of Cellulose Nanocrystals via Solid Acid Hydrolysis: A Mini-Review. Int. J. Biol. Macromol. 2023, 253, 127353. [Google Scholar] [CrossRef] [PubMed]
- Leboucher, J.; Bazin, P.; Goux, D.; El Siblani, H.; Travert, A.; Barbulée, A.; Bréard, J.; Duchemin, B. High-Yield Cellulose Hydrolysis by HCl Vapor: Co-Crystallization, Deuterium Accessibility and High-Temperature Thermal Stability. Cellulose 2020, 27, 3085–3105. [Google Scholar] [CrossRef]
- Lourençon, T.; Altgen, M.; Pääkkönen, T.; Guccini, V.; Penttilä, P.; Kontturi, E.; Rautkari, L. Effect of Moisture on Polymer Deconstruction in HCl Gas Hydrolysis of Wood. ACS Omega 2022, 7, 7074–7083. [Google Scholar] [CrossRef]
- Yousefi, N.; Hannonen, J.; Fliri, L.; Peljo, P.; Kontturi, E. Highly Charged Cellulose Nanocrystals via Electrochemical Oxidation. Nano Lett. 2024, 24, 14610–14614. [Google Scholar] [CrossRef]
- Li, X.; Xiang, Z.; Dang, W.; Lin, Z.; Wang, H.; Wang, H.; Ye, D.; Yao, R. High-Yield and Scalable Cellulose Nanomesh Preparation via Dilute Acid Vapor and Enzymatic Hydrolysis-Mediated Nanofabrication. Carbohydr. Polym. 2024, 323, 121370. [Google Scholar] [CrossRef]
- Jeong, J.-J.; Kim, J.-H.; Lee, J.-S. Efficient Isolation of Cellulose Nanocrystals from Seaweed Waste via a Radiation Process and Their Conversion to Porous Nanocarbon for Energy Storage System. Molecules 2024, 29, 4844. [Google Scholar] [CrossRef] [PubMed]
- Qian, M.; Lei, H.; Villota, E.; Zhao, Y.; Wang, C.; Huo, E.; Zhang, Q.; Mateo, W.; Lin, X. High Yield Production of Nanocrystalline Cellulose by Microwave-Assisted Dilute-Acid Pretreatment Combined with Enzymatic Hydrolysis. Chem. Eng. Process. 2021, 160, 108292. [Google Scholar] [CrossRef]
- Wu, Q.; Ding, C.; Wang, B.; Rong, L.; Mao, Z.; Feng, X. Green, Chemical-Free, and High-Yielding Extraction of Nanocellulose from Waste Cotton Fabric Enabled by Electron Beam Irradiation. Int. J. Biol. Macromol. 2024, 267, 131461. [Google Scholar] [CrossRef]
- Whba, F.; Mohamed, F.; Whba, R.; Idris, M.I.; Noor, N.M.; Bin Mahmood, M.K. Synthesis and Characterization of Cellulose Nanocrystals/Gd2O3 Nanocomposite as a Dual-Mode Contrast Agent for MRI via Gamma-Ray Irradiation. Radiat. Phys. Chem. 2024, 221, 111727. [Google Scholar] [CrossRef]
- Muscolino, E.; Sabatino, M.A.; Jonsson, M.; Dispenza, C. The Role of Water in Radiation-Induced Fragmentation of Cellulosic Backbone Polysaccharides. Cellulose 2024, 31, 841–856. [Google Scholar] [CrossRef]
- Varshney, S.; Mulpuru, V.; Mishra, N.; Gupta, M.K. Microwave-Irradiated Novel Isolation of Nanocellulose from Waste Rice Husk via Modified Chemo-Mechanical Route: Characterization, in-Silico Prediction, and Its Antibacterial Activity. Mater. Technol. 2022, 37, 2608–2622. [Google Scholar] [CrossRef]


| Organic Acid | Raw Material | Treatment | CNC Characteristics | Ref. |
|---|---|---|---|---|
| FA | Bleached eucalyptus kraft pulp | FA 80–98 wt% (1:30, w/v), 70–100 °C, 0–24 h. catalyst 8 wt% of FeCl3, 180 rpm. Then, washed, centrifugated, and high-pressure homogenized. | Thermal stability (maximal weight loss temperature of 375 °C) and crystallinity index of 79%; lengths from 345 to 124 nm and diameter of 5–21 nm. | [8] |
| Pretreated mango seed husk pulp | Optimum FA-to-pulp ratio: 30:1 mL/g; 8 h. | CNC with 66.40% crystallinity index; formate content: 0.92 mmol/g; particle size: 24.13 nm; spherical shape; polydispersity index: 0.488. | [26] | |
| Bleached eucalyptus kraft pulp | H2SO4/FA/H2O, 80 °C, paddle stirring for 3 h. Then, diluted, centrifuged, washed, dialyzed, and sonicated. | Rod-like shape CNC; maximum crystallinity index of 82.08%; maximum sulfate group content of 0.288 mmol/g; maximum formyl group content of 1.087 mmol/g; good dis persibility in aqueous medium; thermal stability around 350 °C. | [9] | |
| CA | Bleached eucalyptus kraft pulp | A 65 wt%, 120 °C, 450 rpm, 1.5–6 h. Then, diluted, centrifuged, dialyzed, and ultrasonicated. | CNC with 73–83% crystallinity index, length 270–215 nm, diameter 11–9 nm, degree of substitution 0.15–0.27, maximum degradation temperature 368 °C. | [6] |
| Bleached eucalyptus kraft pulp | CA 80–85 wt% plus FeCl3 (0.01–0.03 mmol/g CA), 80–100 °C, 6 h with mechanical stirring at 400 rpm. Then, centrifuged, dialyzed, and sonicated. | The highest carboxylic group content reached up to 1.04 mmol/g, lengths from 214–144 nm and diameter of 14–8 nm, the highest crystalline index of 79.72%, excellent dispersibility in an aqueous solution, thermal stability around 350 °C. | [7] | |
| Microcrystalline cellulose (MCC) | CA/HCl ratio of 9:1 (v/v), 80 °C, 350 rpm, 4 h. After cooling to room temperature, the mixture was filtered, washed, and dialyzed. | Well-dispersed and possessed a rod-like morphology, length of 231.8–248.3 nm and diameter of 15.8–18.4 nm, exhibited similar characteristic cellulose I pattern, crystallinity index around 83%, thermal stability 337.2–245.3 °C. | [12] | |
| Blanched Oxytenanthera abyssinica (Ethiopian lowland bamboo) | H2SO4/CA/H2O 9:1 wt% CA/H2SO4, 80 °C, 5 h. Then, diluted, centrifuged, dialyzed, and ultrasonicated. | Maximum carboxylate concentration of 0.75 ± 0.08 mmol/g; stable dispersibility, mostly spherical-like shapes, particle size of 68.06 nm, cellulose crystallinity 60.37 to 81.3%, thermal stability 245 and 400 °C. | [21] | |
| OA | Softwood sulfite dissolving pulp and Softwood kraft pulp | OA dihydrate in a rotary evaporator, 110 °C, 35–60 min. Then, washed, adjusted pH to pH 9–10, and mechanically disintegrated by a microfluidizer. | Particles with similar shape and length (50–500 nm) comparable to CNC shapes, with a considerable number of longer particles (up to 1.1 μm) and shaped like flexible CNF. Crystallinity index of approximately 75%. | [10] |
| Qualitative filter paper | OA 5.75–11.75 g oxalate dihydrate/g filter paper) 10 °C, 300 rpm, 15–120 min. Then diluted, filtrated, washed, neutralized, and ultrasonicated. | Rod-shaped CNC morphology, a length of 151–250 nm and particle size distribution of 5–20 nm, crystallinity 79.62–88.73%. | [27] | |
| Bleached eucalyptus kraft pulp | H2SO4/AA/H2O, 80 °C, with a paddle stirring for 1–10 h. The sample was then washed, centrifuged, and dialyzed. | Rod-like CNC, 150–500 nm length, 5–20 nm diameter, crystallinity index around 80%, thermal stability 270.3–367.0 °C, and excellent dispersion stability in both aqueous and organic phases. | [20] | |
| AA | Alkali-treated microcrystalline cellulose | AA/H2SO4/acetic anhydride and sulfuric acid, 85 °C, 10 min. Then, diluted, centrifugated, and dialyzed. | Rod-like CNC, 60–130 nm length and 12–20 nm width, crystallinity index around 70%, thermal degradation ∼264 °C. | [28] |
| MA | Cellulose from purple sweet potato peels. | Optimum: 75 wt%, 1:10 (g/mL, W/W) MA, ultrasonic-assisted hydrolysis, 60 °C, 1 h, plus 120 °C for 2.5 h in an oil bath. The suspension was then diluted, centrifuged, dialyzed, and sonicated. | Rod-like structure CNC, 10–30 nm width and 60–220 nm length, 58.3% crystallinity index, thermal degradation 346 °C. | [29] |
| Blanched bamboo pulp board | Ball-mill pretreated. Liquor-to-pulp weight ratio of 100:1 in an MA solution of 75 wt% concentration at 110 °C for 3 h. Mechanical stirring. Then, diluted, centrifuged, dialyzed, and ultrasonicated. | Rod-shape CNC particles, 105.6–223.8 nm length, 200−365 °C decomposition temperature range, higher crystallinity index of 91.4% | [30] |
| Methods | Reaction Conditions | CNC Characteristics | Advantages | Disadvantages |
|---|---|---|---|---|
| Conventional Strong acid, H2SO4 | Low temperature and time (64 wt% H2SO4, 45–50 °C, 60 min). | Functionalized CNC. Lower degradation temperature and crystallinity. | Effective; functionalized CNC; short time and low temperatures of reaction. | Damage in equipment, corrosivity, low and moderate yields, and high material degradation. Expensive product purification. Higher cost and difficulty in recovering the reagents. |
| Organic acids | High temperature and time (60–80% v/v, 0.5–6 h, 70–120 °C). | Functionalized CNC. Higher degradation temperature and crystallinity. | Reduced corrosivity, environmentally friendly (possibility of recovery and reuse by evaporation or crystallization), good yields. | Low acidity. Longer reaction time and temperature. It is necessary to recover the reagents. |
| Oxidative methods (H2O2, O3, K2FeO4) | Low temperature and high time, moderate concentrations (60 °C, 72 h, 30% v/v H2O2, O3 30 mg/L). | Functionalized CNC. Lower degradation temperature and crystallinity. | Moderate reaction conditions; environmentally friendly (no harmful chemical uses); minimal equipment corrosion. | Low yields. |
| Solid acids | Low temperature and high time (14–45%, 45 °C, 5 h). | Possibility to obtain functionalized CNC. | Moderate reaction conditions; environmentally friendly (easy to recover by filtration or centrifugation); good yields; minimal equipment corrosion. | Longer reaction times. It is necessary to recover the reagents, non-homogeneous particle size distribution, and high cost for solid acids production. |
| Vapor strategies | Low temperature and variable time (30 min-several days, room temperature). | Non-functionalized CNC. Higher degradation temperature and crystallinity. | Environmentally friendly (possibility of recycling and lower water consumption post treatments), good yields. | High vapor pressure of the reaction, safety risks, and need for recycling. |
| Enzymatic | Low temperature and high time (45 °C, 48–72 h). | Non-functionalized CNC. Higher degradation temperature and crystallinity. | Efficiency; selectivity; low energy consumption; neutral conditions; no corrosion; environmentally friendly (possible recovery and reuse). | Longer reaction times, high enzymes cost, and relatively low yields. |
| ILs and DESs | High temperatures and variable times (70–100 °C, 1.5–20 h). | Functionalized CNC. Higher degradation temperature and crystallinity. | Environmentally friendly (possible recovery and reuse) and moderate yields. Most reagents are biodegradable. | Longer reaction times and high solvent costs. |
| Subcritical water | High temperatures and low times (120–170 °C, 60–120 min). | Functionalized CNC. Higher degradation temperature and crystallinity. | Efficient, environmentally friendly (reduces harsh process chemicals and easy to recover and reuse), good yields, and easy scalability. | High reaction temperatures. |
| Mechanical | Room temperature and variable time. | Non-functionalized CNC. | The use of chemical reagents is not necessary. | High energy consumption and low yields. |
| Radiation-based | Room temperature and lower times. | Non-functionalized CNC. | Less reagent consumption, lower reaction times, higher efficiency, and carboxylic groups increase. | Economic aspects and safety aspects during the process. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dagnino, E.P.; Ehman, N.; Area, M.C. Recent Advances in Cellulose Nanocrystal Production from Green Methods. Processes 2025, 13, 790. https://doi.org/10.3390/pr13030790
Dagnino EP, Ehman N, Area MC. Recent Advances in Cellulose Nanocrystal Production from Green Methods. Processes. 2025; 13(3):790. https://doi.org/10.3390/pr13030790
Chicago/Turabian StyleDagnino, Eliana Paola, Nanci Ehman, and María Cristina Area. 2025. "Recent Advances in Cellulose Nanocrystal Production from Green Methods" Processes 13, no. 3: 790. https://doi.org/10.3390/pr13030790
APA StyleDagnino, E. P., Ehman, N., & Area, M. C. (2025). Recent Advances in Cellulose Nanocrystal Production from Green Methods. Processes, 13(3), 790. https://doi.org/10.3390/pr13030790







