An Analysis of a Cement Hydration Process Using Glass Waste from Household Appliances as a Supplementary Material
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Samples
2.3. Methods Used for the Tests
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HAGw | Household appliance glass waste |
| C-S-H | Calcium silicate hydrates |
| C-A-S-H | Calcium aluminium silicate hydrates |
| UPV | Ultrasound pulse velocity |
References
- Hamada, H.; Alattar, A.; Tayeh, B.; Yahaya, F.; Thomas, B. Effect of Recycled Waste Glass on the Properties of High-Performance Concrete: A Critical Review. Case Stud. Constr. Mater. 2022, 17, e01149. [Google Scholar] [CrossRef]
- Premathilaka, K.K.W.; Liyanapathirana, D.S.; Leo, C.J.; Hu, P. Application of Recycled Waste Glass to Replace Traditional Quarried Aggregates: A Comprehensive Review. J. Build. Eng. 2024, 86, 108846. [Google Scholar] [CrossRef]
- Wang, H.Y. A Study of the Effects of LCD Glass Sand on the Properties of Concrete. Waste Manag. 2009, 29, 335–341. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Value-Added Utilisation of Waste Glass in Concrete. Cem. Concr. Res. 2004, 34, 81–89. [Google Scholar] [CrossRef]
- Mohajerani, A.; Vajna, J.; Cheung, T.H.H.; Kurmus, H.; Arulrajah, A.; Horpibulsuk, S. Practical Recycling Applications of Crushed Waste Glass in Construction Materials: A Review. Constr. Build. Mater. 2017, 156, 443–467. [Google Scholar] [CrossRef]
- Butler, J.H.; Hooper, P. Glass Waste. In Waste: A Handbook for Management; Academic Press: Cambridge, MA, USA, 2011; pp. 151–165. [Google Scholar] [CrossRef]
- Çelik, A.İ.; Özkılıç, Y.O.; Zeybek, Ö.; Karalar, M.; Qaidi, S.; Ahmad, J.; Burduhos-Nergis, D.D.; Bejinariu, C. Mechanical Behavior of Crushed Waste Glass as Replacement of Aggregates. Materials 2022, 15, 8093. [Google Scholar] [CrossRef]
- Surendran, H.; Akhas, P.K. Properties of High-Performance Concrete Incorporating Toughened Glass Waste Coarse Aggregate: An Experimental Study. Structures 2024, 60, 105897. [Google Scholar] [CrossRef]
- Dong, W.; Li, W.; Tao, Z. A Comprehensive Review on Performance of Cementitious and Geopolymeric Concretes with Recycled Waste Glass as Powder, Sand or Cullet. Resour. Conserv. Recycl. 2021, 172, 105664. [Google Scholar] [CrossRef]
- Esmaeili, J.; Oudah Al-Mwanes, A. A Review: Properties of Eco-Friendly Ultra-High-Performance Concrete Incorporated with Waste Glass as a Partial Replacement for Cement. Mater. Today Proc. 2021, 42, 1958–1965. [Google Scholar] [CrossRef]
- Su, N.; Chen, J.S. Engineering Properties of Asphalt Concrete Made with Recycled Glass. Resour. Conserv. Recycl. 2002, 35, 259–274. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, K. A Review on the Use of Waste Glasses in the Production of Cement and Concrete. Resour. Conserv. Recycl. 2007, 52, 234–247. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An Overview on the Reuse of Waste Glasses in Alkali-Activated Materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- Mariaková, D.; Mocová, K.A.; Fořtová, K.; Pavlů, T.; Hájek, P. Waste Glass Powder Reusability in High-Performance Concrete: Leaching Behavior and Ecotoxicity. Materials 2021, 14, 4476. [Google Scholar] [CrossRef] [PubMed]
- Sobolev, K.; Türker, P.; Soboleva, S.; Iscioglu, G. Utilization of Waste Glass in ECO-Cement: Strength Properties and Microstructural Observations. Waste Manag. 2007, 27, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, W.; Manalo, A.; Siddique, R.; Mendis, P.; Zhuge, Y.; Wong, H.S.; Lokuge, W.; Aravinthan, T.; Schubel, P. Recycling of Landfill Wastes (Tyres, Plastics and Glass) in Construction—A Review on Global Waste Generation, Performance, Application and Future Opportunities. Resour. Conserv. Recycl. 2021, 173, 105745. [Google Scholar] [CrossRef]
- Rashid, K.; Hameed, R.; Ahmad, H.A.; Razzaq, A.; Ahmad, M.; Mahmood, A. Analytical Framework for Value Added Utilization of Glass Waste in Concrete: Mechanical and Environmental Performance. Waste Manag. 2018, 79, 312–323. [Google Scholar] [CrossRef]
- Saltan, M.; Öksüz, B.; Uz, V.E. Use of Glass Waste as Mineral Filler in Hot Mix Asphalt. Sci. Eng. Compos. Mater. 2015, 22, 271–277. [Google Scholar] [CrossRef]
- Kazmi, D.; Williams, D.J.; Serati, M. Waste Glass in Civil Engineering Applications—A Review. Int. J. Appl. Ceram. Technol. 2020, 17, 529–554. [Google Scholar] [CrossRef]
- Kazmi, D.; Serati, M.; Williams, D.J.; Qasim, S.; Cheng, Y.P. The Potential Use of Crushed Waste Glass as a Sustainable Alternative to Natural and Manufactured Sand in Geotechnical Applications. J. Clean. Prod. 2021, 284, 124762. [Google Scholar] [CrossRef]
- Stefanidou, M.; Kesikidou, F.; Konopisi, S.; Vasiadis, T. Investigating the Suitability of Waste Glass as a Supplementary Binder and Aggregate for Cement and Concrete. Sustainability 2023, 15, 3796. [Google Scholar] [CrossRef]
- Ibrahim, K.I.M. Recycled Waste Glass Powder as a Partial Replacement of Cement in Concrete Containing Silica Fume and Fly Ash. Case Stud. Constr. Mater. 2021, 15, e00630. [Google Scholar] [CrossRef]
- Stochino, F.; Alibeigibeni, A.; Zucca, M.; Valdes, M.; Concu, G.; Simoncelli, M.; Pisani, M.A.; Bernuzzi, C. Mechanical behavior of composite slabs with recycled concrete aggregates: A preliminary study. Structures 2024, 70, 107838. [Google Scholar] [CrossRef]
- De Castro, S.; De Brito, J. Evaluation of the Durability of Concrete Made with Crushed Glass Aggregates. J. Clean. Prod. 2013, 41, 7–14. [Google Scholar] [CrossRef]
- Chen, G.; Lee, H.; Young, K.L.; Yue, P.L.; Wong, A.; Tao, T.; Choi, K.K. Glass Recycling in Cement Production—An Innovative Approach. Waste Manag. 2002, 22, 747–753. [Google Scholar] [CrossRef]
- Corinaldesi, V.; Gnappi, G.; Moriconi, G.; Montenero, A. Reuse of Ground Waste Glass as Aggregate for Mortars. Waste Manag. 2005, 25, 197–201. [Google Scholar] [CrossRef]
- Tan, K.H.; Du, H. Use of Waste Glass as Sand in Mortar: Part I—Fresh, Mechanical and Durability Properties. Cem. Concr. Compos. 2013, 35, 109–117. [Google Scholar] [CrossRef]
- Wright, J.R.; Cartwright, C.; Fura, D.; Rajabipour, F. Fresh and Hardened Properties of Concrete Incorporating Recycled Glass as 100% Sand Replacement. J. Mater. Civ. Eng. 2014, 26, 1–11. [Google Scholar] [CrossRef]
- Yoo, D.Y.; You, I.; Zi, G. Effects of Waste Liquid–Crystal Display Glass Powder and Fiber Geometry on the Mechanical Properties of Ultra-High-Performance Concrete. Constr. Build. Mater. 2021, 266, 120938. [Google Scholar] [CrossRef]
- Higuchi, A.M.D.; Marques, M.G.; Ribas, L.F.; Vasconcelos, R.P. Use of glass powder residue as an eco-efficient supplementary cementitious material. Constr. Build. Mater. 2021, 304, 124640. [Google Scholar] [CrossRef]
- LST EN 12706; Adhesives-Test Methods for Hydraulic Setting Floor Smoothing and/or Levelling Compounds—Determination of Flow Characteristics. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2004.
- Tian, Z.; Wang, Q.; Hou, S.; Shen, X. Effects of Multi-Sized Glass Fiber-Reinforced Polymer Waste on Hydration and Mechanical Properties of Cement-Based Materials. J. Build. Eng. 2025, 102, 112070. [Google Scholar] [CrossRef]
- Malaiskiene, J.; Costa, C.; Baneviciene, V.; Antonovic, V.; Vaiciene, M. The Effect of Nano SiO2 and Spent Fluid Catalytic Cracking Catalyst on Cement Hydration and Physical Mechanical Properties. Constr. Build. Mater. 2021, 299, 124281. [Google Scholar] [CrossRef]
- LST EN 196-1; Methods of Testing Cement—Part 1: Determination of Strength. Lietuvos Standartizacijos Departamentas: Vilnius, Lithuania, 2016.
- Malaiškienė, J.; Bekerė, K. Impact of Electronic Waste Glass on the Properties of Cementitious Materials. Buildings 2024, 14, 1218. [Google Scholar] [CrossRef]
- Way, S.J.; Shayan, A. Early Hydration of a Portland Cement in Water and Sodium Hydroxide Solutions: Composition of Solutions and Nature of Solid Phases. Cem. Concr. Res. 1989, 19, 759–769. [Google Scholar] [CrossRef]
- Huang, L.; Yang, Z. Early Hydration of Tricalcium Silicate with Potassium Hydroxide and Sulfate from Pore Solution and Solid View. Constr. Build. Mater. 2020, 230, 116988. [Google Scholar] [CrossRef]
- Wagner, D.; Bellmann, F.; Neubauer, J. Impact of KOH on the Interfacial Precipitation Rates of C-S-H during the Early Hydration of C3S. Cem. Concr. Res. 2021, 146, 106488. [Google Scholar] [CrossRef]
- Zheng, Z.; Yang, J.; Fu, H.; Liu, Q.; Li, Y.; Ma, X.; Zhang, P. The Coupling Impact of NaOH and NaNO3 on Hydration Process, Products and Sr2+ Adsorption Ability of the Major Phases in Portland Cement. Constr. Build. Mater. 2025, 459, 139813. [Google Scholar] [CrossRef]
- Beaudoin, J.; Odler, I. Hydration, Setting and Hardening of Portland Cement. In Lea’s Chemistry of Cement and Concrete; Butterworth-Heinemann: Oxford, UK, 2019; pp. 157–250. [Google Scholar] [CrossRef]
- Chen, C.; Habert, G.; Bouzidi, Y.; Jullien, A. Environmental Impact of Cement Production: Detail of the Different Processes and Cement Plant Variability Evaluation. J. Clean. Prod. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on Concrete Containing Ground Waste Glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Properties of Concrete Contains Mixed Colour Waste Recycled Glass as Sand and Cement Replacement. Constr. Build. Mater. 2008, 22, 713–720. [Google Scholar] [CrossRef]
- Mikhailenko, P.; Cassagnabère, F.; Emam, A.; Lachemi, M. Influence of Physico-Chemical Characteristics on the Carbonation of Cement Paste at High Replacement Rates of Metakaolin. Constr. Build. Mater. 2018, 158, 164–172. [Google Scholar] [CrossRef]
- Gabrovšek, R.; Vuk, T.; Kaučič, V. Evaluation of the Hydration of Portland Cement Containing Various Carbonates by Means of Thermal Analysis. Acta Chim. Slov. 2006, 53, 159–165. [Google Scholar]
- Richardson, I.G.; Groves, G.W. Microstructure and Microanalysis of Hardened Ordinary Portland Cement Pastes. J. Mater. Sci. 1993, 28, 265–277. [Google Scholar] [CrossRef]
- Richardson, I.G. Tobermorite/Jennite- and Tobermorite/Calcium Hydroxide-Based Models for the Structure of C-S-H: Applicability to Hardened Pastes of Tricalcium Silicate, β-Dicalcium Silicate, Portland Cement, and Blends of Portland Cement with Blast-Furnace Slag, Metakaolin, or Silica Fume. Cem. Concr. Res. 2004, 34, 1733–1777. [Google Scholar] [CrossRef]

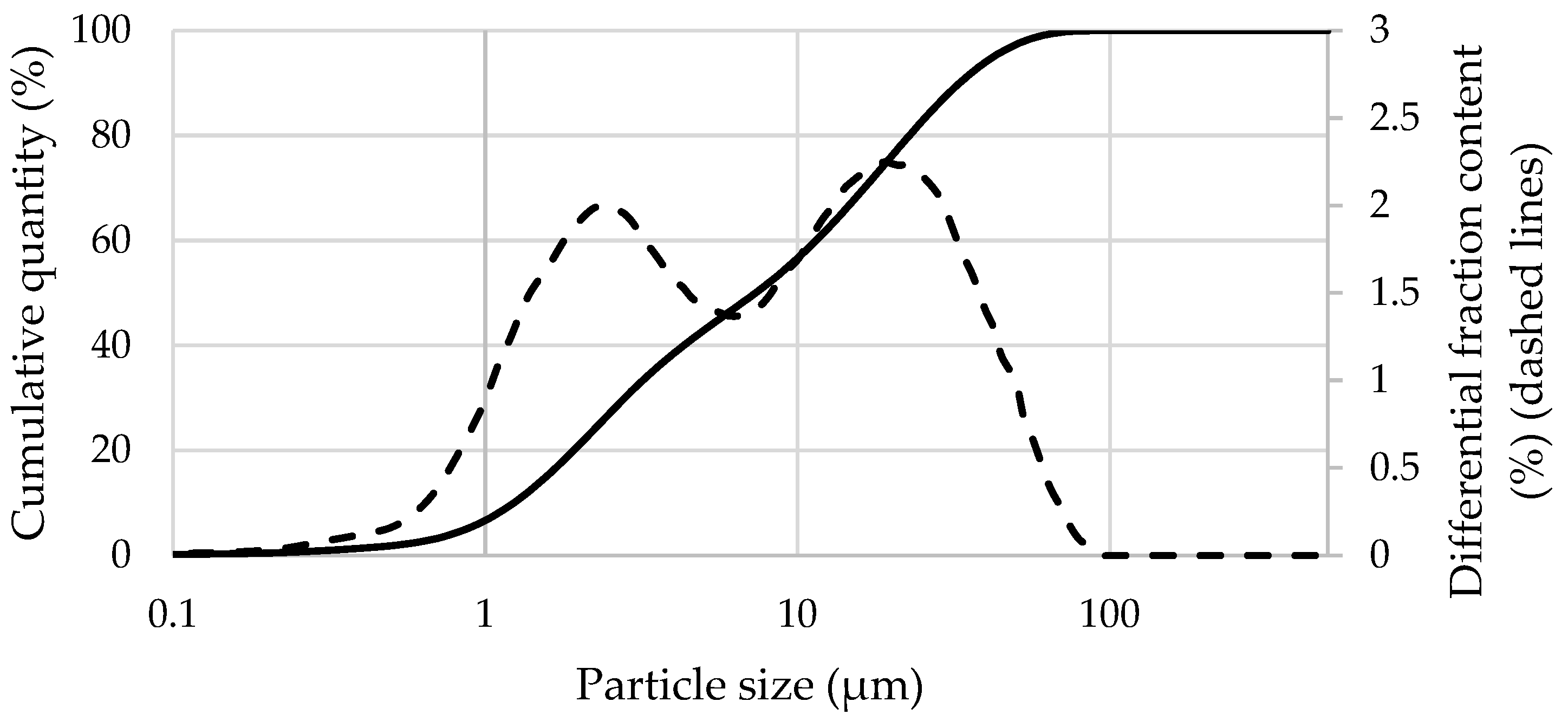
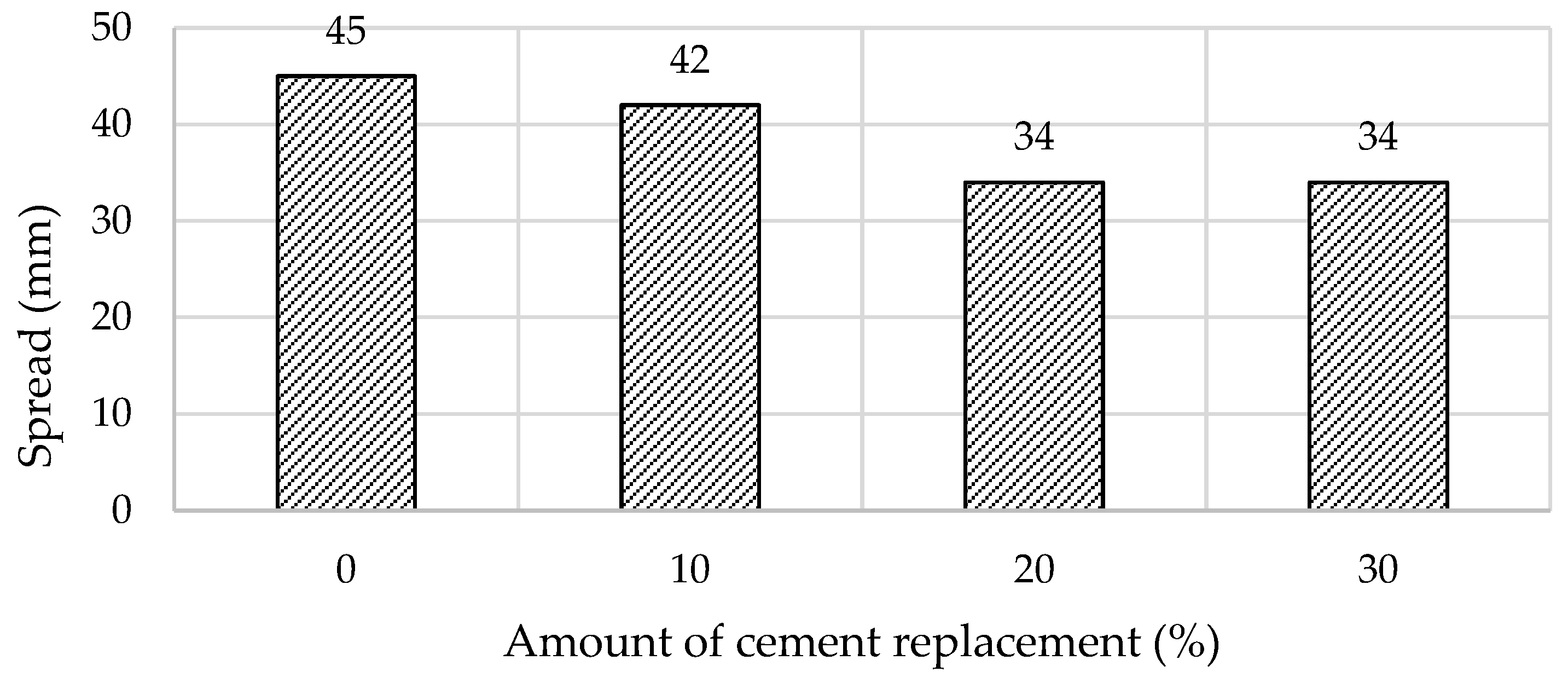

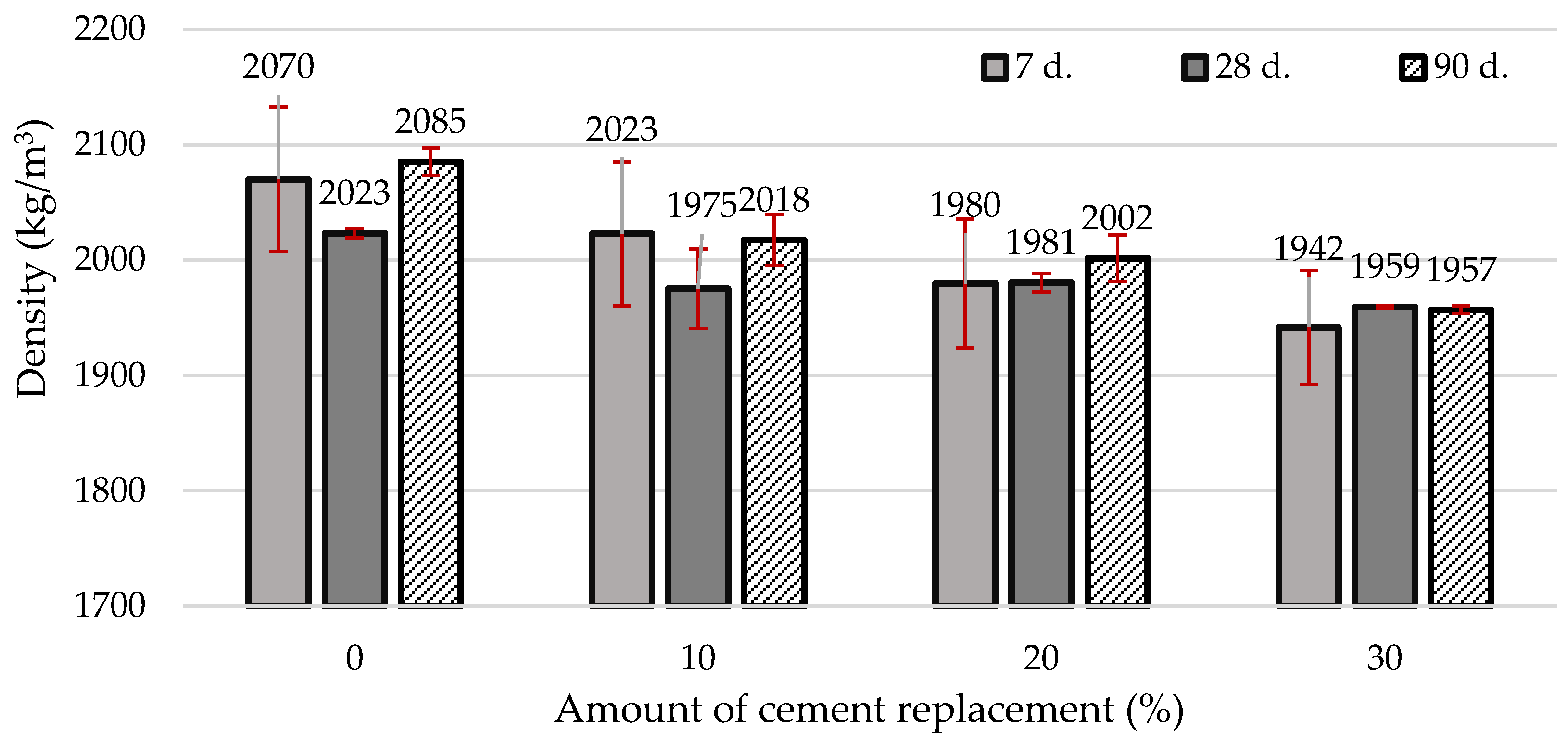
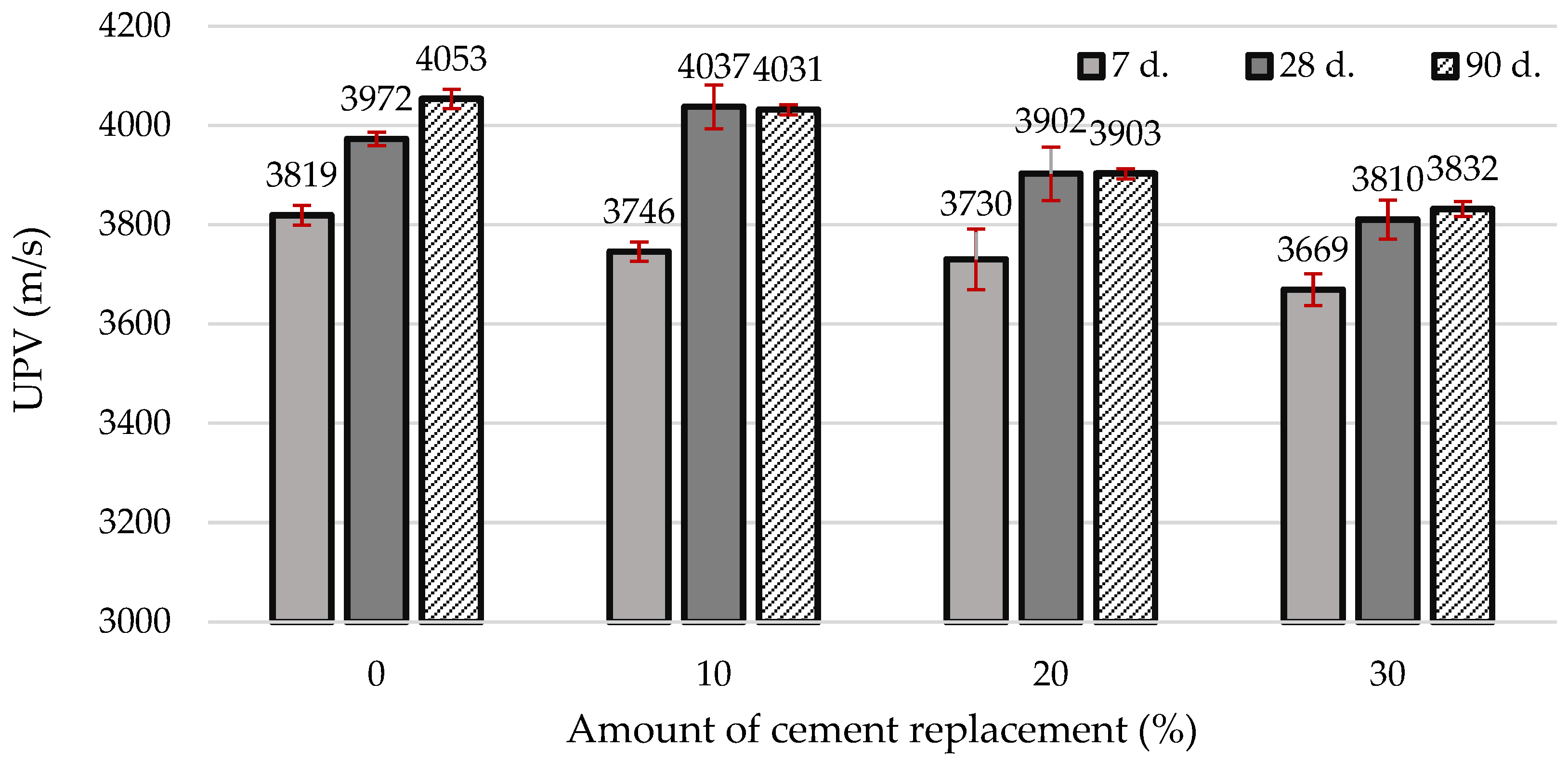
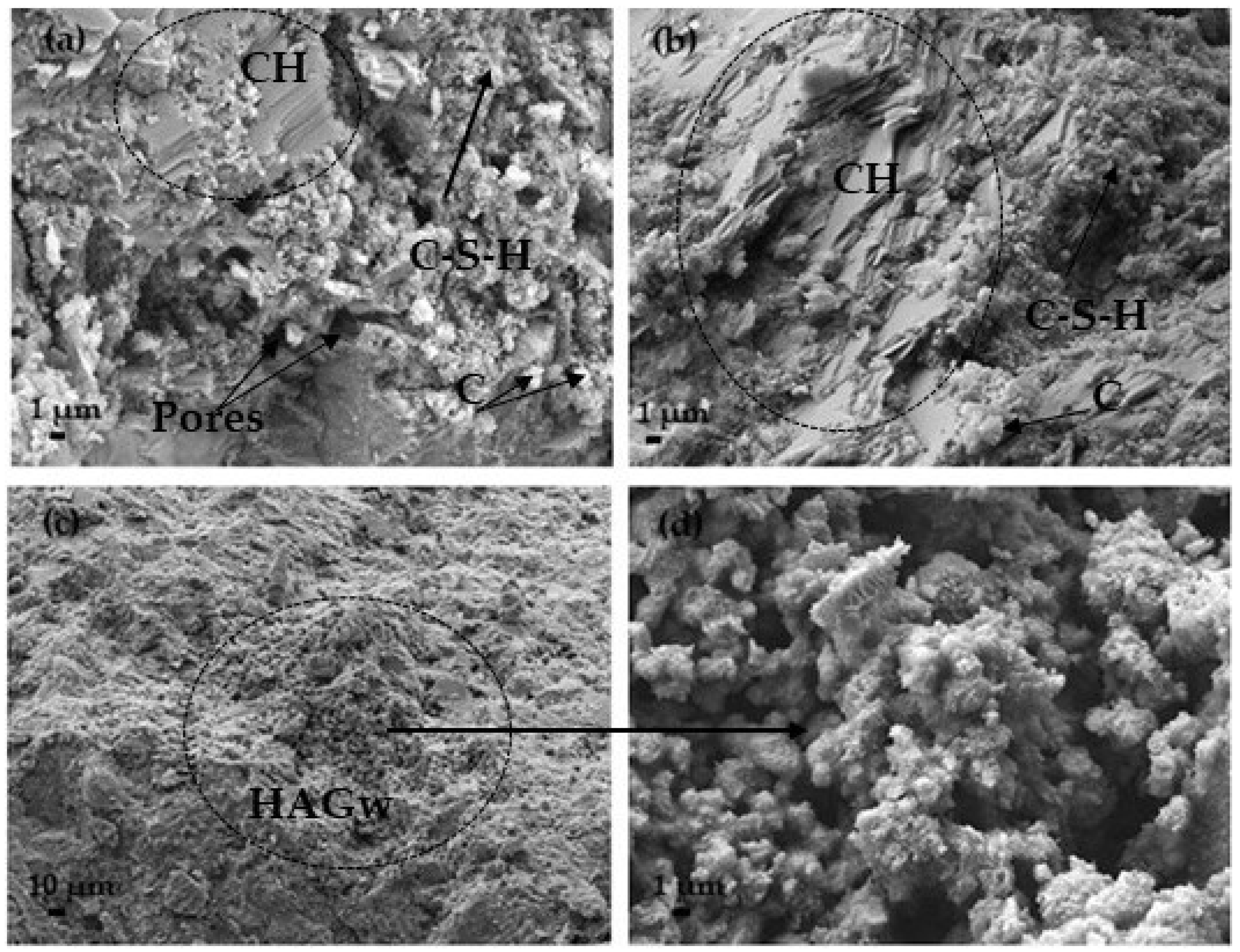



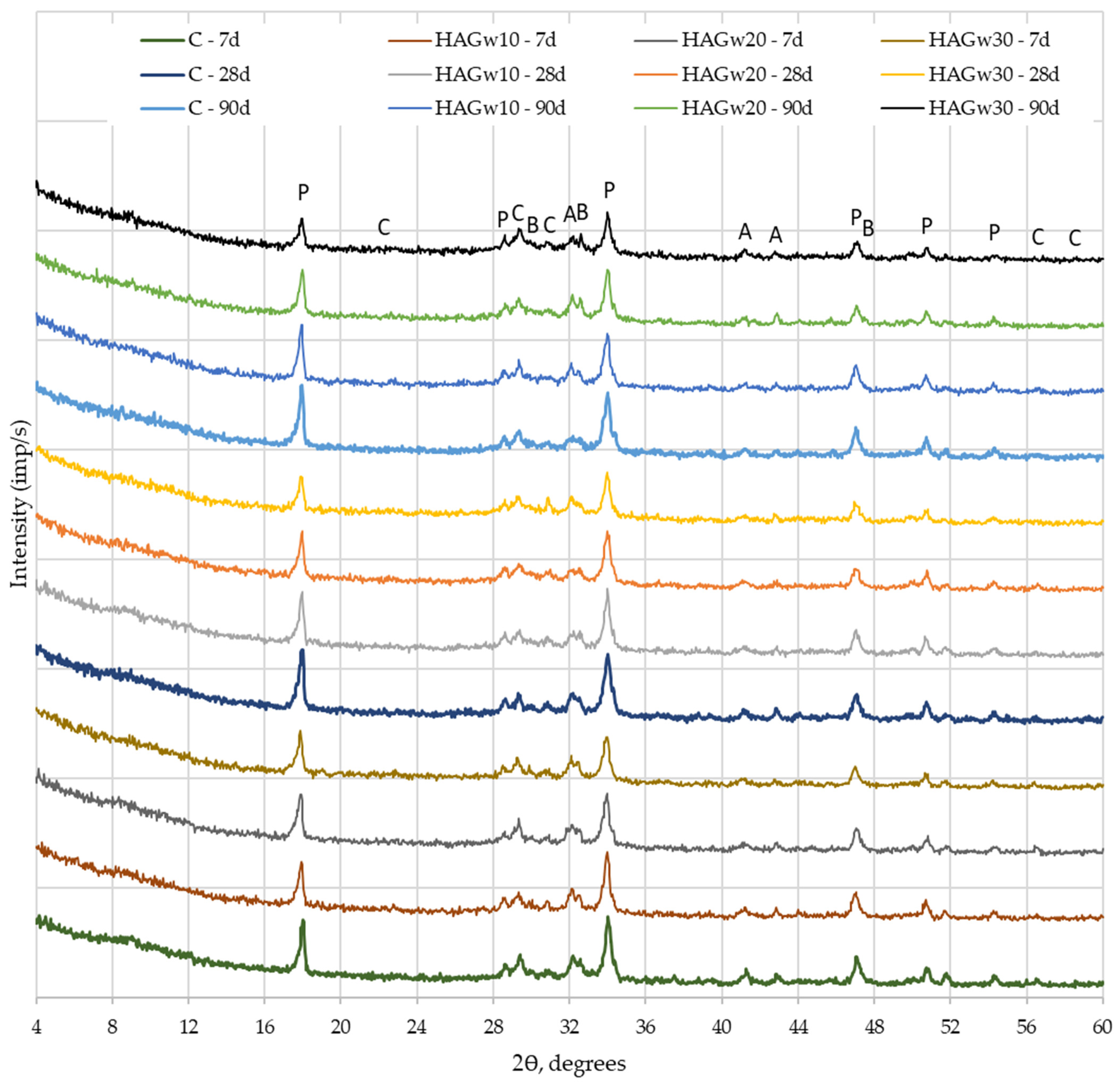
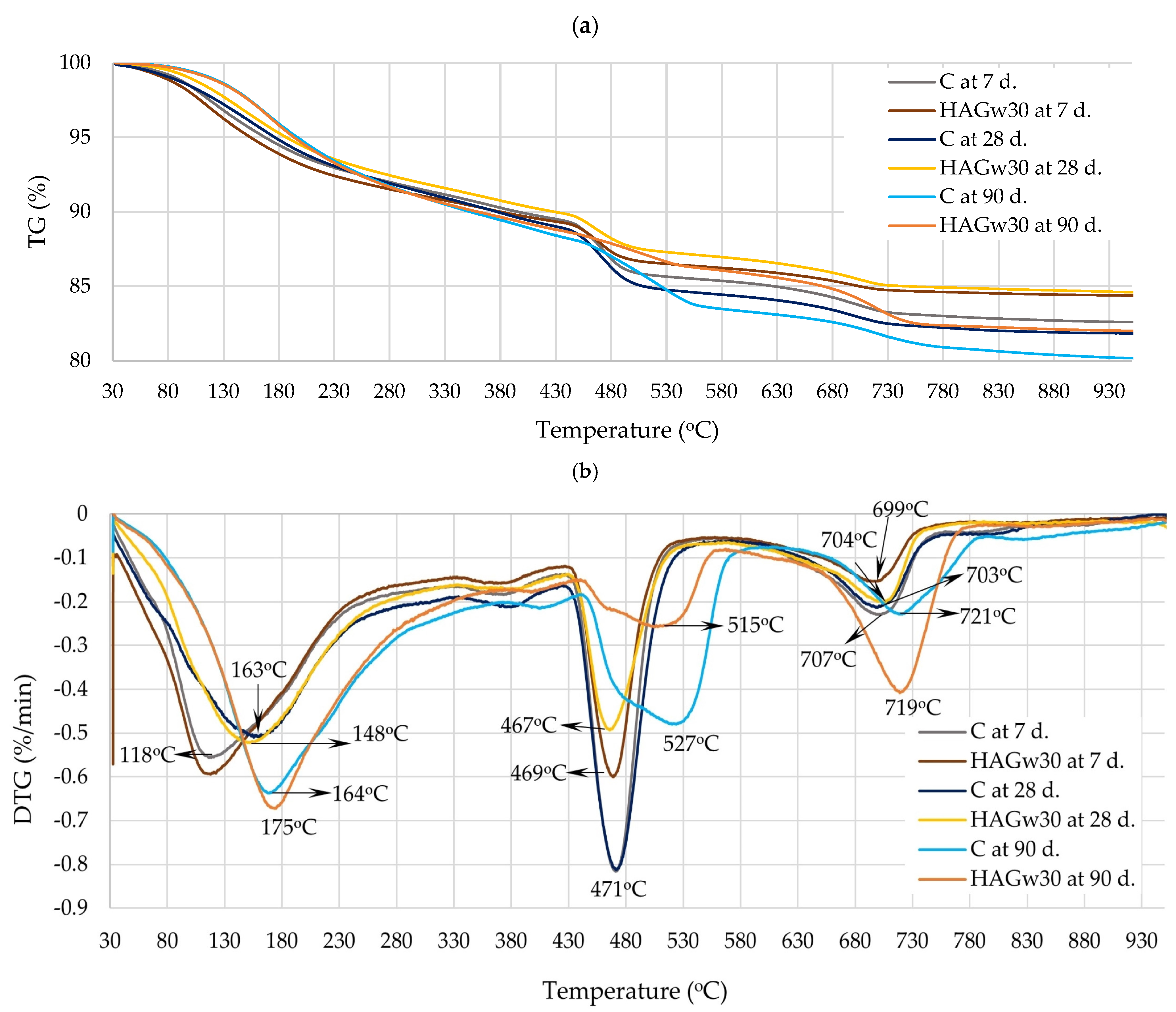
| Materials | CaO | SiO2 | SO3 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | P2O5 | SrO | TiO2 | BaO | CO2 | Cl | ZrO2 | WO3 | ZnO |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 63.4 | 20 | 2.73 | 4.57 | 3.46 | 3.71 | 1.30 | 0.14 | - | - | 0.32 | 0.06 | - | - | - | - | 0.01 |
| HAGw | 3.74 | 81.4 | 0.04 | 2.91 | 0.05 | 0.03 | 1.26 | 6.51 | 0.01 | 0.005 | 0.02 | 0.03 | 3.70 | 0.08 | 0.05 | 0.13 | - |
| Paste Designation | Time of the Second Maximum (h) | Heat After Hours of Hydration (J/g) | |||
|---|---|---|---|---|---|
| 12 | 24 | 36 | 48 | ||
| C | 9.45 | 120.4 | 236.8 | 302.8 | 352.5 |
| HAGw10 | 9.79 | 105.4 | 211.7 | 271.8 | 317.2 |
| HAGw20 | 9.43 | 96.3 | 192.6 | 248.4 | 291.5 |
| HAGw30 | 9.39 | 86.8 | 176.1 | 229.3 | 270.1 |
| Amount of Replaced Cement (%) | After 7 Days | After 28 Days | After 90 Days |
|---|---|---|---|
| 10 | 99.5 (110.6) | 99.4 (110.5) | 109.8 (122.0) |
| 20 | 89.2 (111.4) | 99.2 (124.0) | 108.8 (136.0) |
| 30 | 78.8 (112.6) | 89.0 (127.1) | 98.1 (140.0) |
| Mark | 110–170 °C, % | 180–320 °C, % | 110–350 °C, % at Equal Cement Content | 420–530 °C, % | CH Content in Sample, % | CH Content at Equal Cement Content, % | At 610–770 °C, % |
|---|---|---|---|---|---|---|---|
| After 7 days | |||||||
| C | 3.03 | 3.16 | 6.19 | 4.00 | 16.44 | 16.44 | 2.12 |
| HAGw30 | 3.12 | 2.96 | 8.69 | 2.96 | 12.17 | 17.38 | 1.41 |
| After 28 days | |||||||
| C | 2.75 | 3.76 | 6.51 | 4.42 | 18.17 | 18.17 | 1.96 |
| HAGw30 | 2.85 | 3.59 | 9.20 | 2.83 | 11.63 | 16.62 | 1.79 |
| After 90 days | |||||||
| C | 2.83 | 5.17 | 8.00 | 3.73 | 15.33 | 15.33 | 2.25 |
| HAGw30 | 2.83 | 5.02 | 11.21 | 2.26 | 9.29 | 13.27 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekerė, K.; Malaiškienė, J.; Škamat, J. An Analysis of a Cement Hydration Process Using Glass Waste from Household Appliances as a Supplementary Material. Processes 2025, 13, 840. https://doi.org/10.3390/pr13030840
Bekerė K, Malaiškienė J, Škamat J. An Analysis of a Cement Hydration Process Using Glass Waste from Household Appliances as a Supplementary Material. Processes. 2025; 13(3):840. https://doi.org/10.3390/pr13030840
Chicago/Turabian StyleBekerė, Karolina, Jurgita Malaiškienė, and Jelena Škamat. 2025. "An Analysis of a Cement Hydration Process Using Glass Waste from Household Appliances as a Supplementary Material" Processes 13, no. 3: 840. https://doi.org/10.3390/pr13030840
APA StyleBekerė, K., Malaiškienė, J., & Škamat, J. (2025). An Analysis of a Cement Hydration Process Using Glass Waste from Household Appliances as a Supplementary Material. Processes, 13(3), 840. https://doi.org/10.3390/pr13030840







