The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal
Abstract
:1. Introduction
2. Micropollutants in Aquatic Ecosystems
2.1. Sources of Micropollutants
2.2. Routes of Micropollutants in the Environment
2.3. Environmental Impact of Micropollutants
2.3.1. Effect on Human Health
Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
Antibiotics
Antidepressants
Microplastics
Pesticides
2.3.2. Effects of Microplastics on Aquatic Ecosystems
3. Presence of Micropollutants in the Aquatic Environment
4. Techniques for the Removal of Micropollutants
4.1. Removal Technologies of Microplastics
4.1.1. Physical Techniques for Removal of Microplastics
| Microplastic Types | Physical Methods | Experimental Details | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|
| PS, PS-NH2, and PS-COOH (1 μm) | Adsorption |
| 72.4–89.8 | [188] |
| MPs in sewage treatment plant | Adsorption |
| 92.9 | [191] |
| PE, PET, and PA (48 μm) | Adsorption and magnetization |
| 100 | [192] |
| PS (1 μm) | Adsorption and magnetization |
| 95.02 (MBC) 95.79 (Zn-MBC) 94.60 (Mg-MBC) | [193] |
| MPs | Filtration (disc filter) |
| 89.7 (particle); 75.6 (mass) | [194] |
| MPs | Filtration (disc Filter) | 80–98 | [195] | |
| MPs | Filtration (UF) |
| 75 | [196] |
| MPs | Filtration (sand filter systems with biochar) | >90 | [197] |
4.1.2. Chemical Techniques for Removal of Microplastics
| Microplastics | Chemical Methods | Experimental Details | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|
| PS (1, 6.3 μm) | Coagulation/ flocculation |
| 99.4 (FeCl3) 98.2 (PACl) 65 (polyamine) | [206] |
| PE (10, 140 μm), PS (10, 140 μm), and polyester fibers | Coagulation/ flocculation |
| 82–99 | [207] |
| PE (5–15 μm) and polyester (17.5–50.6 μm) | Coagulation/ flocculation |
| 86–99 | [208] |
| High-density PE | Photocatalytic degradation |
| 71.77 | [209] |
| PS and PE | Photocatalytic degradation |
| 98.40 (400 nm PS) 95.30 (700 nm PS) | [210] |
| MPs | Ozonation |
| 99.2 | [211] |
4.1.3. Biological Techniques for Removal of Microplastics
| Microplastic Types | Biological Methods | Experimental Details | Removal Efficiencies (%) | Ref. |
|---|---|---|---|---|
| MPs | Activated sludge | Media: Wastewater Influent MPs: 47.4 ± 7.0 n/L Effluent MPs: 34.1 ± 9.4 n/L | 16.6 | [218] |
| PE, PET, PS, and PP (75 μm) | Biodegradation | Media: Aqueous phase Microorganism: Bacillus gottheilii Room temperature Time: 40 days | 6.2 (PE) 3.0 (PET) 5.8 (PS) 3.6 (PP) | [222] |
| PP (250 μm–4 mm) | Biodegradation | Media: Aqueous phase Microogranism: Pseudomonas sp. Temperature: 10 °C Time: 40 days | 17.3 | [223] |
| MPs | MBR | Media: Activated sludge | 99.4 | [224] |
4.2. Removal Technologies of Pharmaceuticals
4.2.1. Biological Treatment Technologies
Activated Sludge
Membrane Bioreactor
Phytoremediation
4.2.2. Physical Treatments Technologies
Activated Carbon Adsorption
Membrane Technology
Removal Mechanism of PCs by Membrane Separation Processes
MF and UF
NF and RO
4.2.3. Chemical Treatment Technologies
Ozonation
| Applied Treatment (Concentration and Duration) | Pharmaceuticals | Elimination Efficiencies (%) | Analytical Methods | References |
|---|---|---|---|---|
| O3 (5 mg/L, 15 min) | Carbamazepine Diclofenac Metoprolol Trimethoprim | >90 >90 80–90 >90 | GC-MS/MS, LC-MS/MS | [8] |
| O3 (n/a, n/a) * | Ibuprofen Diclofenac Carbamazepine | 83 99 80 | GC-MS/MS, LC-MS/MS | [349] |
| O3 (33 mg/L, 20 min) | Tetracycline | 95 | UV–Vis at 357 nm and TOC | [350] |
Advanced Oxidation Processes (AOPs)
4.2.4. Hybrid Technologies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | Activated Carbon |
| AnMBRs | Anaerobic Membrane Bioreactors |
| AOP | Advanced Oxidation Products |
| AS | Activated Sludge |
| BEMRs | Bio-electrochemical Membrane Bioreactors |
| BPA | Bisphenol A |
| CAFOs | Concentrated Animal Feeding Operations |
| CAS | Conventional Activated Sludge |
| CW | Constructed Wetlands |
| COD | Chemical Oxidation Demand |
| DAD | Diode Array Detector |
| EAOPs | Electrochemical Processes |
| ECs | Emerging Contaminants |
| EDCs | Endocrine-Disrupting Chemicals |
| FC | Flocculation |
| FLD | Fluorescence Detector |
| FO | Forward Osmosis |
| FWS-CW | Free Water Surface Constructed Wetlands |
| GAC | Granular Activated Carbon |
| GC | Gas Chromatography |
| HFCW | Horizontal Flow Constructed Wetlands |
| HRMBRs | High-Retention Membrane Bioreactors |
| HRMBRs | High-Retention Membrane Bioreactors |
| IC | Inorganic Carbon |
| IT-MS/MS | Ion trap mass spectrometer |
| KOW | Octanol–Water Partition Coefficient |
| LC | Liquid Chromatography |
| MBR | Membrane Bioreactor |
| MD | Membrane Distillation |
| MF | Microfiltration |
| MIPs | Molecular-Imprinted Polymers |
| MLSS | Mixed-Liquor Suspended Solids |
| MOF | Metal–Organic Framework |
| MS/MS | Tandem mass spectrometry |
| MW | Molecular Weight |
| MWCO | Molecular Weight Cut-Off |
| NF | Nanofiltration |
| NP | Nonylphenol |
| NP | Nonylphenol |
| NPs | Nanoplastics |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| OMBRs | Osmotic Membrane Bioreactors |
| PCs | Pharmaceuticals Contaminants |
| PAC | Powdered Activated Carbon |
| PAD | Photodiode Array Detector |
| PCPs | Personal Care Products |
| PE | Polyethylene |
| PET | Polyethylene terephthalate |
| PhACs | Pharmaceutically Active Compounds |
| PKa | The Acid Dissociation Constant |
| PPCPs | Pharmaceutical and Personal Care Products |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| QqQ-MS | Triple-Quadrupole Mass Spectrometry |
| RO | Reverse Osmosis |
| SPE | Solid Phase Extraction |
| SPME | Solid Phase Microextraction |
| SRT | Sludge Retention Time |
| SSF-CW | Subsurface Flow Constructed Wetlands |
| TMP | Transmembrane Pressure |
| TN | Total Nitrogen |
| TOC | Total Organic Carbon |
| TrOCs | Trace Organic Contaminants |
| UF | Ultrafiltration |
| UHPLC | Ultra-High-Performance Liquid Chromatography |
| UPLC | Ultra-Performance Liquid Chromatography |
| UV–Vis | Ultraviolet–Visible Spectrophotometry |
| VFCW | Vertical Flow Constructed Wetlands |
| WWTPs | Wastewater Treatment Plants |
References
- Khanzada, N.K.; Farid, M.U.; Kharraz, J.A.; Choi, J.; Tang, C.Y.; Nghiem, L.D.; Jang, A.; An, A.K. Removal of organic micropollutants using advanced membrane-based water and wastewater treatment: A review. J. Membr. Sci. 2020, 598, 117672. [Google Scholar] [CrossRef]
- Luo, Y.; Guo, W.; Ngo, H.H.; Nghiem, L.D.; Hai, F.I.; Zhang, J.; Liang, S.; Wang, X.C. A review on the occurrence of micropollutants in the aquatic environment and their fate and removal during wastewater treatment. Sci. Total Environ. 2014, 473–474, 619–641. [Google Scholar] [CrossRef] [PubMed]
- Ojajuni, O.; Saroj, D.; Cavalli, G. Removal of organic micropollutants using membrane-assisted processes: A review of recent progress. Environ. Technol. Rev. 2015, 4, 17–37. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging contaminants of high concern for the environment: Current trends and future research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive review of emerging contaminants: Detection technologies, environmental impact, and management strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Gavrilescu, M.; Demnerová, K.; Aamand, J.; Agathos, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Abbasi, N.A.; Shahid, S.U.; Majid, M.; Tahir, A. Introduction to environmental micropollutants. In Environmental Micropollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1–12. ISBN 9780323905558. [Google Scholar]
- Silva, L.L.S.; Moreira, C.G.; Curzio, B.A.; da Fonseca, F.V. Micropollutant Removal from Water by Membrane and Advanced Oxidation Processes—A Review. J. Water Resour. Prot. 2017, 9, 411–431. [Google Scholar] [CrossRef]
- Bila, D.M.; Dezotti, M. Desreguladores endócrinos no meio ambiente: Efeitos e conseqüências. Quím. Nova 2007, 30, 651–666. [Google Scholar] [CrossRef]
- Bila, D.M.; Dezotti, M. Fármacos no meio ambiente. Quím. Nova 2003, 26, 523–530. [Google Scholar] [CrossRef]
- Chhaya; Raychoudhury, T.; Prajapati, S.K. Bioremediation of Pharmaceuticals in Water and Wastewater. In Microbial Bioremediation & Biodegradation; Shah, M.P., Ed.; Springer: Singapore, 2020; pp. 425–446. ISBN 978-981-15-1811-9. [Google Scholar]
- Kim, M.-K.; Zoh, K.-D. Occurrence and removals of micropollutants in water environment. Environ. Eng. Res. 2016, 21, 319–332. [Google Scholar] [CrossRef]
- Altaf, F.; Hashmi, M.Z.; Farooq, U.; Rehman, Z.U.; Hmeed, M.U.; Batool, R.; Pongpiachan, S. Nanotechnology to treat the environmental micropollutants. In Environmental Micropollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 407–441. ISBN 9780323905558. [Google Scholar]
- Fent, K.; Weston, A.A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef]
- Pruden, A.; Pei, R.; Storteboom, H.; Carlson, K.H. Antibiotic resistance genes as emerging contaminants: Studies in northern Colorado. Environ. Sci. Technol. 2006, 40, 7445–7450. [Google Scholar] [CrossRef] [PubMed]
- Backhaus, T. Commentary on the EU Commission’s proposal for amending the Water Framework Directive, the Groundwater Directive, and the Directive on Environmental Quality Standards. Env. Sci. Eur. 2023, 35, 1. [Google Scholar] [CrossRef]
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj/eng (accessed on 27 January 2025).
- Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy Text with EEA Relevance. Available online: https://eur-lex.europa.eu/eli/dir/2013/39/oj/eng (accessed on 27 January 2025).
- Regulation (EC) No 805/2004 of the European Parliament and of the Council of 21 April 2004 Creating a European Enforcement Order for Uncontested Claims. Available online: https://eur-lex.europa.eu/eli/reg/2004/805/oj/eng (accessed on 27 January 2025).
- Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 Establishing a Framework for Community Action in the Field of Water Policy. Available online: https://eur-lex.europa.eu/eli/dir/2000/60/oj/eng (accessed on 27 January 2025).
- Dosis, I.; Ricci, M.; Majoros, L.; Lava, R.; Emteborg, H.; Held, A.; Emons, H. Addressing Analytical Challenges of the Environmental Monitoring for the Water Framework Directive: ERM-CE100, a New Biota Certified Reference Material. Anal. Chem. 2017, 89, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Ahting, M.; Brauer, F.; Duffek, A.; Ebert, I.; Eckhardt, A.; Hassold, E.; Helmecke, M.; Kirst, I.; Krause, B.; Lepom, P.; et al. Recommendations for Reducing Micropollutants in Waters; German Environment Agency: Dessau-Roßlau, Germany, 2017. [Google Scholar]
- Barbosa, M.O.; Moreira, N.F.F.; Ribeiro, A.R.; Pereira, M.F.R.; Silva, A.M.T. Occurrence and removal of organic micropollutants: An overview of the watch list of EU Decision 2015/495. Water Res. 2016, 94, 257–279. [Google Scholar] [CrossRef]
- Eggen, R.I.L.; Hollender, J.; Joss, A.; Schärer, M.; Stamm, C. Reducing the discharge of micropollutants in the aquatic environment: The benefits of upgrading wastewater treatment plants. Environ. Sci. Technol. 2014, 48, 7683–7689. [Google Scholar] [CrossRef]
- de Oliveira, M.; Atalla, A.A.; Frihling, B.E.F.; Cavalheri, P.S.; Migliolo, L.; Filho, F.J.C.M. Ibuprofen and caffeine removal in vertical flow and free-floating macrophyte constructed wetlands with Heliconia rostrata and Eichornia crassipes. Chem. Eng. J. 2019, 373, 458–467. [Google Scholar] [CrossRef]
- Verlicchi, P.; Al Aukidy, M.; Zambello, E. Occurrence of pharmaceutical compounds in urban wastewater: Removal, mass load and environmental risk after a secondary treatment—A review. Sci. Total Environ. 2012, 429, 123–155. [Google Scholar] [CrossRef]
- Hejna, M.; Kapuścińska, D.; Aksmann, A. Pharmaceuticals in the Aquatic Environment: A Review on Eco-Toxicology and the Remediation Potential of Algae. Int. J. Environ. Res. Public Health 2022, 19, 7717. [Google Scholar] [CrossRef]
- Ortúzar, M.; Esterhuizen, M.; Olicón-Hernández, D.R.; González-López, J.; Aranda, E. Pharmaceutical Pollution in Aquatic Environments: A Concise Review of Environmental Impacts and Bioremediation Systems. Front. Microbiol. 2022, 13, 869332. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Jyoti, D.; Sinha, R. Physiological impact of personal care product constituents on non-target aquatic organisms. Sci. Total Environ. 2023, 905, 167229. [Google Scholar] [CrossRef]
- Chakraborty, A.; Adhikary, S.; Bhattacharya, S.; Dutta, S.; Chatterjee, S.; Banerjee, D.; Ganguly, A.; Rajak, P. Pharmaceuticals and Personal Care Products as Emerging Environmental Contaminants: Prevalence, Toxicity, and Remedial Approaches. ACS Chem. Health Saf. 2023, 30, 362–388. [Google Scholar] [CrossRef]
- Krishnan, R.Y.; Manikandan, S.; Subbaiya, R.; Biruntha, M.; Govarthanan, M.; Karmegam, N. Removal of emerging micropollutants originating from pharmaceuticals and personal care products (PPCPs) in water and wastewater by advanced oxidation processes: A review. Environ. Technol. Innov. 2021, 23, 101757. [Google Scholar] [CrossRef]
- Narwal, N.; Katyal, D.; Kataria, N.; Rose, P.K.; Warkar, S.G.; Pugazhendhi, A.; Ghotekar, S.; Khoo, K.S. Emerging micropollutants in aquatic ecosystems and nanotechnology-based removal alternatives: A review. Chemosphere 2023, 341, 139945. [Google Scholar] [CrossRef]
- Gubó, E.; Plutzer, J.; Molnár, T.; Pordán-Háber, D.; Szabó, L.; Szalai, Z.; Gubó, R.; Szakál, P.; Szakál, T.; Környei, L.; et al. A 4-year study of bovine reproductive hormones that are induced by pharmaceuticals and appear as steroid estrogenic pollutants in the resulting slurry, using in vitro and instrumental analytical methods. Environ. Sci. Pollut. Res. Int. 2023, 30, 125596–125608. [Google Scholar] [CrossRef] [PubMed]
- Vadiraj, K.T.; Ram Achar, R.; Siriger, S. A review on emerging micropollutants: Sources, environmental concentration and toxicity. RB 2021, 6, 2305–2325. [Google Scholar] [CrossRef]
- Campo, J.; Masiá, A.; Blasco, C.; Picó, Y. Occurrence and removal efficiency of pesticides in sewage treatment plants of four Mediterranean River Basins. J. Hazard. Mater. 2013, 263 Pt 1, 146–157. [Google Scholar] [CrossRef]
- Köck-Schulmeyer, M.; Villagrasa, M.; López de Alda, M.; Céspedes-Sánchez, R.; Ventura, F.; Barceló, D. Occurrence and behavior of pesticides in wastewater treatment plants and their environmental impact. Sci. Total Environ. 2013, 458–460, 466–476. [Google Scholar] [CrossRef]
- Martin Ruel, S.; Esperanza, M.; Choubert, J.-M.; Valor, I.; Budzinski, H.; Coquery, M. On-site evaluation of the efficiency of conventional and advanced secondary processes for the removal of 60 organic micropollutants. Water Sci. Technol. 2010, 62, 2970–2978. [Google Scholar] [CrossRef]
- Gao, D.; Li, Z.; Wen, Z.; Ren, N. Occurrence and fate of phthalate esters in full-scale domestic wastewater treatment plants and their impact on receiving waters along the Songhua River in China. Chemosphere 2014, 95, 24–32. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Dinsdale, R.M.; Guwy, A.J. The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res. 2009, 43, 363–380. [Google Scholar] [CrossRef] [PubMed]
- Ateia, M.; Ersan, G.; Alalm, M.G.; Boffito, D.C.; Karanfil, T. Emerging investigator series: Microplastic sources, fate, toxicity, detection, and interactions with micropollutants in aquatic ecosystems—A review of reviews. Environ. Sci. Process. Impacts 2022, 24, 172–195. [Google Scholar] [CrossRef]
- Ali, N.; Khan, M.H.; Ali, M.; Sidra; Ahmad, S.; Khan, A.; Nabi, G.; Ali, F.; Bououdina, M.; Kyzas, G.Z. Insight into microplastics in the aquatic ecosystem: Properties, sources, threats and mitigation strategies. Sci. Total Environ. 2024, 913, 169489. [Google Scholar] [CrossRef]
- Rossatto, A.; Arlindo, M.Z.F.; de Morais, M.S.; de Souza, T.D.; Ogrodowski, C.S. Microplastics in aquatic systems: A review of occurrence, monitoring and potential environmental risks. Environ. Adv. 2023, 13, 100396. [Google Scholar] [CrossRef]
- Martinho, S.D.; Fernandes, V.C.; Figueiredo, S.A.; Delerue-Matos, C. Microplastic Pollution Focused on Sources, Distribution, Contaminant Interactions, Analytical Methods, and Wastewater Removal Strategies: A Review. Int. J. Environ. Res. Public Health 2022, 19, 5610. [Google Scholar] [CrossRef] [PubMed]
- Mamta; Bhushan, S.; Rana, M.S.; Raychaudhuri, S.; Simsek, H.; Prajapati, S.K. Algae- and bacteria-driven technologies for pharmaceutical remediation in wastewater. In Removal of Toxic Pollutants Through Microbiological and Tertiary Treatment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 373–408. ISBN 9780128210147. [Google Scholar]
- Gil, A.; García, A.M.; Fernández, M.; Vicente, M.A.; González-Rodríguez, B.; Rives, V.; Korili, S.A. Effect of dopants on the structure of titanium oxide used as a photocatalyst for the removal of emergent contaminants. J. Ind. Eng. Chem. 2017, 53, 183–191. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Cho, S.J.; Li, Y.; Venkatesh, S. Prediction of aqueous solubility of organic compounds using a quantitative structure-property relationship. J. Pharm. Sci. 2002, 91, 1838–1852. [Google Scholar] [CrossRef]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Aherne, G.W.; Briggs, R. The relevance of the presence of certain synthetic steroids in the aquatic environment. J. Pharm. Pharmacol. 1989, 41, 735–736. [Google Scholar] [CrossRef]
- Feier, B.; Gui, A.; Cristea, C.; Săndulescu, R. Electrochemical determination of cephalosporins using a bare boron-doped diamond electrode. Anal. Chim. Acta 2017, 976, 25–34. [Google Scholar] [CrossRef] [PubMed]
- aus der Beek, T.; Weber, F.-A.; Bergmann, A.; Hickmann, S.; Ebert, I.; Hein, A.; Küster, A. Pharmaceuticals in the environment—Global occurrences and perspectives. Environ. Toxicol. Chem. 2016, 35, 823–835. [Google Scholar] [CrossRef]
- aus der Beek, T.; Weber, F.-A.; Bergmann, A. Pharmaceuticals in the Environment: Global Occurrence and Potential Cooperative Action Under the Strategic Approach to International Chemicals Management (SAICM): Project No. (FKZ) 3712 65 408, Report No. (UBA-FB) 002331/ENG; Environmental Research of the Federal Ministry for the Environment, Nature Conservation, Building and Nuclear Safety; German Environment Agency: Dessau-Roßlau, Germany, 2015. [Google Scholar]
- Carmichael, W. The Cyanotoxins. In Incorporating in Plant Pathology—Classic Papers; Elsevier: Amsterdam, The Netherlands, 1997; pp. 211–256. ISBN 9780120059270. [Google Scholar]
- Costanzo, S.D.; Murby, J.; Bates, J. Ecosystem response to antibiotics entering the aquatic environment. Mar. Pollut. Bull. 2005, 51, 218–223. [Google Scholar] [CrossRef]
- Yahaya, A.; Sale, F.J.; Salehdeen, U.M. Analytical Methods for Determination of Regulated and Unregulated Disinfection By- Products in Drinking Water: A Review. CaJoST 2019, 2, 25–36. [Google Scholar]
- Samal, K.; Mahapatra, S.; Hibzur Ali, M. Pharmaceutical wastewater as Emerging Contaminants (EC): Treatment technologies, impact on environment and human health. Energy Nexus 2022, 6, 100076. [Google Scholar] [CrossRef]
- Tiwari, B.; Sellamuthu, B.; Ouarda, Y.; Drogui, P.; Tyagi, R.D.; Buelna, G. Review on fate and mechanism of removal of pharmaceutical pollutants from wastewater using biological approach. Bioresour. Technol. 2017, 224, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; van Hullebusch, E.D.; Rodrigo, M.A.; Esposito, G.; Oturan, M.A. Removal of residual anti-inflammatory and analgesic pharmaceuticals from aqueous systems by electrochemical advanced oxidation processes. A review. Chem. Eng. J. 2013, 228, 944–964. [Google Scholar] [CrossRef]
- Taheran, M.; Brar, S.K.; Verma, M.; Surampalli, R.Y.; Zhang, T.C.; Valero, J.R. Membrane processes for removal of pharmaceutically active compounds (PhACs) from water and wastewaters. Sci. Total Environ. 2016, 547, 60–77. [Google Scholar] [CrossRef]
- Liu, J.-L.; Wong, M.-H. Pharmaceuticals and personal care products (PPCPs): A review on environmental contamination in China. Environ. Int. 2013, 59, 208–224. [Google Scholar] [CrossRef]
- Esplugas, S.; Bila, D.M.; Krause, L.G.T.; Dezotti, M. Ozonation and advanced oxidation technologies to remove endocrine disrupting chemicals (EDCs) and pharmaceuticals and personal care products (PPCPs) in water effluents. J. Hazard. Mater. 2007, 149, 631–642. [Google Scholar] [CrossRef]
- Zhang, W.; Fourcade, F.; Amrane, A.; Geneste, F. Removal of Iodine-Containing X-ray Contrast Media from Environment: The Challenge of a Total Mineralization. Molecules 2023, 28, 341. [Google Scholar] [CrossRef]
- Loke, M.-L.; Tjørnelund, J.; Halling-Sørensen, B. Determination of the distribution coefficient (log Kd) of oxytetracycline, tylosin A, olaquindox and metronidazole in manure. Chemosphere 2002, 48, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Bazrafshan, E.; Esrafili, A.; Yang, J.-K.; Shirzad-Siboni, M. Photocatalytic degradation of Metronidazole with illuminated TiO2 nanoparticles. J. Environ. Health Sci. Eng. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Olivera, M.E.; Manzo, R.H.; Junginger, H.E.; Midha, K.K.; Shah, V.P.; Stavchansky, S.; Dressman, J.B.; Barends, D.M. Biowaiver monographs for immediate release solid oral dosage forms: Ciprofloxacin hydrochloride. J. Pharm. Sci. 2011, 100, 22–33. [Google Scholar] [CrossRef]
- dos Santos, E.C.; Gates, W.P.; Michels, L.; Juranyi, F.; Mikkelsen, A.; da Silva, G.J.; Fossum, J.O.; Bordallo, H.N. The pH influence on the intercalation of the bioactive agent ciprofloxacin in fluorohectorite. Appl. Clay Sci. 2018, 166, 288–298. [Google Scholar] [CrossRef]
- Leal, J.F.; Santos, E.B.H.; Esteves, V.I. Oxytetracycline in intensive aquaculture: Water quality during and after its administration, environmental fate, toxicity and bacterial resistance. Rev. Aquac. 2019, 11, 1176–1194. [Google Scholar] [CrossRef]
- Daghrir, R.; Drogui, P. Tetracycline antibiotics in the environment: A review. Env. Chem. Lett. 2013, 11, 209–227. [Google Scholar] [CrossRef]
- Fujioka, T.; Khan, S.J.; McDonald, J.A.; Nghiem, L.D. Nanofiltration of trace organic chemicals: A comparison between ceramic and polymeric membranes. Sep. Purif. Technol. 2014, 136, 258–264. [Google Scholar] [CrossRef]
- Vieira, Y.; Lima, E.C.; Foletto, E.L.; Dotto, G.L. Microplastics physicochemical properties, specific adsorption modeling and their interaction with pharmaceuticals and other emerging contaminants. Sci. Total Environ. 2021, 753, 141981. [Google Scholar] [CrossRef]
- Rodríguez-Seijo, A.; Pereira, R. Morphological and Physical Characterization of Microplastics. In Characterization and Analysis of Microplastics; Elsevier: Amsterdam, The Netherlands, 2017; pp. 49–66. ISBN 9780444638984. [Google Scholar]
- Teck Kim, Y.; Min, B.; Won Kim, K. General Characteristics of Packaging Materials for Food System. In Innovations in Food Packaging; Elsevier: Amsterdam, The Netherlands, 2014; pp. 13–35. ISBN 9780123946010. [Google Scholar]
- Jürgen Buschow, K.H.; Cahn, R.W.; Flemings, M.C.; Ilschner, B.; Kramer, E.J.; Mahajan, S.; Veyssière, P. (Eds.) Encyclopedia of Materials: Science and Technology; Elsevier: Oxford, UK, 2001. [Google Scholar]
- Glaser, A.; Biological, J. Degradation of Polymers in the Environment. In Plastics in the Environment; Gomiero, A., Ed.; IntechOpen: London, UK, 2019; ISBN 978-1-83880-492-3. [Google Scholar]
- Birch, Q.T.; Potter, P.M.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Sources, transport, measurement and impact of nano and microplastics in urban watersheds. Rev. Environ. Sci. Biotechnol. 2020, 19, 275–336. [Google Scholar] [CrossRef]
- Gottfried Wilhelm Ehrenstein. Polymeric Materials: Structure, Properties, Applications; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2012; ISBN 9783446434134. [Google Scholar]
- Hu, K.; Tian, W.; Yang, Y.; Nie, G.; Zhou, P.; Wang, Y.; Duan, X.; Wang, S. Microplastics remediation in aqueous systems: Strategies and technologies. Water Res. 2021, 198, 117144. [Google Scholar] [CrossRef]
- Khan, N.H.; Rahman, A.u.; Zuljalal, F.; Saeed, T.; Aziz, S.; Ilyas, M. Food additives as environmental micropollutants. In Environmental Micropollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 63–79. ISBN 9780323905558. [Google Scholar]
- Panda, B.P.; Majhi, B.K.; Parida, S.P. Occurrence and fate of micropollutants in water bodies. In Environmental Micropollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 271–293. ISBN 9780323905558. [Google Scholar]
- Stefanakis, A.I.; Becker, J.A. A Review of Emerging Contaminants in Water: Classification, Sources, and Potential Risks. In Waste Management; Management Association Information Reso, Ed.; IGI Global: Hershey, PA, USA, 2020; pp. 177–202. ISBN 9781799812104. [Google Scholar]
- Lamastra, L.; Balderacchi, M.; Trevisan, M. Inclusion of emerging organic contaminants in groundwater monitoring plans. MethodsX 2016, 3, 459–476. [Google Scholar] [CrossRef]
- Ternes, T.; Joss, A. Human Pharmaceuticals, Hormones and Fragrances—The Challenge of Micropollutants in Urban Water Management. Water Intell. Online 2006, 5, 9781780402468. [Google Scholar] [CrossRef]
- Besse, J.-P.; Kausch-Barreto, C.; Garric, J. Exposure Assessment of Pharmaceuticals and Their Metabolites in the Aquatic Environment: Application to the French Situation and Preliminary Prioritization. Hum. Ecol. Risk Assess. Int. J. 2008, 14, 665–695. [Google Scholar] [CrossRef]
- Ortiz de García, S.; Pinto Pinto, G.; García Encina, P.; Irusta Mata, R. Consumption and occurrence of pharmaceutical and personal care products in the aquatic environment in Spain. Sci. Total Environ. 2013, 444, 451–465. [Google Scholar] [CrossRef]
- Küster, A.; Adler, N. Pharmaceuticals in the environment: Scientific evidence of risks and its regulation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130587. [Google Scholar] [CrossRef]
- Ikehata, K.; Jodeiri Naghashkar, N.; Gamal El-Din, M. Degradation of Aqueous Pharmaceuticals by Ozonation and Advanced Oxidation Processes: A Review. Ozone Sci. Eng. 2006, 28, 353–414. [Google Scholar] [CrossRef]
- Das, S.; Ray, N.M.; Wan, J.; Khan, A.; Chakraborty, T.; Ray, M.B. Micropollutants in Wastewater: Fate and Removal Processes. In Physico-Chemical Wastewater Treatment and Resource Recovery; Farooq, R., Ahmad, Z., Eds.; InTech: Bronx, NY, USA, 2017; ISBN 978-953-51-3129-8. [Google Scholar]
- Jiang, J.-Q.; Zhou, Z.; Sharma, V.K. Occurrence, transportation, monitoring and treatment of emerging micro-pollutants in waste water—A review from global views. Microchem. J. 2013, 110, 292–300. [Google Scholar] [CrossRef]
- Capdeville, M.J.; Budzinski, H. Trace-level analysis of organic contaminants in drinking waters and groundwaters. TrAC Trends Anal. Chem. 2011, 329, 99. [Google Scholar] [CrossRef]
- Margot, J.; Rossi, L.; Barry, D.A.; Holliger, C. A review of the fate of micropollutants in wastewater treatment plants. WIREs Water 2015, 2, 457–487. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P.; Aziz, H.A. Fate of common pharmaceuticals in the environment. In The Treatment of Pharmaceutical Wastewater; Elsevier: Amsterdam, The Netherlands, 2023; pp. 69–148. ISBN 9780323991605. [Google Scholar]
- Pereira, A.; Silva, L.; Laranjeiro, C.; Lino, C.; Pena, A. Selected Pharmaceuticals in Different Aquatic Compartments: Part I-Source, Fate and Occurrence. Molecules 2020, 25, 1026. [Google Scholar] [CrossRef]
- Roberts, P.H.; Thomas, K.V. The occurrence of selected pharmaceuticals in wastewater effluent and surface waters of the lower Tyne catchment. Sci. Total Environ. 2006, 356, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wu, L.; Chang, A.C. Seasonal variation of endocrine disrupting compounds, pharmaceuticals and personal care products in wastewater treatment plants. Sci. Total Environ. 2013, 442, 310–316. [Google Scholar] [CrossRef]
- Yan, J.; Lin, W.; Gao, Z.; Ren, Y. Use of selected NSAIDs in Guangzhou and other cities in the world as identified by wastewater analysis. Chemosphere 2021, 279, 130529. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Jaimes, J.A.; Postigo, C.; Melgoza-Alemán, R.M.; Aceña, J.; Barceló, D.; López de Alda, M. Study of pharmaceuticals in surface and wastewater from Cuernavaca, Morelos, Mexico: Occurrence and environmental risk assessment. Sci. Total Environ. 2018, 613–614, 1263–1274. [Google Scholar] [CrossRef]
- Ali, A.M.; Rønning, H.T.; Alarif, W.; Kallenborn, R.; Al-Lihaibi, S.S. Occurrence of pharmaceuticals and personal care products in effluent-dominated Saudi Arabian coastal waters of the Red Sea. Chemosphere 2017, 175, 505–513. [Google Scholar] [CrossRef]
- Pereira, C.D.S.; Maranho, L.A.; Cortez, F.S.; Pusceddu, F.H.; Santos, A.R.; Ribeiro, D.A.; Cesar, A.; Guimarães, L.L. Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci. Total Environ. 2016, 548–549, 148–154. [Google Scholar] [CrossRef]
- Mukhtar, A.; Manzoor, M.; Gul, I.; Zafar, R.; Jamil, H.I.; Niazi, A.K.; Ali, M.A.; Park, T.J.; Arshad, M. Phytotoxicity of different antibiotics to rice and stress alleviation upon application of organic amendments. Chemosphere 2020, 258, 127353. [Google Scholar] [CrossRef]
- Zafar, R.; Bashir, S.; Nabi, D.; Arshad, M. Occurrence and quantification of prevalent antibiotics in wastewater samples from Rawalpindi and Islamabad, Pakistan. Sci. Total Environ. 2021, 764, 142596. [Google Scholar] [CrossRef]
- Zhang, Q.-Q.; Ying, G.-G.; Pan, C.-G.; Liu, Y.-S.; Zhao, J.-L. Comprehensive evaluation of antibiotics emission and fate in the river basins of China: Source analysis, multimedia modeling, and linkage to bacterial resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.; Chu, L.M. Fate of antibiotics in soil and their uptake by edible crops. Sci. Total Environ. 2017, 599–600, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- Cardoso, O.; Porcher, J.-M.; Sanchez, W. Factory-discharged pharmaceuticals could be a relevant source of aquatic environment contamination: Review of evidence and need for knowledge. Chemosphere 2014, 115, 20–30. [Google Scholar] [CrossRef]
- Combalbert, S.; Bellet, V.; Dabert, P.; Bernet, N.; Balaguer, P.; Hernandez-Raquet, G. Fate of steroid hormones and endocrine activities in swine manure disposal and treatment facilities. Water Res. 2012, 46, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2014, 69, 827–834. [Google Scholar] [CrossRef]
- Prosser, R.S.; Sibley, P.K. Human health risk assessment of pharmaceuticals and personal care products in plant tissue due to biosolids and manure amendments, and wastewater irrigation. Environ. Int. 2015, 75, 223–233. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Tomova, A.; López, A.; Maldonado, M.A.; Henríquez, L.A.; Ivanova, L.; Moy, F.; Godfrey, H.P.; Cabello, F.C. Salmon aquaculture and antimicrobial resistance in the marine environment. PLoS ONE 2012, 7, e42724. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial use in aquaculture re-examined: Its relevance to antimicrobial resistance and to animal and human health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, R.; Wang, Y.; Pan, X.; Tang, J.; Zhang, G. Occurrence and distribution of antibiotics in the Beibu Gulf, China: Impacts of river discharge and aquaculture activities. Mar. Environ. Res. 2012, 78, 26–33. [Google Scholar] [CrossRef]
- He, X.; Wang, Z.; Nie, X.; Yang, Y.; Pan, D.; Leung, A.O.W.; Cheng, Z.; Yang, Y.; Li, K.; Chen, K. Residues of fluoroquinolones in marine aquaculture environment of the Pearl River Delta, South China. Environ. Geochem. Health 2012, 34, 323–335. [Google Scholar] [CrossRef]
- Rico, A.; Phu, T.M.; Satapornvanit, K.; Min, J.; Shahabuddin, A.M.; Henriksson, P.J.G.; Murray, F.J.; Little, D.C.; Dalsgaard, A.; van den Brink, P.J. Use of veterinary medicines, feed additives and probiotics in four major internationally traded aquaculture species farmed in Asia. Aquaculture 2013, 412–413, 231–243. [Google Scholar] [CrossRef]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef]
- Iglesias, A.; Nebot, C.; Miranda, J.M.; Vázquez, B.I.; Cepeda, A. Detection and quantitative analysis of 21 veterinary drugs in river water using high-pressure liquid chromatography coupled to tandem mass spectrometry. Environ. Sci. Pollut. Res. Int. 2012, 19, 3235–3249. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.J.; Kluenter, A.M. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed Sci. Technol. 2019, 250, 81–92. [Google Scholar] [CrossRef]
- Klein, E.Y.; van Boeckel, T.P.; Martinez, E.M.; Pant, S.; Gandra, S.; Levin, S.A.; Goossens, H.; Laxminarayan, R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 2018, 115, E3463–E3470. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- de Ilurdoz, M.S.; Sadhwani, J.J.; Reboso, J.V. Antibiotic removal processes from water & wastewater for the protection of the aquatic environment—A review. J. Water Process Eng. 2022, 45, 102474. [Google Scholar] [CrossRef]
- Obimakinde, S.; Fatoki, O.; Opeolu, B.; Olatunji, O. Veterinary pharmaceuticals in aqueous systems and associated effects: An update. Environ. Sci. Pollut. Res. Int. 2017, 24, 3274–3297. [Google Scholar] [CrossRef]
- Du Plessis, M.; Fourie, C.; Stone, W.; Engelbrecht, A.-M. The impact of endocrine disrupting compounds and carcinogens in wastewater: Implications for breast cancer. Biochimie 2023, 209, 103–115. [Google Scholar] [CrossRef]
- Thanh, P.N.; Phung, V.-D.; Nguyen, T.B.H. Recent advances and future trends in metal oxide photocatalysts for removal of pharmaceutical pollutants from wastewater: A comprehensive review. Environ. Geochem. Health 2024, 46, 364. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.M.I.; Ong, H.C.; Chia, W.Y.; et al. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Tyumina, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.d.P.; Ivshina, I.B. Nonsteroidal Anti-inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Dolliver, H.; Gupta, S. Antibiotic losses in leaching and surface runoff from manure-amended agricultural land. J. Environ. Qual. 2008, 37, 1227–1237. [Google Scholar] [CrossRef]
- Lajeunesse, A.; Smyth, S.A.; Barclay, K.; Sauvé, S.; Gagnon, C. Distribution of antidepressant residues in wastewater and biosolids following different treatment processes by municipal wastewater treatment plants in Canada. Water Res. 2012, 46, 5600–5612. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Chu, S.; Judt, C.; Li, H.; Oakes, K.D.; Servos, M.R.; Andrews, D.M. Antidepressants and their metabolites in municipal wastewater, and downstream exposure in an urban watershed. Environ. Toxicol. Chem. 2010, 29, 79–89. [Google Scholar] [CrossRef]
- Gabriel, M.; Sharma, V. Antidepressant discontinuation syndrome. CMAJ 2017, 189, E747. [Google Scholar] [CrossRef]
- Wilson, E.; Lader, M. A review of the management of antidepressant discontinuation symptoms. Ther. Adv. Psychopharmacol. 2015, 5, 357–368. [Google Scholar] [CrossRef]
- Cho, Y.M.; Choi, K.-H. The current status of studies of human exposure assessment of microplastics and their health effects: A rapid systematic review. Environ. Anal. Health Toxicol. 2021, 36, e2021004-0. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. The interactions of microplastics and chemical pollutants. In Microplastic Pollutants; Elsevier: Amsterdam, The Netherlands, 2017; pp. 131–157. ISBN 9780128094068. [Google Scholar]
- Kirstein, I.V.; Kirmizi, S.; Wichels, A.; Garin-Fernandez, A.; Erler, R.; Löder, M.; Gerdts, G. Dangerous hitchhikers? Evidence for potentially pathogenic Vibrio spp. on microplastic particles. Mar. Environ. Res. 2016, 120, 1–8. [Google Scholar] [CrossRef]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Duarte, A.C.; Rocha-Santos, T. Environmental exposure to microplastics: An overview on possible human health effects. Sci. Total Environ. 2020, 702, 134455. [Google Scholar] [CrossRef]
- Yang, X.; Man, Y.B.; Wong, M.H.; Owen, R.B.; Chow, K.L. Environmental health impacts of microplastics exposure on structural organization levels in the human body. Sci. Total Environ. 2022, 825, 154025. [Google Scholar] [CrossRef]
- Campanale, C.; Massarelli, C.; Savino, I.; Locaputo, V.; Uricchio, V.F. A Detailed Review Study on Potential Effects of Microplastics and Additives of Concern on Human Health. Int. J. Environ. Res. Public Health 2020, 17, 1212. [Google Scholar] [CrossRef]
- Marcharla, E.; Vinayagam, S.; Gnanasekaran, L.; Soto-Moscoso, M.; Chen, W.-H.; Thanigaivel, S.; Ganesan, S. Microplastics in marine ecosystems: A comprehensive review of biological and ecological implications and its mitigation approach using nanotechnology for the sustainable environment. Environ. Res. 2024, 256, 119181. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.d.A.; Silva, D.d.M.E.; Pedroso, T.M.A.; Ramos, J.S.A.; Parise, M.R. Farmers exposed to pesticides have almost five times more DNA damage: A meta-analysis study. Environ. Sci. Pollut. Res. Int. 2022, 29, 805–816. [Google Scholar] [CrossRef]
- Long, M.; Moriceau, B.; Gallinari, M.; Lambert, C.; Huvet, A.; Raffray, J.; Soudant, P. Interactions between microplastics and phytoplankton aggregates: Impact on their respective fates. Mar. Chem. 2015, 175, 39–46. [Google Scholar] [CrossRef]
- Naselli-Flores, L.; Padisák, J. Ecosystem services provided by marine and freshwater phytoplankton. Hydrobiologia 2023, 850, 2691–2706. [Google Scholar] [CrossRef]
- Sumaila, U.R.; Tai, T.C. End Overfishing and Increase the Resilience of the Ocean to Climate Change. Front. Mar. Sci. 2020, 7, 2739. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.; Love, D.C.; Rochman, C.M.; Neff, R.A. Microplastics in Seafood and the Implications for Human Health. Curr. Environ. Health Rep. 2018, 5, 375–386. [Google Scholar] [CrossRef]
- Yuan, K.; Jurado-Sánchez, B.; Escarpa, A. Nanomaterials meet surface-enhanced Raman scattering towards enhanced clinical diagnosis: A review. J. Nanobiotechnol. 2022, 20, 537. [Google Scholar] [CrossRef]
- Ghosh, S.; Sinha, J.K.; Ghosh, S.; Vashisth, K.; Han, S.; Bhaskar, R. Microplastics as an Emerging Threat to the Global Environment and Human Health. Sustainability 2023, 15, 10821. [Google Scholar] [CrossRef]
- Wołowicz, A.; Munir, H.M.S. Emerging organic micropollutants as serious environmental problem: A comprehensive review. Sci. Total Environ. 2025, 958, 177948. [Google Scholar] [CrossRef] [PubMed]
- Ashiwaju, B.I.; Uzougbo, C.G.; Orikpete, O.F. Environmental Impact of Pharmaceuticals: A Comprehensive Review. Matrix Science Pharma 2023, 7, 85–94. [Google Scholar] [CrossRef]
- Paut Kusturica, M.; Jevtic, M.; Ristovski, J.T. Minimizing the environmental impact of unused pharmaceuticals: Review focused on prevention. Front. Environ. Sci. 2022, 10, 16211. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Thakur, K.; Mahajan, D.; Brar, B.; Sharma, D.; Kumar, S.; Sharma, A.K. Impact of Pesticides Application on Aquatic Ecosystem and Biodiversity: A Review. Biol Bull Russ Acad Sci 2023, 50, 1362–1375. [Google Scholar] [CrossRef]
- Özkara, A.; Akyil, D.; Konuk, M. Pesticides, Environmental Pollution, and Health. In Environmental Health Risk—Hazardous Factors to Living Species; Larramendy, M., Soloneski, S., Eds.; InTech: London, UK, 2016; ISBN 978-953-51-2401-6. [Google Scholar]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; de Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2011, 127, 64–73. [Google Scholar] [CrossRef]
- Uddin, M.H.; Shahjahan, M.; Ruhul Amin, A.K.M.; Haque, M.M.; Islam, M.A.; Azim, M.E. Impacts of organophosphate pesticide, sumithion on water quality and benthic invertebrates in aquaculture ponds. Aquac. Rep. 2016, 3, 88–92. [Google Scholar] [CrossRef]
- Maurya, P.K.; Malik, D.S.; Sharma, A. Impacts of pesticide application on aquatic environments and fish diversity. In Contaminants in Agriculture and Environment: Health Risks and Remediation; Agro Environ Media—Agriculture and Ennvironmental Science Academy: Haridwar, India, 2019; pp. 111–128. ISBN 9788194201700. [Google Scholar]
- Sarkar, D.J.; Das Sarkar, S.; Das, B.K.; Praharaj, J.K.; Mahajan, D.K.; Purokait, B.; Mohanty, T.R.; Mohanty, D.; Gogoi, P.; Kumar V, S.; et al. Microplastics removal efficiency of drinking water treatment plant with pulse clarifier. J. Hazard. Mater. 2021, 413, 125347. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Song, B.; Zhu, Y.; Zeng, G.; Zhang, Y.; Yang, Y.; Wen, X.; Chen, M.; Yi, H. Removal of microplastics via drinking water treatment: Current knowledge and future directions. Chemosphere 2020, 251, 126612. [Google Scholar] [CrossRef]
- Lam, T.W.L.; Ho, H.T.; Ma, A.T.H.; Fok, L. Microplastic Contamination of Surface Water-Sourced Tap Water in Hong Kong—A Preliminary Study. Appl. Sci. 2020, 10, 3463. [Google Scholar] [CrossRef]
- Fram, M.S.; Belitz, K. Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci. Total Environ. 2011, 409, 3409–3417. [Google Scholar] [CrossRef]
- Li, S.; Wen, J.; He, B.; Wang, J.; Hu, X.; Liu, J. Occurrence of caffeine in the freshwater environment: Implications for ecopharmacovigilance. Environ. Pollut. 2020, 263, 114371. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Davoli, E.; Riva, F.; Palmiotto, M.; Camporini, P.; Manenti, A.; Zuccato, E. Mass balance of emerging contaminants in the water cycle of a highly urbanized and industrialized area of Italy. Water Res. 2018, 131, 287–298. [Google Scholar] [CrossRef]
- Loos, R.; Locoro, G.; Comero, S.; Contini, S.; Schwesig, D.; Werres, F.; Balsaa, P.; Gans, O.; Weiss, S.; Blaha, L.; et al. Pan-European survey on the occurrence of selected polar organic persistent pollutants in ground water. Water Res. 2010, 44, 4115–4126. [Google Scholar] [CrossRef] [PubMed]
- Deo, R.; Halden, R. Pharmaceuticals in the Built and Natural Water Environment of the United States. Water 2013, 5, 1346–1365. [Google Scholar] [CrossRef]
- Shruti, V.C.; Pérez-Guevara, F.; Kutralam-Muniasamy, G. Metro station free drinking water fountain- A potential “microplastics hotspot” for human consumption. Environ. Pollut. 2020, 261, 114227. [Google Scholar] [CrossRef]
- Borrull, J.; Colom, A.; Fabregas, J.; Borrull, F.; Pocurull, E. Presence, behaviour and removal of selected organic micropollutants through drinking water treatment. Chemosphere 2021, 276, 130023. [Google Scholar] [CrossRef]
- Tröger, R.; Klöckner, P.; Ahrens, L.; Wiberg, K. Micropollutants in drinking water from source to tap—Method development and application of a multiresidue screening method. Sci. Total Environ. 2018, 627, 1404–1432. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-W.; Jo, B.-I.; Yoon, Y.; Zoh, K.-D. Occurrence and removal of selected micropollutants in a water treatment plant. Chemosphere 2014, 95, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Ayman, Z.; Işık, M. Pharmaceutically active compounds in water, Aksaray, Turkey. CLEAN–Soil Air Water 2015, 43, 1381–1388. [Google Scholar] [CrossRef]
- Adomat, Y.; Grischek, T. Occurrence, fate and potential risks of pharmaceuticals and personal care products (PPCPs) in Elbe river water during water treatment in Dresden, Germany. Environ. Chall. 2024, 15, 100938. [Google Scholar] [CrossRef]
- Simazaki, D.; Kubota, R.; Suzuki, T.; Akiba, M.; Nishimura, T.; Kunikane, S. Occurrence of selected pharmaceuticals at drinking water purification plants in Japan and implications for human health. Water Res. 2015, 76, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Meena, R.A.A.; Palanisami, T.; Ashokkumar, V.; Palvannan, T.; Gu, F.L. Occurrence, interactive effects and ecological risk of diclofenac in environmental compartments and biota—A review. Sci. Total Environ. 2020, 698, 134057. [Google Scholar] [CrossRef]
- Vulliet, E.; Cren-Olivé, C. Screening of pharmaceuticals and hormones at the regional scale, in surface and groundwaters intended to human consumption. Environ. Pollut. 2011, 159, 2929–2934. [Google Scholar] [CrossRef]
- Zhou, X.J.; Wang, J.; Li, H.Y.; Zhang, H.M.; Zhang, D.L. Microplastic pollution of bottled water in China. J. Water Process Eng. 2021, 40, 101884. [Google Scholar] [CrossRef]
- Samandra, S.; Mescall, O.J.; Plaisted, K.; Symons, B.; Xie, S.; Ellis, A.V.; Clarke, B.O. Assessing exposure of the Australian population to microplastics through bottled water consumption. Sci. Total Environ. 2022, 837, 155329. [Google Scholar] [CrossRef]
- Makhdoumi, P.; Amin, A.A.; Karimi, H.; Pirsaheb, M.; Kim, H.; Hossini, H. Occurrence of microplastic particles in the most popular Iranian bottled mineral water brands and an assessment of human exposure. J. Water Process Eng. 2021, 39, 101708. [Google Scholar] [CrossRef]
- Loos, R.; Carvalho, R.; António, D.C.; Comero, S.; Locoro, G.; Tavazzi, S.; Paracchini, B.; Ghiani, M.; Lettieri, T.; Blaha, L.; et al. EU-wide monitoring survey on emerging polar organic contaminants in wastewater treatment plant effluents. Water Res. 2013, 47, 6475–6487. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H.-S. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef]
- Ben, W.; Zhu, B.; Yuan, X.; Zhang, Y.; Yang, M.; Qiang, Z. Occurrence, removal and risk of organic micropollutants in wastewater treatment plants across China: Comparison of wastewater treatment processes. Water Res. 2018, 130, 38–46. [Google Scholar] [CrossRef]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Rogowska, J.; Cieszynska-Semenowicz, M.; Ratajczyk, W.; Wolska, L. Micropollutants in treated wastewater. Ambio 2020, 49, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Kamdi, P.; Chakraborty, S.; Das, S.; Bafana, A.; Krishnamurthi, K.; Sivanesan, S. Characterization and removal of microplastics in a sewage treatment plant from urban Nagpur, India. Environ. Monit. Assess. 2022, 195, 47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, X.; Ren, H.; Cao, G.; Xie, G.; Xing, D.; Liu, B. Investigation and fate of microplastics in wastewater and sludge filter cake from a wastewater treatment plant in China. Sci. Total Environ. 2020, 746, 141378. [Google Scholar] [CrossRef]
- Hajji, S.; Ben-Haddad, M.; Abelouah, M.R.; De-la-Torre, G.E.; Alla, A.A. Occurrence, characteristics, and removal of microplastics in wastewater treatment plants located on the Moroccan Atlantic: The case of Agadir metropolis. Sci. Total Environ. 2023, 862, 160815. [Google Scholar] [CrossRef]
- Park, H.; Park, B. Review of Microplastic Distribution, Toxicity, Analysis Methods, and Removal Technologies. Water 2021, 13, 2736. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of microplastics in water: Technology progress and green strategies. Green Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Chen, Z.; Yang, F.; Xie, Y.; Yao, W. Current status of microplastics and nanoplastics removal methods: Summary, comparison and prospect. Sci. Total Environ. 2022, 851, 157991. [Google Scholar] [CrossRef] [PubMed]
- Dey, T.K.; Uddin, M.E.; Jamal, M. Detection and removal of microplastics in wastewater: Evolution and impact. Environ. Sci. Pollut. Res. Int. 2021, 28, 16925–16947. [Google Scholar] [CrossRef]
- Sharma, H.B.; Vanapalli, K.R.; Cheela, V.S.; Ranjan, V.P.; Jaglan, A.K.; Dubey, B.; Goel, S.; Bhattacharya, J. Challenges, opportunities, and innovations for effective solid waste management during and post COVID-19 pandemic. Resour. Conserv. Recycl. 2020, 162, 105052. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Ramirez Arenas, L.; Ramseier Gentile, S.; Zimmermann, S.; Stoll, S. Nanoplastics adsorption and removal efficiency by granular activated carbon used in drinking water treatment process. Sci. Total Environ. 2021, 791, 148175. [Google Scholar] [CrossRef]
- Zhou, G.; Huang, X.; Xu, H.; Wang, Q.; Wang, M.; Wang, Y.; Li, Q.; Zhang, Y.; Ye, Q.; Zhang, J. Removal of polystyrene nanoplastics from water by CuNi carbon material: The role of adsorption. Sci. Total Environ. 2022, 820, 153190. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Park, S. Enhancing Microplastics Removal from Wastewater Using Electro-Coagulation and Granule-Activated Carbon with Thermal Regeneration. Processes 2021, 9, 617. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, S.; Su, Y.; Wu, D.; Zhao, Y.; Xie, B. Removal of microplastics from aqueous solutions by magnetic carbon nanotubes. Chem. Eng. J. 2021, 406, 126804. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.-X.; Chi, Y.; Yan, J.-H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Simon, M.; Vianello, A.; Vollertsen, J. Removal of >10 µm Microplastic Particles from Treated Wastewater by a Disc Filter. Water 2019, 11, 1935. [Google Scholar] [CrossRef]
- Komorowska-Kaufman, M.; Marciniak, W. Removal of microplastic particles during municipal wastewater treatment: A current review. Desalination Water Treat. 2024, 317, 100006. [Google Scholar] [CrossRef]
- Zhang, Z.; Su, Y.; Zhu, J.; Shi, J.; Huang, H.; Xie, B. Distribution and removal characteristics of microplastics in different processes of the leachate treatment system. Waste Manag. 2021, 120, 240–247. [Google Scholar] [CrossRef]
- Wang, Z.; Sedighi, M.; Lea-Langton, A. Filtration of microplastic spheres by biochar: Removal efficiency and immobilisation mechanisms. Water Res. 2020, 184, 116165. [Google Scholar] [CrossRef] [PubMed]
- Malankowska, M.; Echaide-Gorriz, C.; Coronas, J. Microplastics in marine environment: A review on sources, classification, and potential remediation by membrane technology. Environ. Sci. Water Res. Technol. 2021, 7, 243–258. [Google Scholar] [CrossRef]
- Ali, I.; Ding, T.; Peng, C.; Naz, I.; Sun, H.; Li, J.; Liu, J. Micro- and nanoplastics in wastewater treatment plants: Occurrence, removal, fate, impacts and remediation technologies—A critical review. Chem. Eng. J. 2021, 423, 130205. [Google Scholar] [CrossRef]
- Michielssen, M.R.; Michielssen, E.R.; Ni, J.; Duhaime, M.B. Fate of microplastics and other small anthropogenic litter (SAL) in wastewater treatment plants depends on unit processes employed. Environ. Sci. Water Res. Technol. 2016, 2, 1064–1073. [Google Scholar] [CrossRef]
- Talvitie, J.; Mikola, A.; Koistinen, A.; Setälä, O. Solutions to microplastic pollution—Removal of microplastics from wastewater effluent with advanced wastewater treatment technologies. Water Res. 2017, 123, 401–407. [Google Scholar] [CrossRef]
- Enfrin, M.; Lee, J.; Le-Clech, P.; Dumée, L.F. Kinetic and mechanistic aspects of ultrafiltration membrane fouling by nano- and microplastics. J. Membr. Sci. 2020, 601, 117890. [Google Scholar] [CrossRef]
- Li, J.; Wang, B.; Chen, Z.; Ma, B.; Chen, J.P. Ultrafiltration membrane fouling by microplastics with raw water: Behaviors and alleviation methods. Chem. Eng. J. 2021, 410, 128174. [Google Scholar] [CrossRef]
- Iwuozor, K.O. Prospects and Challenges of Using Coagulation-Flocculation method in the treatment of Effluents. Adv. J. Chem. A 2019, 2, 105–127. [Google Scholar] [CrossRef]
- Akinnawo, S.O.; Ayadi, P.O.; Oluwalope, M.T. Chemical coagulation and biological techniques for wastewater treatment. Ovidius Univ. Ann. Chem. 2023, 34, 14–21. [Google Scholar] [CrossRef]
- Rajala, K.; Grönfors, O.; Hesampour, M.; Mikola, A. Removal of microplastics from secondary wastewater treatment plant effluent by coagulation/flocculation with iron, aluminum and polyamine-based chemicals. Water Res. 2020, 183, 116045. [Google Scholar] [CrossRef] [PubMed]
- Lapointe, M.; Farner, J.M.; Hernandez, L.M.; Tufenkji, N. Understanding and Improving Microplastic Removal during Water Treatment: Impact of Coagulation and Flocculation. Environ. Sci. Technol. 2020, 54, 8719–8727. [Google Scholar] [CrossRef]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Kleinberg, K.A. Removal of micron-sized microplastic particles from simulated drinking water via alum coagulation. Chem. Eng. J. 2020, 386, 123807. [Google Scholar] [CrossRef]
- Ariza-Tarazona, M.C.; Villarreal-Chiu, J.F.; Hernández-López, J.M.; La Rivera De Rosa, J.; Barbieri, V.; Siligardi, C.; Cedillo-González, E.I. Microplastic pollution reduction by a carbon and nitrogen-doped TiO2: Effect of pH and temperature in the photocatalytic degradation process. J. Hazard. Mater. 2020, 395, 122632. [Google Scholar] [CrossRef]
- Nabi, I.; Bacha, A.-U.-R.; Li, K.; Cheng, H.; Wang, T.; Liu, Y.; Ajmal, S.; Yang, Y.; Feng, Y.; Zhang, L. Complete Photocatalytic Mineralization of Microplastic on TiO2 Nanoparticle Film. iScience 2020, 23, 101326. [Google Scholar] [CrossRef]
- Hidayaturrahman, H.; Lee, T.-G. A study on characteristics of microplastic in wastewater of South Korea: Identification, quantification, and fate of microplastics during treatment process. Mar. Pollut. Bull. 2019, 146, 696–702. [Google Scholar] [CrossRef]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef]
- Zhu, K.; Jia, H.; Zhao, S.; Xia, T.; Guo, X.; Wang, T.; Zhu, L. Formation of Environmentally Persistent Free Radicals on Microplastics under Light Irradiation. Environ. Sci. Technol. 2019, 53, 8177–8186. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Zeng, H.; Lin, H.; Li, H.; Chai, Y.-Y.; Choong, M.-K.; Mohamed, A.R. Green synthesis of Fe-ZnO nanoparticles with improved sunlight photocatalytic performance for polyethylene film deterioration and bacterial inactivation. Mater. Sci. Semicond. Process. 2021, 123, 105574. [Google Scholar] [CrossRef]
- Surana, M.; Pattanayak, D.S.; Yadav, V.; Singh, V.K.; Pal, D. An insight decipher on photocatalytic degradation of microplastics: Mechanism, limitations, and future outlook. Environ. Res. 2024, 247, 118268. [Google Scholar] [CrossRef] [PubMed]
- Tofa, T.S.; Kunjali, K.L.; Paul, S.; Dutta, J. Visible light photocatalytic degradation of microplastic residues with zinc oxide nanorods. Environ. Chem. Lett. 2019, 17, 1341–1346. [Google Scholar] [CrossRef]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastics removal in wastewater treatment plants: A critical review. Environ. Sci. Water Res. Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, W.; Di, M.; Li, Z.; Wang, J. Transfer and fate of microplastics during the conventional activated sludge process in one wastewater treatment plant of China. Chem. Eng. J. 2019, 362, 176–182. [Google Scholar] [CrossRef]
- Tursi, A.; Baratta, M.; Easton, T.; Chatzisymeon, E.; Chidichimo, F.; de Biase, M.; de Filpo, G. Microplastics in aquatic systems, a comprehensive review: Origination, accumulation, impact, and removal technologies. RSC Adv. 2022, 12, 28318–28340. [Google Scholar] [CrossRef] [PubMed]
- Miri, S.; Saini, R.; Davoodi, S.M.; Pulicharla, R.; Brar, S.K.; Magdouli, S. Biodegradation of microplastics: Better late than never. Chemosphere 2022, 286, 131670. [Google Scholar] [CrossRef] [PubMed]
- Rubiños, S.L.; Grados, J.H.; Medina, J.T.; Chávez, E.N.; Huarcaya, E.; Rocha, V.E.; Grados, H.J.; Neyra, M.R. Microplastics in the Ecosystem: A Systematic Review of the Methods for Their Detection and Removal. Int. J. Ecol. 2023, 2023, 1–21. [Google Scholar] [CrossRef]
- Auta, H.S.; Emenike, C.U.; Fauziah, S.H. Screening of Bacillus strains isolated from mangrove ecosystems in Peninsular Malaysia for microplastic degradation. Environ. Pollut. 2017, 231, 1552–1559. [Google Scholar] [CrossRef]
- Habib, S.; Iruthayam, A.; Abd Shukor, M.Y.; Alias, S.A.; Smykla, J.; Yasid, N.A. Biodeterioration of Untreated Polypropylene Microplastic Particles by Antarctic Bacteria. Polymers 2020, 12, 2616. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, identification and removal of microplastic particles and fibers in conventional activated sludge process and advanced MBR technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Pervez, M.N.; Balakrishnan, M.; Hasan, S.W.; Choo, K.-H.; Zhao, Y.; Cai, Y.; Zarra, T.; Belgiorno, V.; Naddeo, V. A critical review on nanomaterials membrane bioreactor (NMs-MBR) for wastewater treatment. NPJ Clean Water 2020, 3, 37. [Google Scholar] [CrossRef]
- Gurung, K.; Ncibi, M.C.; Fontmorin, J.M. Incorporating Submerged MBR in Conventional Activated Sludge Process for Municipal Wastewater Treatment: A Feasibility and Performance Assessment. J. Membra Sci. Technol. 2016, 6, e1000158. [Google Scholar] [CrossRef]
- Rahman, T.U.; Roy, H.; Islam, M.R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, M.N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, M.; Tarigan, M.; Schmitz, O.; Schütz, S.; Gießelmann, S.; Czermak, P. Keramische Membranen für die effiziente Abtrennung von organischen Mikroschadstoffen aus wässrigen Lösungen am Beispiel pharmazeutischer Wirkstoffe. Chem. Ing. Tech. 2023, 95, 1381–1387. [Google Scholar] [CrossRef]
- Freitas, B.d.O.; Leite, L.d.S.; Daniel, L.A. Chlorine and peracetic acid in decentralized wastewater treatment: Disinfection, oxidation and odor control. Process Saf. Environ. Prot. 2021, 146, 620–628. [Google Scholar] [CrossRef]
- Albolafio, S.; Marín, A.; Allende, A.; García, F.; Simón-Andreu, P.J.; Soler, M.A.; Gil, M.I. Strategies for mitigating chlorinated disinfection byproducts in wastewater treatment plants. Chemosphere 2022, 288, 132583. [Google Scholar] [CrossRef] [PubMed]
- Voigt, M.; Wirtz, A.; Hoffmann-Jacobsen, K.; Jaeger, M. Prior art for the development of a fourth purification stage in wastewater treatment plant for the elimination of anthropogenic micropollutants—A short-review. AIMS Environ. Sci. 2020, 7, 69–98. [Google Scholar] [CrossRef]
- Fernandes, J.; Ramísio, P.J.; Puga, H. A Comprehensive Review on Various Phases of Wastewater Technologies: Trends and Future Perspectives. Eng 2024, 5, 2633–2661. [Google Scholar] [CrossRef]
- Jelic, A.; Gros, M.; Ginebreda, A.; Cespedes-Sánchez, R.; Ventura, F.; Petrovic, M.; Barcelo, D. Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res. 2011, 45, 1165–1176. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, C.E.; Marco-Urrea, E.; Caminal, G. Degradation of naproxen and carbamazepine in spiked sludge by slurry and solid-phase Trametes versicolor systems. Bioresour. Technol. 2010, 101, 2259–2266. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Rivera-Utrilla, J.; Sánchez-Polo, M.; Ferro-García, M.Á.; Prados-Joya, G.; Ocampo-Pérez, R. Pharmaceuticals as emerging contaminants and their removal from water. A review. Chemosphere 2013, 93, 1268–1287. [Google Scholar] [CrossRef]
- Oulton, R.L.; Kohn, T.; Cwiertny, D.M. Pharmaceuticals and personal care products in effluent matrices: A survey of transformation and removal during wastewater treatment and implications for wastewater management. J. Environ. Monit. 2010, 12, 1956–1978. [Google Scholar] [CrossRef] [PubMed]
- Tiedeken, E.J.; Tahar, A.; McHugh, B.; Rowan, N.J. Monitoring, sources, receptors, and control measures for three European Union watch list substances of emerging concern in receiving waters—A 20year systematic review. Sci. Total Environ. 2017, 574, 1140–1163. [Google Scholar] [CrossRef]
- Rout, P.R.; Zhang, T.C.; Bhunia, P.; Surampalli, R.Y. Treatment technologies for emerging contaminants in wastewater treatment plants: A review. Sci. Total Environ. 2021, 753, 141990. [Google Scholar] [CrossRef] [PubMed]
- Derco, J.; Žgajnar Gotvajn, A.; Guľašová, P.; Šoltýsová, N.; Kassai, A. Selected Micropollutant Removal from Municipal Wastewater. Processes 2024, 12, 888. [Google Scholar] [CrossRef]
- Suarez, S.; Lema, J.M.; Omil, F. Removal of pharmaceutical and personal care products (PPCPs) under nitrifying and denitrifying conditions. Water Res. 2010, 44, 3214–3224. [Google Scholar] [CrossRef]
- Casas, M.E.; Chhetri, R.K.; Ooi, G.; Hansen, K.M.S.; Litty, K.; Christensson, M.; Kragelund, C.; Andersen, H.R.; Bester, K. Biodegradation of pharmaceuticals in hospital wastewater by staged Moving Bed Biofilm Reactors (MBBR). Water Res. 2015, 83, 293–302. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Grillini, V.; Mutavdžić Pavlović, D.; Verlicchi, P. Activated carbon coupled with advanced biological wastewater treatment: A review of the enhancement in micropollutant removal. Sci. Total Environ. 2021, 790, 148050. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ryu, K.; Kim, E.J.; Choe, W.S.; Cha, G.C.; Yoo, I.-K. Degradation of bisphenol A and nonylphenol by nitrifying activated sludge. Process Biochem. 2007, 42, 1470–1474. [Google Scholar] [CrossRef]
- Bhatt, P.; Bhandari, G.; Bilal, M. Occurrence, toxicity impacts and mitigation of emerging micropollutants in the aquatic environments: Recent tendencies and perspectives. J. Environ. Chem. Eng. 2022, 10, 107598. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Varjani, S.; Taherzadeh, M.J. A Critical Review on the Ubiquitous Role of Filamentous Fungi in Pollution Mitigation. Curr. Pollut. Rep. 2020, 6, 295–309. [Google Scholar] [CrossRef]
- Monsalvo, V.M.; McDonald, J.A.; Khan, S.J.; Le-Clech, P. Removal of trace organics by anaerobic membrane bioreactors. Water Res. 2014, 49, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Luo, W.; McDonald, J.; Khan, S.J.; Hai, F.I.; Price, W.E.; Nghiem, L.D. An anaerobic membrane bioreactor—Membrane distillation hybrid system for energy recovery and water reuse: Removal performance of organic carbon, nutrients, and trace organic contaminants. Sci. Total Environ. 2018, 628–629, 358–365. [Google Scholar] [CrossRef]
- Mehling, S.; Schnabel, T.; Dutschke, M.; Londong, J. Floating immobilized TiO2 catalyst for the solar photocatalytic treatment of micro-pollutants within the secondary effluent of wastewater treatment plants. Water Sci. Technol. 2023, 87, 1082–1095. [Google Scholar] [CrossRef]
- Chow, C.-H.; Leung, K.S.-Y. Removing acesulfame with the peroxone process: Transformation products, pathways and toxicity. Chemosphere 2019, 221, 647–655. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef] [PubMed]
- Mansour, F.; Al-Hindi, M.; Yahfoufi, R.; Ayoub, G.M.; Ahmad, M.N. The use of activated carbon for the removal of pharmaceuticals from aqueous solutions: A review. Rev. Env. Sci. Biotechnol. 2018, 17, 109–145. [Google Scholar] [CrossRef]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater-A meta-analysis of pilot- and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef]
- Pei, Z.; Li, L.; Sun, L.; Zhang, S.; Shan, X.-Q.; Yang, S.; Wen, B. Adsorption characteristics of 1,2,4-trichlorobenzene, 2,4,6-trichlorophenol, 2-naphthol and naphthalene on graphene and graphene oxide. Carbon 2013, 51, 156–163. [Google Scholar] [CrossRef]
- de La Cruz, N.; Giménez, J.; Esplugas, S.; Grandjean, D.; de Alencastro, L.F.; Pulgarín, C. Degradation of 32 emergent contaminants by UV and neutral photo-fenton in domestic wastewater effluent previously treated by activated sludge. Water Res. 2012, 46, 1947–1957. [Google Scholar] [CrossRef]
- Oladipo, A.A.; Gazi, M.; Yilmaz, E. Single and binary adsorption of azo and anthraquinone dyes by chitosan-based hydrogel: Selectivity factor and Box-Behnken process design. Chem. Eng. Res. Des. 2015, 104, 264–279. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Petrinic, I.; Korenak, J.; Povodnik, D.; Hélix-Nielsen, C. A feasibility study of ultrafiltration/reverse osmosis (UF/RO)-based wastewater treatment and reuse in the metal finishing industry. J. Clean. Prod. 2015, 101, 292–300. [Google Scholar] [CrossRef]
- İlbay, Z.; Şahin, S.; Kerkez, Ö.; Bayazit, Ş.S. Isolation of naproxen from wastewater using carbon-based magnetic adsorbents. Int. J. Environ. Sci. Technol. 2015, 12, 3541–3550. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Ricart, M.; Köck-Schulmeyer, M.; Guasch, H.; Bonnineau, C.; Proia, L.; de Alda, M.L.; Sabater, S.; Barceló, D. Pharmaceuticals and pesticides in reclaimed water: Efficiency assessment of a microfiltration-reverse osmosis (MF-RO) pilot plant. J. Hazard. Mater. 2015, 282, 165–173. [Google Scholar] [CrossRef]
- Madondo, N.I.; Tetteh, E.K.; Rathilal, S.; Bakare, B.F. Synergistic Effect of Magnetite and Bioelectrochemical Systems on Anaerobic Digestion. Bioengineering 2021, 8, 198. [Google Scholar] [CrossRef]
- Chandran, P.; Suresh, S.; Balasubramain, B.; Gangwar, J.; Raj, A.S.; Aarathy, U.L.; Meyyazhagan, A.; Pappuswamy, M.; Sebastian, J.K. Biological treatment solutions using bioreactors for environmental contaminants from industrial waste water. J. Umm Al-Qura Univ. Appl. Sci. 2023, 20, 84. [Google Scholar] [CrossRef]
- Sravan, J.S.; Matsakas, L.; Sarkar, O. Advances in Biological Wastewater Treatment Processes: Focus on Low-Carbon Energy and Resource Recovery in Biorefinery Context. Bioengineering 2024, 11, 281. [Google Scholar] [CrossRef]
- Xue, J.; Lei, D.; Zhao, X.; Hu, Y.; Yao, S.; Lin, K.; Wang, Z.; Cui, C. Antibiotic residue and toxicity assessment of wastewater during the pharmaceutical production processes. Chemosphere 2022, 291, 132837. [Google Scholar] [CrossRef]
- Peng, J.; Wang, X.; Yin, F.; Xu, G. Characterizing the removal routes of seven pharmaceuticals in the activated sludge process. Sci. Total Environ. 2019, 650, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Rosal, R.; Rodríguez, A.; Perdigón-Melón, J.A.; Petre, A.; García-Calvo, E.; Gómez, M.J.; Agüera, A.; Fernández-Alba, A.R. Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res. 2010, 44, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Castiglioni, S.; Bagnati, R.; Fanelli, R.; Pomati, F.; Calamari, D.; Zuccato, E. Removal of pharmaceuticals in sewage treatment plants in Italy. Environ. Sci. Technol. 2006, 40, 357–363. [Google Scholar] [CrossRef]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Zhang, Y.; Geissen, S.-U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Sim, W.-J.; Lee, J.-W.; Oh, J.-E. Occurrence and fate of pharmaceuticals in wastewater treatment plants and rivers in Korea. Environ. Pollut. 2010, 158, 1938–1947. [Google Scholar] [CrossRef]
- Clara, M.; Strenn, B.; Gans, O.; Martinez, E.; Kreuzinger, N.; Kroiss, H. Removal of selected pharmaceuticals, fragrances and endocrine disrupting compounds in a membrane bioreactor and conventional wastewater treatment plants. Water Res. 2005, 39, 4797–4807. [Google Scholar] [CrossRef]
- Zwiener, C.; Frimmel, F.H. Short-term tests with a pilot sewage plant and biofilm reactors for the biological degradation of the pharmaceutical compounds clofibric acid, ibuprofen, and diclofenac. Sci. Total Environ. 2003, 309, 201–211. [Google Scholar] [CrossRef]
- Min, X.; Li, W.; Wei, Z.; Spinney, R.; Dionysiou, D.D.; Seo, Y.; Tang, C.-J.; Li, Q.; Xiao, R. Sorption and biodegradation of pharmaceuticals in aerobic activated sludge system: A combined experimental and theoretical mechanistic study. Chem. Eng. J. 2018, 342, 211–219. [Google Scholar] [CrossRef]
- Huang, S.; Yu, J.; Li, C.; Zhu, Q.; Zhang, Y.; Lichtfouse, E.; Marmier, N. The Effect Review of Various Biological, Physical and Chemical Methods on the Removal of Antibiotics. Water 2022, 14, 3138. [Google Scholar] [CrossRef]
- Inyang, M.; Flowers, R.; McAvoy, D.; Dickenson, E. Biotransformation of trace organic compounds by activated sludge from a biological nutrient removal treatment system. Bioresour. Technol. 2016, 216, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Chen, H.; Reinhard, M.; Mao, F.; Gin, K.Y.-H. Occurrence and removal of multiple classes of antibiotics and antimicrobial agents in biological wastewater treatment processes. Water Res. 2016, 104, 461–472. [Google Scholar] [CrossRef]
- Kagle, J.; Porter, A.W.; Murdoch, R.W.; Rivera-Cancel, G.; Hay, A.G. Biodegradation of pharmaceutical and personal care products. Adv. Appl. Microbiol. 2009, 67, 65–108. [Google Scholar] [CrossRef] [PubMed]
- Ávila, C.; Bayona, J.M.; Martín, I.; Salas, J.J.; García, J. Emerging organic contaminant removal in a full-scale hybrid constructed wetland system for wastewater treatment and reuse. Ecol. Eng. 2015, 80, 108–116. [Google Scholar] [CrossRef]
- Yi, X.; Tran, N.H.; Yin, T.; He, Y.; Gin, K.Y.H. Removal of selected PPCPs, EDCs, and antibiotic resistance genes in landfill leachate by a full-scale constructed wetlands system. Water Res. 2017, 121, 46–60. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Nguyen, L.N.; Phan, H.V.; Ngo, H.H.; Guo, W.; Hai, F. Aerobic membrane bioreactors and micropollutant removal. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 147–162. ISBN 9780128198094. [Google Scholar]
- Al-Asheh, S.; Bagheri, M.; Aidan, A. Membrane bioreactor for wastewater treatment: A review. Case Stud. Chem. Environ. Eng. 2021, 4, 100109. [Google Scholar] [CrossRef]
- Kamaz, M.; Wickramasinghe, S.R.; Eswaranandam, S.; Zhang, W.; Jones, S.M.; Watts, M.J.; Qian, X. Investigation into Micropollutant Removal from Wastewaters by a Membrane Bioreactor. Int. J. Environ. Res. Public Health 2019, 16, 1363. [Google Scholar] [CrossRef] [PubMed]
- Arriaga, S.; de Jonge, N.; Nielsen, M.L.; Andersen, H.R.; Borregaard, V.; Jewel, K.; Ternes, T.A.; Nielsen, J.L. Evaluation of a membrane bioreactor system as post-treatment in waste water treatment for better removal of micropollutants. Water Res. 2016, 107, 37–46. [Google Scholar] [CrossRef]
- Mert, B.K.; Ozengin, N.; Dogan, E.C.; Aydıner, C. Efficient Removal Approach of Micropollutants in Wastewater Using Membrane Bioreactor. In Wastewater and Water Quality; Yonar, T., Ed.; InTech: Bronx, NY, USA, 2018; ISBN 978-1-78923-620-0. [Google Scholar]
- Tadkaew, N.; Hai, F.I.; McDonald, J.A.; Khan, S.J.; Nghiem, L.D. Removal of trace organics by MBR treatment: The role of molecular properties. Water Res. 2011, 45, 2439–2451. [Google Scholar] [CrossRef]
- Blandin, G.; Gautier, C.; Sauchelli Toran, M.; Monclús, H.; Rodriguez-Roda, I.; Comas, J. Retrofitting membrane bioreactor (MBR) into osmotic membrane bioreactor (OMBR): A pilot scale study. Chem. Eng. J. 2018, 339, 268–277. [Google Scholar] [CrossRef]
- Julian, H.; Nurgirisia, N.; Qiu, G.; Ting, Y.-P.; Wenten, I.G. Membrane distillation for wastewater treatment: Current trends, challenges and prospects of dense membrane distillation. J. Water Process Eng. 2022, 46, 102615. [Google Scholar] [CrossRef]
- Wang, Y.-K.; Sheng, G.-P.; Shi, B.-J.; Li, W.-W.; Yu, H.-Q. A novel electrochemical membrane bioreactor as a potential net energy producer for sustainable wastewater treatment. Sci. Rep. 2013, 3, 1864. [Google Scholar] [CrossRef] [PubMed]
- Pathak, N.; van Tran, H.; Merenda, A.; Johir, M.A.H.; Phuntsho, S.; Shon, H. Removal of Organic Micro-Pollutants by Conventional Membrane Bioreactors and High-Retention Membrane Bioreactors. Appl. Sci. 2020, 10, 2969. [Google Scholar] [CrossRef]
- Cornelissen, E.R.; Harmsen, D.; Beerendonk, E.F.; Qin, J.J.; Oo, H.; de Korte, K.F.; Kappelhof, J.W.M.N. The innovative osmotic membrane bioreactor (OMBR) for reuse of wastewater. Water Sci. Technol. 2011, 63, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.N.; Hai, F.I.; Kang, J.; Price, W.E.; Nghiem, L.D. Removal of emerging trace organic contaminants by MBR-based hybrid treatment processes. Int. Biodeterior. Biodegrad. 2013, 85, 474–482. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Q.; Campos, L.C. Removal of selected emerging PPCP compounds using greater duckweed (Spirodela polyrhiza) based lab-scale free water constructed wetland. Water Res. 2017, 126, 252–261. [Google Scholar] [CrossRef]
- Sochacki, A.; Nowrotek, M.; Felis, E.; Kalka, J.; Ziembińska-Buczyńska, A.; Bajkacz, S.; Ciesielski, S.; Miksch, K. The effect of loading frequency and plants on the degradation of sulfamethoxazole and diclofenac in vertical-flow constructed wetlands. Ecol. Eng. 2018, 122, 187–196. [Google Scholar] [CrossRef]
- Ruppelt, J.P.; Tondera, K.; Wallace, S.J.; Button, M.; Pinnekamp, J.; Weber, K.P. Assessing the role of microbial communities in the performance of constructed wetlands used to treat combined sewer overflows. Sci. Total Environ. 2020, 736, 139519. [Google Scholar] [CrossRef]
- Vymazal, J.; Březinová, T.; Koželuh, M. Occurrence and removal of estrogens, progesterone and testosterone in three constructed wetlands treating municipal sewage in the Czech Republic. Sci. Total Environ. 2015, 536, 625–631. [Google Scholar] [CrossRef]
- Nageeb, M. Adsorption Technique for the Removal of Organic Pollutants from Water and Wastewater. In Organic Pollutants—Monitoring, Risk and Treatment; Rashed, M.N., Ed.; InTech: Bronx, NY, USA, 2013; ISBN 978-953-51-0948-8. [Google Scholar]
- Dhangar, K.; Kumar, M. Tricks and tracks in removal of emerging contaminants from the wastewater through hybrid treatment systems: A review. Sci. Total Environ. 2020, 738, 140320. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Yuan, Q.; Huang, F. Synthesis and characterization of kraft lignin-based epoxy resins. BioRes 2011, 6, 2492–2503. [Google Scholar] [CrossRef]
- Ghazal, H.; Koumaki, E.; Hoslett, J.; Malamis, S.; Katsou, E.; Barcelo, D.; Jouhara, H. Insights into current physical, chemical and hybrid technologies used for the treatment of wastewater contaminated with pharmaceuticals. J. Clean. Prod. 2022, 361, 132079. [Google Scholar] [CrossRef]
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Rostvall, A.; Zhang, W.; Dürig, W.; Renman, G.; Wiberg, K.; Ahrens, L.; Gago-Ferrero, P. Removal of pharmaceuticals, perfluoroalkyl substances and other micropollutants from wastewater using lignite, Xylit, sand, granular activated carbon (GAC) and GAC+Polonite® in column tests—Role of physicochemical properties. Water Res. 2018, 137, 97–106. [Google Scholar] [CrossRef]
- Finn, M.; Giampietro, G.; Mazyck, D.; Rodriguez, R. Activated Carbon for Pharmaceutical Removal at Point-of-Entry. Processes 2021, 9, 1091. [Google Scholar] [CrossRef]
- Moreno-Castilla, C. Adsorption of organic molecules from aqueous solutions on carbon materials. Carbon. 2004, 42, 83–94. [Google Scholar] [CrossRef]
- de Luna, M.D.G.; Murniati; Budianta, W.; Rivera, K.K.P.; Arazo, R.O. Removal of sodium diclofenac from aqueous solution by adsorbents derived from cocoa pod husks. J. Environ. Chem. Eng. 2017, 5, 1465–1474. [Google Scholar] [CrossRef]
- Baghdadi, M.; Ghaffari, E.; Aminzadeh, B. Removal of carbamazepine from municipal wastewater effluent using optimally synthesized magnetic activated carbon: Adsorption and sedimentation kinetic studies. J. Environ. Chem. Eng. 2016, 4, 3309–3321. [Google Scholar] [CrossRef]
- Chu, L.; Wang, J. Regeneration of sulfamethoxazole-saturated activated carbon using gamma irradiation. Radiat. Phys. Chem. 2017, 130, 391–396. [Google Scholar] [CrossRef]
- Aksu, Z.; Tunç, Ö. Application of biosorption for penicillin G removal: Comparison with activated carbon. Process Biochem. 2005, 40, 831–847. [Google Scholar] [CrossRef]
- Haro, N.K.; Del Vecchio, P.; Marcilio, N.R.; Féris, L.A. Removal of atenolol by adsorption—Study of kinetics and equilibrium. J. Clean. Prod. 2017, 154, 214–219. [Google Scholar] [CrossRef]
- Benstoem, F.; Pinnekamp, J. Characteristic numbers of granular activated carbon for the elimination of micropollutants from effluents of municipal wastewater treatment plants. Water Sci. Technol. 2017, 76, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Sturm, M.T.; Myers, E.; Schober, D.; Thege, C.; Korzin, A.; Schuhen, K. Adaptable Process Design as a Key for Sustainability Upgrades in Wastewater Treatment: Comparative Study on the Removal of Micropollutants by Advanced Oxidation and Granular Activated Carbon Processing at a German Municipal Wastewater Treatment Plant. Sustainability 2022, 14, 11605. [Google Scholar] [CrossRef]
- González-García, P. Activated carbon from lignocellulosics precursors: A review of the synthesis methods, characterization techniques and applications. Renew. Sustain. Energy Rev. 2018, 82, 1393–1414. [Google Scholar] [CrossRef]
- Margot, J.; Kienle, C.; Magnet, A.; Weil, M.; Rossi, L.; de Alencastro, L.F.; Abegglen, C.; Thonney, D.; Chèvre, N.; Schärer, M.; et al. Treatment of micropollutants in municipal wastewater: Ozone or powdered activated carbon? Sci. Total Environ. 2013, 461–462, 480–498. [Google Scholar] [CrossRef]
- Mailler, R.; Gasperi, J.; Coquet, Y.; Deshayes, S.; Zedek, S.; Cren-Olivé, C.; Cartiser, N.; Eudes, V.; Bressy, A.; Caupos, E.; et al. Study of a large scale powdered activated carbon pilot: Removals of a wide range of emerging and priority micropollutants from wastewater treatment plant effluents. Water Res. 2015, 72, 315–330. [Google Scholar] [CrossRef]
- Altmann, J.; Ruhl, A.S.; Zietzschmann, F.; Jekel, M. Direct comparison of ozonation and adsorption onto powdered activated carbon for micropollutant removal in advanced wastewater treatment. Water Res. 2014, 55, 185–193. [Google Scholar] [CrossRef]
- Issaoui, M.; Jellali, S.; Zorpas, A.A.; Dutournie, P. Membrane technology for sustainable water resources management: Challenges and future projections. Sustain. Chem. Pharm. 2022, 25, 100590. [Google Scholar] [CrossRef]
- Dharupaneedi, S.P.; Nataraj, S.K.; Nadagouda, M.; Reddy, K.R.; Shukla, S.S.; Aminabhavi, T.M. Membrane-based separation of potential emerging pollutants. Sep. Purif. Technol. 2019, 210, 850–866. [Google Scholar] [CrossRef]
- Maryam, B.; Buscio, V.; Odabasi, S.U.; Buyukgungor, H. A study on behavior, interaction and rejection of Paracetamol, Diclofenac and Ibuprofen (PhACs) from wastewater by nanofiltration membranes. Environ. Technol. Innov. 2020, 18, 100641. [Google Scholar] [CrossRef]
- Khan, A.; Ali, J.; Jamil, S.U.U.; Zahra, N.; Tayaba, T.B.; Iqbal, M.J.; Waseem, H. Removal of micropollutants. In Environmental Micropollutants; Elsevier: Amsterdam, The Netherlands, 2022; pp. 443–461. ISBN 9780323905558. [Google Scholar]
- Sui, Q.; Huang, J.; Deng, S.; Yu, G.; Fan, Q. Occurrence and removal of pharmaceuticals, caffeine and DEET in wastewater treatment plants of Beijing, China. Water Res. 2010, 44, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bellona, C.; Drewes, J.E.; Xu, P.; Amy, G. Factors affecting the rejection of organic solutes during NF/RO treatment—A literature review. Water Res. 2004, 38, 2795–2809. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Nghiem, L.D.; Le-Clech, P.; Khan, S.J.; Drewes, J.E. Effects of membrane degradation on the removal of pharmaceutically active compounds (PhACs) by NF/RO filtration processes. J. Membr. Sci. 2009, 340, 16–25. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Johir, M.A.H.; Nguyen, T.V.; Kandasamy, J.; Vigneswaran, S. Experimental evaluation of microfiltration–granular activated carbon (MF–GAC)/nano filter hybrid system in high quality water reuse. J. Membr. Sci. 2015, 476, 1–9. [Google Scholar] [CrossRef]
- Licona, K.P.M.; Geaquinto, L.d.O.R.; Nicolini, J.V.; Figueiredo, N.G.; Chiapetta, S.C.; Habert, A.C.; Yokoyama, L. Assessing potential of nanofiltration and reverse osmosis for removal of toxic pharmaceuticals from water. J. Water Process Eng. 2018, 25, 195–204. [Google Scholar] [CrossRef]
- Albergamo, V.; Blankert, B.; Cornelissen, E.R.; Hofs, B.; Knibbe, W.-J.; van der Meer, W.; de Voogt, P. Removal of polar organic micropollutants by pilot-scale reverse osmosis drinking water treatment. Water Res. 2019, 148, 535–545. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Yang, P. Membrane adsorption of endocrine disrupting compounds and pharmaceutically active compounds. J. Membr. Sci. 2007, 303, 267–277. [Google Scholar] [CrossRef]
- Acero, J.L.; Benitez, F.J.; Teva, F.; Leal, A.I. Retention of emerging micropollutants from UP water and a municipal secondary effluent by ultrafiltration and nanofiltration. Chem. Eng. J. 2010, 163, 264–272. [Google Scholar] [CrossRef]
- Jermann, D.; Pronk, W.; Boller, M.; Schäfer, A.I. The role of NOM fouling for the retention of estradiol and ibuprofen during ultrafiltration. J. Membr. Sci. 2009, 329, 75–84. [Google Scholar] [CrossRef]
- Yoon, Y.; Westerhoff, P.; Snyder, S.A.; Wert, E.C. Nanofiltration and ultrafiltration of endocrine disrupting compounds, pharmaceuticals and personal care products. J. Membr. Sci. 2006, 270, 88–100. [Google Scholar] [CrossRef]
- Dolar, D.; Košutić, K. Removal of Pharmaceuticals by Ultrafiltration (UF), Nanofiltration (NF), and Reverse Osmosis (RO). In Analysis, Removal, Effects and Risk of Pharmaceuticals in the Water Cycle—Occurrence and Transformation in the Environment; Elsevier: Amsterdam, The Netherlands, 2013; pp. 319–344. ISBN 9780444626578. [Google Scholar]
- Yangali Quintanilla, V.A. Rejection of Emerging Organic Contaminants by Nanofiltration and Reverse Osmosis Membranes; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9780429063107. [Google Scholar]
- Ghaffour, N.; Missimer, T.M.; Amy, G.L. Technical review and evaluation of the economics of water desalination: Current and future challenges for better water supply sustainability. Desalination 2013, 309, 197–207. [Google Scholar] [CrossRef]
- Bellona, C.; Marts, M.; Drewes, J.E. The effect of organic membrane fouling on the properties and rejection characteristics of nanofiltration membranes. Sep. Purif. Technol. 2010, 74, 44–54. [Google Scholar] [CrossRef]
- Alturki, A.A.; Tadkaew, N.; McDonald, J.A.; Khan, S.J.; Price, W.E.; Nghiem, L.D. Combining MBR and NF/RO membrane filtration for the removal of trace organics in indirect potable water reuse applications. J. Membr. Sci. 2010, 365, 206–215. [Google Scholar] [CrossRef]
- Azaïs, A.; Mendret, J.; Gassara, S.; Petit, E.; Deratani, A.; Brosillon, S. Nanofiltration for wastewater reuse: Counteractive effects of fouling and matrice on the rejection of pharmaceutical active compounds. Sep. Purif. Technol. 2014, 133, 313–327. [Google Scholar] [CrossRef]
- Vergili, I. Application of nanofiltration for the removal of carbamazepine, diclofenac and ibuprofen from drinking water sources. J. Environ. Manag. 2013, 127, 177–187. [Google Scholar] [CrossRef]
- Wavhal, D.S.; Fisher, E.R. Hydrophilic modification of polyethersulfone membranes by low temperature plasma-induced graft polymerization. J. Membr. Sci. 2002, 209, 255–269. [Google Scholar] [CrossRef]
- Park, M.; Snyder, S.A. Attenuation of contaminants of emerging concerns by nanofiltration membrane: Rejection mechanism and application in water reuse. In Contaminants of Emerging Concern in Water and Wastewater; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–206. ISBN 9780128135617. [Google Scholar]
- Zhao, Y.-y.; Wang, X.-m.; Yang, H.-w.; Xie, Y.-f.F. Effects of organic fouling and cleaning on the retention of pharmaceutically active compounds by ceramic nanofiltration membranes. J. Membr. Sci. 2018, 563, 734–742. [Google Scholar] [CrossRef]
- Radeva, J.; Roth, A.G.; Göbbert, C.; Niestroj-Pahl, R.; Dähne, L.; Wolfram, A.; Wiese, J. Hybrid Ceramic Membranes for the Removal of Pharmaceuticals from Aqueous Solutions. Membranes 2021, 11, 280. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and Applications for Low-Cost Ceramic Membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Testolin, R.C.; Mater, L.; Sanches-Simões, E.; Dal Conti-Lampert, A.; Corrêa, A.X.R.; Groth, M.L.; Oliveira-Carneiro, M.; Radetski, C.M. Comparison of the mineralization and biodegradation efficiency of the Fenton reaction and Ozone in the treatment of crude petroleum-contaminated water. J. Environ. Chem. Eng. 2020, 8, 104265. [Google Scholar] [CrossRef]
- Silva, L.M.d.; Jardim, W.F. Trends and strategies of ozone application in environmental problems. Quím. Nova 2006, 29, 310–317. [Google Scholar] [CrossRef]
- Kovalova, L.; Siegrist, H.; von Gunten, U.; Eugster, J.; Hagenbuch, M.; Wittmer, A.; Moser, R.; McArdell, C.S. Elimination of micropollutants during post-treatment of hospital wastewater with powdered activated carbon, ozone, and UV. Environ. Sci. Technol. 2013, 47, 7899–7908. [Google Scholar] [CrossRef]
- Lee, Y.; Gerrity, D.; Lee, M.; Bogeat, A.E.; Salhi, E.; Gamage, S.; Trenholm, R.A.; Wert, E.C.; Snyder, S.A.; Gunten, U. von. Prediction of micropollutant elimination during ozonation of municipal wastewater effluents: Use of kinetic and water specific information. Environ. Sci. Technol. 2013, 47, 5872–5881. [Google Scholar] [CrossRef]
- Bourgin, M.; Beck, B.; Boehler, M.; Borowska, E.; Fleiner, J.; Salhi, E.; Teichler, R.; von Gunten, U.; Siegrist, H.; McArdell, C.S. Evaluation of a full-scale wastewater treatment plant upgraded with ozonation and biological post-treatments: Abatement of micropollutants, formation of transformation products and oxidation by-products. Water Res. 2018, 129, 486–498. [Google Scholar] [CrossRef]
- Farzaneh, H.; Loganathan, K.; Saththasivam, J.; McKay, G. Ozone and ozone/hydrogen peroxide treatment to remove gemfibrozil and ibuprofen from treated sewage effluent: Factors influencing bromate formation. Emerg. Contam. 2020, 6, 225–234. [Google Scholar] [CrossRef]
- de Boer, S.; González-Rodríguez, J.; Conde, J.J.; Moreira, M.T. Benchmarking tertiary water treatments for the removal of micropollutants and pathogens based on operational and sustainability criteria. J. Water Process Eng. 2022, 46, 102587. [Google Scholar] [CrossRef]
- Östman, M.; Björlenius, B.; Fick, J.; Tysklind, M. Effect of full-scale ozonation and pilot-scale granular activated carbon on the removal of biocides, antimycotics and antibiotics in a sewage treatment plant. Sci. Total Environ. 2019, 649, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Polińska, W.; Kotowska, U.; Kiejza, D.; Karpińska, J. Insights into the Use of Phytoremediation Processes for the Removal of Organic Micropollutants from Water and Wastewater; A Review. Water 2021, 13, 2065. [Google Scholar] [CrossRef]
- Wang, C.; Lin, C.-Y.; Liao, G.-Y. Degradation of antibiotic tetracycline by ultrafine-bubble ozonation process. J. Water Process Eng. 2020, 37, 101463. [Google Scholar] [CrossRef]
- Singh, A.; Majumder, A.; Saidulu, D.; Bhattacharya, A.; Bhatnagar, A.; Gupta, A.K. Oxidative treatment of micropollutants present in wastewater: A special emphasis on transformation products, their toxicity, detection, and field-scale investigations. J. Environ. Manag. 2024, 354, 120339. [Google Scholar] [CrossRef]
- Lutterbeck, C.A.; Wilde, M.L.; Baginska, E.; Leder, C.; Machado, Ê.L.; Kümmerer, K. Degradation of 5-FU by means of advanced (photo)oxidation processes: UV/H2O2, UV/Fe2+/H2O2 and UV/TiO2—Comparison of transformation products, ready biodegradability and toxicity. Sci. Total Environ. 2015, 527–528, 232–245. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Sutton, N.B.; Rijnaarts, H.H.H.; Langenhoff, A.A.M. Degradation of pharmaceuticals in wastewater using immobilized TiO2 photocatalysis under simulated solar irradiation. Appl. Catal. B Environ. 2016, 182, 132–141. [Google Scholar] [CrossRef]
- Miranda-García, N.; Suárez, S.; Sánchez, B.; Coronado, J.M.; Malato, S.; Maldonado, M.I. Photocatalytic degradation of emerging contaminants in municipal wastewater treatment plant effluents using immobilized TiO2 in a solar pilot plant. Appl. Catal. B Environ. 2011, 103, 294–301. [Google Scholar] [CrossRef]
- Yuan, Q.; Qu, S.; Li, R.; Huo, Z.-Y.; Gao, Y.; Luo, Y. Degradation of antibiotics by electrochemical advanced oxidation processes (EAOPs): Performance, mechanisms, and perspectives. Sci. Total Environ. 2023, 856, 159092. [Google Scholar] [CrossRef]
- Carlesi Jara, C.; Fino, D.; Specchia, V.; Saracco, G.; Spinelli, P. Electrochemical removal of antibiotics from wastewaters. Appl. Catal. B Environ. 2007, 70, 479–487. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Vakili, S.; Zandifar, A.; Pourebrahimi, S. From wastewater to clean water: Recent advances on the removal of metronidazole, ciprofloxacin, and sulfamethoxazole antibiotics from water through adsorption and advanced oxidation processes (AOPs). Environ. Res. 2024, 252, 119029. [Google Scholar] [CrossRef]
- Loganathan, P.; Kandasamy, J.; Ratnaweera, H.; Vigneswaran, S. Submerged membrane/adsorption hybrid process in water reclamation and concentrate management—A mini review. Environ. Sci. Pollut. Res. Int. 2023, 30, 42738–42752. [Google Scholar] [CrossRef]
- Baresel, C.; Harding, M.; Fång, J. Ultrafiltration/Granulated Active Carbon-Biofilter: Efficient Removal of a Broad Range of Micropollutants. Appl. Sci. 2019, 9, 710. [Google Scholar] [CrossRef]
- Stoquart, C.; Servais, P.; Bérubé, P.R.; Barbeau, B. Hybrid Membrane Processes using activated carbon treatment for drinking water: A review. J. Membr. Sci. 2012, 411–412, 1–12. [Google Scholar] [CrossRef]
- Shanmuganathan, S.; Loganathan, P.; Kazner, C.; Johir, M.A.H.; Vigneswaran, S. Submerged membrane filtration adsorption hybrid system for the removal of organic micropollutants from a water reclamation plant reverse osmosis concentrate. Desalination 2017, 401, 134–141. [Google Scholar] [CrossRef]
- Lou, Y.; Zhang, S.; Zhu, T. Research on the current situation of ultrafiltration combined process in treatment of micro-polluted surface water. E3S Web Conf. 2020, 194, 4041. [Google Scholar] [CrossRef]
- Vigneswaran, S.; Chaudhary, D.S.; Ngo, H.H.; Shim, W.G.; Moon, H. Application of a PAC-Membrane Hybrid System for Removal of Organics from Secondary Sewage Effluent: Experiments and Modelling. Sep. Sci. Technol. 2003, 38, 2183–2199. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, X.; Zhang, X.; Peng, S. The change of NOM in a submerged UF membrane with three different pretreatment processes compared to an individual UF membrane. Desalination 2015, 360, 118–129. [Google Scholar] [CrossRef]
- Jamil, S.; Loganathan, P.; Khan, S.J.; McDonald, J.A.; Kandasamy, J.; Vigneswaran, S. Enhanced nanofiltration rejection of inorganic and organic compounds from a wastewater-reclamation plant’s micro-filtered water using adsorption pre-treatment. Sep. Purif. Technol. 2021, 260, 118207. [Google Scholar] [CrossRef]
- Yangali-Quintanilla, V.; Sadmani, A.; McConville, M.; Kennedy, M.; Amy, G. A QSAR model for predicting rejection of emerging contaminants (pharmaceuticals, endocrine disruptors) by nanofiltration membranes. Water Res. 2010, 44, 373–384. [Google Scholar] [CrossRef]
- Melo-Guimarães, A.; Torner-Morales, F.J.; Durán-Álvarez, J.C.; Jiménez-Cisneros, B.E. Removal and fate of emerging contaminants combining biological, flocculation and membrane treatments. Water Sci. Technol. 2013, 67, 877–885. [Google Scholar] [CrossRef]
- Asheghmoalla, M.; Mehrvar, M. Integrated and Hybrid Processes for the Treatment of Actual Wastewaters Containing Micropollutants: A Review on Recent Advances. Processes 2024, 12, 339. [Google Scholar] [CrossRef]

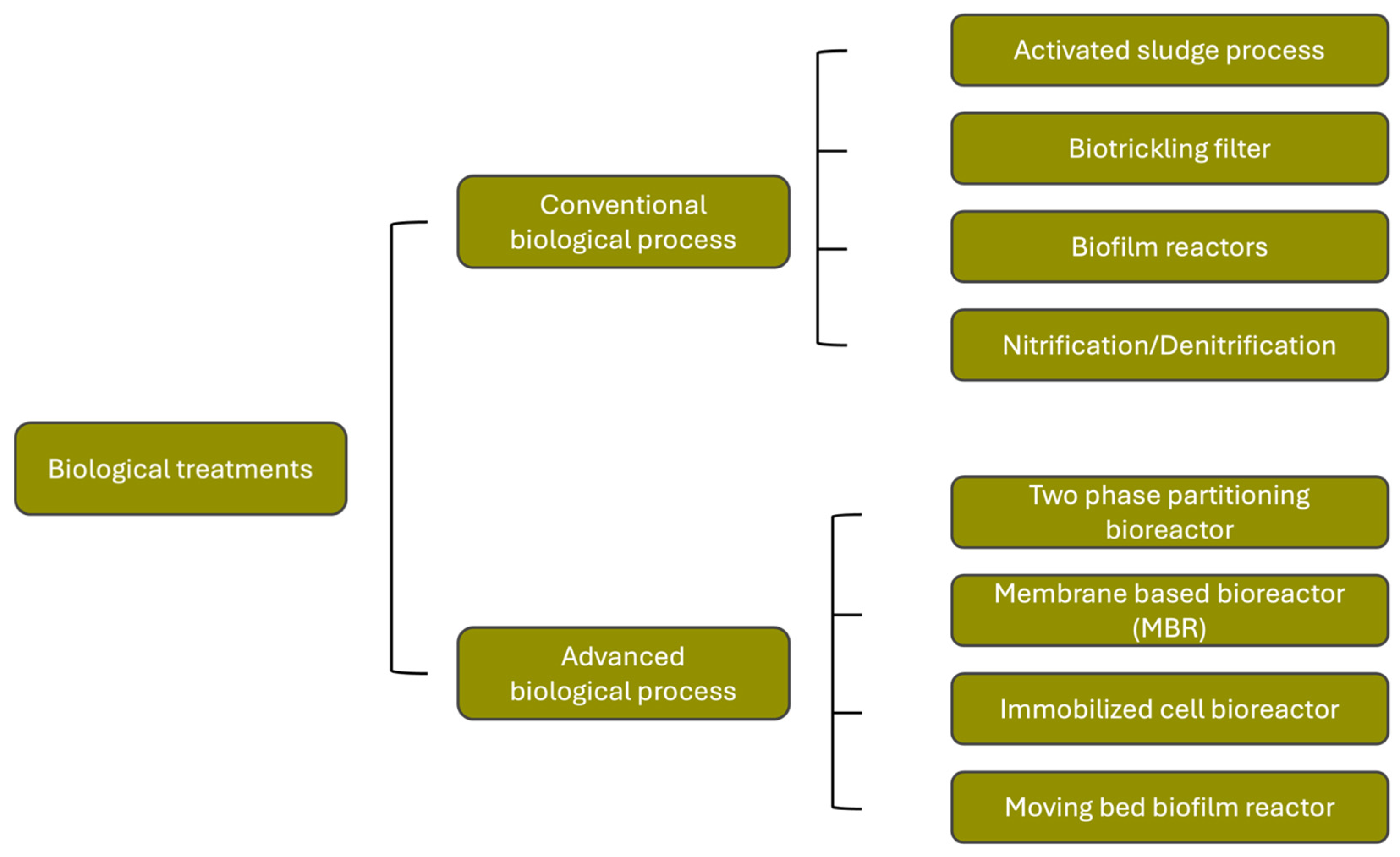
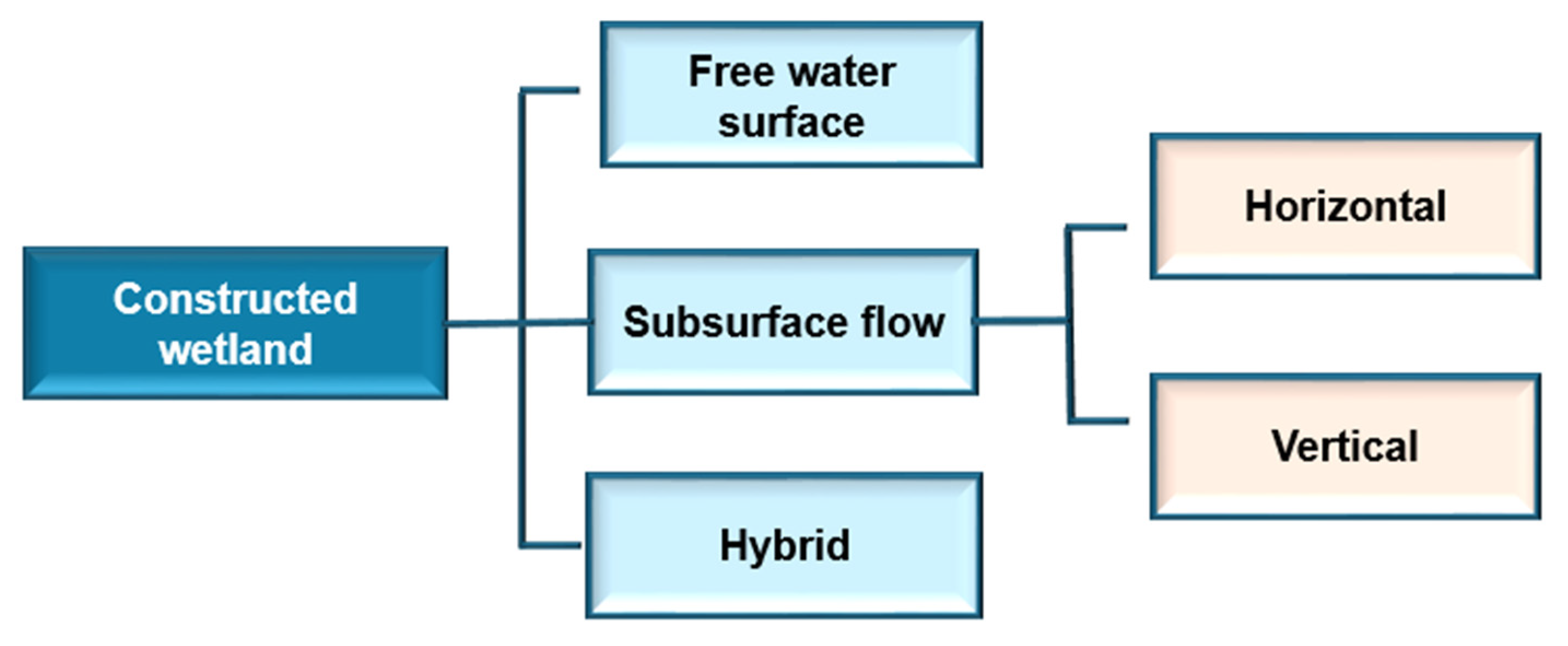
| Categories | Key Subcategories | Primary Sources | References |
|---|---|---|---|
| Pharmaceuticals | NSAIDs *, lipid regulators, antibiotics, β-blockers, contrast media, and anticonvulsants | Wastewater from domestic households, effluents from hospitals, pharmaceutical production plants, aquaculture, and concentrated animal feeding operations (CAFO) * | [2,3,27,28,29] |
| Personal care products | Fragrances, disinfectants, and insect repellents | Household sewage (from activities such as bathing, shaving, and using sprays) | [2,3,30,31,32] |
| Steroid hormones | Estrogens | Wastewater from domestic households, aquaculture, and CAFO * | [3,33,34,35] |
| Pesticides | Insecticides, herbicides and fungicides | Garden runoff and agriculture | [1,2,3,36,37,38] |
| Industrial chemicals | Plasticizers, fire retardants | Domestic wastewater generated from the leaching of materials and industrial discharges | [1,2,39,40] |
| Microplastics | Microfibers, plastic pellets, synthetic fibers | Domestic wastewater resulting from urban runoff | [41,42,43,44] |
| Pharmaceutical Categories | Pharmaceutical Pollutants | Chemical Formulas | Mass (gmol−1) | pKa | Log Kow | Ionization State at pH 7 |
|---|---|---|---|---|---|---|
| Analgesics and Anti-inflammatories | Aspirin | C9H8O4 | 280 | 3.5 | 1.2 | Negative |
| Diclofenac | C14H11Cl2NO2 | 296.2 | 4.91 | 4.51 | Negative | |
| Ibuprofen | C13H18O2 | 206.3 | 4.15 | 4.51 | Negative | |
| Paracetamol | C8H9NO2 | 151.2 | 9.38 | 0.46 | Neutral | |
| Naproxen | C14H14O3 | 230.3 | 4.15 | 3.18 | Negative | |
| Antibiotics | Sulfamethoxazole | C10H11N3O3S | 253.279 | 5.6–5.7 | 0.89 | Negative |
| Erythromycin | C37H67NO13 | 733.93 | 8.88 | 3.06 | Neutral | |
| Trimethoprim | C14H18N4O3 | 290.32 | 7.12 | 0.73 | Neutral | |
| Metronidazole | C6H9N3O3 | 171.2 | 2.5 | −0.1 | Negtative | |
| Ciprofolxacine | C17H18FN3O3 | 331.3 | 6.1; 8.7 | −1.70 | Neutral | |
| Oxytetracycline | C22H24N2O9 | 496.9 | 3.2; 7.46; 8.9 | −1.12 | Neutral/Zwitterion | |
| Anticonvulsants | primidone | C12H14N2O2 | 218 | −1; 12.2 | 0.91 | Negative |
| Carbamazepine | C15H12N2O | 236.27 | 13 | 2.45 | Neutral | |
| ß-blockers | Propranolol | C16H21NO2 | 259.34 | 9.6 | 3.48 | Neutral |
| Metoprolol | C15H25NO3 | 276.37 | 9.49 | 1.88 | Positive | |
| Contrast media | Iopromide | C18H24I3N3O8 | 790.0 | 2;13 | −2.10 | Neutral |
| Iopamidol | C17H22I3N3O8 | 777.1 | 10.7 | −2.42 | Neutral | |
| Iohexol | C19H26I3N3O9 | 821.1 | 11.7 | −3.05 | Neutral | |
| Blood lipid regulators | Clofibric acid | C10H11ClO3 | 214.65 | 3.35 | 2.57 | Negative |
| Gemfibrozil | C15H22O3 | 250.34 | 4.45 | 4.77 | Negative | |
| Bezafibrate | C19H20ClNO4 | 361.82 | 3.44 | 4.25 | Negative | |
| Pravastatin | C23H36O7 | 24.53 | 4.2 | 3.1 | Negative | |
| Herbicide | Atrazine | C8H14ClN5 | 215.7 | 3.20 | 1.32 | Neutral |
| Simazine | C7H12ClN5 | 201.7 | 3.20 | 1.78 | Neutral | |
| Industrial chemicals | N-nitrosodimethylamine (NDMA) | C2H6N2O | 74.1 | 3.50 | 0.04 | Neutral |
| Bisphenol A | C15H16O2 | 228.3 | 9.8; 10.4 | 4.04 | Neutral |
| Type of MPs | PS | PE | PP | PVC | PET |
|---|---|---|---|---|---|
| Chemical formula | (C8H8)n [70] | (C2H4)n [70] | (C3H6)n [70] | (C2H3Cl)n [70] | (C10H8O4)n [70] |
| Specific density (g cm−3) | 0.07–1.05 [71] | 0.85–0.94 [71] | 0.905 [71] | 1.35–1.39 [71] | 0.96–1.45 (average 1.38–1.41) [71] |
| Tensile strength (MPa) | 36–52 [72] | 8–32 [72] | 31–41 [72] | 41–52 [72] | 48 [72] |
| Melting point (°C) | 270 (sydiotactic) [73] | 98–110 [72] | 168–175 [72] | 115–245 [72] | 245 [72] |
| Crytallinity (%) | 0 [74] | 45–95 [75] | 50–80 [75] | 5–15 [74] | 30–40 [76] |
| Lifespan (year) | 50–80 [74] | 10–600 [74] | 10–600 [74] | 50–100 [74] | 450 [74] |
| Main use | Plastic cups, food packaging [70,71,77] | Bottles food, packaging shopping bags, cosmetic products [70,71,77] | Folders, food packaging, hinged caps, car bumper, medicine bottles [71] | Window frames, flooring and pipes, clothes [70,71] | Plastic beverage bottles, food packaging, personal care products [70,71,77] |
| Water Type | Micropollutants | Country | Conc. [ng/L] | Ref. |
|---|---|---|---|---|
| Surface Water | Caffeine | Germany | 65–6798 | [140] |
| Denmark | 65–382 | [141] | ||
| Korea | 268.7 | [142] | ||
| China | 865 | [143] | ||
| Butylbenzylphthalate (BBP) | Mexico | 5–201 | [144] | |
| Diclofenac | UK | 20–91 | [145] | |
| Sweden | 680 | [146] | ||
| Korea | 3 | [147] | ||
| China | <147 | [148] | ||
| Carbamazepine | USA | 6.8 | [149] | |
| Korea | 5–36 | [142] | ||
| Estrone | Germany | 71 | [140] | |
| Korea | 3.6 | [147] | ||
| Ibuprofen | Germany | 60–152 | [150] | |
| Denmark | 9–22 | [141] | ||
| China | 1,417,000 | [151] | ||
| Naproxen | Germany | 70 | [150] | |
| Sweden | 90–250 | [152] | ||
| Denmark | 17–36 | [141] | ||
| China | <118 | [148] | ||
| Bisphenol A | Europe | 10 | [153] | |
| USA | 80 | [153] | ||
| Korea | 59 | [154] | ||
| Perfluorooctane sulfonic acid (PFOS) | Germany | 5–101 | [140] | |
| Atrazine | Korea | 0.61 | [154] | |
| Triclosan | USA | 10–600 | [12] | |
| Mexico | 16–19 | [144] | ||
| Denmark | 10–60 | [141] | ||
| Nonylphenol | China | 36–33.231 | [2] | |
| Microplastics | India | 2.75 ± 0.92 p·L−1 | [155] | |
| China | 266 ± 56 p·L−1 | [156] | ||
| Hongkong | 2.18 ± 0.165 p·L−1 | [157] | ||
| Ground Water | Caffeine | USA | 290 | [158] |
| Germany | 102 | [159] | ||
| China | 42.5 | [159] | ||
| Italy | 84–683 | [160] | ||
| Estrone | Europe | 4 | [161] | |
| BBP | Mexico | 1–82 | [144] | |
| Ibuprofen | USA | 3110 | [162] | |
| Europe | 3–395 | [151] | ||
| Carbamazepine | Europe | 390 | [161] | |
| USA | 42 | [158] | ||
| Atrazine | Europe | 253 | [161] | |
| Bisphenol A | Europe | 79–2299 | [161] | |
| Mexico | 1–10 | [144] | ||
| PFOS | Europe | 135 | [161] | |
| Triclosan | Mexico | 1–345 | [144] | |
| Microplastics | Mexico | 18.7 p·L−1 | [163] | |
| Drinking Water | Caffeine | Spain | 9.10 | [164] |
| Sweden | 5.5 | [165] | ||
| USA | 52.3 | [159] | ||
| Korea | 34.3–95.5 | [166] | ||
| Turkey | 3390 | [167] | ||
| Germany | 611 | [168] | ||
| Diclofenac | Japan | 16 | [169] | |
| Spain | 25 | [170] | ||
| Sweden | 8 | [165] | ||
| France | 56 | [171] | ||
| Carbamazepine | Japan | 25 | [169] | |
| France | 41.6 | [171] | ||
| Ibuprofen | Japan | 6 | [169] | |
| France | 14 | [171] | ||
| Germany | 244 | [168] | ||
| Atrazine | Spain | 1.19 | [164] | |
| Naproxen | France | 6 | [171] | |
| Metoprolol | France | 1 | [171] | |
| Bisphenol A | Germany | 72 | [168] | |
| PFOS | Spain | 0.55 | [164] | |
| 4-methyl−1,2,3-benzotriazole | Germany | 45 | [168] | |
| Terbuthylazine | Spain | 4.56 | [164] | |
| Sucralose | Sweden | 12 | [165] | |
| Microplastics | China | 2–23 p/bottle | [172] | |
| Australia | 13 ± 19 | [173] | ||
| Iran | 8.5 ± 10.2 | [174] | ||
| WWTP Effluent | Caffeine | Europe | 3002 | [175] |
| Korea | 60 | [176] | ||
| China | 376.5 | [177] | ||
| Diclofenac | Europe | 174 | [175] | |
| Korea | 49 | [176] | ||
| Carbamazepine | Europe | 4609 | [175] | |
| Korea | 74 | [176] | ||
| China | 55 | [177] | ||
| PFOS | Europe | 2101 | [175] | |
| Ibuprofen | Europe | 2129 | [175] | |
| Korea | 75 | [176] | ||
| Acesulfame | Europe | 30.000 | [90] | |
| Atrazine | Europe | 36.6 | [175] | |
| Bisphenol A | Europe | 200 | [90] | |
| China | 623.6 | [177] | ||
| Dimethylphthalate (DMP) | Europe | 340 | [90] | |
| Estrone | Europe | 217 | [90] | |
| Asia | <51.2 | [178] | ||
| North America | 56 | [178] | ||
| Sucralose | Europe | 10.000 | [90] | |
| North America | 18.700–48.900 | [178] | ||
| Asia | 1300–5490 | [178] | ||
| 1H-benzotriazole | Europe | 2,210,000 | [179] | |
| Diethyl Phthalate (DEP) | Europe | 800 | [90] | |
| Microplastics | India | 148 ± 51 | [180] | |
| China | 30.6 ± 7.8 | [181] | ||
| Africa | 86 ± 7.49 | [182] |
| Treatment Processes | Advantages | Disadvantages | Category | Analytical Methods |
|---|---|---|---|---|
| Conventional biological treatmen [241,242,243] | Reduced initial investment Versatile and straightforward technology Environmentally sustainable | Inefficient removal of low-biodegradable pharmaceutical contaminants Generation of toxic metabolites Inability to target specific pharmaceutical contaminants High sludge production | EDCs Bisphenol A (BPA) Nonylphenol (NP) | Centrifugation → Acidification → SPE → HPLC-UV at 280 nm [244,245] Liquid Extraction → Centrifugation → HPLC-UV at 274 [244,245] |
| Organic matter, nitrate, and phosphorous, e.g., ammonium, natriumacetate | TC-IC (TOC) [241] | |||
| PCs, e.g., carbamazepine and diazepam | SPE → LC-MS or GC-MS [241] | |||
| Advanced biological treatment [242,243,246] | Focused removal of contaminants, High adaptability to diverse wastewater characteristics Space-efficient design Improved removal of pharmaceutical contaminants Effective operation at elevated suspended solids concentrations | High energy and initial investment costs Membrane fouling issues Challenges in degrading persistent PCs Necessitate effective strategies for managing microbial activity | Organic Matter, e.g., meat extract, peptone, sodium acetate, and glucose | TOC [247] |
| PPCPs, EDCs, and pesticides | SPE → Derivatization → GC-MS [247] | |||
| Organic and inorganic (C, N, and P) | TOC, TN and COD [248] | |||
| Pharmaceuticals and herbicides | SPE → HPLC-MS [248] | |||
| Advanced oxidation processes (AOPs) [249,250,251] | Environmental compatibility Synergy with other processes (biological or physical treatments) Rapid processing and high efficiency Effective in removing a broad spectrum of organic compounds | Generation of toxic byproducts High energy and chemical requirements Limited scalability due to cost and technical constraints Need for specialized equipment and expertise | Pharmaceuticals, e.g., diclofenac | UHPLC-QqQ-MS [249] |
| Food additives and artificial sweeteners, e.g., acesulfame | UHPLC-QqQ-MS [250] | |||
| Adsorptive treatment [251,252,253] | Low operational costs Simple operation Flexibility in using a wide range of adsorbents for specific requirements Effluent with low dissolved solids | Adsorbent saturation Gradual capacity decline after several treatment cycles Column blockage Limited selectivity Challenges in regeneration and production of secondary waste | Industrial chemicals | HPLC-UV at 230 nm and HPLC-FLD at 275 and 225 nm [245,254] |
| Corrosion inhibitors and biocides/pesticides | SPE → UPLC-MS/MS and TOC [255] | |||
| Anionic dyes, e.g., azo and anthraquinone | UV-Vis spectrophotometer [245,256] | |||
| Pharmaceuticals, e.g., naproxen, ibuprofen, diclofenac, ketoprofen | HPLC-UV at 230 nm [245,257] | |||
| Membrane technology [250,255,256] | High removal efficiency Selective separation Compact design, requiring less space Versatility (able to treat a wide range of water matrices) | High installation and material costs High energy consumption Membrane fouling issues Frequent membrane cleaning required Necessitates brine disposal and toxicity assessment | Heavy metals, organic and inorganic compounds | UV-Vis spectrophotometer, titrimetric determination, and oximeter [245,258] |
| Pharmaceuticals, e.g., naproxen | UV-Vis spectrophotometer [259] | |||
| Pesticides | SPE → LC-MS/MS [260] |
| CW Design | Plant Used | PPCPs | Removal (%) | Ref. |
|---|---|---|---|---|
| Free water constructed wetland (lab-scale) | Spirodela polyrhiza | Paracetamol, Caffeine, Triclosan | >95 >95 >95 | [292] |
| VFCW (lab-scale) | Phalaris arundinacea | Diclofenac, Sulfamethoxazole | 52–91 47–74 | [293] |
| VFCW | Phalaris australis | Bisphenol A (BPA), Metoprolol, Diclofenac | 60–73 77 28–30 | [294] |
| Subsurface flow CW | Phragmites australis | 17α-ethinylestradiol | 81.4 | [295] |
| Pharmaceuticals | Water Type | Concentration (mg/L) | AC | Removal Efficiencies (%) | Analytical Methods | Ref. |
|---|---|---|---|---|---|---|
| Diclofenac | Various | 10–30 | AC from cocoa pod husks | 76.0–93.6 | UV-Vis at 380 nm | [304] |
| Carbamazepine | Wastewater | 2 | PAC | 93 | HPLC-UV | [305] |
| Naproxen | Wastewater | 1–30 | PAC | 67.2–89.2 | UV–Vis at 230 nm | [259] |
| Sulfamethoxazole | Distilled | 50–500 | AC | 90 | HPLC-DAD at 254 nm | [306] |
| Penicillin G | Distilled | 50–1000 | AC | 12.0–78.3 | Hydroxylamine method → UV-Vis at 515 nm | [307] |
| Atenolol | Various | 5–900 | GAC | 88 | UV-Vis at 224 nm | [308] |
| Membrane Processes | Matrix | Target MP (PPCPs) | Removal Efficiencies (%) | Analytical Methods | Ref. |
|---|---|---|---|---|---|
| UF (2000–20,000 Da) | Secondary effluent spiked with various compounds | 11 contaminants: Acetaminophen, metoprolol, caffeine, antipyrine, sulfamethoxazole, flumequine, ketorolac, atrazine, isoproturon, hydroxybiphenyl, diclofenac | <50%, except for hydroxybiphenyl | HPLC-PAD at 220, 250, and 280 nm | [326] |
| UF (8000 Da) | Synthetic (model) water and natural freshwater sources | 52 EDCs/PPCPs | Up to 80% removal efficiency for hydrophobic compounds | LC/MS/MS and GC/MS/MS | [328] |
| UF (100 kDa) | Synthetic (model) water | Estradiol and ibuprofen | 25% ibuprofen, 80% estradiol | UV spectrometry + TOC + scintillation counter | [327] |
| PCs | pH | Membrane Name | Materials | MWCO (Da) | Removal Efficiencies (%) | Analytical Methods | Ref. |
|---|---|---|---|---|---|---|---|
| PARA * | 6.5 | NF270 | Polyamide | 200–300 | 44 | HPLC-DAD | [332] |
| PARA IBU * PARA IBU | 7 6–7 | NF 200 NF 90 | Aromatic polyamide | ~300 ~200 | 22 89 75 96 | HPLC-MS | [330] |
| PARA IBU DIC * PARA IBU DIC | 7.4–7.6 | NF 270 NF 90 | Thin aromatic or semi-aromatic polyamide | 200–300 ~200 | 0 99 95 99 99 90 | TOC, TN and HPLC-MS | [333,334] |
| PARA | 7 | NF 270 NF 90 | Polypiperazine with polymeric active layer polyamide supported by polysulfone | 220 102 | 20–30 ~90 | LC-MS/MS | [334] |
| DIC IBU | 8 8 | FM NP010 | Hydrophilic polyethersulfone | 1000 | 61 55 | LC-MS/MS | [335] |
| PARA DIC IBU | 12 3 6–7 | NF 50 | Sulfonated polyethersulfone | 1000 | 36.16 99.74 80.54 | UV-Vis at 243, 222 and 276 nm | [317] |
| Membranes | MWCO (Da) | PCs | Removal Efficiencies (%) | Analytical Methods | Ref. |
|---|---|---|---|---|---|
| TiO2 | 200 | 41 organic compounds (N-nitrosamine and PCs, e.g., IBU, DIC, and CARBA *) | 95–100 | Nitrosamine: SPE → GC.MS/MS PCs: SPE → HPLC-MS/MS | [69] |
| LC1/LC2 * | 630/440 | PCs, e.g., SUL * and CBZ | 50–80 | UPLC-MS/MS | [338] |
| TiO2 (UF-membrane) | 3.0 nm | DIC and IBU | 32–47 | HPLC-MS-MS | [228] |
| Al2O3/LBL coating with polyelectrolytes (polystyrene sulfonic acid) | ~200 | IBU, DIC, SUL, and Clofibric Acid | 56% für SUL, up to 84% für DIC | HPLC | [339] |
| AOPs-Type | Micropollutants | Removal Efficiencies of PCs (%) | Analytical Methods | References |
|---|---|---|---|---|
| Peroxone | Artificial sweetener, acesulfame | ~100 | UHPLC-QqQ-MS | [250] |
| UV/H2O2 | Anticancer drug, fluorouracil (5-FU) | >99 | LC-IT-MS/MS | [352] |
| Photo-Fenton | Pharmaceuticals, corrosion inhibitors, and biocides/pesticides | 97–98 | SPE → UPLC-MS/MS and TOC | [255] |
| Electrochemical Oxidation | Antibiotics, e.g., ofloxacin | ~90 | UV Spectrophotometry | [356] |
| TiO2-Solar Photocatalysis | Pharmaceuticals, diclofenac | ~90 | HPLC-QqQ-MS | [249] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarigan, M.; Raji, S.; Al-Fatesh, H.; Czermak, P.; Ebrahimi, M. The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal. Processes 2025, 13, 843. https://doi.org/10.3390/pr13030843
Tarigan M, Raji S, Al-Fatesh H, Czermak P, Ebrahimi M. The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal. Processes. 2025; 13(3):843. https://doi.org/10.3390/pr13030843
Chicago/Turabian StyleTarigan, Meilia, Samir Raji, Heyam Al-Fatesh, Peter Czermak, and Mehrdad Ebrahimi. 2025. "The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal" Processes 13, no. 3: 843. https://doi.org/10.3390/pr13030843
APA StyleTarigan, M., Raji, S., Al-Fatesh, H., Czermak, P., & Ebrahimi, M. (2025). The Occurrence of Micropollutants in the Aquatic Environment and Technologies for Their Removal. Processes, 13(3), 843. https://doi.org/10.3390/pr13030843















