Electrochemical-Based Technologies for Removing NSAIDs from Wastewater: Systematic Review with Bibliometric Analysis
Abstract
1. Introduction
2. Search Strategy
3. Results and Discussion
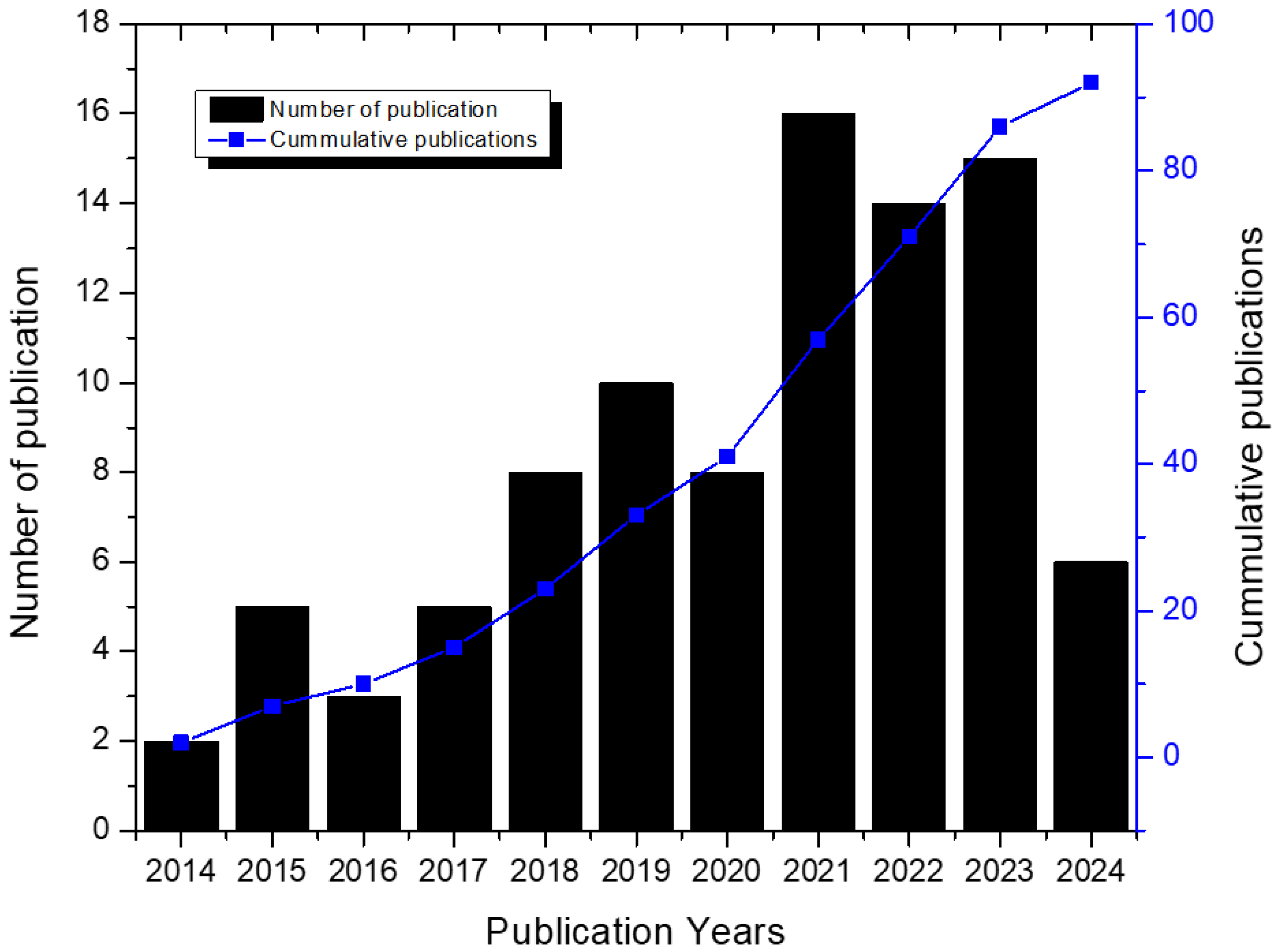
3.1. Research Metrics
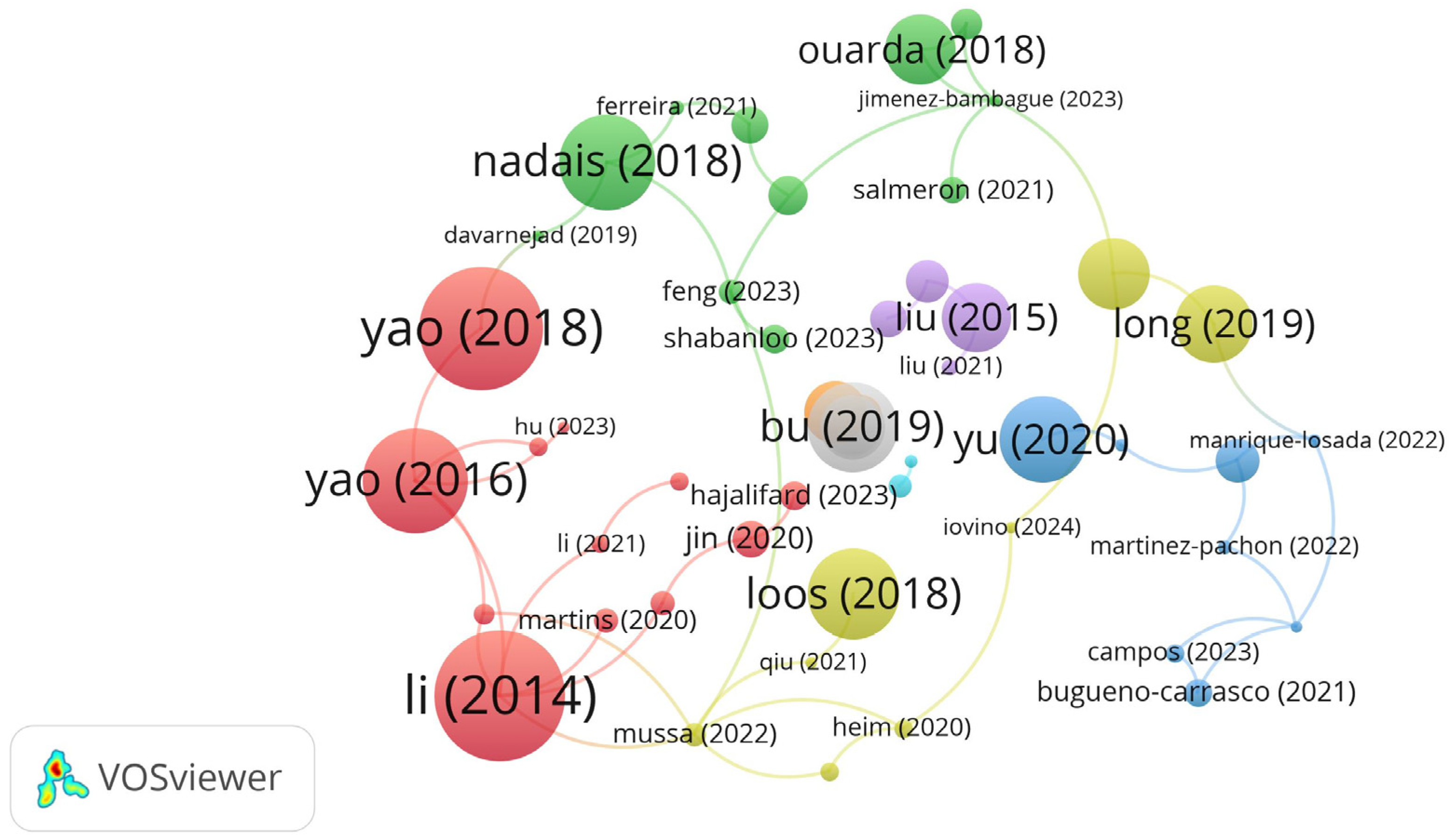
3.2. The Collaboration Network Analysis of Countries, Organizations, and Authors
3.3. Most-Cited Articles
4. Mechanisms and Recent Progress in Electrochemical Technologies for NSAIDs Removal
4.1. Electrochemical Oxidation
4.1.1. Bio-Electrooxidation (Bio-EO) Process
4.1.2. Sono-Electrooxidation (Sono-EO) Process
4.1.3. Solar-Electrooxidation (Solar–EO) Process
4.1.4. EO and Ion Exchange Resins
4.1.5. Photoelectrochemical (PEC) Process
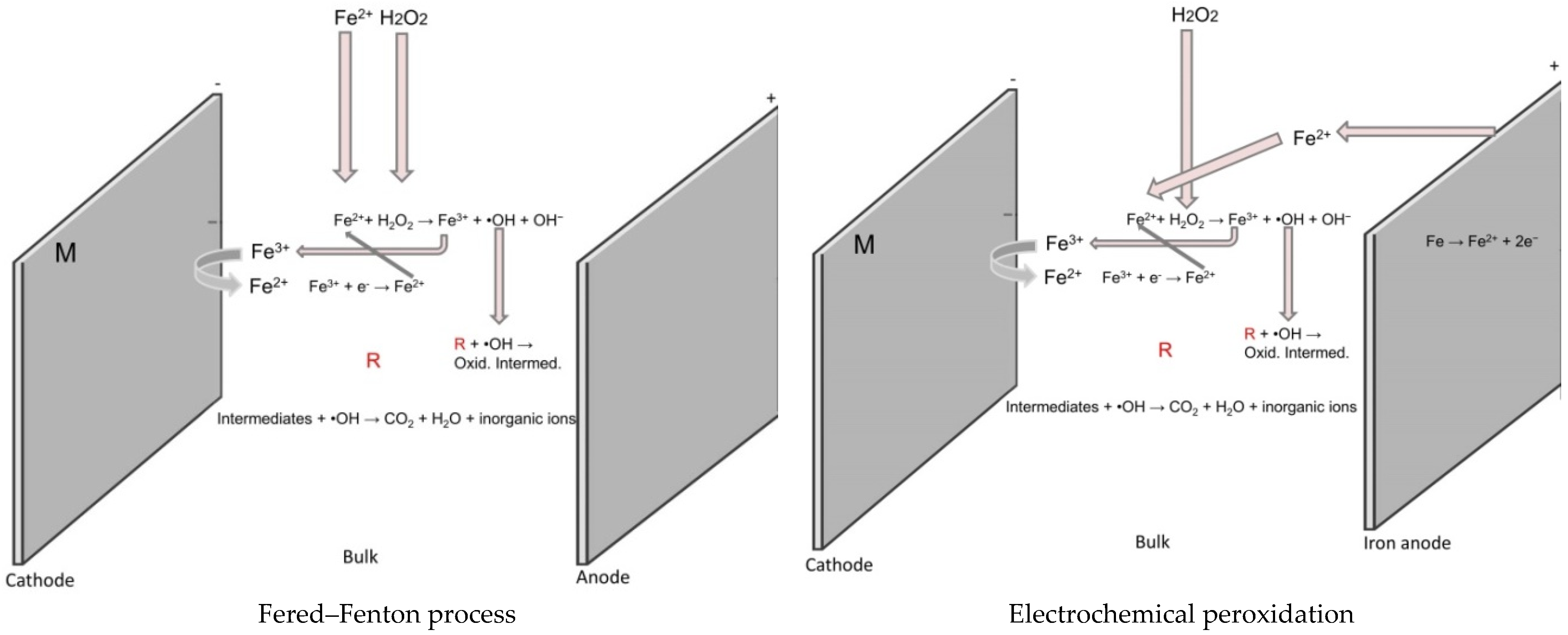
4.2. Electro-Fenton
4.2.1. The Bio-Electro-Fenton
4.2.2. Photo-Electro-Fenton Process and Solar Photo-Electro-Fenton
4.3. The Electro-Peroxone Process
4.4. Environmental Implications and Potential Applications
5. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brennan, R.; Wazaify, M.; Shawabkeh, H.; Boardley, I.; McVeigh, J.; Van Hout, M.C. A Scoping Review of Non-Medical and Extra-Medical Use of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). Drug Saf. 2021, 44, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.D.; Geetha, B.V.; Vibha, M. From prescription to pollution: The ecological consequences of NSAIDs in aquatic ecosystems. Toxicol. Rep. 2024, 13, 101775. [Google Scholar] [CrossRef]
- Li, W.; Zheng, X.; Tu, G.; Zhang, S.; Zhang, P. Novel aqueous biphasic system based on ionic liquid for the simultaneous extraction of seven active pharmaceutical ingredients in aquatic environment. Environ. Sci. Pollut. Res. 2021, 28, 17853–17864. [Google Scholar] [CrossRef]
- The European Commission. Commission Implementing Decision (EU) 2018/840 of 5 June 2018 establishing a watch list of substances for Union-wide monitoring in the field of water policy pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Comm. Off. J. Eur. Union 2018, 50, 9–12. [Google Scholar]
- Huynh, N.C.; Nguyen, T.T.T.; Nguyen, D.T.C.; Tran, T. Van Occurrence, toxicity, impact and removal of selected non-steroidal anti-inflammatory drugs (NSAIDs): A review. Sci. Total Environ. 2023, 898, 165317. [Google Scholar] [CrossRef]
- Schwaiger, J.; Ferling, H.; Mallow, U.; Wintermayr, H.; Negele, R.D. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Aquat. Toxicol. 2004, 68, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Näslund, J.; Asker, N.; Fick, J.; Larsson, D.G.J.; Norrgren, L. Naproxen affects multiple organs in fish but is still an environmentally better alternative to diclofenac. Aquat. Toxicol. 2020, 227, 105583. [Google Scholar] [CrossRef]
- Mathias, F.T.; Fockink, D.H.; Disner, G.R.; Prodocimo, V.; Ribas, J.L.C.; Ramos, L.P.; Cestari, M.M.; Silva de Assis, H.C. Effects of low concentrations of ibuprofen on freshwater fish Rhamdia quelen. Environ. Toxicol. Pharmacol. 2018, 59, 105–113. [Google Scholar] [CrossRef]
- Erhunmwunse, N.O.; Tongo, I.; Ezemonye, L.I. Acute effects of acetaminophen on the developmental, swimming performance and cardiovascular activities of the African catfish embryos/larvae (Clarias gariepinus). Ecotoxicol. Environ. Saf. 2021, 208, 111482. [Google Scholar] [CrossRef]
- Saravanan, M.; Ramesh, M. Short and long-term effects of clofibric acid and diclofenac on certain biochemical and ionoregulatory responses in an Indian major carp, Cirrhinus mrigala. Chemosphere 2013, 93, 388–396. [Google Scholar] [CrossRef]
- Rocco, L.; Frenzilli, G.; Fusco, D.; Peluso, C.; Stingo, V. Evaluation of zebrafish DNA integrity after exposure to pharmacological agents present in aquatic environments. Ecotoxicol. Environ. Saf. 2010, 73, 1530–1536. [Google Scholar] [CrossRef]
- Gómez-Oliván, L.M.; Galar-Martínez, M.; García-Medina, S.; Valdés-Alanís, A.; Islas-Flores, H.; Neri-Cruz, N. Genotoxic response and oxidative stress induced by diclofenac, ibuprofen and naproxen in Daphnia magna. Drug Chem. Toxicol. 2014, 37, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Finnin, J.; Tait, C.; Quirk, S.; Chekhtman, I.; Donohue, A.C.; Ng, S.; D’Souza, A.; Tait, R.; Prankerd, R. The Hydrolysis of Diclofenac Esters: Synthetic Prodrug Building Blocks for Biodegradable Drug–Polymer Conjugates. J. Pharm. Sci. 2016, 105, 773–785. [Google Scholar] [CrossRef] [PubMed]
- Parolini, M.; Binelli, A.; Provini, A. Chronic effects induced by ibuprofen on the freshwater bivalve Dreissena polymorpha. Ecotoxicol. Environ. Saf. 2011, 74, 1586–1594. [Google Scholar] [CrossRef] [PubMed]
- Fontes, M.K.; Gusso-Choueri, P.K.; Maranho, L.A.; de Souza Abessa, D.M.; Mazur, W.A.; de Campos, B.G.; Guimarães, L.L.; de Toledo, M.S.; Lebre, D.; Marques, J.R.; et al. A tiered approach to assess effects of diclofenac on the brown mussel Perna perna: A contribution to characterize the hazard. Water Res. 2018, 132, 361–370. [Google Scholar] [CrossRef]
- Stancová, V.; Ziková, A.; Svobodová, Z.; Kloas, W. Effects of the non-steroidal anti-inflammatory drug(NSAID) naproxen on gene expression of antioxidant enzymes in zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 2015, 40, 343–348. [Google Scholar] [CrossRef]
- Lucero, G.-M.A.; Marcela, G.-M.; Sandra, G.-M.; Manuel, G.-O.L.; Celene, R.-E. Naproxen-Enriched Artificial Sediment Induces Oxidative Stress and Genotoxicity in Hyalella azteca. Water Air Soil Pollut. 2015, 226, 195. [Google Scholar] [CrossRef]
- Alkimin, G.D.; Soares, A.M.V.M.; Barata, C.; Nunes, B. Evaluation of ketoprofen toxicity in two freshwater species: Effects on biochemical, physiological and population endpoints. Environ. Pollut. 2020, 265, 114993. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Fattorini, D.; D’Errico, G.; Consolandi, G.; Milan, M.; Bargelloni, L.; Regoli, F. Long-term exposure of Mytilus galloprovincialis to diclofenac, Ibuprofen and Ketoprofen: Insights into bioavailability, biomarkers and transcriptomic changes. Chemosphere 2018, 198, 238–248. [Google Scholar] [CrossRef]
- Pires, P.; Pereira, A.M.P.T.; Pena, A.; Silva, L.J.G. Non-Steroidal Anti-Inflammatory Drugs in the Aquatic Environment and Bivalves: The State of the Art. Toxics 2024, 12, 415. [Google Scholar] [CrossRef]
- Jin, S.; Kang, P.M. A Systematic Review on Advances in Management of Oxidative Stress-Associated Cardiovascular Diseases. Antioxidants 2024, 13, 923. [Google Scholar] [CrossRef]
- Kataoka, C.; Kashiwada, S. Ecological Risks Due to Immunotoxicological Effects on Aquatic Organisms. Int. J. Mol. Sci. 2021, 22, 8305. [Google Scholar] [CrossRef] [PubMed]
- Oyesola, O.O.; Tait Wojno, E.D. Prostaglandin regulation of type 2 inflammation: From basic biology to therapeutic interventions. Eur. J. Immunol. 2021, 51, 2399–2416. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Noya, V.M.; Gómez-Oliván, L.M.; del Carmen Ramírez-Montero, M.; Islas-Flores, H.; Galar-Martínez, M.; Dublán-García, O.; Romero, R. Ibuprofen at environmentally relevant concentrations alters embryonic development, induces teratogenesis and oxidative stress in Cyprinus carpio. Sci. Total Environ. 2020, 710, 136327. [Google Scholar] [CrossRef] [PubMed]
- Kwak, K.; Ji, K.; Kho, Y.; Kim, P.; Lee, J.; Ryu, J.; Choi, K. Chronic toxicity and endocrine disruption of naproxen in freshwater waterfleas and fish, and steroidogenic alteration using H295R cell assay. Chemosphere 2018, 204, 156–162. [Google Scholar] [CrossRef]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Almeida, H.F.D.; Marrucho, I.M.; Freire, M.G. Removal of Nonsteroidal Anti-Inflammatory Drugs from Aqueous Environments with Reusable Ionic-Liquid-Based Systems. ACS Sustain. Chem. Eng. 2017, 5, 2428–2436. [Google Scholar] [CrossRef]
- Minella, M.; Bertinetti, S.; Hanna, K.; Minero, C.; Vione, D. Degradation of ibuprofen and phenol with a Fenton-like process triggered by zero-valent iron (ZVI-Fenton). Environ. Res. 2019, 179, 108750. [Google Scholar] [CrossRef]
- Sétifi, N.; Debbache, N.; Sehili, T.; Halimi, O. Heterogeneous Fenton-like oxidation of naproxen using synthesized goethite-montmorillonite nanocomposite. J. Photochem. Photobiol. A Chem. 2019, 370, 67–74. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Briguglio, S.; Mattiussi, M.; Gelao, V.; Cabras, I.; Zorzenon, L.; Trovarelli, A.; Goi, D. Enhanced ibuprofen removal by heterogeneous-Fenton process over Cu/ZrO2 and Fe/ZrO2 catalysts. J. Environ. Chem. Eng. 2020, 8, 103586. [Google Scholar] [CrossRef]
- Kråkström, M.; Saeid, S.; Tolvanen, P.; Kumar, N.; Salmi, T.; Kronberg, L.; Eklund, P. Identification and Quantification of Transformation Products Formed during the Ozonation of the Non-steroidal Anti-inflammatory Pharmaceuticals Ibuprofen and Diclofenac. Ozone Sci. Eng. 2022, 44, 157–171. [Google Scholar] [CrossRef]
- Du, M.-S.; Chen, K.-P.; Lin, Y.-P. Degradation of ibuprofen and acetylsulfamethoxazole by multi-walled carbon nanotube catalytic ozonation: Surface properties, kinetics and modeling. Environ. Sci. Water Res. Technol. 2019, 5, 1758–1768. [Google Scholar] [CrossRef]
- Skalska-Tuomi, K.; Kaijanen, L.; Monteagudo, J.M.; Mänttäri, M. Efficient removal of pharmaceuticals from wastewater: Comparative study of three advanced oxidation processes. J. Environ. Manag. 2025, 375, 124276. [Google Scholar] [CrossRef]
- Abdi, S.; Nasiri, M. Enhanced performance of thin film nanocomposite (TFN) membranes by incorporating hydrophilic MOF-808 into the organic phase: Towards eliminating non-steroidal anti-inflammatory drugs (NSAIDs) from aqueous solutions. Desalination 2025, 601, 118590. [Google Scholar] [CrossRef]
- Bohdziewicz, J.; Kudlek, E.; Dudziak, M. Removal of selected pharmaceutical compounds from the simulated municipal secondary effluent using the nanofiltration process. In Membranes and Membrane Processes in Environmental Protection, Red; Polish Academy of Sciences: Warsaw, Poland, 2014; Volume 119, pp. 219–228. ISBN 9788363714185. [Google Scholar]
- Al-Khateeb, L.A.; Hakami, W.; Abdel Salam, M.; Sanari, J.A.; El-Shaheny, R.; El-Maghrabey, M. Solid phase-fabrication of magnetically separable Fe3O4@graphene nanoplatelets nanocomposite for efficient removal of NSAIDs from wastewater. Perception of adsorption kinetics, thermodynamics, and extra-thermodynamics. Anal. Chim. Acta 2022, 1223, 340158. [Google Scholar] [CrossRef]
- Xiong, P.; Zhang, H.; Li, G.; Liao, C.; Jiang, G. Adsorption removal of ibuprofen and naproxen from aqueous solution with Cu-doped Mil-101(Fe). Sci. Total Environ. 2021, 797, 149179. [Google Scholar] [CrossRef]
- de Souza, R.M.; Quesada, H.B.; Cusioli, L.F.; Fagundes-Klen, M.R.; Bergamasco, R. Adsorption of non-steroidal anti-inflammatory drug (NSAID) by agro-industrial by-product with chemical and thermal modification: Adsorption studies and mechanism. Ind. Crops Prod. 2021, 161, 113200. [Google Scholar] [CrossRef]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: Review. J. Environ. Manag. 2017, 190, 274–282. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lee, X.J.; Gan, S.; Thangalazhy-Gopakumar, S.; Lim, S.S.; Pan, G.; Yang, T.C. Adsorptive removal of diclofenac by graphene oxide: Optimization, equilibrium, kinetic and thermodynamic studies. J. Taiwan Inst. Chem. Eng. 2019, 98, 150–162. [Google Scholar] [CrossRef]
- Du, Y.; Zhang, X.; Shu, L.; Feng, Y.; Lv, C.; Liu, H.; Xu, F.; Wang, Q.; Zhao, C.; Kong, Q. Safety evaluation and ibuprofen removal via an Alternanthera philoxeroides-based biochar. Environ. Sci. Pollut. Res. 2021, 28, 40568–40586. [Google Scholar] [CrossRef]
- Martín, J.; del Mar Orta, M.; Medina-Carrasco, S.; Santos, J.L.; Aparicio, I.; Alonso, E. Evaluation of a modified mica and montmorillonite for the adsorption of ibuprofen from aqueous media. Appl. Clay Sci. 2019, 171, 29–37. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Nishat, A.; Yusuf, M.; Qadir, A.; Ezaier, Y.; Vambol, V.; Ijaz Khan, M.; Ben Moussa, S.; Kamyab, H.; Sehgal, S.S.; Prakash, C.; et al. Wastewater treatment: A short assessment on available techniques. Alex. Eng. J. 2023, 76, 505–516. [Google Scholar] [CrossRef]
- Martínez-Alcalá, I.; Guillén-Navarro, J.M.; Fernández-López, C. Pharmaceutical biological degradation, sorption and mass balance determination in a conventional activated-sludge wastewater treatment plant from Murcia, Spain. Chem. Eng. J. 2017, 316, 332–340. [Google Scholar] [CrossRef]
- Reungoat, J.; Escher, B.I.; Macova, M.; Keller, J. Biofiltration of wastewater treatment plant effluent: Effective removal of pharmaceuticals and personal care products and reduction of toxicity. Water Res. 2011, 45, 2751–2762. [Google Scholar] [CrossRef] [PubMed]
- Devault, D.A.; Amalric, L.; Bristeau, S.; Cruz, J.; Tapie, N.; Karolak, S.; Budzinski, H.; Lévi, Y. Removal efficiency of emerging micropollutants in biofilter wastewater treatment plants in tropical areas. Environ. Sci. Pollut. Res. 2021, 28, 10940–10966. [Google Scholar] [CrossRef]
- Ehoussou, K.; Akotto, A.G.; Assémian, A.S.; Adou, K.E.; Kpidi, Y.H.; Ossehein, A.; Fanou, G.D.; Adouby, K.; Yao, K.B.; Drogui, P. Elimination of ibuprofen and diclofenac by biofiltration on an organic support made from coconut fibers and husks. J. Mater. Environ. Sci. 2024, 15, 1104–1126. [Google Scholar]
- Lin, J.; Zhang, Y.; Bian, Y.; Zhang, Y.; Du, R.; Li, M.; Tan, Y.; Feng, X. Non-steroidal anti-inflammatory drugs (NSAIDs) in the environment: Recent updates on the occurrence, fate, hazards and removal technologies. Sci. Total Environ. 2023, 904, 166897. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Tiwari, M.K.; Ghangrekar, M.M. A review on environmental occurrence, toxicity and microbial degradation of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs). J. Environ. Manag. 2021, 300, 113694. [Google Scholar] [CrossRef]
- Chen, Y. CiteSpace II: Detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 2006, 57, 359. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Yuan, S.; Li, Z.; Wang, B.; Huang, J.; Deng, S.; Yu, G. Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process. Water Res. 2014, 63, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Ur Rehman, S.W.; Wang, H.; Yang, H.; Yu, G.; Wang, Y. Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process. Water Res. 2018, 138, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Wang, X.; Yang, H.; Yu, G.; Deng, S.; Huang, J.; Wang, B.; Wang, Y. Removal of pharmaceuticals from secondary effluents by an electro-peroxone process. Water Res. 2016, 88, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Nadais, H.; Li, X.; Alves, N.; Couras, C.; Andersen, H.R.; Angelidaki, I.; Zhang, Y. Bio-electro-Fenton process for the degradation of Non-Steroidal Anti-Inflammatory Drugs in wastewater. Chem. Eng. J. 2018, 338, 401–410. [Google Scholar] [CrossRef]
- Loos, G.; Scheers, T.; Van Eyck, K.; Van Schepdael, A.; Adams, E.; Van der Bruggen, B.; Cabooter, D.; Dewil, R. Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode. Sep. Purif. Technol. 2018, 195, 184–191. [Google Scholar] [CrossRef]
- Bu, L.; Ding, J.; Zhu, N.; Kong, M.; Wu, Y.; Shi, Z.; Zhou, S.; Dionysiou, D.D. Unraveling different mechanisms of persulfate activation by graphite felt anode and cathode to destruct contaminants of emerging concern. Appl. Catal. B Environ. 2019, 253, 140–148. [Google Scholar] [CrossRef]
- Yu, F.; Wang, Y.; Ma, H.; Zhou, M. Hydrothermal synthesis of FeS2 as a highly efficient heterogeneous electro-Fenton catalyst to degrade diclofenac via molecular oxygen effects for Fe(II)/Fe(III) cycle. Sep. Purif. Technol. 2020, 248, 117022. [Google Scholar] [CrossRef]
- Yu, Y. Chemosphere Removal of diclofenac by three-dimensional electro-Fenton-persulfate. Chemosphere 2019, 219, 1024–1031. [Google Scholar]
- García-Montoya, M.F.; Gutiérrez-Granados, S.; Alatorre-Ordaz, A.; Galindo, R.; Ornelas, R.; Peralta-Hernández, J.M. Application of electrochemical/BDD process for the treatment wastewater effluents containing pharmaceutical compounds. J. Ind. Eng. Chem. 2015, 31, 238–243. [Google Scholar] [CrossRef]
- Ouarda, Y.; Tiwari, B.; Azaïs, A.; Vaudreuil, M.-A.; Ndiaye, S.D.; Drogui, P.; Tyagi, R.D.; Sauvé, S.; Desrosiers, M.; Buelna, G.; et al. Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: Kinetic study and toxicity assessment. Chemosphere 2018, 193, 160–169. [Google Scholar] [CrossRef]
- Liu, Y.J.; Hu, C.Y.; Lo, S.L. Comparison of the degradation of multiple amine-containing pharmaceuticals during electroindirect oxidation and electrochlorination processes in continuous system. Water Res. 2021, 203, 117517. [Google Scholar] [CrossRef] [PubMed]
- Coria, G.; Nava, J.L.; Carreño, G. Electrooxidation of Diclofenac in Synthetic Pharmaceutical Wastewater Using an Electrochemical Reactor Equipped with a Boron Doped Diamond Electrode. ECS Meet. Abstr. 2014, MA2014-02, 937. [Google Scholar] [CrossRef]
- Ferreira, M.; Güney, S.; Kuźniarska-Biernacka, I.; Soares, O.S.G.P.; Figueiredo, J.L.; Pereira, M.F.R.; Neves, I.C.; Fonseca, A.M.; Parpot, P. Electrochemical oxidation of diclofenac on CNT and M/CNT modified electrodes. New J. Chem. 2021, 45, 12622–12633. [Google Scholar] [CrossRef]
- Martins, V.L.; Ogden, M.D.; Jones, M.R.; Trowsdale, S.A.; Hall, P.J.; Jensen, H.S. Opportunities for coupled electrochemical and ion-exchange technologies to remove recalcitrant micropollutants in water. Sep. Purif. Technol. 2020, 239, 116522. [Google Scholar] [CrossRef]
- Shabanloo, A.; Akbari, H.; Adibzadeh, A.; Akbari, H. Synergistic activation of persulfate by ultrasound/PbO2 anodic oxidation system for effective degradation of naproxen, a toxic and bio-recalcitrant pollutant: Process optimization and application for pharmaceutical wastewater. J. Water Process Eng. 2023, 54, 103915. [Google Scholar] [CrossRef]
- Kouskouki, A.; Chatzisymeon, E.; Mantzavinos, D.; Frontistis, Z. Electrochemical Degradation of Piroxicam on a Boron-Doped Diamond Anode: Investigation of Operating Parameters and Ultrasound Synergy. ChemElectroChem 2019, 6, 841–847. [Google Scholar] [CrossRef]
- Jiménez-Bambague, E.M.; Villarreal-Arias, D.S.; Ramírez-Vanegas, O.D.; Gómez-Gómez, D.D.; Madera-Parra, C.A.; Peña-Salamanca, E.J.; Mota-Filho, C.R.; Machuca-Martínez, F. Removal of pharmaceutical compounds from real urban wastewater by a continuous bio-electrochemical process at pilot scale. J. Environ. Chem. Eng. 2023, 11, 110130. [Google Scholar] [CrossRef]
- Salmerón, I.; Rivas, G.; Oller, I.; Martínez-Piernas, A.; Agüera, A.; Malato, S. Nanofiltration retentate treatment from urban wastewater secondary effluent by solar electrochemical oxidation processes. Sep. Purif. Technol. 2021, 254, 11–13. [Google Scholar] [CrossRef]
- Tran, N.; Drogui, P.; Nguyen, L.; Brar, S.K. Optimization of sono-electrochemical oxidation of ibuprofen in wastewater. J. Environ. Chem. Eng. 2015, 3, 2637–2646. [Google Scholar] [CrossRef]
- Feitosa, M.H.A.; Prado, T.M.; Santos, A.M.; Silva, L.P.; Grosseli, G.M.; Fadini, P.S.; Fatibello-Filho, O.; Moraes, F.C. Titanium dioxide/cadmium sulfide photoanode applied to photoelectrodegradation of naproxen in wastewater. J. Electroanal. Chem. 2021, 897, 115571. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, X.; Ma, Q.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q.; Li, B. Kinetics of photoelectrocatalytic degradation of diclofenac using N, S CO-doped TIO2 nano-crystallite decorated TIO2 nanotube arrays photoelectrode. Environ. Prot. Eng. 2018, 44, 117–130. [Google Scholar] [CrossRef]
- Changanaqui, K.; Alarcón, H.; Brillas, E.; Sirés, I. Blue LED light-driven photoelectrocatalytic removal of naproxen from water: Kinetics and primary by-products. J. Electroanal. Chem. 2020, 867, 114192. [Google Scholar] [CrossRef]
- Chen, H.; Peng, Y.-P.; Chen, T.-Y.; Chen, K.-F.; Chang, K.-L.; Dang, Z.; Lu, G.-N.; He, H. Enhanced photoelectrochemical degradation of Ibuprofen and generation of hydrogen via BiOI-deposited TiO2 nanotube arrays. Sci. Total Environ. 2018, 633, 1198–1205. [Google Scholar] [CrossRef]
- Muzenda, C.; Arotiba, O.A. Improved Magnetite Nanoparticle Immobilization on a Carbon Felt Cathode in the Heterogeneous Electro-Fenton Degradation of Aspirin in Wastewater. ACS Omega 2022, 7, 19261–19269. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Pachón, D.; Echeverry-Gallego, R.A.; Serna-Galvis, E.A.; Villarreal, J.M.; Botero-Coy, A.M.; Hernández, F.; Torres-Palma, R.A.; Moncayo-Lasso, A. Treatment of wastewater effluents from Bogotá–Colombia by the photo-electro-Fenton process: Elimination of bacteria and pharmaceutical. Sci. Total Environ. 2021, 772, 144890. [Google Scholar] [CrossRef]
- Manrique-Losada, L.; Quimbaya-Ñañez, C.; Serna-Galvis, E.A.; Oller, I.; Torres-Palma, R.A. Enhanced solar photo-electro-Fenton by Theobroma grandiflorum addition during pharmaceuticals elimination in municipal wastewater: Action routes, process improvement, and biodegradability of the treated water. J. Environ. Chem. Eng. 2022, 10, 107489. [Google Scholar] [CrossRef]
- Campos, S.; Lorca, J.; Vidal, J.; Calzadilla, W.; Toledo-Neira, C.; Aranda, M.; Miralles-Cuevas, S.; Cabrera-Reina, A.; Salazar, R. Removal of contaminants of emerging concern by solar photo electro-Fenton process in a solar electrochemical raceway pond reactor. Process Saf. Environ. Prot. 2023, 169, 660–670. [Google Scholar] [CrossRef]
- Bugueño-Carrasco, S.; Monteil, H.; Toledo-Neira, C.; Sandoval, M.Á.; Thiam, A.; Salazar, R. Elimination of pharmaceutical pollutants by solar photoelectro-Fenton process in a pilot plant. Environ. Sci. Pollut. Res. 2021, 28, 23753–23766. [Google Scholar] [CrossRef]
- Wang, H.; Su, L.; Zhu, S.; Zhu, W.; Han, X.; Cheng, Y.; Yu, G.; Wang, Y. Optimization of the Electro-Peroxone Process for Micropollutant Abatement Using Chemical Kinetic Approaches. Molecules 2019, 24, 2638. [Google Scholar] [CrossRef]
- Srinivasan, R.; Nambi, I.M. An electro-peroxone-based multi-pronged strategy for the treatment of ibuprofen and an emerging pharmaceutical wastewater using a novel graphene-coated nickel foam electrode. Chem. Eng. J. 2022, 450, 137618. [Google Scholar] [CrossRef]
- Jin, X.; Xie, X.; Liu, Y.; Wang, Y.; Wang, R.; Jin, P.; Yang, C.; Shi, X.; Wang, X.C.; Xu, H. The role of synergistic effects between ozone and coagulants (SOC) in the electro-hybrid ozonation-coagulation process. Water Res. 2020, 177, 115800. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Lo, S.-L.; Liou, Y.-H.; Hu, C.-Y. Removal of nonsteroidal anti-inflammatory drugs (NSAIDs) by electrocoagulation–flotation with a cationic surfactant. Sep. Purif. Technol. 2015, 152, 148–154. [Google Scholar] [CrossRef]
- Jin, X.; Xie, X.; Zhang, S.; Yang, C.; Xu, L.; Shi, X.; Jin, P.; Wang, X.C. Insights into the electro-hybrid ozonation-coagulation process–Significance of connection configurations and electrode types. Water Res. 2021, 204, 117600. [Google Scholar] [CrossRef] [PubMed]
- Brdarić, T.P.; Aćimović, D.D.; Savić Rosić, B.G.; Simić, M.D.; Stojanović, K.D.; Vranješ, Z.M.; Vasić Anićijević, D. Bibliometric Analysis of Nanostructured Anodes for Electro-Oxidative Wastewater Treatment. Sustainability 2024, 16, 3982. [Google Scholar] [CrossRef]
- Brdarić, T.P.; Aćimović, D.D.; Švorc, Ľ.; Vasić Anićijević, D.D. Bibliometric Study of Electrochemical Advanced Oxidation Processes (EAOPs) for Wastewater Treatment. Coatings 2024, 14, 1060. [Google Scholar] [CrossRef]
- Ječmenica Dučić, M.; Krstić, A.; Zdolšek, N.; Aćimović, D.; Savić, B.; Brdarić, T.; Vasić Anićijević, D. Low-Cost Graphene-Based Composite Electrodes for Electrochemical Oxidation of Phenolic Dyes. Crystals 2023, 13, 125. [Google Scholar] [CrossRef]
- Savić, B.G.; Stanković, D.M.; Živković, S.M.; Ognjanović, M.R.; Tasić, G.S.; Mihajlović, I.J.; Brdarić, T.P. Electrochemical oxidation of a complex mixture of phenolic compounds in the base media using PbO2-GNRs anodes. Appl. Surf. Sci. 2020, 529, 147120. [Google Scholar] [CrossRef]
- Simić, M.D.; Savić, B.G.; Ognjanović, M.R.; Stanković, D.M.; Relić, D.J.; Aćimović, D.D.; Brdarić, T.P. Degradation of bisphenol A on SnO2-MWCNT electrode using electrochemical oxidation. J. Water Process Eng. 2023, 51, 103416. [Google Scholar] [CrossRef]
- Acimovic, D.D.; Nikolic, Z.M.; Tosic, M.S.; Milovanovic, D.S.; Nikolic, V.M.; Brdaric, T.P.; Marceta-Kaninski, M.P. Validation and uncertainty estimation of UPLC-PDA method for the analysis of polycyclic aromatic hydrocarbons in concrete. J. Hazard. Mater. 2017, 325, 271–278. [Google Scholar] [CrossRef]
- Aćimović, D.D.; Karić, S.D.; Nikolić, Ž.M.; Brdarić, T.P.; Tasić, G.S.; Marčeta Kaninski, M.P.; Nikolić, V.M. Electrochemical oxidation of the polycyclic aromatic hydrocarbons in polluted concrete of the residential buildings. Environ. Pollut. 2016, 220, 393–399. [Google Scholar] [CrossRef]
- Ječmenica Dučić, M.; Aćimović, D.; Savić, B.; Rakočević, L.; Simić, M.; Brdarić, T.; Vasić Anićijević, D. Is It Possible to Restrain OER on Simple Carbon Electrodes to Efficiently Electrooxidize Organic Pollutants? Molecules 2022, 27, 5203. [Google Scholar] [CrossRef] [PubMed]
- Simić, M.D.; Brdarić, T.P.; Savić Rosić, B.G.; Švorc, Ľ.; Relić, D.J.; Aćimović, D.D. Degradation of bisphenol A via the electro–Fenton process using nanostructured carbon-metal oxide anodes: Intermediates and reaction mechanisms study. J. Environ. Chem. Eng. 2024, 12, 113369. [Google Scholar] [CrossRef]
- Aćimović, D.; Vasić Anićijević, D. Organophosphates: Detection, Exposure and Occurrence. Volume 1: Impact on Health and the Natural Environment; Lazarević-Pašti, T., Ed.; Nova Science Publishers: New York, NY, USA, 2022; Volume 1, ISBN 9781685076528. [Google Scholar]
- Moradi, M.; Vasseghian, Y.; Khataee, A.; Kobya, M.; Arabzade, H.; Dragoi, E.-N. Service life and stability of electrodes applied in electrochemical advanced oxidation processes: A comprehensive review. J. Ind. Eng. Chem. 2020, 87, 18–39. [Google Scholar] [CrossRef]
- Gandini, D.; Michaud, P.-A.; Duo, I.; Mahe, E.; Haenni, W.; Perret, A.; Comninellis, C. Electrochemical behavior of synthetic boron-doped diamond thin film anodes. New Diam. Front. Carbon Technol. 1999, 9, 303–316. [Google Scholar]
- Comninellis, C.; Chen, G. Electrochemistry for the Environment; Springer: Cham, Switzerland, 2010; Volume 2015. [Google Scholar] [CrossRef]
- Brillas, E.; Sirés, I.; Arias, C.; Cabot, P.L.; Centellas, F.; Rodríguez, R.M.; Garrido, J.A. Mineralization of paracetamol in aqueous medium by anodic oxidation with a boron-doped diamond electrode. Chemosphere 2005, 58, 399–406. [Google Scholar] [CrossRef]
- Brillas, E.; Garcia-Segura, S.; Skoumal, M.; Arias, C. Electrochemical incineration of diclofenac in neutral aqueous medium by anodic oxidation using Pt and boron-doped diamond anodes. Chemosphere 2010, 79, 605–612. [Google Scholar] [CrossRef]
- Zhao, X.; Hou, Y.; Liu, H.; Qiang, Z.; Qu, J. Electro-oxidation of diclofenac at boron doped diamond: Kinetics and mechanism. Electrochim. Acta 2009, 54, 4172–4179. [Google Scholar] [CrossRef]
- Murugananthan, M.; Latha, S.S.; Bhaskar Raju, G.; Yoshihara, S. Anodic oxidation of ketoprofen—An anti-inflammatory drug using boron doped diamond and platinum electrodes. J. Hazard. Mater. 2010, 180, 753–758. [Google Scholar] [CrossRef]
- Ambuludi, S.L.; Panizza, M.; Oturan, N.; Özcan, A.; Oturan, M.A. Kinetic behavior of anti-inflammatory drug ibuprofen in aqueous medium during its degradation by electrochemical advanced oxidation. Environ. Sci. Pollut. Res. 2013, 20, 2381–2389. [Google Scholar] [CrossRef]
- Qiu, H.; Fan, P.; Li, X.; Hou, G. Electrochemical degradation of DCF by boron-doped diamond anode: Degradation mechanism, pathways and influencing factors. Water Sci. Technol. 2021, 84, 431–444. [Google Scholar] [CrossRef]
- Iovino, P.; Lavorgna, M.; Orlo, E.; Russo, C.; De Felice, B.; Campolattano, N.; Muscariello, L.; Fenti, A.; Chianese, S.; Isidori, M.; et al. An integrated approach for the assessment of the electrochemical oxidation of diclofenac: By-product identification, microbiological and eco-genotoxicological evaluation. Sci. Total Environ. 2024, 909, 168511. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Song, W.; Oturan, N.; Karbasi, M.; van Hullebusch, E.D.; Esposito, G.; Giannakis, S.; Oturan, M.A. Electrochemical oxidation of Naproxen in aqueous matrices: Elucidating the intermediates’ eco-toxicity, by assessing its degradation pathways via experimental and density functional theory (DFT) approaches. Chem. Eng. J. 2023, 451, 138483. [Google Scholar] [CrossRef]
- Dang, Q.; Wang, L.; Liu, J.; Wang, D.; Chai, J.; Wu, M.; Tang, L. Recent progress of photoelectrocatalysis systems for wastewater treatment. J. Water Process Eng. 2023, 53, 103609. [Google Scholar] [CrossRef]
- Gozzi, F.; Guelfi, D.R.V.; da Silva, T.F.; de Oliveira, S.C.; Machulek Junior, A. Recent developments on the application of photoelectrochemical processes for sustainable water treatment. Curr. Opin. Electrochem. 2024, 46, 101502. [Google Scholar] [CrossRef]
- Razzaq, U.; Nguyen, T.-B.; Saleem, M.U.; Le, V.-R.; Chen, C.-W.; Bui, X.-T.; Dong, C.-D. Recent progress in electro-Fenton technology for the remediation of pharmaceutical compounds in aqueous environments. Sci. Total Environ. 2024, 946, 174253. [Google Scholar] [CrossRef]
- Shi, K.; Wang, Y.; Xu, A.; Zhou, X.; Zhu, H.; Wei, K.; Liu, X.; Shen, J.; Han, W. Efficient degradation of ibuprofen by electro-Fenton with microtubular gas- diffusion electrodes synthesized by wet-spinning method. J. Electroanal. Chem. 2021, 897, 115615. [Google Scholar] [CrossRef]
- Martone, L.; Minella, M.; Minero, C.; Sordello, F.; Vione, D. Effective degradation of ibuprofen through an electro-Fenton process, in the presence of zero-valent iron (ZVI-EF). J. Clean. Prod. 2022, 367, 132894. [Google Scholar] [CrossRef]
- Chen, Z.; Xia, P.; Wang, D.; Niu, X.; Ao, L.; He, Q.; Wang, S.; Ye, Z.; Sirés, I. New insights into the mechanism of Fered-Fenton treatment of industrial wastewater with high chloride content: Role of multiple reactive species. Sci. Total Environ. 2023, 882, 163596. [Google Scholar] [CrossRef]
- Le, T.X.H.; Bechelany, M.; Lacour, S.; Oturan, N.; Oturan, M.A.; Cretin, M. High removal efficiency of dye pollutants by electron-Fenton process using a graphene based cathode. Carbon N. Y. 2015, 94, 1003–1011. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, S.; Huang, Y. Removing organic contaminants by an electro-Fenton system constructed with graphene cathode. Toxicol. Environ. Chem. 2016, 98, 530–539. [Google Scholar] [CrossRef]
- Le, T.X.H.; Charmette, C.; Bechelany, M.; Cretin, M. Facile Preparation of Porous Carbon Cathode to Eliminate Paracetamol in Aqueous Medium Using Electro-Fenton System. Electrochim. Acta 2016, 188, 378–384. [Google Scholar] [CrossRef]
- Gao, Y.; Xie, F.; Bai, H.; Zeng, L.; Zhang, J.; Liu, M.; Zhu, W. A carbon felt cathode modified by acidic oxidised carbon nanotubes for the high H2O2 generation and its application in electro-Fenton. Environ. Technol. 2024, 45, 1669–1682. [Google Scholar] [CrossRef] [PubMed]
- Karatas, O.; Gengec, N.A.; Gengec, E.; Khataee, A.; Kobya, M. High-performance carbon black electrode for oxygen reduction reaction and oxidation of atrazine by electro-Fenton process. Chemosphere 2022, 287, 132370. [Google Scholar] [CrossRef] [PubMed]
- Daniel, G.; Zhang, Y.; Lanzalaco, S.; Brombin, F.; Kosmala, T.; Granozzi, G.; Wang, A.; Brillas, E.; Sirés, I.; Durante, C. Chitosan-Derived Nitrogen-Doped Carbon Electrocatalyst for a Sustainable Upgrade of Oxygen Reduction to Hydrogen Peroxide in UV-Assisted Electro-Fenton Water Treatment. ACS Sustain. Chem. Eng. 2020, 8, 14425–14440. [Google Scholar] [CrossRef]
- Zhong, S.; Zhu, Z.-S.; Duan, X.; Wang, S. Electro-Fenton-Based Membrane System for Organic Micropollutant Removal: New Trend and Prospect. ACS EST Eng. 2023, 3, 2147–2160. [Google Scholar] [CrossRef]
- Nair, K.M.; Kumaravel, V.; Pillai, S.C. Carbonaceous cathode materials for electro-Fenton technology: Mechanism, kinetics, recent advances, opportunities and challenges. Chemosphere 2021, 269, 129325. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Wang, Y.; Cao, T.; Zhao, G. Electro-Fenton oxidation of pesticides with a novel Fe3O4@Fe2O3/activated carbon aerogel cathode: High activity, wide pH range and catalytic mechanism. Appl. Catal. B Environ. 2012, 125, 120–127. [Google Scholar] [CrossRef]
- Birjandi, N.; Younesi, H.; Ghoreyshi, A.A.; Rahimnejad, M. Electricity generation through degradation of organic matters in medicinal herbs wastewater using bio-electro-Fenton system. J. Environ. Manag. 2016, 180, 390–400. [Google Scholar] [CrossRef]
- Hidalgo, D.; Tommasi, T.; Bocchini, S.; Chiolerio, A.; Chiodoni, A.; Mazzarino, I.; Ruggeri, B. Surface modification of commercial carbon felt used as anode for Microbial Fuel Cells. Energy 2016, 99, 193–201. [Google Scholar] [CrossRef]
- Vigil-Castillo, H.H.; Ruiz-Ruiz, E.J.; López-Velázquez, K.; Hinojosa-Reyes, L.; Gaspar-Ramírez, O.; Guzmán-Mar, J.L. Assessment of photo electro-Fenton and solar photo electro-Fenton processes for the efficient degradation of asulam herbicide. Chemosphere 2023, 338, 139585. [Google Scholar] [CrossRef]
- Villanueva-Rodríguez, M.; Bello-Mendoza, R.; Hernández-Ramírez, A.; Ruiz-Ruiz, E.J. Degradation of anti-inflammatory drugs in municipal wastewater by heterogeneous photocatalysis and electro-Fenton process. Environ. Technol. 2019, 40, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Aliste, M.; León-Morán, L.O.; Martínez-Escudero, C.M.; Garrido, I.; Contreras, F.; Hellín, P.; Flores, P.; Fenoll, J. Solar photo-Fenton with Fe3+-EDDS and Fe3+-NTA at neutral pH for removal of ibuprofen, diclofenac and their main transformation products in wastewater. Catal. Today 2025, 446, 115139. [Google Scholar] [CrossRef]
- Farzaneh, H.; Loganathan, K.; Saththasivam, J.; McKay, G. Ozone and ozone/hydrogen peroxide treatment to remove gemfibrozil and ibuprofen from treated sewage effluent: Factors influencing bromate formation. Emerg. Contam. 2020, 6, 225–234. [Google Scholar] [CrossRef]
- Öztürk, H.; Barışçı, S.; Turkay, O. Paracetamol degradation and kinetics by advanced oxidation processes (AOPs): Electro-peroxone, ozonation, goethite catalyzed electro-fenton and electro-oxidation. Environ. Eng. Res. 2020, 26, 180332. [Google Scholar] [CrossRef]
- Feng, L.; Watts, M.J.; Yeh, D.; Esposito, G.; van Hullebusch, E.D. The Efficacy of Ozone/BAC Treatment on Non-Steroidal Anti-Inflammatory Drug Removal from Drinking Water and Surface Water. Ozone Sci. Eng. 2015, 37, 343–356. [Google Scholar] [CrossRef]
- Farzaneh, H.; Loganathan, K.; Saththasivam, J.; McKay, G. Selectivity and competition in the chemical oxidation processes for a binary pharmaceutical system in treated sewage effluent. Sci. Total Environ. 2021, 765, 142704. [Google Scholar] [CrossRef]
- Snyder, S.A.; Wert, E.C.; Rexing, D.J.; Zegers, R.E.; Drury, D.D. Ozone Oxidation of Endocrine Disruptors and Pharmaceuticals in Surface Water and Wastewater. Ozone Sci. Eng. 2006, 28, 445–460. [Google Scholar] [CrossRef]
- Merényi, G.; Lind, J.; Naumov, S.; Sonntag, C. von Reaction of Ozone with Hydrogen Peroxide (Peroxone Process): A Revision of Current Mechanistic Concepts Based on Thermokinetic and Quantum-Chemical Considerations. Environ. Sci. Technol. 2010, 44, 3505–3507. [Google Scholar] [CrossRef]





| Nb. | Document | Citations | Links | Title | Journal |
|---|---|---|---|---|---|
| 1 | Li et al. (2014) [52] | 149 | 6 | Degradation of the anti-inflammatory drug ibuprofen by electro-peroxone process | Water Research |
| 2 | Yao et al. (2018) [53] | 139 | 2 | Pilot-scale evaluation of micropollutant abatements by conventional ozonation, UV/O3, and an electro-peroxone process | Water Research |
| 3 | Yao et al. (2016) [54] | 114 | 5 | Removal of pharmaceuticals from secondary effluents by an electro-peroxone process | Water Research |
| 4 | Nadais et al. (2018) [55] | 102 | 3 | Bio-electro-Fenton process for the degradation of Non-Steroidal Anti-Inflammatory Drugs in wastewater | Chemical Engineering Journal |
| 5 | Loos et al. (2018) [56] | 96 | 1 | Electrochemical oxidation of key pharmaceuticals using a boron doped diamond electrode | Separation and Purification Technology |
| 6 | Bu et al. (2019) [57] | 95 | 0 | Unraveling different mechanisms of persulfate activation by graphite felt anode and cathode to destruct contaminants of emerging concern | Applied Catalysis B: Environmental |
| 7 | Yu et al. (2020) [58] | 91 | 1 | Hydrothermal synthesis of FeS2 as a highly efficient heterogeneous electro-Fenton catalyst to degrade diclofenac via molecular oxygen effects for Fe(II)/Fe(III) cycle | Separation and Purification Technology |
| 8 | Yu et al. (2019) [59] | 79 | 2 | Removal of diclofenac by three-dimensional electro-Fenton-persulfate (3D electro-Fenton-PS) | Chemosphere |
| 9 | Fernanda Garcia-Montoya et al. (2015) [60] | 72 | 3 | Application of electrochemical/BDD process for the treatment wastewater effluents containing pharmaceutical compounds | Journal of Industrial and Engineering Chemistry |
| 10 | Ouarda et al. (2018) [61] | 70 | 2 | Synthetic hospital wastewater treatment by coupling submerged membrane bioreactor and electrochemical advanced oxidation process: Kinetic study and toxicity assessment | Chemosphere |
| Technologies Type | NSAID | Experimental Conditions | Removal, % | References |
|---|---|---|---|---|
| EO | Diclofenac | Electrolyte: Synthetic pharmaceutical wastewater (NaCl or Na2SO4 and amine containing pharmaceuticals); Pt/Ti electrodes; Current density of 0.8 mA cm−2; | 56.1% | [62] |
| EO | Diclofenac | Electrolyte: 0.5 M NaClO4; Anode: BDD; Cathode: stainless steel plate; Current density of 10, 15 i 20 mA cm−2; | 100% | [63] |
| EO | Diclofenac | Electrolyte: 0.1 M NaOH; CNT, Pt/CNT and Ru/CNT modified electrodes; | 48% | [64] |
| EO | Diclofenac | Electrolyte: Na2SO4; Anode: boron-doped diamond; Cathode: stainless steel; Current densities: 1.56 to 6.25 mA cm−2; | 80% | [60] |
| EO | Ibuprofen | Electrolyte: synthetic hospital wastewater; Anode: BDD; Cathode: Nb; Current: 0.5 A | >97% | [61] |
| EO | Ibuprofen | Electrolyte: 0.1 M NaCl; Anode: glassy carbon, carbon fibre; Cathode: TiO2, stainless steel mesh; Voltage: 1.5 V and 2.5 V; | 86% | [65] |
| EO | Naproxen | Electrolyte: 0.5 M Pb(NO3)2, 0.1 M HNO3, and 0.05 M NaF; Anode: graphite sheet; Cathode: stainless steel sheet; Current density: 7.5 mA cm−2; | 98.5% | [66] |
| EO | Piroxicam | Electrolyte: 0.1 M Na2SO4; Anode: BDD; Cathode: Pt; Current density: 26.7 mA cm−2; | >98% | [67] |
| Bio-EO | Diclofenac | Electrolyte: wastewater sample and a mixed culture BDD anode and cathode; Current density of between 20 and 30 mA cm−2 | 73.1–96.6% | [68] |
| Bio-EO | Diclofenac | Electrolyte: synthetic wastewater; Anode: cylindrical aluminum; Cathode: stainless-steel mesh; Current density of 0.5 mA cm−2 | 70% | [68] |
| Bio-EO | Ibuprofen | Electrolyte: wastewater sample and a mixed culture; BDD anode and cathode; Current density of between 20 and 30 mA cm−2 | 79–100% | [68] |
| Bio-EO | Ketoprofen | Electrolyte: wastewater sample and a mixed culture BDD anode and cathode; Current density of between 20 and 30 mA cm−2; | 72–96% | [68] |
| Bio-EO | Naproxen | Electrolyte: wastewater sample and a mixed culture BDD anode and cathode; Current density of between 20 and 30 mA cm−2 | 82–100% | [68] |
| Solar-EO | Diclofenac | Electrolyte: 50 mM of Na2SO4; Anode: BDD film on a niobium mesh substrate; Cathode: carbon-PTFE; Current density of 74 mA cm−2 | >80% | [69] |
| Sono-EO | Ibuprofen | Electrolyte: syntetic solution and wastewater sample with IBU; AnodeTi/PbO2; Cathode:Ti; Current: 70 A at an open circuit potential of 40 V | 90% | [70] |
| PEC | Naproxen | Electrolyte: 200 mM phosphate buffer pH 7.0; photoanode TiO2/CdS; UV Lamp: 9 W; Potential: +2.0 V | 95.3% | [71] |
| PEC | Diclofenac | Electrolyte: 0.1 M Na2SO4; photoanode: N, S co-doped TiO2 nanocrystals on TiO2 nanotube arrays; 35 W Xenon lamp; Potential: 0.4 V | 71.4% | [72] |
| PEC | Naproxen | Electrolyte: 70 mM NaCl, photoanode: ZnO/TiO2/Ag2Se; 36 W blue LED lamp; Potential: 1.0 V | 100% | [73] |
| PEC | Ibuprofen | Electrolyte: water solutions, photoanode: BiOI/TNTAs; 100 W Hg lamp; Potential: 1.2 V | 100% | [74] |
| EF | Diclofenac | Electrolyte: sodium persulfate addition 1.50 mM and the Fe0 addition 0.20 mM. Anode: titanium mesh; Cathode: carbon fiber; Voltage: 8 V | 96.3% | [59] |
| EF | Diclofenac | Eletrolyte: synthetic pharmaceutical wastewater; Two parallel plate ferrous electrodes; power supply (30 V and 10 A), Current density of 58.47 mA cm−2 | 97.21% | [65] |
| EF | Diclofenac | Electrolyte: 0,05 M Na2SO4; Anode: Pt mesh; Cathode: GDE; Current density: 47.77 mA cm−2, | 88% | [58] |
| EF | Diclofenac | Electrolyte: Na2SO4; Anode: boron-doped diamond; Cathode: stainless steel; Current densities: 1.56 to 6.25 mA cm−2; | 50% | [60] |
| EF | Aspirin | Electrolyte: 0.05 M Na2SO4; Anode: Ti4O7; Cathode: carbon felt; Voltage: 8 V | 100% | [75] |
| Bio-EF | Diclofenac | Electrolyte: 0.05 M Na2SO4 and 0.012 M sodium acetate solution; Anode: carbon brush; Cathode: graphite plate; Voltage: 0.3 V | 87–97% | [55] |
| Bio-EF | Ibuprofen | Electrolyte: 0.05 M Na2SO4 and 0.012 M sodium acetate solution; Anode: carbon brush; Cathode: graphite plate Voltage: 0.3 V | 80–86% | [55] |
| Bio-EF | Ketoprofen | Electrolyte: 0.05 M Na2SO4 and 0.012 M sodium acetate solution; Anode: carbon brush; Cathode: graphite plate; Voltage: 0.3 V | 59–61% | [55] |
| Bio-EF | Naproxen | Electrolyte: 0.05 M Na2SO4 and 0.012 M sodium acetate solution Anode: carbon brush; Cathode: graphite plate; Voltage: 0.3 V | 75–81% | [55] |
| PEF | Diclofenac | Electrolyte: 50 mM NaCl; Anode: Ti/IrO2 doped with SnO2; Cathode: carbon-felt; Current density of 6.92 mA cm−2; | >80% | [76] |
| SPEF | Diclofenac | Electrolyte: Na2SO4 or NaCl; Anode: BDD or IrO2; Cathode: gas diffusion or inert Ti; Current density: 3.25 mA cm−2; The electric potential remained between 3.5 and 4.0 V; | >80% | [77] |
| SPEF | Diclofenac | Electrolyte: 0.05 M Na2SO4; Anode: Ti/Ru0.3Ti0.7O2 plate; Cathode: carbon-PTFE ADC; Current density of 20 mA cm−2; | 55% | [78] |
| SPEF | Diclofenac | Electrolyte: 0.05 M Na2SO4 and heptahydrated FeSO4; Anode: Ti/Ru0.3Ti0.7O2 plate; Cathode: carbon-PTFE ADC; Current densities: 10, 25, and 50 mA cm−2; | Almost 100% | [79] |
| SPEF | Ibuprofen | Electrolyte: 0.05 M Na2SO4; Anode: Ti/Ru0.3Ti0.7O2 plate; Cathode: PTFE ADC; Current density of 20 mA cm−2; | 55% | [78] |
| SPEF | Salicylic acid | Electrolyte: 0. 05 M Na2SO4 and heptahydrated FeSO4; Anode: Ti/Ru0.3Ti0.7O2 plate; Cathode: carbon-PTFE ADC; Current densities: 10, 25, and 50 mA cm−2; | Almost 100% | [79] |
| E-peroxone | Diclofenac | Electrolyte: surface water; Anode: Pt plate; Cathode: carbon-PTFE; Applied currents: 10–50 mA; | >90% | [80] |
| E-peroxone | Diclofenac | Electrolyte: groundwater, surface water, and secondary wastewater; Anode: RuO2/IrO2 coated Ti plate; Cathode: carbon C-PTFE; Current: 50–200 mA; | >90% | [53] |
| E-peroxone | Ibuprofen | Electrolyte: 0.05 M Na2SO4; Anode: platinum-coated titanium (Pt-Ti); Cathode: Graphene-coated nickel foam (Gr-NF);Current of 400 mA; | 87% | [81] |
| E-peroxone | Ibuprofen | Electrolyte: 0.05 M Na2SO4; Anode: Pt plate; Cathode: carbon-PTFE; Current of 300 mA; | 100% | [52] |
| E-peroxone | Ibuprofen | Electrolyte: surface water; Anode: Pt; Cathode: carbon-PTFE; Currents: 10–50 mA; | 90% | [80] |
| E-peroxone | Ibuprofen | Electrolyte: 0.05 M Na2SO4; Anode: Pt plate; Cathode: carbon-PTFE; Current 80 mA; | >90% | [54] |
| E-peroxone | Naproxen | Electrolyte: groundwater, surface water, and secondary wastewater; Anode: RuO2/IrO2 coated Ti plate; Cathode: carbon-PTFE; Current: 50–200 mA; | 90% | [53] |
| EC | Diclofenac | Electrolyte: synthetic water samples and real water samples;Each electrode was made of aluminum;Current: 0.5 A; | 14% | [62] |
| EC | Ibuprofen | Electrolyte: WWTP effluent collected from a secondary sedimentation tank Anode: Al; Cathode: stainless steel; Current densities: 5, 10 and 15 mA cm−2 | <25% | [82] |
| EC | Ibuprofen | Electrolyte: synthetic water samples and real water samples; Each electrode was made of aluminum; Current density of 0.5 A; | 44% | [83] |
| EC | Ibuprofen | Electrolyte: raw water; Anode: Al; Cathode: stainless steel; Current density: 15 mA cm−2; | 58.6% | [84] |
| EC | Ketoprofen | Electrolyte: synthetic water samples and real water samples; Each electrode was made of aluminum; Current density of 0.5 A; | 10% | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stojanović, K.D.; Aćimović, D.D.; Brdarić, T.P. Electrochemical-Based Technologies for Removing NSAIDs from Wastewater: Systematic Review with Bibliometric Analysis. Processes 2025, 13, 1272. https://doi.org/10.3390/pr13051272
Stojanović KD, Aćimović DD, Brdarić TP. Electrochemical-Based Technologies for Removing NSAIDs from Wastewater: Systematic Review with Bibliometric Analysis. Processes. 2025; 13(5):1272. https://doi.org/10.3390/pr13051272
Chicago/Turabian StyleStojanović, Katarina D., Danka D. Aćimović, and Tanja P. Brdarić. 2025. "Electrochemical-Based Technologies for Removing NSAIDs from Wastewater: Systematic Review with Bibliometric Analysis" Processes 13, no. 5: 1272. https://doi.org/10.3390/pr13051272
APA StyleStojanović, K. D., Aćimović, D. D., & Brdarić, T. P. (2025). Electrochemical-Based Technologies for Removing NSAIDs from Wastewater: Systematic Review with Bibliometric Analysis. Processes, 13(5), 1272. https://doi.org/10.3390/pr13051272









