Heavy Metal Pollution in a Cu Mine Dump and in Close Agricultural Soils and Crops in Mozambique
Abstract
:1. Introduction
2. Materials and Methods
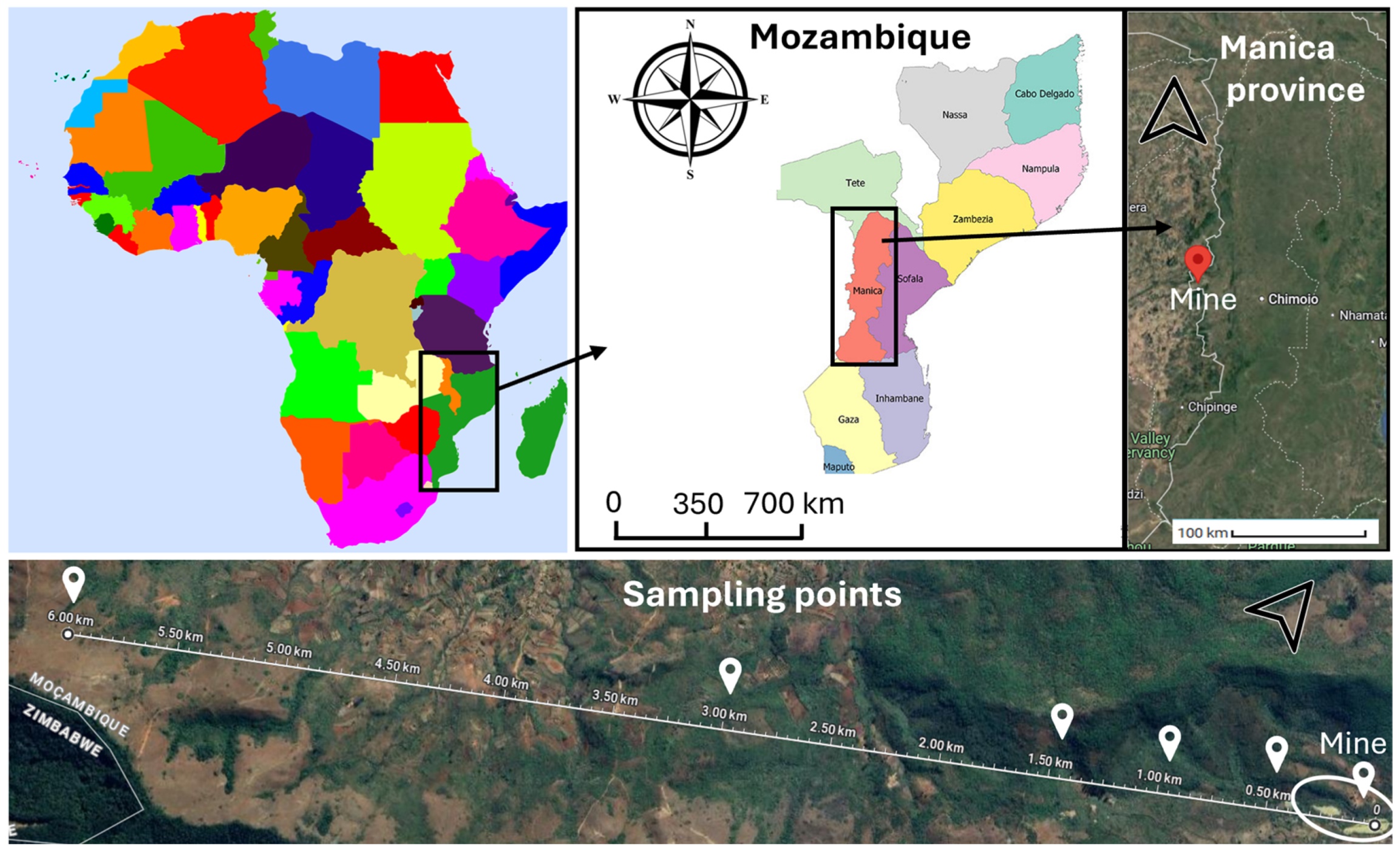
2.1. The Study Area
2.2. Characteristics of the Mundonguara Mine
2.3. Collection of Soil, Dump Waste Material, Water and Plant Samples
2.4. Analytical Methods
2.5. Statistical Analyses
3. Results and Discussion
3.1. General Characteristics of the Mine Dump Materials and the Surrounding Soils
3.2. Heavy Metals in the Mine Dump and in the Surrounding Soils
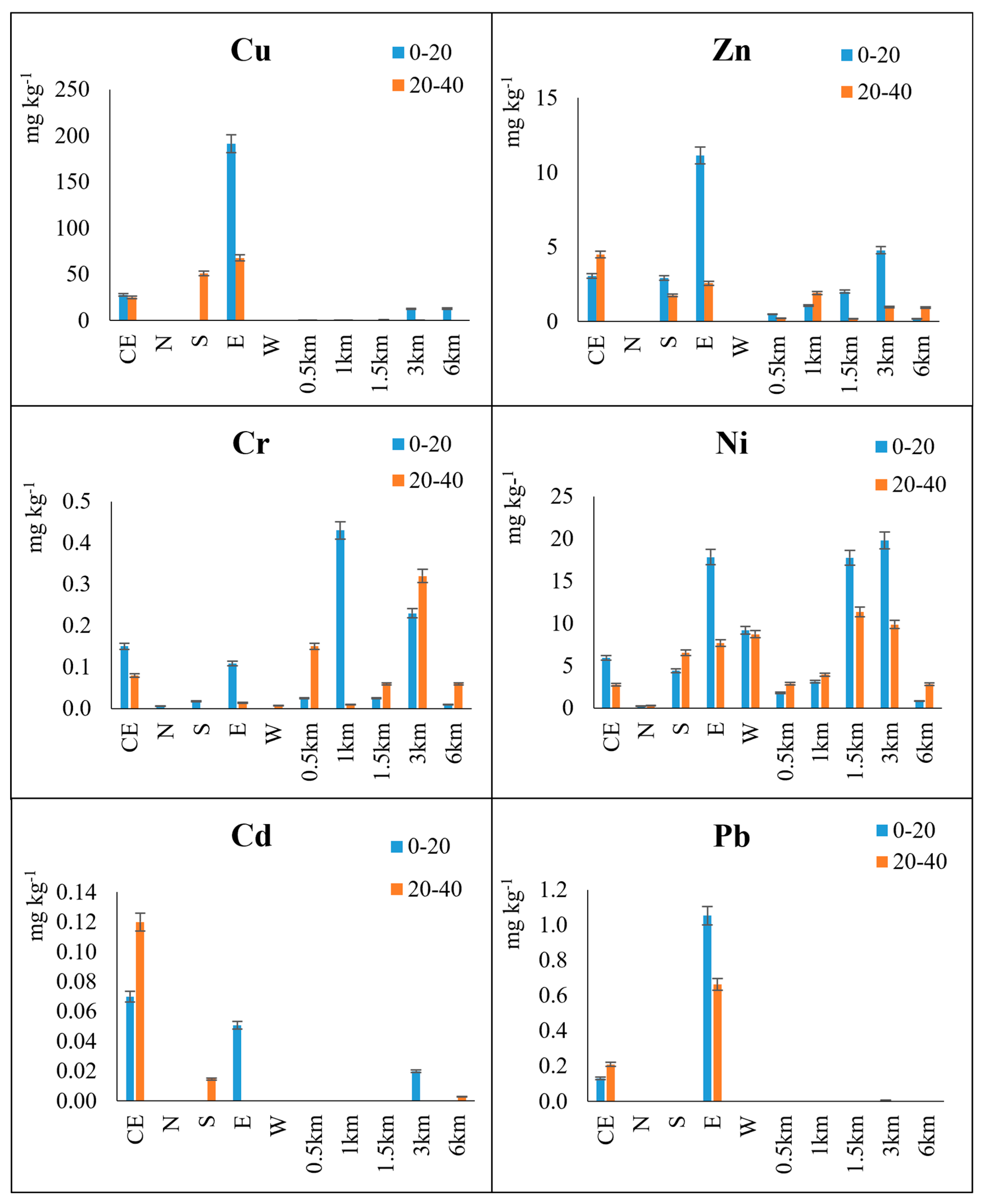
3.2.1. Total Heavy Metal Contents
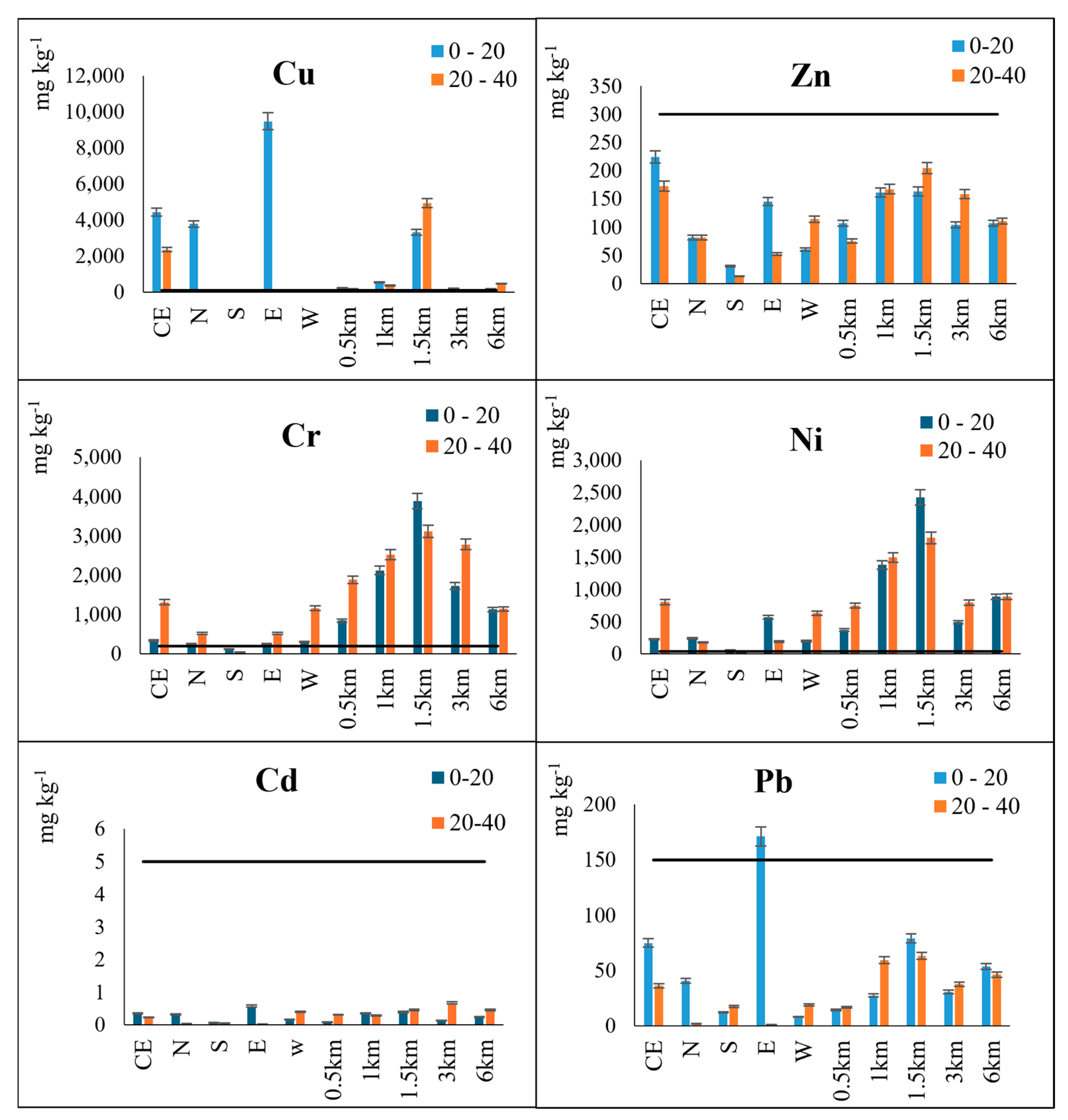
3.2.2. Available Heavy Metal Contents
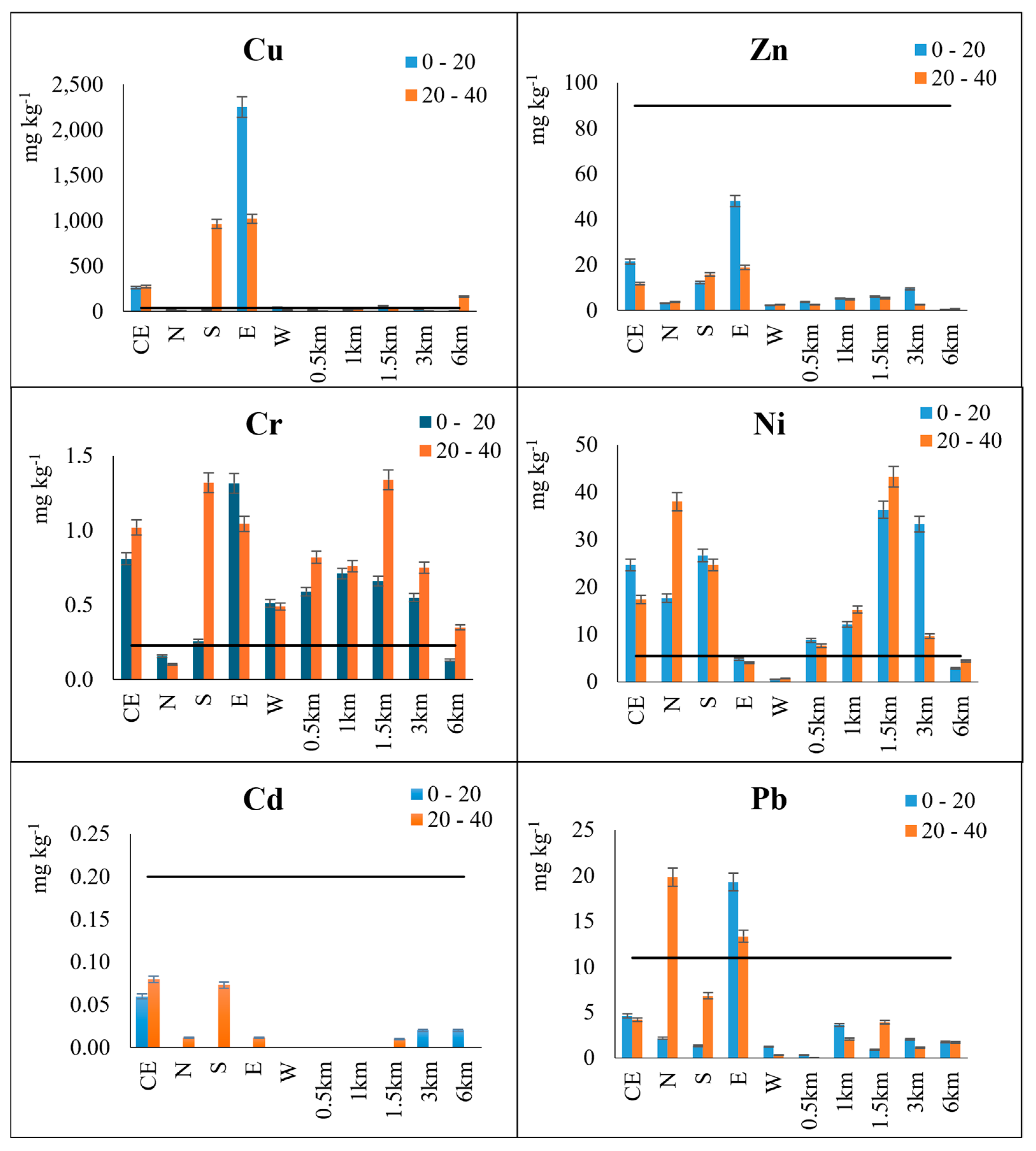
3.2.3. Exchangeable Heavy Metal Contents
3.3. Heavy Metals in Vegetation Samples
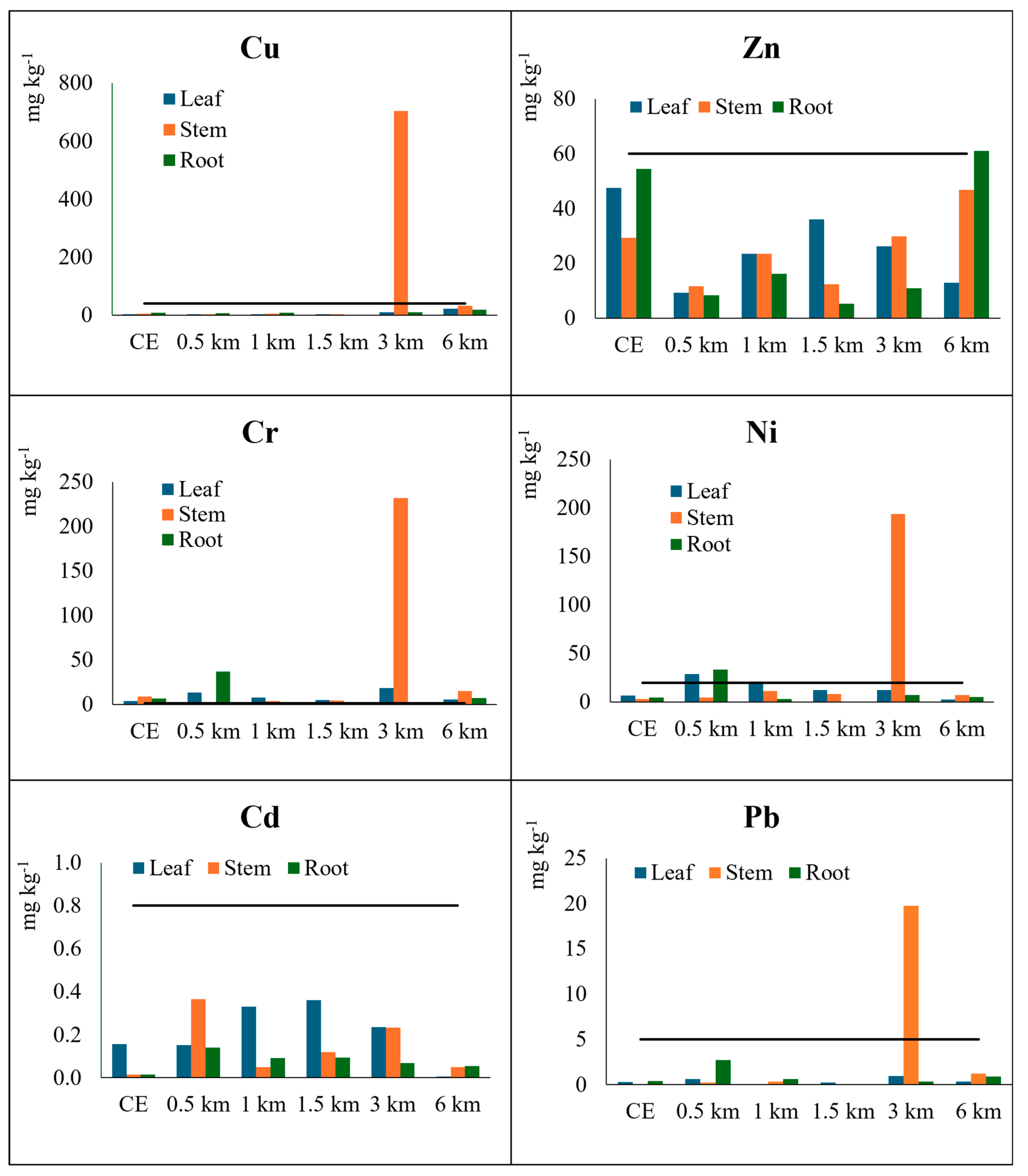
3.4. Assessment of Pollution Risks as a Function of Soil and Plant Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Punia, A. Role of temperature wind and precipitation in heavy metal contamination at copper mines: A review. Environ. Sci. Pollut. Res. 2021, 28, 4056–4072. [Google Scholar] [CrossRef] [PubMed]
- Conesa, H.M.; Schulin, R. The Cartagena–La Unión mining district (SE Spain): A review of environmental problems and emerging phytoremediation solutions after fifteen years research. J. Environ. Monit. 2010, 12, 1225–1233. [Google Scholar] [CrossRef]
- Rivas-Pérez, I.M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Macías, F.; Monterroso, C.; Álvarez-Rodríguez, E. Aluminium fractionation and speciation in a coal mine dump: Twenty years of time-course evolution. Geoderma 2016, 273, 45–53. [Google Scholar] [CrossRef]
- Rivas-Pérez, I.M.; Fernández-Sanjurjo, M.J.; Núñez-Delgado, A.; Monterroso-Martínez, C.; Macías-Vázquez, F.; Álvarez-Rodríguez, E. Efficacy of two different reclamation strategies to improve chemical properties and to reduce Al toxicity in a lignite mine dump during a 20-year period. Land Degrad. Dev. 2019, 30, 658–669. [Google Scholar] [CrossRef]
- Asare, M.O.; Afriyie, J.O. Ancient mining and metallurgy as the origin of Cu, Ag, Pb, Hg, and Zn contamination in soils: A review. Water Air Soil Pollut. 2021, 232, 240. [Google Scholar] [CrossRef]
- Qin, C.; Luo, C.; Chen, Y.; Shen, Z. Spatial-based assessment of metal contamination in agricultural soils near an abandoned copper mine of eastern China. Bull. Environ. Contam. Toxicol. 2012, 89, 113–118. [Google Scholar] [CrossRef]
- Punia, A.; Siddaiah, N.S. Assessment of heavy metal contamination in groundwater of Khetri copper mine region, India and health risk assessment. Asian J. Water Environ. Pollut. 2017, 14, 9–19. [Google Scholar] [CrossRef]
- Palanivel, T.M.; Victor, R. Contamination assessment of heavy metals in the soils of an abandoned copper mine in Lasail, Northern Oman. Int. J. Environ. Stud. 2019, 77, 432–446. [Google Scholar] [CrossRef]
- Cutillas-Barreiro, L.; Pérez-Rodríguez, P.; Gómez-Armesto, A.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Arias-Estévez, M.; Nóvoa-Muñoz, J.C. Lithological and land-use based assessment of heavy metal pollution in soils surrounding a cement plant in SW Europe. Sci. Total Environ. 2016, 562, 179–190. [Google Scholar] [CrossRef]
- Adamo, P.; Agrelli, D.; Zampella, M.; Caporale, A.G. Chapter 10—Chemical speciation to assess bioavailability, bioaccessibility, and geochemical forms of potentially toxic metals (PTMs) in polluted soils. In Environmental Geochemistry, 3rd ed.; De Vivo, B., Belkin, H.E., Lima, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 211–269. [Google Scholar] [CrossRef]
- Álvarez, E.; Fernández Marcos, M.L.; Vaamonde, C.; Fernández-Sanjurjo, M.J. Heavy metals in the dump of an abandoned mine in Galicia (NW Spain) and in the spontaneously occurring vegetation. Sci. Total Environ. 2003, 313, 185–197. [Google Scholar] [CrossRef]
- Mapaure, I.; Chimwamurombe, P.M.; Mapani, B.S.; Kamona, F.A. Impacts of mine dump pollution on plant species diversity, composition and structure of a semiarid savanna in Namibia. Afr. J. Range Forage Sci. 2011, 28, 149–154. [Google Scholar] [CrossRef]
- Ale, T.O.; Ogunribido, T.H.; Ademila, O.; Akingboye, A.S. Soil pollution status due to potentially toxic elements in active open dumpsites: Insights from different Nigerian geological environments. Environ. Earth Sci. 2024, 83, 535. [Google Scholar] [CrossRef]
- Jung, H.; Kim, J.; Lee, J. Seasonal and spatial contributions of sulfate and trace elements in river water in mining districts: Insights from hydrogeochemical and isotopic analysis based on statistical models. J. Hazard. Mat. 2025, 488, 137246. [Google Scholar] [CrossRef] [PubMed]
- Lopes, T.O.M.; Silveira, C.R.D.; Araujo da Silva, J.; Guedes, T.; Tavella, R.A.; Rola, R.C.; Marques, J.A.; Vieira, C.E.D.; Bianchini, A.; Martins, C.D.M.G. A six-year ecotoxicological assessment of the Doce river and coastal marine areas impacted by the Fundão tailings dam failure, Brazil. Environ. Pollut. 2025, 371, 125897. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, M.; Wang, J.; Zhang, Z.; Duan, C.; Wang, X.; Zhao, S.; Bai, X.; Li, Z.; Fang, L. A global meta-analysis of heavy metal(loid)s pollution in soils near copper mines: Evaluation of pollution level and probabilistic health risks. Sci. Total Environ. 2022, 835, 155441. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, B.G.; Kidd, K.A.; Marcarelli, A.M.; Paterson, G.; Walters, D.M. Effects of chronic metal exposure and metamorphosis on the microbiomes of larval and adult insects and riparian spiders through the aquatic-riparian food web. Environ. Pollut. 2025, 371, 125867. [Google Scholar] [CrossRef]
- Schlesinger, M.E.; Sole, K.C.; Davenport, W.G.; Flores, G.R.A. Extractive Metallurgy of Copper; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Monterroso, C.; Macías, F. Prediction of the acid generating potential of coal mining spoils. Int. J. Surf. Mining Reclam. Environ. 1998, 12, 5–9. [Google Scholar] [CrossRef]
- Vriens, B.; Peterson, H.; Laurenzi, L.; Smith, L.; Aranda, C.; Mayer, K.U.; Beckie, R.D. Long-term monitoring of waste-rock weathering at the Antamina mine, Peru. Chemosphere 2019, 215, 858–869. [Google Scholar] [CrossRef]
- Punia, A.; Siddaiah, N.S.; Singh, S.K. Source and assessment of heavy metal pollution at Khetri copper mine tailings and surrounding soil, Rajasthan, India. Bull. Environ. Contam. Toxicol. 2017, 99, 633–641. [Google Scholar] [CrossRef]
- Wu, F.; Liu, Y.; Xia, Y.; Shen, Z.; Chen, Y. Copper contamination of soils and vegetables in the vicinity of Jiuhuashan copper mine, China. Environ. Earth Sci. 2011, 64, 761–769. [Google Scholar] [CrossRef]
- Arroyo-Díaz, F.; Sarmiento-Villagrana, A.; Villegas-Torres, O.G.; Calderón-Segura, M.E.; Aguirre-Noyola, J.L.; Hernández-Castro, E.; Rodríguez-Alviso, C.; Rosas-Acevedo, J.L.; Talavera-Mendoza, O. Elemental partitioning, morpho-physiological effects, genotoxicity, and health risk assessment associated with tomato (Solanum lycopersicum L.) grown in soil contaminated with mining tailings. Environ. Res. 2025, 270, 120939. [Google Scholar] [CrossRef] [PubMed]
- Gitari, M.W.; Akinyemi, S.A.; Ramugondo, L.; Matidza, M.; Mhlongo, S.E. Geochemical fractionation of metals and metalloids in tailings and appraisal of environmental pollution in the abandoned Musina Copper Mine, South Africa. Environ. Geochem. Health 2018, 40, 2421–2439. [Google Scholar] [CrossRef]
- Marove, C.A.; Sotozono, R.; Tangviroon, P.; Tabelin, C.B.; Igarashi, T. Assessment of soil, sediment and water contaminations around open-pit coal mines in Moatize, Tete province, Mozambique. Environ. Adv. 2022, 8, 100215. [Google Scholar] [CrossRef]
- MAE (Ministry of State Administration). Profile of the District of Manica, Province of Manica; MAE: Maputo, Mozambique, 2005; pp. 2–18. [Google Scholar]
- Ruotoistenmäki, T. Geophysical maps and petrophysical data of Mozambique. Geol. Surv. Finl. Spec. Pap. 2008, 48, 65–80. [Google Scholar]
- Sumburane, E.I. Evolução Crustal dos Terrenos Granito Greenstone de Manica Região Centro—Oeste de Moçambique. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2011. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; APHA/AWWA/WPCF, American Public Health Association, American Water Works Association and Water Environmental Federation: Washington, DC, USA, 1998; 1134p. [Google Scholar]
- Peech, L.; Alexander, L.T.; Dean, L.A. Methods of Soil Analysis for Fertility Investigations; U.S. Department of Agriculture: Washington, DC, USA, 1947; pp. 1–25. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; EEUU: Madison, WI, USA, 1982; pp. 403–427. [Google Scholar]
- USEPA. Environmental Sampling and Analytical Methods (ESAM) Program. In Method 3051A: Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils; United States Environmental Protection Agency: Washington, DC, USA, 2007; Revision 1. [Google Scholar]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1985, 15, 1409–1416. [Google Scholar] [CrossRef]
- López Tabar, C. Efecto de la Eficiencia de Distintos Tipos de Manejo de la Cobertura Vegetal en la Retención de agua y la Calidad del Suelo en la Provincia de Cabo Delgado (Mozambique). Bachelor’s Thesis, Universidad de Navarra, Pamplona, Spain, 2011. [Google Scholar]
- Oshunsanya, S. Relevance of Soil pH to Agriculture. In Soil pH for Nutrient Availability and Crop Performance; Oshunsanya, S., Ed.; BoD–Books on Demand: Norderstedt, Germany, 2019. [Google Scholar]
- Covre, W.P.; Ramos, S.J.; Da Silveira Pereira, W.V.; De Souza, E.S.; Martins, G.C.; Teixeira, O.M.M.; Fernandes, A.R. Impact of copper mining wastes in the Amazon: Properties and risks to environment and human health. J. Hazard. Mater. 2022, 421, 126688. [Google Scholar] [CrossRef]
- Buol, S.W.; Sanchez, P.A.; Cate, R.B., Jr.; Granger, M.A. Soil fertility capability classification. In Soil Management in Tropical America: Proceedings of the Seminar, CIAT, Cali, Colombia, 10–14 February 1974; Soil Science Department, North Carolina State University: Raleigh, NC, USA, 1975; pp. 126–141. [Google Scholar]
- Álvarez, E.; Fernández-Sanjurjo, M.J.; Otero, X.L.; Macías, E. Aluminium geochemistry in the bulk and rhizospheric soil of the species colonising an abandoned copper mine in Galicia (NW Spain). J. Soils Sediments 2010, 10, 1236–1245. [Google Scholar] [CrossRef]
- Todorova, E.; Kostadinova, A. Risks of environmental pollution from mining waste from ore-containing copper processing. J. Environ. Prot. Ecol. 2019, 20, 397–403. [Google Scholar]
- Lopes, A.S. Fertilidade de Solo: Manual Internacional de Fertilidade de solo. Tradução e adaptação de Alfredo Scheid Lopes, 2nd ed.; Revisão Ampliada; Potafos: Piracicadas, Brazil, 1989; p. 153. [Google Scholar]
- Domínguez Vivancos, A. Tratado de Fertilización; Mundi Prensa: Madrid, Spain, 1997. [Google Scholar]
- Syers, J.K.; Johnston, A.E.; Curtin, D. Efficiency of soil and fertilizer phosphorus use. FAO Fertil. Plant Nutr. Bull. 2008, 18, 108. [Google Scholar]
- Galán, E.H.; Romero, A.B. Contaminación de suelos por metales pesados. Rev. Soc. Española Mineral. 2008, 10, 48–60. [Google Scholar]
- Manceau, A.; Matynia, A. The nature of Cu bonding to natural organic matter. Geochim. Cosmochim. Acta 2010, 74, 2556–2580. [Google Scholar] [CrossRef]
- Ramírez-Pérez, A.M.; Paradelo, M.; Nóvoa-Muñoz, J.C.; Arias-Estévez, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A. Heavy metal retention in copper mine soil treated with mussel shells: Batch and column experiments. J. Hazard. Mater. 2013, 248–249, 122–130. [Google Scholar] [CrossRef]
- Monterroso, C.; Álvarez, E.; Fernández-Marcos, M.L.; Macías, F. Evaluation of Mehlich 3 reagent as a multielement extractant in mine soils. Land Degrad. Dev. 1999, 10, 35–48. [Google Scholar] [CrossRef]
- Macías, F.; Calvo de Anta, R. Niveles Genéricos de Referencia de Metales Pesados y Otros Elementos Traza en Suelos de Galicia. Xunta de Galicia; Consellería de Medio Ambiente e Desenvolvemento Sostible: Santiago de Compostela, Spain, 2009; 232p. [Google Scholar]
- Dusengemungu, L.; Mubemba, B.; Gwanama, C. Evaluation of heavy metal contamination in copper mine tailing soils of Kitwe and Mufulira, Zambia, for reclamation prospects. Sci. Rep. 2022, 12, 11283. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; Taylor and Francis: Boca Raton, FL, USA, 2011. [Google Scholar]
- Bowie, S.H.U.; Thornton, I. Environmental Geochemistry and Health; Kluwer Academic Publishers: Hingham, MA, USA, 1985. [Google Scholar]
- Belmonte Serrato, F.B.; Díaz, A.R.; Sarría, F.A.; Brotóns, J.M.; López, S.R. Afección de suelos agrícolas por metales pesados en áreas limítrofes a explotaciones mineras del sureste de España. Papeles Geogr. 2010, 51–52, 45–54. [Google Scholar]
- Saha, A.; Gupta, B.S.; Patidar, S.; Martínez-Villegas, N. Evaluation of potential ecological risk index of toxic metals contamination in the soils. Chem. Proc. 2022, 10, 59. [Google Scholar] [CrossRef]
- Tietjen, C. Principal problems of the use of city wastes for crop production and soil conservation. FAO Soils Bull. 1975, 27, 221–226. [Google Scholar]
- Lago-Vila, M.; Rodríguez-Seijo, A.; Arenas-Lago, D.; Andrade, L.; Vega, M.F.A. Heavy metal content and toxicity of mine and quarry soils. J. Soils Sediments 2017, 17, 1331–1348. [Google Scholar] [CrossRef]
- Kierczak, J.; Pietranik, A.; Pędziwiatr, A. Ultramafic geoecosystems as a natural source of Ni, Cr, and Co to the environment: A review. Sci. Total Environ. 2021, 755, 142620. [Google Scholar] [CrossRef]
- Alloway, B.J. (Ed.) Heavy Metals in Soils; John Wiley & Sons: New York, NY, USA, 1990; pp. 1–39. [Google Scholar]
- Brooks, R.R. Serpentine and Its Vegetation, a Multidisciplinary Approach; Croom Helm: London, UK, 1987. [Google Scholar]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; FAO Irrigation and Drainage; Food and Agriculture and Organization: Rome, Italy, 1985; 29p. [Google Scholar]
- Malakar, A.; Snow, D.D.; Chittaranjan, R. Irrigation water quality—A contemporary perspective. Water 2019, 11, 1482–1504. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Drechsel, P.; Keraita, B.; Itanna, F.; Gebrekidan, H. Heavy metal accumulation and health risk assessment in wastewater-irrigated urban vegetable farming sites of Addis Ababa, Ethiopia. Int. J. Food Contam. 2017, 4, 9. [Google Scholar] [CrossRef]
- Abii, T.A.; Okorie, D.O. Assessment of the level of heavy metals (Cu, Pb, Cd and Cr) contamination in four popular vegetables sold in urban and rural markets of Abia State Nigeria. J. Water Air Soil Pollut. 2011, 2, 42–47. [Google Scholar]
- Singh, S.; Kumar, M. Heavy metal load of soil, water and vegetables in peri-urban Delhi. Environ. Monit. Assess. 2006, 120, 79–91. [Google Scholar] [CrossRef]
- Otero, X.L.; Álvarez, E.; Fernández-Sanjurjo, M.J.; Macías, F. Micronutrients and toxic trace metals in the bulk and rhizospheric soil of the spontaneous vegetation at an abandoned copper mine in Galicia (NW Spain). J. Geochem. Explor. 2012, 112, 84–92. [Google Scholar] [CrossRef]
- Nielsen, M.T.; Scott-Fordsmand, J.J.; Murphy, M.W.; Kristiansen, S.M. Speciation and solubility of copper along a soil contamination gradient. J. Soils Sediments 2015, 15, 1558–1570. [Google Scholar] [CrossRef]
- Brunetto, G.; De Melo, G.W.B.; Terzano, R.; Del Buono, D.; Astolfi, S.; Tomasi, N.; Cesco, S. Copper accumulation in vineyard soils: Rhizosphere processes and agronomic practices to limit its toxicity. Chemosphere 2016, 162, 293–307. [Google Scholar] [CrossRef]
- Moreno-Lora, A.; Delgado, A. Factors determining Zn availability and uptake by plants in soils developed under Mediterranean climate. Geoderma 2020, 376, 114509. [Google Scholar] [CrossRef]
- Soares, M.R.; Casagrande, J.C.; Mouta, E.R. Nickel adsorption by variable charge soils: Effect of pH and ionic strength. Braz. Arch. Biol. Technol. 2011, 54, 207–220. [Google Scholar] [CrossRef]
- Cong, L.U.; Yaoguo, W.U.; Sihai, H.U.; Zhang, X.; Yilin, F.U. Distribution and transport of residual lead and copper along soil profiles in a mining region of North China. Pedosphere 2016, 26, 848–860. [Google Scholar]
- Manyiwa, T.; Ultra, V.U.; Rantong, G.; Opaletswe, K.A.; Gabankitse, G.; Taupedi, S.B.; Gajaje, K. Heavy metals in soil, plants, and associated risk on grazing ruminants in the vicinity of Cu–Ni mine in Selebi-Phikwe, Botswana. Environ. Geochem. Health 2022, 44, 1633–1648. [Google Scholar] [CrossRef]
- Ghaderian, S.M.; Ravandi, A.A.G. Accumulation of copper and other heavy metals by plants growing on Sarcheshmeh copper mining area, Iran. J. Geochem. Explor. 2012, 123, 25–32. [Google Scholar] [CrossRef]
- Iwegbue, C.M.A.; Nwajei, G.E.; Ogala, J.E.; Overahm, C.L. Determination of trace metal concentrations in soil profiles of municipal waste dumps in Nigeria. Environ. Geochem. Health 2010, 32, 415–430. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Jiang, C. Heavy metal concentrations in soils and plants in Rongxi Manganese Mine of Chongqing, Southwest of China. Acta Ecol. Sin. 2015, 35, 46–51. [Google Scholar] [CrossRef]
- Farooq, M.; Anwar, F.; Rashid, U. Appraisal of heavy metal contents in different vegetables grown in the vicinity of an industrial area. Pak. J. Bot. 2008, 40, 2099–2106. [Google Scholar]
- Pehoiu, G.; Murarescu, O.; Radulescu, C.; Dulama, I.D.; Teodorescu, S.; Stirbescu, R.M.; Stanescu, S.G. Heavy metals accumulation and translocation in native plants grown on tailing dumps and human health risk. Plant Soil 2020, 456, 405–424. [Google Scholar] [CrossRef]
- Kloke, A.; Sauerbeck, D.R.; Vetter, H. The contamination of plants and soils with heavy metals and the transport of metals in terrestrial food chains. In Changing Metal Cycles and Human Health: Report of the Dahlem Workshop on Changing Metal Cycles and Human Health; Nriagu, J.O., Ed.; Springer: Berlin, Germany, 1984. [Google Scholar]
- Kastori, R.; Petrović, N.; Arsenijević-Maksimović, I. Heavy Metals and Plants; Naučni Institut za Ratarstvo i Povrtarstvo: Novi Sad, Serbia, 1997; pp. 197–225. [Google Scholar]
- Zhu, G.; Xiao, H.; Guo, Q.; Song, B.; Zheng, G.; Zhang, Z.; Zhao, J.; Okoli, C.P. Heavy metal contents and enrichment characteristics of dominant plants in wasteland of the downstream of a lead-zinc mining area in Guangxi, Southwest China. Ecotoxicol. Environ. Saf. 2018, 151, 266–271. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
| Situation in the Dump | Lithological Material | Vegetation (Dominant Species) |
|---|---|---|
| Center | Gneiss | Bauhinia galpinii |
| North | Gneiss | Bauhinia galpinii |
| South | Gneiss | Bauhinia galpinii |
| East | Gneiss | Bauhinia galpinii |
| West | Gneiss | Bauhinia galpinii |
| 0.5 km | Granite | Zingiber officinale |
| 1 km | Granite | Brassica oleracea |
| 1.5 km | Serpentinite | Psidium guajava |
| 3 km | Schist | Phaseolus vulgaris |
| 6 km | Schist | Mangifera indica |
| Sample | Parameter | Method | Equipment |
|---|---|---|---|
| Soils | pH, water | H2O (ratio 1:2.5) | 2202 pH-meter (Crison, Barcelona, Spain) |
| pH, KCl | KCl 0.1 mol L−1 (ratio 1:2.5) | ||
| Total C | - | Autoanalyzer TRUSPEC CHNS (LECO, St. Joseph, MI, USA) | |
| Total N | - | ||
| Texture | Robison pipette | ||
| Exchangeable cations | 1 mol L−1 NH4Cl [30] | - | |
| Effective cation exchange capacity | [30] | - | |
| Available phosphorus | [31] | - | |
| Total Cd, Cr, Cu, Ni, Pb and Zn | Microwave acid digestion EPA method 3051A [32] | ICP-MS 7900 (Agilent, Santa Clara, CA, USA). Detection limit: Cu = 5.4 µg L−1; Zn = 1.8 µg L−1; Ni = 10.0 µg L−1; Pb = 42.0 µg L−1; Cd = 2.7 µg L−1; Cr = 7.1 µg L−1 | |
| Bioavailable Cd, Cr, Cu, Ni, Pb and Zn | Mehlich 3 extracting solution (0.015 mol L−1 NH4F, 0.25 mol L−1 NH4NO3, 0.2 mol L−1 CH3COOH, 0.013 mol L−1 HNO3, 0.001 mol L−1 EDTA) [33] | ||
| Exchangeable Cd, Cr, Cu, Ni, Pb and Zn | 1 mol L−1 NH4Cl | ||
| Plants | Total Fe, Mn, Zn, Cu, Cr, Ni, Pb and Cd | Acid digestion | ICP-MS (Agilent, Santa Clara, CA, USA). |
| Water | pH, water | - | 2202 pH-meter (Crison, Barcelona, Spain) |
| Electrical conductivity | - | 2202 conductimeter (Crison, Barcelona, Spain) | |
| Total Al, Ca, Mg, Na, K Cu, Zn, Cr, Ni, Cd, Pb, Fe, Mn | - | Absorption and emission spectrometry, ICP-OES (Agilent, Santa Clara, CA, USA). |
| Depth (cm) | Dump Zone | Distance of Soil Sampling Point from the Dump (km) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CE | N | S | E | W | 0.5 | 1 | 1.5 | 3 | 6 | ||
| Sand (%) | 0–20 | 19.1 | 20.2 | 29.6 | 27.4 | 41.7 | 31.6 | 44.8 | 18.8 | 17.8 | 7.6 |
| 20–40 | 16.1 | 18.9 | 26.3 | 25.1 | 42.1 | 19.8 | 52.8 | 24.8 | 22.7 | 7.7 | |
| Clay (%) | 0–20 | 75 | 78.1 | 69.1 | 71.1 | 57.6 | 62 | 53.1 | 81.4 | 81.9 | 90.4 |
| 20–40 | 78.4 | 79.7 | 72.4 | 73.3 | 55.7 | 72.3 | 45.4 | 73.3 | 74 | 90.4 | |
| OM (%) | 0–20 | 3.04 | 4.15 | 2.92 | 4.28 | 3.5 | 1.86 | 1.82 | 1.97 | 2.81 | 1.49 |
| 20–40 | 2.07 | 2.3 | 2.29 | 3.94 | 2.24 | 1.01 | 1.34 | 1.57 | 1.22 | 1.51 | |
| pH H2O | 0–20 | 5.4 | 5.4 | 5.2 | 6.2 | 5.5 | 6.2 | 6.2 | 6 | 6 | 6.3 |
| 20–40 | 6 | 5.3 | 6.5 | 6.4 | 6.3 | 6.1 | 6.4 | 6.2 | 5.6 | 5.9 | |
| pH KCl | 0–20 | 4.5 | 5.3 | 5.2 | 6.5 | 4.8 | 5.1 | 5.5 | 4.9 | 5 | 5.3 |
| 20–40 | 5.2 | 5.2 | 6.6 | 6.2 | 5.1 | 4.8 | 5.3 | 5.1 | 4.8 | 4.6 | |
| Ca | 0–20 | 5.09 | 6.97 | 4.72 | 5.96 | 3.76 | 0.66 | 1.38 | 1.24 | 1.31 | 1.04 |
| 20–40 | 4.25 | 6.64 | 5.61 | 5.62 | 2.59 | 0.3 | 0.92 | 0.62 | 0.4 | 1.04 | |
| Mg | 0–20 | 3.92 | 12.78 | 4.32 | 3.52 | 6.1 | 1.43 | 0.83 | 2.45 | 1.23 | 0.72 |
| 20–40 | 3.48 | 12.84 | 2.4 | 3.35 | 6.09 | 1.68 | 0.8 | 1 | 1.64 | 0.83 | |
| Na | 0–20 | 0.14 | 0.12 | 0.14 | 0.13 | 0.18 | 0.05 | 0.06 | 0.02 | 0.03 | 0.06 |
| 20–40 | 0.13 | 0.28 | 0.15 | 0.17 | 0.24 | 0.04 | 0.05 | 0.02 | 0.04 | 0.045 | |
| K | 0–20 | 0.44 | 0.37 | 1.08 | 0.28 | 0.21 | 0.09 | 0.2 | 0.08 | 0.13 | 0.11 |
| 20–40 | 0.28 | 0.23 | 0.15 | 0.15 | 0.13 | 0.04 | 0.1 | 0.07 | 0.07 | 0.06 | |
| Al | 0–20 | 0.00 | 0.00 | 0.02 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 20–40 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.13 | 0.02 | |
| eCEC | 0–20 | 19.5 | 0.36 | 0.31 | 0.09 | 0.54 | 1.01 | 1.67 | 0.22 | 1.26 | 0.45 |
| 20–40 | 3.9 | 0.19 | 0.12 | 0.14 | 0.07 | 0.75 | 0.87 | 0.29 | 1.28 | 0.53 | |
| P | 0–20 | 29.09 | 20.6 | 10.6 | 9.98 | 10.79 | 3.24 | 4.14 | 4.01 | 3.96 | 2.38 |
| 20–40 | 12.04 | 20.18 | 8.43 | 9.43 | 9.12 | 2.82 | 2.74 | 2 | 3.56 | 2.52 | |
| In the Dump | Semiquantitative (%) |
|---|---|
| Talc | 27 |
| Kaolinite | 15 |
| Microcline | 15 |
| Clinochlore | 12 |
| Hornblende | 11 |
| Albite | 8 |
| Quartz | 8 |
| Biotite | 4 |
| Distance: 1 km | |
| Quartz | 42 |
| Albite | 13 |
| Talc | 12 |
| Clinochlore | 7 |
| Microcline | 7 |
| Biotite | 7 |
| Antigorite | 7 |
| Hornblende | 5 |
| Gibbsite | 2 |
| Distance: 6 km | |
| Quartz | 68 |
| Kaolinite | 12 |
| Albite | 8 |
| Talc | 7 |
| Biotite | 4 |
| pH | 8.37 |
| EC (dS m−1) | 299 |
| Ca (mg L−1) | 12.09 |
| Mg (mg L−1) | 35.90 |
| Na (mg L−1) | 2.390 |
| K (mg L−1) | 1.07 |
| Al (µg L−1) | 77.10 |
| Cu (µg L−1) | 186.88 |
| Zn (µg L−1) | 7.10 |
| Cr (µg L−1) | 8.21 |
| Ni (µg L−1) | 116.55 |
| Cd (µg L−1) | 0.11 |
| Pb (µg L−1) | 0.07 |
| R2 | ||
|---|---|---|
| Cu | pH | 0.095 |
| pH, eCEC | 0.225 | |
| Zn | eCEC | 0.152 |
| eCEC, pH | 0.267 | |
| Cr | MO | 0.123 |
| Ni | MO | 0.092 |
| MO, eCEC | 0.189 | |
| MO, eCEC, pH | 0.318 | |
| Pb | Clay | 0.159 |
| Clay, MO | 0.214 |
| CE | N | S | E | W | 0.5 km | 1 km | 1.5 km | 3 km | 6 km | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | ||||||||||
| Cu | ||||||||||
| Zn | ||||||||||
| Cr | ||||||||||
| Ni | ||||||||||
| Cd | ||||||||||
| Pb | ||||||||||
| Available | ||||||||||
| Cu | ||||||||||
| Zn | ||||||||||
| Cr | ||||||||||
| Ni | ||||||||||
| Cd | ||||||||||
| Pb | ||||||||||
| CE | 0.5 km | 1 km | 1.5 km | 3 km | 6 km | |
|---|---|---|---|---|---|---|
| Root | ||||||
| Cu | ||||||
| Zn | ||||||
| Cr | ||||||
| Ni | ||||||
| Cd | ||||||
| Pb | ||||||
| Stem | ||||||
| Cu | ||||||
| Zn | ||||||
| Cr | ||||||
| Ni | ||||||
| Cd | ||||||
| Pb | ||||||
| Leaf | ||||||
| Cu | ||||||
| Zn | ||||||
| Cr | ||||||
| Ni | ||||||
| Cd | ||||||
| Pb | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Savaio, S.; Barreiro, A.; Núñez-Delgado, A.; Suluda, A.; Álvarez-Rodríguez, E.; Fernández-Sanjurjo, M.J. Heavy Metal Pollution in a Cu Mine Dump and in Close Agricultural Soils and Crops in Mozambique. Processes 2025, 13, 902. https://doi.org/10.3390/pr13030902
dos Santos Savaio S, Barreiro A, Núñez-Delgado A, Suluda A, Álvarez-Rodríguez E, Fernández-Sanjurjo MJ. Heavy Metal Pollution in a Cu Mine Dump and in Close Agricultural Soils and Crops in Mozambique. Processes. 2025; 13(3):902. https://doi.org/10.3390/pr13030902
Chicago/Turabian Styledos Santos Savaio, Severino, Ana Barreiro, Avelino Núñez-Delgado, Antonio Suluda, Esperanza Álvarez-Rodríguez, and María J. Fernández-Sanjurjo. 2025. "Heavy Metal Pollution in a Cu Mine Dump and in Close Agricultural Soils and Crops in Mozambique" Processes 13, no. 3: 902. https://doi.org/10.3390/pr13030902
APA Styledos Santos Savaio, S., Barreiro, A., Núñez-Delgado, A., Suluda, A., Álvarez-Rodríguez, E., & Fernández-Sanjurjo, M. J. (2025). Heavy Metal Pollution in a Cu Mine Dump and in Close Agricultural Soils and Crops in Mozambique. Processes, 13(3), 902. https://doi.org/10.3390/pr13030902











