Topical and Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Inflammation and Pain: Current Trends and Future Directions in Delivery Systems
Abstract
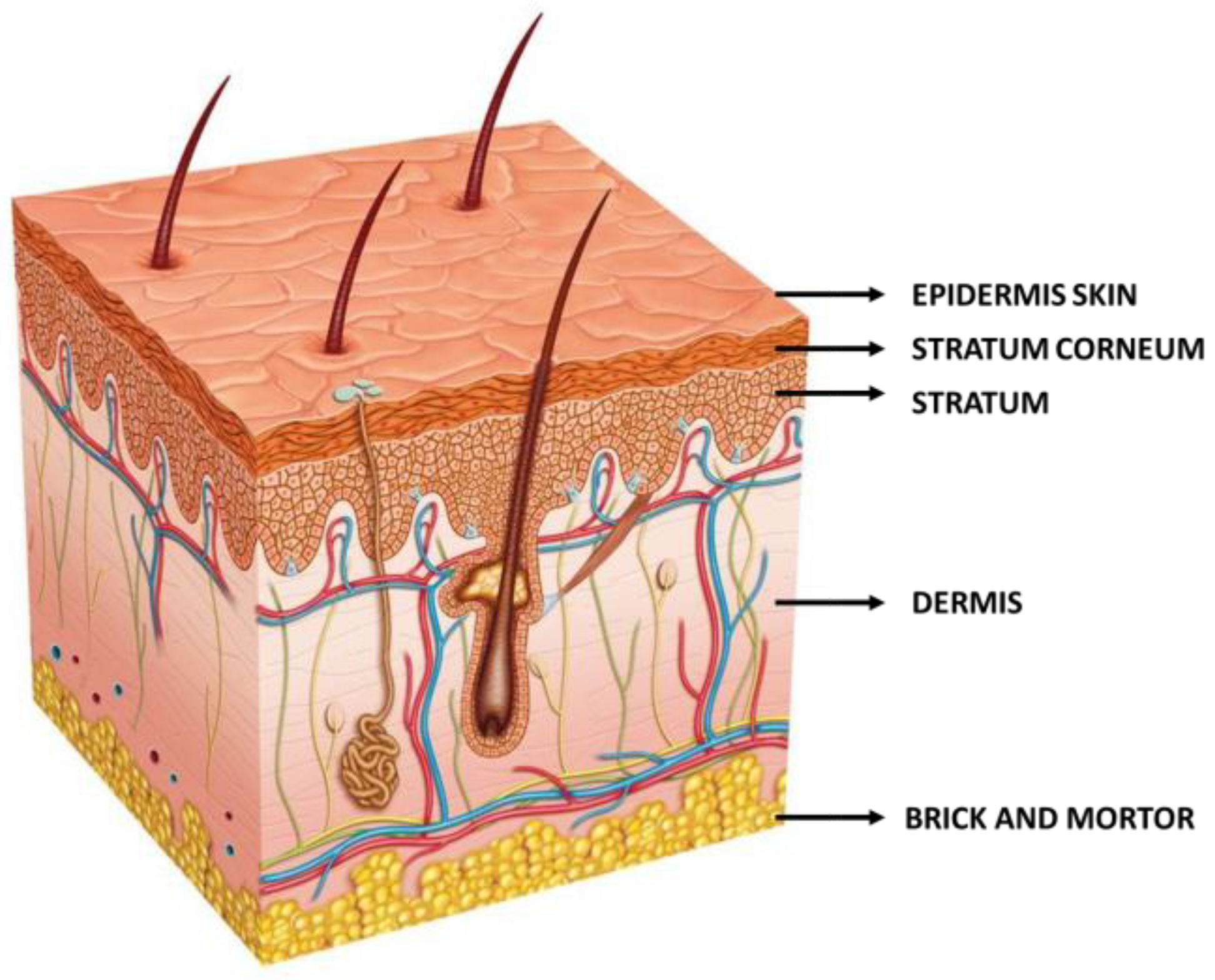
:1. Introduction
- i.
- The intercellular, which enables a winding penetration, like a “zig-zag” route, through the lipids located between corneocyte cells.
- ii.
- The transcellular, which allows penetration through the keratinized corneocytes.
- iii.
- The intrafollicular, which enables drug passage via hair follicles.
- iv.
- The polar, meaning through pores with polar properties located between cells and encased by polar lipids.
2. Materials and Methods
3. Processes for Increasing the Efficacy of NSAIDs Through Topical and Transdermal Applications
3.1. NSAIDs
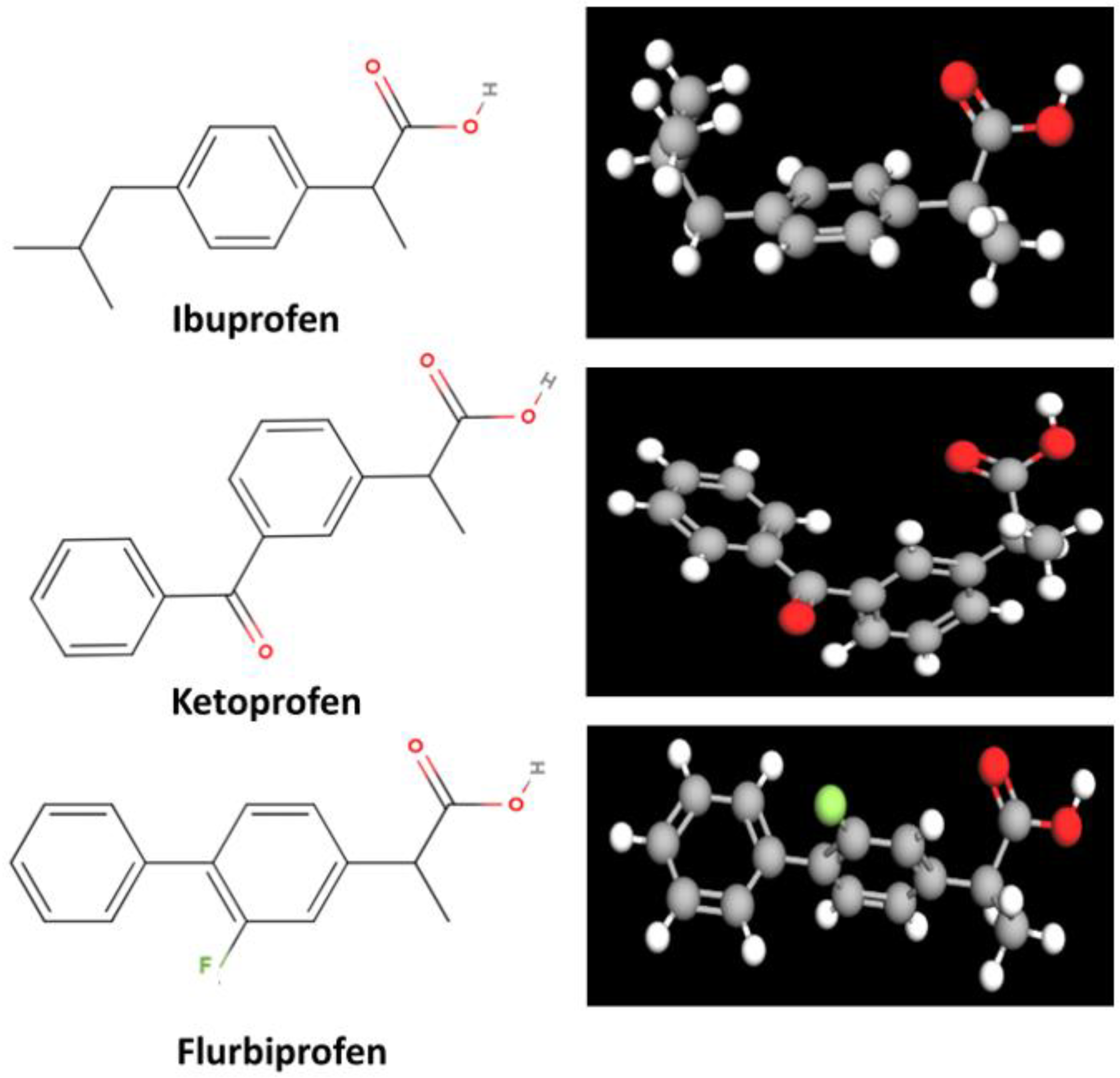
3.1.1. Ibuprofen
3.1.2. Ketoprofen
3.1.3. Flurbiprofen
| Drug | Skin Permeability | Anti-Inflammatory Efficacy | Optical Formulation | References |
|---|---|---|---|---|
| IBU | Variable; formulation-dependent | Effective; varies with formulation | Gel/Spray/Mousse | [38] |
| KTP | High | Superior | Patch/Gel | [54] |
| FB | Moderate to high; effective deeper tissue penetration | Effective | Patch | [58] |
| Drug | General Side Effects | Topical Related Side Effects | References |
|---|---|---|---|
| IBU | Gastrointestinal issues | Impaired wound healing, infection, reduced collagen synthesis | [19,31,32] |
| Suppressed local immune responses | [19,31] | ||
| KTP | Rare systemic adverse events | Photosensitivity reactions | [39,41,50] |
| Gastrointestinal disorders | Chronic dermatitis in rare cases | [51,52] | |
| FB | Gastrointestinal discomfort | Poor skin permeability limits systemic absorption | [55,57] |
| Limited topical effectiveness due to low permeability | [57] |
| Drug | Local Skin Reactions | Systemic Absorption and Toxicity | Delayed Wound Healing and Skin Barrier Damage | Allergic Reactions | Potential Drug Interactions | References |
|---|---|---|---|---|---|---|
| IBU | Mild irritation, redness, and itching | Can lead to renal impairment, especially in prolonged use or high doses | May slow down skin repair due to prostaglandin inhibition | Possible cross-reactivity in aspirin-sensitive individuals | Can interact with anticoagulants | [19,31,32] |
| KTP | Higher risk of photosensitivity reactions | Risk of kidney and liver toxicity in patients with preexisting conditions | Can impair wound healing and cause dryness or sensitization | Higher chance of photoallergic reactions, leading to long-term skin sensitivity | Risk of bleeding and kidney damage when combined with other NSAIDs | [39,41,50,51,52] |
| FB | Moderate irritation; risk of allergic contact dermatitis | Higher cardiovascular risks, linked to hypertension, heart attack, and stroke | Moderate risk of skin barrier disruption | May cause hypersensitivity reactions | Can interfere with blood pressure medications and increase cardiovascular strain | [55,57] |
3.2. Hydrogels
3.3. Topical and Transdermal Drug Delivery
3.3.1. Topical Drug Delivery
3.3.2. Transdermal Drug Delivery
3.4. Enhancement of Skin Permeability
3.4.1. Physical Enhancement Techniques
Iontophoresis
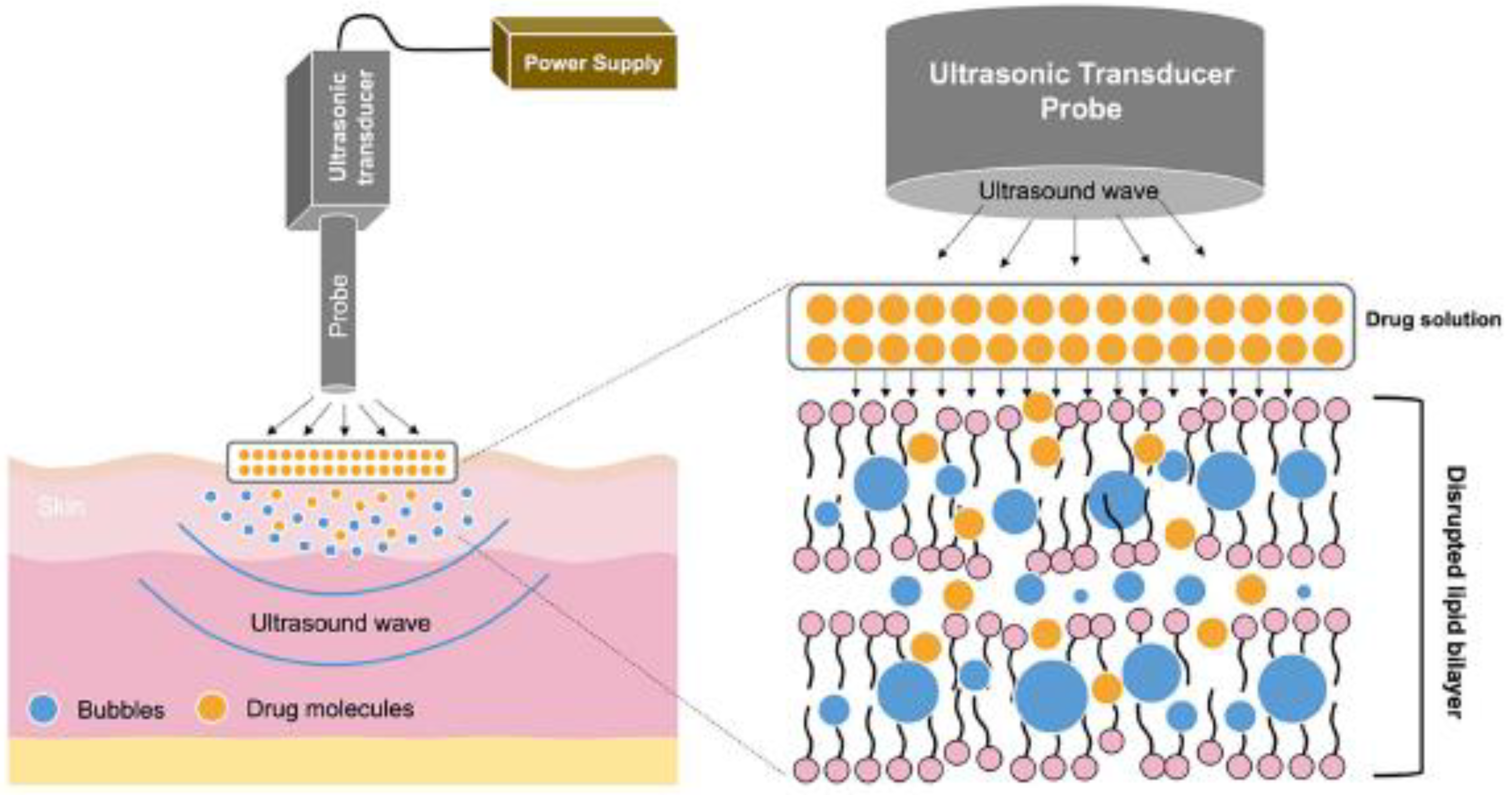
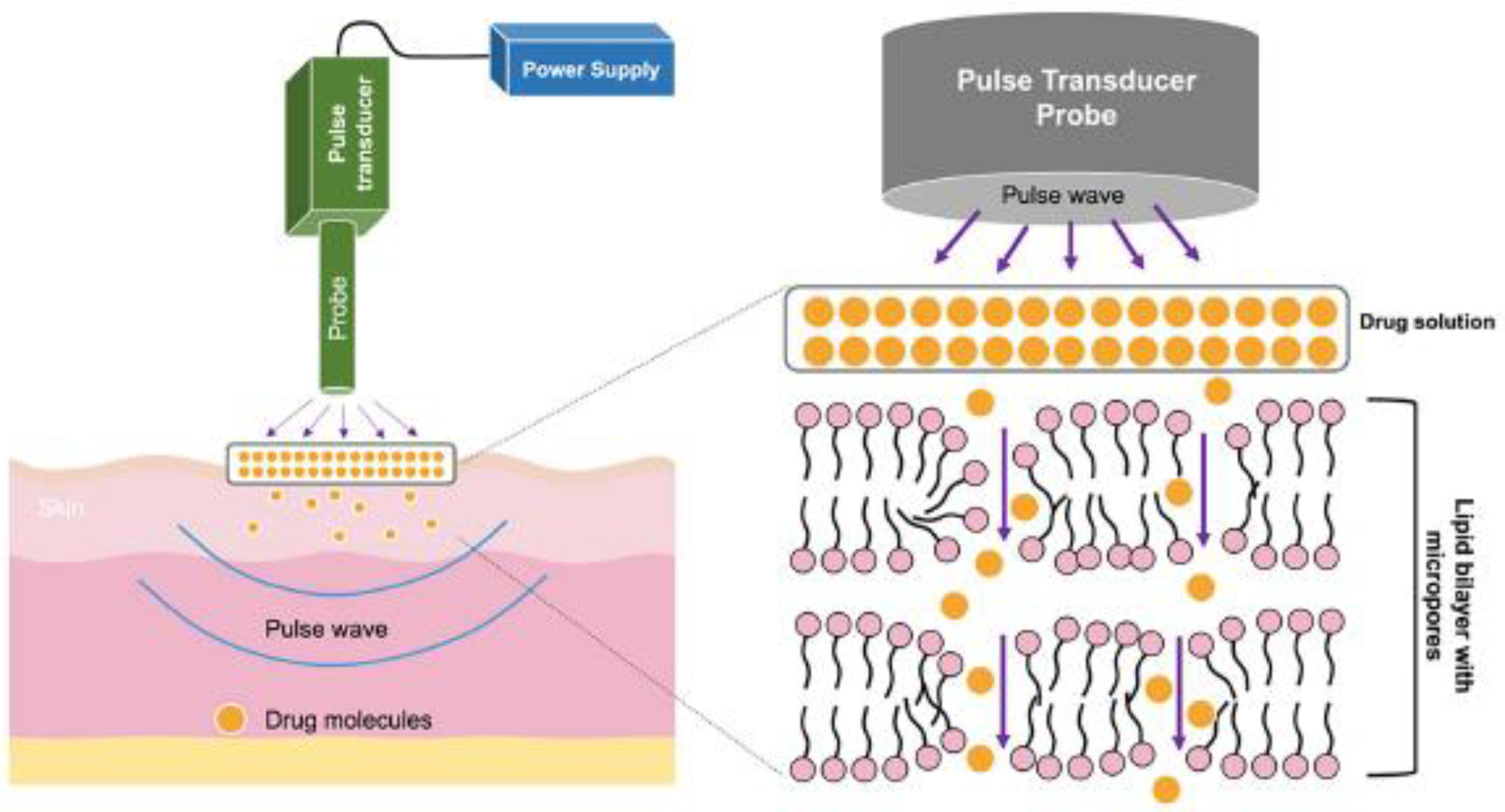
Sonophoresis
Electroporation
3.4.2. Chemical Enhancement Techniques
Chemical Penetration Enhancers
Solid Lipid Nanoparticles (SLNs)
Vesicles
Polymeric Nanoparticles (PNPTs)
Nanoemulsions (NEs)
4. Clinical Trials
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NSAIDs | Nonsteroidal Anti-Inflammatory Drugs |
| SC | Stratum Corneum |
| COX | Cyclooxygenase |
| PGs | Prostaglandins |
| IBU | Ibuprofen |
| KTP | Ketoprofen |
| FB | Flurbiprofen |
| TDD | Transdermal Drug Delivery |
| CPEs | Chemical Penetration Enhancers |
| PG | Propylene Glycol |
| PEG | Polyethylene Glycol |
| NLCs | Nanostructured Lipid Carriers |
| SLNs | Solid Lipid Nanoparticles |
| PNPTs | Polymeric Nanoparticles |
| NEs | Nanoemulsions |
| NBs | Nanobubbles |
| HA | Hyaluronic Acid |
References
- Schaefer, H.; Redelmeier, T.E. Skin Barrier: Principles of Percutaneous Absorption; Karger: Basel, Switzerland, 1996. [Google Scholar]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials Based on Chitin and Chitosan in Wound Dressing Applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Marks, R. The Stratum Corneum Barrier: The Final Frontier. J. Nutr. 2004, 134, 2017S–2021S. [Google Scholar] [CrossRef]
- Elias, P.M. Epidermal Lipids, Barrier Function, and Desquamation. J. Investig. Dermatol. 1983, 80, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Scheuplein, R.J.; Blank, I.H. Permeability of the Skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef]
- Pouillot, A.; Dayan, N.; Polla, A.S.; Polla, L.L.; Polla, B.S. The Stratum Corneum: A Double Paradox. J. Cosmet. Dermatol. 2008, 7, 143–148. [Google Scholar] [CrossRef]
- Barry, B.W. Lipid-Protein-Partitioning Theory of Skin Penetration Enhancement. J. Control. Release 1991, 15, 237–248. [Google Scholar] [CrossRef]
- Barry, B.W. Novel Mechanisms and Devices to Enable Successful Transdermal Drug Delivery. Eur. J. Pharm. Sci. 2001, 14, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Wokovich, A.M.; Prodduturi, S.; Doub, W.H.; Hussain, A.S.; Buhse, L.F. Transdermal Drug Delivery System (TDDS) Adhesion as a Critical Safety, Efficacy and Quality Attribute. Eur. J. Pharm. Biopharm. 2006, 64, 1–8. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Langer, R. Transdermal Drug Delivery. Nat. Biotechnol. 2008, 26, 1261–1268. [Google Scholar] [CrossRef]
- Alexander, A.; Dwivedi, S.; Ajazuddin; Giri, T.K.; Saraf, S.; Saraf, S.; Tripathi, D.K. Approaches for Breaking the Barriers of Drug Permeation through Transdermal Drug Delivery. J. Control. Release 2012, 164, 26–40. [Google Scholar] [CrossRef]
- Nastiti, C.; Ponto, T.; Abd, E.; Grice, J.; Benson, H.; Roberts, M. Topical Nano and Microemulsions for Skin Delivery. Pharmaceutics 2017, 9, 37. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.; Mohammed, Y.; Pastore, M.; Namjoshi, S.; Yousef, S.; Alinaghi, A.; Haridass, I.; Abd, E.; Leite-Silva, V.; Benson, H.; et al. Topical and Cutaneous Delivery Using Nanosystems. J. Control. Release 2017, 247, 86–105. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical Applications of Hydrogels: A Review of Patents and Commercial Products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Ahsan, A.; Tian, W.-X.; Farooq, M.A.; Khan, D.H. An Overview of Hydrogels and Their Role in Transdermal Drug Delivery. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 574–584. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; Tomic-Canic, M. PERSPECTIVE ARTICLE: Growth Factors and Cytokines in Wound Healing. Wound Repair Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Nathan, C. Points of Control in Inflammation. Nature 2002, 420, 846–852. [Google Scholar] [CrossRef]
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef]
- Anderson, K.; Hamm, R.L. Factors That Impair Wound Healing. J. Am. Coll. Clin. Wound Spec. 2012, 4, 84–91. [Google Scholar] [CrossRef]
- Elias, P.M.; Wood, L.C.; Feingold, K.R. Epidermal Pathogenesis of Inflammatory Dermatoses. Am. J. Contact Dermat. 1999, 10, 119–126. [Google Scholar] [CrossRef]
- Fukumoto, A.; Tajima, K.; Hori, M.; Toda, Y.; Kaku, S.; Matsumoto, H. Analgesic Effect of S (+)-Flurbiprofen Plaster in a Rat Model of Knee Arthritis: Analysis of Gait and Synovial Fluid Prostaglandin E2 Levels. J. Pharm. Pharmacol. 2018, 70, 929–936. [Google Scholar] [CrossRef]
- Irvine, J.; Afrose, A.; Islam, N. Formulation and Delivery Strategies of Ibuprofen: Challenges and Opportunities. Drug Dev. Ind. Pharm. 2018, 44, 173–183. [Google Scholar] [CrossRef] [PubMed]
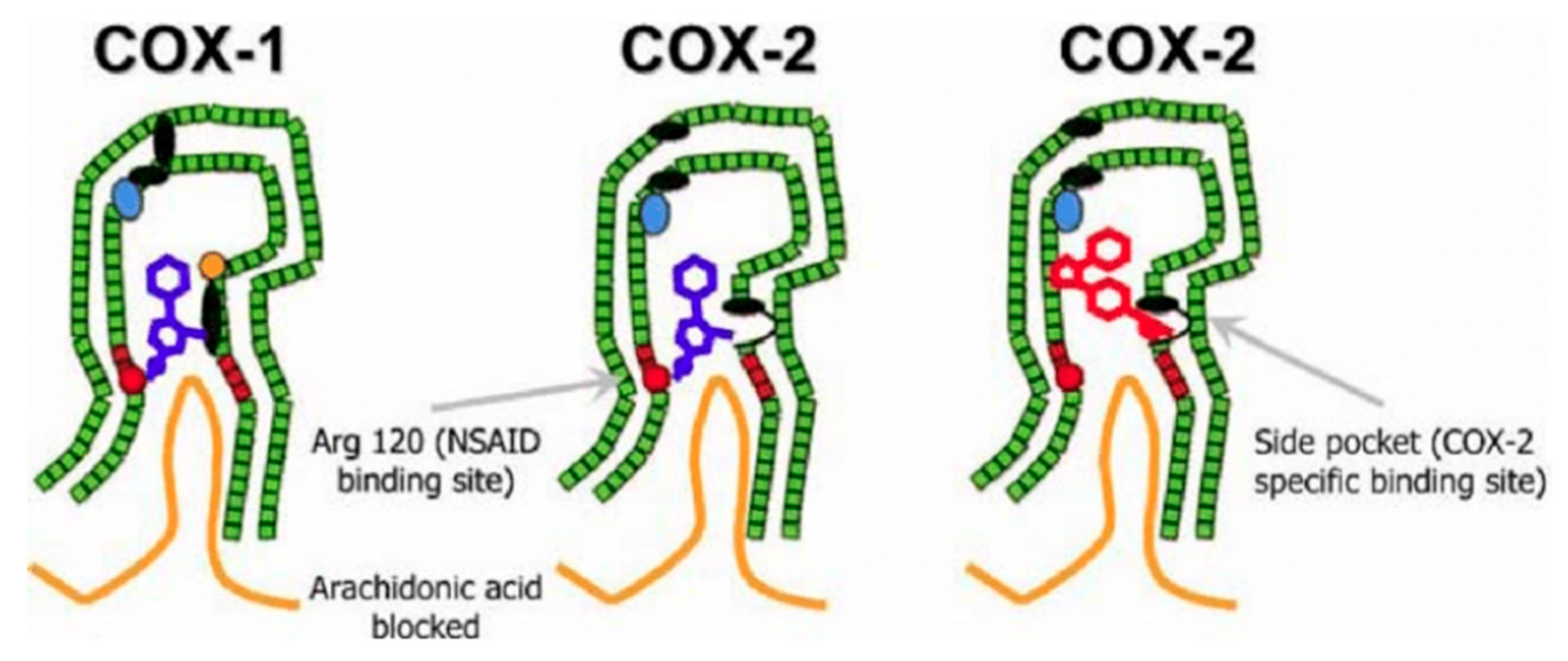
- DuBois, R.N.; Abramson, S.B.; Crofford, L.; Gupta, R.A.; Simon, L.S.; van de Putte, L.B.A.; Lipsky, P.E. Cyclooxygenase in Biology and Disease. FASEB J. 1998, 12, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Kuczyńska, J.; Nieradko-Iwanicka, B. Future Prospects of Ketoprofen in Improving the Safety of the Gastric Mucosa. Biomed. Pharmacother. 2021, 139, 111608. [Google Scholar] [CrossRef] [PubMed]
- Warner, T.D.; Giuliano, F.; Vojnovic, I.; Bukasa, A.; Mitchell, J.A.; Vane, J.R. Nonsteroid Drug Selectivities for Cyclo-Oxygenase-1 Rather than Cyclo-Oxygenase-2 Are Associated with Human Gastrointestinal Toxicity: A Full in Vitro Analysis. Proc. Natl. Acad. Sci. USA 1999, 96, 7563–7568. [Google Scholar] [CrossRef]
- Yuan, C.; Sidhu, R.S.; Kuklev, D.V.; Kado, Y.; Wada, M.; Song, I.; Smith, W.L. Cyclooxygenase Allosterism, Fatty Acid-Mediated Cross-Talk between Monomers of Cyclooxygenase Homodimers. J. Biol. Chem. 2009, 284, 10046–10055. [Google Scholar] [CrossRef]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and Inflammation. ATVB 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Meek, I.L.; Van de Laar, M.A.; Vonkeman, H.E. Non-Steroidal Anti-Inflammatory Drugs: An Overview of Cardiovascular Risks. Pharmaceuticals 2010, 3, 2146–2162. [Google Scholar] [CrossRef]
- Sánchez-Borges, M.; Capriles-Hulett, A.; Caballero-Fonseca, F. Risk of Skin Reactions When Using Ibuprofen-Based Medicines. Expert. Opin. Drug Saf. 2005, 4, 837–848. [Google Scholar] [CrossRef]
- Noize, P.; Bénard-Laribière, A.; Aulois-Griot, M.; Moore, N.; Miremont-Salamé, G.; Haramburu, F. Cutaneous Adverse Effects of Ketoprofen for Topical Use. Am. J. Clin. Dermatol. 2010, 11, 131–136. [Google Scholar] [CrossRef]
- Busti, A.J.; Hooper, J.S.; Amaya, C.J.; Kazi, S. Effects of Perioperative Antiinflammatory and Immunomodulating Therapy on Surgical Wound Healing. Pharmacotherapy 2005, 25, 1566–1591. [Google Scholar] [CrossRef]
- Ulbrich, H.; Dannhardt, G. A heterogenous drug class. NSAID: Classification and spectrum of action. Pharm. Unserer Zeit 2002, 31, 146–154. [Google Scholar] [CrossRef]
- Patel, A.; Bell, M.; O’Connor, C.; Inchley, A.; Wibawa, J.; Lane, M.E. Delivery of Ibuprofen to the Skin. Int. J. Pharm. 2013, 457, 9–13. [Google Scholar] [CrossRef]
- Seth, P.L. Percutaneous Absorption of Ibuprofen from Different Formulations. Comparative Study with Gel, Hydrophilic Ointment and Emulsion Cream. Arzneimittelforschung 1993, 43, 919–921. [Google Scholar]
- Berner, G.; Engels, B.; Vögtle-Junkert, U. Percutaneous Ibuprofen Therapy with Trauma-Dolgit Gel: Bioequivalence Studies. Drugs Exp. Clin. Res. 1989, 15, 559–564. [Google Scholar] [PubMed]
- Tegeder, I.; Muth-Selbach, U.; Lötsch, J.; Rüsing, G.; Oelkers, R.; Brune, K.; Meller, S.; Kelm, G.R.; Sörgel, F.; Geisslinger, G. Application of Microdialysis for the Determination of Muscle and Subcutaneous Tissue Concentrations after Oral and Topical Ibuprofen Administration. Clin. Pharmacol. Ther. 1999, 65, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.R.; Carter, W.C. (Eds.) Ibuprofen: Clinical Pharmacology, Medical Uses and Adverse Effects; Pharmacology-research, safety testing and regulation; Nova Science Publishers, Inc.: New York, NY, USA, 2013; ISBN 978-1-62618-660-6. [Google Scholar]
- Hadgraft, J.; Whitefield, M.; Rosher, P.H. Skin Penetration of Topical Formulations of Ibuprofen 5%: An in Vitro Comparative Study. Ski. Pharmacol. Physiol. 2003, 16, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Lhiaubet, V.; Montastruc, J.L.; Chouini-Lalanne, N. Photosensitivity to Ketoprofen. Drug-Safety 2000, 22, 339–349. [Google Scholar] [CrossRef]
- Carbone, C.; Rende, P.; Comberiati, P.; Carnovale, D.; Mammí, M.; De Sarro, G. The Safety of Ketoprofen in Different Ages. J. Pharmacol. Pharmacother. 2013, 4, S99–S103. [Google Scholar] [CrossRef]
- Coaccioli, S. Ketoprofen 2.5% Gel: A Clinical Overview. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 943–949. [Google Scholar]
- Rafanan, B.S., Jr.; Valdecañas, B.F.; Lim, B.P.; Malairungsakul, A.; Tassanawipas, W.; Shiyi, C.; Tse, L.F.; Luong, T.K. Consensus Recommendations for Managing Osteoarthritic Pain with Topical NSAIDs in Asia-Pacific. Pain Manag. 2018, 8, 115–128. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, J.; Meng, X.; Cui, Y.; Zhu, T.; Sun, F.; Li, Y.; Teng, L. Polyketal Nanoparticles Co-Loaded With miR-124 and Ketoprofen for Treatment of Rheumatoid Arthritis. J. Pharm. Sci. 2021, 110, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Atzeni, F.; Masala, I.F.; Bagnasco, M.; Lanata, L.; Mantelli, F.; Sarzi-Puttini, P. Comparison of Efficacy of Ketoprofen and Ibuprofen in Treating Pain in Patients with Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Pain Ther. 2021, 10, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, S.; Zhang, M.; He, B. Targeted Eco-Pharmacovigilance for Ketoprofen in the Environment: Need, Strategy and Challenge. Chemosphere 2018, 194, 450–462. [Google Scholar] [CrossRef]
- Veys, E.M. 20 Years’ Experience with Ketoprofen. Scand. J. Rheumatol. Suppl. 1991, 19 (Suppl. S90), 3–44. [Google Scholar] [CrossRef]
- Altman, R.; Barkin, R.L. Topical Therapy for Osteoarthritis: Clinical and Pharmacologic Perspectives. Postgrad. Med. 2009, 121, 139–147. [Google Scholar] [CrossRef]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dinçer, F.; Dziedzic, K.; Häuselmann, H.J.; Herrero-Beaumont, G.; et al. EULAR Evidence Based Recommendations for the Management of Hand Osteoarthritis: Report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann. Rheum. Dis. 2007, 66, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Kantor, T.G. Ketoprofen: A Review of Its Pharmacologic and Clinical Properties. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1986, 6, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Lahoz, A.; Hernández, D.; Miranda, M.A.; Pérez-Prieto, J.; Morera, I.M.; Castell, J.V. Antibodies Directed to Drug Epitopes to Investigate the Structure of Drug−Protein Photoadducts. Recognition of a Common Photobound Substructure in Tiaprofenic Acid/Ketoprofen Cross-Photoreactivity. Chem. Res. Toxicol. 2001, 14, 1486–1491. [Google Scholar] [CrossRef]
- Hindsén, M.; Isaksson, M.; Persson, L.; Zimersson, E.; Bruze, M. Photoallergic Contact Dermatitis from Ketoprofen Induced by Drug-Contaminated Personal Objects. J. Am. Acad. Dermatol. 2004, 50, 215–219. [Google Scholar] [CrossRef]
- Matthieu, L.; Meuleman, L.; Van Hecke, E.; Blondeel, A.; Dezfoulian, B.; Constandt, L.; Goossens, A. Contact and Photocontact Allergy to Ketoprofen. The Belgian Experience. Contact Dermat. 2004, 50, 238–241. [Google Scholar] [CrossRef]
- Sarzi- Puttini, P.; Atzeni, F.; Lanata, L.; Bagnasco, M.; Colombo, M.; Fischer, F.; D’Imporzano, M. Pain and Ketoprofen: What Is Its Role in Clinical Practice? Reumatismo 2011, 62, 172–188. [Google Scholar] [CrossRef]
- Komatsu, T.; Sakurada, T. Comparison of the Efficacy and Skin Permeability of Topical NSAID Preparations Used in Europe. Eur. J. Pharm. Sci. 2012, 47, 890–895. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, K.; Anbu, J.; Ravichandiran, V.; Venkateswarlu, V.; Rao, Y.M. Lipid Nanoparticles for Transdermal Delivery of Flurbiprofen: Formulation, in Vitro, Ex Vivo and in Vivo Studies. Lipids Health Dis. 2009, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A.; E-Rabbani, M.; Akhtar, M.F.; Akhtar, B.; Saleem, A.; Farzana, K.; Usman, A.; Murtaza, G. Design and Evaluation of Modified Release Bilayer Tablets of Flurbiprofen. Adv. Clin. Exp. Med. 2011, 20, 343–349. [Google Scholar]
- Morimoto, Y.; Hatanaka, T.; Sugibayashi, K.; Omiya, H. Prediction of Skin Permeability of Drugs: Comparison of Human and Hairless Rat Skin. J. Pharm. Pharmacol. 1992, 44, 634–639. [Google Scholar] [CrossRef]
- Goi, N.; Morishita, K.; Taniguchi, A.; Ishii, T.; Saitoh, K. Evaluation of Percutaneous Permeation of Flurbiprofen and Ketoprofen after Application of Transdermal Patches Using a Lateral Sectioning Approach in Hairless Rats. Pharm. Dev. Technol. 2010, 15, 658–665. [Google Scholar] [CrossRef]
- Arakelova, E. New Drug Delivery System for Cancer Therapy. Int. J. Med. Sci. Eng. 2013, 7, 1075–1080. [Google Scholar]
- Tibbitt, M.W.; Dahlman, J.E.; Langer, R. ChemInform Abstract: Emerging Frontiers in Drug Delivery. ChemInform 2016, 47. [Google Scholar] [CrossRef]
- Mohan, Y.M.; Premkumar, T.; Lee, K.; Geckeler, K.E. Fabrication of Silver Nanoparticles in Hydrogel Networks. Macromol. Rapid Commun. 2006, 27, 1346–1354. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R. Designing Hydrogels for Controlled Drug Delivery; MDPI: Basel, Switzerland, 2020; ISBN 978-3-03928-357-6. [Google Scholar]
- Corrente, F.; Matricardi, P.; Paolicelli, P.; Tita, B.; Vitali, F.; Casadei, M.A. Physical Carboxymethylscleroglu-can/Calcium Ion Hydrogels as Modified Drug Delivery Systems in Topical Formulations. Molecules 2009, 14, 2684–2698. [Google Scholar] [CrossRef]
- Mahmood, S.; Almurisi, S.H.; AL-Japairai, K.; Hilles, A.R.; Alelwani, W.; Bannunah, A.M.; Alshammari, F.; Alheibshy, F. Ibuprofen-Loaded Chitosan–Lipid Nanoconjugate Hydrogel with Gum Arabic: Green Synthesis, Characterisation, In Vitro Kinetics Mechanistic Release Study and PGE2 Production Test. Gels 2021, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Mikušová, V.; Ferková, J.; Žigrayová, D.; Krchňák, D.; Mikuš, P. Comparative Study of Polysaccharide-Based Hydrogels: Rheological and Texture Properties and Ibuprofen Release. Gels 2022, 8, 168. [Google Scholar] [CrossRef] [PubMed]
- Mohseni, M.; Shokrollahi, P.; Barzin, J. Gelatin/O-Carboxymethyl Chitosan Injectable Self-Healing Hydrogels for Ibuprofen and Naproxen Dual Release. Int. J. Biol. Macromol. 2024, 263, 130266. [Google Scholar] [CrossRef]
- Chen, Y.; Quan, P.; Liu, X.; Wang, M.; Fang, L. Novel Chemical Permeation Enhancers for Transdermal Drug Delivery. Asian J. Pharm. Sci. 2014, 9, 51–64. [Google Scholar] [CrossRef]
- Escobar-Chávez, J.J.; Díaz-Torres, R.; Rodríguez-Cruz, I.M.; Domínguez-Delgado, C.L.; Morales, R.S.; Ángeles-Anguiano, E.; Melgoza-Contreras, L.M. Nanocarriers for Transdermal Drug Delivery. RRTD 2012, 1, 3–17. [Google Scholar] [CrossRef]
- Bolla, P.K.; Meraz, C.A.; Rodriguez, V.A.; Deaguero, I.; Singh, M.; Yellepeddi, V.K.; Renukuntla, J. Clotrimazole Loaded Ufosomes for Topical Delivery: Formulation Development and In-Vitro Studies. Molecules 2019, 24, 3139. [Google Scholar] [CrossRef]
- Cheruvu, H.S.; Liu, X.; Grice, J.E.; Roberts, M.S. Modeling Percutaneous Absorption for Successful Drug Discovery and Development. Expert. Opin. Drug Discov. 2020, 15, 1181–1198. [Google Scholar] [CrossRef]
- Roy, S.D.; Gutierrez, M.; Flynn, G.L.; Cleary, G.W. Controlled Transdermal Delivery of Fentanyl: Characterizations of Pressure-Sensitive Adhesives for Matrix Patch Design. J. Pharm. Sci. 1996, 85, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, V.; Yadav, P. Transdermal Drug Delivery System: An Overview. Asian J. Pharm. 2012, 6, 161. [Google Scholar] [CrossRef]
- Zhai, Y.; Zhai, G. Advances in Lipid-Based Colloid Systems as Drug Carrier for Topic Delivery. J. Control. Release 2014, 193, 90–99. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef] [PubMed]
- Richard, C.; Cassel, S.; Blanzat, M. Vesicular Systems for Dermal and Transdermal Drug Delivery. RSC Adv. 2020, 11, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Jain, V.; Mahajan, S.C. Lipid Based Vesicular Drug Delivery Systems. Adv. Pharm. 2014, 2014, 574673. [Google Scholar] [CrossRef]
- Ramadon, D.; McCrudden, M.T.C.; Courtenay, A.J.; Donnelly, R.F. Enhancement Strategies for Transdermal Drug Delivery Systems: Current Trends and Applications. Drug Deliv. Transl. Res. 2022, 12, 758–791. [Google Scholar] [CrossRef]
- Jasim, S. Innovative Strategies for Enhancing Topical and Transdermal Drug Delivery. AACE Clin. Case Rep. 2021, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Dhote, V.; Bhatnagar, P.; Mishra, P.K.; Mahajan, S.C.; Mishra, D.K. Iontophoresis: A Potential Emergence of a Transdermal Drug Delivery System. Sci. Pharm. 2012, 80, 1. [Google Scholar] [CrossRef]
- Zuo, J.; Du, L.; Li, M.; Liu, B.; Zhu, W.; Jin, Y. Transdermal Enhancement Effect and Mechanism of Iontophoresis for Non-Steroidal Anti-Inflammatory Drugs. Int. J. Pharm. 2014, 466, 76–82. [Google Scholar] [CrossRef]
- Dhal, S.; Pal, K.; Giri, S. Transdermal Delivery of Gold Nanoparticles by a Soybean Oil-Based Oleogel under Iontophoresis. ACS Appl. Bio Mater. 2020, 3, 7029–7039. [Google Scholar] [CrossRef]
- Vranić, E. Iontophoresis: Fundamentals, Developments and Application. Bosn. J. Basic. Med. Sci. 2003, 3, 54–58. [Google Scholar] [CrossRef]
- Nguyen, H.X.; Banga, A.K. Electrically and Ultrasonically Enhanced Transdermal Delivery of Methotrexate. Pharmaceutics 2018, 10, 117. [Google Scholar] [CrossRef]
- Park, D.; Song, G.; Jo, Y.; Won, J.; Son, T.; Cha, O.; Kim, J.; Jung, B.; Park, H.; Kim, C.-W.; et al. Sonophoresis Using Ultrasound Contrast Agents: Dependence on Concentration. PLoS ONE 2016, 11, e0157707. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Rani, A.; Singh, V.; Murari, B.M. Prospects and Limitations of Non-Invasive Blood Glucose Monitoring Using near-Infrared Spectroscopy. Biomed. Signal Process. Control 2015, 18, 214–227. [Google Scholar] [CrossRef]
- Herwadkar, A.; Sachdeva, V.; Taylor, L.F.; Silver, H.; Banga, A.K. Low Frequency Sonophoresis Mediated Transdermal and Intradermal Delivery of Ketoprofen. Int. J. Pharm. 2012, 423, 289–296. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, L.; Li, R.; Pang, L.; Zhu, S.; Ma, J.; Du, L.; Jin, Y. Electroporation-Enhanced Transdermal Drug Delivery: Effects of logP, pKa, Solubility and Penetration Time. Eur. J. Pharm. Sci. 2020, 151, 105410. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kwon, M.; Choi, H.E.; Kim, K.S. Recent Advances in Transdermal Drug Delivery Systems: A Review. Biomater. Res. 2021, 25, 24. [Google Scholar] [CrossRef] [PubMed]
- Stanekzai, A.; Sudhakar, C.K.; Zhakfar, A.M.; Karan, V.S. Recent Approaches in Transdermal Drug Delivery System. Rese. Jour. Pharm. Technol. 2019, 12, 4550. [Google Scholar] [CrossRef]
- Lane, M.E. Skin Penetration Enhancers. Int. J. Pharm. 2013, 447, 12–21. [Google Scholar] [CrossRef]
- Karande, P.; Mitragotri, S. Enhancement of Transdermal Drug Delivery via Synergistic Action of Chemicals. Biochim. Biophys. Acta (BBA)—Biomembr. 2009, 1788, 2362–2373. [Google Scholar] [CrossRef]
- Williams, A.C.; Barry, B.W. Penetration Enhancers. Adv. Drug Deliv. Rev. 2012, 64, 128–137. [Google Scholar] [CrossRef]
- Herkenne, C.; Naik, A.; Kalia, Y.N.; Hadgraft, J.; Guy, R.H. Effect of Propylene Glycol on Ibuprofen Absorption into Human Skin in Vivo. J. Pharm. Sci. 2008, 97, 185–197. [Google Scholar] [CrossRef]
- Bednarczyk, P.; Nowak, A.; Duchnik, W.; Kucharski, Ł.; Ossowicz-Rupniewska, P. Enhancing Transdermal Delivery: Investigating the Impact of Permeation Promoters on Ibuprofen Release and Penetration from Medical Patches—In Vitro Research. IJMS 2023, 24, 15632. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, R.K.; Murtaza, G.; Imran, M.; Khan, S.A.; Mir, S.; Khan, A.K.; Azhar, S.; Mehmood, Z.; Sajjad, A.; Shah, S.N.H. Formulation and Permeation Kinetic Studies of Flurbiprofen Gel. Trop. J. Pharm. Res. 2015, 14, 195–203. [Google Scholar] [CrossRef]
- González-Mira, E.; Nikolić, S.; García, M.L.; Egea, M.A.; Souto, E.B.; Calpena, A.C. Potential Use of Nanostructured Lipid Carriers for Topical Delivery of Flurbiprofen. J. Pharm. Sci. 2011, 100, 242–251. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Hwang, T.-L.; Fang, C.-L.; Chiu, H.-C. In Vitro and in Vivo Evaluations of the Efficacy and Safety of Skin Permeation Enhancers Using Flurbiprofen as a Model Drug. Int. J. Pharm. 2003, 255, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Panchaxari Gadad, A.; Patil, A.S.; Singh, Y.; Mallappa Dandagi, P.; Bolmal, U.B.; Basu, A. Development and Evaluation of Flurbiprofen Loaded Transethosomes to Improve Transdermal Delivery. IJPER 2020, 54, 954–962. [Google Scholar] [CrossRef]
- Wissing, S.A.; Müller, R.H. Cosmetic Applications for Solid Lipid Nanoparticles (SLN). Int. J. Pharm. 2003, 254, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Dingler, A.; Krohs, S.; Lukowski, G.; Gohla, S.; Müller, R.H. SLN (Solid Lipid Nanoparticles) as New Solid Carrier of Active Ingredients in Cosmetics. Eur. J. Pharm. Sci. 1996, 4, S148. [Google Scholar] [CrossRef]
- Geszke-Moritz, M.; Moritz, M. Solid Lipid Nanoparticles as Attractive Drug Vehicles: Composition, Properties and Therapeutic Strategies. Mater. Sci. Eng. C 2016, 68, 982–994. [Google Scholar] [CrossRef]
- Roy, E.; Pallandre, A.; Zribi, B.; Horny, M.C.; Delapierre, F.D.; Cattoni, A.; Gamby, J.; Haghiri-Gosnet, A.M. Overview of Materials for Microfluidic Applications. In Advances in Microfluidics—New Applications in Biology, Energy, and Materials Sciences; IntechOpen: London, UK, 2016; ISBN 978-953-51-2786-4. [Google Scholar]
- Hou, D.; Xie, C.; Huang, K.; Zhu, C. The Production and Characteristics of Solid Lipid Nanoparticles (SLNs). Biomaterials 2003, 24, 1781–1785. [Google Scholar] [CrossRef]
- Zoabi, A.; Touitou, E.; Margulis, K. Recent Advances in Nanomaterials for Dermal and Transdermal Applications. Colloids Interfaces 2021, 5, 18. [Google Scholar] [CrossRef]
- Mancini, G.; Gonçalves, L.M.D.; Marto, J.; Carvalho, F.A.; Simões, S.; Ribeiro, H.M.; Almeida, A.J. Increased Therapeutic Efficacy of SLN Containing Etofenamate and Ibuprofen in Topical Treatment of Inflammation. Pharmaceutics 2021, 13, 328. [Google Scholar] [CrossRef]
- Hung, C.-F.; Chen, W.-Y.; Hsu, C.-Y.; Aljuffali, I.A.; Shih, H.-C.; Fang, J.-Y. Cutaneous Penetration of Soft Nanoparticles via Photodamaged Skin: Lipid-Based and Polymer-Based Nanocarriers for Drug Delivery. Eur. J. Pharm. Biopharm. 2015, 94, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wen, J.; Sharma, M. Solid Lipid Nanoparticles for Topical Drug Delivery: Mechanisms, Dosage Form Perspectives, and Translational Status. Curr. Pharm. Des. 2020, 26, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Beloqui, A.; Solinís, M.Á.; Rodríguez-Gascón, A.; Almeida, A.J.; Préat, V. Nanostructured Lipid Carriers: Promising Drug Delivery Systems for Future Clinics. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Uner, M.; Damgali, S.; Ozdemir, S.; Celik, B. Therapeutic Potential of Drug Delivery by Means of Lipid Nanoparticles: Reality or İllusion? Curr. Pharm. Des. 2017, 23, 6573–6591. [Google Scholar] [CrossRef]
- Khurana, S.; Bedi, P.M.S.; Jain, N.K. Preparation and Evaluation of Solid Lipid Nanoparticles Based Nanogel for Dermal Delivery of Meloxicam. Chem. Phys. Lipids 2013, 175–176, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Gumustas, M.; Sengel-Turk, C.T.; Badilli, U.; Amasya, G.; Ozkan, S.A.; Tarimci, N. Optimization of Stability Indicating LC Method for the Sensitive In Vitro Determination from Solid Lipid Nanoparticles and Ex Vivo Analysis from Rat Skin of Etofenamate. Curr. Pharm. Anal. 2017, 13, 63–71. [Google Scholar] [CrossRef]
- Pham, C.V.; Van, M.C.; Thi, H.P.; Thanh, C.Đ.; Ngoc, B.T.; Van, B.N.; Le Thien, G.; Van, B.N.; Nguyen, C.N. Development of Ibuprofen-Loaded Solid Lipid Nanoparticle-Based Hydrogels for Enhanced in Vitro Dermal Permeation and in Vivo Topical Anti-Inflammatory Activity. J. Drug Deliv. Sci. Technol. 2020, 57, 101758. [Google Scholar] [CrossRef]
- Burki, F.A.; Shah, K.U.; Razaque, G.; Shah, S.U.; Nawaz, A.; Saeed, M.D.; Rehman, M.U.; Bibi, H.; Alfatama, M.; Elsayed, T.M. Optimization of Chitosan-Decorated Solid Lipid Nanoparticles for Improved Flurbiprofen Transdermal Delivery. ACS Omega 2023, 8, 19302–19310. [Google Scholar] [CrossRef]
- Bagde, A.; Kouagou, E.; Singh, M. Formulation of Topical Flurbiprofen Solid Lipid Nanoparticle Gel Formulation Using Hot Melt Extrusion Technique. AAPS PharmSciTech 2022, 23, 257. [Google Scholar] [CrossRef]
- Opatha, S.A.T.; Titapiwatanakun, V.; Chutoprapat, R. Transfersomes: A Promising Nanoencapsulation Technique for Transdermal Drug Delivery. Pharmaceutics 2020, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid Vesicles: A Versatile Drug Delivery Platform for Dermal and Transdermal Applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Bakhshayesh, A.R.D.; Ha, J.W.; Hamishehkar, H.; Kim, K.H. Invasome: A Novel Nanocarrier for Transdermal Drug Delivery. Nanomaterials 2020, 10, 341. [Google Scholar] [CrossRef]
- Sudhakar, K.; Fuloria, S.; Subramaniyan, V.; Sathasivam, K.V.; Azad, A.K.; Swain, S.S.; Sekar, M.; Karupiah, S.; Porwal, O.; Sahoo, A.; et al. Ultraflexible Liposome Nanocargo as a Dermal and Transdermal Drug Delivery System. Nanomaterials 2021, 11, 2557. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Sandeep, K.; Pandey, D.; Dutta, R.K. Liposomes for Drug Delivery. J. Biotechnol. Biomater. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, Y.; Meng, Y.; Wu, L.; Chen, J.; Cao, W.; Chu, X. Liposome and Microemulsion Loaded with Ibuprofen: From Preparation to Mechanism of Drug Transport. J. Microencapsul. 2022, 39, 539–551. [Google Scholar] [CrossRef]
- Kumar, T.M.; Reddy, V.A.; Kumar, N.P.; Nivedita, J.R.; Shravani, J.; Rajeshwari, J.; Mounika, K.L.; Kumar, K.M.; Vishnuvardhan, K. Formulation & Evaluation of Flubriprofen Drug Loaded Liposomes. JIDPTS 2024, 7, 26–31. [Google Scholar]
- Rajan, R.; Jose, S.; Mukund, V.P.B.; Vasudevan, D.T. Transferosomes—A Vesicular Transdermal Delivery System for Enhanced Drug Permeation. J. Adv. Pharm. Technol. Res. 2011, 2, 138. [Google Scholar] [CrossRef]
- Lymberopoulos, A.; Demopoulou, C.; Kyriazi, M.; Katsarou, M.; Demertzis, N.; Hatziandoniou, S.; Maswadeh, H.; Papaioanou, G.; Demetzos, C.; Maibach, H.; et al. Liposome Percutaneous Penetration in Vivo. Toxicol. Res. Appl. 2017, 1, 2397847317723196. [Google Scholar] [CrossRef]
- Cevc, G.; Blume, G. Lipid Vesicles Penetrate into Intact Skin Owing to the Transdermal Osmotic Gradients and Hydration Force. Biochim. Biophys. Acta (BBA)—Biomembr. 1992, 1104, 226–232. [Google Scholar] [CrossRef]
- Cevc, G.; Schätzlein, A.; Richardsen, H. Ultradeformable Lipid Vesicles Can Penetrate the Skin and Other Semi-Permeable Barriers Unfragmented. Evidence from Double Label CLSM Experiments and Direct Size Measurements. Biochim. Biophys. Acta (BBA)—Biomembr. 2002, 1564, 21–30. [Google Scholar] [CrossRef]
- Rai, S.; Pandey, V.; Rai, G. Transfersomes as Versatile and Flexible Nano-Vesicular Carriers in Skin Cancer Therapy: The State of the Art. Nano Rev. Exp. 2017, 8, 1325708. [Google Scholar] [CrossRef]
- Rother, M.; Lavins, B.J.; Kneer, W.; Lehnhardt, K.; Seidel, E.J.; Mazgareanu, S. Efficacy and Safety of Epicutaneous Ketoprofen in Transfersome (IDEA-033) versus Oral Celecoxib and Placebo in Osteoarthritis of the Knee: Multicentre Randomised Controlled Trial. Ann. Rheum. Dis. 2007, 66, 1178–1183. [Google Scholar] [CrossRef] [PubMed]
- Vashist, A.; Vashist, A.; Gupta, Y.K.; Ahmad, S. Recent Advances in Hydrogel Based Drug Delivery Systems for the Human Body. J. Mater. Chem. B 2013, 2, 147–166. [Google Scholar] [CrossRef]
- Zhang, Z.; Tsai, P.; Ramezanli, T.; Michniak-Kohn, B.B. Polymeric Nanoparticles-based Topical Delivery Systems for the Treatment of Dermatological Diseases. WIREs Nanomed. Nanobiotechnol. 2013, 5, 205–218. [Google Scholar] [CrossRef]
- Suriya Prabha, A.; Dorothy, R.; Jancirani, S.; Rajendran, S.; Singh, G.; Senthil Kumaran, S. Chapter 7—Recent Advances in the Study of Toxicity of Polymer-Based Nanomaterials. In Nanotoxicity; Rajendran, S., Mukherjee, A., Nguyen, T.A., Godugu, C., Shukla, R.K., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–165. ISBN 978-0-12-819943-5. [Google Scholar]
- Al-Nemrawi, N.; Nimrawi, S. A Novel Formulation of Chitosan Nanoparticles Functionalized with Titanium Dioxide Nanoparticles. J. Adv. Pharm. Technol. Res. 2021, 12, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for Biomedical and Healthcare Applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Dhal, S.; Gavara, R.R.; Pal, K.; Banerjee, I.; Mishra, M.; Giri, S. Facile Transdermal Delivery of Upconversion Nanoparticle by Iontophoresis-Responsive Magneto-Upconversion Oleogel. Nano Ex. 2020, 1, 010012. [Google Scholar] [CrossRef]
- Jeong, W.Y.; Kim, S.; Lee, S.Y.; Lee, H.; Han, D.W.; Yang, S.Y.; Kim, K.S. Transdermal Delivery of Minoxidil Using HA-PLGA Nanoparticles for the Treatment in Alopecia. Biomater. Res. 2019, 23, 16. [Google Scholar] [CrossRef]
- Jijie, R.; Barras, A.; Boukherroub, R.; Szunerits, S. Nanomaterials for Transdermal Drug Delivery: Beyond the State of the Art of Liposomal Structures. J. Mater. Chem. B 2017, 5, 8653–8675. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, J.T.; Barua, S.; Yoo, S.-Y.; Hong, S.-C.; Lee, K.B.; Lee, J. Seeking Better Topical Delivery Technologies of Moisturizing Agents for Enhanced Skin Moisturization. Expert Opin. Drug Deliv. 2018, 15, 17–31. [Google Scholar] [CrossRef]
- Solans, C.; Izquierdo, P.; Nolla, J.; Azemar, N.; Garcia-Celma, M.J. Nano-Emulsions. Curr. Opin. Colloid Interface Sci. 2005, 10, 102–110. [Google Scholar] [CrossRef]
- Sinha, P.; Srivastava, S.; Mishra, N.; Singh, D.K.; Luqman, S.; Chanda, D.; Yadav, N.P. Development, Optimization, and Characterization of a Novel Tea Tree Oil Nanogel Using Response Surface Methodology. Drug Dev. Ind. Pharm. 2016, 42, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Zhang, Y.; Wen, N.; Sun, Z.; Li, C.; Zhang, B. W/O Nanoemulsion-Based Intranasal Drug Delivery System of Panax Notoginseng Saponins for Brain Targeting. J. Control. Release 2015, 213, e11. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, F.; Shafiq, S.; Haq, N.; Alanazi, F.K.; Alsarra, I.A. Nanoemulsions as Potential Vehicles for Transdermal and Dermal Delivery of Hydrophobic Compounds: An Overview. Expert Opin. Drug Deliv. 2012, 9, 953–974. [Google Scholar] [CrossRef]
- Zoumpanioti, M.; Stamatis, H.; Xenakis, A. Microemulsion-Based Organogels as Matrices for Lipase Immobilization. Biotechnol. Adv. 2010, 28, 395–406. [Google Scholar] [CrossRef]
- Esmaeili, F.; Baharifar, H.; Amani, A. Improved Anti-Inflammatory Activity and Minimum Systemic Absorption from Topical Gels of Ibuprofen Formulated by Micelle or Nanoemulsion. J. Pharm. Innov. 2022, 17, 1314–1321. [Google Scholar] [CrossRef]
- Lucca, L.G.; De Matos, S.P.; Weimer, P.; Teixeira, H.F.; Koester, L.S. Improved Skin Delivery and Validation of Novel Stability-Indicating HPLC Method for Ketoprofen Nanoemulsion. Arab. J. Chem. 2020, 13, 4505–4511. [Google Scholar] [CrossRef]
- Hamzah, M. Formulation and Evaluation of Flurbiprofen Nanogel. Res. J. Pharm. Tech. 2020, 13, 5183–5188. [Google Scholar]
- Akgun, A.; Alkin, M. Pain Management with Topical Ibuprofen in Partial-Thickness Burn Wounds and Effects on Wound Healing: A Prospective Randomized Clinical Study. Wound. Manag. Prev. 2023, 69, 32–48. [Google Scholar] [CrossRef] [PubMed]
- McCormack, K.; Kidd, B.L.; Morris, V. Assay of Topically Administered Ibuprofen Using a Model of Post-Injury Hypersensitivity. Eur. J. Clin. Pharmacol. 2000, 56, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Osterwalder, A.; Reiner, V.; Reiner, G.; Lualdi, P. Tissue Absorption and Distribution of Ketoprofen after Patch Application in Subjects Undergoing Knee Arthroscopy or Endoscopic Carpal Ligament Release. Arzneimittelforschung 2011, 52, 822–827. [Google Scholar] [CrossRef]
- Ozaki, M.; Minami, K.; Sata, T.; Shigematsu, A. Transdermal Ketoprofen Mitigates the Severity of Postoperative Sore Throat. Can. J. Anesth. 2001, 48, 1080–1083. [Google Scholar] [CrossRef]
- Takeshi, M.; Ebihara, T.; Maekawa, K.; Yamamoto, K.; Higo, N. Elimination of Ketoprofen from the Stratum Corneum after Topical Administration with Ketoprofen Formulations in Human Subjects. Int. J. Pharm. 2014, 465, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Serinken, M.; Eken, C.; Tünay, K.; Gölcük, Y. Topical Ketoprofen Versus Placebo in Children Presenting with Ankle Sprain to the Emergency Department: A Randomized Controlled Study. Pediatr. Emer. Care 2020, 36, e447–e450. [Google Scholar] [CrossRef]
- Kai, S.; Kondo, E.; Kawaguchi, Y.; Kitamura, N.; Yasuda, K. Flurbiprofen Concentration in Soft Tissues Is Higher after Topical Application than after Oral Administration. Br. J. Clin. Pharmacol. 2013, 75, 799–804. [Google Scholar] [CrossRef]
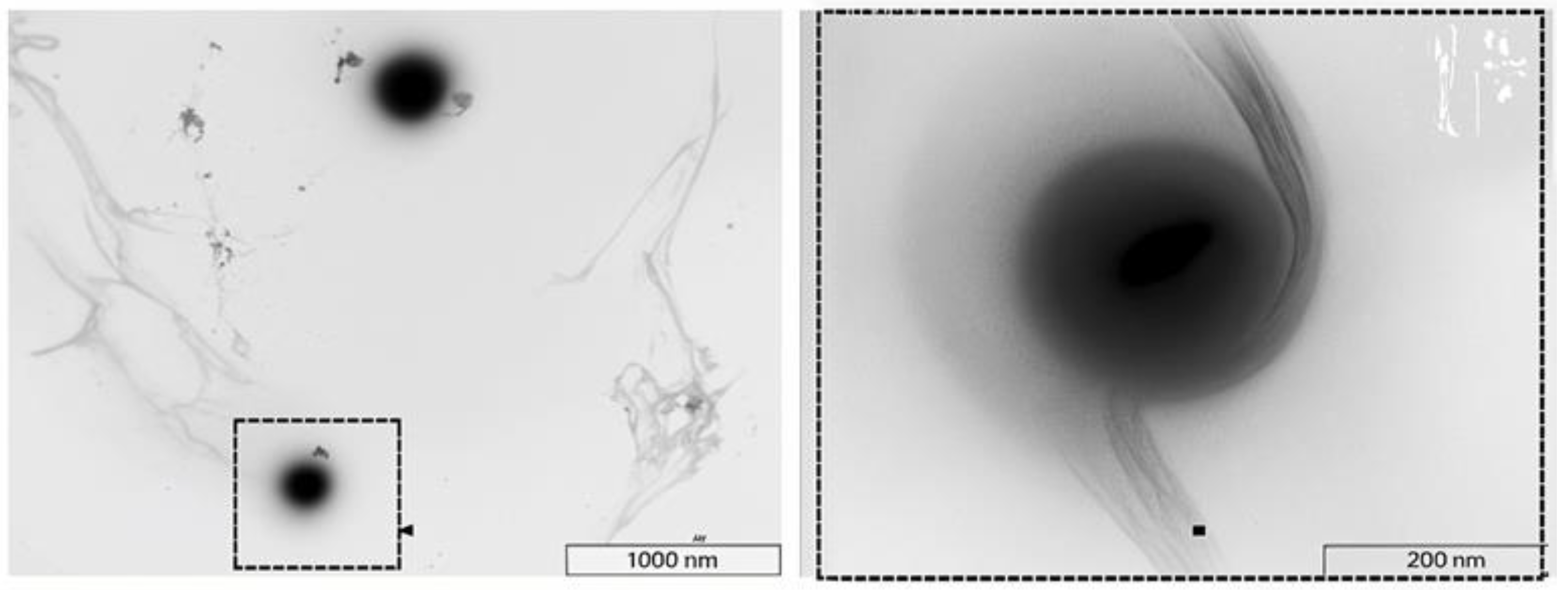
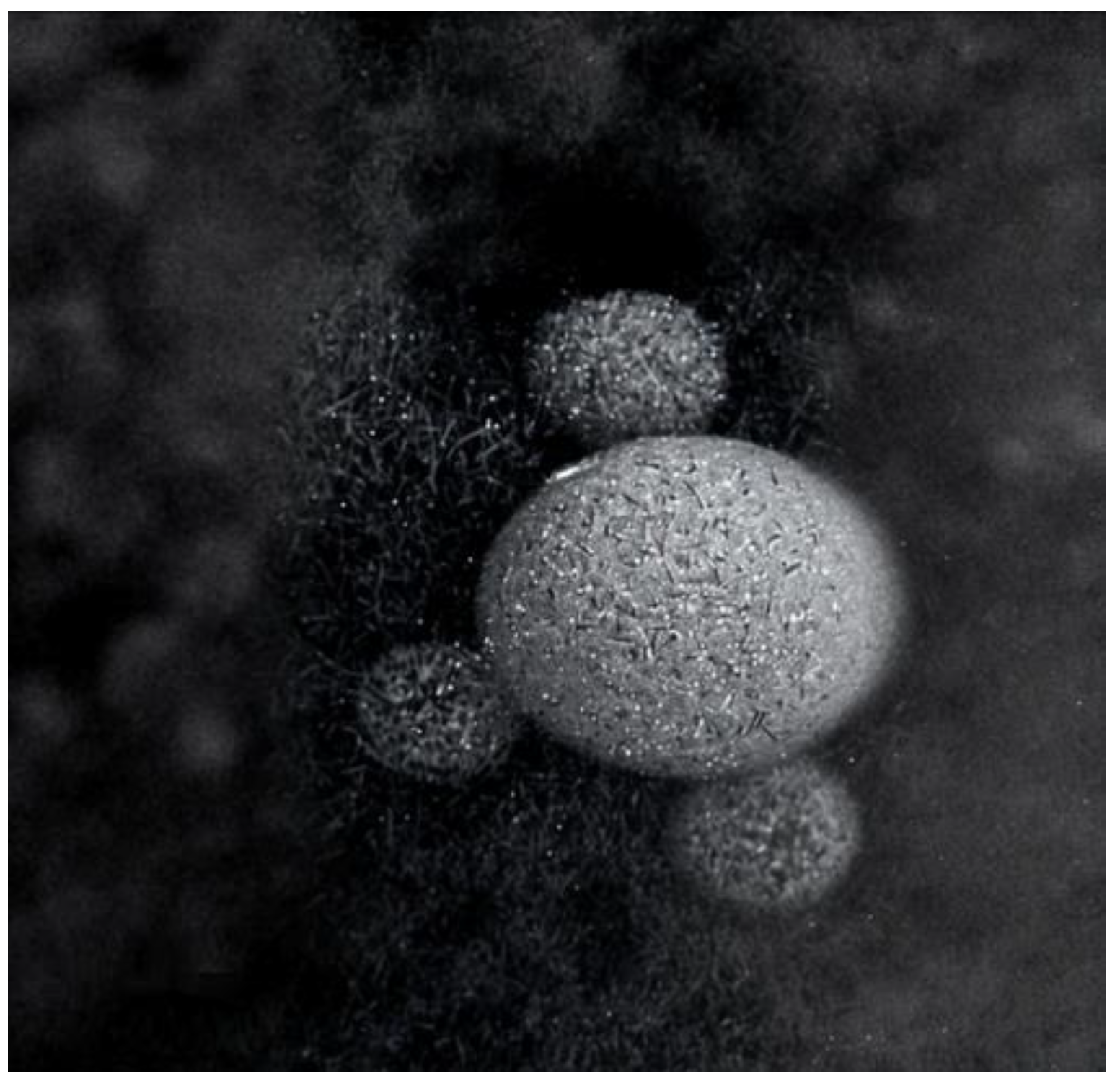
- Park, Y.; Shin, S.; Shukla, N.; Kim, K.; Park, M.-H. Effects of Nanobubbles in Dermal Delivery of Drugs and Cosmetics. Nanomaterials 2022, 12, 3286. [Google Scholar] [CrossRef]
- Mitropoulos, A.C.; Pappa, C.; Kosheleva, R.I.; Kyzas, G.Z. The Effect of Nanobubbles on Transdermal Applications. Nanomaterials 2023, 13, 2600. [Google Scholar] [CrossRef]
- Ebina, K.; Shi, K.; Hirao, M.; Hashimoto, J.; Kawato, Y.; Kaneshiro, S.; Morimoto, T.; Koizumi, K.; Yoshikawa, H. Oxygen and Air Nanobubble Water Solution Promote the Growth of Plants, Fishes, and Mice. PLoS ONE 2013, 8, e65339. [Google Scholar] [CrossRef]
- Meegoda, J.N.; Aluthgun Hewage, S.; Batagoda, J.H. Stability of Nanobubbles. Environ. Eng. Sci. 2018, 35, 1216–1227. [Google Scholar] [CrossRef]
- Nirmalkar, N.; Pacek, A.W.; Barigou, M. On the Existence and Stability of Bulk Nanobubbles. Langmuir 2018, 34, 10964–10973. [Google Scholar] [CrossRef] [PubMed]
- Koshoridze, S.I.; Levin, Y.K. Thermodynamic analysis of the stability of nanobubbles in water. Nano Sci. Technol. Int. J. 2019, 10, 21–27. [Google Scholar] [CrossRef]
- Li, M.; Ma, X.; Eisener, J.; Pfeiffer, P.; Ohl, C.-D.; Sun, C. How Bulk Nanobubbles Are Stable over a Wide Range of Temperatures. J. Colloid Interface Sci. 2021, 596, 184–198. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, L.; Gao, Y. Can Bulk Nanobubbles Be Stabilized by Electrostatic Interaction? Phys. Chem. Chem. Phys. 2021, 23, 16501–16505. [Google Scholar] [CrossRef]
- Ettoumi, F.; Zhang, R.; Belwal, T.; Javed, M.; Xu, Y.; Li, L.; Weide, L.; Luo, Z. Generation and Characterization of Nanobubbles in Ionic Liquid for a Green Extraction of Polyphenols from Carya Cathayensis Sarg. Food Chem. 2022, 369, 130932. [Google Scholar] [CrossRef] [PubMed]
- Chandan, R.; Banerjee, R. Pro-Apoptotic Liposomes-Nanobubble Conjugate Synergistic with Paclitaxel: A Platform for Ultrasound Responsive Image-Guided Drug Delivery. Sci. Rep. 2018, 8, 2624. [Google Scholar] [CrossRef]
- Prabhakar, A.; Banerjee, R. Nanobubble Liposome Complexes for Diagnostic Imaging and Ultrasound-Triggered Drug Delivery in Cancers: A Theranostic Approach. ACS Omega 2019, 4, 15567–15580. [Google Scholar] [CrossRef]






| NSAID | Study Focus | Outcome | Conclusion | Ref. |
|---|---|---|---|---|
| IBU | The efficacy of IBU-containing foam dressing in partial-thickness burns in N = 50 patients with superficial second-degree burn wounds was evaluated. |
|
| [146] |
| The quantification of the analgesic efficacy of NSAIDs in a model of clinical pain applied in N = 15 volunteers. |
|
| [147] | |
| KTP | The study of the diffusion into the target tissues of 100 mg KTP from a new topical patch in N = 10 patients. |
|
| [148] |
| To assess the possible postoperative sore throat attenuation by treatment with transdermal KTP on the anterior skin of the neck during operation in N = 63 patients |
|
| [149] | |
| To determine the drug concentrations and elimination rate of KTP in the SC following topical administration of two different formulations (tape or gel) in N = 10 human subjects. |
|
| [150] | |
| To evaluate the analgesic effect of 2.5% KTP gel administered in a 5 cm area on children between 7- and 18 years old presenting with ankle sprain versus a placebo gel. |
|
| [151] | |
| FB | To compare the tissue FB levels observed after skin application and oral intake in N = 16 patients. |
|
| [152] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drosopoulou, K.; Kosheleva, R.I.; Ofrydopoulou, A.; Tsoupras, A.; Mitropoulos, A. Topical and Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Inflammation and Pain: Current Trends and Future Directions in Delivery Systems. Processes 2025, 13, 907. https://doi.org/10.3390/pr13030907
Drosopoulou K, Kosheleva RI, Ofrydopoulou A, Tsoupras A, Mitropoulos A. Topical and Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Inflammation and Pain: Current Trends and Future Directions in Delivery Systems. Processes. 2025; 13(3):907. https://doi.org/10.3390/pr13030907
Chicago/Turabian StyleDrosopoulou, Kalliopi, Ramonna I. Kosheleva, Anna Ofrydopoulou, Alexandros Tsoupras, and Athanassios Mitropoulos. 2025. "Topical and Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Inflammation and Pain: Current Trends and Future Directions in Delivery Systems" Processes 13, no. 3: 907. https://doi.org/10.3390/pr13030907
APA StyleDrosopoulou, K., Kosheleva, R. I., Ofrydopoulou, A., Tsoupras, A., & Mitropoulos, A. (2025). Topical and Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) for Inflammation and Pain: Current Trends and Future Directions in Delivery Systems. Processes, 13(3), 907. https://doi.org/10.3390/pr13030907







